Summary
Mutations in the X-linked MECP2, which encodes the transcriptional regulator methyl-CpG-binding protein 2 (MeCP2) cause Rett syndrome (RTT) and several neurodevelopmental disorders including cognitive disorders, autism, juvenile-onset schizophrenia, and encephalopathy with early lethality. RTT is characterized by apparently normal early development followed by regression, motor abnormalities, seizures, and features of autism, especially stereotyped behaviors. The mechanisms mediating these striking features are poorly understood. Here we show that mice lacking Mecp2 from γ-amino-butyric-acid-(GABA)-ergic neurons recapitulate numerous RTT and autistic features, including repetitive behaviors. Loss of MeCP2 from a subset of forebrain GABAergic neurons also recapitulates many features of RTT. MeCP2-deficient GABAergic neurons show reduced inhibitory quantal size consistent with presynaptic reduction in glutamic acid decarboxylase-1 and -2 levels and GABA immunoreactivity. These data demonstrate that MeCP2 is critical for normal GABAergic neuronal function and that subtle dysfunction of GABAergic neurons contributes to numerous neuropsychiatric phenotypes.
Introduction
Mutations in MECP2 cause Rett syndrome as well as a variety of neuropsychiatric syndromes including autism, bipolar disorder with cognitive deficits, and childhood onset schizophrenia with intellectual disabilities1-6. RTT is typically characterized by loss of language skills and hand use, cognitive deficits, stereotyped behaviors, impaired coordination, apraxia, ataxia, seizures, respiratory dysrhythmias and sometimes premature lethality1, 6-13. Mice either lacking MeCP2 or engineered to express an allele mimicking some RTT-causing mutations reproduce many of the phenotypes of RTT14-16. In search of the anatomical basis of RTT, early studies showed that deleting MeCP2 from nearly all post-mitotic neurons and glia using Cre-recombinase (Cre) driven by the Nestin promoter (Nestin-Cre) reproduce neurological phenotypes of RTT14, 15. In contrast, subsequent neuron-specific or regional Mecp2 deletion studies reproduce some aspects of Rett syndrome17-20. Loss of MeCP2 from dopaminergic neurons causes motor incoordination whereas loss from serotoninergic neurons leads to increased aggression19; loss from the amygdala impairs amygdala-dependent learning and memory20; and Mecp2 deletion in the hypothalamic Sim1-expressing neurons leads to alterations in feeding behavior, aggression, and stress response18. Postnatal loss of MeCP2 from forebrain excitatory neurons produce motor incoordination, increased anxiety-like behaviors, and impaired fear conditioning and social behavior17. These genetic studies suggest discrete features of RTT are associated with dysfunction of discrete neuronal populations (Supplemental Table 1); nonneuronal cells, such as glia, may also contribute to RTT21, 22. Importantly, deletions of MeCP2 from specific neurons frequently provide novel insight into the role of the targeted neurons in mediating certain behaviors and phenotypes. In this study we explore the consequences of loss of MeCP2 from GABAergic neurons and demonstrate that subtle perturbation of GABAergic neuronal function contributes to neuropsychiatric phenotypes.
Generation and characterization of Viaat-Cre mice
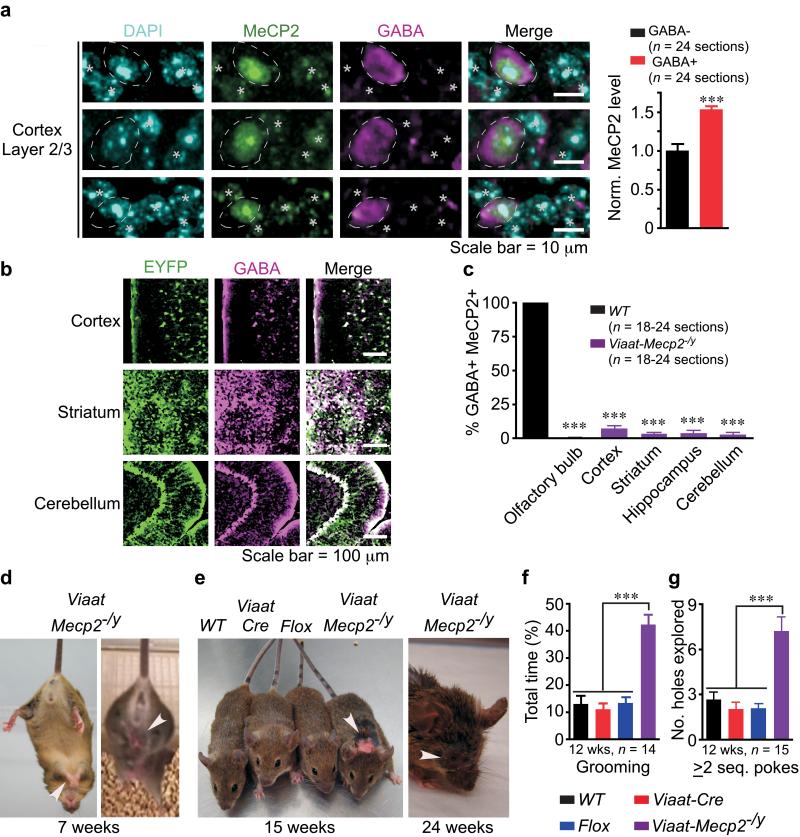
Our observation that cortical wild-type GABAergic neurons express ~50% more MeCP2 than non-GABAergic neurons (Fig.1a, p < 0.02) hinted that MeCP2 might be particularly important to GABAergic function. To assess the role of MeCP2 in GABAergic neurons we used a bacterial artificial chromosome (BAC) containing the Viaat (vesicular inhibitory amino acid transporter) promoter to target Cre expression in global GABAergic neurons (Supplemental Fig. 1a-c)23. Viaat encodes a transporter required for loading GABA, as well as glycine, into synaptic vesicles24, 25. Since the BAC contained 77.5 kb upstream and 23 kb downstream of the Viaat locus that includes the truncated coding sequence for Actin-related protein 5 homolog (Actr5), we performed quantitative real-time reverse-transcription polymerase chain reaction (Q-RTPCR) and found neither Viaat nor Actr5 is overexpressed in Viaat-Cre mice (Supplemental Fig. 1d). Characterization of Viaat-Cre expression using ROSA26R-EYFP reporter mice26 showed >98% colocalization between GABA and EYFP labeled cells and no colocalization with the astrocytic marker, glial fibrillary acidic protein (GFAP) (Fig.1b and Supplemental Fig. 2a-i, 3a).
Figure 1. Viaat-Mecp2−/y mice lose MeCP2 in GABA+ neurons and develop stereotypies, self-injury, and compulsive behavior.
(a) WT cortex layer 2/3 neurons from 17-week old mice labeled with DAPI, MeCP2, and GABA reveal 50% higher MeCP2 levels in GABA+ (circled) than in GABA− cells (asterisk). Data normalized to MeCP2 level in GABA− cells; n = 3 mice per genotype. (b) Viaat-Cre expression as assessed by ROSA26R-EYFP reporter and colocalization of EYFP and GABA in 14-week old mice. (c) More than 90% of GABA+ cells in Viaat-Mecp2−/y mice lack MeCP2. Data from n = 3 mice per genotype. (d) Seven-week old Viaat-Mecp2−/y mice displaying forepaw and hindlimb clasping (arrowhead). (e) Viaat-Mecp2−/y mice showing fur loss at 15 weeks of age and self-injury, including ocular damage, at 24 weeks (arrowhead). (f,g) Viaat-Mecp2−/y mice show ~300% increase in grooming time (f) and in number of holes explored with ≥ 2 sequential nose-pokes (g).
Viaat-Mecp2−/y mice display nearly all RTT features
We generated conditional Mecp2 deletion mice by crossing Viaat-Cre to Mecp2flox/+ mice14. Male Viaat-Mecp2−/y mice lost MeCP2 from >90% GABAergic neurons (Fig.1c, p < 0.0001). We compared male Viaat-Mecp2−/y mice to male littermate controls: wildtype (WT), Cre transgene (Viaat-Cre), and conditional allele (Flox, with constitutive 50% reduction in MeCP2 levels27).
Viaat-Mecp2−/y mice were indistinguishable from controls until ~5 weeks of age, when they exhibited hindlimb clasping repetitive behaviors including forelimb stereotypies reminiscent of mid-line hand-wringing (Fig. 1d, Supplemental Videos 1 and 2). These repetitive behaviors were not seen in controls (Supplemental Videos 3-5). Viaat-Mecp2−/y mice spent 300% more time grooming than wildtype mice leading to fur loss and epidermal lesion in group- and single-housed mice (Fig. 1e,f, p <0.0001). The self-injury was not due to impaired nociception (Supplemental Fig. 4a,b). Another compulsive behavior was revealed in the holeboard assay for head-dipping stereotypy: Viaat-Mecp2−/y mice showed a greater tendency than controls to poke their nose into the same hole two or more sequential times (Fig. 1g, p <0.0001).
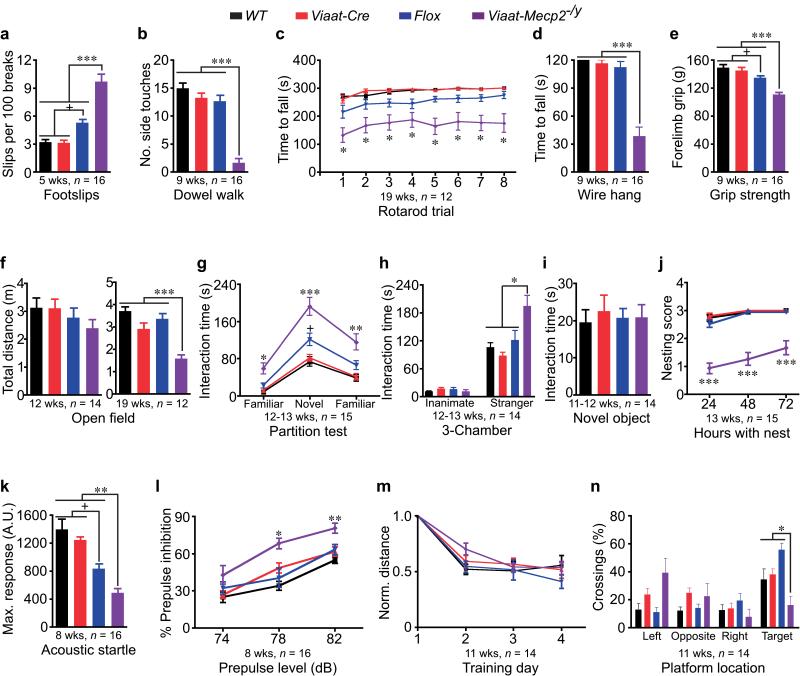
Viaat-Mecp2−/y mice showed progressive motor dysfunction with more footslips on a wire grid at 5 weeks of age (Fig. 2a, p < 0.0001), impaired dowel walk at 9 weeks (Fig. 2b, p < 0.001), and shorter latency to fall on accelerating rotarod at 19 weeks (Supplemental Fig. 4c and Fig. 2c, p <0.05). The mice also developed motor weakness with decreased latency to fall on wire hang and reduced forelimb grip strength at 9 weeks (Fig. 2d, p < 0.001 and 2e, p < 0.0001). Twelve-week old mice showed a trend toward reduced activity (Fig. 2f), but became clearly hypoactive by 19 weeks (Fig. 2f, p <0.0001). We found no evidence of anxiety-like phenotypes, indicating hypoactivity did not reflect increased anxiety (Supplemental Fig. 5a-h).
Figure 2. MeCP2 deficiency in GABAergic neurons causes several RTT-like features.
(a-f) Viaat-Mecp2−/y mice have more footslips (a), reduced number of side touches on dowel (b), shorter latency to fall on rotarod (c) and wire (d), reduced forelimb grip strength (e), and pronounced hypoactivity (f). (g) Viaat-Mecp2−/y mice have intact social recognition but increased social interaction with novel and familiar partners. (h) Viaat-Mecp2−/y mice spend 60% more time interacting with the unfamiliar stranger mouse than controls. The wire cup served as a familiar inanimate control without social valence. (i) Viaat-Mecp2−/y mice exhibit similar interaction time with a novel inanimate Lego™ object compared to controls. (j) Viaat-Mecp2−/y mice are poor nest-builders. (k,l) Viaat-Mecp2−/y mice show impaired maximum ASR to 120 dB (k) and increased PPI at 78 and 82 dB prepulses (l). (m,n) Viaat-Mecp2−/y mice have similar learning rate during training (m) but reduced crossings over the target platform location during the probe test (n).
Partition test and modified 3-chamber assay for quantifying social interaction showed that 12-13-week old Viaat-Mecp2−/y mice spent significantly more time exhibiting directed interest through sniffing, pawing, or rearing near the partner mouse (Fig. 2g, p <0.0001; Fig. 2h, p <0.0001; Supplemental Fig. 6c-f), but in a separate novel object interaction test they showed no more interest in a novel Lego™ inanimate object, than controls (Fig. 2i). The mice were poor nest-builders (Fig. 2j, p <0.0001 and Supplemental Fig. 6a,b, p < 0.0001), which could be due to alterations in social behavior or forepaw apraxia. Since we found no signs of anxiety-like phenotypes, these alterations in social behavior are independent of anxiety (Supplemental Fig. 5a-h).
We assessed olfactory recognition and learning and found that Viaat-Mecp2−/y mice recognized and habituated to a novel vanilla odorant, but spent more time sniffing vanilla than controls on the first test day without differences on the second test day (Supplemental Fig. 7a,b, p < 0.01). This increased sniffing time could be a manifestation of repetitive behavior and may contribute to the increase in social interaction.
We examined the acoustic startle response (ASR) and percent of prepulse inhibition (PPI) as indicators of sensorimotor arousal and gating28. Viaat-Mecp2−/y mice showed significantly lower ASR to 120 dB than controls (Fig. 2k, p < 0.001) and significantly increased PPI at 78 and 82 dB prepulses (Fig. 2l, p < 0.05), revealing that GABAergic regulation of sensorimotor gating and arousal requires MeCP2 function.
To determine whether MeCP2 deficiency in GABAergic neurons impairs hippocampal learning and memory, we evaluated Viaat-Mecp2−/y mice in the Morris water maze paradigm. Despite a similar rate of learning during the four training days, Viaat-Mecp2−/y mice had difficulty locating the platform during the probe trial (Fig. 2m,n, p < 0.05).
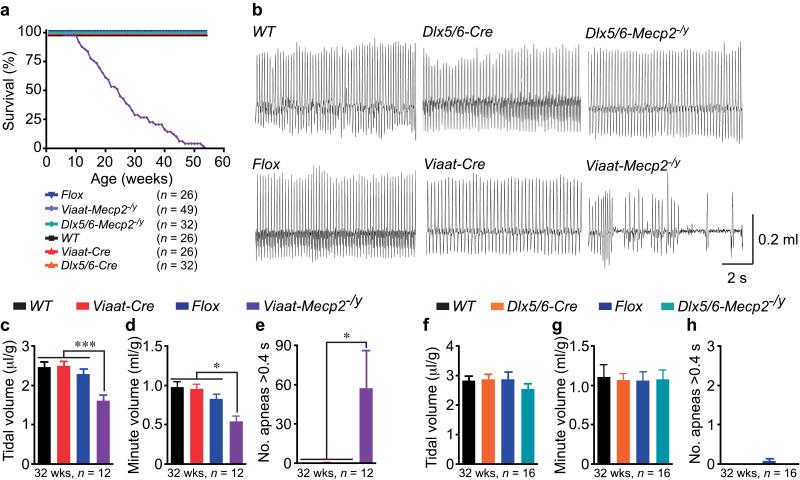
Premature lethality is associated with MeCP2 deficiency in all human males, in some RTT females, and in mouse models lacking MeCP2 in all neurons1, 14, 15. Roughly half of Viaat-Mecp2−/y mice died by 26 weeks of age after a period of dramatic weight loss (Fig. 3a and Supplemental Fig. 8). Coinciding with the weight loss, Viaat-Mecp2−/y mice developed severe respiratory dysfunction, with 42% reduced tidal volume, 45% reduced minute volume, and frequent apneas lasting more than 0.4 s (Fig. 3b, 3c, p <0.0001, 3d, p <0.02, and 3e, p < 0.05). Similar respiratory abnormalities are prominent in RTT and could contribute to early death8, 9. We note that Flox males, which display some respiratory alterations19, 27, show neither premature lethality nor apneas, thus constitutive partial reduction of MeCP2 levels is insufficient to shorten lifespan.
Figure 3. Loss of MeCP2 in inhibitory GABAergic neurons compromises respiration and survival.
(a) Viaat-Mecp2−/y mice exhibit premature lethality with 50% survival by 26-weeks. Dlx5/6-Mecp2−/y mice survive up to at least 80-weeks. (b) Representative plethysmography traces from 32-week old WT, Flox, Dlx5/6-Cre, Viaat-Cre, Dlx5/6-Mecp2−/y, and Viaat-Mecp2−/y mice. Only Viaat-Mecp2−/y mice exhibit pronounced apneas and abnormal respiratory pattern. (c-e) Viaat-Mecp2−/y mice show 42% reduction in tidal volume (c), 45% reduction in minute volume (d), and increased number of apneas longer than 0.4 s (e). (f-h) Dlx5/6-Mecp2−/y mice show no alterations in tidal volume, minute volume, and no apneas.
Dlx5/6-Mecp2−/y mice reproduce many RTT features
Since dysfunction of forebrain structures, such as the striatum and cortex, could be responsible for the stereotypies, compulsive behavior, motor dysfunction, and social behavioral alterations, we employed a forebrain-specific GABAergic Cre, Dlx5/6-Cre29, 30, to remove MeCP2 from a subset of forebrain GABAergic neurons and generate male conditional deletion mice referred to as Dlx5/6-Mecp2−/y. Dlx5/6-Mecp2−/y mice displayed repetitive behavior, impaired motor coordination, increased social interaction preference, reduced ASR, and enhanced PPI. Complete details of the characterization of Dlx5/6-Cre expression pattern and Dlx5/6-Mecp2−/y phenotypes can be found in Supplemental Fig. 3b, Supplemental Fig. 7c, 7d, and Supplemental Figures 9-12. In contrast to Viaat-Mecp2−/y mice, Dlx5/6-Mecp2−/y mice survive to at least 80 weeks without apparent alterations in respiratory function (Fig. 3a,b,f-h), suggesting that respiratory dysfunction and premature lethality could result from either loss of MeCP2 in only hindbrain GABAergic neurons or synergism between MeCP2 deficiency in hindbrain and forebrain GABAergic neurons.
Reduced GABA and Gad1/2 in Viaat-Mecp2−/y mice
Given the repetitive behaviors, motor deficits, altered sensorimotor gating and arousal, and altered social behavior observed in Viaat-Mecp2−/y and Dlx5/6-Mecp2−/y mice, we focused on cortex and striatum to determine a mechanism by which selective MeCP2 deficiency impacts GABAergic function.
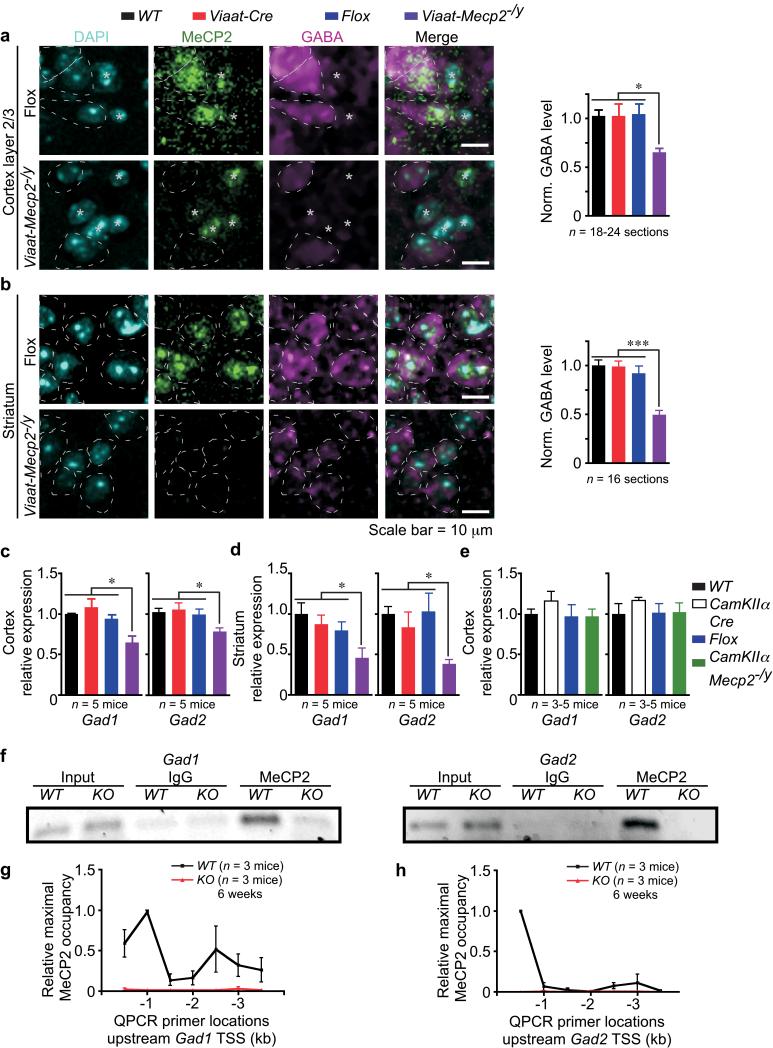
Immunolabeling showed that somatic GABA content in layer 2/3 cortical neurons and in striatal medium spiny neurons was reduced by 37% and 50%, respectively, in Viaat-Mecp2−/y mice (Fig. 4a, p <0.0005; Fig. 4b, p < 0.0001). Given that somatic GABA content is indicative of intracellular GABA level, which is determined by the biosynthetic activity of glutamic acid decarboxylase-67 (Gad67) and -65 (Gad65)31, we assessed whether mRNA level of Gad1 (encoding Gad67) and Gad2 (encoding Gad65) is affected by MeCP2 deficiency in GABAergic neurons. Q-RTPCR revealed that mRNA level of Gad1 and Gad2 was reduced by 36% and 28%, respectively, in Viaat-Mecp2−/y cortex (Fig. 4c, p <0.05) and reduced by 54% and 62%, respectively, in striatum (Fig. 4d, p < 0.05). To determine whether the reduction in Gad1 and Gad2 expression is specific to MeCP2 deletion from GABAergic neurons, we used CamKIIα-Cre32 to selectively delete Mecp2 from forebrain excitatory neurons (denoted as CamKIIα-Mecp2−/y mice). Neither Gad1 nor Gad2 expression is altered in CamKIIα -Mecp2−/y cortex (Fig. 4e), demonstrating the cell-autonomous specificity of reduced Gad1 and Gad2 expression in Viaat-Mecp2−/y mice.
Figure 4. MeCP2 deficiency in GABAergic neurons reduces Gad1, Gad2, and GABA levels.
(a, b) Somatic GABA immunoreactivity in GABA+ cells from 15-17-week old Flox and Viaat-Mecp2−/y mice labeled with DAPI, MeCP2, and GABA. GABA− cells are labeled with asterisks. Images show loss of MeCP2 and reduced GABA immunoreactivity in GABA+ cells (circled) of Viaat-Mecp2−/y mice by 37% in cortical layer 2/3 neurons (a) and 50% in striatal neurons (b). Data normalized to GABA level in WT; n = 2-4 mice per genotype. (c, d) Gad1 and Gad2 mRNA levels are reduced in Viaat-Mecp2−/y cortex by 36% and 28%, respectively (c), and in Viaat-Mecp2−/y striatum by 54% and 62%, respectively (d). (e) Gad1 and Gad2 mRNA levels are unaltered in CamKIIα-Mecp2−/y cortex. (f) ChIP reveals MeCP2 occupancy of Gad1 and Gad2 promoters in WT, which is absent in IgG and KO. (g, h) Mapping MeCP2 occupancy upstream of TSS, after normalization with IgG, reveals increased occupancy in WT (black line) across Gad1 (g) and Gad2 (h) promoters without enhanced MeCP2 binding in KO (red line).
Chromatin immunoprecipitation (ChIP) on WT brain tissue showed that MeCP2 occupies the region 1 kb upstream of the transcriptional start site (TSS) of Gad1 and Gad2 (Fig. 4f), and this binding is absent in constitutive null (Mecp2−/y, denoted as KO)14 and IgG controls. Using primers spanning the region from 500 bp to 3.5 kb upstream of Gad1 and Gad2 TSS showed selective enrichment of MeCP2 occupancy upstream of TSS for both genes and one intergenic region for Gad1 (Fig. 4g,h) demonstrating enrichment of MeCP2 at the Gad1 and Gad2 promoters. Promoter occupancy for Gad1 and Gad2 was minimal in KO (Fig. 4g,h), confirming the binding specificity. The reduced GABA immunoreactivity and Gad1/2 expression predict that MeCP2 deficiency in GABAergic neurons may reduce GABAergic synaptic quantal size.
Quantal size, excitability, and plasticity are altered in Viaat-Mecp2−/y mice
Recording of miniature inhibitory postsynaptic currents (mIPSCs) from layer 2/3 pyramidal neurons of the somatosensory (S1) cortex in acute brain slices showed that the amplitude and charge of mIPSCs were reduced in Viaat-Mecp2−/y mice, with no alterations in frequency (Fig. 5a,b, p < 0.0001). However, the miniature excitatory postsynaptic current (mEPSC) quantal size and frequency were similar to controls (Fig. 5c,d), suggesting that MeCP2 deficiency from GABAergic neurons does not have non-cell-autonomous impact on quantal release from glutamatergic neurons.
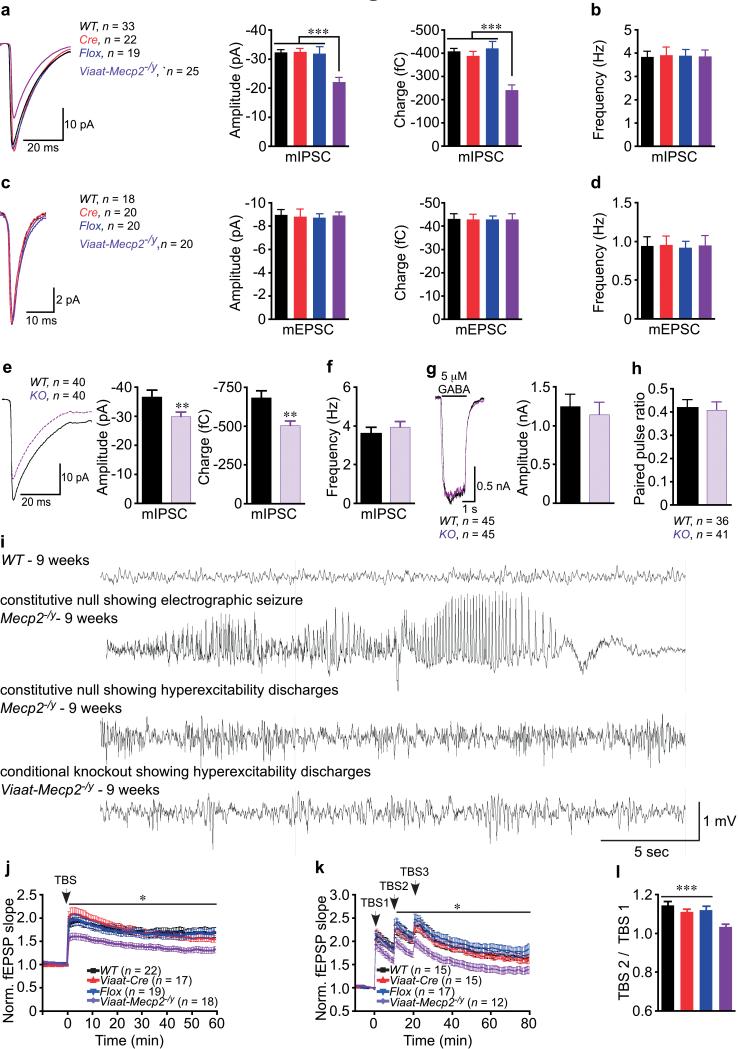
Figure 5. MeCP2 deficiency in GABAergic neurons results in reduced mIPSC quantal size in cortical layer 2/3 and striatal neurons, EEG hyperexcitability, and impaired hippocampal LTP.
(a-d) Data from three mice per genotype and the number of neurons recorded is shown. (a, b) mIPSC amplitude and charge are reduced in Viaat-Mecp2−/y cortical slices. Average traces for each genotype are overlaid and graphs show mIPSC amplitude and charge (a), and frequency (b). (c, d) mEPSC amplitude and charge are unaltered in Viaat-Mecp2−/y cortical slices. Average traces for each genotype are overlaid and graphs show mEPSC amplitude and charge (c), and frequency (d). (e-h) Data from two independent autaptic striatal cultures and the number of neurons recorded is shown. Average traces for each genotype are overlaid and bar graphs show KO neurons have reduced mIPSC amplitude and charge (e), but no differences in frequency (f). (g) 5 μM GABA evokes similarly sized responses from WT and KO. (h) PPR is similar between WT and KO. (i) EEG recordings from constitutive null and Viaat-Mecp2−/y mice compared to WT. Constitutive null Mecp2−/y mice (n = 4) occasionally develop electrographic seizures, but predominantly exhibit hyperexcitability discharges. Viaat-Mecp2−/y mice (n = 7) frequently exhibit hyperexcitability discharges, but do not show electrographic seizures. (j-l) Acute hippocampal slices from 11-13-week old mice, six mice per genotype, reveal reduced magnitude of TBS-LTP (j) and saturating LTP shows no further increases in synaptic potentiation (k) in Viaat-Mecp2−/y slices. Number of slices recorded shown in the figure. (l) Significant increase in potentiation with the second TBS in controls but not in Viaat-Mecp2−/y slices.
To determine whether the reduction in mIPSC quantal size is due to pre- or postsynaptic alterations, we used cultured neurons for pharmacological manipulation. We confirmed that MeCP2-deficient autaptic striatal GABAergic neurons showed reduced mIPSC amplitude and charge, with no alterations in frequency (Fig. 5e, p < 0.02, 5f). Next we examined responsiveness of GABAA receptors by applying a 2-second pulse of 5 μM GABA and found no differences in the magnitude of the response between WT and KO neurons (Fig. 5g), indicating that MeCP2 deficiency does not significantly alter postsynaptic response to GABA. MeCP2 deficiency also does not significantly alter the paired pulse ratio, indicating presynaptic release probability is similar between WT and KO neurons (Fig. 5h). These results show that MeCP2 deficiency in GABAergic neurons leads to functional presynaptic reduction in GABA release and are consistent with the reduced Gad1/2 levels and GABA immunoreactivity. Altogether, these data indicate that loss of MeCP2 in GABAergic neurons compromises intracellular GABA levels via cell-autonomous effect on Gad1/2 mRNA levels.
The reduction in GABA content and inhibitory neurotransmission could cause hyperexcitable network activity. Electroencephographic (EEG) recordings revealed that constitutive null mice suffer both electrographic seizure and non-seizure hyperexcitability discharges (Fig. 5i). Viaat-Mecp2−/y mice displayed frequent hyperexcitability discharges (Fig. 5i), but no electrographic seizures despite prolonged continuous monitoring. Remarkably, EEG recordings from 39-week old Dlx5/6-Mecp2−/y mice showed neither electrographic seizures nor hyperexcitability discharges (Supplemental Fig. 10l), indicating that loss of MeCP2 in a subset of GABAergic neurons is insufficient to cause hyperexcitability.
To determine whether impairments in synaptic plasticity can result from loss of MeCP2 in GABAergic neurons, we examined Schaffer-collateral (SC) synapses in acute hippocampal slices from Viaat-Mecp2−/y mice and controls. We observed no significant differences in input-output curves or paired-pulse ratio (Supplemental Fig. 13a,b), but we did observe impaired long-term potentiation (LTP) induced by theta-burst-stimulation (TBS) of SC synapses in Viaat-Mecp2−/y mice (Fig.5j, p <0.05). To determine whether additional stimulation could increase LTP in Viaat-Mecp2−/y slices, we used a saturating LTP paradigm with three separate TBS. In contrast with control slices, Viaat-Mecp2−/y slices resisted further increases in potentiation (Fig. 5k,l, p < 0.05), possibly because the basal state of the circuit in Viaat-Mecp2−/y slices is closer to maximal potentiation and therefore cannot respond with further potentiation upon subsequent stimulations. These results also suggest that cellular disruptions or circuit alterations in addition to altered GABAergic transmission may contribute to impaired LTP.
Discussion
Previous electrophysiological studies using mice constitutively lacking MeCP2 showed reduction in excitatory synaptic strength and glutamatergic synapse numbers in both isolated autaptic neurons and slices33,34. Additionally, some studies indicated that the strength of inhibition in the cortex, hippocampus, and brainstem is also altered upon constitutive loss of MeCP235-37. Whether these alterations in GABAergic inhibition were cell-autonomous or had specific behavioral consequences remained unknown. We now demonstrate that GABAergic dysfunction is a critical mediator of RTT phenotypes. Mice with MeCP2 deficiency in GABAergic neurons initially exhibit normal behavior, then develop forepaw stereotyped movements, compulsive grooming, increased sociability (similar to RTT Stage III or IV)12, impaired motor coordination, learning/memory deficits, abnormal EEG hyperexcitability, severe respiratory dysrhythmias, and premature lethality. Sensorimotor gating and arousal are also altered. It is noteworthy that MeCP2 deficiency in a subset of forebrain GABAergic neurons is sufficient to cause some repetitive behaviors, impaired motor coordination, increased social interest, and altered sensorimotor gating and arousal. The phenotypic similarity between Viaat-Mecp2−/y mice and RTT strongly suggests that disrupting MeCP2 function in GABAergic neurons alone perturbs the neuronal network in a way similar to what occurs in RTT.
It is intriguing that subtle disruption of GABAergic neuronal function by 30-40% reduction in GABA neurotransmitter release, as assayed neurophysiologically, leads to significant functional abnormalities and neuropsychiatric features. Prior studies implicated a role for GABAergic dysfunction in isolated behaviors, such as altered learning and memory38, 39 and impaired social behavior40 (but see also41). To our knowledge loss of MeCP2 in GABAergic neurons, both globally and in a subset, is unique in that it reveals a multitude of neuropsychiatric phenotypes encompassing social behavior, learning/memory, motor function, stereotyped behaviors, and sensorimotor gating.
The discovery that MeCP2 deficiency in GABAergic neurons alters Gad1/2 expression and results in a change in neuronal GABA content with corresponding alterations in synaptic physiology provides critical insight into how MeCP2 supports GABAergic function. Although MeCP2 binds broadly throughout the genome with selectivity to methylated sites42, there are sites where binding is increased relative to neighboring regions, including alternative promoters at the Bdnf locus42-45, and as shown in this study for Gad1/2. Exactly how the broad and region specific binding of MeCP2 affects specific gene expression remains to be elucidated but certainly the reduction in Bdnf mRNA levels (in Mecp2 constitutive null mice) and Gad1/2 (in this study) demonstrate that such binding is consequential to gene expression. It is worth noting in this context that some post-mortem studies have observed reduced Gad1/2 expression in schizophrenia, bipolar disorder, or autism patients46-50, three disorders found, in rare instances, to be caused by MECP2 mutations2-5. It may be that GABAergic dysregulation, from any of a variety of causes, is central to the pathogenesis of these neuropsychiatric disorders. Our study supports the critical role of the GABAergic system in modulating neuropsychiatric behaviors and suggests that manipulations of this system might be a promising avenue for therapeutic interventions.
Methods Summary.
The mice used in all behavioral, molecular, and electrophysiological studies were still active, still gaining weight, had no signs of seizures (before, during, or after behavioral testing): did not display any staring-freezing episodes and had no signs of delay in performing the behavioral tasks. All data are presented as mean ± standard error of mean with n and ages as shown in figures or stated in text. Full statistical tests and values for behavioral studies are presented in Supplemental Tables 2 and 3. * p < 0.05, ** p < 0.01, and *** p < 0.001
Supplementary Material
Acknowledgements
We thank G. Schuster for pronuclear injections; C. Spencer and R. Paylor for advice on behavioral assays; M. Albright for advice on slice electrophysiology; R. Atkinson, Y. Sun, J. Tang, and S. Vaishnav for technical advice. This work was supported by HHMI, NINDS HD053862, Simons Foundation, RSRT (H.Y.Z.); IDDRC HD024064 (H.Y.Z., C.R., and J.L.Noebels.); NINDS 29709 (J.L.Noebels.); IRSF (C.R.); Autism Speaks (R.C.S.); NIMH F31MH078678, BRASS, and McNair Fellowships (H.-T.C.).
Footnotes
Full Methods, associated references, and supplemental figures are available in supplemental materials.
References
- 1.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–37. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Lam CW, et al. Spectrum of mutations in the MECP2 gene in patients with infantile autism and Rett syndrome. J Med Genet. 2000;37:E41. doi: 10.1136/jmg.37.12.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klauck SM, et al. A mutation hot spot for nonspecific X-linked mental retardation in the MECP2 gene causes the PPM-X syndrome. Am J Hum Genet. 2002;70:1034–7. doi: 10.1086/339553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen D, et al. MECP2 mutation in a boy with language disorder and schizophrenia. Am J Psychiatry. 2002;159:148–9. doi: 10.1176/appi.ajp.159.1.148-a. [DOI] [PubMed] [Google Scholar]
- 5.Carney RM, et al. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr Neurol. 2003;28:205–11. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- 6.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 7.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14:471–9. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 8.Weese-Mayer DE, et al. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60:443–9. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- 9.Weese-Mayer DE, et al. Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr Pulmonol. 2008;43:1045–60. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- 10.Deidrick KM, Percy AK, Schanen NC, Mamounas L, Maria BL. Rett syndrome: pathogenesis, diagnosis, strategies, therapies, and future research directions. J Child Neurol. 2005;20:708–17. doi: 10.1177/08830738050200090201. [DOI] [PubMed] [Google Scholar]
- 11.Jedele KB. The overlapping spectrum of rett and angelman syndromes: a clinical review. Semin Pediatr Neurol. 2007;14:108–17. doi: 10.1016/j.spen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Hagberg B. Clinical manifestations and stages of Rett syndrome. Ment Retard Dev Disabil Res Rev. 2002;8:61–5. doi: 10.1002/mrdd.10020. [DOI] [PubMed] [Google Scholar]
- 13.Neul JL, et al. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70:1313–21. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–6. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 15.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–31. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 16.Shahbazian M, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–54. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 17.Gemelli T, et al. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry. 2006;59:468–76. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Fyffe SL, et al. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59:947–58. doi: 10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samaco RC, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adachi M, Autry AE, Covington HE, 3rd, Monteggia LM. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J Neurosci. 2009;29:4218–27. doi: 10.1523/JNEUROSCI.4225-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maezawa I, Swanberg S, Harvey D, LaSalle JM, Jin LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–61. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong S, et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–23. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhry FA, et al. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–50. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojcik SM, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–87. doi: 10.1016/j.neuron.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samaco RC, et al. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–27. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 29.Monory K, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–66. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohwi M, et al. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–91. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsien JZ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–26. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 33.Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett Syndrome. J Neurosci. 2009;29:11263–70. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dani VS, et al. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–5. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medrihan L, et al. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol. 2008;99:112–21. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, He J, Jugloff DG, Eubanks JH. The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus. 2008;18:294–309. doi: 10.1002/hipo.20389. [DOI] [PubMed] [Google Scholar]
- 38.Cui Y, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–60. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez F, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–3. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 40.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–6. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chadman KK, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–58. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skene PJ, et al. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 37:457–68. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasui DH, et al. Integrated epigenomic analyses of neuronal MeCP2 reveal a role for long-range interaction with active genes. Proc Natl Acad Sci U S A. 2007;104:19416–21. doi: 10.1073/pnas.0707442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–9. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 45.Martinowich K, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–3. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 46.Akbarian S, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–66. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 47.Fatemi SH, et al. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–10. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 48.Addington AM, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10:581–8. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- 49.Lundorf MD, et al. Mutational screening and association study of glutamate decarboxylase 1 as a candidate susceptibility gene for bipolar affective disorder and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:94–101. doi: 10.1002/ajmg.b.30137. [DOI] [PubMed] [Google Scholar]
- 50.Fatemi SH, Stary JM, Earle JA, Araghi-Niknam M, Eagan E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr Res. 2005;72:109–22. doi: 10.1016/j.schres.2004.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.