Abstract
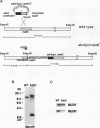
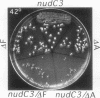
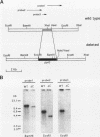
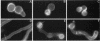
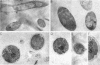
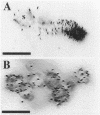
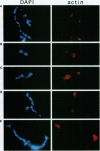
Nuclear migration is required for normal development in both higher and lower eukaryotes. In fungi this process is mediated by cytoplasmic dynein. It is believed that this motor protein is anchored to the cell membrane and moves nuclei by capturing and pulling on spindle pole body microtubules. To date, four genes have been identified and shown to be required for this process in Aspergillus nidulans. The nudA and nudG genes, respectively, encode the heavy and light chains of cytoplasmic dynein, and the nudF and nudC gene products encode proteins of 49 and 22 kDa. The precise biochemical functions of the nudF and nudC genes have not yet been identified. In this report we further investigate NUDC protein function by deleting the nudC gene. Surprisingly, although deletion of nudA and nudF affect nuclear migration, deletion of nudC profoundly affected the morphology and composition of the cell wall. Spores of the strain deleted for nudC grew spherically and lysed. The thickness of the cell wall was increased in the deletion mutant and wall polymer composition was abnormal. This phenotype could be repressed by growth on osmotically buffered medium at low temperature. Similar, but less severe, effects were also noted in a strain depleted for NUDC by down-regulation. These results suggest a possible relationship between fungal cell wall biosynthesis and nuclear migration.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnicki-Garcia S., Persson J., Chanzy H. An electron microscope and electron diffraction study of the effect of calcofluor and congo red on the biosynthesis of chitin in vitro. Arch Biochem Biophys. 1994 Apr;310(1):6–15. doi: 10.1006/abbi.1994.1133. [DOI] [PubMed] [Google Scholar]
- Borgia P. T., Dodge C. L. Characterization of Aspergillus nidulans mutants deficient in cell wall chitin or glucan. J Bacteriol. 1992 Jan;174(2):377–383. doi: 10.1128/jb.174.2.377-383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgia P. T. Roles of the orlA, tsE, and bimG genes of Aspergillus nidulans in chitin synthesis. J Bacteriol. 1992 Jan;174(2):384–389. doi: 10.1128/jb.174.2.384-389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruno K. S., Tinsley J. H., Minke P. F., Plamann M. Genetic interactions among cytoplasmic dynein, dynactin, and nuclear distribution mutants of Neurospora crassa. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4775–4780. doi: 10.1073/pnas.93.10.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulawa C. E., Osmond B. C. Chitin synthase I and chitin synthase II are not required for chitin synthesis in vivo in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7424–7428. doi: 10.1073/pnas.87.19.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H. Cell shape determination: a pivotal role for Rho. Science. 1996 Apr 12;272(5259):224–225. doi: 10.1126/science.272.5259.224. [DOI] [PubMed] [Google Scholar]
- Chiu Y. H., Morris N. R. Extragenic suppressors of nudC3, a mutation that blocks nuclear migration in Aspergillus nidulans. Genetics. 1995 Oct;141(2):453–464. doi: 10.1093/genetics/141.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y. H., Morris N. R. Genetic and molecular analysis of a tRNA(Leu) missense suppressor of nudC3, a mutation that blocks nuclear migration in Aspergillus nidulans. Genetics. 1997 Mar;145(3):707–714. doi: 10.1093/genetics/145.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Smith K. W., Gustin M. C. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J Cell Biol. 1992 Aug;118(3):561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. W., Meyer D. I. ACT3: a putative centractin homologue in S. cerevisiae is required for proper orientation of the mitotic spindle. J Cell Biol. 1994 Oct;127(1):129–138. doi: 10.1083/jcb.127.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D. Biophysical control of plant cell growth. Annu Rev Plant Physiol. 1986;37:377–405. doi: 10.1146/annurev.pp.37.060186.002113. [DOI] [PubMed] [Google Scholar]
- Drgonová J., Drgon T., Tanaka K., Kollár R., Chen G. C., Ford R. A., Chan C. S., Takai Y., Cabib E. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996 Apr 12;272(5259):277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- Engle A. R., Purdie N., Hyatt J. A. Induced circular dichroism study of the aqueous solution complexation of cello-oligosaccharides and related polysaccharides with aromatic dyes. Carbohydr Res. 1994 Dec 16;265(2):181–195. doi: 10.1016/0008-6215(94)00235-5. [DOI] [PubMed] [Google Scholar]
- Eshel D., Urrestarazu L. A., Vissers S., Jauniaux J. C., van Vliet-Reedijk J. C., Planta R. J., Gibbons I. R. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson H. V., Anderson B. L., Warrick H. M., Pon L. A., Spudich J. A. Synthetic lethality screen identifies a novel yeast myosin I gene (MYO5): myosin I proteins are required for polarization of the actin cytoskeleton. J Cell Biol. 1996 Jun;133(6):1277–1291. doi: 10.1083/jcb.133.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Adachi H., Tsujimoto M., Arai H., Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase [corrected]. Nature. 1994 Jul 21;370(6486):216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- Holleran E. A., Tokito M. K., Karki S., Holzbaur E. L. Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J Cell Biol. 1996 Dec;135(6 Pt 2):1815–1829. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E. L., Vallee R. B. DYNEINS: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- Karki S., Holzbaur E. L. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995 Dec 1;270(48):28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Katz D., Rosenberger R. F. Hyphal wall synthesis in Aspergillus nidulans: effect of protein synthesis inhibition and osmotic shock on chitin insertion and morphogenesis. J Bacteriol. 1971 Oct;108(1):184–190. doi: 10.1128/jb.108.1.184-190.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K. E., Morris N. R. Either alpha-tubulin isogene product is sufficient for microtubule function during all stages of growth and differentiation in Aspergillus nidulans. Mol Cell Biol. 1993 Aug;13(8):4465–4476. doi: 10.1128/mcb.13.8.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecká M., Gabriel M. The influence of congo red on the cell wall and (1----3)-beta-D-glucan microfibril biogenesis in Saccharomyces cerevisiae. Arch Microbiol. 1992;158(2):115–126. doi: 10.1007/BF00245214. [DOI] [PubMed] [Google Scholar]
- Käfer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet. 1977;19:33–131. doi: 10.1016/s0065-2660(08)60245-x. [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Yeh E., Hays T., Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham P., Bainbridge B. W. Effect of restrictive conditions on the growth and morphology of a temperature-sensitive mannose-requiring mutant of Aspergillus nidulans. FEMS Microbiol Lett. 1992 Aug 1;74(1):115–120. doi: 10.1016/0378-1097(92)90746-b. [DOI] [PubMed] [Google Scholar]
- McGoldrick C. A., Gruver C., May G. S. myoA of Aspergillus nidulans encodes an essential myosin I required for secretion and polarized growth. J Cell Biol. 1995 Feb;128(4):577–587. doi: 10.1083/jcb.128.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R. Mitotic mutants of Aspergillus nidulans. Genet Res. 1975 Dec;26(3):237–254. doi: 10.1017/s0016672300016049. [DOI] [PubMed] [Google Scholar]
- Morris N. R., Xiang X., Beckwith S. M. Nuclear migration advances in fungi. Trends Cell Biol. 1995 Jul;5(7):278–282. doi: 10.1016/s0962-8924(00)89039-x. [DOI] [PubMed] [Google Scholar]
- Muhua L., Karpova T. S., Cooper J. A. A yeast actin-related protein homologous to that in vertebrate dynactin complex is important for spindle orientation and nuclear migration. Cell. 1994 Aug 26;78(4):669–679. doi: 10.1016/0092-8674(94)90531-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985 Feb;40(2):405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Morris N. R. Nuclear movement is beta--tubulin-dependent in Aspergillus nidulans. Cell. 1980 Jan;19(1):255–262. doi: 10.1016/0092-8674(80)90407-9. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Rinehart J. E., Mitchell B. L., Oakley C. E., Carmona C., Gray G. L., May G. S. Cloning, mapping and molecular analysis of the pyrG (orotidine-5'-phosphate decarboxylase) gene of Aspergillus nidulans. Gene. 1987;61(3):385–399. doi: 10.1016/0378-1119(87)90201-0. [DOI] [PubMed] [Google Scholar]
- Ortega J. K., Zehr E. G., Keanini R. G. In vivo creep and stress relaxation experiments to determine the wall extensibility and yield threshold for the sporangiophores of phycomyces. Biophys J. 1989 Sep;56(3):465–475. doi: 10.1016/S0006-3495(89)82694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
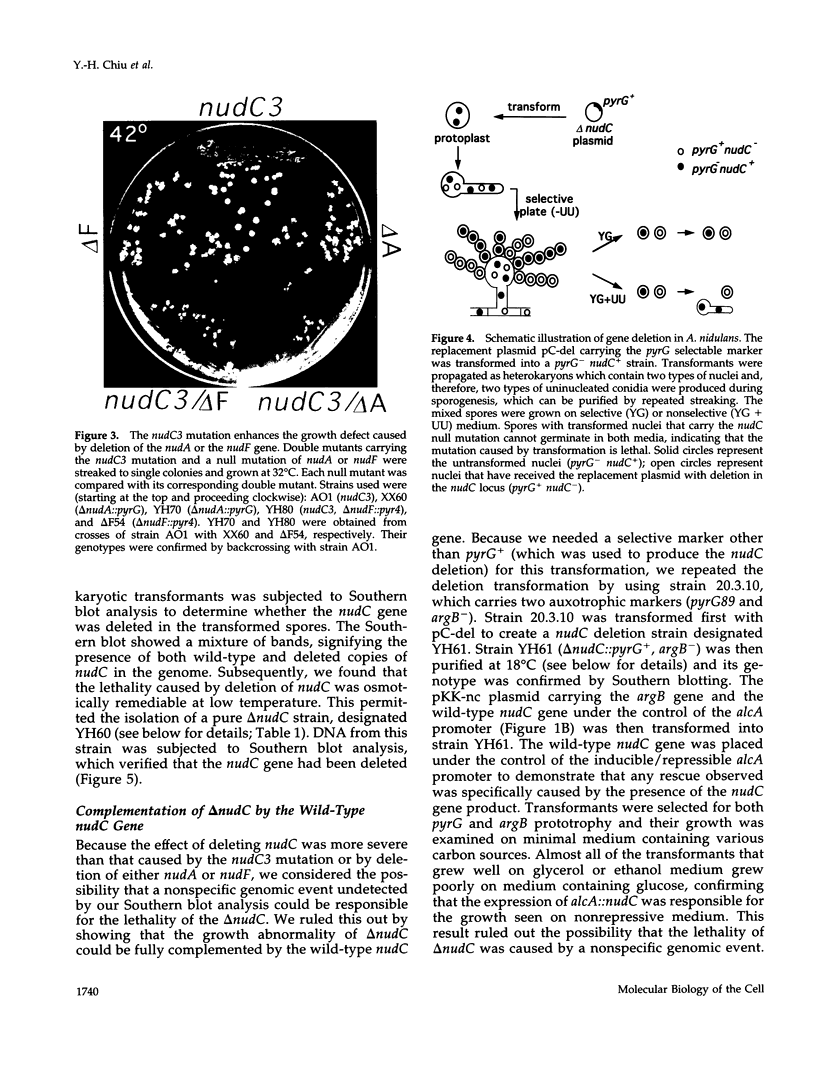
- Osmani A. H., Osmani S. A., Morris N. R. The molecular cloning and identification of a gene product specifically required for nuclear movement in Aspergillus nidulans. J Cell Biol. 1990 Aug;111(2):543–551. doi: 10.1083/jcb.111.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
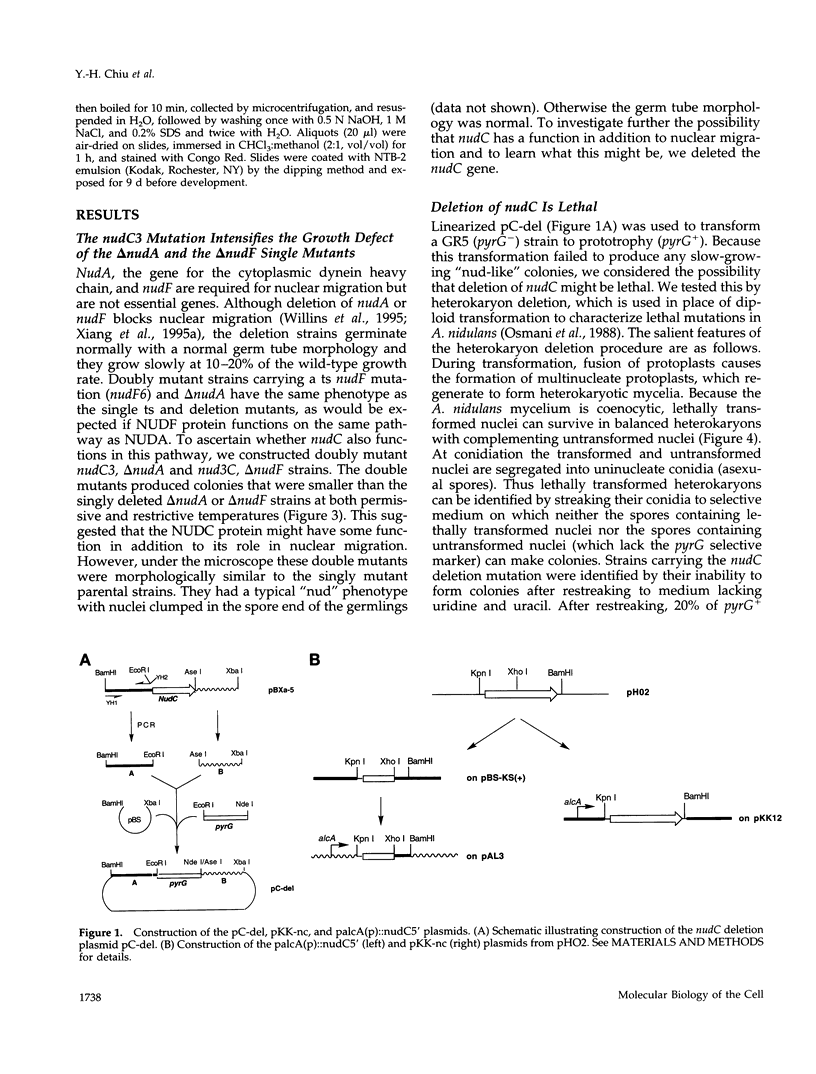
- Osmani S. A., Engle D. B., Doonan J. H., Morris N. R. Spindle formation and chromatin condensation in cells blocked at interphase by mutation of a negative cell cycle control gene. Cell. 1988 Jan 29;52(2):241–251. doi: 10.1016/0092-8674(88)90513-2. [DOI] [PubMed] [Google Scholar]
- Pancaldi S., Poli F., Dall'Olio G., Vannini G. L. Morphological anomalies induced by Congo red in Aspergillus niger. Arch Microbiol. 1984 Mar;137(3):185–187. doi: 10.1007/BF00414540. [DOI] [PubMed] [Google Scholar]
- Plamann M., Minke P. F., Tinsley J. H., Bruno K. S. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J Cell Biol. 1994 Oct;127(1):139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadota H., Python C. P., Inoue S. B., Arisawa M., Anraku Y., Zheng Y., Watanabe T., Levin D. E., Ohya Y. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science. 1996 Apr 12;272(5259):279–281. doi: 10.1126/science.272.5259.279. [DOI] [PubMed] [Google Scholar]
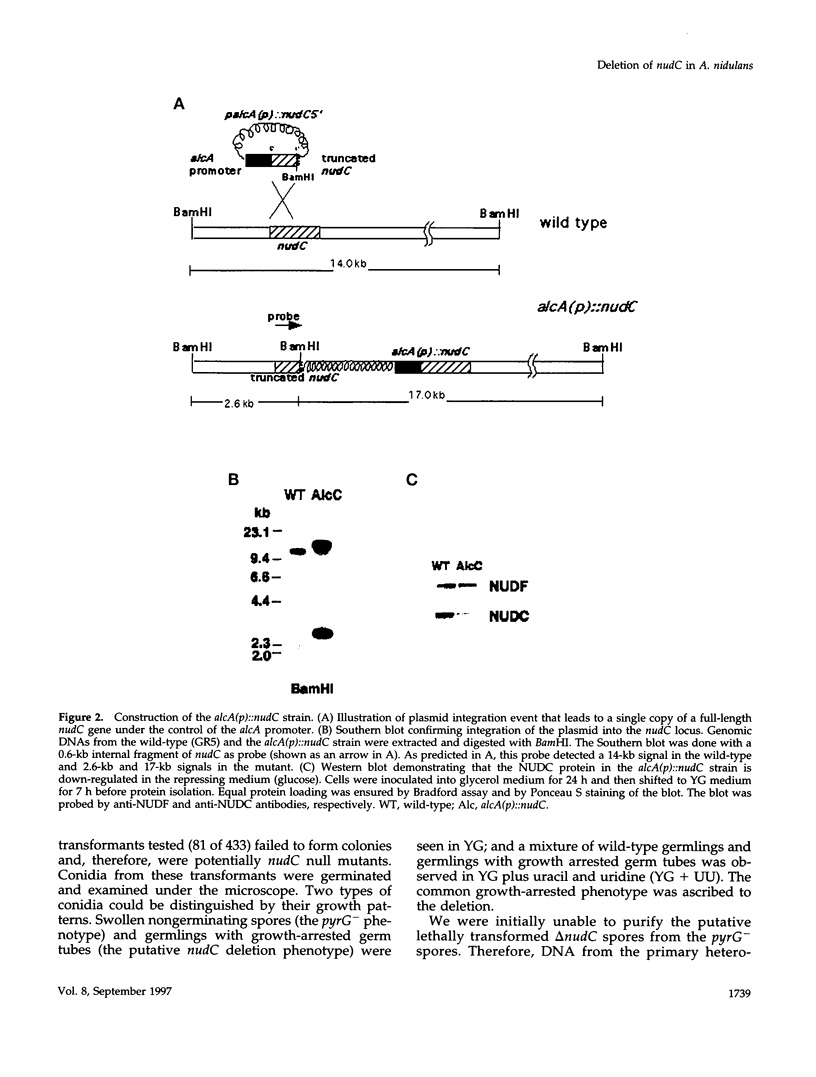
- Read E. B., Okamura H. H., Drubin D. G. Actin- and tubulin-dependent functions during Saccharomyces cerevisiae mating projection formation. Mol Biol Cell. 1992 Apr;3(4):429–444. doi: 10.1091/mbc.3.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W. B., Caskey C. T., Ledbetter D. H. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993 Aug 19;364(6439):717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Robb M. J., Wilson M. A., Vierula P. J. A fungal actin-related protein involved in nuclear migration. Mol Gen Genet. 1995 Jun 10;247(5):583–590. doi: 10.1007/BF00290350. [DOI] [PubMed] [Google Scholar]
- Roncero C., Durán A. Effect of Calcofluor white and Congo red on fungal cell wall morphogenesis: in vivo activation of chitin polymerization. J Bacteriol. 1985 Sep;163(3):1180–1185. doi: 10.1128/jb.163.3.1180-1185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer D. A., Gill S. R., Cooper J. A., Heuser J. E., Schroer T. A. Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J Cell Biol. 1994 Jul;126(2):403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A. Structure, function and regulation of cytoplasmic dynein. Curr Opin Cell Biol. 1994 Feb;6(1):69–73. doi: 10.1016/0955-0674(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Shaw J. A., Mol P. C., Bowers B., Silverman S. J., Valdivieso M. H., Durán A., Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1991 Jul;114(1):111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Payton M. A. Hyphal tip extension in Aspergillus nidulans requires the manA gene, which encodes phosphomannose isomerase. Mol Cell Biol. 1994 Sep;14(9):6030–6038. doi: 10.1128/mcb.14.9.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G., Schliwa M. Organelle movements in the wild type and wall-less fz;sg;os-1 mutants of Neurospora crassa are mediated by cytoplasmic microtubules. J Cell Sci. 1993 Oct;106(Pt 2):555–564. doi: 10.1242/jcs.106.2.555. [DOI] [PubMed] [Google Scholar]
- Sullivan D. S., Huffaker T. C. Astral microtubules are not required for anaphase B in Saccharomyces cerevisiae. J Cell Biol. 1992 Oct;119(2):379–388. doi: 10.1083/jcb.119.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela J. C., van Beekvelt C., Planta R. J., Mager W. H. Osmostress-induced changes in yeast gene expression. Mol Microbiol. 1992 Aug;6(15):2183–2190. doi: 10.1111/j.1365-2958.1992.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Vaughan K. T., Vallee R. B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995 Dec;131(6 Pt 1):1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring R. B., May G. S., Morris N. R. Characterization of an inducible expression system in Aspergillus nidulans using alcA and tubulin-coding genes. Gene. 1989 Jun 30;79(1):119–130. doi: 10.1016/0378-1119(89)90097-8. [DOI] [PubMed] [Google Scholar]
- Willins D. A., Liu B., Xiang X., Morris N. R. Mutations in the heavy chain of cytoplasmic dynein suppress the nudF nuclear migration mutation of Aspergillus nidulans. Mol Gen Genet. 1997 Jun;255(2):194–200. doi: 10.1007/s004380050489. [DOI] [PubMed] [Google Scholar]
- Willins D. A., Xiang X., Morris N. R. An alpha tubulin mutation suppresses nuclear migration mutations in Aspergillus nidulans. Genetics. 1995 Dec;141(4):1287–1298. doi: 10.1093/genetics/141.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Beckwith S. M., Morris N. R. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Osmani A. H., Osmani S. A., Xin M., Morris N. R. NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol Biol Cell. 1995 Mar;6(3):297–310. doi: 10.1091/mbc.6.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Roghi C., Morris N. R. Characterization and localization of the cytoplasmic dynein heavy chain in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9890–9894. doi: 10.1073/pnas.92.21.9890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A., DeWald D. B., Boronenkov I. V., Anderson R. A., Emr S. D., Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell. 1995 May;6(5):525–539. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamochi W., Tanaka K., Nonaka H., Maeda A., Musha T., Takai Y. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J Cell Biol. 1994 Jun;125(5):1077–1093. doi: 10.1083/jcb.125.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]