Abstract
Parvulins are a group of peptidyl-prolyl isomerases (PPIases) responsible for important biological processes in all kingdoms of life. The PinA protein from the psychrophilic archaeon Cenarchaeum symbiosum is a parvulin-like PPIase. Due to its striking similarity to the human parvulins Pin1 and Par14, PinA constitutes an interesting subject for structural and functional studies. Here, we present the first high resolution NMR structure of an archaeal parvulin, PinA, based on 1798 conformational restraints. Structure calculation yields an ensemble of 20 convergent low energy structures with a backbone r.m.s.d. value of 0.6 Å within the secondary structure elements. The overall fold of PinA comprises the β-α3-β-α-β2 fold typical for all parvulin structures known so far, but with helix III being a short 310-helix. A detailed comparison of this high resolution structure of the first archaeal PinA protein with bacterial and eukaryotic parvulin PPIase structures reveals an atypically large catalytic binding site. This feature provides an explanation for cold-adapted protein function. Moreover, the residues in and around 310-helix III exhibit strong intramolecular dynamics on a microsecond to millisecond timescale and display structural heterogeneity within the NMR ensemble. A putative peptide ligand was found for PinA by phage display and was used for 1H-15N-HSQC titrations. Again, the flexible region around 310-helix III as well as residues of the peptide binding pocket showed the strongest chemical shift perturbations upon peptide binding. The local flexibility of this region also was modulated by ligand binding. A glycine and two positively charged residues are conserved in most parvulin proteins in this flexible loop region, which may be of general functional importance for parvulin-type PPIases.
Keywords: Archaebacteria, NMR, Prolyl Isomerase, Protein Folding, Protein Structure, Thaumarchaeota, Cold-adapted Protein
Introduction
Peptidyl-prolyl isomerases (PPIases)5 catalyze the cis/trans rotation around the Xaa-Pro peptide bonds in target proteins. Pin proteins are small parvulin-type PPIases found in bacteria and all eukaryotes (1) and are involved in key steps of cell cycle regulation and protein quality control. This latter function relates parvulin proteins to folding disorders in human brain tissues such as Alzheimer and Parkinson diseases (2, 3). Very little is known about the archaeal members of this highly conserved protein family (4).
The genome of the psychrophilic archaeon Cenarchaeum symbiosum (5, 6) living in association with the marine sponge Axinella mexicana encodes a small protein isomerase (PinA), the first archaeal member of the parvulin PPIase family. The symbiotic archaeon C. symbiosum belongs to a large group of marine Archaea that eluded cultivation (7). It was initially classified as a member of the phylum of Crenarchaeota, but its optimal growth temperature at 10 °C differed strongly from the ones of any other cultivated member of that phylum (5). Recently, this species was grouped into a new deep-branching archaeal phylum, Thaumarchaeota (8).
There is nothing yet known about cold-optimized parvulins or about archaeal Pin proteins in general but only the PinA protein sequence from C. symbiosum. This protein comprises 92 amino acids without N- or C-terminal extensions to its PPIase domain similar to Escherichia coli Par10. Such extensions are found in eukaryotic parvulins or bacterial members of the SurA/PrsA-type (1, 9, 10). As seen in the alignment of several parvulins (Fig. 1), PinA does not contain an extended phosphoryl-binding loop typical for Ser(P)/Thr(P)-Pro specific PPIases such as human Pin1 (11, 12).
FIGURE 1.
Alignment of parvulin sequences from different organisms. The available structures of parvulins were aligned onto the CsPinA structure using DaliLite (50). Therefore, representative structures were chosen by NMRCLUST (51) and indicated within the alignment. For the loop region of 1J6Y and 1M5Y, an additional alignment on 1NMV_08 was used. Aligned residues are written as uppercase letters. Structures represent: 1JNS, E. coli Par10; 1J6Y, Arabidopsis thaliana Pin1; 1NMV, human Pin1; 1ZK6, B. subtilis PrsA; 1EQ3, human Par14; The structure of E. coli SurA contains two PPIase domains and 1M5Y_1 and 1M5Y_2 denote the N-terminal and C-terminal PPIase domain, respectively. Information for α-helical and β-sheet conformations from the PDB codes is given in light gray below the protein sequences. Residues believed to be important for PPIase activity are labeled in white and highlighted in black when conserved in most parvulins; otherwise, residues are colored gray. Other conserved residues relative to CsPinA are highlighted in gray. 1NMV and 1J6Y contain an extended N-terminal loop typical for phosphorylation-specific PPIases. A C-terminal loop specific for eukaryotic parvulins is contained in the 1EQ3 sequence. The Xaa-Pro motif conserved in many parvulins is underlined.
Though the function of this conserved protein within the psychrophilic endosymbiont is not known, it is tempting to assume a role in cold adaptation like that seen with the FKBP-type PPIase in the psychrotrophic bacterium Shewanella (13). As a bacterium living at ambient temperatures, E. coli displays a cold shock response. Its ribosome-associated cold shock response protein YfiA inhibits translation at temperatures lower than 16 °C (14, 15). Enzymatically active PPIases should be highly important for the cold-loving archaeon C. symbiosum as the rates for the catalyzed and spontaneous cis/trans isomerization differ dramatically at lower temperatures (4). Of note, the C. symbiosum genome only contains two cyclophilins (RefSeq accession nos. YP_876752 and YP_876758), one FK506-binding protein (FKBP) (YP_876474), and the parvulin PinA under study. This is in stark contrast to the PPIase repertoire of E. coli with a total of eight PPIase genes.6 Instead of facilitating the Xaa-Pro bond rotation at low temperatures, PPIases with little isomerization activity can function as binding modules for important peptidic structures or play as yet unknown cellular functions (1).
The current work aims to understand the first member of archaeal parvulins in terms of structure and dynamics as well as interaction with a ligand. We report the first high resolution structure of an archaeal parvulin, PinA, from the cold-loving archaeon C. symbiosum. The putative peptide binding site is clearly larger than that of any eukaryotic or bacterial parvulins known so far. Backbone dynamics studies indicate amino acids in and around 310-helix III to be significantly more flexible than those in the rest of the protein. A potential ligand for PinA was derived from phage display and binding involved residues Ser44, Gly55, Gly57, Lys58, Val60, and Phe83 located around 310-helix III and the catalytic binding pocket. Considering that C. symbiosum has fewer PPIases than E. coli, our study represents a step forward in explaining the molecular basis of cold adaptation of cis/trans isomerization in psychrophilic Archaea. Moreover, the flexible loop contributing to ligand binding might be a general feature in all parvulins known to date.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
A coding sequence of the PinA protein from C. symbiosum (SwissProt database no. O74049; RefSeq accession no. YP_876111) with E. coli adapted codon usage was synthesized (Entelechon, Regensburg, Germany) and BamHI/EcoRI cloned into a modified pET41 expression vector with GST fusion and a PreScission protease cleavage site (16), followed by expression in E. coli Rosetta and purification upon cell disruption by GSH affinity chromatography. The GST fusion was cleaved off by PreScission protease digestion and removed by size exclusion chromatography on a Superdex75 column. The same protocol was used to purify isotopically labeled PinA protein grown in M9 minimal medium with addition of 15NH4Cl and/or [U-13C]glucose.
NMR Spectroscopy and Resonance Assignment
Samples of 13C,15N-double labeled CsPinA were prepared by dissolving 0.8 mm protein in 50 mm Tris, pH 7.5, 50 mm NaCl. All spectra were recorded at 289 K on a Varian VNMRS 800 NMR spectrometer (Varian, Inc.) equipped with four channels, z axis pulsed field gradient unit and triple (1H/13C/15N) cryogenic probe head with inverse detection. Heteronuclear NMR data were acquired using the States-TPPI quadrature method (17) followed by sensitivity enhanced detection (18). All chemical shifts are reported relative to 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) as external reference. Acquired spectra were processed with NMRPipe (19) and analyzed using Sparky.
Sequence-specific assignments of backbone 1H, 13C, and 15N resonances were obtained by analyzing three-dimensional heteronuclear HNCACB, CBCA(CO)NH, HNCA, HN(CO)CA, and HNCO spectra by standard methods (20) and confirmed by analyzing a 15N-edited NOESY-HSQC spectrum. The 1H and 13C resonances in aliphatic side chains were assigned based on heteronuclear two-dimensional 1H-13C-HSQC, three-dimensional C(CO)NH, H(CCO)NH, and HCCH-TOCSY experiments. Aromatic side chain 13Cδ, 13Cϵ, and 13Cζ resonances were assigned from two-dimensional 1H-13C-HSQC with tuned offset, spectral widths, and 1H-13C couplings to aromatic carbons, and three-dimensional 13C-edited NOESY-HSQC spectra.
Structure Calculation
Distance constraints were derived from three NOESY spectra; the three-dimensional 15N-edited NOESY-HSQC spectrum was recorded with a mixing time of 150 ms and two three-dimensional 13C-edited NOESY-HSQC data sets with parameters tuned into either aliphatic or aromatic regions were acquired with a mixing time of 90 ms. The initial structure calculations were performed by CYANA (21) following the automated NOE assignment procedure (22). Stereospecific assignments for 92 chiral groups in side chains were derived by the program GLOMSA (23) included in CYANA software. Further structural calculations were performed in the CNS program (version 1.2) (24). Additionally, 76 distance constraints for 38 hydrogen bonds were defined as rHN–O = 1.5–2.8 Å and rN–O = 2.4–3.5 Å and included for structure calculations at final refining stage. Secondary structure was defined for PinA on the basis of 2JN(i)Cα(i−1) couplings, which are in good agreement with predicted secondary structure deduced from 1H, 13C, and 15N chemical shifts using TALOS+ software (25). Analyzing experimental J couplings yield 70 restraints for ψ backbone torsion angles defined as ψ = −40 ± 40 degrees (37 restraints), and ψ = 140 ± 40 degrees (33 restraints) for α-helices and β-sheets, respectively. Finally, 20 structures of 200 calculated were selected according to lowest energy and deposited in the Protein Data Bank (PDB code 2RQS). Due to the five additional residues GPMGS remaining at the N terminus after PreScission protease cleavage, numbering in that PDB file has an offset of five relative to all numbering in the article. Quality of the ensemble was analyzed with PROCHECK-NMR (26) and WhatIf (27). Figures were generated with MOLMOL (28) or Chimera (29).
Relaxation Measurements
The 15N longitudinal (R1) and transverse (R2) relaxation rates were measured at 289 K on Varian Unity+ 500 and Varian VNMRS 700 NMR spectrometers at 11.7 and 16.1 T, respectively. Both spectrometers were equipped with three channels (1H, 13C, 15N), inverse probe heads, and a gradient unit. Relaxation rates were measured using pulse sequences included in BioPack (Varian, Inc.) with an optional measurement of either R1 or R2 relaxation on 15N nuclei. Ten evolution times 10, 90, 170, 290, 410, 550, 690, 850, 1010, and 1250 ms were used in the 15N R1 experiments. The 15N R2 experiments were performed with eight evolution times 10, 30, 50, 90, 130, 170, 210, and 250 ms. The Carr-Parcell-Meiboom-Gill pulse train with a refocusing time of 650 μs was used in this case. The cross-correlation effect was suppressed by using π (1H) pulses every 5 or 10 ms in R1 and R2 measurements, respectively (18). A relaxation delay of 3.0 s was employed in both experiments. R1 and R2 relaxation rates were calculated using two parameter, nonlinear least-square fit of cross-peak heights to a single exponential decay. Accuracies of R1 and R2 rates were determined from variance-covariance matrices. The {1H}-15N NOE were obtained from ratios of intensity cross-peaks in spectra with and without saturation and were also measured with a pulse sequence included in BioPack (Varian, Inc.). The relaxation delay of 8 s and a 3-s saturation delay were employed for recording these spectra. Experimental errors in {1H}-15N NOE were evaluated as described in (30).
Analysis of Relaxation Data
Relaxation data for PinA protein were analyzed using the model-free approach (31) combined with axially anisotropic overall molecular tumbling (32). Four global parameters D‖, D⊥, θ, and ϕ, denote parallel and perpendicular components of the rotational diffusion tensor and direction of unique axis of the diffusion tensor relative to the molecule fixed coordinate system, respectively. Three local, residue-specific parameters comprise a generalized order parameter S, which is a measure of the degree of spatial restriction of the motion, an effective correlation time τint corresponding to the rate of this motion, and Rex describing conformational exchange contribution to R2 resulting from the dynamic processes on a microsecond to millisecond time scale (33). All model parameters were determined simultaneously from the relaxation data and known orientations of N–H bonds calculated from the atomic coordinates of the NMR-derived structure of PinA protein. The least-squares procedure used to optimize model parameters consisted of a minimization through a grid search of the appropriate target functions using a Fortran routine written in-house. Model parameter uncertainties derived in the minimization of target function were obtained as S.D. from 200 Monte Carlo simulations. The values of N–H distance, rNH = 0.103 nm and 15N chemical-shift anisotropy Δσ = −160 ppm were used in our calculations.
Phage Display and PinA Peptide Titration Experiments
Purified PinA protein was diluted in 0.1 m NaHCO3, pH 8.6, to 0.1 mg/ml and immobilized on Petri dishes. Unbound protein was removed, and the plate was blocked with 5 mg/ml BSA in 0.1 m NaHCO3, pH 8.6. A 7-mer M13 phage display peptide library (New England Biolabs) was used according to the manufacturer's protocol for three rounds of panning with increasing concentrations of Tween 20 (0.1% to 0.5%) within the washing buffer (Tween 20-containing Tris-buffered saline). Bound phages were eluted with 0.2 m glycine, pH 2.2. Following the third round of panning, clonal phage DNA was isolated and sequenced.
7-mer sequences that have been obtained several times were commercially synthesized at ChemCube (Bochum, Germany). Titration experiments were performed as series two-dimensional 1H-15N-HSQC spectra on a Varian Unity+ 500 NMR spectrometer at 283 K on samples containing 0.4 mm 13C,15N-doubly labeled protein in 50 mm Tris, pH 7.4, 50 mm NaCl, with 10% D2O. Protein-peptide complexes were obtained by the addition of an equivalent amount of 6 mm stock solution prepared in the same buffer and pH; pH was additionally checked after titration. Spectra for four PinA-HQSPWHH ratios corresponding to 1:0, 1:1, 1:4, and 1:8 were acquired (supplemental Fig. S12). Chemical shift differences of backbone 1H and 15N were calculated according to Equation 1 (34).
Dynamics measurements of the protein-peptide complex were performed as described above with an 0.8 mm protein sample and 7.6 mm HQSPWHH peptide (1:9.5 ratio) in the same buffer. Eight points for R2 and 10 points for R1 were collected and analyzed as described above.
RESULTS
Sequence-specific Resonance Assignment
Sequence-specific assignment of backbone 1H, 13C, and 15N resonances of PinA from C. symbiosum was obtained from analyzing triple-resonance HNCACB, CBCA(CO)NH, HNCO, HNCA, and HN(CO)CA spectra. Existing cross-peaks were confirmed using a 15N-edited three-dimensional NOESY-HSQC spectrum recorded with a 150-ms mixing time. Assignments of aliphatic 1H and 13C resonances of the protein side chains were achieved on the basis of three-dimensional HBHA(CO)NH, C(CO)NH-TOCSY, and HCCH-TOCSY experiments. More than 96% of all resonances could be assigned by this procedure with the exception of signals from aromatic side chains and from six N-terminal residues (Met1 to Asp3 as well as residual amino acids from the N-terminal extension). Finally, all obtained 1H, 13C, and 15N chemical shifts for the PinA protein were deposited in the Biological Magnetic Resonance Bank (BMRB accession no. 11080). A fully assigned 1H-15N-HSQC spectrum is shown in Fig. 2.
FIGURE 2.
Two-dimensional 1H-15N-HSQC spectrum of PinA recorded at 289 K on a Varian VNMRS 800 NMR spectrometer. Obtained sequential assignments are indicated by the one-letter amino acid code and residue number.
Information about the redox state of the unique cysteine residue (Cys7) in PinA was deduced from the chemical shift of its 13Cβ nucleus (35). The obtained value for this 13Cβ chemical shift was 33.6 ppm, clearly indicating a reduced state of this thiol group.
Secondary Structure
Known structures of parvulin-like PPIases from various prokaryotic and eukaryotic organisms typically exhibit a β-α3-β-α-β2 fold (1, 36, 37). In the case of the PinA protein from C. symbiosum, the existence of three α-helices and four β-strands were predicted from inspection of 2JN(i)Cα(i−1) couplings measured in a three-dimensional HNCO Cα-coupling experiment (38). The 2JN(i)Cα(i−1) scalar couplings are strongly correlated with the ψ backbone torsion angle of a preceding residue and could be used for secondary structure prediction in proteins (39, 40). The extended or helical conformation could be predicted from deviation of 2JN(i)Cα(i−1) scalar coupling constants from the delineation value of 7.2 Hz (39), taking into account the negative sign of this coupling (40). Evaluation of 2JN(i)Cα(i−1) couplings of CsPinA confirms the existence of four β-sheets by a series of at least three residues with 2JN(i)Cα(i−1) values smaller than −7.2 Hz within the secondary structure elements (Lys4–Lys13, Tyr53–Gly57, Val75–Ser81, and Tyr85–Leu91).
At the same time, four regions forming α-helices were postulated on the basis of values higher than −7.2 Hz (Glu17–Glu29, Glu32–Ser39, Ser44–Arg47, and Phe63–Leu70). According to these data, the organization of the secondary structure elements of PinA could be described as a β-α3-β-α-β2 topology. This topology is in good agreement with the analysis of chemical shift data using the TALOS+ software (25), where backbone conformations for 78 of 94 residues were classified as “good.” Nevertheless, the exact positions of α-helices and β-sheets were justified only after full analysis of NOE connectivities obtained from the three-dimensional 15N-edited NOESY-HSQC spectrum. Three α-helices (Gln15–Leu25, Phe31–Glu37, and Lys61–Ala66) were confirmed on the basis of HN-Hα (i, i+4) and (i, i+3) NOE contacts. The four β-sheets (Ile5–Leu11, Ser50–Phe54, Val79–Ser81, and Gly84–Arg90) were identified on the basis of HN-HN and HN-HA NOE contacts. Finally, the proposed α-helix III turned out to be a short one-turn 310-helix between Gly43 and Arg47, as confirmed by HN-Hα (i, i+2) NOE contacts.
Structure Calculations and Tertiary Structure of CsPinA Protein
The high resolution solution structure of PinA was determined with 1585 distance constraints (777 intraresidual and sequential, 294 short range, and 514 long range NOEs) and 175 restraints for backbone torsion angles using CNS software (version 1.2) (24). Moreover, 38 hydrogen bonds, which yielded an additional 76 distance constraints (rNH–O 1.5–2.8 Å and rN–O 2.5–3.5 Å), were defined on the basis of NOESY cross-peaks and the previously determined secondary structures that were introduced during the refinement procedure. The statistics of distance constraints and analysis of the NMR ensemble containing the 20 lowest energy structures of PinA protein are presented in Table 1; the ensemble was deposited in the Protein Data Bank (PDB code 2RQS).
TABLE 1.
NMR constraints and structural statistic for the ensemble of the 20 lowest energy PinA conformers of C. symbiosum
| NOE distance constraintsa | 1585 | |
| Intraresidual and sequential (| i − j| ≥ 1) | 777 | |
| Medium range (1 < |i − j| < 5) | 294 | |
| Long range (|i − j| ≥ 5) | 514 | |
| Hydrogen bonds | 38 | |
| Restraints per residue | 17.1 | |
| Torsion angle constraints | ||
| Backbone (ϕ/ψ) | 70 | |
| r.m.s.d. from idealized covalent geometry (±S.D.) | ||
| Bonds (Å) | 0.0020 ± 0.0001 | 0.0018 |
| Angles | 0.338 ± 0.005° | 0.330° |
| Impropers | 0.198 ± 0.010° | 0.177° |
| Ramachandran plot (region 2–92)b | ||
| Residues in most favored regions (%) | 71.3 ± 3.4 | 78.8 |
| Residues in additional allowed regions (%) | 27.0 ± 3.4 | 32.5 |
| Residues in generously allowed regions (%) | 1.1 ± 1.1 | 3.8 |
| Residues in disallowed regions | 0.6 ± 1.0 | 0.0 |
| r.m.s.d. to the mean structure (2–92) (Å) | ||
| Ordered backbone atoms | 0.62 ± 0.17 | |
| Ordered heavy atoms | 1.34 ± 0.15 | |
| Equivalent x-ray resolution (2–92) | 2.1 Å | |
| r.m.s. Z-scoresc | ||
| Bond lengths | 1.035 ± 0.001 | |
| Bond angles | 0.287 ± 0.003 | |
| ω angle restraints | 0.157 ± 0.010 | |
| Side chain planarity | 0.081 ± 0.023 | |
| Improper dihedral distribution | 0.381 ± 0.019 | |
| Inside/outside distribution | 0.986 ± 0.018 | |
a None of the 20 structures had a distance violation more than 0.2 Å or a dihedral angle violation >5°.
b Quality of the ensemble consisting of the 20 lowest energy structures of PinA was checked by PROCHECK-NMR (version 3.4).
c Ensemble of structures was validated using WhatIf (27).
The globular fold of the PinA protein from C. symbiosum exhibits a central four-stranded β-sheet motif wrapped around the C-terminal α-helix IV (Fig. 3A). Hydrophobic residues (Leu51, Phe54, and Ile92) and the two histidines (His9 and His86) forming the hydrophobic core are located on the concave side of the β-sheet cluster. The other, convex side is defined by the hydrophilic residues Cys7, Ser8, Lys89, and Arg90. The determined three-dimensional fold is very similar to the other PPIase structures from various organisms known to date. An overlay of PinA with the structures of human Pin1, E. coli Par10 and Bacillus subtilis PrsA shows an r.m.s.d. over backbone heavy atoms of ∼1.2 Å (Fig. 4). Residues that are postulated as important for catalyzing the Xaa-Pro peptide bond isomerization (His9, Asp41, Met59, Phe63, Phe83, and His86) are essentially at the same positions.
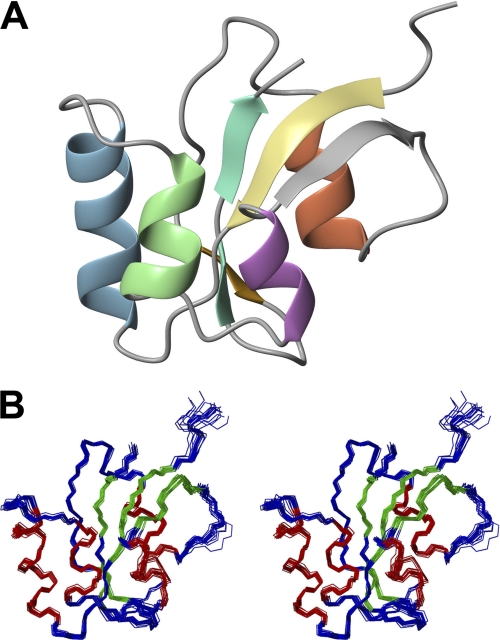
FIGURE 3.
NMR solution structure of the parvulin protein PinA from C. symbiosum. Only the structured parts (residues 2–92) are shown. A, ribbon representation of PinA protein. Four helices and four β-sheets are presented by different colors demonstrating the globular β-α3-β-α-β2 fold characteristic for parvulins. B, stereo view of backbone ensemble of the 20 lowest energy structures. Residues in helical and extended conformation are shown in red and green, respectively.
FIGURE 4.
Structural similarities between various parvulins. Overlay of the PinA structure from C. symbiosum (black) with Homo sapiens Pin1 PPIase domain (A; PDB 1PIN), E. coli Par10 (B; PDB 1JNS), and B. subtilis PrsA (C; PDB 1ZK6).
Conformation of a Conserved Xaa-Pro Peptide Bond
One intriguing structural question in parvulin-type PPIases surrounds the conformation of a conserved Xaa-Pro peptide bond within the catalytic domain. In E. coli Par10, the Gly76–Pro77 bond has been reported to be in cis conformation (37) and the corresponding Asp113–Pro114–Pro115 in human Par14 was assigned a cis conformation for the Asp113–Pro114 bond (36). Initially, 13Cβ and 13Cγ chemical shift data for the two proline residues, Pro62 and Pro78 of CsPinA, were used to determine the probability of cis conformation using the program PROMEGA (41), which uses both chemical shift data and sequence context for prediction. The trans conformation was postulated for both prolines in our structure. In the case of the Gly77–Pro78, the conserved Xaa-Pro bond in parvulins, the trans conformation was additionally validated by the analysis of three-dimensional NOESY-HSQC 13C-edited spectra, where cross-peaks between Glu77 Hα and Pro78 Hδ as well as between Pro78 Hα and Val79 HN were clearly detected. This conformation is similar to the PrsA structures from B. subtilis (42), Staphylococcus aureus (43), and other parvulins. Our PROMEGA prediction for this conserved motif was then extended to all other parvulins whose chemical 1H, 15N, and 13C shifts were available in the BMRB. The probabilities for the conserved Xaa–Pro bond to be in cis conformation are listed in Table 2 for six of them. Following this prediction, a cis Xaa–Pro bond is found only in E. coli Par10 and human Par14 (see also supplemental Fig. S11).
TABLE 2.
Probability (Pcis) for a Xaa-Pro peptide bond being in cis conformation calculated by the program PROMEGA
Gly75–Pro76 and Pro114-Pro115 with a high probability for the cis conformation are highlighted in bold.
| Protein | Peptide bond | Pcis |
|---|---|---|
| E. coli Par10 (1JNS)a | Glu72–Pro73 | 0.124 |
| Gly75–Pro76 | 0.987 | |
| H. sapiens Par14 (1EQ3)b | Asp113–Pro114 | 0.092 |
| Pro114–Pro115 | 0.991 | |
| B. substitilis PrsA (1ZK6)c | Asp77–Pro78 | 0.000 |
| Mycoplasma genitalium PpiD (1HXV)d | Lys101–Pro102 | 0.051 |
| C. symbiosum PinA (2RQS)e | Glu82–Pro83 | 0.022 |
| H. sapiens Pin1 (1PIN)f | Lys152–Pro153 | 0.000 |
| Gly168–Pro169 | 0.056 |
a BMRB accession no. 5225.
b BMRB accession no. 4768.
c BMRB accession no. 6601.
d BMRB accession no. 4953.
e BMRB accession no. 11080.
f BMRB accession no. 5305.
Backbone Dynamics of PinA from Relaxation Data
To study the global and local backbone dynamics of CsPinA, 15N relaxation rates, R1 and R2, were determined at two magnetic fields, 11.7 and 16.4 T. Additionally, {1H}-15N NOEs were measured at the lower magnetic field. Relaxation data were obtained for 79 backbone amide groups. Missing data comprise two prolines (Pro62 and Pro78), N-terminal residues Met1–Asp3, together with residues from the N-terminal extension experiencing fast exchange of their HN protons with water as well as several residues scattered along the polypeptide chain with strongly superposed cross-peaks. These data are shown in Fig. 5.
FIGURE 5.
Relaxation data for backbone amide 15N nuclei. R1, R2, and {1H}-15N NOE of CsPinA protein at 11.7 T (open circles) and 16.4 T (closed circles), respectively. The mean values for R1 rates are 2.05 s−1 and 1.50 s−1 at 11.7 and 16.4 T, respectively. The mean values for R2 rates are 9.40 s−1 and 11.21 s−1 at 11.7 and 16.4 T, respectively. Finally, the {1H}-15N NOE mean value was 0.75 at 11.7 T. For definition of the error bars, please refer to the “Relaxation Measurements” under “Experimental Procedures.”
The inertia tensor of the PinA protein was calculated from the PDB coordinates. Its principal value ratios were 1.51:1.66:1.00, allowing the approximation of the protein molecule as a prolate ellipsoid. Rotational diffusion constants and local model parameters were determined after rejection of doubtful R1 data at 16.1 T for Lys4 and Ala45. The overall molecular diffusion constants were: D‖ = (4.09 ± 0.08) 107 s−1 and D⊥ = (1.70 ± 0.04) 107 s−1.
Anisotropy of the overall motion is considerable with the anisotropy ratio D‖/D⊥ = 2.41 ± 0.07, indicating a substantial deviation of the protein structure from a spherical shape. An effective correlation time of τR = 1/(2D‖ + 4D⊥) = 6.7 ± 0.1 ns (32) fits well with the value expected for a 97-residue protein (44, 45).
All determined values of local parameters, S2 and Rex, are included in the BMRB deposition (BMRB accession no. 11080) and are shown in Fig. 6. Correlation times for internal motions, τint, are very sensitive to experimental errors resulting in large inaccuracies, as often observed (46). Therefore, their values are hardly informative and not presented here.
FIGURE 6.
Model-free approach parameters, S2 (top) and Rex at 11. 7 T (middle) and R1R2 product values at 16.4 T (bottom) for CsPinA protein. Note the different Rex and R1R2 values for the region from amino acids Ser44 to Gly49. For definition of the error bars, please refer to “Analysis of Relaxation Data” under “Experimental Procedures.”
Fast and simple identification of the residues undergoing conformational exchange slower than the overall molecular tumbling can be derived from larger than average values of R1R2 product (47). It is superior to the R1/R2 ratio, which is prone to errors in the case of anisotropic overall tumbling. R1R2 values calculated from relaxation rates determined at 16.4 T are shown in Fig. 6. On the other hand, smaller than average values of the R1R2 product indicate residues with increased mobility on a sub-nanosecond timescale. It has to be stressed that R1R2 values cannot be used uncritically instead of full analysis of all available relaxation data. Residues undergoing both fast and slow motions can display average R1R2 product values due to mutual cancellation of the two opposite effects.
Residues with larger R1R2 values also displayed elevated Rex values, unequivocally pointing to increased mobility on a microsecond to millisecond time scale. Large Rex values for Gly42, Gly43, Ser44, Ala45, Lys46, Asp48, and Gly49 at 11.7 T were equal to 1.8 ± 0.7 s−1, 1.7 ± 0.4 s−1, 3.5 ± 0.2 s−1, 3.7 ± 0.3 s−1, 3.5 ± 0.2 s−1, 1.7 ± 0.6 s−1, and 2.1 ± 0.1 s−1, respectively. Residues Gly43–Arg47 constitute the short one turn 310-helix III sandwiched between the two nonstructured loops. Analysis of the ensemble of the 20 lowest energy structures of the protein confirms a relatively high r.m.s.d. value of 0.35 ± 0.22 Å for this 310-helix relative to the other secondary structure elements (0.09 ± 0.06 Å for β-sheet I and 0.18 ± 0.07 Å for α-helix I). This structural heterogeneity is in agreement with the flexibility detected in this region. Other residues displaying chemical exchange, Val60 (2.3 ± 0.2 s−1), Gln71 (1.7 ± 0.1 s−1), and Ser81 (2.0 ± 0.1 s−1), are located in unstructured parts of the molecule.
The weighted mean of the S2 parameter for residues forming three α-helices and four β-sheets (0.88 ± 0.04) does not differ from the weighted mean calculated for all residues (0.87 ± 0.05) within the accuracy limit. Residues involved in secondary structure elements, except 310-helix, are very rigid; they exhibit no significant internal mobility. Several residues dispersed along the polypeptide chain display relatively small values of the S2 parameter, which are usually associated with their high S.D. due to low accuracy of experimental data and cannot be attributed to the increased mobility on a fast timescale. Lys4 located at the flexible N terminus (S2 = 0.78 ± 0.1) is a unique prominent exception.
Phage Display and 1H-15N-HSQC Titrations Identify Peptide HQSPWHH as a Ligand of PinA
To identify interacting partners of PinA from the archaeon C. symbiosum that cannot be cultivated, we screened a 7-mer peptide phage display using our recombinant protein as bait. The heptamer sequence HQSPWHH was selected several times during screening and hence was obtained as a peptide for titration purposes. 1H-15N-HSQC spectra for four protein:peptide ratios (1:0, 1:1, 1:4, and 1:8) were recorded for CsPinA and the HQSPWHH peptide. A plot of the chemical shift perturbation shows that the selected HQSPWHH peptide has an affinity in the high micromolar/low millimolar range. Structural mapping of the chemical shift perturbation (supplemental Fig. S12) demonstrates that peptide binding primarily affects residues of the catalytic binding pocket (Gly57, Lys58, Val60, and Phe83) and within the 310-helix III (Gly42 and Ser44). Monitoring HQSPWHH peptide binding with 1H-13C correlation spectra allowed the detection of chemical shift changes for the Cϵ1/Hϵ1 correlation of His9 and His86 within the PinA protein, residues that are part of the substrate binding pocket (supplemental Fig. S13).
This peptide ligand for CsPinA then allowed the investigation of protein dynamics and their changes upon ligand binding. Therefore, R1 and R2 relaxation parameters were again measured for 15N amide groups in the presence and absence of the HQSPWHH peptide (Fig. 7). Clearly, the region from Ala44 to Gly49 showed a different behavior from the rest of the protein, indicating increased mobility on a microsecond to millisecond time scale despite of strongly anisotropic overall tumbling.
FIGURE 7.
Changes in dynamics upon binding of the peptide HQSPWHH. Differences for the R1 and R2 relaxation parameters and the R1R2 product are shown for free PinA protein and complexed to the peptide HQSPWHH. Formal statistical analysis includes averaging over all amino acids as well as the flexible region from Ser44 to Gly49 (black bars). av., average; st. dev., S.D. For definition of the error bars, please refer to “Analysis of Relaxation Data” under “Methods.”
DISCUSSION
The overall three-dimensional structure of the PinA protein from C. symbiosum is characterized by the typical parvulin-like β-α3-β-α-β2 fold (four α-helices exposed to the solvent and four β-sheets with a 3-4-1-2 strand order), classifying CsPinA as a member of the FKBP-superfold family. Superpositions of backbone heavy atoms between the PPIase domain of human Pin1 (48), the E. coli Par10 (37) and B. subtilis PrsA (42) with the PinA as a reference molecule show r.m.s.d. values of 1.05, 1.22, and 1.09 Å, respectively. These values clearly indicate that parvulins are structurally and evolutionarily well conserved among different species. Moreover, the structural similarities dictate the geometry of the catalytic center, which remains the same among all known PPIases from different organisms. The residues that are strongly conserved and postulated as important for Xaa-Pro substrate binding, His9, Asp41, Met59, Phe63, Phe83, and His86, occupy essentially the same positions (Fig. 8). Nevertheless, there are some structural features occurring exclusively in cold-adapted parvulins that may be responsible for cold adaptation of parvulins from Thaumarchaeota.
FIGURE 8.
The hydrophobicity surface of selected parvulins (left) together with their ribbon representations of secondary motifs (right). The side chains for evolutionary conserved residues that are important for cis/trans isomerization are shown on the right. Black circles indicate opening of the catalytic substrate binding site. The radius of this binding pocket was calculated using MOLMOL (28) with an error of 0.4 Å. C. symbiosum PinA (PDB 2RQS; A), E. coli Par10 (PDB 1JNS; B), H. sapiens Pin1 (PDB 1PIN; C), and H. sapiens Par14 (PDB 1EQ3; D).
An Unusually Large Catalytic Binding Pocket Is Observed in PinA from C. symbiosum
When comparing the structure of CsPinA with those of the other parvulins, it becomes evident that the hydrophobic catalytic center and its surrounding environment are remarkably different. Despite the structural build-up of the catalytic center that is essentially identical to other homologous parvulins, the accessibility of Xaa-Pro substrates to the active center in CsPinA is dramatically different from other parvulins.
We analyzed the diameter of the entrance to the substrate binding pocket (Fig. 8). In the case of the archaeal PinA protein, the radius of the catalytic center is 8.4 ± 0.5 Å in size. For the atomic structures of other parvulins known to date, this parameter lies between 4.5 and 5.8 Å, irrespective of their different substrate specificities. A large substrate binding pocket may indicate a preference for bulky, hydrophobic residues similar to the substrate specificity of E. coli Par10, but even then, there is a significant difference between these two proteins. Enlarged catalytic clefts have been previously reported for other proteins of psychrophilic origin (49). In light of these data, the catalytic center of PinA from C. symbiosum can be regarded as an evolutionary adaptation of this protein for functioning in a cold environment.
Slow Molecular Dynamic Processes Are Especially Important for Protein-Substrate Binding
Molecular dynamics on microsecond to millisecond time scales (conformational exchange) is another important factor for protein-substrate interaction. The stretch of residues Gly42–Gly49 as well as Val60, Gln71, and Ser80 showed significantly elevated Rex values (Fig. 6). Out of these, Ser44, Val60, and Ser80 are located around the ligand binding site, which is conserved between different parvulins (Fig. 9). Our results extend former observations of some local flexibility in the helix III region of other parvulins. Elevated flexibility in this region was contained in the relaxation data reported for the PrsA parvulin from S. aureus (43). As a glycine and two positively charged residues within this loop are conserved in most parvulin proteins, this flexible loop may be of general importance for parvulin function.
FIGURE 9.
Dynamic residues of the PinA protein and residues affected by ligand binding are found around the catalytic center. A, mapping of residues exhibiting strong dynamics on the μs-ms time scale on the three-dimensional structure of PinA. The residues His9, Gln20, Phe31, Gly32, Asp41–Ser50, Val60, Lys61, Glu82, and Phe83 are shown in red, orange-red, orange, and yellow depending on the intensity of their R1R2 product derived from 15N relaxation data. B, residues demonstrating chemical shift perturbations larger than 0.022 ppm upon HQSPWHH peptide binding are shown in red (Gly55, Val60), orange (Gly32, Lys58), yellow (Phe31, Gly42, Gly84), and green (Ser44, Gly53, Gly57, Phe83, Lys89), respectively. See supplemental Fig. S12 for more information.
Next, peptides were selected for PinA binding by phage display. This screening resulted only in relatively weak binders, which might indicate that PinA from C. symbiosum is an isomerase with little specificity for the primary sequence of its substrates. The peptide HQSPWHH was used for chemical shift perturbation studies with PinA. Interestingly, the residues most affected by ligand binding are located in a groove formed by helix II, β-sheet II, and the highly mobile short 310-helix III. Flexibility of this stretch of five residues within and around the short 310-helix III has not been reported previously for any other parvulin.
Conclusions
The high resolution three-dimensional structure of PinA from the psychrophilic archaeon C. symbiosum was determined from NMR data. Relative to other known parvulin structures, PinA has an atypically large catalytic binding site providing an explanation for cold-adapted protein function in Archaea. The structure of PinA is relatively rigid; only one stretch of residues comprising 310-helix III and the following turn displayed significant mobility on a microsecond to millisecond time scale and showed structural heterogeneity within the NMR ensemble. The peptide HQSPWHH was identified as a CsPinA ligand and used for HSQC titrations and dynamics measurements of the complex. In addition to known residues involved in cis/trans isomerization, the flexible region around 310-helix III showed the strongest chemical shift perturbations and a change in flexibility upon peptide binding. The extraordinary flexibility of this region and its involvement in ligand binding has not previously been recognized for parvulin-type PPIases.
Supplementary Material
Acknowledgments
We thank Drs W. Kozminski, K. Kazimierczuk, and A. Zawadzka-Kazimierczuk (Chemistry Department, University of Warsaw) for technical assistance in recording and processing of three-dimensional HNCO 13Cα-coupled ultrahigh resolution NMR data sets. Christoph Lederer is acknowledged for searching a variety of genomes for PPIase genes, and Alma Rueppel performed excellent sample preparation. The measurements on the Varian VNMRS 700 NMR spectrometer were performed in the Structural Research Laboratory (Chemistry Department, University of Warsaw).
This work was supported in part by Polish Ministry of Science and Higher Education Grant N301 07131/2159, Slovenian Research Agency Grant P1-0242-0104 (to I. Z.), Polish Ministry of Science and Higher Education luventus Plus program (project nr IP2010014570, to L. J.) .

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. S10–S13.
The atomic coordinates and structure factors (code 2RQS) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
C. Lederer, personal communication.
- PPIase
- peptidyl-prolyl isomerase
- r.m.s.d.
- root mean square deviation
- HSQC
- heteronuclear single-quantum correlation spectroscopy
- CsPinA
- PinA protein from C. symbiosum
- PDB
- Protein Data Bank
- BMRB
- Biological Magnetic Resonance Bank
- TOCSY
- total correlated spectroscopy
- T
- Tesla
- FKBP
- FK506-binding protein.
REFERENCES
- 1. Mueller J. W., Bayer P. (2008) Perspect. Medicin. Chem. 2, 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryo A., Togo T., Nakai T., Hirai A., Nishi M., Yamaguchi A., Suzuki K., Hirayasu Y., Kobayashi H., Perrem K., Liou Y. C., Aoki I. (2006) J. Biol. Chem. 281, 4117–4125 [DOI] [PubMed] [Google Scholar]
- 3. Rudrabhatla P., Zheng Y. L., Amin N. D., Kesavapany S., Albers W., Pant H. C. (2008) J. Biol. Chem. 283, 26737–26747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maruyama T., Suzuki R., Furutani M. (2004) Front Biosci. 9, 1680–1720 [DOI] [PubMed] [Google Scholar]
- 5. Preston C. M., Wu K. Y., Molinski T. F., DeLong E. F. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 6241–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hallam S. J., Mincer T. J., Schleper C., Preston C. M., Roberts K., Richardson P. M., DeLong E. F. (2006) PLoS. Biol. 4, e95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karner M. B., DeLong E. F., Karl D. M. (2001) Nature 409, 507–510 [DOI] [PubMed] [Google Scholar]
- 8. Brochier-Armanet C., Boussau B., Gribaldo S., Forterre P. (2008) Nat. Rev. Microbiol. 6, 245–252 [DOI] [PubMed] [Google Scholar]
- 9. Mueller J. W., Kessler D., Neumann D., Stratmann T., Papatheodorou P., Hartmann-Fatu C., Bayer P. (2006) BMC. Mol. Biol. 7, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kessler D., Papatheodorou P., Stratmann T., Dian E. A., Hartmann-Fatu C., Rassow J., Bayer P., Mueller J. W. (2007) BMC Biol. 5, 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bayer E., Goettsch S., Mueller J. W., Griewel B., Guiberman E., Mayr L. M., Bayer P. (2003) J. Biol. Chem. 278, 26183–26193 [DOI] [PubMed] [Google Scholar]
- 12. Yaffe M. B., Schutkowski M., Shen M., Zhou X. Z., Stukenberg P. T., Rahfeld J. U., Xu J., Kuang J., Kirschner M. W., Fischer G., Cantley L. C., Lu K. P. (1997) Science 278, 1957–1960 [DOI] [PubMed] [Google Scholar]
- 13. Suzuki Y., Haruki M., Takano K., Morikawa M., Kanaya S. (2004) Eur. J. Biochem. 271, 1372–1381 [DOI] [PubMed] [Google Scholar]
- 14. Rak A., Kalinin A., Shcherbakov D., Bayer P. (2002) Biochem. Biophys. Res. Commun. 299, 710–714 [DOI] [PubMed] [Google Scholar]
- 15. Ye K., Serganov A., Hu W., Garber M., Patel D. J. (2002) Eur. J. Biochem. 269, 5182–5191 [DOI] [PubMed] [Google Scholar]
- 16. Grum D., van den Boom J., Neumann D., Matena A., Link N. M., Mueller J. W. (2010) Biochem. Biophys. Res. Commun. 395, 420–425 [DOI] [PubMed] [Google Scholar]
- 17. Marion D., Ikura M., Tschudin R., Bax A. (1989) J. Magn. Reson. 85, 393–399 [Google Scholar]
- 18. Kay L. E., Nicholson L. K., Delaglio F., Bax A., Torchia D. A. (1992) J. Magn. Reson. 97, 359–375 [Google Scholar]
- 19. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 20. Ikura M., Kay L. E., Bax A. (1990) Biochemistry 29, 4659–4667 [DOI] [PubMed] [Google Scholar]
- 21. Güntert P., Mumenthaler C., Wüthrich K. (1997) J. Mol. Biol. 273, 283–298 [DOI] [PubMed] [Google Scholar]
- 22. Herrmann T., Güntert P., Wüthrich K. (2002) J. Mol. Biol. 319, 209–227 [DOI] [PubMed] [Google Scholar]
- 23. Güntert P., Qian Y. Q., Otting G., Müller M., Gehring W., Wüthrich K. (1991) J. Mol. Biol. 217, 531–540 [DOI] [PubMed] [Google Scholar]
- 24. Brunger A. T. (2007) Nat. Protoc. 2, 2728–2733 [DOI] [PubMed] [Google Scholar]
- 25. Shen Y., Delaglio F., Cornilescu G., Bax A. (2009) J. Biomol. NMR 44, 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laskowski R. A., Rullmannn J. A., MacArthur M. W., Kaptein R., Thornton J. M. (1996) J. Biomol. NMR 8, 477–486 [DOI] [PubMed] [Google Scholar]
- 27. Vriend G. (1990) J. Mol. Graph. 8, 52–56 [DOI] [PubMed] [Google Scholar]
- 28. Koradi R., Billeter M., Wüthrich K. (1996) J. Mol. Graph. 14, 51–55 [DOI] [PubMed] [Google Scholar]
- 29. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 30. Fushman D. (2003) in BioNMR in Drug Research (Zerbe O. ed) Wiley-VCH, Weinheim, Germany [Google Scholar]
- 31. Lipari G., Szabo A. (1982) J. Am. Chem. Soc. 104, 4546–4559 [Google Scholar]
- 32. Barbato G., Ikura M., Kay L. E., Pastor R. W., Bax A. (1992) Biochemistry 31, 5269–5278 [DOI] [PubMed] [Google Scholar]
- 33. Stone M. J., Fairbrother W. J., Palmer A. G., 3rd, Reizer J., Saier M. H., Jr., Wright P. E. (1992) Biochemistry 31, 4394–4406 [DOI] [PubMed] [Google Scholar]
- 34. Ayed A., Mulder F. A., Yi G. S., Lu Y., Kay L. E., Arrowsmith C. H. (2001) Nat. Struct. Biol. 8, 756–760 [DOI] [PubMed] [Google Scholar]
- 35. Sharma D., Rajarathnam K. (2000) J. Biomol. NMR 18, 165–171 [DOI] [PubMed] [Google Scholar]
- 36. Sekerina E., Rahfeld J. U., Müller J., Fanghänel J., Rascher C., Fischer G., Bayer P. (2000) J. Mol. Biol. 301, 1003–1017 [DOI] [PubMed] [Google Scholar]
- 37. Kühlewein A., Voll G., Hernandez, Alvarez B., Kessler H., Fischer G., Rahfeld J. U., Gemmecker G. (2004) Protein Sci. 13, 2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kazimierczuk K., Zawadzka A., Koźmiński W., Zhukov I. (2008) J. Am. Chem. Soc. 130, 5404–5405 [DOI] [PubMed] [Google Scholar]
- 39. Ding K., Gronenborn A. M. (2004) J. Am. Chem. Soc. 126, 6232–6233 [DOI] [PubMed] [Google Scholar]
- 40. Koźmiński W., Zhukov I., Pecul M., Sadlej J. (2005) J. Biomol. NMR 31, 87–95 [DOI] [PubMed] [Google Scholar]
- 41. Shen Y., Bax A. (2010) J. Biomol. NMR 46, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tossavainen H., Permi P., Purhonen S. L., Sarvas M., Kilpeläinen I., Seppala R. (2006) FEBS Lett. 580, 1822–1826 [DOI] [PubMed] [Google Scholar]
- 43. Heikkinen O., Seppala R., Tossavainen H., Heikkinen S., Koskela H., Permi P., Kilpeläinen I. (2009) BMC. Struct. Biol. 9, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buck M., Boyd J., Redfield C., MacKenzie D. A., Jeenes D. J., Archer D. B., Dobson C. M. (1995) Biochemistry 34, 4041–4055 [DOI] [PubMed] [Google Scholar]
- 45. Wong K. B., Fersht A. R., Freund S. M. (1997) J. Mol. Biol. 268, 494–511 [DOI] [PubMed] [Google Scholar]
- 46. Ghalebani L., Kotsyubynskyy D., Kowalewski J. (2008) J. Magn Reson. 195, 1–8 [DOI] [PubMed] [Google Scholar]
- 47. Kneller J. M., Lu M., Bracken C. (2002) J. Am. Chem. Soc. 124, 1852–1853 [DOI] [PubMed] [Google Scholar]
- 48. Ranganathan R., Lu K. P., Hunter T., Noel J. P. (1997) Cell 89, 875–886 [DOI] [PubMed] [Google Scholar]
- 49. Siddiqui K. S., Cavicchioli R. (2006) Annu. Rev. Biochem. 75, 403–433 [DOI] [PubMed] [Google Scholar]
- 50. Holm L., Park J. (2000) Bioinformatics. 16, 566–567 [DOI] [PubMed] [Google Scholar]
- 51. Yongye A. B., Bender A., Martinez-Mayorga K. (2010) J. Comput. Aided Mol. Des. 24, 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.