SUMMARY
Defective transepithelial electrolyte transport is thought to initiate cystic fibrosis (CF) lung disease. Yet, how loss of CFTR affects electrolyte transport remains uncertain. CFTR−/− pigs spontaneously develop lung disease resembling human CF. At birth, their airways exhibit a bacterial host defense defect, but are not inflamed. Therefore, we studied ion transport in newborn nasal and tracheal/bronchial epithelia in tissue, cultures, and in vivo. CFTR−/− epithelia showed markedly reduced Cl− and HCO3− transport. However, in contrast to a widely held view, lack of CFTR did not increase transepithelial Na+ or liquid absorption or reduce periciliary liquid depth. Like human CF, CFTR−/− pigs showed increased amiloride-sensitive voltage and current, but lack of apical Cl− conductance caused the change, not increased Na+ transport. These results indicate that CFTR provides the predominant transcellular pathway for Cl− and HCO3− in porcine airway epithelia, and reduced anion permeability may initiate CF airway disease.
INTRODUCTION
Loss of cystic fibrosis transmembrane conductance regulator (CFTR) function causes CF (Davis, 2006; Quinton, 1999; Rowe et al., 2005; Welsh et al., 2001). Disease manifestations appear in many organs, but most morbidity and mortality currently arise from airway disease, where inflammation and infection destroy the lung. Understanding the pathogenesis of lung disease has been difficult, and there are many theories to explain how deficient CFTR function causes airway disease (Boucher, 2007; Davis, 2006; Quinton, 1999; Rowe et al., 2005; Verkman et al., 2003; Welsh et al., 2001; Wine, 1999). One factor impeding progress in identifying the events that initiate airway disease has been lack of an animal model that replicates features of the disease; mice with mutated CFTR genes do not develop gastrointestinal or lung disease typical of human CF (Grubb and Boucher, 1999). Therefore, we recently developed CFTR−/− pigs (hereafter referred to as CF pigs) (Rogers et al., 2008b). At birth, they manifest features typically observed in patients with CF, including pancreatic destruction, meconium ileus, early focal biliary cirrhosis, and microgallbladder (Meyerholz et al., 2010b). Within a few months of birth, CF pigs spontaneously develop lung disease with the hallmark features of CF including inflammation, infection, mucus accumulation, tissue remodeling, and airway obstruction (Stoltz et al., 2010).
Finding that CF pigs develop airway disease like that in humans provided an opportunity to explore very early events in the disease. We previously showed that within hours of birth, CF pigs have a reduced ability to eliminate bacteria that either enter the lung spontaneously or that are introduced experimentally (Stoltz et al., 2010). However, like newborn human babies with CF, CF pigs lack airway inflammation at birth. Those data indicate that impaired bacterial elimination is the pathogenic event that begins a cascade of inflammation, remodeling and pathology in CF lungs. Thus, these newborn animals provide an ideal model in which to evaluate ion transport processes because they possess the host defense defect, but they do not yet exhibit inflammation, tissue remodeling or other features of progressive CF. Hence, electrolyte transport defects can be attributed to loss of CFTR rather than to secondary manifestations of the disease.
Abnormal electrolyte transport across airway epithelia has frequently been hypothesized to cause the initial CF host defense defect (Boucher, 2007; Davis, 2006; Quinton, 1999; Rowe et al., 2005; Verkman et al., 2003; Welsh et al., 2001; Wine, 1999). In CF epithelia, loss of CFTR decreases airway Cl− and HCO3− transport. This result is consistent with the anion channel activity of CFTR (Sheppard and Welsh, 1999). Some have also concluded that CFTR negatively regulates epithelial Na+ channels (ENaC); hence CFTR mutations are proposed to eliminate that ENaC inhibition, increase Na+ permeability, and cause Na+ hyperabsorption, which is widely viewed as the initial event in CF lung disease pathogenesis (Boucher, 2007).
To understand how CF affects airway epithelial ion transport, we asked if loss of CFTR would disrupt transepithelial Cl−, HCO3−, and Na+ transport in CF pigs. We studied newborn animals to identify defects prior to the onset of inflammation. Knowledge of the extent to which these processes are disrupted is key to understanding CF airway disease and is important for developing mechanism-based treatments and preventions.
RESULTS
CF pig airways lack cAMP-stimulated Cl− and HCO3− transport
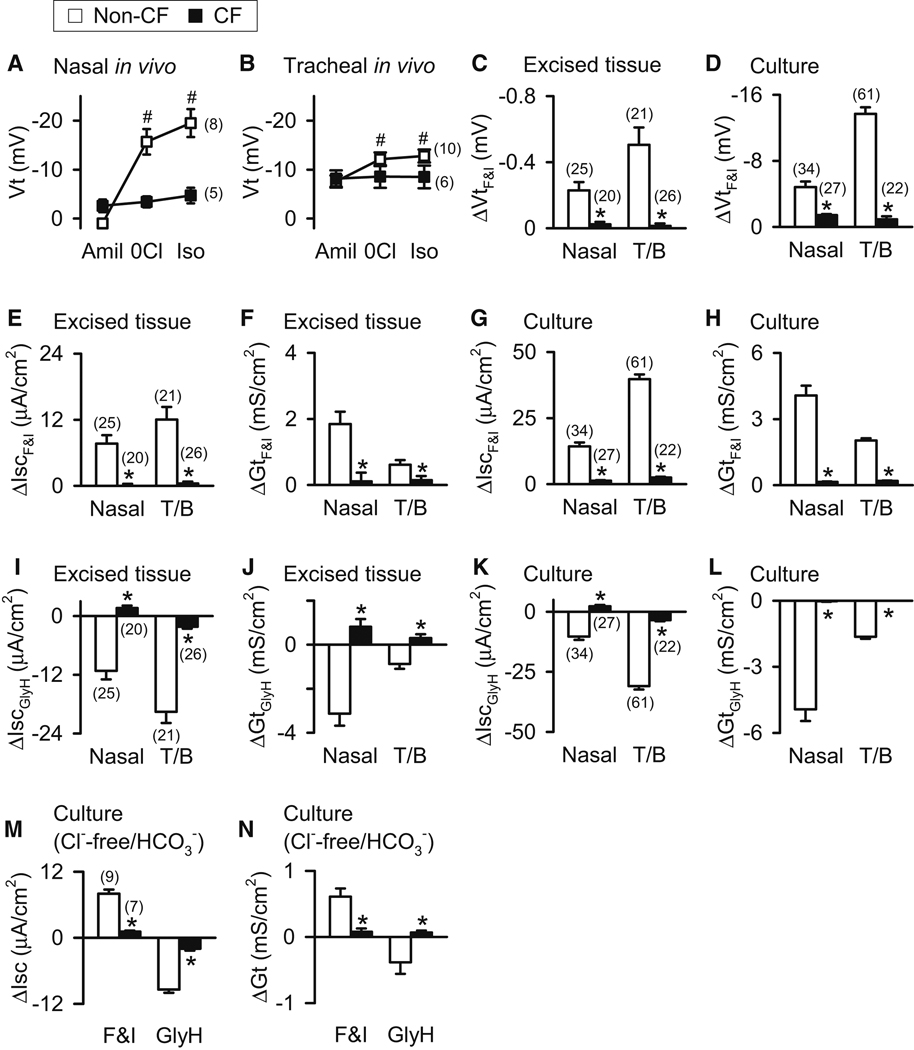
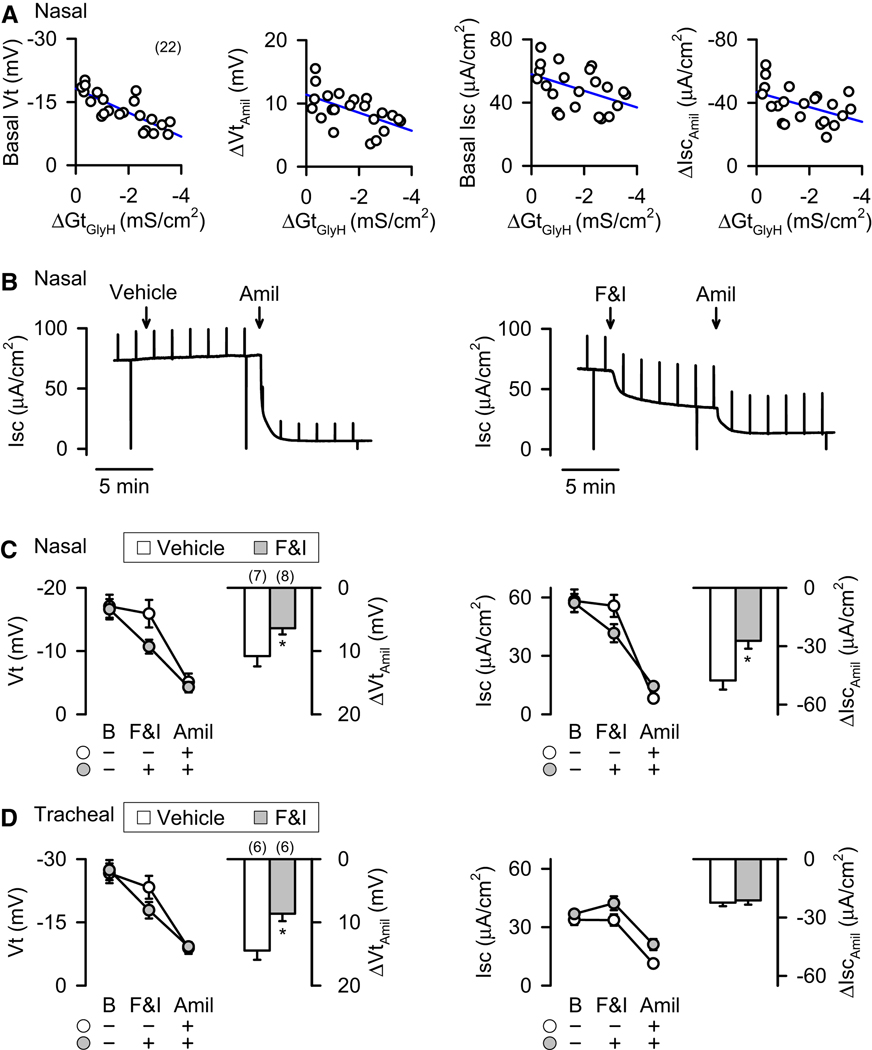
We measured the nasal and tracheal transepithelial voltage (Vt) in vivo in newborn pigs. Perfusion of the apical surface of epithelia with a Cl−-free solution and isoproterenol (to increase cellular cAMP levels) hyperpolarized Vt in non-CF pigs (Fig. 1A,1B) (Rogers et al., 2008b). In contrast, Vt failed to hyperpolarize in CF pigs. These data suggest a lack of cAMP-stimulated Cl− permeability in CF.
Figure 1. Loss of CFTR decreases anion transport in CF airway epithelia.
Data are means ± SE from newborn CFTR+/+ (open symbols and bars) and CFTR−/− (closed symbols and bars) pigs. Amiloride (100 µM) was present on the apical surface in all cases. Numbers in parentheses indicate n, * indicates P<0.05 between CF and non-CF, and T/B indicates tracheal/bronchial. A,B. Vt measured in vivo in nasal and tracheal epithelia in the presence of amiloride (100 µM), during perfusion with a Cl−-free solution (0Cl) containing amiloride, and during perfusion with a Cl−-free solution containing isoproterenol (10 µM) and amiloride. Nasal epithelia include data from 4 non-CF and 4 CF pigs that were previously reported (Rogers et al., 2008b). # indicates P<0.05 compared to initial value. C–H. Change in Vt, Isc, and Gt induced by adding 10 µM forskolin and 100 µM IBMX (ΔVtF&I, ΔIscF&I, and ΔGtF&I) to excised and cultured nasal and tracheal/bronchial epithelia. I–L. Change in Isc (ΔIscGlyH) and Gt (ΔGtGlyH) following addition of GlyH-101 (100 µM) to excised and cultured nasal and tracheal/bronchial epithelia. M–N. CFTR-mediated HCO3− transport in cultured tracheal epithelia. Solution was Cl−-free and contained 25 mM HCO3−. Data are ΔIsc and ΔGt following addition of forskolin and IBMX and GlyH-101. See also Fig. S1.
When non-CF nasal, tracheal, and bronchial epithelia were excised or cultured as differentiated airway epithelia and studied in Ussing chambers, adding forskolin and isobutylmethylxanthine (IBMX) to elevate cellular cAMP levels increased absolute values of Vt (Fig. 1C,1D), short-circuit current (Isc) (Fig. 1E,1G), and transepithelial electrical conductance (Gt) (Fig. 1F,1H). Adding GlyH-101, which inhibits CFTR (Fig. S1) (Muanprasat et al., 2004), had the opposite effects (Fig. 1I–1L). In contrast, CF epithelia failed to respond to either forskolin and IBMX or GlyH-101 (Fig. 1C–1L). CFTR has a significant HCO3− conductance, and human non-CF airway epithelia transport HCO3− (Poulsen et al., 1994; Smith and Welsh, 1992). When we studied non-CF tracheal epithelia in Cl−-free bathing solution containing 25 mM HCO3−, forskolin and IBMX stimulated and then GlyH-101 inhibited Isc and Gt (Fig. 1M–1N), revealing electrically conductive HCO3− transport. CF epithelia lacked these responses.
These data indicate that porcine CF airway epithelia extending from nose to bronchi lack cAMP-stimulated Cl− and HCO3− permeability. Our findings agree with studies of human airway epithelia, which have consistently demonstrated a loss of Cl− and HCO3− permeability in CF airway epithelia (Knowles et al., 1983; Smith and Welsh, 1992; Standaert et al., 2004; Widdicombe et al., 1985). Moreover, our results indicate that in wild-type porcine airway epithelia, CFTR provides an important transepithelial pathway for Cl− and HCO3−.
Vt is abnormal in CF nasal, but not tracheal epithelia in vivo
The first indication of abnormal electrolyte transport in CF airways was the finding that nasal Vt was more electrically negative in CF than non-CF subjects and that amiloride produced a greater reduction in Vt (ΔVtamiloride) in CF (Knowles et al., 1981). Those and additional observations led the authors to conclude that CF epithelia have increased Na+ absorption that depletes periciliary liquid, which in turn impairs mucociliary clearance and initiates lung disease (Boucher, 2007; Donaldson and Boucher, 2007).
There is evidence that changes in Na+ transport can affect the lung. For example, transgenic mice overexpressing the β subunit of the epithelial Na+ channel (βENaC) had lung disease that shared some features with CF (Mall et al., 2004). Mutations have also been reported in human ENaC genes, and they may contribute to lung disease with some CF-like features. However, the ENaC mutations are associated with both decreases and increases in ENaC activity (Azad et al., 2009; Baker et al., 1998; Huber et al., 2010; Kerem et al., 1999; Schaedel et al., 1999; Sheridan et al., 2005). Thus, while alterations in Na+ permeability can contribute to lung disease, those results do not indicate whether Na+ absorption is increased, reduced, or unchanged in CF.
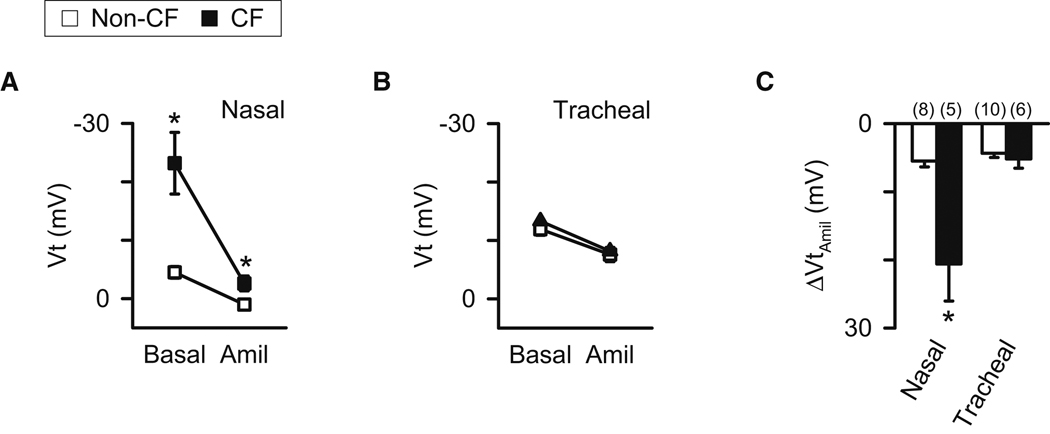
Therefore, we measured Vt and the response to amiloride in vivo in newborn pigs. In the nose, Vt and ΔVtamiloride were greater in CF than non-CF pigs (Fig. 2A,2C) (Rogers et al., 2008b). Remarkably, this was not the case in tracheal epithelia; Vt and ΔVtamiloride were similar in non-CF and CF pigs (Fig. 2B,2C).
Figure 2. Vt in vivo is abnormal in CF nasal epithelia, but not tracheal epithelia.
Data are means ± SE from CFTR+/+ (open symbols and bars) and CFTR−/− (closed symbols and bars) pigs. A,B. Effects of amiloride (100 µM) on nasal and tracheal Vt in vivo. C. Amiloride-sensitive change in Vt (ΔVtamiloride) in vivo. * indicates P<0.05 compared to non-CF.
Earlier studies showed that Vt and ΔVtamiloride are more negative in nasal and tracheal epithelia of CF patients than in non-CF controls (Davies et al., 2005; Knowles et al., 1981; Standaert et al., 2004). Our data in porcine nasal epithleia parallel those results. However, interestingly, when measurements were made in main bronchi and distal airways of children, Vt values were similar in CF and non-CF (Davies et al., 2005). Those results are like the data in porcine trachea. It seems that airway region and age, and perhaps inflammation and infection influence the activity of epithelial ion channels and thereby whether a Vt difference exists between CF and non-CF epithelia. It will also be important to study electrolyte transport in vivo, in excised tissue, and in cultures from older CF pigs as the disease progresses.
Absorptive Na+ fluxes are not increased in porcine CF airway epithelia
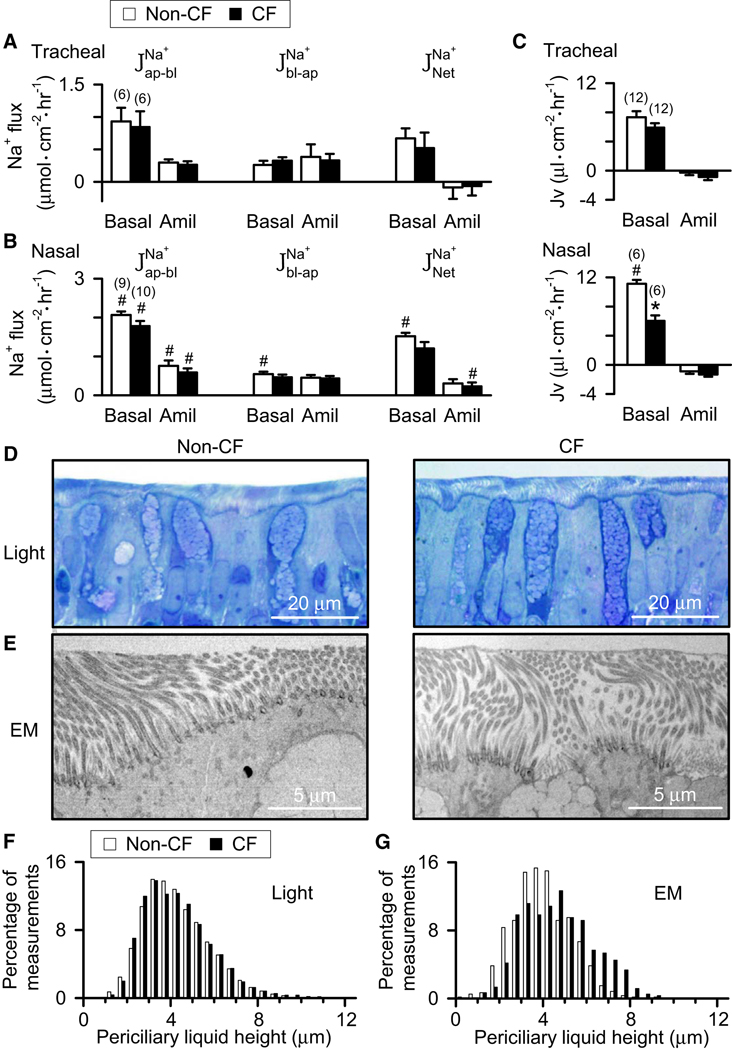
The difference in Vt between CF and non-CF nasal epithelia could relate to differences in Na+ transport. Therefore, we directly examined Na+ transport by measuring transepithelial 22Na+ fluxes. We studied primary cultures of differentiated airway epithelia and used open-circuit conditions to mimic the in vivo situation. There were three main observations. First, in tracheal epithelia, unidirectional and net Na+ fluxes did not differ between CF and non-CF epithelia (Fig. 3A, Table S2). Adding amiloride decreased the unidirectional absorptive (apical to basolateral) and net Na+ fluxes, indicating the importance of apical Na+ channels for Na+ absorption. Second, in nasal epithelia, Na+ fluxes and the response to amiloride were also similar in CF and non-CF epithelia (Fig. 3B). Third, nasal epithelia had greater unidirectional absorptive fluxes and net Na+ absorption than tracheal epithelia (compare Fig. 3A and 3B).
Figure 3. Porcine CF epithelia do not hyperabsorb Na+.
Data are means ± SE from newborn CFTR+/+ (open bars) and CFTR−/− (closed bars) pigs. Numbers in parentheses indicate n; * indicates P<0.05. A,B. Isotopic 22Na+ unidirectional and net Na+ flux rates under basal conditions and after adding 100 µM amiloride apically. indicates Na+ flux from the apical (ap) to the basolateral (bl) surface, indicates flux in the opposite direction, and indicates net flux. # indicates that value in nasal epithelia differed from that in tracheal epithelia, p < 0.05. C. Rate of liquid absorption (Jv) in differentiated primary cultures of nasal and tracheal epithelia under basal conditions and after adding 100 µM amiloride apically. # indicates that value in nasal epithelia differed from that in tracheal epithelia, p < 0.05. In panels A–C, the basal electrophysiological properties of matched epithelia are shown in Table S2. D. Examples of light microscopic images of tracheal epithelia. Note heterogeneity in depth of periciliary liquid in both non-CF and CF epithelia. E. Examples of transmission electron microscopic images of tracheal epithelia showing periciliary liquid. F. Histogram of periciliary liquid depth over tracheal epithelia obtained from light microscopic images. N=9,140 non-CF and 6,260 CF measurements. Multiple images were made from each of 4 segments of trachea obtained from 8 non-CF and 5 CF animals. See Methods for additional details. Three observers unaware of genotype then measured periciliary liquid depth using a standardized protocol. A linear mixed model and maximum likelihood estimation were used to calculate means and standard errors allowing for variability between observers, measurements, images, segments and pigs. There was no significant difference between periciliary liquid depth in non-CF and CF epithelia (p=0.96), and the difference was 0.71 µm or less with 95% confidence. The residual variability on the same image had an estimated standard deviation of 1.29 µm and between images was 0.60 µm. For comparison, non-CF trachea was air-exposed and showed a reduced height of periciliary liquid (2.81 µm). G. Histogram of periciliary liquid depth measured from transmission electron microscopic images. N=600 measurements for each genotype and 5 animals per genotype. There was no significant difference in periciliary liquid depth between non-CF and CF, p=0.12. For comparison the standard deviations of measurements on an image and between images were both 0.95 µm.
Like our data in pigs, in human nasal epithelia, 22Na+ fluxes measured under open-circuit conditions revealed no difference between non-CF and CF (Willumsen and Boucher, 1991a, b). Under short-circuited conditions, which differ from the in vivo situation, net 22Na+ fluxes were reported to be either the same or increased in CF vs. non-CF (Boucher et al., 1986; Knowles et al., 1983).
Liquid absorption is not increased in porcine CF epithelia
We also measured rates of transepithelial liquid absorption, which is driven by Na+ absorption. Liquid absorption rates were greater in nasal than tracheal epithelia, consistent with the 22Na+ fluxes (Fig. 3C). However, CF epithelia did not absorb liquid at a greater rate than non-CF epithelia. In fact, in nasal epithelia, the absorption rate was less in CF than non-CF epithelia.
In studies of cultured human airway epithelia, the initial rate of liquid absorption has been reported to be increased (Matsui et al., 1998), similar (Van Goor et al., 2009), or reduced (Zabner et al., 1998) in CF compared to non-CF. The reason for the differences is uncertain, but might relate to variations in basal CFTR activity (Zabner et al., 1998).
The depth of periciliary liquid is not altered by lack of CFTR
Transepithelial ion and H2O movement contribute to the depth of liquid covering the airway surface. Twenty-four hr after adding liquid to the apical surface of cultured epithelia, the depth of periciliary liquid has been reported to be less in CF compared to non-CF epithelia (Matsui et al., 1998; Van Goor et al., 2009). A study that obtained bronchoscopic biopsies from patients with CF reported that although not statistically significant, there was a trend toward reduced periciliary liquid height in CF (Griesenbach et al., 2010). However, the authors noted that inflammation (most patients were experiencing a respiratory exacerbation) and the methods used (periciliary liquid height could not be measured in half the patients or over the majority of cells) limit the interpretation.
To test the hypothesis that loss of CFTR alters periciliary liquid depth in the absence of infection, inflammation, and tissue remodeling, we studied pigs 6–12 hr after birth. In <1 min following euthanasia, we removed and placed tracheal segments in a non-aqueous fixative containing osmium tetroxide to rapidly preserve the morphology of the airway surface (Matsui et al., 1998; Satir, 1963; Sims and Horne, 1997). The depth of periciliary liquid showed substantial variability in both non-CF and CF epithelia, with areas of deeper liquid and outstretched cilia and shallower areas with cilia that appeared bent over (Fig. 3D). Therefore, we examined multiple portions of trachea, prepared multiple sections from each portion, and made many measurements from each section. Observers unaware of genotype measured periciliary liquid depth. A histogram of periciliary liquid depth is shown in Figure 3F; the mean depths of non-CF (4.5±0.3 µm, n=8 pigs) and CF (4.4±0.2 µm, n=5 pigs) periciliary liquid did not differ statistically. In addition, we prepared thin sections from the same blocks and examined them with transmission electron microscopy. The transmission electron microscopic images provided a smaller area for observation than light microscopic images and the number of samples was lower. These images also revealed both erect and bent cilia and heterogeneity in the depth of periciliary liquid covering airways of both genotypes (Fig. 3E). The periciliary liquid depth was not statistically different between non-CF (4.0±0.3 µm, n=5 pigs) and CF (4.7±0.3 µm, n=5 pigs) epithelia (Fig. 3G).
Compared to earlier studies, our measurements of periciliary liquid depth have the advantages that the epithelia were in vivo rather than cultured, they were immediately prepared without other manipulations, the epithelia did not demonstrate inflammation from chronic infection, and the experiments were performed at a time point when bacterial eradication was impaired. Our data also agree with an earlier study of maximal cilia length in formalin fixed/paraffin embedded newborn porcine airway epithelia, which showed no difference between CF and non-CF (Meyerholz et al., 2010a). Potential differences with a study of bronchoscopic biopsies in patients with acute and chronic disease (Griesenbach et al., 2010) raise interesting questions of whether inflammation with its associated effects on surface epithelium and submucosal glands might change ion transport or periciliary liquid height. Although our data show no difference in periciliary liquid depth between CF and non-CF newborn pigs, it is possible that with time and progression of disease, the depth of periciliary liquid might differ between the genotypes. In addition, although we measured periciliary liquid depth in trachea because of the speed with which we could remove and prepare the tissue, it will also be important to study its depth in distal airways.
All these measurements indicated that Na+ absorption by CF tracheal/bronchial epithelia did not exceed that in non-CF. Strikingly, this was also true in nasal epithelia. So why in nasal epithelia are Vt and ΔVtamiloride increased in CF? To answer this question, we first studied cultured and excised epithelia and examined electrophysiological properties (Vt, Isc, and Gt) that are influenced by apical Na+ conductance. Those results, considered together with an equivalent circuit model of the epithelium suggested an explanation for why electrophysiological properties differ between CF and non-CF nasal epithelia even though Na+ absorption is not increased. We then tested predictions of that analysis.
Vt, Isc, Gt and the response to amiloride under basal conditions in nasal epithelia
Transepithelial voltage
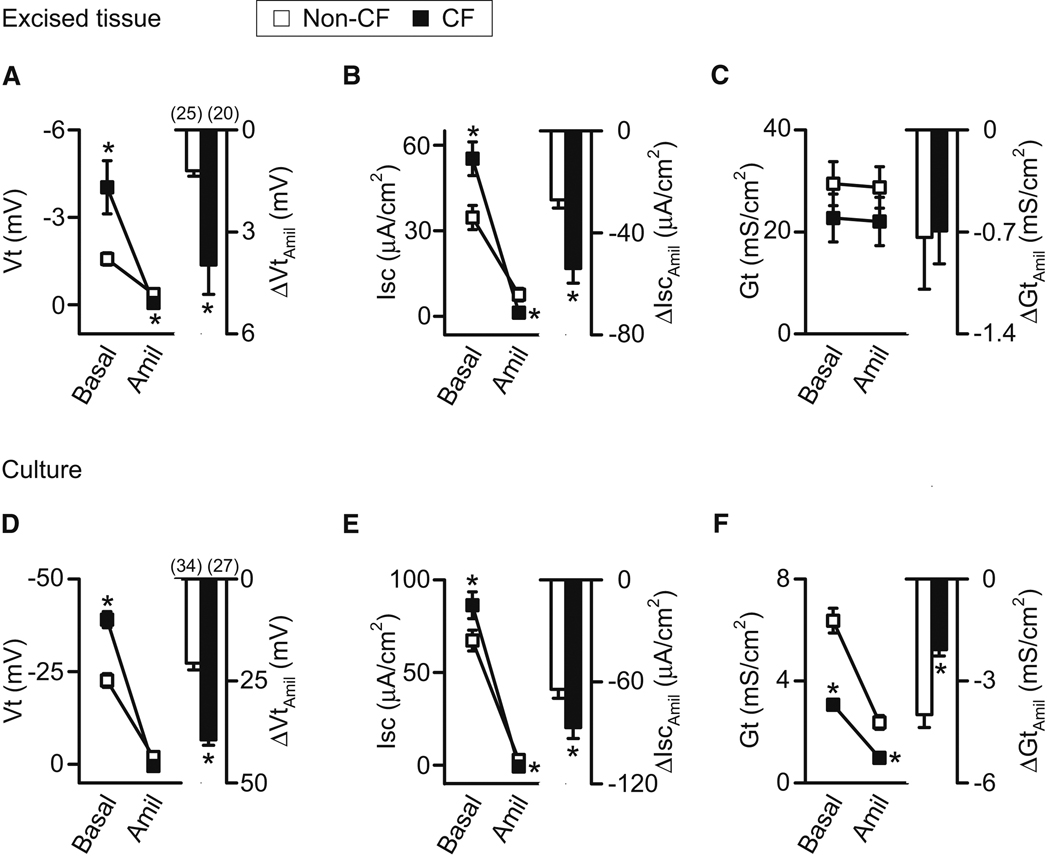
In nasal epithelia, basal Vt was greater in CF than non-CF, both in excised and cultured epithelia (Fig. 4A,4D). ΔVtamiloride was also greater in CF than non-CF nasal epithelia. Absolute values of Vt were less in excised than in vivo and cultured epithelia, because of damage caused by clamping epithelia in Ussing chambers, i.e., “edge damage” (Helman and Miller, 1973). Excised and cultured tracheal/bronchial epithelia showed smaller or no differences between CF and non-CF (Fig. S2A,S2D).
Figure 4. Amiloride alters electrical properties in non-CF and CF nasal epithelia.
Data are means ± SE from CFTR+/+ (open symbols and bars) and CFTR−/− (closed symbols and bars) pigs. Numbers in parentheses indicate n, and * indicates P<0.05. A–F. Effects of adding amiloride (100 µM) to the apical solution on Vt, Isc and Gt of freshly excised (A–C) and differentiated primary cultures (D–F) of nasal epithelia. ΔVtamil, ΔIscamil, and ΔGtamil indicate changes induced by amiloride. See also Fig. S2.
Thus, like in vivo measurements, airway location influenced whether Vt differed between CF and non-CF epithelia. This similarity suggests that cultured and excised epithelia reflect in vivo transport. However, Vt does not measure rates of ion transport.
Short-circuit current
In nasal epithelia, basal Isc and the amiloride-induced reduction in Isc (ΔIscamiloride) were greater in CF than non-CF (Fig. 4B,4E). This was the case for both excised and cultured epithelia. In tracheal/bronchial epithelia, basal Isc did not significantly differ between CF and non-CF; ΔIscamiloride was greater in excised but not cultured CF epithelia (Fig. S2B,S2E).
These results largely parallel the Vt measurements, indicating a strong effect of airway region on these electrical measurements. Studies of excised human epithelia reported that CF nasal epithelia had either higher or the same Isc values as non-CF epithelia (Boucher et al., 1986; Knowles et al., 1983; Mall et al., 1998).
Transepithelial conductance
In excised nasal epithelia, there was little difference between CF and non-CF basal Gt, perhaps because the large Gt values associated with edge damage obscured small differences (Fig. 4C). However in cultured epithelia, basal Gt was greater in non-CF epithelia (Fig. 4F); this can be explained by the presence of CFTR anion channels. Most importantly for assessing Na+ permeability, the amiloride-induced decrease in Gt (ΔGtamiloride) in CF did not exceed that in non-CF epithelia (Fig. 4C,4F). Likewise, ΔGtamiloride of CF tracheal/bronchial epithelia did not exceed that in non-CF (Fig. S2C,S2F).
Electrical conductance is directly related to the ion permeability of channels, and ΔGtamiloride is directly influenced by the Na+ conductance. Gt is also a more direct function of permeability than Vt or Isc, both of which are much more strongly determined by ion concentration gradients and membrane voltages. If CF epithelia had a greater apical Na+ conductance than non-CF epithelia, and other conductances (except for the CFTR Cl− conductance) were equal, then ΔGtamiloride should have been greater in CF. That was not the case (Fig. 4C,4F). Indeed, even if apical Na+ conductance were equal in CF and non-CF epithelia, then ΔGtamiloride should have been greater in CF than non-CF epithelia (Note S1). Thus, finding that ΔGtamiloride was not greater in CF epithelia suggests that Na+ conductance might be less in CF than non-CF epithelia.
The lack of a greater ΔGtamiloride in CF than non-CF nasal epithelia is consistent with the lack of greater Na+ absorption measured with Na+ fluxes and volume absorption. However, basal Vt, ΔVtamiloride, basal Isc, and ΔIscamiloride were greater in CF than non-CF nasal epithelia. Because those differences are commonly interpreted to demonstrate that CF epithelia have an increased Na+ permeability and hyperabsorb Na+ (Boucher, 2007; Boucher et al., 1988; Donaldson and Boucher, 2007; Knowles et al., 1981), it was important to understand what causes the CF/non-CF difference in electrical properties in nasal epithelia.
Equivalent electrical circuit analyses indicate that apical Cl− conductance can alter Vt, Isc, and the response to amiloride without a change in Na+ permeability
Could loss of CFTR increase Vt, ΔVtamiloride, Isc, and ΔIscamiloride without increasing apical Na+ permeability? A simple explanation for how this could occur arises from the fact that placing a second ion channel (i.e., a CFTR anion conductance) in the apical membrane in parallel with a Na+ conductance changes apical membrane voltage in three ways. First, it introduces another electromotive force generated by transmembrane ion concentration gradients. Second, it introduces a conductance that can shunt the voltage generated by other apical membrane channels, transporters, and pumps. Third, it alters the effect on apical voltage of current that is generated at the basolateral membrane. The resulting changes in apical voltage (as well as basolateral voltage) can then alter transepithelial Vt and Isc.
Horisberger (Horisberger, 2003) developed an equivalent electrical circuit model to simulate the effect of an apical membrane Cl− conductance (CFTR) on electrical properties that are influenced by ENaC-mediated Na+ conductance. He showed that activating CFTR reduced ΔVtamiloride and ΔIscamiloride even when Na+ conductance was held constant. He concluded that a decrease in ΔIscamiloride or ΔVtamiloride upon CFTR activation could not be interpreted to indicate a regulatory interaction between CFTR and ENaC. Another mathematical model also showed that increasing apical Cl− permeability could reduce Na+ transport under short-circuited conditions even though apical Na+ permeability remained unchanged (Duszyk and French, 1991). Thus, increasing apical Cl− conductance can reduce Vt and Isc without a change in Na+ conductance. Conversely, eliminating an apical Cl− conductance, as in CF, can increase Vt, ΔVtamiloride, Isc and ΔIscamiloride even without changing Na+ conductance. Of course, in these models, changes in electrophysiological properties will depend on the absolute values of the Cl− and Na+ conductances and electromotive forces relative to that of all the other channels and transporters.
CFTR-mediated Cl− conductance is greater in nasal vs. tracheal/bronchial epithelia
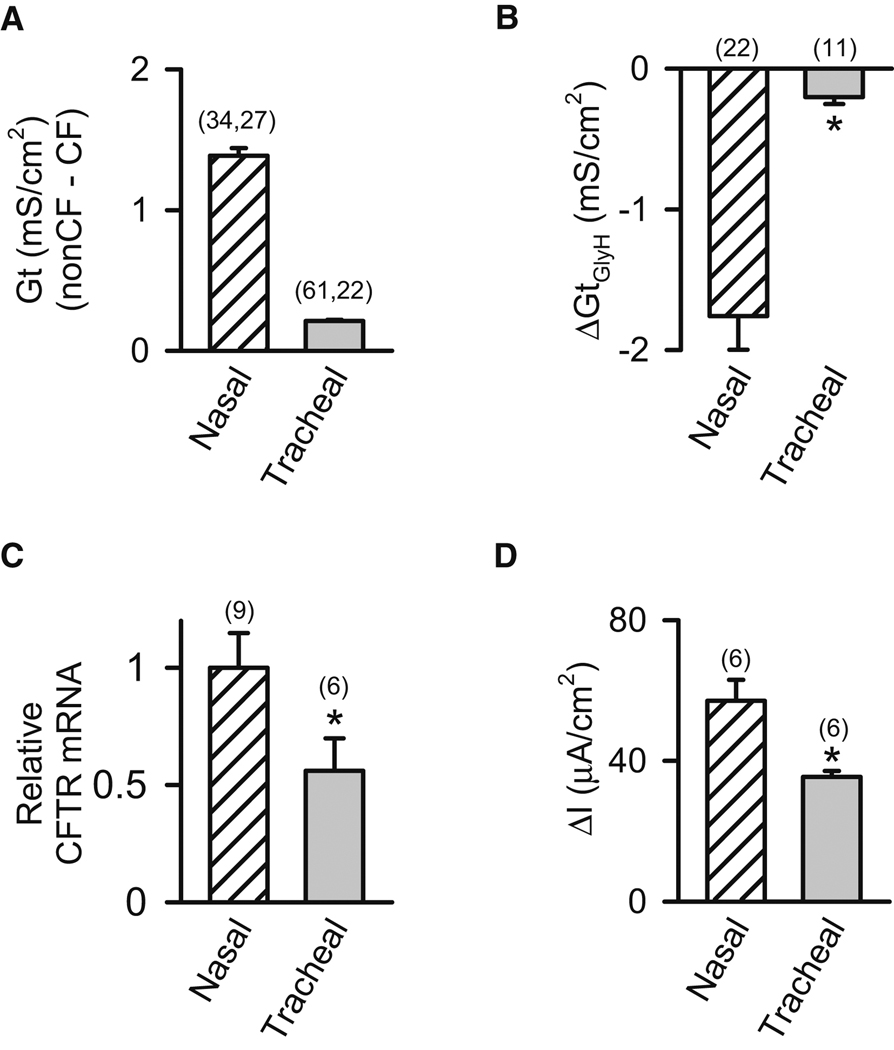
Based on the equivalent circuit analysis, we reasoned that if nasal epithelia had a greater basal Cl− conductance than tracheal/bronchial epithelia, it might explain the CF/non-CF difference in Vt, ΔVtamiloride, Isc and ΔIscamiloride even though nasal epithelia did not hyperabsorb Na+. To further test this possibility, we added amiloride to eliminate the Na+ conductance and then compared Gt in non-CF and CF epithelia. The difference between non-CF and CF Gt was much greater in nasal than tracheal epithelia (Fig. 5A), indicating that nasal epithelia have a greater Cl− conductance under basal conditions. As another test, we added amiloride to inhibit Na+ channels and DIDS to inhibit other Cl− channels, and we then examined the response to GlyH-101 (Fig. 5B). GlyH-101 reduced Gt more in nasal than tracheal epithelia, indicating that nasal epithelia have a greater CFTR Cl− conductance under basal conditions. In addition, quantitative RT-PCR (q-RT-PCR) revealed relatively more CFTR transcripts in cultured nasal than tracheal epithelia (Fig. 5C).
Figure 5. Non-CF nasal epithelia have a larger Cl− conductance than tracheal/bronchial epithelia.
Data are means ± SE from nasal (cross-hatched bars) and tracheal/bronchial (shaded bars) epithelia. Amiloride (100 µM) was present on the apical surface in panels A, B, and D. Numbers in parentheses indicate n, and * indicates P<0.05. A. Difference between Gt in cultured non-CF and CF epithelia. B. Change in Gt (ΔGtGlyH) following addition of 100 µM GlyH-101 to cultured non-CF epithelia. C. Relative CFTR mRNA by q-RT-PCR in primary cultures of non-CF epithelia. D. Apical Cl− currents measured in nasal and tracheal epithelia from non-CF cultured epithelia. Apical solution was Cl−-free with 100 µM amiloride, 100 µM DIDS, 10 µM forskolin, and 100 µM IBMX, and basolateral solution contained 139.8 mM Cl−. Data are current following permeabilization of basolateral membrane with nystatin (0.36 mg.ml−1). See also Fig. S3.
The data suggested that apical Cl− conductance under stimulated conditions was also greater in nasal than tracheal/bronchial epithelia. First, forskolin and IBMX increased Gt approximately twice as much in nasal as in tracheal/bronchial epithelia from normal pigs (Fig. 1F,1H). Second, adding GlyH-101 (after forskolin and IBMX) caused a greater Gt reduction in nasal epithelia (Fig. 1J,1L). The Gt response to cAMP-dependent stimulation and GlyH-101 inhibition showed similar trends in excised and cultured epithelia. Third, as an additional test of apical Cl− conductance, we imposed a transepithelial Cl− concentration gradient, added forskolin and IBMX, permeabilized the basolateral membrane with nystatin, and measured Cl− current (Fig. 5D). Cl− current was greater in nasal than tracheal epithelia, indicating a greater Cl− conductance. We noticed that although nasal epithelia have a greater Cl− conductance than tracheal/bronchial epithelia, tracheal/bronchial epithelia have a greater Isc response to forskolin and IBMX when studied in the presence of amiloride; this difference appears to result from a greater driving force for Cl− secretion that is generated by a greater basolateral K+ conductance (Note S2 and Fig. S3).
Thus, basal CFTR Cl− conductance was greater in nasal than tracheal/bronchial epithelia. That result plus equivalent circuit analyses may explain why in nasal epithelia, Vt, ΔVtamiloride, Isc and ΔIscamiloride are greater in CF than non-CF epithelia.
Altering apical Cl− conductance changes Vt, Isc, ΔVtamiloride and ΔIscamiloride
Our conclusion that Cl− conductance affects these electrical parameters in nasal epithelia together with the equivalent circuit analysis make four testable predictions.
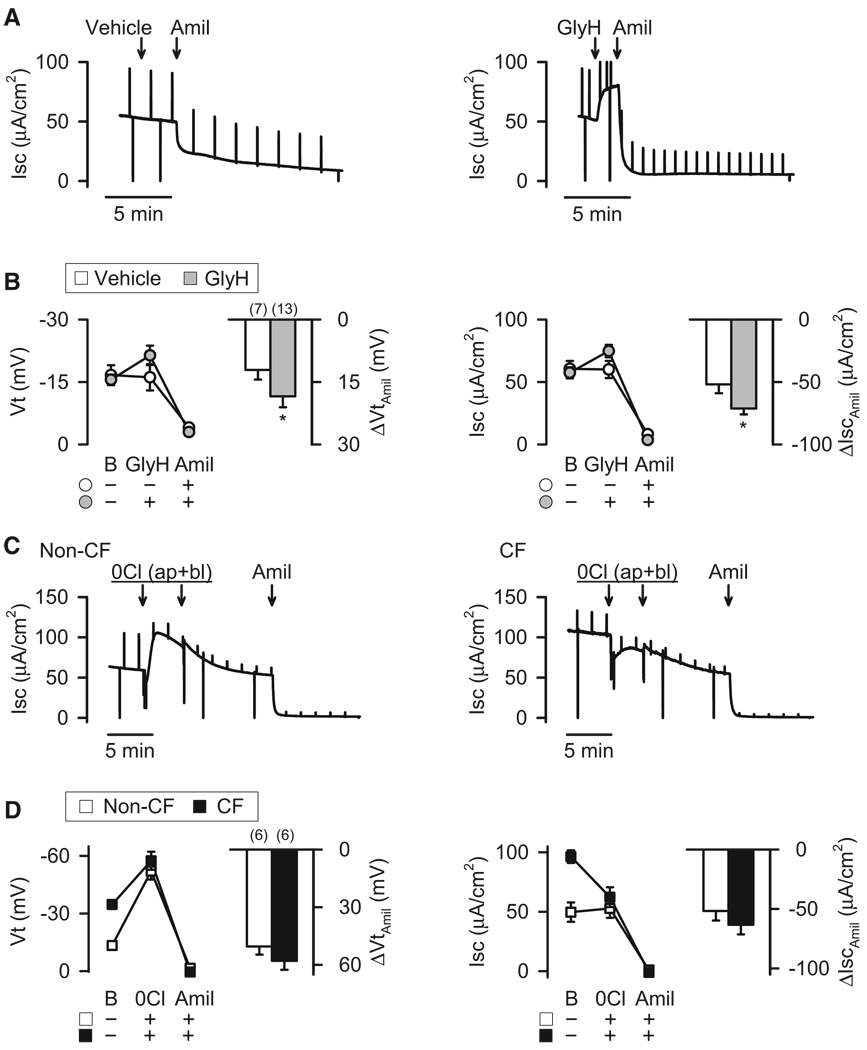
First, if Vt, ΔVtamiloride, Isc and ΔIscamiloride were increased in CF compared to non-CF nasal epithelia because of a lack of Cl− conductance, then there should be an inverse relationship between these values and basal Cl− conductance. To test this prediction, we measured Vt and Isc in nasal epithelia before and after adding amiloride. Then, to obtain an approximation of Cl− conductance, we measured the decrease in Gt following GlyH-101 addition (ΔGtGlyH). We plotted these values, which varied spontaneously from epithelium to epithelium, and found an inverse relationship (Fig. 6A).
Figure 6. Increased Cl− conductance is associated with reduced basal and amiloride-sensitive Vt and Isc.
A. Relationship between basal Vt, ΔVtamil, basal Isc, and ΔIscamil and the change in Gt produced by adding apical 100 µM GlyH-101 (ΔGtGlyH) in the presence of amiloride. Epithelia were cultured non-CF nasal epithelia. Each data point represents a different epithelium. Blue lines indicate linear regression fits to data. Correlation coefficients and p values were: basal Vt, R = −0.831, p < 0.001; ΔVtamil, R = 0.592, p < 0.005; basal Isc, R = −0.495, p < 0.02; and ΔIscamil, R = 0.450, p < 0.05. Spearman rank order correlation was used to test statistical significance. B,C. Effect of 10 µM forskolin and 100 µM IBMX (F&I) or vehicle control on basal Vt and Isc and on changes induced by 100 µM amiloride in cultured nasal epithelia. Panel B shows representative experiments, and panel C shows means ± SE. “B” indicates basal. * indicates P<0.05 vs. vehicle controls. D. Same as panel C, except tracheal epithelia.
Second, further increasing apical Cl− conductance in nasal epithelia should reduce Vt, Isc, ΔVtamiloride, and ΔIscamiloride. To test this prediction, we added forskolin and IBMX and found that compared to vehicle control, it decreased these electrophysiological properties in non-CF nasal epithelia (Fig. 6B,6C). The cAMP-induced reductions in Isc and Vt were opposite to the increases observed when forskolin and IBMX were added after first blocking Na+ channels with amiloride (Fig. 1C,1D,1E,1G). Because cAMP can increase Na+ transport with a slow time course (Boucher et al., 1988; Cullen and Welsh, 1987), we tested this possibility by adding forskolin and IBMX to CF epithelia, where Cl− channel activity would not confound interpretation; with this protocol, forskolin and IBMX did not alter ΔIscamiloride (−104±19 µA.cm−2 after forskolin and IBMX vs. −106±11 µA.cm−2 with vehicle control, n=6 each).
Although tracheal epithelia showed little difference in electrophysiological properties between CF and non-CF, we also tested the effect of forskolin and IBMX in non-CF tracheal epithelia. As in nasal epithelia, increasing cAMP reduced ΔVtamiloride (Fig. 6D), due largely to an increase in Gt (change in Gt with vehicle +0.26±0.04 mS.cm−2 and with forskolin and IBMX +2.40±0.25 mS.cm−2, n=6, P<0.001). However, ΔIscamiloride did not change, perhaps because tracheal epithelia may have greater membrane driving forces for Cl− secretion under short-circuit conditions (Note S2). These results further indicate that the electrophysiological properties are affected by factors other than just Na+ permeability. These results may also explain two earlier studies that reported that increasing cAMP increased Isc or calculated current in human nasal epithelia (Boucher et al., 1988; Boucher et al., 1986).
Third, decreasing apical Cl− conductance in nasal epithelia should increase Vt, Isc, ΔVtamiloride, and ΔIscamiloride. Adding GlyH-101 to reduce the Cl− conductance of non-CF epithelia acutely increased these properties (Fig. 7A,7B). Note that the increase in Isc and Vt are opposite to what occurs when we added GlyH-101 in the presence of amiloride, which eliminates the Na+ conductance (Fig. 1I,1K).
Figure 7. A decreased Cl− conductance reduces the difference between CF and non-CF Vt and Isc.
Epithelia were cultured non-CF nasal epithelia. A,B. Effect of GlyH-101 (100 µM) on Vt and Isc and the response to 100 µM amiloride. Panel A shows representative experiments, and panel B shows means ± SE. “B” indicates basal. * indicates P<0.05 vs. vehicle controls. C,D. Effect of Cl−-free apical (ap) and basolateral (bl) solutions on the response to amiloride in non-CF and CF epithelia. Panel C shows representative experiments in non-CF (left) and CF (right) epithelia, and panel D shows means ± SE. The two arrows for the change to Cl−-free solution in panel C indicate two exchanges of bathing solution. * indicates P<0.05 vs. non-CF controls.
Fourth, non-CF and CF epithelia should show similar properties when Cl− conductance is eliminated by replacing Cl− with gluconate, an impermeant anion. In CF nasal epithelia, Vt and Isc were approximately double the values of non-CF epithelia (Fig. 7C,7D). However, in a Cl−-and HCO3−-free solution, those values and ΔVtamiloride and ΔIscamiloride did not differ between genotypes.
These data further clarify how electrophysiological measurements (increased Vt, ΔVtamiloride, Isc and ΔIscamiloride) that are often interpreted to demonstrate increased CF Na+ absorption may simply reflect the lack of a Cl− conductance.
DISCUSSION
Advantages, limitations, and considerations of this study
Our work has the advantage that we studied airway epithelia in vivo, in freshly excised tissue, and in primary cultures of differentiated airway epithelia, and we obtained similar results. In this regard, most studies of CF ion transport have relied either on in vivo nasal Vt or on cultured airway epithelia or cell lines. However, the relationship between the quantitative and qualitative aspects of ion transport in vivo and those measured in cultured airway epithelia have been uncertain. In addition, chronic infection and inflammation may influence measures of ion transport in nasal Vt, in excised tissue, and perhaps in epithelia cultured from patients (Fu et al., 2007; Gray et al., 2004; Kunzelmann et al., 2006). Thus, it is encouraging that our data from newborn pigs indicate that primary airway cultures retain many of the properties of in vivo and excised airways. For example, like excised nasal epithelia, cultured epithelia derived from nasal tissue had a greater CFTR Cl− conductance than tracheal/bronchial epithelia. In addition, the response to interventions was also similar in vivo, in excised tissue, and in differentiated cultures. These data suggest that cultured epithelia provide a valuable model for studying electrolyte transport by porcine airway.
In this study, we primarily investigated CFTR-mediated anion conductance and amiloride-sensitive Na+ conductance. However, numerous other channels and transporters may contribute to electrolyte transport across airway epithelia, including SLC26 transporters, other HCO3− transporters, electrically neutral Na+ transporters, K+ channels and the Na+/K+-ATPase. Ca2+-activated Cl− channels are also of interest because it has been speculated that they might compensate for the loss of CFTR anion channels in CFTR−/− mice, thereby accounting for lack of a typical CF phenotype (Clarke et al., 1994). Although our data do not indicate whether or not these other transport processes are altered by loss of CFTR, their function remains an important area for investigation.
Our conclusions also have limitations. In comparing how loss of CFTR function affects Na+ absorption in pigs and humans, we acknowledge that regulation of Na+ absorption might differ between the two species, i.e., human CF airway epithelia might hyperabsorb Na+, whereas porcine airway epithelia do not. In addition, although we studied newborn pigs that exhibit a bacterial host defense defect, it is possible that epithelial transport properties differ in older animals and adults. We also studied CFTR−/− pigs, whereas most patients have at least one ΔF508 allele (Welsh et al., 2001). We previously showed that human, porcine, and murine CFTR-ΔF508 show some differences in processing (Ostedgaard et al., 2007), and thus, it should be interesting to learn how CFTRΔF508/ΔF508 pigs compare to CFTR−/− pigs.
There are additional considerations from our studies. First, although we found that CF epithelia do not hyperabsorb Na+, in vivo measures of basal Vt and ΔVtamiloride can be valuable assays in the diagnosis of CF and for assessing the response to interventions designed to increase CFTR activity in patients with CF (Standaert et al., 2004). Second, our conclusions do not mean that increased Na+ absorption could not occur at a later time-point as disease progresses or under some conditions (Myerburg et al., 2006). Third, improving airway surface liquid hydration may benefit patients with CF (Elkins et al., 2006; Donaldson et al., 2006; Robinson et al., 1997); our study did not address that issue. Fourth, it may seem paradoxical that CF nasal epithelia have a greater ΔVtamiloride and ΔIscamiloride than non-CF epithelia, and yet Na+ absorption is not increased in CF. As one example, consider that in non-CF epithelia studied under short-circuit conditions, adding amiloride will hyperpolarize apical membrane voltage, thereby increasing the driving force for Cl− secretion, whereas lack of CFTR precludes Cl− secretion in CF epithelia. Thus, adding amiloride under short-circuit conditions will inhibit Na+ absorption and increase Cl− secretion in non-CF epithelia, and therefore ΔIscamiloride will be greater in CF than non-CF epithelia when apical Na+ conductance is the same. While this is not the only factor involved in determining the response to amiloride (see above), it provides an example of the complexity of interpreting electrical properties in assessing epithelial ion transport.
Implications for CF pathogenesis and treatments
Our data indicate that Na+ absorption is not increased in airway epithelia from newborn CF compared to non-CF pigs. We also explain how loss of CFTR can alter electrophysiological properties that have been construed to indicate enhanced Na+ absorption in CF. These results conflict with the widely held view that CFTR negatively regulates ENaC, and that the loss of this regulation in CF causes airway epithelia to hyperabsorb Na+ (Boucher, 2007; Donaldson and Boucher, 2007). Although we studied electrolyte transport by airway epithelia of pigs shortly after birth, data from that time-point is germane to the issue because newborn CF pigs have an impaired ability to eliminate bacteria (Stoltz et al., 2010). Nevertheless, assaying these properties in older animals as the disease progresses will also be important.
Elucidating the first steps leading to CF lung disease is key if we are to understand pathogenesis and develop mechanism-based treatments and preventions. CF pigs provide a unique opportunity to investigate those initiating steps, because they spontaneously develop lung disease like humans, and at birth they already manifest a bacterial host defense defect, but they do not have the secondary consequences of infection. Our studies using this model identify loss of CFTR anion permeability as the predominant transport defect at birth. In this regard, porcine CF airway epithelia are similar to two other tissues that express both CFTR and ENaC channels, sweat gland ducts and submucosal glands, where loss of anion transport and not Na+ hyperabsorption is the CF defect (Joo et al., 2006; Quinton, 1999; Quinton, 2007). Thus, our data emphasize the role that loss of Cl− and HCO3− permeability may play in impairing bacterial eradication and the subsequent development of airway disease.
EXPERIMENTAL PROCEDURES
For a detailed description of all the methods, please see the Supplemental Material.
CFTR−/− and CFTR+/+ pigs
We previously reported generation of CFTR−/− pigs (Rogers et al., 2008a; Rogers et al., 2008b; Stoltz et al., 2010). The University of Iowa Animal Care and Use Committee approved the animal studies. Animals were produced by mating CFTR+/− male and female pigs. Newborn littermates were obtained from Exemplar Genetics. Animals were studied and/or euthanized 8–15 hours after birth (Euthasol, Virbac).
Measurement of transepithelial voltage in vivo
Transepithelial voltage (Vt) was measured in the nose and trachea of newborn pigs using a standard protocol as described previously (Rogers et al., 2008b; Standaert et al., 2004).
Preparation of differentiated primary cultures of airway epithelia
Epithelial cells were isolated from the various tissues by enzymatic digestion, seeded onto permeable filter supports, and grown at the air-liquid interface as previously described (Karp et al., 2002). Differentiated epithelia were used at least 14 days after seeding.
Electrophysiological measurements in freshly excised and cultured epithelia
Epithelial tissues were excised from the nasal turbinate and septum, and from trachea through 2nd generation bronchi immediately after animals were euthanized. Tissues and cultured epithelia were studied in modified Ussing chambers. Epithelia were bathed on both surfaces with solution containing (mM): 135 NaCl, 2.4 K2HPO4, 0.6 KH2PO4, 1.2 CaCl2, 1.2 MgCl2, 10 dextrose, 5 HEPES (pH = 7.4) at 37 °C and gassed with compressed air. For Cl−-free solution, Cl− was replaced with gluconate and Ca2+ was increased to 5 mM. For the high K+ and Na+-free solution, Na+ was replaced with K+. To study HCO3− transport, we used Cl−-free Kreb’s solution containing (mM): 118.9 NaGluconate, 25 NaHCO3, 2.4 K2HPO4, 0.6 KH2PO4, 5 CaGluconate, 1 MgGluconate, and 5 dextrose and gassed with 5% CO2.
Vt was maintained at 0 mV to measure short-circuit current (Isc). Transepithelial electrical conductance (Gt) was measured by intermittently clamping Vt to +5 and/or −5 mV. Spontaneous values of Vt were measured by transiently removing the voltage clamp. At the beginning of these experiments, we used cultured, non-CF tracheal epithelia to test the dose-response relationship for the agents used in this study (Fig. S1).
Measurement of Na+ flux and fluid transport
Transepithelial Na+ flux and liquid absorption were measured using methods similar to those we previously reported (Flynn et al., 2009; Zabner et al., 1998). The supplemental methods describe the detailed methods.
Measurement of periciliary liquid depth
Newborn pigs (8–10 hrs old) were sedated with ketamine and xylazine (15–20 mg/kg and 1.5 mg/kg, IM, respectively) and immediately euthanized with intravenous Euthasol. A 1–2 cm portion of the trachea was immediately removed, immersed in 2% osmium tetroxide dissolved in FC-72 perfluorocarbon (3M, St Paul, MN), and fixed for 90 to 120 min. The trachea was then rinsed in FC-72 and dehydrated in three changes of 100% ethanol, one hr each. During the second ethanol step, the samples were hand-trimmed into 4 pieces with a scalpel to 1 mm slices. Both open ends of the tracheas were removed and discarded to avoid areas possibly disturbed during removal from the animal. Tissue near the trachealis muscle was avoided. After dehydration, samples were placed in 2:1 100% ethanol:Eponate 12 resin (Ted Pella, Inc., Redding, CA) followed by 1:2 100% ethanol:Eponate 12 for one hr each. Tracheal segments were then infiltrated in 3 changes of 100% Eponate 12 for at least 2 hrs each and polymerized for 24 hrs at 60°C.
Following processing, 4 tissue blocks from each trachea were trimmed and thick-sectioned for light-level PCL thickness determination after staining with Toluidine Blue. Imaging was performed on an Olympus BX-51 equipped with a DP-72 CCD camera (Olympus America Inc., Center Valley, PA) using a 100× NA 1.35 PlanApo lens. Five random images were taken from each block and PCL measured using ImageJ (NIH, Bethesda, MD). PCL height was determined by drawing a line perpendicular to the apical membrane of the epithelial cell surface. On each image, PCL height measurements were performed at 20 random locations. Three observers who were unaware of the CFTR genotype made independent measurements on every image (number of approximate measurements per trachea: 4 tracheal blocks/animal × 5 images/block × 20 measurements/image ~ 400 PCL measurements/piglet trachea/observer). Measurements were made by three independent observers; therefore ~ 1,200 PCL measurements/piglet trachea were obtained. A linear mixed model and maximum likelihood estimation were used to estimate means and standard errors (Bates and Maechler, 2009; R Development Core Team, 2009). The model included fixed effects for observers and genotype and random effects for pigs, segments of the trachea within a pig, images within a segment, and inter-observer variability of measurements on the same image. Tissue blocks used for light microscopy were also trimmed and sectioned at 80 nm for transmission electron microscopy. Non post-stained grids were imaged in a JEOL 1230 TEM (JEOL USA Inc., Peabody, MA) equipped with a Gatan 2k × 2k camera (Gatan Inc., Pleasanton, CA). The transmission electron microscope data were analyzed similarly to the light microscopic images.
Quantitative real-time RT-PCR
Total RNA from excised tissue and cultures was isolated and prepared using standard techniques. Table S1 shows the PCR primers. Real-time RT-PCR was performed using standard methodology and analysis.
Statistical analysis
Data are presented as means ± standard error (SE). Spearman rank order correlation was used to test statistical significance of relationships shown in Fig. 6A. The methods for statistical evaluation of periciliary liquid depth are described in that section of the methods. All other statistical analysis used an unpaired t test. Differences were considered statistically significant at P < 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Lisaurie Lopez Rivera, Paula Ludwig, Theresa Mayhew, Peter Taft, Jingyang Zhang, and Yuping Zhang for excellent assistance. We thank Drs. John B Stokes and Peter M Snyder for helpful discussions. GlyH-101 was a generous gift from the Cystic Fibrosis Foundation Therapeutics and R. Bridges. This work was supported by the National Heart Lung and Blood Institute (grants HL51670, HL091842, and HL097622), the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK54759), and the Cystic Fibrosis Foundation. DAS is a Parker B. Francis Fellow and was supported by the National Institute of Allergy and Infectious Diseases (grant AI076671). MJW is an Investigator of the HHMI. MJW was a co-founder of Exemplar Genetics, a company that is licensing materials and technology related to this work.
REFERENCES
- Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, Bassinet L, Fichou Y, des Georges M, Stanke F, et al. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat. 2009;30:1093–1103. doi: 10.1002/humu.21011. [DOI] [PubMed] [Google Scholar]
- Baker E, Jeunemaitre X, Portal AJ, Grimbert P, Markandu N, Persu A, Corvol P, MacGregor G. Abnormalities of nasal potential difference measurment in Liddle's syndrome. J Clin Invest. 1998;102:10–14. doi: 10.1172/JCI1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M. Ime4: Linear mixed-effects models using S4 classes. R package version. 2009 R package version 0999375-32 http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- Boucher RCJ, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR. Evidence for reduced Cl− and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol. 1988;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RCJ, Stutts MJ, Knowles MR, Cantley LC, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986;78:1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RCJ. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(−/−) mice. Proc Natl Acad Sci U S A. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen JJ, Welsh MJ. Regulation of sodium absorption by canine tracheal epithelium. J Clin Invest. 1987;79:73–79. doi: 10.1172/JCI112811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JC, Davies M, McShane D, Smith S, Chadwick S, Jaffe A, Farley R, Collins L, Bush A, Scallon M, et al. Potential difference measurements in the lower airway of children with and without cystic fibrosis. Am J Respir Crit Care Med. 2005;171:1015–1019. doi: 10.1164/rccm.200408-1116OC. [DOI] [PubMed] [Google Scholar]
- Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;19:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- Duszyk M, French AS. An analytical model of ionic movements in airway epithelial cells. J Theor Biol. 1991;151:231–247. doi: 10.1016/s0022-5193(05)80362-5. [DOI] [PubMed] [Google Scholar]
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- Flynn AN, Itani OA, Moninger TO, Welsh MJ. Acute regulation of tight junction ion selectivity in human airway epithelia. Proc Natl Acad Sci U S A. 2009;106:3591–3596. doi: 10.1073/pnas.0813393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Bettega K, Carroll S, Buchholz KR, Machen TE. Role of Ca2+ in responses of airway epithelia to Pseudomonas aeruginosa, flagellin, ATP, and thapsigargin. Am J Physiol Lung Cell Mol Physiol. 2007;292:L353–L364. doi: 10.1152/ajplung.00042.2006. [DOI] [PubMed] [Google Scholar]
- Gray T, Coakley R, Hirsh A, Thornton D, Kirkham S, Koo JS, Burch L, Boucher R, Nettesheim P. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L320–L330. doi: 10.1152/ajplung.00440.2002. [DOI] [PubMed] [Google Scholar]
- Griesenbach U, Soussi S, Larsen MB, Casamayor I, Dewar A, Regamey N, Bush A, Shah PL, Davies JC, Alton EW. Quantification of periciliary fluid (PCL) height in human airway biopsies is feasible, but not suitable as a biomarker. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2009-0265OC. as. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- Helman SI, Miller DA. Edge damage effect on electrical measurements of frog skin. Am J Physiol Cell Physiol. 1973;225:972–977. doi: 10.1152/ajplegacy.1973.225.4.972. [DOI] [PubMed] [Google Scholar]
- Horisberger JD. ENaC-CFTR interactions: the role of electrical coupling of ion fluxes explored in an epithelial cell model. Pflugers Arch. 2003;445:522–528. doi: 10.1007/s00424-002-0956-0. [DOI] [PubMed] [Google Scholar]
- Huber R, Krueger B, Diakov A, Korbmacher J, Haerteis S, Einsiedel J, Gmeiner P, Azad AK, Cuppens H, Cassiman JJ, et al. Functional characterization of a partial loss-of-function mutation of the epithelial sodium channel (ENaC) associated with atypical cystic fibrosis. Cell Physiol Biochem. 2010;25:145–158. doi: 10.1159/000272059. [DOI] [PubMed] [Google Scholar]
- Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem. 2006;281:7392–7398. doi: 10.1074/jbc.M512766200. [DOI] [PubMed] [Google Scholar]
- Karp PH, Moninger T, Weber SP, Nesselhauf TS, Launspach J, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia: methods and evaluation of primary cultures. In: Wise C, editor. Epithelial Cell Culture Protocols. Totowa, NJ: Humana Press, Inc.; 2002. pp. 115–137. [DOI] [PubMed] [Google Scholar]
- Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett WD, MacLaughlin E, Barker P, Nash M, Quittell MD, et al. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med. 1999;341:156–162. doi: 10.1056/NEJM199907153410304. [DOI] [PubMed] [Google Scholar]
- Knowles M, Gatzy JT, Boucher RCJ. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Stutts MJ, Spock A, Fischer N, Gatzy JT, Boucher RCJ. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Scheidt K, Scharf B, Ousingsawat J, Schreiber R, Wainwright B, McMorran B. Flagellin of Pseudomonas aeruginosa inhibits Na+ transport in airway epithelia. FASEB J. 2006;20:545–546. doi: 10.1096/fj.05-4454fje. [DOI] [PubMed] [Google Scholar]
- Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RCJ. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Stoltz DA, Namati E, Ramachandran S, Pezzulo AA, Smith AR, Rector MV, Suter MJ, Kao S, McLennan G, et al. Loss of CFTR function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010a doi: 10.1164/rccm.201004-0643OC. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010b;176 doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem. 2006;281:27942–27949. doi: 10.1074/jbc.M606449200. [DOI] [PubMed] [Google Scholar]
- Ostedgaard LS, Rogers CS, Dong Q, Randak CO, Vermeer DW, Rokhlina T, Karp PH, Welsh MJ. Processing and function of CFTR-ΔF508 are species-dependent. Proc Natl Acad Sci U S A. 2007;104:15370–15375. doi: 10.1073/pnas.0706974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton P. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Cystic fibrosis: lessons from the sweat gland. Physiology (Bethesda) 2007;22:212–225. doi: 10.1152/physiol.00041.2006. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. Vol. Vienna Austria: R Foundation for Statistical Computing; 2009. A language and environment for statistical computing. http://www.R-project.org. [Google Scholar]
- Robinson M, Hemming AL, Regnis JA, Wong AG, Bailey DL, Bautovich GJ, King M, Bye PT. Effect of increasing doses of hypertonic saline on mucosciliary clearance in patients with cystic fibrosis. Thorax. 1997;52:900–903. doi: 10.1136/thx.52.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, Petroff E, Vermeer DW, Kabel AC, Yan Z, et al. Production of CFTR null and ΔF508 heterozygous pigs by AAV-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008a;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008b;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- Satir P. Studies on Cilia. The Fixation of the Metachronal Wave. J Cell Biol. 1963;18:345–365. doi: 10.1083/jcb.18.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaedel C, Marthinsen L, Kristoffersson AC, Kornfalt R, Nilsson KO, Orlenius B, Holmberg L. Lung symptoms in pseudohypoaldosteronism type 1 are associated with deficiency of the alpha-subunit of the epithelial sodium channel. J Pediatr. 1999;135:739–745. doi: 10.1016/s0022-3476(99)70094-6. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Structure and function of the CFTR Cl− channel. Physiol Rev. 1999;79:S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- Sheridan MB, Fong P, Groman JD, Conrad C, Flume P, Diaz R, Harris C, Knowles M, Cutting GR. Mutations in the beta-subunit of the epithelial Na+ channel in patients with a cystic fibrosis-like syndrome. Hum Mol Genet. 2005;14:3493–3498. doi: 10.1093/hmg/ddi374. [DOI] [PubMed] [Google Scholar]
- Sims DE, Horne MM. Heterogeneity of the composition and thickness of tracheal mucus in rats. Am J Physiol. 1997;273:L1036–L1041. doi: 10.1152/ajplung.1997.273.5.L1036. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert TA, Boitano L, Emerson J, Milgram LJ, Konstan MW, Hunter J, Berclaz PY, Brass L, Zeitlin PL, Hammond K, et al. Standardized procedure for measurement of nasal potential difference: an outcome measure in multicenter cystic fibrosis clinical trials. Pediatr Pulmonol. 2004;37:385–392. doi: 10.1002/ppul.10448. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane CL, Dohrn CL, Bartlett JA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Science Translational Medicine. 2010;2 doi: 10.1126/scitranslmed.3000928. 29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Song Y, Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol Cell Physiol. 2003;284:C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic Fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 2001. pp. 5121–5189. [Google Scholar]
- Widdicombe JH, Welsh MJ, Finkbeiner WE. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci U S A. 1985;82:6167–6171. doi: 10.1073/pnas.82.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RCJ. Sodium transport and intracellular sodium activity in cultured human nasal epithelium. Am J Physiol. 1991a;261:C319–C331. doi: 10.1152/ajpcell.1991.261.2.C319. [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RCJ. Transcellular sodium transport in cultured cystic fibrosis human nasal epithelium. Am J Physiol. 1991b;261:C332–C341. doi: 10.1152/ajpcell.1991.261.2.C332. [DOI] [PubMed] [Google Scholar]
- Wine JJ. The genesis of cystic fibrosis lung disease. J Clin Invest. 1999;103:309–312. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J, Smith JJ, Karp PH, Widdicombe JH, Welsh MJ. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol Cell. 1998;2:397–403. doi: 10.1016/s1097-2765(00)80284-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.