Abstract
Prenatal exposure to endocrine disruptors has the potential to impact early brain development. Neurodevelopmental toxicity in utero may manifest as psychosocial deficits later in childhood. This study investigates prenatal exposure to two ubiquitous endocrine disruptors, the phthalate esters and bisphenol A (BPA), and social behavior in a sample of adolescent inner-city children. Third trimester urines of women enrolled in the Mount Sinai Children's Environmental Health Study between 1998 and 2002 (n = 404) were analyzed for phthalate metabolites and BPA. Mother-child pairs were asked to return for a follow-up assessment when the child was between the ages of 7 to 9 years. At this visit, mothers completed the Social Responsiveness Scale (SRS) (n = 137), a quantitative scale for measuring the severity of social impairment related to Autistic Spectrum Disorders (ASD) in the general population. In adjusted general linear models increasing log-transformed low molecular weight phthalate (LMW) metabolite concentrations were associated with greater social deficits (β = 1.53, 95% CI 0.25-2.8). Among the subscales, LMWP were also associated with poorer Social Cognition (β = 1.40, 95% CI 0.1-2.7); Social Communication (β = 1.86, 95% CI 0.5-3.2) and Social Awareness (β = 1.25, 95% CI 0.1-2.4), but not for Autistic Mannerisms or Social Motivation. No significant association with BPA was found (β = 1.18, 95% CI: -0.75, 3.11). Prenatal phthalate exposure was associated with childhood social impairment in a multiethnic urban population. Even mild degrees of impaired social functioning in otherwise healthy individuals can have very important adverse effects over a child's lifetime. These results extend our previous finding of atypical neonatal and early childhood behaviors in relation to prenatal phthalate exposure.
Keywords: Endocrine Disruptors, Phthalates, BPA, SRS, Autism Spectrum Disorder, Environmental Exposure
Introduction
Phthalates and bisphenol A (BPA) are high volume synthetic chemicals with annual production in the billions of pounds worldwide. They are commonly found in consumer products and human exposure arises from inhalation, ingestion, and dermal contact with such products. The Centers for Disease Control and Prevention (CDC) as part of the National Health and Nutrition Examination Survey (NHANES) has consistently found urinary concentrations of phthalates and BPA in a representative sample of the U.S. general population aged 6 years and older (CDC 2009). The heightened concern surrounding exposure to these ubiquitous chemicals relates to their classification as endocrine disruptors (EDs), hormonally-active compounds linked to reproductive toxicity in both animals and humans (CERHR 2007; NRC 2008). High molecular weight (HMW) phthalates such as di(2-ethylhexyl) phthalate (DEHP) are plasticizers found in polyvinyl chloride (PVC) plastics, some food packaging, intravenous tubing, building materials, and children's toys. Low molecular weight (LMW) phthalates, including diethyl phthalate (DEP) and dibutyl phthalate (DBP), are common constituents of personal care products (e.g., cosmetics, shampoos and nail polish), fragrances, and in the coating of certain medications (NRC 2008). BPA is a monomer used in the manufacture of polycarbonate re-usable bottles among other consumables, in epoxy resins that coat and protect the insides of metal food containers, and in dental composites and sealants (CERHR 2007). Based on NHANES data, BPA and phthalates have been detected in over 90% of the US general population (CDC 2009). Additional biomonitoring studies have found detectable levels of these industrial compounds in a wide range of body tissues including urine, blood, placenta, amniotic fluid and breast milk (CERHR 2007; NRC 2008).
Phthalates exhibit anti-androgenic or weakly estrogenic activity while BPA acts as a weak estrogen; both compounds appear to adversely impact thyroid hormone regulation (Crofton 2008; Boas et al. 2010). The neuroendocrine actions of these EDs may interfere with hormone-sensitive periods of neural development including neuronal differentiation, growth, and synapse formation and subsequent behavior (Colborn 2004). Prenatal BPA exposure in rodent studies is associated with aggressive behavior, memory impairment, increased anxiety, and hyperactivity (Kawai et al., 2003; Miyagawa et al., 2007; Tian et al., 2010). These effects may underlie maladaptive behaviors in humans as well, although epidemiological data is limited. In our own cohort, we have observed associations between higher maternal phthalate exposure and more atypical neonatal behaviors, specifically poorer scores for the Orientation and Motor scales and overall Quality of Alertness, as well as more externalizing behavior problems (i.e., hyperactivity, aggressiveness) and poorer executive functioning in later childhood (Engel et al., 2009; Engel et al., 2010).
Social responsiveness is based in large part on how the brain processes and responds to external social cues (Adolphs 2001). Some pediatric clinical disorders that commonly present with impaired reciprocal social behavior include ASD, Attention-Deficit Hyperactivity Disorder (ADHD), Oppositional Defiant Disorder (ODD) and learning disabilities (Reiersen et al., 2007; Friedman et al., 2003; Carpenter et al., 2009; Bhaumik et al., 1997). Subclinical forms of social impairment, often referred to as autistic-like traits, appear to extend into the general population (Constnantino and Todd 2003; Hoekstra et al., 2007; Ronald et al., 2005). The Social Responsiveness Scale (SRS) is the first widely used quantitative method of identifying autistic traits and problems with social-relational skills related to ASDs in the general population (Constantino and Gruber 2005). Impaired social functioning and autistic traits may serve as markers of maladaptive development associated with early environmental toxicant exposures. We hypothesize that prenatal environmental toxicants such as phthalates and BPA may contribute to some forms of childhood social impairment.
Materials and Methods
The Mount Sinai Children's Environmental Health study is a prospective multiethnic cohort of primiparous women who delivered at Mount Sinai Hospital between May 1998 and July 2002 (Berkowitz et al., 2003; Berkowitz et al., 2004). Of the 479 mothers recruited, seventy-five were excluded for medical complications (n = 3), infant or fetal demise (n = 2), very premature births (delivery at < 32 completed weeks or < 1,500 g) (n = 5), miscarriage (n = 1), delivery of an infant with genetic abnormalities or malformations (n = 5), inability to collect biologic specimens before birth (n = 12), change of hospital or residence outside New York City (n = 28), or loss to follow-up or refusal to continue to participate (n = 19) (Wolff et al., 2008) leaving 404 for whom birth data were available. Recruitment occurred through a prenatal care center serving the predominantly minority East Harlem population and from one of two private practices on the Upper East Side of Manhattan. Participants completed a questionnaire regarding sociodemographic characteristics, medical history, and lifestyle factors during the third trimester of pregnancy. Women were invited to return for three follow-up visits when their children were between 4 and 9 years of age. At the last childhood evaluation, which occurred between the ages of 7 to 9, mothers completed the Social Responsiveness Scale (SRS) (n = 137), a 65-item caregiver/educator rating scale of social behaviors characteristic of autism spectrum and related disorders. The study was approved by the Institutional Review Board of Mount Sinai School of Medicine; participants provided written informed consent before the study.
The SRS is a well-validated quantitative scale for detecting and measuring the severity of autistic behavior. Each SRS item rates the frequency of a particular behavior using a 4-point Likert scale (0 to 3 points for each item), with higher scores indicating a higher degree of autistic symptoms (Constantino and Gruber 2005). The SRS generates a clinically-relevant standardized total score (total T-score); the subscale T-scores include Social Awareness, Social Cognition, Social Communication, Social Motivation, and Autistic Mannerisms provide more in-depth analysis of the total score when the instrument is used in clinical settings, but they are not diagnostic. The Social Awareness subdomain measures one's ability to discern social cues and sensory aspects of social interactions. The Social Cognition subdomain measures deficits in interpreting social cues and other cognitive functions. The Social Communication subdomain captures deficits in pragmatic and expressive communication, which are the primary deficits seen in high-functioning children with ASDs. The cumulative deficits in these subdomains may reflect higher level socialization difficulties and impairments in both engaging in and interpreting fast-paced social interactions. In contrast, the more classically autistic behaviors captured by the Social Motivation (e.g. avoiding social interactions) and Autistic Mannerisms (e.g. highly restricted interests, stereotypical motor activity) subdomains reflect more severe ASD symptoms.
T-Scores have a mean of 50 and a standard deviation of 10 and have been calculated separately for males and females. Higher total T-scores on the SRS indicate greater severity of social impairment in the autism spectrum. T-scores between 60 and 75 indicate deficiencies in social behavior that are clinically significant and may interfere with everyday social interactions; scores greater than 75 are strongly associated with a clinical diagnosis of Autistic Disorder, Asperger's Disorder, or more severe cases of Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS) and severe interference with everyday social interactions (Constantino and Gruber 2005). The standardization sample showed no evidence of an age effect and the various ages groups differed by no more than 0.2 standard deviations from the overall group mean. The SRS has good retest temporal stability, parent-parent and parent-teacher interrater agreement and discriminate and concurrent validity (Constantino and Gruber 2005). The parent-report SRS exhibits substantial agreement with the Autism Diagnostic Interview-Revised (ADI-R), a widely recognized gold standard for establishing a research diagnosis of autistic disorder (Constantino et al., 2003).
Maternal spot urine samples were collected during pregnancy between 25 and 40 weeks (mean of 31.2 weeks) gestation and analyzed by the CDC for 10 individual phthalate metabolites and the phenolic compound, BPA as described in detail elsewhere (Kato et al., 2005; Ye et al., 2005). Urinary concentrations of the biomarkers were examined both as micrograms per liter and corrected for urine dilution as micrograms per gram creatinine (μg/gC). To limit the influence of multiple comparisons on our findings, phthalate metabolites were grouped into micromolar sums (μmol/L) of high-molecular-weight (> 250 Da) monoester metabolites (HMW) and low molecular-weight (< 250 Da) monoester metabolites (LMW). The phthalate metabolites within each grouping represent similar molecular structure, biological activity, and sources of exposure as the parent diester (Wolff et al., 2008). The individual phthalate metabolites and the high and low molecular weight micromolar sums were used as continuous variables in multivariable analysis. Limits of detection (LOD) were in the low microgram per liter (μg/L) range. Phthalate metabolites (except for monomethyl phthalate, MMP) and BPA were detectable in over 90% of our subjects; those below the LOD were assigned the value of LOD divided by the square root of 2 (Hornung and Reed 1990). Continuous biomarker values and creatinine were natural log transformed (ln) to produce more normal distributions. We accounted for urine dilution by including log-creatinine in models where biomarkers were continuous log-transformed variables.
Data were analyzed using SAS version 9.2 (Cary, NC). Phthalate metabolites and BPA urinary concentrations were transformed using the natural log to approximate a normal distribution. After examining unadjusted correlations of concentrations of BPA and phthalate metabolites to SRS total score, general linear models (using PROC GLM) were used to analyze relationships between natural log transformed biomarker concentrations and continuous SRS total and subscale scores. The following were considered as potential confounders or covariates: maternal age (continuous variable), maternal IQ, marital status at the time of follow-up (single caretaker versus living with both parents), maternal education (less than high school versus more than a high school), child race (non-Hispanic white, non-Hispanic black, or Hispanic), sex, child IQ, exact age at examination, and urinary creatinine. Backward elimination was used to arrive at the final adjusted models. Covariates were eliminated from the full model if their exclusion caused less than a 10% change in the exposure beta coefficient of the full model for the SRS Total Score. This final parsimonious model was applied to the SRS subscales for continuity and comparability. Maternal age was not associated with SRS score. Maternal IQ and child IQ were negatively associated with total SRS score, but they did not change the main effect of the model. Interactions of prenatal phthalate and phenols exposure with child sex were tested and found to be nonsignificant (p > 0.10). In BPA analyses, models were run with and without outliers. Outliers were detected using an approach that detects influential observations according to their effect on predicted values (implemented in SAS via the Difference in Fit Statistic (DFFITS)) and observations that appear to be inconsistent with the rest of the data (implemented in SAS via the studentized deleted residuals (RSTUDENT)). The specific criteria were DFFITS greater than 2, and RSTUDENT greater than the absolute value of 2.
Results
Maternal and infant characteristics of the original cohort have been previously reported (Engel et al., 2007). Slight differences were observed in the characteristics of women who completed the SRS versus the original birth cohort (Table 1). Compared with the original birth cohort (n=404), the women who returned for follow-up (n=137) were more likely to be single or divorced and had attained a higher level of education; median urinary concentrations of the low and high molecular phthalates metabolites were similar between the cohorts while BPA was slightly lower in the follow up cohort (Table 2). The median LMW phthalate metabolite concentration for our population was 419 μg/L. The median urine BPA concentration for our study population was1.2 μg/L, lower than the NHANES 2003-2004 median level of 2.6 μg/L (Calafat et al., 2008). The LMW phthalate metabolite concentrations for thirty-one (22.6%) children who met the threshold for “Mild to Moderate” (SRS T-score of 60 to 74) or “Severe” (SRS T-score ≥ 75) social impairment in this population were 460 μg/L (n = 25) and 1260 μg/L (n = 6), respectively (Table 3). Although for phthalates and BPA the median exposure level in the Severe Social Impairment category was the highest, there was a wide range of exposure in this group and few cases of Severe Impairment overall.
Table 1. Comparison of Maternal Characteristics in Original Birth Cohort (N = 404) with those at 7 to 9 Year Follow Up (N = 137).
| Characteristics of Population | Original Cohort | Follow Up Cohort | ||
|---|---|---|---|---|
| N | % | N | % | |
| Maternal Age at Enrollment (years) | ||||
| < 20 | 142 | 35.1 | 47 | 34.3 |
| 20 – 24 | 132 | 32.7 | 50 | 36.5 |
| 25 – 29 | 44 | 10.9 | 16 | 11.7 |
| ≥ 30 | 86 | 21.3 | 24 | 17.5 |
| Maternal Race/Ethnicity | ||||
| White | 86 | 21.3 | 21 | 15.3 |
| Black | 112 | 27.7 | 45 | 32.9 |
| Hispanic | 200 | 49.5 | 71 | 51.8 |
| Other | 6 | 1.5 | 0 | 0 |
| Maternal Education | ||||
| < High School | 118 | 29.2 | 18 | 13.1 |
| High School | 83 | 20.5 | 26 | 19.0 |
| Some College | 103 | 25.5 | 58 | 42.3 |
| ≥ College Degree | 100 | 24.8 | 30 | 21.9 |
| Marital Status at Enrollment | ||||
| Married | 117 | 29.0 | 36 | 26.3 |
| Living w/ Baby's Father | 98 | 24.3 | 23 | 16.8 |
| Single/Divorced/Widowed | 189 | 46.8 | 76 | 55.5 |
| Smoke during Pregnancy (ever) | 67 | 16.6 | 8 | 5.2 |
| Alcohol during Pregnancy (ever) | 59 | 14.9 | 23 | 15.0 |
| Mother is Primary Caretaker | 112 | 73.2 | ||
| Breastfeeding | ||||
| < 1 month | 61 | 43.9 | ||
| 1-4 months | 43 | 30.9 | ||
| >4 months | 35 | 25.2 | ||
Table 2.
Comparison of Median Maternal Biomarker Concentrations in Original Birth Cohort with those at 7 to 9 Year Follow Up.
| Urine Biomarker | N | Original Cohort, μg/L (IQR) | N | Follow Up Cohort, μg/L (IQR) |
|---|---|---|---|---|
| BPA | 404 | 1.3 (0.7–2.3) | 134 | 1.2 (0.5–2.0) |
| ΣHMW Phthalatesa | 404 | 120 (61–250) | 137 | 125 (49–278) |
| ΣLMW Phthalatesb | 404 | 430 (175–1,090) | 137 | 419 (158–1,015) |
| MEP | 380 (137-1,010) | 372 (130–964) | ||
| MBP | 36 (16-75) | 33 (15–87) | ||
| MiBP | 6.2 (2.7-12) | 6.5 (2.9–15) | ||
| MMP | 1.6 (1-3.8) | 1.8 (0.7–3.9) |
IQR = Interquartile Range;
ΣHMW Phthalates = DEHP metabolites [MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxylhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate and MEHP, mono(2-ethylhexyl) phthalate], MBzP, monobenzyl phthalate and MCPP, mono(3-carboxypropyl) phthalate.
ΣLMW phthalates = MMP, monomethyl phthalate; MEP, monoethyl phthalate; MBP, monobutyl phthalate; and MiBP, mono-isobutyl phthalate).
Table 3. Prenatal Biomarker Concentrations (μg/L) in Relation to Severity of Social Impairmenta.
| Clinical Category (SRS T-Score) | N | LMW Phthalates Median, μg/L(IQR) | HMW Phthalates Median, μg/L(IQR) | N | BPA Median, μg/L(IQR) |
|---|---|---|---|---|---|
| Normal Range (< 60) | 106 | 328 (147 - 831) | 170 (65 - 362) | 103 | 1.10 (0.50 - 2.10) |
| Mild Social Impairment (60–74) | 25 | 460 (224 - 1124) | 159 (92 - 316) | 25 | 1.40 (0.90 - 2.00) |
| Severe Social Impairment (≥ 75) | 6 | 1260 (544 - 2863) | 318 (160 - 433) | 6 | 1.45 (1.00 - 2.00) |
IQR = Interquartile Range
Wilcoxon rank-sum tests comparing the medians of mild + severe social impairment versus normal range were not significant. (LMW phthalates, p = 0.09; HMW phthalates, p = 0.54; BPA, p = 0.24).
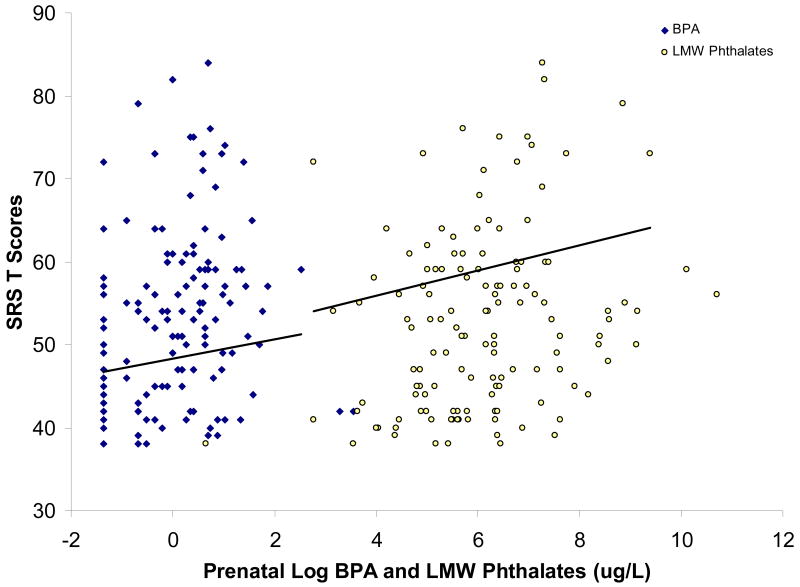
In unadjusted models, we found a positive correlation of LMW phthalate (Spearman rho = 0.24. p = 0.005) and BPA level with total SRS (Spearman rho = 0.25, p = 0.004) (Figure 1). Positive correlations reflect poorer SRS scores with increasing BPA and phthalate metabolite concentrations. In multivariable adjusted linear models, we examined the association of continuous urinary concentrations of phthalate molar sums, individual phthalate metabolites and BPA with SRS total T-score (Table 3). LMW phthalate concentrations were strongly related to total SRS score and a number of subscales. Each log-unit increase in LMW phthalate metabolite concentration was associated with higher SRS scores. Among the subscales, LMW phthalate metabolite concentrations were positively associated with poorer scores on Social Cognition, Social Communication, and Social Awareness, but not for Social Motivation or Autistic Mannerisms (Table 4). Furthermore, the regression coefficients for total and subscale scores were generally consistent in direction and comparable in precision. Of the individual LMW phthalate metabolites, only MEP was statistically significant, consistent with the major contribution of MEP to the total LMW phthalate sum; however, the effect size was, in general, consistent for monobutyl phthalate (MBP), MEP and MMP. There were no consistent associations of SRS scores with HMW phthalate metabolites. For BPA, regression coefficients were consistent with LMW phthalates in direction, but with a weaker main effect estimate and measured with substantially less precision. Additionally, although there was no significant interaction between creatinine concentration and BPA, the BPA effect appeared to be sensitive to urinary dilution and BPA concentration, suggesting a higher likelihood of exposure misclassification when urines were dilute or exposure values were very low. The large number of participants with BPA concentrations near the LOD and the lower BPA exposure levels in our study population may partly explain the weak overall association we observe for BPA in relation to total SRS score. When influential outliers are excluded from the full BPA model (n = 6), however, the magnitude of the BPA associations for total and subscale scores are quite similar to those for LMW phthalates although the precision is unchanged (Table 4).
Figure 1. Prenatal BPA and LMW Phthalate urine biomarkers in relation to SRS T Scores at age 7 to 9 years.
Scatter plot of total SRS scores and log urinary LMW phthalate and BPA levels (both in μg/L) for each study participant; the regression lines are adjusted for child race, sex, caretaker marital status and urinary creatinine as described in Table 4. The intercept represents the total SRS score among white women who are married or living with partner, and the index child is female. The urinary creatinine and toxicant levels are centered at the geometric mean.
Table 4.
Beta Coefficients and 95% Confidence Intervals for Prenatal Urine Biomarkers in Relation to Social Responsiveness Scale Scores (N = 137).
| SRS T-Scores | ΣLMWPa | MBP | MEP | MMP | ΣHMWPb | ΣDEHPc | BPA (N = 134) | BPA (N = 128)d |
|---|---|---|---|---|---|---|---|---|
| Total Score | 1.53 (0.25, 2.82)* | 1.37 (-0.43, 3.17) | 1.38 (0.23, 2.53)* | 1.29 (-0.65, 3.24) | 0.84 (-0.81, 2.48) | 0.83 (-0.69, 2.35) | 1.18 (-0.75, 3.11) | 1.73 (0.02, 3.45)* |
| Subscale Scores | ||||||||
| Cognition | 1.40 (0.07, 2.74)* | 1.24 (-0.62, 3.10) | 1.28 (0.10, 2.47)* | 1.69 (-0.31, 3.68) | 0.64 (-1.06, 2.34) | 0.76 (-0.81, 2.33) | 0.82 (-1.16, 2.81) | 1.50 (-0.22, 3.22) |
| Communication | 1.86 (0.48, 3.24)* | 1.85 (-0.08, 3.78) | 1.67 (0.44, 2.90)* | 1.32 (-0.77, 3.42) | 0.87 (-0.89, 2.64) | 0.83 (-0.81, 2.47) | 1.00 (-1.09, 3.09) | 1.77 (-0.01, 3.56) |
| Mannerisms | 0.88 (-0.50, 2.26) | 1.3 (-0.60, 3.21) | 0.77 (-0.46, 2.00) | 1.59 (-0.46, 3.64) | 0.73 (-1.01, 2.47) | 0.46 (-1.16, 2.07) | 1.17 (-0.88, 3.22) | 1.39 (-0.52, 3.30) |
| Motivation | 0.83 (-0.35, 2.02) | 0.28 (-1.36, 1.92) | 0.77 (-0.28, 1.83) | 0.25 (-1.52, 2.03) | 0.59 (-0.90, 2.08) | 0.56 (-0.82, 1.94) | 0.82 (-0.93, 2.57) | 0.95 (-0.79, 2.68) |
| Awareness | 1.25 (0.09, 2.42)* | 0.63 (-1.01, 2.26) | 1.10 (0.06, 2.14)* | 0.19 (-1.58, 1.95) | 0.66 (-0.82, 2.15) | 0.90 (-0.47, 2.28) | 1.35 (-0.41, 3.10) | 1.62 (-0.17, 3.40) |
Adjusted for child race, sex, caretaker marital status and urinary creatinine.
ZLMW phthalates = MMP, MEP, MBP and MiBP.
ΣHMW = MBzP, MCPP and
ΣDEHP [MECPP, MEHHP, MEOHP and MEHP].
excluded outliers.
p < 0.05
Discussion
Our findings add to the growing body of literature examining industrial chemical exposures and developmental neurotoxicity in humans. A few studies have suggested that there may be an association between postnatal phthalate exposure and atypical social functioning in school-aged children. One ecological study found that children 1 to 3 years of age who lived in homes with PVC flooring, an important indoor source of phthalate dust, had higher rates of ASD when followed up 5 years later (Larson et al., 2009). A cross-sectional study in Korean children demonstrated a strong positive association between the urinary concentrations of some phthalate metabolites and ADHD symptoms among school-age children (Kim et al., 2009). This is the first study to focus on social behavior in school-aged children in relation to prenatal exposure to BPA and phthalates.
The prenatal period is uniquely vulnerable to the effects of EDs on maternal and fetal sex hormones in brain development (Zoeller 2007; Gore and Crews 2009). Both estrogen and testosterone are major modulators of brain biochemistry and behavior. They regulate and interact with neurotransmitters and influence the structural and functional organization of the brain (Rubinow 1996). They also influence the differentiation of sexually dimorphic brain regions involved in behavior, learning, memory, mood and socialization (Breedlove 1994). Some studies have suggested that the child's gender modifies the effect of prenatal exposure to BPA and phthalates. Prenatal exposure to some phthalates was found to be associated with less male-typical behavior (i.e., toy and activity preferences, play styles) in 3 to 6 year old boys (Swan et al., 2009). Higher prenatal BPA urinary concentrations were associated with externalizing behaviors in female offspring at 2 years of age (Braun et al., 2009). We found no substantial interaction between sex and phthalate or BPA exposures. Our analysis was conducted using samples collected in the third trimester whereas the findings reported by Braun et al. and Swan et al. were in relation to urine metabolites measured at mid-pregnancy. We also believe the prenatal hormonal milieu affects broader aspects of cognition and memory that may overshadow sex differences (MacLusky and Naftolin, 1981; McCarthy, 2009).
Phthalates and BPA appear to interfere with thyroid hormone regulation, which may also explain the lack of sex-specific associations between prenatal exposure and childhood behavior (Crofton et al. 2008; Ghisari et al. 2009; Zoeller et al. 2005). Meeker et al. (2007) reported an inverse association between phthalates and thyroxine (T4) levels in adult men (Meeker et al., 2007). Low serum free T4 in a cohort of pregnant women was associated with high urinary concentration of monobutyl phthalate, a metabolite of dibutyl phthalate during the second trimester (Huang et al., 2007). BPA appears to bind to the thyroid receptor (TR) and significantly inhibits TR-mediated gene activation at doses as low as 10 μM (Moriyama et al. 2002); BPA acted as a selective thyroid receptor antagonist in both rat pups (Zoeller et al. 2005) and in mouse oligodendrocyte precursor cells (Seiwa et al. 2004). Nakamura et al. (2006) found that prenatal exposure to low-doses of BPA in pregnant mice altered thyroid receptor expression in the fetal neocortex. The consequences of subclinical perinatal hypothyroxinemia on neurological outcomes in the offspring are well described (Haddow and Thyroid Study Group 2005; Pop and Vulsma 2005; Berbel et al. 2009). Experimental animal models have shown that transient intrauterine deficits of thyroid hormones result in permanent alterations of cerebral cortical architecture consistent with those observed in brains of patients with autism (Roman 2007; Sadamatsu et al., 2006).
Many of the families recruited into the original cohort were difficult to locate at the 7 to 9 year visit due to changes in residence or contact information. Despite the attrition that occurred over the ten year study period, we do not believe selection bias would account for our findings. There were no significant differences with respect to median urine concentrations of phthalate metabolites although BPA urine concentrations were slightly lower in the follow up cohort. One of the inherent limitations of using a parent rating survey is the potential for parental reporting bias. Although parents may over- or underreport symptoms on the SRS, misclassification on the SRS alone is not likely to result in bias of the effect estimates unless it is also differential by exposure (i.e. highly exposed more likely to over-report). This may be true, but would be predicated on the assumption that women knew their exposure levels. Mothers in our study were not aware of their phthalate metabolite or BPA urine concentrations; moreover, the study staff conducting interviews was also unaware of maternal exposure levels. An alternative explanation could be that our study population was in some way uniquely susceptible to the effects of endocrine disruption, i.e., a population at higher risk for childhood neurobehavioral or neuropsychiatric disorders. If mothers who noted problems with their children were more likely to return for clinic assessments (perhaps in order to receive free and independent evaluations of their child) and these children were more uniquely susceptible to the effects of EDs, then our effect estimates may be over-estimated (i.e. exposure-outcome effect of those who returned is different than that of the study base). This rationale is at best speculative, however, given that very little is known about susceptibility factors underlying ED exposures and effects. It is, therefore, essential that these results are replicated in an independent population, preferably one at lower risk for neurobehavioral disorders in childhood.
Both BPA and phthalates have short biological half-lives, therefore a single urine sample in third trimester may not adequately reflect long-term exposure levels to these chemicals and only represents one time point along a continuum of brain development; however, in the case of phthalates, urine biomarker measurements appear to be stable over periods of days to months perhaps due to consistent patterns of exposure to phthalate-containing personal care products (Adibi et al., 2008). Furthermore, the proliferation, migration and differentiation of certain cell populations within the cerebellum and hippocampus and ongoing developmental processes such as myelination and synaptogenesis are particularly sensitive to thyroid hormone status during the latter half of pregnancy (Howdeshell 2002; Bernal 2005). The neurodevelopmental endpoints we observed may also characterize exposures occurring during the major brain growth spurt that begins in the third trimester (Dobbing 1974).
Our study did not rely on the clinical diagnosis of ASD but only on symptoms common to the disorder. We can only report that phthalate metabolites were associated with autistic symptomatology in a non-referred cohort of healthy adolescents. With respect to MEP, for which we observed our strongest associations, there are currently four human studies suggesting its reproductive toxicity (Colon et al., 2000; Duty et al., 2003; Main et al., 2006; Swan et al., 2005). The parent diester of MEP, diethyl phthalate, is widely used in personal care products with fragrances and therefore MEP may serve as a marker of overall phthalate exposure or as a surrogate for other potentially toxic ingredients present in the same products.
Linkage studies and monozygotic twin concordance rates for autism attest to a predominantly genetic mode of inheritance; however, broader phenotypes that include communication and social disorders suggest that epigenetic and environmental factors may contribute to the variable expression of autistic-like traits (Muhle et al. 2004). Even when the level of social impairment falls below the threshold for an ASD, any existing impairment can intensify behavior problems and psychiatric conditions other than autism and PDD-NOS (Constantino, Hudziak, & Todd 2003). Social impairment is considered a reliable predictor of long-term outcome in both ASD and ADHD and without targeted interventions these social deficits often increase with age (Soorya and Halpern 2009). While the effect sizes we observed were relatively modest, even small perturbations in neurocognitive development at the individual level translate into clinically evident impairment across the population (Bellinger 2007).
Our findings also suggest an association between LMW phthalates and more autistic-like behavior on the subscales of Social Communication, Social Cognition, and Social Awareness. These subscales represent facets of the same underlying deficit in reciprocal social behavior. The milder behavior problems encompassed by these subdomains may be more relevant to an otherwise healthy population such as our study sample, while classic autistic behaviors captured by the Social Motivation and Autistic Mannerisms subscales may be more characteristic of a referred population (Constantino and Gruber 2005).
BPA-containing baby bottles are being phased out in many parts of the country and the 2008 Consumer Product Safety Improvement Act banned several phthalates from children's toys; however, BPA and phthalates are still widely used in many consumer goods and are rarely listed on labels of product ingredients (CERHR 2007; NRC 2008). Furthermore, mixtures of phthalate compounds and repeated daily exposures to both phthalates and BPA may potentiate their individual effects (Gray et al. 2006; NRC 2008). Finally, ongoing exposure to these endocrine disruptors in the postnatal period may be independently associated or may act cumulatively with prenatal exposure to increase the risk of atypical childhood behavior. Regulatory policies must account for the aggregate of mechanisms, pathways and mixtures that may give rise to these subtle health effects.
Acknowledgments
This research was supported by NIEHS/EPA Children's Center grants ES09584 and R827039, The New York Community Trust, The Mount Sinai Children's Environmental Health Center, ATSDR/CDC/ATPM and NICHD 5T32HD049311. The authors would like to thank Rebecca Bausell for assistance with chart reviews and Ella Samandar, James Preau, Amber Bishop and John A. Reidy (CDC, Atlanta, GA) for technical assistance in measuring the concentrations of phthalate and bisphenol A.
Abbreviations
- ASD
Autism Spectrum Disorder
- BBP
Benzylbutyl Phthalate
- BPA
Bisphenol A
- CI
Confidence Interval
- DEHP
Di(2-ethylhexyl) Phthalate
- DEP
Diethyl phthalate
- DBP
Dibutyl phthalate
- ED
Endocrine disruptor
- HMW
High molecular weight/ Phthalate
- LMW
Low molecular weight/ Phthalate
- MBP
Monobutyl Phthalate
- MiBP
Mono-iso-butyl Phthalate
- MBzP
Monobenzyl Phthalate
- MCPP
Mono(3-carboxypropyl) Phthalate
- MEP
Monoethyl Phthalate
- MECPP
Mono(2-ethyl-5-carboxypentyl) Phthalate
- MEHP
Mono(2-ethylhexyl) Phthalate
- MEHHP
Mono(2-ethyl-5-hydroxylhexyl)-Phthalate
- MEOHP
Mono(2-ethyl-5-oxohexyl) Phthalate
- MMP
Monomethyl Phthalate
- NHANES
National Health and Nutrition Examination Survey
- PDD-NOS
Pervasive Developmental Disorder-Not Otherwise Specified
- SD
Standard Deviation
- SRS
Social Responsiveness Scale
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Conflict of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, et al. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 2008;116:467–473. doi: 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Interpretation of small effect sizes in occupational and environmental neurotoxicology: Individual versus population risk. Neurotoxicology. 2007;28:245–251. doi: 10.1016/j.neuro.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Berbel P, Mestre JL, Santamaría A, Palazón I, Franco A, Graells M, et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid. 2009;19:511–9. doi: 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- Bernal J. Thyroid hormones and brain development. Vitam Horm. 2005;71:95–122. doi: 10.1016/S0083-6729(05)71004-9. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Obel J, Deych E, et al. Exposure to indoor pesticides during pregnancy in a multiethnic urban cohort. Environ Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz GS, Wetmur JG, Birman-Deych E, Obel J, Lapinski RH, Godbold JH, et al. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environ Health Perspect. 2004;112:388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S, Branford D, McGrother C, et al. Autistic traits in adults with learning disabilities. British Journal of Psychiatry. 1997;170:502–506. doi: 10.1192/bjp.170.6.502. [DOI] [PubMed] [Google Scholar]
- Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, Hilsted L, et al. Childhood exposure to phthalates - associations with thyroid function, insulin-like growth factor I (IGF-I) and growth. Environ Health Perspect. 2010;118:1458–1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braissant O, Wahli W. Differential expression of peroxisome proliferator-activated receptor-alpha, -beta, and -gamma during rat embryonic development. Endocrinology. 1998;139:2748–2754. doi: 10.1210/endo.139.6.6049. [DOI] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–52. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM. Sexual differentiation of the human nervous system. Annu Rev Psychol. 1994;45:389–418. doi: 10.1146/annurev.ps.45.020194.002133. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter Rich E, Loo SK, Yang M, Dang J, Smalley SL. Social functioning difficulties in ADHD: Association with PDD risk. Clin Child Psychol and Psychiatry. 2009;14:329–344. doi: 10.1177/1359104508100890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Fourth National Report on Human Exposure to Environmental Chemicals. [January 1, 2010]; Available at: http://www.cdc.gov/exposurereport/
- Center for the Evaluation of Risks to Human Reproduction . NTP-CERHR Expert Panel Report on the Reproductive and Developmental Toxicity of Bisphenol A. Research Triangle Park, NC: National Toxicology Program; 2007. [January 1, 2010]. Available at: http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPAFinalEPVF112607.pdf. [Google Scholar]
- Colborn T. Neurodevelopment and endocrine disruption. Environ Health Perspect. 2004;112:944–9. doi: 10.1289/ehp.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon I, Caro D, Bourdony CJ, Rosario O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ Health Perspect. 2000;108:895–900. doi: 10.1289/ehp.108-2556932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM. Thyroid disrupting chemicals: mechanisms and mixtures. Int J Androl. 2008;31:209–23. doi: 10.1111/j.1365-2605.2007.00857.x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: A twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. J Am Acad Child Adolesc Psychiatry. 2003;42:458–467. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the Autism Diagnostic Interview-Revised. J Autism Dev Disord. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. The Social Responsiveness Scale Manual. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Dobbing J. The later growth of the brain and its vulnerability. Pediatrics. 1974;53:2–6. [PubMed] [Google Scholar]
- Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003;14:269–277. [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165:1397–404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, et al. Prenatal phthalate exposure and performance on the neonatal behavioral assessment scale in a multiethnic birth cohort. Neurotoxicology. 2009;30:522–8. doi: 10.1016/j.neuro.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S, Miodovnik A, Canfield R, Zhu C, Calafat A, et al. Prenatal exposure to low molecular weight phthalates and childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, Rapport LJ, Lumley M, Tzelepis A, VanVoorhis A, Stettner L, et al. Aspects of social and emotional competence in adult attention-deficit/hyperactivity disorder. Neuropsychology. 2003;17:50–58. [PubMed] [Google Scholar]
- Ghisari M, Bonefeld-Jorgensen EC. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett. 2009;189:67–77. doi: 10.1016/j.toxlet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Gore AC, Crews D. Environmental endocrine disruption of brain and behavior. In: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, Rubin RT, editors. Hormones, brain and behavior. San Diego: Academic Press; 2009. pp. 1789–1816. [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Wilson VS, Stoker T, Lambright C, Furr J, Noriega N, et al. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Thyroid Study Group Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;106:198. doi: 10.1097/01.AOG.0000169596.53498.7a. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimeier RA, Das B, Buchholz DR, Shi Y. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology. 2009;150:2964–73. doi: 10.1210/en.2008-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RH, Mitchell FE, Mann A, Chescoe D, Price SC, Nunn A, et al. Effects of phthalic acid esters on the liver and thyroid. Environ Health Perspect. 1986;70:195–210. doi: 10.1289/ehp.8670195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Verweij CJH, Boomsma DI. Heritability of autistic traits in the general population. Arch Pediatr Adolesc Med. 2007;161:372–377. doi: 10.1001/archpedi.161.4.372. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Kawai K, Nozaki T, Nishikata H, Aou S, Takii M, Kubo C. Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol A. Environ Health Perspect. 2003;111:175–8. doi: 10.1289/ehp.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology. 2009;30:822–31. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang Z, Ma M, Peng X. Analysis of environmental endocrine disrupting activities using recombinant yeast assay in wastewater treatment plant effluents. Bull Environ Contam Toxicol. 2010;84:529–35. doi: 10.1007/s00128-010-0004-2. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. The two faces of estradiol: effects on the developing brain. Neuroscientist. 2009;15:599–610. doi: 10.1177/1073858409340924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa K, Narita M, Narita M, Akama H, Suzuki T. Memory impairment associated with a dysfunction of the hippocampal cholinergic system induced by prenatal and neonatal exposures to bisphenol-A. Neurosci Lett. 2007;418:236–41. doi: 10.1016/j.neulet.2007.01.088. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–90. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- National Research Council . Committee on the Health Risks of Phthalates. Washington D.C: The National Academies Press; 2008. Phthalates and Cumulative Risk Assessment: the Tasks Ahead. [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48:464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Ribas-Fito N, Torrent M, Carrizo D, Julvez J, Grimalt JO, Sunyer J. Exposure to hexachlorobenzene during pregnancy and children's social behavior at 4 years of age. Environ Health Perspect. 2007;115:447–450. doi: 10.1289/ehp.9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. Developing brain as a target of toxicity. Environ Health Perspect. 1995;103:73–6. doi: 10.1289/ehp.95103s673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22:2715–2722. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77(9):2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Kim B, Cho S, Kim Y, Shin M, Yoo H, Kim J, et al. Phthalates exposure and attention-Deficit/Hyperactivity disorder in school-age children. Biol Psychiatry. 2009;66:958–963. doi: 10.1016/j.biopsych.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114(2):270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115(7):1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The Genetics of Autism. Pediatrics. 2004;113:e472–486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Itoh K, Yaoi T, Fujiwara Y, Sugimoto T, Fushiki S. Murine neocortical histogenesis is perturbed by prenatal exposure to low doses of Bisphenol A. J Neurosci Res. 2006;84:1197–205. doi: 10.1002/jnr.21020. [DOI] [PubMed] [Google Scholar]
- Pereira C, Mapuskar K, Vaman Rao C. A two-generation chronic mixture toxicity study of ClophenA60 and diethyl phthalate on histology of adrenal cortex and thyroid of rats. Acta Histochem. 2007;109:29–36. doi: 10.1016/j.acthis.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Vulsma T. Maternal hypothyroxinaemia during (early) gestation. Lancet. 2005;365:1604–1606. doi: 10.1016/S0140-6736(05)66489-6. [DOI] [PubMed] [Google Scholar]
- Roman GC. Autism: Transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci. 2007;262:15–26. doi: 10.1016/j.jns.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happé F, Plomin R. The genetic relationship between individual differences in social and nonsocial behaviours characteristic of autism. Dev Sci. 2005;8:444–458. doi: 10.1111/j.1467-7687.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- Rubinow DR. Androgens, brain, and behavior. Am J Psychiatry. 1996;153:974. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- Sadamatsu M, Kanai H, Xu X, Liu Y, Kato N. Review of animal models for autism: Implication of thyroid hormone. Congenit Anom (Kyoto) 2006;46:1–9. doi: 10.1111/j.1741-4520.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Seiwa C, Nakahara J, Komiyama T, Katsu Y, Iguchi T, Asou H. Bisphenol A exerts thyroid-hormonelike effects on mouse oligodendrocyte precursor cells. Neuroendocrinology. 2004;80:21–30. doi: 10.1159/000080663. [DOI] [PubMed] [Google Scholar]
- Shearer BG, Hoekstra WJ. Recent advances in peroxisome proliferator-activated receptor science. Curr Med Chem. 2003;10:267–80. doi: 10.2174/0929867033368295. [DOI] [PubMed] [Google Scholar]
- Shen O, Du G, Sun H, Wu W, Jiang Y, Song L, et al. Comparison of in vitro hormone activities of selected phthalates using reporter gene assays. Toxicol Lett. 2009;191:9–14. doi: 10.1016/j.toxlet.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Soorya LV, Halpern D. Psychosocial Interventions for Motor Coordination, Executive Functions, and Socialization Deficits in ADHD and ASD. Primary Psychiatry. 2009;16:48–54. [Google Scholar]
- Sugiyama S, Shimada N, Miyoshi H, Yamauchi K. Detection of thyroid system-disrupting chemicals using in vitro and in vivo screening assays in Xenopus laevis. Toxicol Sci. 2005;88:367–374. doi: 10.1093/toxsci/kfi330. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Liu F, Hines M, Kruse RL, Wang C, Redmon JB, Sparks A, Weiss Prenatal phthalate exposure and reduced masculine play in boys. International Journal of Andrology. 2009;33:259–269. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol Teratol. 2005;27:771–780. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Tian YH, Baek JH, Lee SY, Jang CG. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse. 2010;64:432–9. doi: 10.1002/syn.20746. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Agrawal S, Cook TJ, Knipp GT. Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure. Arch Toxicol. 2007;81:57–62. doi: 10.1007/s00204-006-0143-8. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. Quantification of urinary conjugates of bisphenol A, 2,5-dichlorophenol, and 2-hydroxy-4-methoxybenzophenone in humans by online solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2005;383:638–644. doi: 10.1007/s00216-005-0019-4. [DOI] [PubMed] [Google Scholar]
- Zoeller RT. Environmental chemicals impacting the thyroid: Targets and consequences. Thyroid. 2007;17:811–817. doi: 10.1089/thy.2007.0107. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]