Abstract
The aim of this study is to demonstrate T1ρ MRI’s capability for measuring intervertebral disc osmotic pressure. Self-coregistered sodium and T1ρ-weighted MR images were acquired on ex vivo bovine intervertebral discs (N = 12) on a 3 T clinical MRI scanner. The sodium MR images were used to calculate effective nucleus pulposus fixed-charge-density (mean = 138.2±27.6 mM) and subsequently osmotic pressure (mean = 0.53±0.18 atm), while the T1ρ-weighted images were used to compute T1ρ relaxation maps. A significant linear correlation (R=0.56, p<0.01) between nucleus pulposus fixed-charge-density and T1ρ relaxation time constant was observed. More importantly, a significant power correlation (R=0.72, p<0.01) between nucleus pulposus osmotic pressure as predicted by sodium MRI and T1ρ relaxation time constant was also observed. The current clinical method for assessing disc pressure is discography, which is an invasive procedure that has been shown to have negative effects on disc biomechanical and biochemical properties. In contrast, T1ρ MRI is non-invasive and can be easily implemented in a clinical setting due to its superior signal-to-noise ratio compared to sodium MRI. Therefore, T1ρ MRI may serve as a non-invasive clinical tool for the longitudinal evaluation of disc osmotic pressure.
Keywords: Sodium, T1ρ, osmotic pressure, intervertebral disc, nucleus pulposus
INTRODUCTION
Degeneration of the intervertebral disc (IVD) is the most common cause of back-related disability among North American adults(1). More than 80% of the population will experience an episode of low back pain in their lifetime(2), with recurrence rate as high as 85%(3). Current clinical diagnosis of IVD degeneration is based on radiography and conventional proton T1 and T2-weighted MRI. These techniques are useful for observing morphological changes in IVD structure. However, IVD morphological changes tend to occur in the later stages of IVD degeneration(4). The earlier stage of IVD degeneration is typified by the breakdown of extracellular proteoglycan (PG) aggrecans in the nucleus pulposus (NP). As the aggrecans degrade into smaller fragments, they are more likely to diffuse out of the NP extracellular matrix and into the surrounding fluid. The loss of PG’s negatively charged GAG side chains decreases the fixed-charge-density (FCD) of the NP, resulting in the subsequent loss of sodium ions (Na+). Since the Na+ are the main extracellular solutes responsible for generating an osmotic pressure within the IVD, the aforementioned PG depletion in the IVD’s extracellular matrix leads to decreased IVD hydration and hydrostatic pressure(5,6). Both healthy and mildly degenerated IVDs have been shown to behave hydrostatically(7), and osmotic pressure in healthy IVDs linearly correlate with their hydrostatic pressure(8).
Previous studies have demonstrated that sodium MRI can accurately quantify PG concentration ([PG]) and FCD in articular cartilage under ideal Donnan equilibrium condition(9-11). A recent study has concluded a significant correlation between IVD [PG] measured using dimethylmethylene blue assay and IVD sodium concentration ([Na+]) measured with sodium MRI(12). More importantly, the FCD value computed from sodium MRI can be used to calculate IVD osmotic pressure according to a model developed by Urban et al.(13). While sodium MRI is specific for FCD and osmotic pressure, its low signal-to-noise ratio (SNR) significantly limits its role in in vivo applications. Recent studies on T1ρ MRI have shown great potential for the non-invasive evaluation of [PG] in articular cartilage and in IVD(14-16). Since T1ρ MRI targets proton nuclei as conventional MRI techniques, it has significantly higher SNR efficiency than sodium MRI, which makes T1ρ MRI a more clinically viable technique. T1ρ MRI is implemented as a spin-locking radiofrequency pulse cluster, which can be readily appended to a wide array of radiofrequency pulse sequences on most clinical MRI scanners. During the application of the spin-locking radiofrequency pulse, the transverse spin magnetization undergoes T1ρ relaxation. During T1ρ relaxation, spin-dephasing due to processes occurring at frequencies below the spin-locking amplitude is refocused. The resulting relaxation becomes sensitive to the interactions between PG molecules and motionally restricted protons, provided that the frequency of this interaction is close to the spin-locking nutational frequency (γB1)(17). Numerous studies have already demonstrated that water T1ρ relaxation (γB1=0.1~10 kHz) is sensitive to the interactions between macromolecules and motionally restricted protons(18-20). Despite previous works that correlated T1ρ relaxation and [PG] in fibrocartilage(14-16), the relationship between T1ρ relaxation and sodium MRI remains to be investigated in a quantitative fashion in the IVDs. More importantly, T1ρ relaxation time constant has yet to be correlated with IVD osmotic pressure, an important biomechanical property, in intact IVD samples and in a non-invasive fashion.
The current clinical method for assessing IVD pressure is discography, which is an invasive and painful procedure. In addition, discography has potential negative side effects that may warrant additional scrutiny. A previous study has shown that the needle puncture injury caused by discography has both immediate and progressive mechanical and biological consequences on the IVD(21). Therefore, there is a need for a noninvasive method to measure IVD pressure.
In this study, we demonstrated T1ρ MRI’s clinical significance as a potential non-invasive diagnostic tool for IVD osmotic pressure by establishing a correlation between T1ρ relaxation time constant and osmotic pressure measured via sodium MRI.
MATERIALS AND METHODS
Bovine Specimen
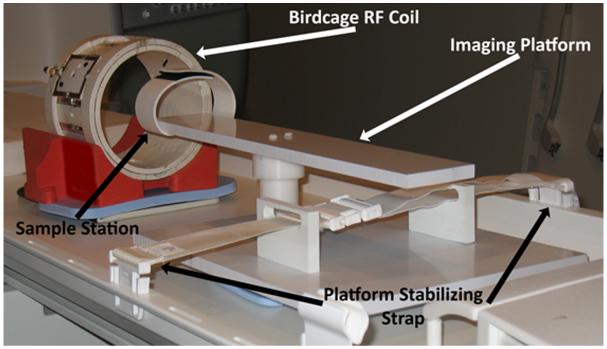
Four fresh whole veal lumbar spines were obtained from a local abattoir (Bierig Brothers, Vineland NJ), within a few hours of slaughter. The last three caudal IVDs of each spine sample were surgically removed. The vertebral body on each side of the IVD specimen was trimmed with a bone saw to include the endplate and approximately 1 cm of bony tissue, thus each processed IVD specimen contained a single IVD sandwiched between vertebral endplates, preserving the integrity of the motion segment. Next, the specimens were secured to a custom-made platform attached to the scanner bed, as shown in Figure 1. This platform allowed for the interchange of a pair of sodium and proton radiofrequency (RF) coils while the sample remained stationary inside the scanner bore. As a result, the same FOV was preserved between the proton and sodium MR scans, which allowed for the pixel-to-pixel quantitative comparison of sodium and proton MR images.
Figure 1.
Diagram of the imaging platform used for the self-coregistered sodium and T1ρ MRI. The body of the platform is secured onto the MRI scanner bed using a strap. The IVDs samples are placed at the sample station at the end of imaging platform, where a Velcro strap firmly secures the IVD sample to the imaging platform. The sodium and proton birdcage RF coils can then be slipped over the IVD sample in succession, without disturbing the sample itself. Additional sandbags are placed at the base of the imaging platform to further dampen vibrations contributed by gradient activity during the imaging sessions.
Sodium MR Imaging Protocol for Bovine IVDs ex vivo
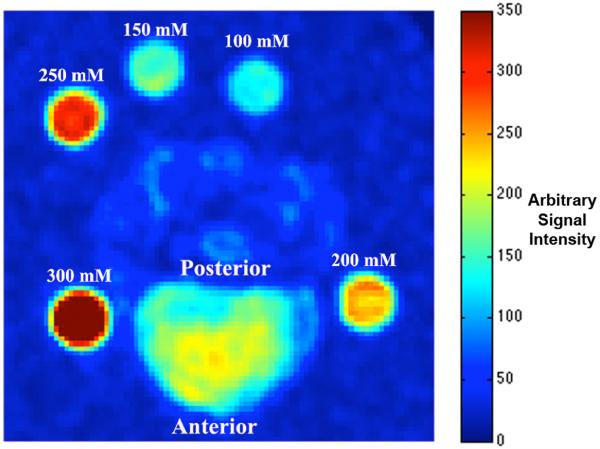
Both sodium and T1ρ-weighted MRI were performed on a 3 T Siemens Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a broadband transmitter and receiver at the Hospital of the University of Pennsylvania. All experimental procedures were in accordance with IACUC regulations at our institution. First the custom-made sodium low-pass quadrature birdcage RF coil (ωo = 32.6 MHz) was slipped over the previously mentioned imaging platform. The sodium RF coil was 17 cm in diameter and 12.5 cm long, containing 16 struts. Its two receiver ports were inductively coupled to the coil and spatially oriented 90° relative to each other. Five 10% agarose gel phantoms containing 300 mM, 250 mM, 200 mM, 150 mM, and 100 mM [Na+] were imaged alongside each specimen for eventual [Na+] calibration (Figure 2). A standard gradient-echo pulse sequence was used to acquire all sodium images. A low-resolution sodium image was acquired prior to the actual 3D sodium MR image acquisition in order to localize the desired FOV. The 3D imaging parameters were as follows: TE/TR = 6/30 ms, Ernst angle = 75.2°, FOV = 15 × 15 cm, matrix size = 128 × 128, slices = 128, slice thickness = 1.2 mm, BW = 60 Hz/Pixel, signal average = 75, imaging time = 10 hours and 14 minutes. Sodium nuclei exhibit biexponential T2* decay, with both a short and a long T2* component. However, the relatively long TE in this protocol minimized signal contribution from sodium’s short T2* component. Thus the sodium signal measured in this study can be approximately modeled as a single exponential. In addition, the broadening of the point-spread-function is minimized by not acquiring sodium signal undergoing the short T2* relaxation. The small acquisition BW was chosen to maximize the image SNR. The above parameters were chosen to obtain a minimum SNR of 15:1 for an isotropic voxel size of 1.2 mm3. T1ρ MRI was carried out immediately after sodium MRI during the same imaging session.
Figure 2.
An axial plane sodium image of a representative IVD, surrounded by five sodium phantoms containing different [Na+].
Mapping [Na+] in Bovine IVDs
The sodium MR images were first smoothed using a 3×3×3 pixel boxcar filter. Next, the pixel-wise [Na+] computation based on sodium phantom signals was carried out according to the method described by Shapiro, et al(9). The sodium signals from the sample and the phantoms were corrected separately for T1 and T2* decays according to Equation 1.
| Equation 1 |
In Equation 1, θ is the flip angle and So is the thermal equilibrium magnetization. Sodium T1 and T2* of the IVD and phantoms were computed using progressive saturation experiments, yielding T1 of 22 ms and 23 ms for the IVD and phantoms, respectively. The T2* of the IVD and phantoms were determined by varying the TE parameter, yielding T2* of 16ms and 8ms for IVD and phantoms, respectively. After compensating for T1 and T2* relaxation, the average sodium signal from each phantom of known [Na+] was plotted on a calibration curve of [Na+] versus sodium signal. The slope and y-intercept of the linear fit of the calibration curve was then used to compute a 3D [Na+] map of the IVD.
Calculating Bovine IVD FCD and Osmotic Pressure
FCD can be directly calculated form tissue [Na+] measurement ([Na+]t) and surrounding fluid [Na+] measurement ([Na+]f) using a method developed by Lesperance et al.(22). The expression that relates FCD to [Na+]t and [Na+]f is shown in Equation 2.
| Equation 2 |
In the context of this study, the FCD is computed using a [Na+]f value of 150 mM, which is the normal [Na+] level in serum fluid. Osmotic pressure was subsequently calculated from Equation 3 empirically derived by Urban et al.(13). However, the FCD value computed according to Equation 2 is an average FCD measurement of the total NP extracellular sodium. The NP extracellular sodium is in fact composed of both intrafibrillar (relative to the type II collagen fibers) and extrafibrillar water compartments. Intrafibrillar water resides in the space within the collagen fibrils, thus the PGs are excluded from the intrafibrillar volume due to their large size(23). Instead, the PGs are confined within the extrafibrillar water, where they attract the positively charged Na+ ions to generate the osmotic pressure necessary to resist loads. Therefore, the effective calculation of FCD should reflect the compartmentalization of Na+ ions to the smaller volume of the extrafibrillar water. In fact, the effective FCD (FCDeffective) is always higher than the FCD value calculated from Equation 2, assuming that the extrafibrillar water is always less than the total water content of the IVD NP tissue. With the knowledge of FCDeffective, the actual osmotic pressure of the IVD can be accurately calculated according to Equation 3.
| Equation 3 |
In Equation 3, P is osmotic pressure in unit of atm, B is a constant of 26.6 atm/M2, and FCDeffective is the FCD specific to NP extrafibrillar water. In order to compute FCDeffective from FCD calculated directly from sodium MRI, the relative fractions of intrafibrillar and extrafibrillar water compartments in the NP need to be determined first. The relationship between FCD and FCDeffective is defined in Equation 4(23).
| Equation 4 |
In Equation 4, the FCD value calculated using Equation 2 is compensated by the ratio of the tissue’s total wet weight (Wtotal) and the weight of the extrafibrillar water, which is defined as the subtraction of the total water weight (Wwater) by the intrafibrillar water weight (Wintrafibrillar). Since Wintrafibrillar is not easily measured directly, it is expanded in Equation 4 to form Equation 5(23).
| Equation 5 |
In Equation 5, the Wintrafibrillar term from Equation 4 is expanded as the product of Wtotal, (dry weight of collagen normalized against Wtotal) and (weight of intrafibrillar water normalized per dry weight of collagen). A previous study has concluded that water content constitutes 80% of a healthy adult’s IVD NP(5). Therefore, Wwater = 0.8 after normalizing it against Wtotal. NP collagen amounts to 20% of the dry weight of the NP tissue (24), which yields 0.04 for . At last, for a healthy adult has been shown to be 0.98 g water/g collagen(25). Substituting these values back in Equation 5 and Equation 3 results in the final expression that relates osmotic pressure measurement to the FCD values measured from sodium MRI directly, as shown in Equation 6.
| Equation 6 |
Equation 6 takes into account the contribution of intrafibrillar water to the total water volume in IVD NP.
Mapping T1ρ Relaxation Rate in Bovine IVD
Following the sodium MR scan, the sodium coil was removed and a Siemens 8-channel proton birdcage radiofrequency coil was slipped over the imaging platform. T1ρ MRI was carried out using a custom spin-locking prepared 3D SPGR pulse sequence. The FOV and resolution parameters were identical to those of the sodium MRI. The imaging parameters were as follows: TE/TR = 4.5/120 ms, Flip Angle = 15°, FOV = 15×15 cm, matrix size = 128×128, slices = 64, slice thickness = 1.2 mm. The small flip angle was chosen to allow both the AF and the NP longitudinal magnetizations to recover to their thermal equilibrium during the given TR, which mitigated the need to separately correct for T1 relaxation in the AF and the NP compartments. Spin-locking preparation was applied once for every 16 phase-encodes, and the phase-encoding was centrically ordered to preserve T1ρ weighting in the center of the k-space. T1ρ-weighted images at four spin-locking times (TSL = 10, 20, 30, 40 ms) were collected, with a spin-locking amplitude of 500 Hz, for a total imaging time of 1 hour and 20 minutes. The highest TSL of 40 ms was limited by the SAR restriction on the clinical MRI scanner used. The FOV center and spatial resolution of the T1ρ scans were copied directly from the previous sodium MRI scans, thus the pixel-to-pixel coregistration between the sodium and T1ρ MR scans was maintained. A T1ρ map was computed on a pixel-by-pixel basis from the four T1ρ-weighted images according to Equation 7.
| Equation 7 |
In Equation 7, S is the image signal intensity and So is the intensity of the thermal equilibrium magnetization.
Image Processing and Data Analysis for Bovine IVD
All sodium T1ρ-weighted MR images were transferred to a Macbook Pro computer (Apple, Cupertino, CA) for processing and ROI analysis, which were carried out using algorithms developed with MATLAB software (Mathworks, Natick, MA). For each IVD, a single user (CW) chose a 4mm diameter circular ROI on three mid-axial slices in the T1ρ-weighted image. The ROIs were then used to extract average [Na+] and T1ρ relaxation time constant values from the self-coregistered [Na+] and T1ρ maps. The average ROI FCDeffective and osmotic pressure measurements were computed from [Na+] using Equation 5 and Equation 6 accordingly.
Statistical Analysis
Linear regression analysis was applied to the T1ρ relaxation time constant versus FCDeffective data. Bivariate correlation of the same data pairs was also carried out, and Pearson correlation coefficient and Spearman’s rank correlation coefficient were computed to determine if there was a direct linear relationship between T1ρ relaxation time constant and FCDeffective in the NP regions of the IVDs. Regression analysis was applied to the T1ρ relaxation time constant versus osmotic pressure data. However, due to the non-linear relationship between FCDeffective and osmotic pressure as shown in Equation 6, a power regression analysis was applied to the T1ρ relaxation time constant versus osmotic pressure data instead of a linear regression analysis. Both the bivariate correlation analysis between T1ρ relaxation time constant and FCDeffective measurements, and the power regression analysis between T1ρ relaxation time constant and osmotic pressure data were carried out with the significance level of p<0.01.
RESULTS
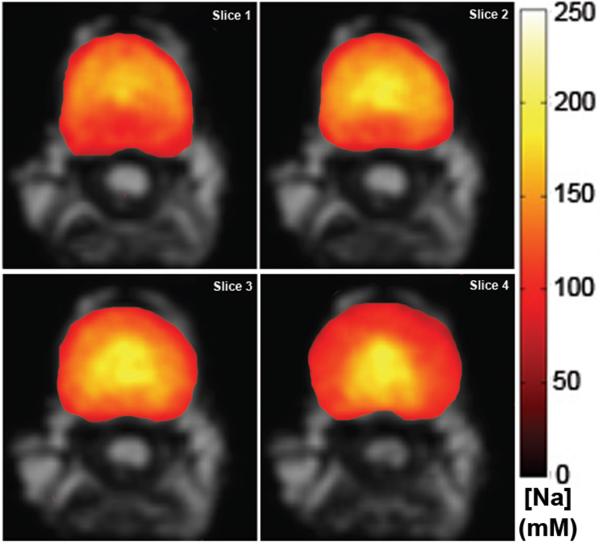
Figure 3 illustrates a series of four consecutive axial slices of an IVD [Na+] color maps overlaid on top of their grayscale anatomical images. The center of the IVD typically has the highest [Na+] value around roughly 250 mM. Since the NP contains the majority of PGs in the IVD, the regions of high [Na+] represent the NP region. In contrast, the peripheries of the IVD [Na+] maps in Figure 3 indicate a much lower [Na+] at around 150 mM, which is close to the theoretical serum fluid level [Na+]. The peripheries of the IVD [Na+] maps represent the AF region with lower [Na+], due to the lack of PGs.
Figure 3.
Four consecutive axial slices of an IVD’s [Na+] map. Note the decrease in [Na+] going from the center of the NP toward the AF. Ventral side of the IVD faces up.
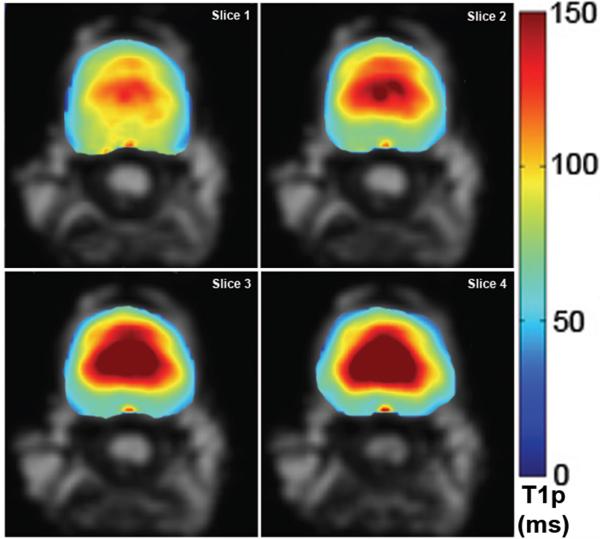
Figure 4 illustrates four consecutive axial slice T1ρ maps of the same IVD for which the [Na+] maps are shown in Figure 3. As in the case of the [Na+] maps, T1ρ appeared to be the highest in the center of the IVD, where the PG-rich NP is located. In the IVD peripheries composed of the AF, T1ρ decreased to approximately 30% of the maximum value observed in the center of the NP. In addition, the T1ρ maps in Figure 4 exhibit superior spatial resolution when compared to the corresponding [Na+] maps, which have a blurry appearance.
Figure 4.
Four consecutive axial slices of an IVD’s T1ρ map. Note the decrease in T1ρ going from the center of the NP toward the AF. Ventral side of the IVD faces up.
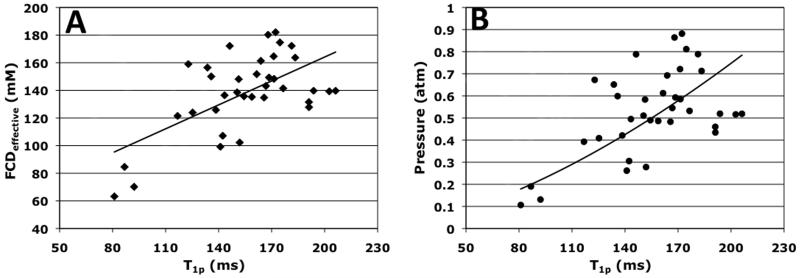
ROI analysis of the NP [Na+] maps and the NP T1ρ maps yielded average FCDeffective (from Equation 5), osmotic pressure (from Equation 6), and T1ρ relaxation time constant. T1ρ relaxation time constants were separately compared to first the FCDeffective values and then to the osmotic pressure values. Figure 5(A) contains the scatter plot of average NP FCDeffective vs. T1ρ relaxation time constant. The solid line marks the linear regression fit of the data points, and it shows a positive relationship between NP FCDeffective and T1ρ relaxation time constant. A one-tailed bivariate correlation analysis of the FCDeffective vs. T1ρ data yielded a significant Pearson correlation coefficient of 0.56 with p<0.01, as well as a significant Spearman’s rank correlation coefficient of 0.44 with p<0.01. Due to the squared relationship between FCDeffective and osmotic pressure as shown in Equation 6, a power regression analysis was applied to the osmotic pressure vs. T1ρ relaxation time constant scatter plot in Figure 5(B), resulting in the expression relating NP T1ρ relaxation time constant in unit of ms to IVD osmotic pressure in unit of atm, as shown in Equation 8.
| Equation 8 |
Figure 5.
(A) A scatter plot of IVD NP FCDeffective measurement vs. the corresponding T1ρ relaxation time constant. The solid line represents the linear regression line of the scatter plot, with a correlation coefficient of 0.56 at p<0.01. (B) A scatter plot of IVD NP osmotic pressure measurement vs. the T1ρ relaxation time constant. A power regression fit was applied to the scatter plot, yielding a correlation coefficient of 0.72 at p<0.01.
Equation 8 was obtained with a significant correlation coefficient of 0.72 with p<0.01. Note that T1ρ was raised to the power of 1.6 in Equation 8, in contrast to the power of 2 for the FCDeffective variable in Equation 6.
DISCUSSION AND CONCLUSIONS
The high [Na+] in IVD NP compared to the lower [Na+] in IVD AF observed from the colored [Na+] maps in Figure 3 is consistent with a previous study that measured IVD [Na+] directly and demonstrated significantly lower sodium content in the AF compared to the NP(26). In a similar fashion, the observation of elevated T1ρ relaxation time constant in the IVD NP and a much lower T1ρ in the IVD AF supports previous studies that concluded a positive relationship between IVD [PG] and T1ρ relaxation time constant(16,27). In contrast to IVDs, articular cartilage has been shown to exhibit an opposite relationship between [PG] and T1ρ relaxation time constant(14,28,29). The discrepancy between the trend of T1ρ in articular cartilage and IVD degenerations may be due to their different possible mechanisms of degeneration. In articular cartilage degeneration, PG depletion creates space for synovial fluid infiltration, resulting in elevated T1ρ relaxation time constant. In contrast, PG depletion in IVD leads to decreased hydration and hence decreased T1ρ relaxation time constant.
The quadrupolar nature of sodium results in a biexponential T2* relaxation when the rotational correlation time of sodium is long enough to not satisfy the extreme narrowing condition(30). The IVD NP Na+ reside in a motion-restricted macromolecular environment composed of cross-linked type II collagen fibers, which results in an increase in sodium’s rotational correlation time. Therefore, the same IVD NP sodium undergoes both short and long T2* relaxations simultaneously. Thus the measurement of sodium signal undergoing long T2* relaxation alone along with calibrated phantoms can be used to quantify total sodium content. This technique has been previously demonstrated by a sodium MRI study of articular cartilage, which confirmed that the FCD value computed from sodium images acquired at long TE correlated strongly with FCD value obtained from dimethylmethylene blue PG assay(9).
Moreover, FCD has been previously used to calculate the osmotic pressure of IVD and articular cartilage(13). However, the FCD value obtained from [Na+] measured using sodium MRI is in fact a spatially averaged value taking into account both the extrafibrillar sodium and the intrafibrillar sodium of the IVD NP. Since the PGs responsible for generating IVD osmotic pressure are restricted to the extrafibrillar space due to their large size, the FCD calculated according to the expression (Equation 2) developed by Lesperance et al. in theory would result in an underestimation of the effective FCD (FCDeffective) and subsequently an underestimation of the IVD osmotic pressure. In order to address this issue, we determined the relationship between FCDeffective and the FCD value calculated from sodium MRI using literature values of IVD NP intrafibrillar water content and collagen content. The expression of this relationship (Equation 6) was then utilized in the calculation of IVD osmotic pressure. As a result, we demonstrated that the FCD measurement computed using sodium MRI also offers a potentially useful tool for the non-invasive quantification of IVD osmotic pressure, which is an important IVD biomechanical property.
Despite sodium MRI’s promising capability in monitoring IVD NP FCDeffective and IVD osmotic pressure, it has inherently low SNR. Thus, the spatial resolution of the sodium MR scan is often lowered in combination with increased scanning time in order to compensate for its low SNR. Together both the long scanning time and low spatial resolution limit the clinical applicability of sodium MRI. The three primary factors contributing to sodium MRI’s low SNR are listed as follows, in the order of importance. The first factor is the low natural concentration of sodium in tissue. In human body, typical proton density ([H]) is around 110 M while [Na+] in healthy IVD is only around 250 mM. The second factor leading to low SNR of sodium MRI is sodium’s lower gyromagnetic ratio (γ=11.26 MHz/T) compared to proton’s (γ=42.57 MHz/T), which results in a smaller measurable thermal equilibrium magnetization. The third factor is the short T2* of sodium nuclei, which causes rapid relaxation of sodium’s measurable transverse magnetization.
In comparison to sodium MRI, T1ρ MRI yields significantly higher SNR because it targets proton nuclear spin. Moreover, T1ρ contrast is sensitive to the interactions between protons on PG and free water protons. Free water proton spin-spin relaxation in IVD NP is partially influenced by the residual dipolar interaction between type II collagen fibers and free water protons. T1ρ relaxation effectively refocuses the spin relaxation due to residual dipolar interaction, which renders T1ρ relaxation relatively insensitive to collagen content and more sensitive to PG content and hydration in the IVD. A previous ex vivo IVD study has indeed demonstrated a strong correlation between IVD PG content and T1ρ relaxation(31). However, to the best of our knowledge, there has been no previous attempt to use non-invasive MRI techniques such as sodium MRI and T1ρ MRI to compute IVD osmotic pressure in intact IVD specimens.
From the results of our regression and bivariate correlation analyses between T1ρ relaxation time constant value, FCDeffective, and osmotic pressure, we concluded that T1ρ is not only linearly correlated with FCDeffective, but also correlated with osmotic pressure measurements, which has been shown to be linearly correlated with the hydrostatic pressure produced by IVDs under normal loading conditions(8). The power regression analysis of T1ρ relaxation time constant and osmotic pressure yielded Equation 8, which showed that the osmotic pressure measurement was related to T1ρ raised to the power of 1.6. Note in Equation 6, the FCDeffective variable was raised to the power of 2 for the calculation of osmotic pressure. Assuming a linear relationship between T1ρ relaxation time constant and FCDeffective, as demonstrated by the significant Pearson and Spearman’s correlation coefficients of their bivariate analysis, the discrepancy between Equation 8 and Equation 6 might be attributed to the fact that Equation 6 was derived from an experiment conducted at 4 °C(13), which is significantly lower than both the body temperature as well as the room temperature at our imaging facility.
In conclusion, we demonstrated that T1ρ MRI of IVD correlates well with FCDeffective and osmotic pressure measurements obtained from sodium MRI. Due to its non-invasive nature and high SNR, T1ρ MRI can potentially be readily applied in clinical setting. Therefore, we have shown that T1ρ MRI has significant potential as a non-invasive clinical tool for the evaluation of IVD osmotic pressure.
ACKNOWLEDGEMENTS
This project was supported by partial support from the following grants: NIH-NCRR RR02305, NIHR01AR45404, NIHR01AR051041, AO Spine Research grant, and NIBIB T32 training grant (T32-EB000814).
REFERENCES
- 1.ERRICO TJ. LUMBAR DISC ARTHROPLASTY. CLIN ORTHOP RELAT RES. 2005;(435):106–117. doi: 10.1097/01.blo.0000165718.22159.d9. [DOI] [PubMed] [Google Scholar]
- 2.RUBIN DI. EPIDEMIOLOGY AND RISK FACTORS FOR SPINE PAIN. NEUROLOGIC CLINICS. 2007;25(2):353. doi: 10.1016/j.ncl.2007.01.004. + [DOI] [PubMed] [Google Scholar]
- 3.VAN TULDER M, KOES B, BOMBARDIER C. LOW BACK PAIN. BEST PRACT RES CLIN RHEUMATOL. 2002;16(5):761–775. doi: 10.1053/berh.2002.0267. [DOI] [PubMed] [Google Scholar]
- 4.SAIFUDDIN A, MITCHELL R, TAYLOR BA. EXTRADURAL INFLAMMATION ASSOCIATED WITH ANNULAR TEARS: DEMONSTRATION WITH GADOLINIUM-ENHANCED LUMBAR SPINE MRI. EUR SPINE J. 1999;8(1):34–39. doi: 10.1007/s005860050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ANTONIOU J, STEFFEN T, NELSON F, WINTERBOTTOM N, HOLLANDER AP, POOLE RA, AEBI M, ALINI M. THE HUMAN LUMBAR INTERVERTEBRAL DISC: EVIDENCE FOR CHANGES IN THE BIOSYNTHESIS AND DENATURATION OF THE EXTRACELLULAR MATRIX WITH GROWTH, MATURATION, AGEING, AND DEGENERATION. J CLIN INVEST. 1996;98(4):996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.URBAN JP, MCMULLIN JF. SWELLING PRESSURE OF THE LUMBAR INTERVERTEBRAL DISCS: INFLUENCE OF AGE, SPINAL LEVEL, COMPOSITION, AND DEGENERATION. SPINE. 1988;13(2):179–187. doi: 10.1097/00007632-198802000-00009. [DOI] [PubMed] [Google Scholar]
- 7.NACHEMSON A. SOME MECHANICAL PROPERTIES OF THE LUMBAR INTERVERTEBRAL DISCS. BULL HOSP JOINT DIS. 1962;23:130–143. [PubMed] [Google Scholar]
- 8.JAYSON MIV. THE LUMBAR SPINE AND BACK PAIN. SECTOR PUBLISHING LTD. FOR PITMAN MEDICAL; LONDON: 1976. p. 400. [Google Scholar]
- 9.SHAPIRO EM, BORTHAKUR A, GOUGOUTAS A, REDDY R. 23NA MRI ACCURATELY MEASURES FIXED CHARGE DENSITY IN ARTICULAR CARTILAGE. MAGN RESON MED. 2002;47(2):284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.REGATTE RR, KAUFMAN JH, NOYSZEWSKI EA, REDDY R. SODIUM AND PROTON MR PROPERTIES OF CARTILAGE DURING COMPRESSION. JOURNAL OF MAGNETIC RESONANCE IMAGING. 1999;10(6):961–967. doi: 10.1002/(sici)1522-2586(199912)10:6<961::aid-jmri8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.WHEATON AJ, BORTHAKUR A, SHAPIRO EM, REGATTE RR, AKELLA SV, KNEELAND JB, REDDY R. PROTEOGLYCAN LOSS IN HUMAN KNEE CARTILAGE: QUANTITATION WITH SODIUM MR IMAGING--FEASIBILITY STUDY. RADIOLOGY. 2004;231(3):900–905. doi: 10.1148/radiol.2313030521. [DOI] [PubMed] [Google Scholar]
- 12.WANG C, MCARDLE E, FENTY M, WITSCHEY W, ELLIOTT M, SOCHOR M, REDDY R, BORTHAKUR A. VALIDATION OF SODIUM MAGNETIC RESONANCE IMAGING OF INTERVERTEBRAL DISC. SPINE (PHILA PA 1976) 2010;35(5):505–510. doi: 10.1097/BRS.0b013e3181b32d3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.URBAN JP, MAROUDAS A, BAYLISS MT, DILLON J. SWELLING PRESSURES OF PROTEOGLYCANS AT THE CONCENTRATIONS FOUND IN CARTILAGINOUS TISSUES. BIORHEOLOGY. 1979;16(6):447–464. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- 14.WHEATON AJ, DODGE GR, ELLIOTT DM, NICOLL SB, REDDY R. QUANTIFICATION OF CARTILAGE BIOMECHANICAL AND BIOCHEMICAL PROPERTIES VIA T1RHO MAGNETIC RESONANCE IMAGING. MAGN RESON MED. 2005;54(5):1087–1093. doi: 10.1002/mrm.20678. [DOI] [PubMed] [Google Scholar]
- 15.AKELLA SV, REGATTE RR, GOUGOUTAS AJ, BORTHAKUR A, SHAPIRO EM, KNEELAND JB, LEIGH JS, REDDY R. PROTEOGLYCAN-INDUCED CHANGES IN T1RHO-RELAXATION OF ARTICULAR CARTILAGE AT 4T. MAGN RESON MED. 2001;46(3):419–423. doi: 10.1002/mrm.1208. [DOI] [PubMed] [Google Scholar]
- 16.AUERBACH JD, JOHANNESSEN W, BORTHAKUR A, WHEATON AJ, DOLINSKAS CA, BALDERSTON RA, REDDY R, ELLIOTT DM. IN VIVO QUANTIFICATION OF HUMAN LUMBAR DISC DEGENERATION USING T(1RHO)-WEIGHTED MAGNETIC RESONANCE IMAGING. EUR SPINE J. 2006;15(SUPPL 15):338–344. doi: 10.1007/s00586-006-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DUVVURI U, GOLDBERG AD, KRANZ JK, HOANG L, REDDY R, WEHRLI FW, WAND AJ, ENGLANDER SW, LEIGH JS. WATER MAGNETIC RELAXATION DISPERSION IN BIOLOGICAL SYSTEMS: THE CONTRIBUTION OF PROTON EXCHANGE AND IMPLICATIONS FOR THE NONINVASIVE DETECTION OF CARTILAGE DEGRADATION. PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES OF THE UNITED STATES OF AMERICA. 2001;98(22):12479–12484. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.VIRTA A, KOMU M, KORMANO M. T1RHO OF PROTEIN SOLUTIONS AT VERY LOW FIELDS: DEPENDENCE ON MOLECULAR WEIGHT, CONCENTRATION, AND STRUCTURE. MAGN RESON MED. 1997;37(1):53–57. doi: 10.1002/mrm.1910370109. [DOI] [PubMed] [Google Scholar]
- 19.SEPPONEN RE, TANTTU JI. MEASUREMENT OF T1RHO AND T1RHO DISPERSION INVIVO AT 0.1 T. RADIOLOGY. 1992;185:253–253. [Google Scholar]
- 20.KNISPEL RR, THOMPSON RT, PINTAR MM. DISPERSION OF PROTON SPIN-LATTICE RELAXATION IN TISSUES. JOURNAL OF MAGNETIC RESONANCE. 1974;14(1):44–51. [Google Scholar]
- 21.KORECKI CL, COSTI JJ, IATRIDIS JC. NEEDLE PUNCTURE INJURY AFFECTS INTERVERTEBRAL DISC MECHANICS AND BIOLOGY IN AN ORGAN CULTURE MODEL. SPINE (PHILA PA 1976) 2008;33(3):235–241. doi: 10.1097/BRS.0b013e3181624504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LESPERANCE LM, GRAY ML, BURSTEIN D. DETERMINATION OF FIXED CHARGE DENSITY IN CARTILAGE USING NUCLEAR MAGNETIC RESONANCE. J ORTHOP RES. 1992;10(1):1–13. doi: 10.1002/jor.1100100102. [DOI] [PubMed] [Google Scholar]
- 23.MAROUDAS A. METHODS IN CARTILAGE RESEARCH. Vol. 1. ACADEMIC PRESS; LONDON: 1990. [Google Scholar]
- 24.EYRE DR, MUIR H. QUANTITATIVE ANALYSIS OF TYPES I AND II COLLAGENS IN HUMAN INTERVERTEBRAL DISCS AT VARIOUS AGES. BIOCHIM BIOPHYS ACTA. 1977;492(1):29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 25.SIVAN S, MERKHER Y, WACHTEL E, EHRLICH S, MAROUDAS A. CORRELATION OF SWELLING PRESSURE AND INTRAFIBRILLAR WATER IN YOUNG AND AGED HUMAN INTERVERTEBRAL DISCS. J ORTHOP RES. 2006;24(6):1292–1298. doi: 10.1002/jor.20144. [DOI] [PubMed] [Google Scholar]
- 26.URBAN JP, WINLOVE CP. PATHOPHYSIOLOGY OF THE INTERVERTEBRAL DISC AND THE CHALLENGES FOR MRI. J MAGN RESON IMAGING. 2007;25(2):419–432. doi: 10.1002/jmri.20874. [DOI] [PubMed] [Google Scholar]
- 27.WANG C, AUERBACH JD, WITSCHEY WR, BALDERSTON RA, REDDY R, BORTHAKUR A. ADVANCES IN MAGNETIC RESONANCE IMAGING FOR THE ASSESSMENT OF DEGENERATIVE DISC DISEASE OF THE LUMBAR SPINE. SEMIN SPINE SURG. 2007;19(2):65–71. doi: 10.1053/j.semss.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.REGATTE RR, AKELLA SV, BORTHAKUR A, KNEELAND JB, REDDY R. PROTEOGLYCAN DEPLETION-INDUCED CHANGES IN TRANSVERSE RELAXATION MAPS OF CARTILAGE: COMPARISON OF T2 AND T1RHO. ACAD RADIOL. 2002;9(12):1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 29.LI X, BENJAMIN MAC, LINK TM, CASTILLO DD, BLUMENKRANTZ G, LOZANO J, CARBALLIDOGAMIO J, RIES M, MAJUMDAR S. IN VIVO T(1RHO) AND T(2) MAPPING OF ARTICULAR CARTILAGE IN OSTEOARTHRITIS OF THE KNEE USING 3 T MRI. OSTEOARTHRITIS CARTILAGE. 2007;15(7):789–797. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PEKAR J, LEIGH JS. DETECTION OF BIEXPONENTIAL RELAXATION IN NA-23 FACILITATED BY DOUBLE-QUANTUM FILTERING. JOURNAL OF MAGNETIC RESONANCE. 1986;69(3):582–584. [Google Scholar]
- 31.JOHANNESSEN W, AUERBACH JD, WHEATON AJ, KURJI A, BORTHAKUR A, REDDY R, ELLIOTT DM. ASSESSMENT OF HUMAN DISC DEGENERATION AND PROTEOGLYCAN CONTENT USING T1RHO-WEIGHTED MAGNETIC RESONANCE IMAGING. SPINE (PHILA PA 1976) 2006;31(11):1253–1257. doi: 10.1097/01.brs.0000217708.54880.51. [DOI] [PMC free article] [PubMed] [Google Scholar]