Abstract
An abscess in a gum pocket, resulting from bacterial infection, is a common source of chronic halitosis. Although antibiotics are generally prescribed for abscesses, they require multiple treatments with risks of creating resistant bacterial strains. Here we develop a novel vaccine using ultraviolet-inactivated Fusobacterium nucleatum (F. nucleatum), a representative oral bacterium for halitosis. A gum pocket model, established by continuous inoculation of F. nucleatum, was employed to validate the vaccine potency. Mice immunized with inactivated F. nucleatum effectively minimized the progression of abscesses, measured by swollen tissues of gum pockets. Most notably, the immunized mice were capable of eliciting neutralizing antibodies against the production of volatile sulfur compounds of F. nucleatum. The novel vaccine inducing protective immunity provides an alternative option to conventional antibiotic treatments for chronic halitosis associated with abscesses.
Keywords: Vaccine, F. nucleatum, Abscesses, Halitosis
1. Introduction
Although halitosis (bad breath) is a multi-factorial disease, more than 90% of cases of this disease originate from oral bacterial infections [1]. This disease afflicts up to 50% of the U.S. population. Foul-smelling breath results when bacteria metabolize to produce volatile sulfur compounds (VSCs) including hydrogen sulfide (H2S) and methyl mercaptan, the major substances associated with halitosis. VSCs are produced once sulfur amino acids (such as cysteine or methionine) are degraded by bacterial enzymes [2]. Plaque biofilms are considered to be the principle source generating VSCs [3]. Biofilms form when planktonic cells adhere to surfaces, proliferate and co-aggregate with other bacteria. Furthermore, biofilms comprise a slimy, glue-like substance that allows bacteria to anchor themselves onto the surface of various materials and tissues. During proliferation and co-aggregation, bacteria use amino acids as nutrients and convert them to VSCs. Moreover, gum disease, including abscesses at the base of a tooth, can be the causes of bad breath due to bacteria emitting hydrogen sulfur vapors [4]. The infection spreads to affect the entire periodontium and even lodges in blood vessels, causing cardiovascular disease when gum disease becomes more severe.
Fusobacterium nucleatum (F. nucleatum), a gram-negative anaerobe oral bacterium, has pathogenic potential and is implicated in periodontal diseases. It produces large amounts of VSCs [5,6] and is a representative for the development of halitosis [7]. Current literature shows that the mgl gene encodes an L-methionine-alpha-deamino-gamma-mercaptomethane-lyase (METase) that catalyzes the alpha, gamma-elimination of L-methionine to produce methyl mercaptan in F. nucleatum [7]. Furthermore, it has been demonstrated that F. nucleatum in oral cavity can aggregate with other bacteria to form plaque biofilms that cause bad breath [6,8]. At this time, available treatments for halitosis, including chemical antiseptics, are effective only while treatment is maintained. When treatments are discontinued, increased halitosis becomes clearly apparent. In addition, most of chemical antiseptics fail to cure chronic and severe halitosis. Systemic treatments involving multiple doses of antibiotics to cure infection-induced halitosis risk generating resistant strains and misbalancing the resident body flora [9]. The disease now has no appropriate therapeutic modalities that are long lasting, systemically effective and specifically suppress bacteria-induced pathogenesis. Vaccines targeting oral bacteria [such as Streptococcus mutans (S. mutans) for dental caries; Porphyromonas gingivalis (P. gingivalis) for periodontitis] are being developed [10,11] and may indirectly decrease oral malodors. However, these vaccines cannot combat the pathogenesis (e.g. co-aggregation/biofilms) of F. nucleatum. Based on above information, we propose that F. nucleatum produces VSCs during biofilm development in gum disease, such as abscesses, resulting in chronic halitosis. In the study, we develop a new vaccine by immunizing mice with inactivated F. nucleatum. The vaccine efficiently diminishes F. nucleatum-induced abscesses and elicits neutralizing antibodies against VSC production and biofilm formation.
2. Materials and methods
2.1. Bacterial strains and culture conditions
F. nucleatum (ATCC® 10953) was cultured in 4% (w/v) Trypticase Soy Broth (TSB) (Sigma–Aldrich, St. Louis, MO), and supplemented with 0.5% (w/v) yeast extract (DIFCO, Detroit, MI), 1.0% (v/v) hemin (Remel, Lenexa, KS) and 0.1% (v/v) vitamin K1 (Remel, Lenexa, KS). S. mutant (ATCC® 25175) was grown in 3% (w/v) Todd-Hewitt broth (THB) (Sigma–Aldrich, St. Louis, MO). Both bacteria were cultured under anaerobic conditions using Gas-Pak (BD, Sparks, MD) at 37 °C without shaking.
2.2. VSCs and biofilm detection
F. nucleatum [optical density at 600 nm is 0.9, 4 × 109 colony-forming unit (CFU) in 2 ml] was cultured on a 6-well nonpyrogenic polystyrene plate for 6, 12, 24 and 36 h. An oral hydrogen sulfide-producing organism plate (OHO-C, Anaerobe Systems, CA) containing lead acetate was used for detection of VSCs (mainly H2S). After excising the bottom of each well, attached bacteria on one side of each well were positioned on the surface of an OHO-C agar plate and immediately cultured at anaerobic atmosphere at 37 °C overnight. VSC production was visualized as brown/dark precipitates of lead sulfides on the surfaces of agar plates. For biofilm detection, excised wells on agar plates were removed, gently washed with phosphate-buffered saline (PBS) (pH 7.2), and stained with 0.4% (w/v) crystal violet for 1 min according to a protocol as described [12].
2.3. Establishment of a gum pocket mouse model
Female ICR (Institute of Cancer Research) mice (3–6 weeks old; Harlan, Indianapolis, IN) were used. For establishment of a gum pocket mouse model, an aliquot of 100 µl of live F. nucleatum (4 × 108 CFU) suspended in PBS was inoculated into a mouse oral cavity every day for 3 days in order to induce abscesses. An aliquot of 30 ul was injected into the gums of lower incisors with a 28-gauge needle. An aliquot of 30 ul was directly dropped into the oral cavity. The remaining 40 ul of aliquot was spread over the surface of the tongue. Inoculation with the same volume of PBS served as a negative control. For histological observation, the gum tissues with abscesses were cross-sectioned, stained with hematoxylin and eosin (H&E) (Sigma diagnostics, St Louis, MO), and viewed on a Zeiss Axioskop2 plus microscope (Carl Zeiss, Thornwood, NY).
2.4. Intranasal immunization with ultraviolet (UV)-inactivated F. nucleatum
F. nucleatum and S. mutans were suspended in PBS and inactivated by a UV crosslinker (Spectronics, Westbury, NY) at 7000 J/m2 for 30 min. Inactivation of F. nucleatum or S. mutans was demonstrated by the inability to form colonies on TSB or THB agar plates, respectively (Supplementary Fig. 1). Inactivated bacteria were harvested by centrifuging at 5000 × g for 5 min and re-suspended in PBS. The suspension of inactivated bacteria (1 × 108 CFU/25 µl) or PBS (25 µl) was then intranasally inoculated in ICR mice for nine weeks at a 3-week interval. The second and third inoculations were administered in the same manner as the first immunization.
2.5. Antibody detection by Western blotting
After centrifugation, bacterial pellets were suspended in a sample buffer [125 mM Tris-HCl buffer, pH 6.8, containing 4% (w/v) sodium dodecyl sulfate (SDS), 10% (w/v) glycerol, 5% (v/v) 2-mercaptoethanol and 0.002% (w/v) bromophenol blue] and boiled for 5 min. The protein concentration of the bacteria pellet was determined by Bradford assay (Bio-Rad, Hercules, CA). The sample (20 µg) was electrophoresed in a 10% (w/v) SDS-polyacrylamide gel (SDS-PAGE, Bio-Rad, Hercules, CA) and electrophoretically transferred onto polyvinyliden difluoride membranes (Millipore, Billerica, MA) for 2 h at a current of 75 V. The membranes were pre-incubated for 3 h at 4 °C in Tris-buffered saline [with 0.1% (v/v) Tween 20] containing 5% (w/v) skim milk, and then incubated at 4 °C overnight with serum (1:5000 dilution) obtained from mice immunized with inactivated bacteria for nine weeks. Bound antibodies (IgG) were detected with anti-mouse horseradish peroxidase (HRP)-conjugated IgG (1:5000 dilution, Promega, Madison, WI). The peroxidase activity was developed with a western lighting chemiluminescence kit (PerkinElmer, Boston, MA).
2.6. In vivo protective immunity
Immunized mice were inoculated with live F. nucleatum to induce gum swelling with abscesses as described in Section 2.3. The increased thickness (mm) of gum swelling was measured using a digital caliper (Traceable Digital Caliper, Fisher Scientific, Pittsburgh, PA). For the measurement of an increase in swelling, a transparent piece of parafilm was put on the top of a swollen site. The swollen area was marked on the transparent parafilm by drawing an area that covered the whole swollen site. The swollen area was calculated using ImageJ software, version 1.40 [National Institutes of Health (NIH), http://rsb.info.nih.gov/ij/] [13] and expressed as mm2. The volume of gum swelling in mm3 was calculated by the formula: volume = height × area. Experiments were performed in triplicate at four mice per group. To determine F. nucleatum number in the immunized mice, the bacteria-injected gum tissues were excised 6 h after the third day of F. nucleatum injections. The tissues were homogenized in 1 ml of sterile PBS for 1 min using a vibrating homogenizer (mini-beadbeater, Biospec Products, Bartlesville, OK) in the presence of 0.5 ml of 2.0 mm glass beads. CFUs of F. nucleatum within gums were enumerated by plating serial dilutions (1:10–1:105) of the homogenates on a TSB agar plate. To count colonies, the plate was anaerobically incubated for 24 h at 37 °C.
2.7. Neutralizing antibodies against VSC production
F. nucleatum (4 × 109 CFU) in the culture medium was pre-incubated with 2.5% (v/v) anti-F. nucleatum or anti-S. mutans serum at 37 °C for 2 h. The 2 h incubation did not significantly influence the growth of F. nucleatum (4 ± 0.12 and 4 ± 0.15 × 109 CFU for anti-F. nucleatum and anti-S. mutans serum, respectively). After removing unbound sera with centrifugation, F. nucleatum was cultured on a 6-well plate for 36 h under anaerobic conditions. Sera were obtained from mice immunized with UV-irradiated bacteria for three weeks. Complements in the sera were deactivated by heating at 56 °C for 30 min before incubation with bacteria. VSC production was detected by using OHO-C plates as described in Section 2.2. Experiments were performed three times with similar results. One representative is shown.
3. Results
3.1. Characterization of VSCs and biofilms of F. nucleatum
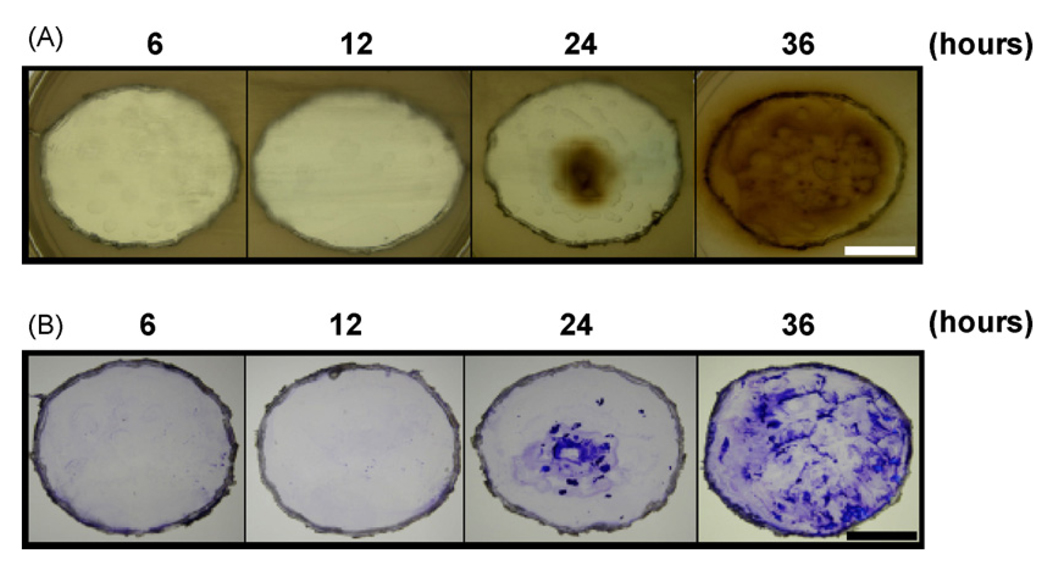
To examine the capability of F. nucleatum to produce VSCs, we cultured bacteria (4 × 109 CFU in 2 ml) on 6 well culture dishes for 6, 12, 24 and 36 h. Afterwards, each well containing attached bacteria was excised and then pasted onto an agar plate supplemented with glutathione and lead acetate. VSC production was visualized as brown/dark precipitates of lead sulfides on the surfaces of agar plates. F. nucleatum grown on dishes for 6 and 12 h did not produce detectable VSCs (Fig. 1A). VSCs were detectable at 24 h after bacterial culture and predominant in a 36 h culture (Fig. 1A). To investigate the association of VSC production with biofilms, each well, pasted on a lead acetate-containing agar, was removed and stained with 0.4% (w/v) crystal violet (Fig. 1B). Consistently, biofilms were clearly apparent in both 24 and 36 h cultures. These results suggest that (1) F. nucleatum is able to produce the VSCs and biofims and (2) an increase in biofilm formation leads to greater VSC production. Although various methods for VSC detection are available [14,15], we reveal for the first time to measure VSC production of F. nucleatum by using lead acetate-containing agars that can detect both VSCs and biofilms at the same bacterial culture. In addition, an optimal protocol by setting a 36 h culture for tremendous production of VSCs and biofilms was obtained.
Fig. 1.
Detection of VSC production and biofilm formation of F. nucleatum. F. nucleatum (4 × 109 CFU/well) was cultured on a 6-well nonpyrogenic polystyrene plate for 6, 12, 24 and 36 h. (A) After removing the media, each well containing attached bacteria was excised and pasted onto the top of OHO-C agar plate overnight under anaerobic conditions using Gas-Pak. (B) For detection of biofilm formation, excised wells were removed from OHO-C agar plates and stained with 0.4% (w/v) crystal violet for 1 min. The brown/dark precipitates of lead sulfides and purple crystal violet stain on the 24 and 36 h-cultured plates indicated the VSCs and biofilms, respectively. Bars = 1.5 cm.
3.2. Modeling a gum pocket with an abscess
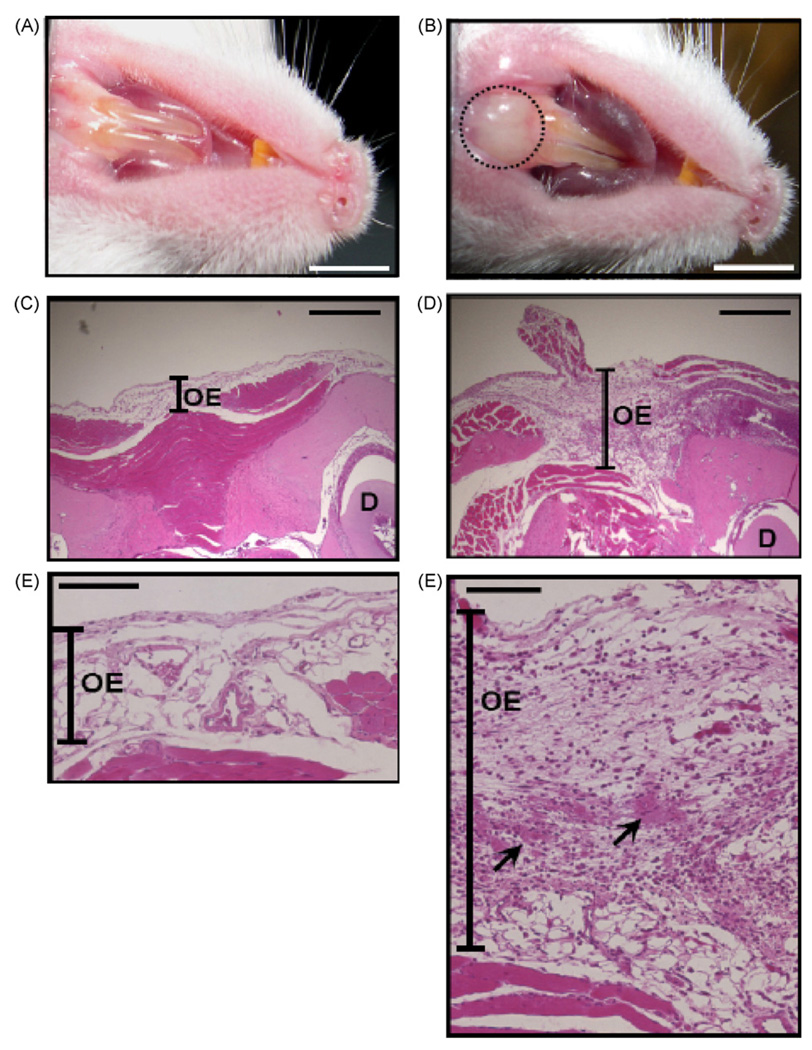
Currently, there are no appropriate mouse models for investigating chronic halitosis caused by F. nucleatum infection in gum pockets. To create gum pockets, ICR mice were orally inoculated with F. nucleatum that naturally does not colonize in mice. Live F. nucleatum (100 µl; 4 × 108 CFU) was injected into the gums of lower incisors and pipeted onto the tongue surfaces as well as epithelium in oral cavity daily to aggravate F. nucleatum infection. Inoculation with 100 µl of PBS served as a control. Three days after inoculation, swelling in gums of lower incisors was observed in the F. nucleatum-, but not in PBS-inoculated mice (Fig. 2A and B), suggesting the swelling was derived from F. nucleatum infection, not from the injection itself. Histological examination by H&E staining verified that gramulomatous inflammation occurred within swollen gum tissues (Fig. 2D and F) compared to PBS-inoculated mice (Fig. 2C and E). Since the mice were infected and inflamed gum tissues were generated, the model reiterates the formation of abscesses by F. nucleatum, and is used for evaluation of potency of vaccines targeting F. nucleatum.
Fig. 2.
An abscess induced by the injection of live F. nucleatum into the gums of lower incisors of ICR mice. One hundred micro liter of PBS (A, C and E) or F. nucleatum (4 × 108 CFU in PBS) (B, D and F) was inoculated into a mouse oral cavity for 3 days, as described in Section 2. An abscess (B, circle) was observed in the gums 3 days after injection with F. nucleatum, but not with PBS (A). H&E magnification 4× (C and D) and magnification 20× (E and F) indicated that gramulomatous inflammation (arrows) occurs in an abscess. D: dentin. OE: oral epithelium. Bars (A and B) = 2.2 mm. Bars (C and D) = 1 mm. Bars (E and F) = 0.5 mm.
3.3. Vaccines using inactivated F. nucleatum
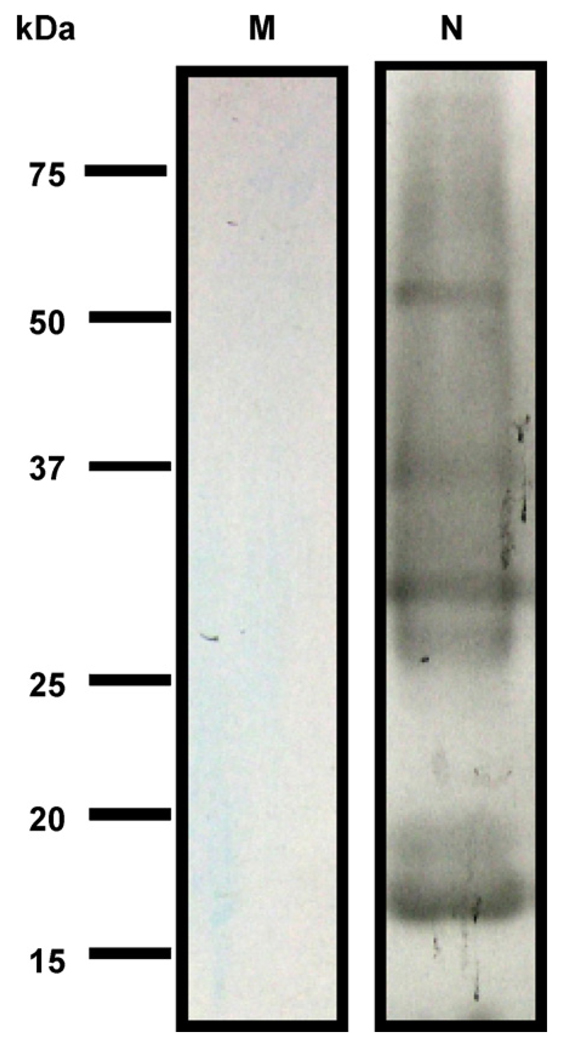
Killed microbes generated via UV have been reported to have the benefit of vaccines to induce an appropriate immune response without the issue of pathogen replication in the host [16]. The capability of replication of F. nucleatum and S. mutans was inactivated by UV irradiation (Supplementary Fig. 1). The inactivated bacteria were utilized for intranasal vaccination. To generate sufficient antibodies, ICR mice were vaccinated with UV-inactivated F. nucleatum or S. mutans for three times at a 3-week interval. No exogenous adjuvants were applied along with bacteria while vaccination occurred. Antibody (IgG) to F. nucleatum was detectable three weeks after vaccination (Fig. 3). Data from Western blot indicated that several F. nucleatum proteins with molecular weights ranging from 15 to 75 kDa were immunoreactive to antibodies elicited by inactivated F. nucleatum (Fig. 3, lane 2). None of the F. nucleatum proteins was recognized by antibodies generated by inactivated S. mutans (Fig. 3, lane 1). These results support the immunogenicity of inactivated F. nucleatum.
Fig. 3.
The production of antibody (IgG) to F. nucleatum. For immunization, cultured F. nucleatum and S. mutans were irradiated by UV at a total energy of 7000 J/m2. F. nucleatum lysate (20 µg) was separated by 10% SDS-PAGE and then subjected to Western blotting using sera (1:5000 dilution) obtained from irradiated F. nucleatum (N) and S. mutans (M)-immunized mice; immunoreactivity was developed using anti-mouse IgG conjugated with HRP complex and a Western lighting chemiluminescence kit. Molecular weights (kDa) are indicated.
3.4. In vivo protective immunity of inactivated F. nucleatum-based vaccines
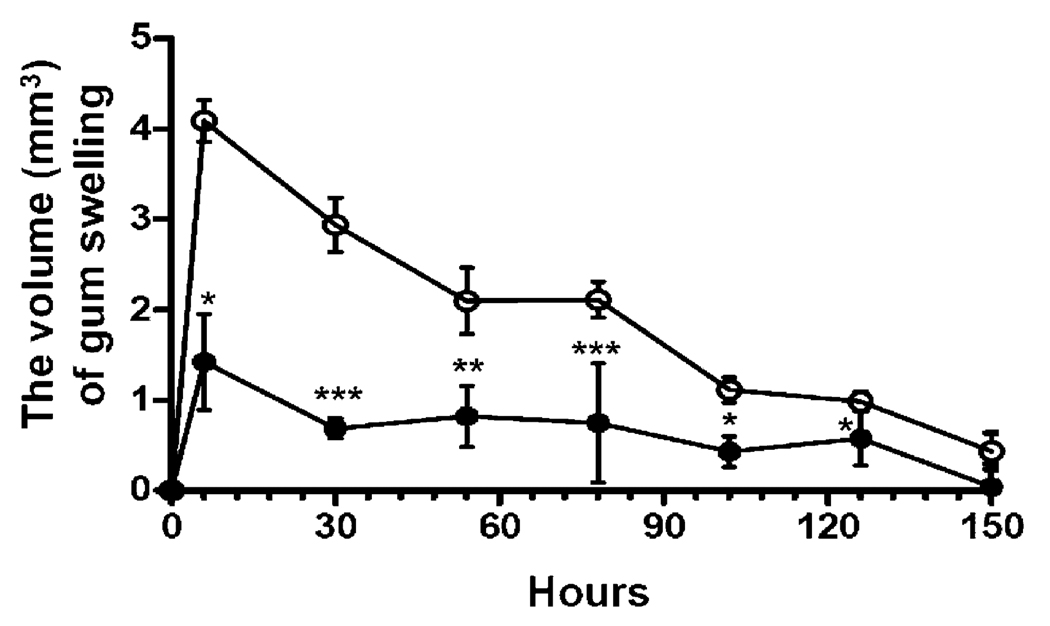
To construct infected gum pockets (Fig. 2), live F. nucleatum (4 × 108 CFU) was continuously inoculated into oral cavities of F. nucleatum-immunized mice for three days (Fig. 4). Inoculation of live F. nucleatum into S. mutans-immunized mice served as a control since antibodies to S. mutans did not cross-react F. nucleatum (Fig. 3, lane 1). To assess the protective immunity, F. nucleatum-induced gum swelling in the F. nucleatum- and S. mutans- immunized mice was recorded. A remarkable swelling (4.09 ± 0.23 mm3) was observed in the S. mutant-immunized mice at 6 h after final inoculation of live F. nucleatum (Fig. 4). The swelling (1.42 ± 0.53 mm3) was dramatically suppressed in the F. nucleatum-immunized mice. The volume of gum swelling in the S. mutans-immunized mice remained above 2.10 mm3 during prior 78 h records. In contrast, the swelling in the F. nucleatum-immunized mice stayed subtle (under 1.5 mm3) and completely subsided 150 h after final bacterial inoculation. The result strongly validates the in vivo protective immunity of an inactivated F. nucleatum-based vaccine in the prevention of abscess progression.
Fig. 4.
In vivo protective immunity. A gum pocket model with abscesses and swollen tissues was created, as described in Fig. 2A and Section 2. After inoculation with live F. nucleatum (4 × 108 CFU) for 3 days, the change in the volume of gum swelling in the F. nucleatum- (solid circles) and S. mutans- (open circles) immunized mice was recorded for 150 h. Bars represent mean ± SE (*p < 0.05, **p < 0.005, ***p < 0.0005 using Student’s t-test).
3.5. Neutralizing antibodies against VSC production and biofilm formation
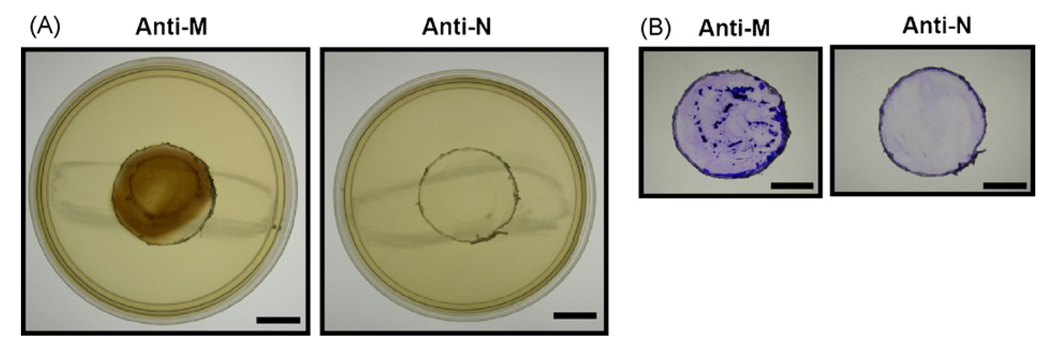
To verify if inactivated F. nucleatum-based vaccines elicit neutralizing antibodies against VSC production and biofilm formation, F. nucleatum (4 × 109 CFU) was incubated with serum obtained from the F. nucleatum- or S. mutans-immunized mice. VSCs (Fig. 5A) and biofilms (Fig. 5B) were detected on lead acetate-contained agar plates as described in Section 2. F. nucleatum retained its ability to generate VSCs and biofilms after incubation with serum from S. mutans-immunized mice. However, the ability was entirely suppressed by incubation with serum from F. nucleatum-immunized mice. The evidence implicates the competency of antibodies generated in F. nucleatum-immunized mice in the prevention of halitosis.
Fig. 5.
Inhibition of VSC production and biofilm formation by anti-F. nucleatum serum. F. nucleatum (4 × 109 CFU) was pre-incubated with 2.5% (v/v) anti-F. nucleatum (Anti-N) or anti-S. mutans (Anti-M) sera without activated complements for 2 h. Afterwards, serum-bound F. nucleatum was cultured on a 6-well for additional 36 h under anaerobic conditions. VSC production (A) and biofilm formation (B) were detected by lead acetate-containing agar plates and crystal violet staining, respectively, as described in Section 2. Bars = 1.5 cm.
4. Discussion
It is well known that the VSCs mainly govern halitosis [17]. VSCs in human oral cavities are produced via digestion of amino acids by bacterial enzymes such as L-cysteine desulfhydrase and METase [7]. However, for vaccine targeting, there are several reasons for not choosing molecules involved in the degradation of amino acids to VSCs. Firstly, VSCs are not the only source of bad breath. Secondly, various oral bacteria utilize different systems to degrade amino acids from diverse sources [18]. Furthermore, most amino acid catabolic enzymes are located within bacteria where antibodies cannot easily reach them. In contrast, a plaque biofilm, a key source of oral malodor, is a common feature for most of oral bacteria [19]. Thus, vaccines suppressing the formation of plaque biofilms may provide an effective modality to eliminate halitosis. More importantly, it has been shown that F. nucleatum serves as a central bridging organism in the architecture of the oral mixed-species biofilms [20]. Immunization targeting the central organism, F. nucleatum, may prevent the formation of mixed-species biofilms and eventually lead to the broad-spectrum elimination of VSC production from various oral species. After adherence of planktonic cells, F. nucleatum takes up nutrients including amino acids to enforce its multiplication and biofilm formation [21]. Although our results suggest that biofilm accumulation leads to greater VSC production (Fig. 1), VSCs were undetectable when F. nucleatum was cultured at the first 6 and 12 h. Although it is unclear if each individual bacterium within a biofilm increases the VSC production rate, it has been known that there was a positive correlection between the bacterial numbers and the VSC production during biofilm formation [22]. The dominance of non-adherent bacteria present at the first 12 h culture may explain the failure in the VSC production. It is also possible that VSC production of non-adherent bacteria at the first 12 h culture is too low to be detected.
The fact that F. nucleatum is not an indigenous bacterium in murine oral cavities has hindered the development of halitosis animal models for evaluation of vaccines targeting F. nucleatum-initiated oral malodor. Although germ-free mice have been used for studying oral infection [23], the immunity of the mice differs from that of humans. ICR outbred mice, with a diversity in gene expression between individuals, closely resemble the human population. Thus, ICR mice were chosen in the study. In humans, the pockets (empty space defined by the root of the tooth, the level of the bone and the top edge of the gum) appear. These pockets trap the bacteria and are the perfect incubators for bacteria to grow biofilms and produce VSCs. We imitated the infection in a gum pocket by inoculating live F. nucleatum into mouse oral cavities to induce abscesses. The gum pocket-created mice (Fig. 2) provide a valuable model for development of anti-halitosis drugs and vaccines in the future. Moreover, neutrophils play an important role in periodontal abscesses. These phagocytic cells are important, acting in conjunction with opsonin antibodies and complements by ingesting and killing the periodontal microflora before or during the early invasive process [24]. Although intranasal immunization of inactivated whole bacteria is associated with biohazard [25] and has a risk of causing neuronal damage [26], the use of inactivated F. nucleatum as nasal vaccines eliminates the time-consuming steps required for antigen purification. Furthermore, intranasal immunization circumvents the intrinsic problems associated with multiple needle injections. Thus, F. nucleatum-based nasal vaccines are beneficial for large-scale and rapid vaccine production. Our data indicates that the immunogenicity of UV-inactivated F. nucleatum could be elicited without adding exogenous adjuvants (Fig. 3). The gamma-irradiation may be an excellent alternative to UV for large-scale bacterial inactivation, since it can penetrate into the deeper layer of bacterial culture [27]. It has been documented that immunization with F. nucleatum did not induce the production of cross-reactive antibodies to other oral micro-organisms [28]. Our results showed that anti-S. mutans serum did not cross-react with F. nucleatum (Fig. 3 and Supplementary Fig. 2). Although which components in F. nucleatum specifically contribute to adjuvant effects has not yet been determined, it has been known that F. nucleatum extract immunologically modulates the humoral immunity [29] and polyclonal B-cell activation [30]. Several proteins with molecular weights ranging from 15 to 75 kDa were highly immunogenic when mice were immunized with F. nucleatum (Fig. 3). Previous findings demonstrated that outer membrane proteins of F. nucleatum with molecular weights at 70, 60, 55 and 40 kDa are major immunogens [31,32]. Identification of these immunogens by proteomic approaches may be critical for development of subunit vaccines against F. nucleatum. Although the antibodies to F. nucleatum were detectable in patients with periodontitis [33], it may be critical to determine if the titers of antibodies to F. nucleatum are proportionate to the severity of periodontitis or halitosis. At 6 h after final inoculation of live F. nucleatum (Fig. 4), the volume of gum swelling in the F. nucleatum-immunized mice was smaller than that in the S. mutant-immunized mice. However, there were no differences in the number of F. nucleatum colonized within the gums of mice immunized with F. nucleatum (9.3 ± 4.1 × 103 CFU) or S. mutant (8.0 ± 5.2 × 103 CFU), suggesting that the swelling subside by F. nucleatum immunization was not correlated to bacterial survival in gums. The data also indicated that F. nucleatum immunization did not enhance host defense systems to eradicate F. nucleatum, which may lower the risk of disturbing the balance of oral microflora. Recently, it has been found that H2S stimulated the activation of human U937 monocytes with the generation of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 [34]. Thus, it is worthwhile to investigate if F. nucleatum immunization decreases VSC production, which leads to a suppression of gum inflammation.
In clinics, increasing circulating antibiotics by intravenous or oral administration is a common treatment to eliminate bacteria-trapped gum pockets, since these bacteria cannot be easily removed by brushing. Vaccination is a means to boost the levels of circulating antibodies that have a chance to reach bacteria in gum pockets. Memory mucosal immune responses also play an imperative role in the prevention of oral infections [35]. In addition to IgG, secretory immunoglobulin A (S-IgA) in saliva was detectable in mice with UV-irradiated F. nucleatum (Supplementary Fig. 3). Although S-IgA in saliva may not obtain access to bacteria accumulated in gum pockets, it is worth investigating whether S-IgA can eliminate the halitosis generated from plaque biofilms on the surface of mouse incisors and/or oral epithelium. Immunization with UV-irradiated F. nucleatum dramatically decreased the F. nucleatum-induced gum swelling (Fig. 4). Although IgG in serum and S-IgA in saliva were measurable in F. nucleatum-immunized mice, determination of other IgG subclasses (such as IgG1 and IgG2a) [36] and cell-mediated immunity may increase understanding of the potency of inactivated F. nucleatum-based vaccines.
Dynamic detection of VSCs in oral cavities using a halitosis detector such as halitometer [RH-17 Halimeter, Interscan Corp., USA and Canada, 15] is a direct method to monitor VSC production. Although halitosis detectors have been used in humans, they are not sensitive enough to sense the change of VSCs in mouse oral cavities. Gas chromatography coupled with flame ionization detection is an alternative for analysis of VSCs. However, the main disadvantages of conventional gas chromatography were unable to quantify VSCs [37]. On the other hand, the use of lead acetate-contained agar plates allows us to compare the levels of VSCs and biofilms under various experimental conditions. Suppression of VSCs and biofilms by serum (Fig. 5) indicates that mice have produced neutralizing antibodies after immunization with inactivated F. nculeatum.
After F. nucleatum inoculation, S. mutant immunization exhibits greater gum swelling than F. nucleatum immunization (Fig. 4). To exclude the possibility that pre-immunization with S. mutans sensitizes the mice to F. nucleatum infection, the gum swelling in the F. nucleatum-immunized mice was compared to that in the PBS-administrated mice (Supplementary Fig. 4A). The gum swelling was substantially induced by F. nucleatum in the PBS-administrated, but not in the F. nucleatum-immunized mice. In addition, the F. nucleatum-immunized, but not the PBS-administrated mice, are able to elicit a neutralizing antibody against VSC production F. nucleatum (Supplementary Fig. 4B). Indeed, it has been reported that mice immunized with F. nucleatum modulated the host susceptibility to P. gingivalis [38]. Thus, concerning the vaccine safety, it is valuable to investigate if F. nucleatum immunization will alter the host sensitivities to other pathogens.
In conclusion, an inactivated F. nucleatum-based vaccine induces systemic immune responses to abrogate gum abscesses, VSC production and biofilm formation. Mounting evidence demonstrates that F. nucleatum can enter the bloodstream and cause endo-carditis [39], urinary tract infections [40] or preterm birth [41]. F. nucleatum has also been implicated in the pathogenesis of several diseases [42], including urinary tract infections, bacteremia, peri-carditis, otitis media, and disorders of the oral cavity, such as pulpal infections and alveolar bone abscesses. Thus, besides halitosis, the vaccines developed in this study may benefit patients with various F. nucleatum-associated diseases. Most importantly, our study provides an alternative strategy to conventional antibiotic therapy for treatment of chronic halitosis.
Acknowledgements
This work was supported by National Institutes of Health Grants (R01-AI067395-01, R21-R022754-01, R21-I58002-01, and 1R41AR056169-01). We thank Rachel Ross for critical reading of the manuscript.
Abbreviations
- ATCC
American Type Culture Collection
- CFU
Colony forming unit
- F. nucleatum
Fusobacterium nucleatum
- H&E
Hematoxylin & eosin
- HRP
Horseradish peroxidase
- H2S
Hydrogen sulfide
- ICR
Institute of Cancer Research
- IgG
Immunoglobulin G
- IL
Interleukin
- METase
L-methionine-alpha-deamino-gamma-mercaptomethane-lyase
- NIH
National Institutes of Health
- OHO-C
Oral hydrogen sulfide-producing organism
- PBS
Phosphate-buffered saline
- P. gingivalis
Porphyromonas gingivalis
- SDS
Sodium dodecyl sulfate
- SDS-PAGE
SDS-polyacrylamide gel electrophoresis
- S-IgA
Secretory immunoglobulin A
- S. mutans
Streptococcus mutans
- THB
Todd-Hewitt broth
- TNF-α
Tumor necrosis factor-α
- TSB
Trypticase soy broth
- VSCs
Volatile sulfur compounds
- UV
Ultraviolet
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2008.12.058.
References
- 1.Feller L, Blignaut E. Halitosis: a review. SADJ. 2005;60(1):17–19. [PubMed] [Google Scholar]
- 2.Wåler SM. On the transformation of sulfur-containing amino acids and peptides to volatile sulfur compounds (VSC) in the human mouth. Eur J Oral Sci. 1997;105(5 Pt 2):534–537. doi: 10.1111/j.1600-0722.1997.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 3.Yaegaki K, Sanada K. Volatile sulphur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodont Res. 1992;27(4 Pt 1):233–238. doi: 10.1111/j.1600-0765.1992.tb01673.x. [DOI] [PubMed] [Google Scholar]
- 4.Scully C, Rosenberg M. Halitosis. Dent Update. 2003;30(4):205–210. doi: 10.12968/denu.2003.30.4.205. [DOI] [PubMed] [Google Scholar]
- 5.Kang MS, Kim BG, Chung J, Lee HC, Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J Clin Periodontol. 2006;33(3):226–232. doi: 10.1111/j.1600-051X.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 6.Hinode D, Fukui M, Yokoyama N, Yokoyama M, Yoshioka M, Nakamura R. Relationship between tongue coating and secretory-immunoglobulin A level in saliva obtained from patients complaining of oral malodor. J Clin Periodontol. 2003;30(12):1017–1023. doi: 10.1046/j.0303-6979.2003.00407.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura M, Nakano Y, Fukamachi H, Koga T. 3-Chloro-DL-alanine resistance by L-methionine-alpha-deamino-gamma-mercaptomethane-lyase activity. FEBS Lett. 2002;523(1–3):119–122. doi: 10.1016/s0014-5793(02)02958-7. [DOI] [PubMed] [Google Scholar]
- 8.Bachrach G, Ianculovici C, Naor R, Weiss EI. Fluorescence based measurements of Fusobacterium nucleatum coaggregation and of fusobacterial attachment to mammalian cells. FEMS Microbiol Lett. 2005;248(2):235–240. doi: 10.1016/j.femsle.2005.05.055. [DOI] [PubMed] [Google Scholar]
- 9.Liébana J, Castillo AM, Alvarez M. Periodontal diseases: microbiological considerations. Med Oral Patol Oral Cir Bucal. 2004;9 Suppl. 82–91:75–82. [PubMed] [Google Scholar]
- 10.Smith DJ. Dental caries vaccines: prospects and concerns. Crit Rev Oral Biol Med. 2002;13(4):335–349. doi: 10.1177/154411130201300404. [DOI] [PubMed] [Google Scholar]
- 11.Sharma DC, Prasad SB, Karthikeyan BV. Vaccination against periodontitis: the saga continues. Expert Rev Vaccines. 2007;6(4):579–590. doi: 10.1586/14760584.6.4.579. [DOI] [PubMed] [Google Scholar]
- 12.Saito Y, Fujii R, Nakagawa KI, Kuramitsu HK, Okuda K, Ishihara K. Stimulation of Fusobacterium nucleatum biofilm formation by Porphyromonas gingivalis. Oral Microbiol Immunol. 2008;23(1):1–6. doi: 10.1111/j.1399-302X.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 13.The National Institue of Health. ImageJ. http://rsb.info.nih.gov/ij/
- 14.Garcia AH, Vazquez EP, Campos JM. Minibioreactor-gas collector for determining bacteria-produced hydrogen sulphide. Electron J Biotechnol. 2003;6(3):223–232. [Google Scholar]
- 15.Delanghe G, Bollen C, Desloovere C. Halitosis–foetor ex ore. Laryngo-Rhino-Otologie. 1999;78(9):521–524. doi: 10.1055/s-2007-996920. [DOI] [PubMed] [Google Scholar]
- 16.Brockstedt DG, Bahjat KS, Giedlin MA, Liu W, et al. Killed but metabolically active microbes: a new vaccine paradigm for eliciting effector T-cell responses and protective immunity. Nat Med. 2005;11(8):853–860. doi: 10.1038/nm1276. [DOI] [PubMed] [Google Scholar]
- 17.Tonzetich J. Direct gas chromatographic analysis of sulphur compounds in mouth air in man. Arch Oral Biol. 1971;16(6):587–597. doi: 10.1016/0003-9969(71)90062-8. [DOI] [PubMed] [Google Scholar]
- 18.González AB, Rodríguez-Inda G, Morlet-Andreu R. Microbiological aspects of halitosis and its treatment. Rev ADM. 1983;40(6):178–180. [PubMed] [Google Scholar]
- 19.Washio J, Sato T, Koseki T, Takahashi N. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J Med Microbiol. 2005;54(Pt 9):889–895. doi: 10.1099/jmm.0.46118-0. [DOI] [PubMed] [Google Scholar]
- 20.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66(3):486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robrish SA, Oliver C, Thompson J. Amino acid-dependent transport of sugars by Fusobacterium nucleatum ATCC 10953. J Bacteriol. 1987;169(9):3891–3897. doi: 10.1128/jb.169.9.3891-3897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratten J, Pasu M, Jackson G, Flanagan A, Wilson M. Modelling oral malodour in a longitudinal study. Arch Oral Biol. 2003;48(11):737–743. doi: 10.1016/s0003-9969(03)00154-7. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro Sobrinho AP, de Melo Maltos SM, Farias LM, de Carvalho MA, Nicoli JR, de Uzeda M, et al. Cytokine production in response to endodontic infection in germ-free mice. Oral Microbiol Immunol. 2002;17(6):344–353. doi: 10.1034/j.1399-302x.2002.170603.x. [DOI] [PubMed] [Google Scholar]
- 24.Tufano MA, Ianniello R, Sanges MR, Rossano F. Neutrophil function in rapidly progressive and adult periodontitis. Eur J Epidemiol. 1992;8(1):67–73. doi: 10.1007/BF03334974. [DOI] [PubMed] [Google Scholar]
- 25.Jertborn M, Ahren C, Holmgren J, Svennerholm AM. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine. 1998;16(2–3):255–260. doi: 10.1016/s0264-410x(97)00169-2. [DOI] [PubMed] [Google Scholar]
- 26.Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine. 2002;20(19–20):2431–2438. doi: 10.1016/s0264-410x(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Shi Z, Kong Fk, Jex E, Huang Z, Watt JM, et al. Topical application of Escherichia coli-vectored vaccine as a simple method for eliciting protective immunity. Infect Immun. 2006;74(6):3607–3617. doi: 10.1128/IAI.01836-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gemmell E, Bird PS, Carter CL, Drysdale KE, Seymour GJ. Effect of Fusobacterium nucleatum on the T and B cell responses to Porphyromonas gingivalis in a mouse model. Clin Exp Immunol. 2002;128(2):238–244. doi: 10.1046/j.1365-2249.2002.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshie H, Mitsuma T, Kozima K, Hara K. Effects of a Fusobacterium nucleatum extract on immunoregulation in mice. J Dent Res. 1985;64(3):431–436. doi: 10.1177/00220345850640030701. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, Inui M, Kawagoe K, Nakata H, Aono M. Adjuvant effects of oral microorganisms on the immunological responses. Arch Oral Biol. 1974;19(9):761–766. doi: 10.1016/0003-9969(74)90163-0. [DOI] [PubMed] [Google Scholar]
- 31.Mallison SM, 3rd, Smith JP, Schenkein HA, Tew JG. Accumulation of plasma cells in inflamed sites: effects of antigen, nonspecific microbial activators, and chronic inflammation. Infect Immun. 1991;59(11):4019–4025. doi: 10.1128/iai.59.11.4019-4025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakken V, Aarø S, Hofstad T, Vasstrand EN. Outer membrane proteins as major antigens of Fusobacterium nucleatum. FEMS Microbiol Immunol. 1989;1(8–9):473–483. doi: 10.1111/j.1574-6968.1989.tb02438.x. [DOI] [PubMed] [Google Scholar]
- 33.Naito Y, Okuda K, Takazoe I. Immunoglobulin. G response to subgingival gram-negative bacteria in human subjects. Infect Immun. 1984;45(1):47–51. doi: 10.1128/iai.45.1.47-51.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol. 2007;81(5):1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 35.Koga T, Oho T, Shimazaki Y, Nakano Y. Immunization against dental caries Vaccine. 2002;20(16):2027–2044. doi: 10.1016/s0264-410x(02)00047-6. [DOI] [PubMed] [Google Scholar]
- 36.Gemmell E, Bird PS, Ford PJ, Ashman RB, Gosling P, Hu Y, et al. Modulation of the antibody response by Porphyromonas gingivalis and Fusobacterium nucleatum in a mouse model. Oral Microbiol Immunol. 2004;19(4):247–251. doi: 10.1111/j.1399-302X.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 37.Lässer R, Grünhagen S, Kawamura Y. Use of micro gas chromatography in the fuel cycle of fusion reactors. Fusion Eng Des. 2003;69(1–4):813–817. [Google Scholar]
- 38.Choi J, Borrello MA, Smith E, Cutler CW, Sojar H, Zauderer M. Prior exposure of mice to Fusobacterium nucleatum modulates host response to Porphyromonas gingivalis. Oral Microbiol Immunol. 2001;16(6):338–344. doi: 10.1034/j.1399-302x.2001.160604.x. [DOI] [PubMed] [Google Scholar]
- 39.Elkaïm R, Dahan M, Kocgozlu L, Werner S, Kanter D, Kretz JG, et al. Prevalence of periodontal pathogens in subgingival lesions, atherosclerotic plaques and healthy blood vessels: a preliminary study. J Periodontal Res. 2008;43(2):224–231. doi: 10.1111/j.1600-0765.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 40.Shenker BJ, Datar S. Fusobacterium nucleatum inhibits human T-cell activation by arresting cells in the mid-G1 phase of the cell cycle. Infect Immun. 1995;63(12):4830–4836. doi: 10.1128/iai.63.12.4830-4836.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72(4):2272–2279. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts GL. Fusobacterial infections: an underestimated threat. Br J Biomed Sci. 2002;57(2):156–162. [PubMed] [Google Scholar]