Abstract
Background
Despite evidence for genetic influences on alcohol use and alcohol-related cognitions, genetic factors and endophenotypes are rarely incorporated in cognitive models of drinking behavior. This study evaluated a model of ALDH2 and drinking behavior stipulating cognitive factors and alcohol sensitivity as accounting for genetic influences on drinking outcomes.
Methods
Participants were Asian-American young adults (n = 171) who completed measures of alcohol cognitions (drinking motives, drinking refusal self-efficacy and alcohol expectancies), alcohol sensitivity, drinking behavior and alcohol-related problems as part a prospective study. Structural equation modeling (SEM) evaluated a model of drinking behavior that stipulated indirect effects of ALDH2 on drinking outcomes through cognitive variables and alcohol sensitivity.
Results
The full model provided an adequate fit to the observed data, with the measurement model explaining 63% of the variance in baseline heavy drinking and 50% of the variance in alcohol-related problems at follow-up. Associations of ALDH2 with cognitive factors and alcohol sensitivity were significant, whereas the association of ALDH2 with drinking was not significant with these factors included in the model. Mediation tests indicated significant indirect effects of ALDH2 through drinking motives, drinking refusal self-efficacy and alcohol sensitivity.
Conclusions
Results are consistent with the perspective that genetic influences on drinking behavior can be partly explained by learning mechanisms and implicate cognitive factors as important for characterizing associations of ALDH2 and drinking.
Keywords: aldehyde dehydrogenase, self-efficacy, drinking motives, alcohol expectancies, college students, structural equation modeling
Theories of alcohol-related learning propose that biological factors provide a substrate for individual variability in the reinforcement value of alcohol, which can manifest as differences in alcohol-related cognitions (e.g., Goldman et al., 1999; 2006; Kuntsche et al., 2005; Wiers et al., 2009). To date, studies evaluating genetic influences on alcohol cognitions are generally consistent with this possibility. For example, twin studies suggest moderate heritability for alcohol expectancies (e.g., Agrawal et al., 2008; Slutske et al., 2002) and drinking motives (Agrawal et al., 2008; Prescott et al., 2004). Additionally, molecular genetic studies provide initial evidence for associations of genetic variants and alcohol cognitions. Most of these studies have examined alcohol expectancies (i.e., anticipated effects of alcohol; Goldman et al., 1999), which have been associated with variation in the alcohol metabolizing genes ALDH2 (Hahn et al., 2006; Hendershot et al., 2009a; McCarthy et al., 2000; 2001) and ADH1B (Ehlers et al., 2003), as well as with GABRB3 (Young et al., 2004).
Drinking motives, which index positive and negative reinforcement reasons for drinking, are also presumed to reflect biological influences (Kuntsche et al., 2005) and appear to show higher heritability than alcohol expectancies (Agrawal et al., 2007). Although few studies have examined genetic associations with drinking motives, a recent study found associations of the OPRM1 A118G polymorphism with enhancement and coping motives for alcohol use in adolescents (Miranda et al., 2010). OPRM1 A118G has also been linked to automatic approach biases toward alcohol cues, suggesting possible associations of OPRM1 with implicit (i.e., automatic) motivational processes (Wiers et al., 2009). Cognitive and learning theories of alcohol use also emphasize the importance of self-efficacy, or the perceived ability to self-regulate drinking behavior. Drinking refusal self-efficacy, which is implicated in the risk for alcohol use disorders (Oei et al., 2005), has been associated with the ANKK1 TaqI A and GABRB3 G1 alleles (Connor et al., 2008; Young et al., 2004). In sum, there is both theoretical (Goldman et al., 1999; 2006) and empirical support that biological factors might account for variance in alcohol-related cognitions. Importantly, there is also some evidence that alcohol cognitions can partly mediate genetic influences on alcohol-related phenotypes (Agrawal et al., 2007; Hendershot et al., 2009a; McCarthy et al., 2000; Miranda et al., 2010).
Whereas cognitive-behavioral theories predict joint influences of expectancies, motives and self-efficacy on drinking behavior (e.g., Engels et al., 2005; Witkiewitz and Marlatt, 2004), genetic influences are rarely modeled in these contexts (e.g., Hendershot et al., 2007; Young et al., 2004). Moreover, few efforts have been made to include theoretically relevant endophenotypes in the context of cognitive-behavioral models. For example, physiological sensitivity to alcohol is a heritable endophenotype that is frequently examined in relation to genetic risk for alcohol dependence (e.g., Joslyn et al., 2008; Schuckit et al., 2004). Because alcohol sensitivity is proposed to influence alcohol expectancies and drinking motives (Goldman et al., 1999; Kuntsche et al., 2005) and may predict drinking in part through expectancies (Schuckit et al., 2005), this construct is an appealing endophenotype for studies addressing biological influences on alcohol-related cognitions.
The current study evaluated a cognitive model of genetic influences on drinking behavior, focusing on ALDH2 genotype. ALDH2 encodes the mitochondrial aldehyde dehydrogenase (ALDH) enzyme, the principal catalyst for oxidation of acetaldehyde during alcohol metabolism. A single nucleotide substitution at exon 12 (rs671) results in a variant allele (ALDH2*2) that encodes a functionally inactive ALDH enzyme subunit, thereby limiting oxidation of acetaldehyde during alcohol metabolism. ALDH2*2 is associated with higher levels of post-drinking acetaldehyde, increased sensitivity to alcohol (Edenberg, 2007; Peng and Yin, 2009), and significantly reduced rates of heavy drinking and alcohol dependence (Luczak et al., 2006). Few studies have evaluated cognitive or learning mechanisms relevant for these associations; however, it is proposed that biological factors (including alcohol sensitivity) can influence the subjective reinforcement value of alcohol by altering rewarding or punishing effects of consumption (e.g., Brown et al. 1999; Goldman et al., 1999). Exposure to these contingencies during drinking events is presumed to result in learned associations that can be indexed as cognitive representations of the reinforcement value of alcohol (c.f. Goldman et al., 1999). Consistent with this model, previous studies have found that ALDH2*2 is associated with alcohol expectancies, which account for significant indirect effects of ALDH2 on drinking outcomes (Hendershot et al., 2009a; McCarthy et al., 2000).
The goal of the current study was to evaluate a relatively more comprehensive cognitive model of ALDH2 and drinking behavior. The current model was informed by social learning theories of alcohol use (e.g., Abrams and Niaura, 1987; Cooper et al., 1988), which emphasize the importance of drinking motives and self-efficacy (in addition to expectancies) in predicting drinking behavior. Drinking motives have been invoked as a final common pathway for alcohol use and are often found to mediate associations of expectancies with drinking (Cooper et al., 1995; Kuntsche et al., 2005; 2007). Drinking refusal self-efficacy is frequently shown to predict drinking independent of alcohol expectancies (Engels et al., 2005; Oei & Jardim, 2007), but is not frequently conceptualized as a mediator of expectancies. Based on these considerations, the current study tested the following hypotheses: a) ALDH2*2 would be associated not only with self-reported alcohol sensitivity and alcohol expectancies, but with lower drinking motives and higher drinking refusal self-efficacy, b) cognitive factors and alcohol sensitivity would account for the association of ALDH2 with drinking behavior, such that the ALDH2-drinking association would not be significant when accounting for these factors in a multivariate model, and c) associations of ALDH2 with drinking motives and alcohol sensitivity would be mediated through expectancies, whereas the association of ALDH2 with drinking refusal self efficacy would not involve expectancies. Structural equation modeling (SEM) was used to evaluate the model in relation to heavy drinking and a prospective measure of alcohol-related problems in a sample of Asian-American young adults.
Methods
Participant recruitment and characteristics
Participants were undergraduates enrolled in a prospective study of genetic and environmental influences on drinking behavior at the University of Washington. Individuals were recruited after they completed a web-based survey on alcohol-related norms and behavior that was sent to 4,000 randomly selected undergraduates as part of a prior study. Students who completed this survey, reported 100% Chinese, Korean or Japanese heritage, and consented to follow-up contact (n = 292) were eligible for the current study. Recruitment continued until a target sample of 200 was obtained. Efforts to establish contact were unsuccessful for 44 students and 48 students declined participation. Participation included an initial laboratory session for DNA collection, followed by two assessments separated by 3 months. Assessments were delivered via the Internet (DatStat Inc., Seattle, WA). Survey completion rates were 96.5% (baseline) and 95.5% (3-month). Additional information about recruitment and baseline descriptive characteristics has been reported previously (Hendershot et al., 2009b). The current analyses excluded lifetime abstainers because some constructs (e.g., alcohol sensitivity, drinking motives) are not readily measured in abstainers. These criteria yielded a sample of 171 (92 females; 79 males; 104 Chinese 53 Korean, 14 Japanese) with a mean age of 20.32 years (SD =1.53).
Measures
Participants completed questionnaires assessing alcohol-related cognitions, drinking behavior, and alcohol-related problems at two assessment points separated by 3 months. The present analyses utilized baseline measures of cognitive and alcohol use variables and follow-up (3 month) measures of drinking problems. Questionnaire subscales/items were used to create latent variables for SEM analyses.
Drinking Motives
Motives for alcohol use were assessed with the 20-item Drinking Motives Questionnaire (DMQ; Cooper, 1994). The DMQ includes four factors reflecting reasons for drinking: enhancement (e.g., because it's exciting), social (e.g., to be sociable), coping (e.g., to forget about your problems), and conformity (e.g., to fit in with a group you like). Items are rated on a scale of 1 (almost never/never), to 5 (almost always/always). Subscale reliabilities (α) were .94 (Social), .90 (Enhancement), .86 (Coping) and .84 (Conformity).
Self-Efficacy
Self-efficacy for resisting alcohol was measured using the 19-item Drinking Refusal Self-Efficacy Questionnaire-Revised (DRSEQ-R; Oei et al., 2005). The DRSEQ-R assesses respondents' confidence for resisting alcohol in specific contexts. The DRSEQ-R has 3 subscales: Social Pressure (SP) (e.g., When my friends are drinking), Emotional Relief (ER) (e.g., When I feel frustrated) and Opportunistic (OP) (e.g., When I am by myself) (Oei et al., 2005). Items are assessed on a scale of 1 (I am very sure I would drink) – 6 (I am very sure I would NOT drink). Subscale alphas were .88 (SP), .96 (ER), and .92 (OP).
Alcohol Expectancies
Participants reported on global alcohol expectancies as well as expectancies specific to physiological effects of alcohol. Global positive and negative expectancies were measured by the 38-item Comprehensive Effects of Alcohol questionnaire (CEOA; Fromme et al., 1993), which includes subscales for positive and negative expectancies. Physiological alcohol expectancies were measured with the 23-item Physiological Effects of Alcohol Questionnaire (PEAQ). This measure was developed in a study of young adults of northeast Asian descent and validated in a prior study (Hendershot et al., 2009a). PEAQ score was calculated by averaging responses to all items. Both CEOA and PEAQ expectancies are rated based on the perceived likelihood of the drinking outcome (1 = disagree, 4 = agree).
Heavy drinking
Heavy drinking was assessed with 3 items: maximum drinks consumed in the past 30 days; number of heavy episodes in the past 30 days (defined as 4+ drinks [females] or 5+ drinks [males] within a two-hour period (NIAAA, 2003); and frequency of consuming 6 or more drinks (as measured by the Alcohol Use Disorders Identification Test; Babor et al., 2001).
Alcohol-related problems
Alcohol-related problems were assessed using two validated measures. The Rutgers Alcohol Problems Index (RAPI; White and Labouvie, 1989) is a 23-item measure that asks respondents to rate the frequency of drinking problems (e.g., tolerance/withdrawal symptoms, social/interpersonal consequences) on a scale of 0 (Never) to 4 (More than 10 times). The Young Adult Alcohol Problems Screening Test (YAAPST; Hurlbut and Sher, 1992) is a 27-item measure assessing negative consequences of drinking common among college students. Questionnaire items addressed a broad range of consequences and their relative frequencies (e.g. Have you ever gotten into trouble at work or school because of drinking?; Have you ever felt guilty about your drinking?), rated on a scale of 0 (Never) to 10 (40 or more times). For both the RAPI and YAAPST participants' responses were averaged across items. Given the 3-month follow-up period, both measures of drinking problems were administered using a reference frame of 3 months.
Alcohol sensitivity
Alcohol sensitivity was measured with the Self-Rating of the Effects of Alcohol (SRE) Form (Schuckit et al., 1997). The SRE assesses sensitivity to alcohol by asking about the number of drinks needed to notice each of four effects (feeling different, dizziness/slurred speech, stumbling/loss of coordination, involuntary sleeping/passing out) for three different drinking periods (first 5 drinking occasions, most recent 3-month period of regular drinking, period of heaviest drinking). SRE score is calculated as the total number of drinks reported across all items divided by the number of items endorsed. Higher scores indicate more drinks needed to achieve given effects (i.e., lower alcohol sensitivity).
Genotyping
Blood samples were genotyped at the Alcohol Research Center at Indiana University. Genomic DNA was isolated with the “HotSHOT” method (Truett et al., 2000) using TaqMan probes for allelic discrimination (Applied Biosystems, Foster City, CA). Specific details on genotyping procedures were reported previously (Hendershot et al., 2009b).
Statistical Analyses
Associations between ALDH2, cognitive variables, alcohol sensitivity, alcohol consumption and drinking related problems were examined using SEM. All models were estimated in Mplus V 5.21 (Muthén and Muthén, 2007) using a maximum likelihood estimator. Maximum likelihood is a favored method for estimation when the dataset contains missing information, assuming it is missing at random (Schafer & Graham, 2002). Attrition analyses revealed no significant differences on any study variables between those with missing data and those with complete data. Model fit of all models was evaluated by χ2 values, the Root Mean Square Error of Approximation [RMSEA; (Browne & Cudeck, 1993)], and the Comparative Fit Index [CFI; (Bentler, 1990)]. Models with non-significant χ2, RMSEA less than 0.06 and CFI greater than 0.95 were considered a good fit to the observed data (Hu & Bentler, 1999).
Preliminary confirmatory factor models were estimated with cognitive variables (DMQ, DRSE, and expectancy variables) to determine whether item-level or subscale-level latent variables would provide a better summary of the observed data. Results from these analyses indicated that subscale level latent variables provided a superior fit to the data, based on RMSEA and CFI. One DM subscale (Conformity) and one DRSE subscale (Opportunistic) resulted in decrement in fit for their respective latent variables; these subscales were therefore dropped from the analyses.
Latent variable measurement models that included ALDH2, cognitive factors and drinking outcomes were estimated using structural equation modeling. Relationships specified in the model were initially dictated by theoretical considerations and were modified based on estimates of model fit across successive models. We first evaluated a model stipulating that expectancies acted as mediators of the association of a) ALDH2 and drinking motives and b) ALDH2 and alcohol sensitivity. Empirically, however, models in which expectancies were included as a mediator provided a poor fit to the data (all CFIs < 0.90, all RMSEAs > 0.05). Also, results did not conform to theoretical expectations or previous research; therefore, global expectancies (CEOA) and physiological expectancies (PEAQ) were dropped from the model.
The product of coefficients method (MacKinnon et al., 2002) was used to evaluate the significance of the indirect effect of ALDH2 on drinking via self-efficacy, alcohol sensitivity, and drinking motives. Indirect effect models were estimated in Mplus (Muthén and Muthén, 2007) using a maximum likelihood estimator and 1000 bootstrap draws to obtain 95% confidence intervals for the indirect effects.
Results
Of the 171 participants, 90 had the ALDH2*1/*1 genotype, 64 were ALDH2*1/*2 and 17 were ALDH2*2/*2. The latter two groups were combined for subsequent analyses to compare individuals with versus without ALDH2*2. Bivariate associations among variables included in the final model are reported in Table 1.
Table 1.
Descriptive Characteristics and Correlations for Variables Included in the SEM
| M(SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. ALDH2 | - | - | |||||||||||
| 2. Maximum drinks | 2.99 (3.28) | −0.27** | - | ||||||||||
| 3. Heavy episodes | 0.82 (1.61) | −0.18* | 0.71** | - | |||||||||
| 4. AUDIT-3 | 0.80 (0.95) | −0.25** | 0.76** | 0.66** | - | ||||||||
| 5. RAPI | 0.12 (0.26) | −0.12 | 0.50** | 0.54** | 0.48** | - | |||||||
| 6. YAAPST | 1.57 (0.86) | −0.19* | 0.53** | 0.53** | 0.58** | 0.71** | - | ||||||
| 7. SRE | 4.68 (2.49) | −0.37** | 0.52** | 0.40** | 0.54** | 0.26** | 0.37** | - | |||||
| 8. Drinking motives–Social | 2.75 (1.22) | −0.23** | 0.52** | 0.33** | 0.48** | 0.24** | 0.29** | 0.30** | - | ||||
| 9. Drinking motives–Coping | 1.61 (0.76) | −0.23** | 0.43** | 0.39** | 0.55** | 0.36** | 0.42** | 0.42** | 0.51** | - | |||
| 10. Drinking motives–Enhancement | 2.05 (1.06) | −0.28** | 0.52** | 0.37** | 0.60** | 0.30** | 0.41** | 0.33** | 0.75** | 0.60** | - | ||
| 11. Self-efficacy–Social Pressure | 3.71 (1.34) | 0.26** | −0.50** | −0.37** | −0.51** | −0.34** | −0.33** | −0.32** | −0.54** | −0.35** | −0.47** | - | |
| 12. Self-efficacy–Emotional relief | 4.84 (1.14) | 0.16* | −0.40** | −0.37** | −0.51** | −0.37** | −0.40** | −0.30** | −0.21** | −0.50** | −0.29** | 0.64** | - |
ALDH2 coded 0 (ALDH2*1/*1), 1 (ALDH2*1/*2 or ALDH2*2/*2) AUDIT-3, Frequency of consuming 6 or more drinks; RAPI, Rutgers Alcohol Problem Index (mean item response); YAAPST, Young Adult Alcohol Problems Screening Test (mean item response); SRE, Self-Rating of the Effects of Alcohol form.
p < 0.01
p < 0.05.
Measurement Model
Preliminary model testing indicated that item-level factor models provided a poor fit to the observed data (i.e., all χ2 were significant, all CFI < .90 and all RMSEA > 1.0). Following from these results we estimated a model whereby the self-efficacy construct was indicated by the emotion regulation and social pressure subscales of the DRSE, the drinking motives construct was indicated by the coping, social and enhancement subscales of the DMQ, and the alcohol sensitivity construct was measured by the observed SRE scores (with higher scores reflecting lower alcohol sensitivity). Covariances between the emotion regulation subscale of the DRSE and coping subscale of the DMQ, as well as the social pressures subscale of the DRSE and social subscale of the DMQ were estimated to allow for shared variance across constructs. The final measurement model combining the drinking motives, self-efficacy, and alcohol sensitivity variables provided an excellent fit to the observed data (χ2 (14) = 22,67, p = 0.07, CFI = 0.99, RMSEA = 0.06, 90% CI =0.00 – 0.10).
Structural Equation Models
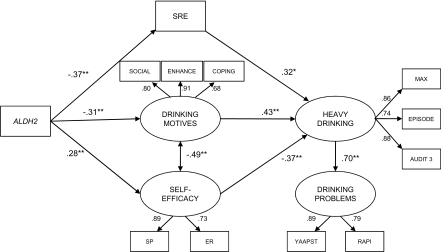
In the final model (Figure 1), alcohol sensitivity, drinking motives and self-efficacy were estimated as explaining indirect effects of ALDH2 on alcohol-related outcomes. This formulation resulted in a better fit than did a model stipulating ALDH2 effects as being fully mediated by alcohol sensitivity. The full model incorporated a) the measurement model described above, b) the heavy drinking latent variable, c) the 3-month problem drinking latent variable, and d) ethnicity and gender as observed covariates. Ethnicity was included as a dummy coded variable (0 = Chinese, 1 = Korean or Japanese), which reflected that the Chinese group had the lowest drinking rates. The full model also provided an adequate fit to the observed data based on the CFI and RMSEA (χ2 (59) = 119.18, p < 0.01, CFI = 0.95, RMSEA = 0.08, 90% CI = 0.06 – 0.09), with the measurement model explaining 63% of the variance of heavy drinking and 50% of the variance of alcohol-related problems. Additionally, heavy drinking significantly predicted drinking related problems 3 months later.
Figure 1.
Structural equation model with standardized regression coefficients for hypothesized effects. Note, some non-significant estimated paths are not shown (see Table 2 for more information). *p < 0.05; **p < 0.005. ALDH2 coded 0 (ALDH2*1/*1), 1 (ALDH2*1/*2 or ALDH2*2/*2) SRE = Self-Rating of the Effects of Alcohol form SOCIAL = Drinking Motives - Social subscale ENHANCE = Drinking Motives – Enhancement subscale COPING = Drinking Motives – Enhancement subscale SP = Drinking Refusal Self-Efficacy – Social Pressure subscale ER = Drinking Refusal Self-Efficacy – Emotional Relief subscale MAX = Maximum drinks, past 30 days EPISODE = Number of heavy episodes, past 30 days AUDIT3 = Frequency of consuming 6 or more drinks RAPI = Rutgers Alcohol Problem Index YAAPST = Young Adult Alcohol Problems Screening Test
The hypothesized paths between ALDH2 and cognitive latent variables were significant (Table 2). Notably, the relation between ALDH2 and heavy drinking was not significant with cognitive and alcohol sensitivity variables included in the model. Thus, the significant correlation between ALDH2 and the heavy drinking variables (Table 1) could be partly explained by the cognitive and alcohol sensitivity constructs. Mediation modeling indicated that the indirect effects of ALDH2 on drinking via self-efficacy, drinking motives and alcohol sensitivity measures were all significant (Self-efficacy: Indirect = −0.56, p = 0.01, 95% CI: −0.99, −0.13, % of direct effect mediated by self-efficacy = 5.8%; Motives: Indirect = −0.73, p = 0.007, 95% CI: −1.26, −0.20, % of direct effect mediated by motives = 7.9%; Alcohol sensitivity: Indirect = −0.65, p = 0.001, 95% CI: −1.03, −0.26 % of direct effect mediated by alcohol sensitivity = 10.6%). Finally, gender was significantly related to the alcohol sensitivity measure, with females reporting significantly higher levels of alcohol sensitivity. Ethnicity was not related to any of the constructs in the full model. To account for the possibility of differential effects for individuals heterozygous versus homozygous for ALDH2*2 (i.e., one copy versus two copies of ALDH2*2), the model was re-estimated eliminating the homozygous group (n = 17). Results (model fit and parameter estimates) were consistent with or without the homozygous group.
Table 2.
Unstandardized regression coefficients for path model, shown in Figure 1.
| Path | B (SE) | |
|---|---|---|
| Heavy drinking → Problems | 0.05 (0.01) | p < 0.005 |
| Motives → Heavy drinking | 1.23 (0.27) | p < 0.005 |
| Self-efficacy → Heavy drinking | −0.84 (0.27) | p < 0.005 |
| Alcohol sensitivity (SRE) → Heavy drinking | 0.36 (0.09) | p < 0.005 |
| ALDH2 → Motives | −0.59 (0.15) | p < 0.005 |
| ALDH2 → Self-efficacy | 0.67 (0.18) | p < 0.005 |
| ALDH2 → Alcohol sensitivity (SRE) | −1.81 (0.34) | p < 0.005 |
| ALDH2 → Heavy drinking | 0.37 (0.30) | p = 0.21 |
| Gender → Motives | 0.08 (0.14) | p = 0.59 |
| Gender → Self-efficacy | −0.06 (0.19) | p = 0.74 |
| Gender → Alcohol sensitivity (SRE) | −1.05 (0.36) | p = 0.004 |
| Gender → Heavy drinking | −0.57 (0.34) | p = 0.10 |
| Ethnicity → Motives | −0.06 (0.22) | p = 0.79 |
| Ethnicity → Self-efficacy | 0.12 (0.31) | p = 0.71 |
| Ethnicity → Alcohol sensitivity (SRE) | −0.59 (0.39) | p = 0.13 |
| Ethnicity → Heavy drinking | −0.18 (0.42) | p = 0.68 |
| Motives with Self-efficacy | −0.51 (0.14) | p < 0.005 |
Discussion
The current study evaluated a conceptual model stipulating that the association of the ALDH2*2 allele with decreased risk for alcohol use can be explained in part by alcohol-related cognitions as well as differences in alcohol sensitivity. The association of ALDH2 and drinking outcomes, which was significant in bivariate analyses, was not significant in a model that included cognitive factors and self-reported alcohol sensitivity. Additionally, mediation tests showed that drinking motives, drinking refusal self-efficacy and alcohol sensitivity each accounted for significant indirect effects of ALDH2 on drinking behavior. Overall, the findings are generally consistent with the perspective that biological influences on drinking behavior are potentially translated in part through alcohol-related learning (Goldman et al., 1999; 2006).
Notably, the final model diverged from theoretical predictions with respect to the importance of alcohol expectancies. Learning theories generally dictate that associations of background factors (in this case, ALDH2) and proximal cognitive factors (i.e., drinking motives) are mediated through expectancies (e.g., Cooper et al., 1995; Kuntsche et al., 2005). Empirically, drinking motives have been found to mediate the effects of expectancies in some studies (Goldsmith et al., 2009; Kuntsche et al., 2007) but not others (Engels et al., 2005). The current results were not consistent with a primary role of expectancies; in contrast, model fit was improved by eliminating expectancy variables. This finding is consistent with other reports that expectancies are not significantly associated with drinking behavior in the context of multivariate analyses that include other cognitive variables such as drinking motives and self-efficacy (e.g., Engels et al., 2005), including one prior study with Asian college students (Oei & Jardim, 2007).
This finding could be attributed in part to measurement overlap across cognitive variables. For example, whereas drinking motives are theoretically distinct from expectancies (Cooper et al., 1995), these constructs are not completely dissociable from a measurement perspective (Goldman et al., 1999). Simultaneous evaluation of motives and expectancies in multivariate models could therefore prove problematic statistically. Additionally, the measure of physiological expectancies that was evaluated in this study was significantly related to self-reported alcohol sensitivity (as measured by the SRE form), perhaps explaining why physiological expectancies did not contribute to the model as an independent measure of alcohol sensitivity.
The associations of ALDH2 with drinking motives and drinking refusal self-efficacy observed in this study have not been reported previously, raising the question of how these relationships arise. In the present sample, individuals with ALDH2*2 reported lower motivation for alcohol use in both positive reinforcement (enhancement, social) and negative reinforcement (coping) contexts. Theoretically, any biobehavioral factor that alters the reward value of alcohol can potentially influence the development of drinking motives (Kuntsche et al., 2005). Thus, a general interpretation of this finding is that aversive physiological effects of acetaldehyde, or related observable symptoms (i.e., skin flushing), render subjective experiences of alcohol use less reinforcing for those with ALDH2*2 compared to ALDH2*1/*1 individuals. If so, individuals with ALDH2*2 might be less likely to use alcohol to enhance positive states or alleviate negative states. It should be noted that this interpretation is potentially inconsistent with some experimental evidence indicating that individuals with the ALDH2*1/*2 genotype report more intense, but not necessarily more aversive responses to alcohol (Wall et al., 1992). In terms of the association of ALDH2 with self-efficacy, one interpretation is that individuals with ALDH2*2, by virtue of being more likely to avoid alcohol, acquire more experience with refusing opportunities to drink. These interpretations are speculative and would require further evaluation. Moreover, these interpretations imply that any associations between ALDH2 genotype and alcohol cognitions emerge as a function of drinking experiences; that is, drinking history presumably serves to mediate these relationships. This possibility is consistent with existing theory (Goldman et al., 1999) but could not be tested in the current study given the use of cross-sectional analyses.
Although the current analyses provide additional evidence about variables that might be important for characterizing associations of ALDH2 and drinking behavior, it is likely that additional mediating factors are important. As one example, individuals with ALDH2*2 might be less likely to affiliate with peers or environments in which drinking is likely, in which case environmental variables could serve as mediators. Additionally, in naturalistic settings, relationships between cognitive or motivational variables and substance use likely involve complex and dynamic processes that are not readily assessed using conventional methods (Witkiewitz and Marlatt, 2004). For instance, studies using ecological momentary assessment (EMA) suggest that transient shifts in outcome expectancies and self-efficacy can be important for predicting substance use behavior (Gwaltney et al., 2005). A promising approach for future studies is therefore to evaluate associations among genetic predictors, cognitive factors and drinking behavior in the context of naturalistic assessments. A recent study offers support for the influence of genetic variables on drinking behavior at the event level by showing that DRD4 and OPRM1 genotypes predicted differences in reported urge and subjective responses to alcohol in the natural environment (Ray et al., 2010). Thus, future research might examine whether genetic differences moderate associations of cognitive variables and drinking outcomes at the level of specific drinking events.
Several limitations to the current study should be noted. First, associations of cognitive variables with drinking behavior were cross-sectional, which obviates conclusions about the mediating role of cognitive factors. Reciprocal effects between cognitive factors and drinking behavior have been demonstrated (Sher et al., 1996) and the current design cannot rule out the possibility that drinking behavior has causal influences on cognitive factors rather than vice versa. However, the fact that ALDH2 predicted cognitive factors while simultaneously controlling for heavy drinking suggests that these associations are not purely due to overlap between cognitive variables and alcohol use. Additionally, the use of a short prospective period for evaluating alcohol-related problems does not allow evaluation of long-term drinking consequences. The study design is also not immune to the effects of gene-environment correlation. Individuals with ALDH2*2 have at least one parent with this allele; therefore, parental modeling of alcohol use could contribute to differences in alcohol cognitions (McCarthy et al., 2000). Ideally, these associations would be tested in a longitudinal study in which cognitive factors are assessed prior to drinking initiation and parental drinking patterns are assessed. While focusing on ALDH2 is useful in that its mechanism of influence is well characterized, examination of one genetic variant and a specific population (Asian-American young adults) limits the extent to which these findings can be generalized to other populations. As genetic variants contributing to differences in the risk for alcohol use disorders are continuality identified (Dick and Foroud, 2003), it will be increasingly important to evaluate multiple variants in relation to alcohol-related outcomes. Finally, whereas the current study focused on explicit cognitive measures assessed via self-report, the importance of implicit (i.e., automatic) cognitive processes in addiction is increasingly apparent (Wiers and Stacy, 2006). These processes would likely be relevant for future investigations of ALDH2 and drinking behavior.
Overall, the current study suggests that cognitive and learning constructs, in addition to physiological differences in alcohol sensitivity, are likely important for characterizing associations of ALDH2 and drinking behavior. Moreover, the results support the notion that relevant genetic factors and endophenotypes can be incorporated in the context of cognitive-behavioral theories of substance use, potentially increasing their predictive ability. Further efforts to identify mechanisms accounting for biological influences on alcohol use should aid in characterizing genetic risk and identifying possible avenues for intervention.
Acknowledgements
This research was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grants F31AA016440 and K02AA00269 and a Small Grant Award from the University of Washington Alcohol and Drug Abuse Institute. Genotyping services were provided by the Genomics and Molecular Biology Core of the Alcohol Research Center Indiana, which is funded by NIAAA grant P60AA07611-20.
References
- Abrams DB, Niaura RS. In: Social learning theory, in Psychological Theories of Drinking and Alcoholism. Blane HT, Leonard KE, editors. Guilford Press; New York: 1987. pp. 131–178. [Google Scholar]
- Agrawal A, Dick DM, Bucholz KK, Madden PAF, Cooper ML, Sher KJ, Heath AC. Drinking expectancies and motives: a genetic study of young adult women. Addiction. 2008;103:194–204. doi: 10.1111/j.1360-0443.2007.02074.x. [DOI] [PubMed] [Google Scholar]
- Babor TF, Biddle-Higgins JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- Bentler PM. Comparative fit indices in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tate SR, Vik PW, Haas AL, Aarons GA. Modeling of alcohol use mediates the effect of family history of alcoholism on adolescent alcohol expectancies. Exp Clin Psychopharmacol. 1999;7:20–27. doi: 10.1037//1064-1297.7.1.20. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Sage Publications; Beverly Hills, CA: 1993. pp. 136–162. [Google Scholar]
- Connor JP, Young RM, Saunders JB, Lawford BR, Ho R, Ritchie TL, Noble EP. The A1 allele of the D2 dopamine receptor gene region, alcohol expectancies and drinking refusal self-efficacy are associated with alcohol dependence severity. Psychiatry Res. 2008;160:94–105. doi: 10.1016/j.psychres.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Cooper ML. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychol Assess. 1994;6:117–128. [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: A test of social learning formulations. J Abnorm Psychol. 1988;97:218–230. doi: 10.1037//0021-843x.97.2.218. [DOI] [PubMed] [Google Scholar]
- Dick DM, Foroud T. Candidate genes for alcohol dependence: A review of genetic evidence from human studies. Alcohol Clin Exp Res. 2003;27:868–879. doi: 10.1097/01.ALC.0000065436.24221.63. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Carr L, Betancourt M, Montane-Jaime K. Association of the ADH2*3 allele with greater alcohol expectancies in African-American young adults. J Stud Alcohol. 2003;64:176–181. doi: 10.15288/jsa.2003.64.176. [DOI] [PubMed] [Google Scholar]
- Engels RCME, Wiers R, Lemmers L, Overbeek G. Drinking motives, alcohol expectancies, self-efficacy, and drinking patterns. J Drug Educ. 2005;35:147–166. doi: 10.2190/6Q6B-3LMA-VMVA-L312. [DOI] [PubMed] [Google Scholar]
- Fromme K, Stroot EA, Kaplan D. Comprehensive effects of alcohol: Development and psychometric assessment of a new expectancy questionnaire. Psychol Assess. 1993;5:19–26. [Google Scholar]
- Goldman MS, Darkes J, Del Boca FK. Expectancy mediation of biopsychosocial risk for alcohol use and alcoholism. In: Kirsch I, editor. How expectancies shape experience. American Psychological Association; Washington, DC: 1999. pp. 233–262. [Google Scholar]
- Goldman MS, Darkes J, Reich RR, Brandon KO. From DNA to conscious thought: The influence of anticipatory processes on human alcohol consumption. In: Munafo M, Albery IP, editors. Cognition and Addiction. Oxford University Press; New York: 2006. pp. 147–184. [Google Scholar]
- Goldsmith AA, Tran GQ, Smith JR, Howe SR. Alcohol expectancies and drinking motives in college drinkers: Mediating effects on the relationship between generalized anxiety and heavy drinking in negative-affect situations. Addict Behav. 2009;34:505–513. doi: 10.1016/j.addbeh.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: Prediction of smoking lapse and relapse. J Abnorm Psychol. 2005;114:661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Hahn CY, Huang SY, Ko HC, Hsieh CH, Lee IH, Yeh TL, Yang YK, Lee JF, Lin WW, Lu RB. Acetaldehyde involvement in positive and negative alcohol expectancies in Han Chinese persons with alcoholism. Arch Gen Psychiatry. 2006;63:817–823. doi: 10.1001/archpsyc.63.7.817. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Neighbors C, George WH, McCarthy DM, Wall TL, Liang T, Larimer ME. ALDH2, ADH1B and alcohol expectancies: Integrating genetic and learning perspectives. Psychol Addict Behav. 2009a;23:452–463. doi: 10.1037/a0016629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Collins SE, George WH, Wall TL, McCarthy DM, Liang T, Larimer ME. Associations of ALDH2 and ADH1B genotypes with alcohol-related phenotypes in Asian young adults. Alcohol Clin Exp Res. 2009b;33:839–847. doi: 10.1111/j.1530-0277.2009.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Marlatt GA, George WH. Relapse prevention and the maintenance of optimal health. In: Shumaker S, Ockene JK, Riekert K, editors. The Handbook of Health Behavior Change. 3rd edn Springer Publishing Co; New York: 2007. pp. 127–150. [Google Scholar]
- Hu LT, Bentler PM. Cut-off criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- Hurlbut SC, Sher KJ. Assessing alcohol problems in college students. J Am College Health. 1992;41:49–58. doi: 10.1080/07448481.1992.10392818. [DOI] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, White RL. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proc Natl Acad Sci U S A. 2008;105:20368–20373. doi: 10.1073/pnas.0810970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Engels R, Gmel G. Drinking motives as mediators of the link between alcohol expectancies and alcohol use among adolescents. J Stud Alcohol Drugs. 2007;68:76–85. doi: 10.15288/jsad.2007.68.76. [DOI] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clin Psychol Rev. 2005;25:841–861. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Glatt SJ, Wall TL. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull. 2006;132:607–621. doi: 10.1037/0033-2909.132.4.607. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Erlbaum; Mahwah, NJ: 2008. [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DM, Brown SA, Carr LG, Wall TL. ALDH2 status, alcohol expectancies, and alcohol response: Preliminary evidence for a mediation model. Alcohol Clin Exp Res. 2001;25:1558–1563. [PubMed] [Google Scholar]
- McCarthy DM, Wall TL, Brown SA, Carr LG. Integrating biological and behavioral factors in alcohol use risk: The role of ALDH2 status and alcohol expectancies in a sample of Asian Americans. Exp Clin Psychopharmacol. 2000;8:168–175. doi: 10.1037//1064-1297.8.2.168. [DOI] [PubMed] [Google Scholar]
- Miranda R, Ray L, Justus A, Meyerson LA, Knopik VS, McGeary J, Monti PM. Initial Evidence of an Association Between OPRM1 and Adolescent Alcohol Misuse. Alcohol Clin Exp Res. 2010;34:112–122. doi: 10.1111/j.1530-0277.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user's guide. 5th ed. Muthén & Muthén; Los Angeles: 2007. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA) Task force on recommended questions of the National council on alcohol abuse and alcoholism. [Accessed June 2009];Recommended sets of alcohol consumption questions. 2003 Available at: http://www.niaaa.nih.gov/Resources/ ResearchResources/TaskForce.htm.
- Oei TPS, Hasking PA, Young RM. Drinking refusal self-efficacy questionnaire-revised (DRSEQ-R): a new factor structure with confirmatory factor analysis. Drug Alcohol Depend. 2005;78:297–307. doi: 10.1016/j.drugalcdep.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Oei TPS, Jardim CL. Alcohol expectancies, drinking refusal self-efficacy and drinking behaviour in Asian and Australian students. Drug Alcohol Depend. 2007;87:281–287. doi: 10.1016/j.drugalcdep.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Peng GS, Yin SJ. Effect of the allelic variants of aldehyde dehydrogenase ALDH2*2 and alcohol dehydrogenase ADH1B*2 on blood acetaldehyde concentrations. Hum Genomics. 2009;3:121–127. doi: 10.1186/1479-7364-3-2-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Cross RJ, Kuhn JW, Horn JL, Kendler KS. Is risk for alcoholism mediated by individual differences in drinking motivations? Alcohol Clin Exp Res. 2004;28:29–39. doi: 10.1097/01.ALC.0000106302.75766.F0. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Tidey JW, McGeary JE, Mackillop J, Gwaltney CJ, Rogsenow DJ, Swift RM, Monti PM. Polymorphisms of the mu-opioid receptor and dopamine D receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol. 2010;119:115–125. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. The search for genes contributing to the low level of response to alcohol: Patterns of findings across studies. Alcohol Clin Exp Res. 2004;28:1449–1458. doi: 10.1097/01.alc.0000141637.01925.f6. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE. The self-rating of the effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction. 1997;92:979–988. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Anderson KG, Brown SA, Kuperman S, Kramer J, Hesselbrock V, Bucholz K. Evaluation of a level of response to alcohol-based structural equation model in adolescents. J Stud Alcohol. 2005;66:174–184. doi: 10.15288/jsa.2005.66.174. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Wood MD, Wood PK, Raskin G. Alcohol outcome expectancies and alcohol use: A latent variable cross-lagged panel study. J Abnorm Psychol. 1996;105:561–574. doi: 10.1037/0021-843X.105.4.561. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Cronk NJ, Sher KJ, Madden PAF, Bucholz KK, Heath AC. Genes, environment, and individual differences in alcohol expectancies among female adolescents and young adults. Psychol Addict Behav. 2002;16:308–317. [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- Wall TL, Thomasson HR, Schuckit MA, Ehlers CL. Subjective feelings of alcohol intoxication in Asians with genetic variations of ALDH2 alleles. Alcohol Clin Exp Res. 1992;16:991–995. doi: 10.1111/j.1530-0277.1992.tb01907.x. [DOI] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. J Stud Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, van den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav. 2009;8:101–106. doi: 10.1111/j.1601-183X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Sage Publications Inc; Thousand Oaks, CA: 2006. [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am Psychol. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Young RMD, Lawford BR, Feeney GF, Ritchie T, Noble EP. Alcohol-related expectancies are associated with the D2 dopamine receptor and GABAA receptor β subunit genes. Psychiatry Res. 2004;127:171–183. doi: 10.1016/j.psychres.2003.11.004. [DOI] [PubMed] [Google Scholar]