Abstract
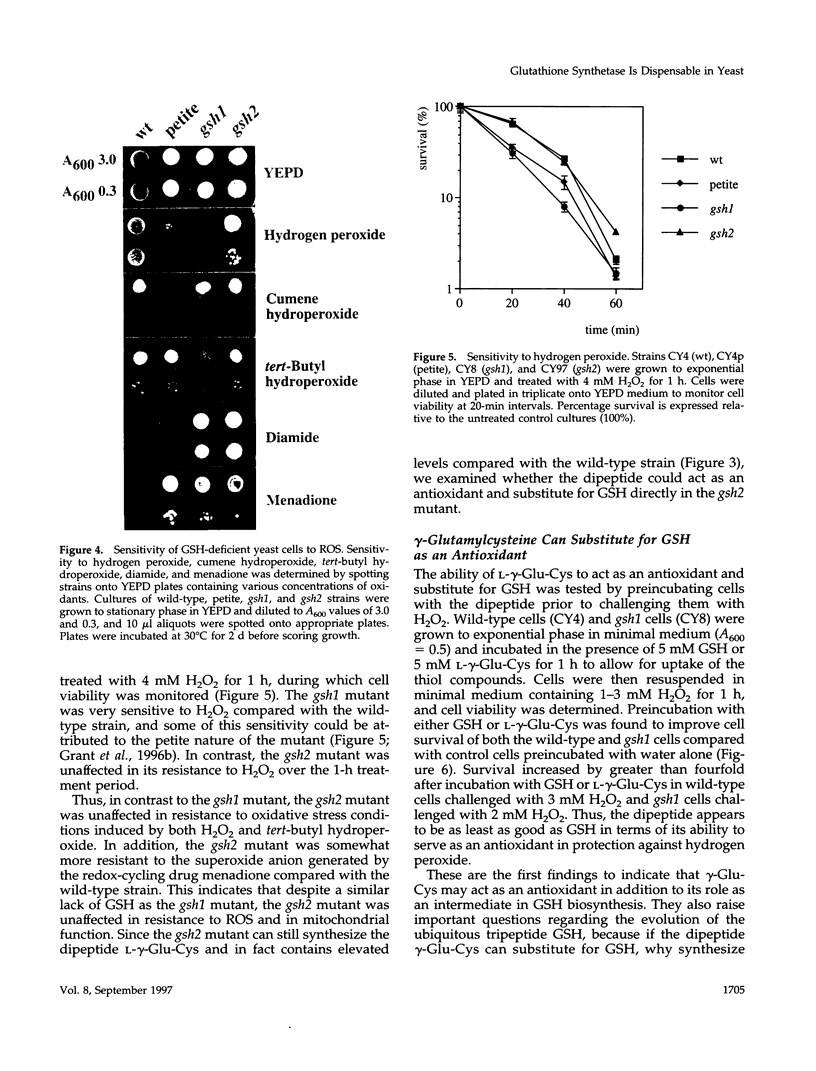
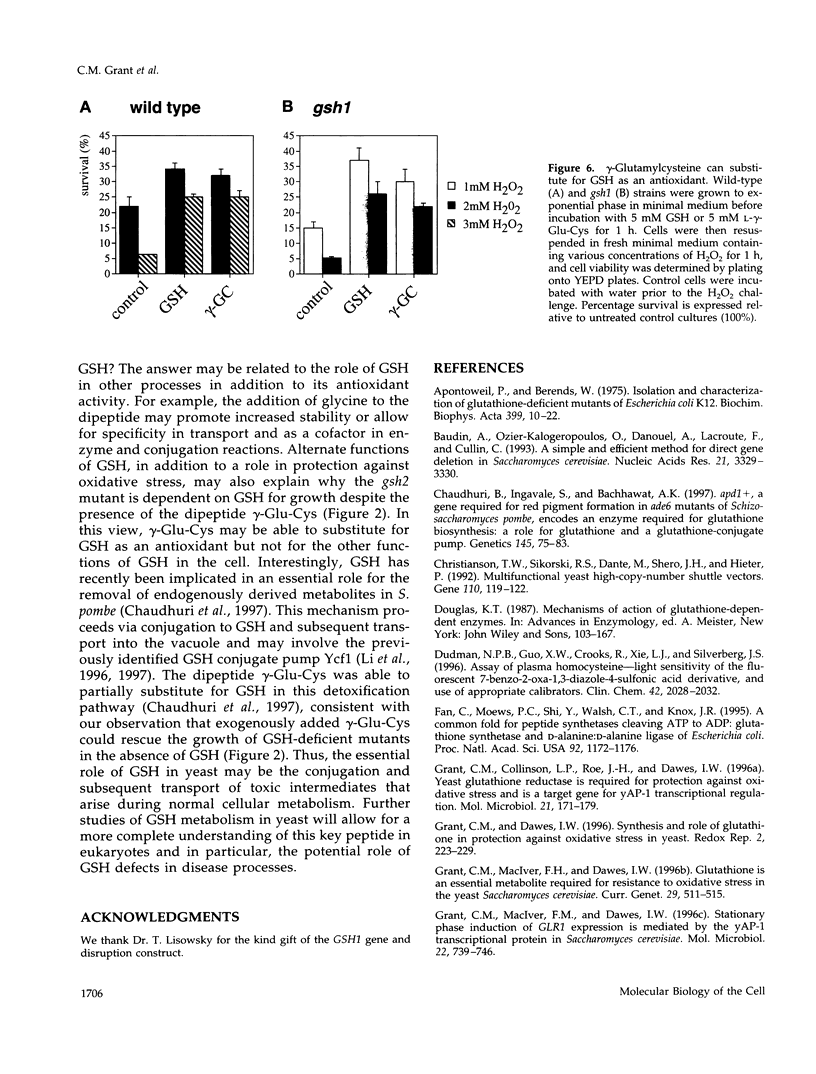
Glutathione (GSH) synthetase (Gsh2) catalyzes the ATP-dependent synthesis of GSH from gamma-glutamylcysteine (gamma-Glu-Cys) and glycine. GSH2, encoding the Saccharomyces cerevisiae enzyme, was isolated and used to construct strains that either lack or overproduce Gsh2. The identity of GSH2 was confirmed by the following criteria: 1) the predicted Gsh2 protein shared 37-39% identity and 58-60% similarity with GSH synthetases from other eukaryotes, 2) increased gene dosage of GSH2 resulted in elevated Gsh2 enzyme activity, 3) a strain deleted for GSH2 was dependent on exogenous GSH for wild-type growth rates, and 4) the gsh2 mutant lacked GSH and accumulated the dipeptide gamma-Glu-Cys intermediate in GSH biosynthesis. Overexpression of GSH2 had no effect on cellular GSH levels, whereas overexpression of GSH1, encoding the enzyme for the first step in GSH biosynthesis, lead to an approximately twofold increase in GSH levels, consistent with Gsh1 catalyzing the rate-limiting step in GSH biosynthesis. In contrast to a strain deleted for GSH1, which lacks both GSH and gamma-Glu-Cys, the strain deleted for GSH2 was found to be unaffected in mitochondrial function as well as resistance to oxidative stress induced by hydrogen peroxide, tert-butyl hydroperoxide, and the superoxide anion. Furthermore, gamma-Glu-Cys was at least as good as GSH in protecting yeast cells against an oxidant challenge, providing the first evidence that gamma-Glu-Cys can act as an antioxidant and substitute for GSH in a eukaryotic cell. However, the dipeptide could not fully substitute for the essential function of GSH in the cell as shown by the poor growth of the gsh2 mutant on minimal medium. We suggest that this function may be the detoxification of harmful intermediates that are generated during normal cellular metabolism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apontoweil P., Berends W. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim Biophys Acta. 1975 Jul 14;399(1):10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- Baudin A., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993 Jul 11;21(14):3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri B., Ingavale S., Bachhawat A. K. apd1+, a gene required for red pigment formation in ade6 mutants of Schizosaccharomyces pombe, encodes an enzyme required for glutathione biosynthesis: a role for glutathione and a glutathione-conjugate pump. Genetics. 1997 Jan;145(1):75–83. doi: 10.1093/genetics/145.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992 Jan 2;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Douglas K. T. Mechanism of action of glutathione-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1987;59:103–167. doi: 10.1002/9780470123058.ch3. [DOI] [PubMed] [Google Scholar]
- Dudman N. P., Guo X. W., Crooks R., Xie L., Silberberg J. S. Assay of plasma homocysteine: light sensitivity of the fluorescent 7-benzo-2-oxa-1, 3-diazole-4-sulfonic acid derivative, and use of appropriate calibrators. Clin Chem. 1996 Dec;42(12):2028–2032. [PubMed] [Google Scholar]
- Fan C., Moews P. C., Shi Y., Walsh C. T., Knox J. R. A common fold for peptide synthetases cleaving ATP to ADP: glutathione synthetase and D-alanine:d-alanine ligase of Escherichia coli. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1172–1176. doi: 10.1073/pnas.92.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C. M., Collinson L. P., Roe J. H., Dawes I. W. Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol Microbiol. 1996 Jul;21(1):171–179. doi: 10.1046/j.1365-2958.1996.6351340.x. [DOI] [PubMed] [Google Scholar]
- Grant C. M., MacIver F. H., Dawes I. W. Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr Genet. 1996 May;29(6):511–515. doi: 10.1007/BF02426954. [DOI] [PubMed] [Google Scholar]
- Grant C. M., Maciver F. H., Dawes I. W. Stationary-phase induction of GLR1 expression is mediated by the yAP-1 transcriptional regulatory protein in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1996 Nov;22(4):739–746. doi: 10.1046/j.1365-2958.1996.d01-1727.x. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986 Nov;168(2):1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gushima H., Yasuda S., Soeda E., Yokota M., Kondo M., Kimura A. Complete nucleotide sequence of the E. coli glutathione synthetase gsh-II. Nucleic Acids Res. 1984 Dec 21;12(24):9299–9307. doi: 10.1093/nar/12.24.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A., Hille S., Knöchel W. Molecular cloning of the large subunit of glutathione synthetase from Xenopus laevis embryos. Biochim Biophys Acta. 1993 Sep 23;1174(3):295–298. doi: 10.1016/0167-4781(93)90202-o. [DOI] [PubMed] [Google Scholar]
- Hara T., Tanaka T., Kato H., Nishioka T., Oda J. Site-directed mutagenesis of glutathione synthetase from Escherichia coli B: mapping of the gamma-L-glutamyl-L-cysteine-binding site. Protein Eng. 1995 Jul;8(7):711–716. doi: 10.1093/protein/8.7.711. [DOI] [PubMed] [Google Scholar]
- Harvey P. R., Ilson R. G., Strasberg S. M. The simultaneous determination of oxidized and reduced glutathiones in liver tissue by ion pairing reverse phase high performance liquid chromatography with a coulometric electrochemical detector. Clin Chim Acta. 1989 Apr 14;180(3):203–212. doi: 10.1016/0009-8981(89)90001-6. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Huang C. S., Moore W. R., Meister A. On the active site thiol of gamma-glutamylcysteine synthetase: relationships to catalysis, inhibition, and regulation. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2464–2468. doi: 10.1073/pnas.85.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Inoue Y., Kimura A. Oxidative stress response in yeast: effect of glutathione on adaptation to hydrogen peroxide stress in Saccharomyces cerevisiae. FEBS Lett. 1995 Jul 10;368(1):73–76. doi: 10.1016/0014-5793(95)00603-7. [DOI] [PubMed] [Google Scholar]
- Kistler M., Maier K., Eckardt-Schupp F. Genetic and biochemical analysis of glutathione-deficient mutants of Saccharomyces cerevisiae. Mutagenesis. 1990 Jan;5(1):39–44. doi: 10.1093/mutage/5.1.39. [DOI] [PubMed] [Google Scholar]
- Kistler M., Summer K. H., Eckardt F. Isolation of glutathione-deficient mutants of the yeast Saccharomyces cerevisiae. Mutat Res. 1986 Feb;173(2):117–120. doi: 10.1016/0165-7992(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M. Diamide: an oxidant probe for thiols. Methods Enzymol. 1995;251:123–133. doi: 10.1016/0076-6879(95)51116-4. [DOI] [PubMed] [Google Scholar]
- Kuge S., Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994 Feb 1;13(3):655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. S., Lu Y. P., Zhen R. G., Szczypka M., Thiele D. J., Rea P. A. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc Natl Acad Sci U S A. 1997 Jan 7;94(1):42–47. doi: 10.1073/pnas.94.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. S., Szczypka M., Lu Y. P., Thiele D. J., Rea P. A. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J Biol Chem. 1996 Mar 15;271(11):6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- Lisowsky T. A high copy number of yeast gamma-glutamylcysteine synthetase suppresses a nuclear mutation affecting mitochondrial translation. Curr Genet. 1993 May-Jun;23(5-6):408–413. doi: 10.1007/BF00312627. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Muller E. G. A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth. Mol Biol Cell. 1996 Nov;7(11):1805–1813. doi: 10.1091/mbc.7.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh N., Nakagawa C. W., Ando S., Tanabe K., Hayashi Y. Cloning and sequencing of the gene encoding the large subunit of glutathione synthetase of Schizosaccharomyces pombe. Biochem Biophys Res Commun. 1991 Nov 27;181(1):430–436. doi: 10.1016/s0006-291x(05)81437-8. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Stole E., Frayer W., Auld P. A., Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake Y., Yabuuchi S. Molecular cloning of the gamma-glutamylcysteine synthetase gene of Saccharomyces cerevisiae. Yeast. 1991 Dec;7(9):953–961. doi: 10.1002/yea.320070907. [DOI] [PubMed] [Google Scholar]
- Oppenheimer L., Wellner V. P., Griffith O. W., Meister A. Glutathione synthetase. Purification from rat kidney and mapping of the substrate binding sites. J Biol Chem. 1979 Jun 25;254(12):5184–5190. [PubMed] [Google Scholar]
- Peters J. M., Dalrymple B. P., Jorgensen W. K. Sequence of a putative glutathione synthetase II gene and flanking regions from Anaplasma centrale. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1040–1046. doi: 10.1016/0006-291x(92)91836-f. [DOI] [PubMed] [Google Scholar]
- Rawlins M. R., Leaver C. J., May M. J. Characterisation of an Arabidopsis thaliana cDNA encoding glutathione synthetase. FEBS Lett. 1995 Nov 27;376(1-2):81–86. doi: 10.1016/0014-5793(95)01253-1. [DOI] [PubMed] [Google Scholar]
- Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993 Jul 15;215(2):213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton H. E., Dawes I. W., Grant C. M. Saccharomyces cerevisiae exhibits a yAP-1-mediated adaptive response to malondialdehyde. J Bacteriol. 1997 Feb;179(4):1096–1101. doi: 10.1128/jb.179.4.1096-1101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann P., Gondet L., Potier S., Bach T. J. Cloning of Arabidopsis thaliana glutathione synthetase (GSH2) by functional complementation of a yeast gsh2 mutant. Eur J Biochem. 1996 Mar 1;236(2):662–669. doi: 10.1111/j.1432-1033.1996.00662.x. [DOI] [PubMed] [Google Scholar]
- Vandeputte C., Guizon I., Genestie-Denis I., Vannier B., Lorenzon G. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturized protocol. Cell Biol Toxicol. 1994 Dec;10(5-6):415–421. doi: 10.1007/BF00755791. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Kato H., Hata Y., Nishioka T., Kimura A., Oda J., Katsube Y. Three-dimensional structure of the glutathione synthetase from Escherichia coli B at 2.0 A resolution. J Mol Biol. 1993 Feb 20;229(4):1083–1100. doi: 10.1006/jmbi.1993.1106. [DOI] [PubMed] [Google Scholar]