Abstract
In yeast, a number of regulatory proteins expressed only in specific cell types interact with general transcription factors in a combinatorial manner to control expression of cell-type-specific genes. We report a detailed analysis of activation and repression events that occur at the promoter of the a-cell-specific STE6 gene fused to a β-galactosidase gene in a yeast minichromosome, as well as factors that control the chromatin structure of this promoter both in the minichromosome and in the genomic STE6 locus. Mcm1p results in chromatin remodeling and is responsible for all transcriptional activity from the STE6 promoter in both wild-type a and α cells. Matα2p cooperates with Tup1p to block both chromatin remodeling and Mcm1p-associated activation. While Matα2p represses only Mcm1p, the Tup1p-mediated repression involves both Mcm1p-dependent and -independent mechanisms. Swi/Snf and Gcn5p, required for full induction of the STE6 gene, do not contribute to chromatin remodeling. We suggest that Tup1p can contribute to repression by blocking transcriptional activators, in addition to interacting with transcription machinery and stabilizing chromatin.
Keywords: a-cell-type-specific gene/chromatin/Mcm1p/Ssn6p–Tup1p/transcriptional regulation
Introduction
Transcriptional regulation in eukaryotes involves a complex interplay of non-histone trans-acting factors functioning in a chromatin environment. Promoter regions of a number of genes are organized in nucleosomal arrays, implicated in transcriptional regulation. Positioned nucleosomes often flank activator or repressor binding sites and occlude other cis-acting elements. Eukaryotic cells have a number of mechanisms to overcome repressive chromatin, including remodeling by ATP-dependent complexes and modification of histones (Pollard and Peterson, 1998; Kingston and Narlikar, 1999). Binding of activators to nucleosome-free sites may alter nearby chromatin, enhancing transcription (Verdone et al., 1996; Beato and Eisfeld, 1997; Lohr, 1997). On the other hand, repressors such as Matα2p may organize or stabilize chromatin (Simpson et al., 1993; Roth, 1995; Bortvin and Winston, 1996).
Seven genes are specifically expressed only in yeast cells of the a mating type. In α cells, Matα2p cooperates with Mcm1p to repress these a-cell-type-specific genes (Johnson, 1992, 1995). Promoters of a-specific genes contain a 32 bp sequence, the α2 operator, which includes two recognition sequences for Matα2p flanking a central Mcm1p binding site. Matα2p binds to DNA cooperatively with a homodimer of Mcm1p (Johnson, 1992) and recruits the Ssn6p–Tup1p general transcriptional repressor complex (Keleher et al., 1992; Komachi et al., 1994). Mcm1p and Ste12p have been implicated in transcriptional activation of a-specific genes in a cells (Dolan and Fields, 1991). Ste12p binds cooperatively with Mcm1p to pheromone response elements (PREs), located downstream of the α2 operator (Errede and Ammerer, 1989; Hwang-Shum et al., 1991; Mueller and Nordheim, 1991). The relative contribution of Mcm1p to transcriptional activation of a-specific genes is unsettled, since MCM1 is an essential gene. In a cells, Mcm1p contributes significantly to STE2 expression (Ammerer, 1990; Elble and Tye, 1991; Hwang-Shum et al., 1991) and deletion of the α2 operator reduces transcription of the BAR1 gene (Kronstad et al., 1987). In addition, α2 operator-dependent activation in a cells markedly decreases as the distance between the operator and the promoter is increased (Patterton and Simpson, 1994). On the other hand, the STE6 α2 operator has only weak upstream activating sequence (UAS) activity when placed upstream of a heterologous reporter gene in a cells (Johnson and Herskowitz, 1985; Keleher et al., 1988; Acton et al., 1997).
Chromatin around all endogenous α2 operators examined to date is organized as a well-defined nucleosomal array in α cells that appears to be disrupted in a cells (Ganter et al., 1993; Simpson et al., 1993; Weiss and Simpson, 1997). It was proposed that the organized chromatin was associated with Matα2p binding and implicated in repression of a-specific genes (Simpson et al., 1993; Roth, 1995). The well-defined nucleosomal organization at the STE6 promoter (Cooper et al., 1994) and the recombination enhancer (Weiss and Simpson, 1997) require Tup1p, which has been suggested to repress transcription through stabilization of chromatin structure by direct interaction with the core histones (Edmondson et al., 1996). Tup1p also associates with STE6 nucleosomes in vivo (Ducker and Simpson, 2000). However, the Matα2p–Mata1p–Tup1p-mediated repression of haploid-specific genes does not require positioned nucleosomes, although histones are involved in repression (Huang et al., 1997). Nor is organized chromatin required for a low level of repression of promoters with an isolated sequence of the α2 operator in vitro (Herschbach et al., 1994) or in vivo (Redd et al., 1996). In this study, we investigated the role of chromatin and other factors involved in transcriptional regulation of a-specific genes by studying both activation and repression events taking place at the STE6 promoter in a and α cells.
Results
Chromatin remodeling and transcriptional activation require Mcm1p binding in a cells
Correspondence between an α2 operator in the native context of a transcriptionally repressed a-cell-specific gene in α cells and an organized nucleosomal array in vivo is well established (Shimizu et al., 1991). Although positioned nucleosomes have been observed abutting the α2 operator in repressed, heterologous genes (Roth et al., 1990; Patterton and Simpson, 1994), recent evidence (Redd et al., 1996; Wu et al., 1998) suggests that the sequence context of the α2 operator may have a significant effect on chromatin structure. Disruption of nucleosome positioning abutting the α2 operator at the promoters of a-specific genes in a cells correlates with but does not require transcription (Ganter et al., 1993; Cooper et al., 1994). The change in STE6 chromatin structure in a cells may be caused by binding of trans-acting factors, e.g. Mcm1p, in the absence of Matα2p.
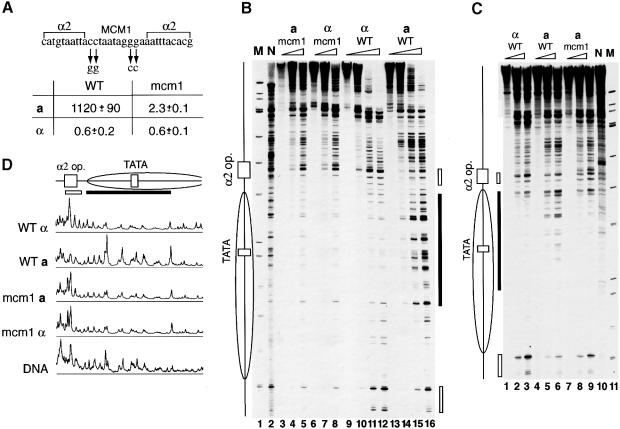
Mcm1p occupies the central P element of the α2 operator in a as well as in α cells in vivo (Keleher et al., 1992; Ganter et al., 1993; Murphy et al., 1993). Muta tions in the P element reduce Mcm1p binding and the ability to affect transcription from heterologous promoters (Ammerer, 1990; Acton et al., 1997). For example, the DNA substitutions shown in Figure 1, at sites that make predominant contacts with the protein (Tan and Richmond, 1998), reduce Mcmlp affinity by at least 100-fold (Smith and Johnson, 1994). We mutated the Mcm1p recognition sequence at the STE6 promoter fused to a lacZ gene in pSTEZL, a plasmid that has chromatin structure similar to the native STE6 locus in both α and a cells (Patterton and Simpson, 1994). Following transformation, β-galactosidase activity and chromatin structure around the α2 operator were determined (Figure 1). For pSTEZL the β-galactosidase activity was 1120 Miller units (M.u.) in a cells and 0.6 M.u. in α cells (Figure 1A). When pSTEZL mutated in the P element was introduced into a cells, the β-galactosidase activity was 2.3 M.u., only 3.6-fold higher than that in α cells. This 500-fold decrease in transcriptional activity from the mutated STE6 promoter suggests that Mcmlp binding is a prerequisite for transcriptional activation of a-cell-specific genes. Slightly higher transcription in a relative to α cells likely results from weak residual Mcm1p binding to the mutated P element (Smith and Johnson, 1994), as transcription is decreased when Matα2p is present. In contrast, in α cells, Mcm1p binding has no effect on repression because no other transcriptional activator is present at the STE6 promoter. We conclude that Mcm1p binding contributes to repression of the STE6 promoter only by enhancing Matα2p binding.
Fig. 1. Mcm1p binding is a prerequisite to chromatin remodeling and transcriptional activation at the STE6 promoter in a cells. (A) Mcm1p binding is responsible for all transcriptional activation from the STE6 promoter in a cells. The β-galactosidase activity (in M.u.) per 10 plasmids per cell is shown for the STE6–lacZ fusion plasmid carrying either the WT α2 operator or the operator bearing four single point mutations in the Mcm1p binding site (mcm1) in a as well as in α cells. Sequence of the α2 operator, positions of Matα2p and Mcm1p binding sites (Smith and Johnson, 1994) as well as mutations introduced into the Mcm1p recognition sequence are shown on the top. (B) Mcm1p remodels chromatin structure at the STE6 promoter in minichromosomes in a cells. Nuclei isolated from cells from experiments shown in (A) were digested with increasing amounts of micrococcal nuclease. Primer corresponding to positions +40 to +17 of the lacZ sequence was extended using DNA purified from digested nuclei as a template. The first chromatin lane from each type of cell corresponds to DNA from undigested nuclei showing Taq polymerase pauses. Purified plasmid DNA digested with MNase (N) is shown as a control for MNase sequence specificity. M is DNA molecular weight standards from HinfI digest of φX174 RF DNA corresponding to 726, 713, 553, 500, 427, 417, 413, 311, 249, 200, 151, 140, 118 and 100 nucleotides. Positions of the α2 operator, TATA element (Patterton and Simpson, 1994) (rectangles) and inferred position of a nucleosome downstream of the α2 operator (an ellipse) are shown on the left. The regions of more (filled bar) and less (open bars) cleavage in a cells compared with α cells are shown on the right. (C) Mcm1p remodels chromatin around the genomic STE6 α2 operator. Primer extension analysis of the STE6 promoter at the chromosomal location using the primer corresponding to +76 to +47 of the coding region is shown. Bars show the same sequences as in (B). (D) Loss of nucleosome positioning downstream of the α2 operator depends on Mcm1p binding. Scanning profiles of the last lanes in the sets of chromatin probes from the gel in (B) are shown to compare the chromatin structure around the TATA box for different cells.
Chromatin structure around the α2 operator was mapped by micrococcal nuclease (MNase) digestion and extension mapping from a primer corresponding to positions +40 to +17 of the lacZ sequence. The 168 bp region downstream of the hypersensitive site at the wild-type (WT) α2 operator is less accessible in α cells compared with naked DNA (Figure 1B and D). In contrast to the genomic locus, where a nuclease-sensitive site 16 bp from the edge of the α2 operator marks the edge of a positioned nucleosome (Figure 1C), this protected region is larger than expected for a nucleosome core. Nuclease cutting sites are present at ∼10 bp intervals in this protected region adjacent to the operator, suggesting several translational positions for rotationally positioned nucleosomes. The differences between this structure for the promoter–lacZ fusion and the genomic copy of STE6 may arise from contributions of prokaryotic sequences, since positioned nucleosomes span the length of the genomic gene but are limited to two for the promoter–lacZ fusion construct. Irrespective of these differences, the minichromosome does provide an example of organized chromatin at the STE6 promoter for study of the relationship between chromatin and repression in an Mcm1p- and Tup1p-dependent system.
In a cells, strong cleavage appears at multiple sites within this region (filled bars in Figure 1B), resembling the digestion pattern of naked DNA. At the same time, the linker regions (open bars) are less accessible to the enzyme, indicating disruption of nucleosome positioning. When the P element was mutated, protection of this region was restored in a cells; chromatin structure is similar to that around the WT α2 operator in α cells (Figure 1B). In α cells, the same mutation had no effect on chromatin structure (Figure 1B). Thus, inability of Mcm1p to bind to the mutated α2 operator restores protection by a nucleosome in a cells, suggesting a role for Mcm1p in chromatin remodeling at the promoter regions of a-specific genes.
To examine whether Mcm1p is responsible for chromatin remodeling of the STE6 genomic promoter, the same mutations were introduced into the α2 operator at the STE6 locus, and chromatin structure was determined using the primer corresponding to +76 to +47 of the STE6 coding region. The positioned nucleosome noted previously is present in α cells (Figure 1C; Shimizu et al., 1991; Cooper et al., 1994). The region downstream of the α2 operator indicated by the filled bar is accessible to MNase in a cells and protected in α cells. The linker region marked by open bars, hypersensitive in α cells, is less accessible in a cells. Point mutations introduced into the genomic P element restored protection due to a positioned nucleosome downstream of the α2 operator and the hypersensitivity of the linker region in a cells (Figure 1C) so that the chromatin structure is similar to that in α cells. Thus, Mcm1p binding to the genomic STE6 promoter disrupts chromatin in a cells as well as in minichromosomes. Presence of the nucleosome downstream of the mutated α2 operator in a cell signals organized chromatin at the STE6 promoter and supports the suggestion that sequences surrounding the α2 operator or other factors may contribute to chromatin organization in α cells.
Matα2p blocks Mcm1p-dependent transcription and chromatin remodeling
Matα2p may block the Mcm1p-dependent transcriptional activation and chromatin remodeling seen in a cells. To explore this possibility, we mutated Matα2p binding sites in pSTEZL and examined both chromatin structure and lacZ expression in both cell types. The four substitutions shown in Figure 2A relieve repression and dramatically reduce Matα2p affinity for the STE6 α2 operator with no apparent decrease in Mcm1p binding to the P element (Smith and Johnson, 1994). When all four mutations were introduced into pSTEZL and the resulting construct transformed into a cells, β-galactosidase activity from the mutated promoter was slightly higher than observed for the WT promoter (Figure 2A), suggesting that these mutations do not affect Mcm1p binding to the α2 operator. In α cells, lacZ expression from the mutated promoter was increased nearly 800-fold and was only 3-fold less than that in a cells. Consistent with the loss of transcriptional repression, the nucleosome downstream of the α2 operator was no longer positioned (Figure 2B and C). Intense cleavage in this previously protected region produced a pattern similar to that observed for the WT α2 operator in a cells (Figures 2B and C, and 1B). Although these results suggest that a role of Matα2p is to block Mcm1p activity, it is formally possible that transcription and chromatin remodeling in the absence of Matα2p in α cells may be activated by an Mcm1p-independent mechanism. Therefore, we made a double Matα2p/Mcm1p binding site mutant and examined both lacZ expression and chromatin structure in α and a cells. Mutation of the Mcm1p binding site reduced lacZ expression in the absence of Matα2p binding in α cells (Figure 2A). Consistent with the loss of transcriptional activation, no chromatin remodeling was observed in this construct (Figures 2B and C, and 1B). Thus, one role of Matα2p in repression of a-specific genes is to block Mcm1p transcriptional and chromatin remodeling activity in α cells.
Fig. 2. Matα2p blocks Mcm1p-dependent transcriptional activation and chromatin remodeling at the STE6 promoter in α cells. (A) Transcriptional activation in α cells in the absence of Matα2p binding is Mcm1p dependent. β-galactosidase assays of transcription from the STE6 promoter bearing point mutations in both α2 half-sites either alone (α2) or in combination with the mutations in Mcm1p recognition sequence (α2/mcm1). Point mutations introduced into the Matα2p sites are shown on the top. The data for the WT a and α cells from Figure 1 are shown for comparison. The remaining β-galactosidase values were obtained from the same experiment. (B) Matα2p binding blocks Mcm1p chromatin remodeling activity in α cells. Primer extension analysis of the STE6 chromatin structure in α cells from experiments shown in (A). (C) Disruption of a nucleosome downstream of the α2 operator in α cells in the absence of Matα2p binding requires Mcm1p. For other details see legend to Figure 1.
Tup1p cooperates with Matα2p to block Mcm1p transcriptional and chromatin remodeling activity
Ssn6p–Tup1p is a pleiotropic corepressor complex, thought to be targeted to promoters by sequence-specific DNA-binding proteins (Balasubramanian et al., 1993; Komachi et al., 1994; Treitel and Carlson, 1995; Huang et al., 1998; Park et al., 1999). The Ssn6p–Tup1p complex is required for Matα2p-mediated repression both in vitro (Redd et al., 1997) and in vivo (Keleher et al., 1992; Cooper et al., 1994). Matα2p interacts with Tup1p in vitro (Komachi et al., 1994; Komachi and Johnson, 1997). Three mechanisms for Ssn6p–Tup1p-mediated repression have been proposed. One suggests that Tup1p organizes a repressive chromatin structure through interaction with H4 and H3 (Cooper et al., 1994; Edmondson et al., 1996; Ducker and Simpson, 2000). A second model invokes direct interaction of Ssn6p–Tup1p with transcriptional machinery (Herschbach et al., 1994; Redd et al., 1996; Kuchin and Carlson, 1998). Finally, we and others have proposed that the Ssn6p–Tup1p complex blocks transcriptional activators (Gavin and Simpson, 1997; Geisberg and Struhl, 2000). Our finding that the Mcm1p-dependent transcriptional activation and chromatin remodeling at the STE6 promoter are blocked by Matα2p implicates Ssn6–Tup1p in blocking Mcm1p activity.
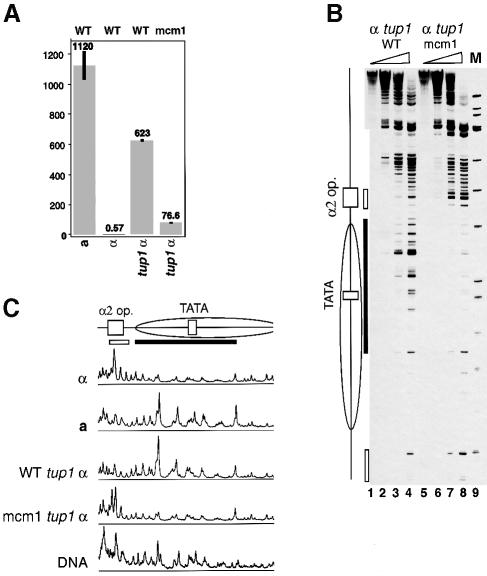
We transformed pSTEZL-based constructs containing the WT or mutant Mcm1p binding site α2 operator into tup1 α cells and examined lacZ expression and chromatin remodeling (Figure 3). The tup1 mutation increases β-galactosidase expression from the WT STE6 promoter in α cells ∼1100-fold, only 2-fold less than in WT a cells. Chromatin structure around the α2 operator in the mutant background is similar to the WT a cells, with disruption of nucleosomes downstream of the α2 operator (Figures 3B and C, and 1B). These data correlate well with previous observations (Cooper et al., 1994) and with the change in expression and chromatin structure of the genomic STE6 locus in tup1 α cells (see below). Mutations in the P element reduced β-galactosidase activity ∼8-fold in tup1 cells, but the expression level was still two orders of magnitude higher than in WT α cells (Figure 3A). The near lack of Mcm1p binding to the mutant P element suggests that transcriptional activation in tup1 mutants also involves an Mcm1p-independent mechanism. In spite of a high level of Mcm1p-independent transcriptional activity in tup1 α cells, no disruption of chromatin structure was observed downstream of the mutated α2 operator (Figures 3B and 1B). Chromatin remodeling downstream of the α2 operator in the absence of Tup1p in α cells requires Mcm1p binding. We suggest that recruitment of Tup1p by Matα2p to the close proximity of Mcm1p blocks its chromatin remodeling activity.
Fig. 3. Tup1p represses Mcm1p transcriptional and chromatin remodeling activity. (A) Transcriptional activation in α tup1 cells is Mcm1p dependent. β-galactosidase assay of transcription from the STE6 promoter bearing either the WT or mutated α2 operator, as indicated on the top, either in tup1 mutants or in WT cells. Standard deviation is shown by the black bars. For comparison, the data for the WT a and α cells from Figure 1 are shown. The remaining β-galactosidase values were obtained from the same experiment. (B) Tup1p blocks chromatin remodeling by Mcm1p in α cells. Primer extension analysis of the chromatin structure around either the WT or mutated α2 operator in α tup1 cells from (A). (C) Disruption of a nucleosome downstream of the α2 operator in α tup1 mutants requires Mcm1p binding. For other details see legend to Figure 1.
High transcriptional activity in tup1 α cells in the absence of Mcm1p binding suggests that Tup1p represses transcription of a-specific genes by an alternative, Mcm1p-independent mechanism. Ste12p seems unlikely to contribute to this since Mcm1p recruits Ste12p to the promoters of a-specific genes (Errede and Ammerer, 1989; Hwang-Shum et al., 1991; Mueller and Nordheim, 1991). Mcm1p-independent Tup1p-mediated repression is difficult to explain in the context of either a model suggesting interaction of the Ssn6p–Tup1p complex with the general transcription machinery or Ssn6p–Tup1p-dependent formation of a repressive chromatin structure. Since we find no Tup1p associated with the STE6 promoter in isolated minichromosomes in the absence of either Mcm1p or Matα2p (Ducker and Simpson, 2000), we think it is likely that the Mcm1p-independent effect of tup1 mutation is indirect. The Ssn6p–Tup1p complex is required for expression of a large number of yeast genes and some of them may be involved in downstream regulation of other genes.
Chromatin disruption at the STE6 locus in a cells is independent of Swi/Snf and Gcn5p
While the chromatin remodeling complex SWI/SNF and Gcn5p-containing histone acetyltransferase complexes are necessary for full activity of the STE6 gene in a cells, they are dispensable for alterations of nucleosome positioning at the promoter of the genomic gene. Data supporting these conclusions are to be found in the Supplementary data, available at The EMBO Journal Online.
Matα2p binds the α2 operator in the absence of Tup1p
The lack of Matα2p expression in a cells or its inability to bind the α2 operator in α cells has the same effect on STE6 transcription and chromatin structure as a tup1 mutation in α cells. Although it has previously been shown that Matα2p can bind the α2 operator in the absence of Ssn6p in α cells (Keleher et al., 1992), Ssn6p and Tup1p have distinct roles in STE6 repression (Cooper et al., 1994). Northern blot analysis shows that ssn6 mutations have a very small effect on STE6 transcription in α cells compared with the deletion of TUP1. Thus, the tup1 mutation might impair Matα2p binding to the STE6 promoter. Therefore, we examined Matα2p binding to the α2 operator in α and a cells that were WT and tup1. These strains also harbored a single, integrated copy of Sss I DNA methyltransferase (M. Sss I), which enables sensitive detection of DNA–protein interactions in vivo (Kladde et al., 1996; Xu et al., 1998). MNase digestion of α cell nuclei (Roth et al., 1992; Cooper et al., 1994) indicated the presence of a positioned nucleosome downstream of the α2 operator at the genomic STE6 locus, and protection against methylation of three CpG M. Sss I target sites confirmed this structural assignment (Figure 4). In contrast, this region was accessible to the methyltransferase in a WT and tup1 strains of both mating types. These data rule out the possibility that the isolation of nuclei for the MNase analyses contributed to disruption of the positioned nucleosome in α tup1 cells. Importantly, a CpG sequence located within the downstream α2 half-site of the operator (marked with an asterisk) was protected against methylation by M. Sss I in both WT and tup1 α cells as compared with a cells (Figure 4). This Matα2p footprint was also present in α tup1 cells at the α2 operator #1 of the recombination enhancer (Wu and Haber, 1996; data not shown). These data demonstrate that a null mutation of TUP1 leads to loss of nucleosome positioning downstream of the α2 operator without affecting Matα2p binding. Therefore, the derepression of STE6 transcription observed in α tup1 cells (Figure 3A) is not due to a lack of binding of Matα2p at the operator.

Fig. 4. Matα2p occupies the STE6 α2 operator in the absence of Tup1p. (A) The Matα2p–operator interaction in haploid cells of each mating type (a or α) was footprinted in vivo in WT (TUP1) or mutant (tup1) by M. Sss I. Expression of the single-copy methyltransferase under control of the GAL1 promoter was achieved by growth in galactose-containing media. Following rapid isolation of DNA, cytosines methylated in CpG target sites (indicated by filled circles) were identified by genomic bisulfite sequencing as described previously (Kladde et al., 1996). Band intensity is directly proportional to the degree of cytosine methylation at each CpG sequence. In vitro methylation of protein-free DNA (D) is shown in lane 1. The α2 operator is demarcated by the open bar and a CpG site within the α2 operator is marked by the asterisk. Note protection of three methylation sites adjacent to the α2 operator in α TUP1 cells (lane 3) due to the presence of a positioned nucleosome (marked by open ellipse). Increased methylation at these three CpG sites in a cells (lanes 2 and 4) or when TUP1 was deleted (lanes 4 and 5) indicates disruption of the nucleosome. (B) Operator binding by Matα2p in the same samples as in (A) was analyzed with a primer that anneals closer to the operator. Note protection of the operator CpG site (asterisk) in α cells (lanes 3 and 5) in the presence (lane 3) and absence (lane 5) of TUP1 as compared with a cells (lanes 2 and 4).
Matα2p has a short half-life and is rapidly degraded by the ubiquitin–proteasome pathway (Chen et al., 1993). Proteins are often degraded when their partners in macromolecular complexes are absent; this type of observation suggests that the specific proteins may be components of a common complex (Peterson et al., 1994). For example, association of Matα2p with Mata1p significantly increases the stability of the former protein in diploid cells (Johnson et al., 1998). In this vein, Ssn6p–Tup1p might stabilize Matα2p, with its rapid degradation in the absence of Tup1p resulting in the loss of a-specific gene repression. Occupancy of the α2 operator by repressor in tup1 mutants rules out this possibility.
Discussion
Mcm1p binding is required for the Ste12p-dependent activation of a-specific genes
Mcm1p is a member of the MADS-box family of transcription factors that is thought to cooperate with other proteins in the regulation of target genes (Shore and Sharrocks, 1995). In the present paper, we show that Mcm1p binding to the STE6 promoter results in chromatin remodeling and transcriptional activation at the promoter of the STE6 gene. Our findings are consistent with previous observations that Mcm1p is responsible for the majority of STE2 expression in a cells (Ammerer, 1990; Elble and Tye, 1991; Hwang-Shum et al., 1991). However, expression of a-specific genes also requires Ste12p. Even in the absence of pheromone, the STE12 deletion reduces mRNA levels from 5-fold for the STE6 and STE2 genes to >50-fold for the MFa1 gene (Fields and Herskowitz, 1985; Fields et al., 1988), suggesting that Mcm1p can not significantly activate transcription on its own and requires Ste12p for full activity. This suggestion is consistent with the findings that an isolated P element or α2 operator displayed weak transcriptional activity in a cells (Johnson and Herskowitz, 1985; Keleher et al., 1988; Acton et al., 1997), while the α2 operator containing flanking sequences was able to activate a reporter gene to a significant level (Errede and Ammerer, 1989; Ammerer, 1990). It has been shown that Ste12p affinity for its DNA site in vitro is dramatically reduced in the absence of Mcm1p (Errede and Ammerer, 1989; Hwang-Shum et al., 1991; Mueller and Nordheim, 1991). This suggests a mechanism for transcriptional activation of a-specific genes in a cells where Mcm1p binds to the P element and recruits Ste12p to activate the gene. This scheme may also involve the Swi/Snf complex and Gcn5-containing acetyltransferase coactivator complexes that are required for maximal transcriptional activity. A similar model was suggested for the activation of α-specific genes (Dolan and Fields, 1991; Johnson, 1995), where cooperative binding of Mcm1p and Matα1p to the PQ elements at promoters of α-specific genes recruits Ste12p through direct protein–protein interaction with Matα1p (Yuan et al., 1993).
Ssn6p–Tup1p represses transcription by interaction with transcriptional activators
Matα2p expression in α cells abolishes both Mcm1p-dependent chromatin remodeling and activation of the STE6 gene. However, Matα2p binding to the α2 operator alone is not sufficient to repress Mcm1p, and requires Tup1p, a component of the Ssn6p–Tup1p general repressor complex. It has been shown that when targeted, Tup1p does not require Ssn6p for repression (Tzamarias and Struhl, 1994), while Ssn6p represses transcription by recruiting Tup1p (Keleher et al., 1992; Tzamarias and Struhl, 1995). These observations are consistent with a small effect of ssn6 mutations on expression and chromatin structure of a-specific genes (Cooper et al., 1994; Tzamarias and Struhl, 1995; this study). When neither Matα2p nor Mcm1p can bind to the α2 operator, no transcriptional activity was observed. These data argue against the model that Mcm1p contributes to the Matα2p-mediated repression of a-specific genes (Johnson, 1992). However, repression of activated transcription from heterologous promoters requires Mcm1p (Keleher et al., 1988; Smith and Johnson, 1994; Acton et al., 1997). This finding may be explained by the fact that Mcm1p binding to the α2 operator increases Matα2p affinity for its recognition sequences at least 500-fold (Keleher et al., 1989). Mcm1p has a high affinity for DNA in vitro (Acton et al., 1997) and binds to the α2 operator in the absence of Matα2p in vivo (Keleher et al., 1992; Ganter et al., 1993; Murphy et al., 1993; this study). In α cells, Mcm1p interacts with Matα2p (Vershon and Johnson, 1993; Mead et al., 1996; Tan and Richmond, 1998) and recruits Matα2p to the promoter. We have shown that Matα2p can bind to the operator in the absence of Tup1p in vivo and therefore ruled out a potential requirement for the Ssn6p–Tup1p complex in stabilization of operator binding. Upon DNA binding, Matα2p may recruit Tup1p through direct protein–protein interactions (Komachi et al., 1994; Komachi and Johnson, 1997). Once targeted, the Ssn6p–Tup1p complex represses activated transcription.
A number of studies have suggested that the Ssn6p–Tup1p complex represses transcription either through interaction with the general transcriptional machinery (Herschbach et al., 1994; Redd et al., 1996; Kuchin and Carlson, 1998) or by organizing a repressive chromatin structure (Simpson et al., 1993; Cooper et al., 1994; Edmondson et al., 1996). Based on the analysis of transcriptional regulation and chromatin structure of SUC2, a glucose repressible gene, we have proposed an alternative model for the Ssn6p–Tup1p-mediated repression that implies an inhibitory effect on pathway-specific chromatin remodeling transcription factors (Gavin and Simpson, 1997). Although the sequence-specific activators that are involved in SUC2 expression and chromatin remodeling are yet to be identified, our finding that Tup1p blocks Mcm1p activation suggests that this mechanism may be realized in transcriptional regulation of other Ssn6p–Tup1p-dependent genes. Geisberg and Struhl (2000) have recently reached a similar conclusion regarding the mechanism of action of corepressor Tup1 based on an analysis of distinctive TATA binding protein mutants.
Role of chromatin structure in regulation of a-specific genes
A current model for chromatin organization at the promoter regions of a-type-specific genes in α cells suggests that Matα2p repressor binding establishes positioned nucleosomes around the α2 operator by recruiting Tup1p, which interacts with the N-termini of histones H3 and H4 (Cooper et al., 1994; Roth, 1995; Edmondson et al., 1996). Alternatively, the positioned nucleosomes observed adjacent to naturally located α2 operators of the STE6 and BAR1 genes and a recombination enhancer region may be positioned by the underlying sequence. In the absence of Mcm1p binding, the chromatin structure around the mutated α2 operator at the STE6 promoter and at the recombination enhancer (Wu et al., 1998) in a cells is organized as arrays of positioned nucleosomes similarly, but not identically, to the array in α cells. This suggests that sequence elements flanking the 32 bp α2 operator may be required for the organization of adjacent chromatin. Although transcriptional repression was observed, organized chromatin was not found in minichromosomes bearing the α2 operator sequence at the promoter regions of a heterologous gene (Redd et al., 1996). On the other hand, the fact that positioned nucleosome arrays were found around all naturally occurring α2 operators in α cells suggests that these regions may have some unidentified elements that organize the chromatin structure in a similar way.
The observation that repression of cell-type-specific genes requires histone H3 and H4 N-terminal regions (Roth et al., 1992; Huang et al., 1997) suggests that chromatin plays an active role in transcriptional regulation of a-specific genes. Mcm1p binding appears to be the key element in STE6 chromatin remodeling and transcriptional initiation. One of the possible scenarios for the events taking place at the STE6 promoter during transcriptional initiation is as follows: when Mcm1p is inactive or not present at the STE6 promoter, the PREs and TATA box are occluded by a positioned nucleosome and the gene is repressed. Following binding to the α2 operator, Mcm1p disorganizes chromatin structure, rendering accessibility to the PREs and the TATA box, and subsequent recruitment of Ste12p assists in preinitiation complex formation. In α cells, Matα2p cooperates with Tup1p to block Mcm1p transcriptional and chromatin remodeling activity. In addition, Tup1p likely stabilizes chromatin structure through interaction with histone N-terminal regions.
Materials and methods
Yeast strains, plasmids and media
Cell cultures were grown either in YPD or in selective media as described by Sherman (1991). Yeast strains FY23(MATa, ura3-52, trp1-Δ63, leu2-Δ1), FY24(MATα, ura3-52, trp1-Δ63, leu2-Δ1), and their derivatives FY24 ssn6, FY24 tup1 and FY24 tup1 swi1, were described earlier (Gavin and Simpson, 1997). Strains FY23 swi1 and FY23 swi1 tup1 were obtained as segregants from the same FY23/FY24 cross followed by SWI1 replacement with LEU2 (Gavin and Simpson, 1997). The YPH499 gcn5 strain is a segregant from a cross of YPH499 (Sikorski and Hieter, 1989) with CY569 (Pollard and Peterson, 1997). Strains for footprinting of the α2 operator are M. Sss I+ segregants from a cross of YPH500ΔL.19-2 (Kladde et al., 1996) with MKY47, which is YPH499ΔL (Kladde et al., 1996) containing a tup1::URA3 allele. All mutations were verified by PCR. Plasmid pSTEZL was described previously (Patterton and Simpson, 1994).
Plasmid construction
All molecular biological manipulations were performed as described (Ausubel et al., 1997). The point mutations were introduced into the α2 operator as follows: the pSTEZL PCR fragments bearing mutations in the α2 operator were obtained using the primer 5′-CAGCTATGACCATGATTACGCCAAGC and either 5′-CAATTGCCAAGGTGCGAAGCAGCGTTAAAATTTCCCTATTAGGTAATTCAATGGCA for mutation of Matα2p binding sites, 5′-CAATTGCCAAGGTGCGAAGCAGCGTGTAAATTTGGCTATTACCTAATTAC for mutation of the Mcm1p binding site or 5′-CAATTGCCAAGGTGCGAAGCAGCGTTAAAATTTGGCTATTACCTAATTCAATGGCA for both α2 and MCM1 mutations. The fragments were cut with NotI and StyI and were ligated into the pSTEZL replacing the WT α2 operator with the mutated one. The resulting plasmids were used to transform either FY23, FY24 or FY24 tup1 to tryptophan prototrophy.
Mutations in the Mcm1p binding site at the endogenous STE6 α2 operator were introduced by transforming FY23 with the pRS406 yeast integration plasmid bearing the pSTEZL MCM1 fragment cloned into the EcoRI and BamHI sites. The plasmid was linearized with MfeI prior to transformation. The integration event was confirmed by PCR using primers to the mutated α2 operator and STE6 coding region.
Micrococcal nuclease digestion and primer extension
Nuclei isolation, micrococcal nuclease digestion and primer extension were carried out according to Gavin and Simpson (1997).
β-galactosidase assay and northern blot analysis
β-galactosidase assays were performed as described by Rose et al. (1990). Activities as reported are normalized to a plasmid copy number of 10 per cell. RNA isolation, northern blotting and hybridization to the STE6 probe corresponding to position +97 to +668, or to the ACT1 probe were carried out as described in Gavin and Simpson (1997) except that RNA samples were electrophoresed in a 1% agarose gel.
In vivo footprinting with M. Sss I
Yeast cells were grown in YP galactose (2%) medium for 16 h to induce expression of the integrated copy of M. Sss I from the GAL1 promoter. DNA was isolated and deaminated by sodium metabisulfite as described previously (Kladde et al., 1996). A 773 bp product encompassing –648 to +124 of the STE6 genomic locus was amplified from the purified, deaminated DNA by PCR with primers STE6a1a (CCaaCACTAaaCCTaTTaCCACaaTAC; corresponding to position +98 to +124 at STE6) and STE6a2a (TAtTAtTTTTttAAGtTATAGGtAAATGGtAttTG; STE6 –614 to –648), respectively, containing G→a or C→t transitions. The 32 bp STE6 α2 operator is located at –183 to –214. The PCR product was purified and directly subjected to thermal cycle sequencing with [γ-32P]-end-labeled primers to identify 5-methylcytosine residues (Kladde et al., 1996). The extension products were analyzed by electrophoresis on a 6% (w/v; 19:1 bisacrylamide:acrylamide), 50% (w/v) urea sequencing gel. For Figure 4A and B the oligonucleotides STE6a1a and STE6a1b (aaAaATAaTTCAaCCATATCCAa; STE6 –75 to –97) were end-labeled and used as extension primers, respectively.
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
The authors wish to thank Dr Hugh Patterton for sharing unpublished data and help in manuscript preparation. This research was supported by NIH grants GM52311 and GM52908.
References
- Acton T.B., Zhong,H. and Vershon,A.K. (1997) DNA-binding specificity of Mcm1: operator mutations that alter DNA-bending and transcriptional activities by a MADS box protein. Mol. Cell. Biol., 17, 1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammerer G. (1990) Identification, purification and cloning of a polypeptide (PRTF/GRM) that binds to mating-specific promoter elements in yeast. Genes Dev., 4, 299–312. [DOI] [PubMed] [Google Scholar]
- Ausubel F., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1997), Short Protocols in Molecular Biology, 3rd edn. John Wiley and Sons, New York, NY. [Google Scholar]
- Balasubramanian B., Lowry,C.V. and Zitomer,R.S. (1993) The Rox1 repressor of the Saccharomyces cerevisiae hypoxic genes is a specific DNA-binding protein with a high-mobility-group motif. Mol. Cell. Biol., 13, 6071–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. and Eisfeld,K. (1997) Transcription factor access to chromatin. Nucleic Acids Res., 25, 3559–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortvin A. and Winston,F. (1996) Evidence that Spt6p controls chromatin structure by direct interactions with histones. Science, 272, 1473–1476. [DOI] [PubMed] [Google Scholar]
- Chen P., Johnson,P., Sommer,T., Jentsch,S. and Hochstrasser,M. (1993) Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MATα2 repressor. Cell, 74, 357–369. [DOI] [PubMed] [Google Scholar]
- Cooper J.P., Roth,S.Y. and Simpson,R.T. (1994) The global transcriptional regulators, SSN6 and TUP1, play distinct roles in the establishment of a repressive chromatin structure. Genes Dev., 8, 1400–1410. [DOI] [PubMed] [Google Scholar]
- Dolan J.W. and Fields,S. (1991) Cell-type-specific transcription in yeast. Biochim. Biophys. Acta, 1088, 155–169. [DOI] [PubMed] [Google Scholar]
- Ducker C.E. and Simpson,R.T. (2000) The organized chromatin domain of the repressed yeast a cell-specific gene STE6 contains two molecules of the corepressor Tup1p per nucleosome. EMBO J., 19, 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D.G., Smith,M.M. and Roth,S.Y. (1996) Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev., 10, 1247–1259. [DOI] [PubMed] [Google Scholar]
- Elble R. and Tye,B.-K. (1991) Both activation and repression of a-mating-type-specific genes in yeast require transcription factor Mcm1. Proc. Natl Acad. Sci. USA, 88, 10966–10970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B. and Ammerer,G. (1989) STE12, a protein involved in cell-type-specific transcription and signal transduction in yeast, is part of protein–DNA complexes. Genes Dev., 3, 1349–1361. [DOI] [PubMed] [Google Scholar]
- Fields S. and Herskowitz,I. (1985) The yeast STE12 product is required for expression of two sets of cell-type-specific genes. Cell, 42, 923–930. [DOI] [PubMed] [Google Scholar]
- Fields S., Chaleff,D.T. and Sprague,G.F.,Jr (1988) Yeast STE7, STE11 and STE12 genes are required for expression of cell-type-specific genes. Mol. Cell. Biol., 8, 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter B., Tan,S. and Richmond,T.J. (1993) Genomic footprinting of the promoter regions of STE2 and STE3 genes in the yeast Saccharomyces cerevisiae. J. Mol. Biol., 234, 975–987. [DOI] [PubMed] [Google Scholar]
- Gavin I.M. and Simpson,R.T. (1997) Interplay of yeast global transcriptional regulators Ssn6p–Tuplp and Swi–Snf and their effect on chromatin structure. EMBO J., 16, 6263–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisberg J.V. and Struhl,K. (2000) TATA-binding protein mutants that increase transcription from enhancerless and repressed promoters in vivo. Mol. Cell. Biol., 20, 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschbach B.M., Arnaud,M.B. and Johnson,A.D. (1994) Transcriptional repression directed by the yeast α2 protein in vitro. Nature, 370, 309–311. [DOI] [PubMed] [Google Scholar]
- Huang L., Zhang,W. and Roth,S.Y. (1997) Amino termini of histones H3 and H4 are required for a1-α2 repression in yeast. Mol. Cell. Biol., 17, 6555–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Zhou,Z. and Elledge,S.J. (1998) The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell, 94, 595–605. [DOI] [PubMed] [Google Scholar]
- Hwang-Shum J.-J., Hagen,D.C., Jarvis,E.E., Westby,C.A. and Sprague,G.F.,Jr (1991) Relative contributions of MCM1 and STE12 to transcriptional activation of a- and α-specific genes from Saccharomyces cerevisiae. Mol. Gen. Genet., 227, 197–204. [DOI] [PubMed] [Google Scholar]
- Johnson A.D. (1992) A combinatorial regulatory circuit in budding yeast. In McKnight,S.L. and Yamamoto,K.R. (eds), Transcriptional Regulation. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 975–1006. [Google Scholar]
- Johnson A.D. (1995) Molecular mechanisms of cell-type determination in yeast. Curr. Opin. Genet. Dev., 5, 552–558. [DOI] [PubMed] [Google Scholar]
- Johnson A.D. and Herskowitz,I. (1985) A repressor (MATα2 product) and its operator control expression of a set of cell type specific genes in yeast. Cell, 42, 237–247. [DOI] [PubMed] [Google Scholar]
- Johnson P.R., Swanson,R., Rakhilina,L. and Hochstrasser,M. (1998) Degradation signal masking by heterodimerization of MATα2 and MATa1 blocks their mutual destruction by the ubiquitin–proteasome pathway. Cell, 94, 217–227. [DOI] [PubMed] [Google Scholar]
- Keleher C.A., Goutte,C. and Johnson,A.D. (1988) The yeast cell-type-specific repressor α2 acts cooperatively with a non-cell-type-specific protein. Cell, 53, 927–936. [DOI] [PubMed] [Google Scholar]
- Keleher C.A., Passmore,S. and Johnson,A.D. (1989) Yeast repressor α2 binds to its operator cooperatively with yeast protein Mcm1. Mol. Cell. Biol., 9, 5228–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher C.A., Redd,M.J., Schultz,J., Carlson,M. and Johnson,A.D. (1992) Ssn6–Tup1 is a general repressor of transcription in yeast. Cell, 68, 709–719. [DOI] [PubMed] [Google Scholar]
- Kingston R.E. and Narlikar,G.J. (1999) ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev., 13, 2339–2352. [DOI] [PubMed] [Google Scholar]
- Kladde M.P., Xu M. and Simpson,R.T. (1996) Direct study of DNA–protein interactions in repressed and active chromatin in living cells. EMBO J., 15, 6290–6300. [PMC free article] [PubMed] [Google Scholar]
- Komachi K. and Johnson,A.D. (1997) Residues in the WD repeats of Tup1 required for interaction with α2. Mol. Cell. Biol., 17, 6023–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komachi K., Redd,M.J. and Johnson,A.D. (1994) The WD repeats of Tup1 interact with the homeo domain protein α2. Genes Dev., 8, 2857–2867. [DOI] [PubMed] [Google Scholar]
- Kronstad J.W., Holly,J.A. and MacKay,V.L. (1987) A yeast operator overlaps an upstream activation site. Cell, 50, 369–377. [DOI] [PubMed] [Google Scholar]
- Kuchin S. and Carlson,M. (1998) Functional relationships of Srb10-Srb11 kinase, carboxy-terminal domain kinase CTDK-I and transcriptional corepressor Ssn6–Tup1. Mol. Cell. Biol., 18, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D. (1997) Nucleosome transactions on the promoter of the yeast GAL and PHO genes. J. Biol. Chem., 272, 26795–26798. [DOI] [PubMed] [Google Scholar]
- Mead J., Zhong,H., Acton,T.B. and Vershon,A.K. (1996) The yeast α2 and Mcml proteins interact through a region similar to a motif found in homeodomain proteins of higher eukaryotes. Mol. Cell. Biol., 16, 2135–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C.G.F. and Nordheim,A. (1991) A protein domain conserved between yeast MCM1 and human SRF directs ternary complex formation. EMBO J., 10, 4219–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.R., Shimizu,M., Roth,S.Y., Dranginis,A.M. and Simpson,R.T. (1993) DNA–protein interactions at the S.cerevisiae α2 operator in vivo. Nucleic Acids Res., 21, 3295–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Koh,S.S., Chun,J.H., Hwang,H.J. and Kang,H.S. (1999) Nrg1 is a transcriptional repressor for glucose repression of STA1 gene expression in Saccharomyces cerevisiae. Mol. Cell. Biol., 19, 2044–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterton H.-G. and Simpson,R.T. (1994) Nucleosomal location of the STE6 TATA box and Matα2p-mediated repression. Mol. Cell. Biol., 14, 4002–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C.L., Dingwall,A. and Scott,M.P. (1994) Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc. Natl Acad. Sci. USA, 91, 2905–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard K. and Peterson,C.L. (1997) Role for ADA/GCN5 products in antagonizing chromatin-mediated transcriptional repression. Mol. Cell. Biol., 17, 6212–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard K. and Peterson,C.L. (1998) Chromatin remodeling: a marriage between two families? BioEssays, 20, 771–780. [DOI] [PubMed] [Google Scholar]
- Redd M.J., Stark,M.R. and Johnson,A.D. (1996) Accessibility of α2-repressed promoters to the activator Gal4. Mol. Cell. Biol., 16, 2865–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redd M.J., Arnaud,M.B. and Johnson,A.D. (1997) A complex composed of Tup1 and Ssn6 represses transcription in vitro. J. Biol. Chem., 272, 11193–11197. [DOI] [PubMed] [Google Scholar]
- Rose M.D., Winston,F. and Hieter,P. (1990) Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Roth S.Y. (1995) Chromatin-mediated transcriptional repression in yeast. Curr. Opin. Genet. Dev., 5, 168–173. [DOI] [PubMed] [Google Scholar]
- Roth S.Y., Dean,A. and Simpson,R.T. (1990) Yeast α2 repressor positions nucleosomes in TRP1/ARS1 chromatin. Mol. Cell. Biol., 10, 2247–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S.Y., Shimizu,M., Johnson,L., Grunstein,M. and Simpson,R.T. (1992) Stable nucleosome positioning and complete repression by the yeast α2 repressor are disrupted by amino-terminal mutations in histone H4. Genes Dev., 6, 411–425. [DOI] [PubMed] [Google Scholar]
- Sherman F. (1991) Getting started with yeast. Methods Enzymol., 194, 281–301. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Roth,S.Y., Szent-Gyorgyi,C. and Simpson,R.T. (1991) Nucleosomes are positioned with base pair precision adjacent to the α2 operator in Saccharomyces cerevisiae. EMBO J., 10, 3033–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore P. and Sharrocks,A.D. (1995) The MADS-box family of transcription factors. Eur. J. Biochem., 229, 1–13. [DOI] [PubMed] [Google Scholar]
- Sikorski R.S. and Hieter,P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics, 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R.T., Roth,S.Y., Morse,R.H., Patterton,H.-G., Cooper,J.P., Murphy,M., Kladde,M.P. and Shimizu,M. (1993) Nucleosome positioning and transcription. Cold Spring Harb. Symp. Quant. Biol., 58, 237–245. [DOI] [PubMed] [Google Scholar]
- Smith D.L. and Johnson,A.D. (1994) Operator-constitutive mutations in a DNA sequence recognized by a yeast homeodomain. EMBO J., 13, 2378–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. and Richmond,T.J. (1998) Crystal structure of the yeast MATα2/MCM1/DNA ternary complex. Nature, 391, 660–666. [DOI] [PubMed] [Google Scholar]
- Treitel M.A. and Carlson,M. (1995) Repression by SSN6–TUP1 is directed by MIG1, a repressor/activator protein. Proc. Natl Acad. Sci. USA, 92, 3132–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzamarias D. and Struhl,K. (1994) Functional dissection of the yeast Cyc8–Tup1 transcriptional co-repressor complex. Nature, 369, 758–761. [DOI] [PubMed] [Google Scholar]
- Tzamarias D. and Struhl,K. (1995) Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8–Tup1 corepressor complex to differentially regulated promoters. Genes Dev., 9, 821–831. [DOI] [PubMed] [Google Scholar]
- Verdone L., Camilloni,G., Di Mauro,E. and Caserta,M. (1996) Chromatin remodeling during Saccharomyces cerevisiae ADH2 gene activation. Mol. Cell. Biol., 16, 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vershon A.K. and Johnson,A.D. (1993) A short, disordered protein region mediates interactions between the homeodomain of the yeast α2 protein and the MCM1 protein. Cell, 72, 105–112. [DOI] [PubMed] [Google Scholar]
- Weiss K. and Simpson,R.T. (1997) Cell type-specific chromatin organization of the region that governs directionality of yeast mating type switching. EMBO J., 16, 4352–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Weiss,K., Yang,C., Harris,M.A., Tye,B.-K., Newlon,C.S., Simpson,R.T. and Haber,J.E. (1998) Mcm1 regulates donor preference controlled by the recombination enhancer in Saccharomyces mating-type switching. Genes Dev., 12, 1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. and Haber,J.E. (1996) A 700 bp cis-acting region controls mating-type dependent recombination along the entire left arm of yeast chromosome III. Cell, 87, 277–285. [DOI] [PubMed] [Google Scholar]
- Xu M., Simpson,R.T. and Kladde,M.P. (1998) Gal4p-mediated chromatin remodeling depends on binding site position in nucleosomes but does not require DNA replication. Mol. Cell. Biol., 18, 1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.O., Stroke,I.L. and Fields,S. (1993) Coupling of cell identity to signal response in yeast: interaction between the α1 and STE12 protein. Genes Dev., 7, 1584–1597. [DOI] [PubMed] [Google Scholar]