Abstract
Ceramide is a key component of intracellular stress responses. Evidence is provided for a novel mechanism of ceramide formation that mediates solar ultraviolet (UV) A radiation-induced expression of the intercellular adhesion molecule (ICAM)-1. Similarly to UVA radiation, ceramide stimulation of human keratinocytes induced ICAM-1 mRNA expression and activated the ICAM-1 promoter through transcription factor AP-2. Ceramide-activated AP-2 and ceramide-induced ICAM-1 reporter gene activation were abrogated through deletion of the AP-2 binding site. UVA radiation increased the level of ceramide in keratinocytes and inhibition of sphingomyelin synthesis prevented UVA radiation-induced ICAM-1 expression. Hitherto, two pathways have been identified for ceramide accumulation: hydrolysis from sphingomyelin through neutral and acid sphingomyelinases, and de novo synthesis by ceramide synthase. UVA radiation did not activate any of these enzymes. Ceramide generation in UVA-irradiated cells, however, was inhibited by singlet oxygen quenchers and mimicked in unirradiated cells by a singlet oxygen-generating system. In addition, UVA radiation and singlet oxygen both generated ceramide in protein-free, sphingomyelin-containing liposomes. This study indicates that singlet oxygen triggers a third, non-enzymatic mechanism of ceramide formation.
Keywords: ceramide/human keratinocytes/signal transduction/singlet oxygen/ultraviolet radiation
Introduction
Long-wavelength ultraviolet (UVA; 320–400 nm) radiation induces gene expression in human cells (Tyrrell, 1996; Bender et al., 1997; Grether-Beck et al., 1997). Analysis of the underlying photobiological and molecular mechanism is important because solar UVA radiation-induced gene expression is involved in photoaging and photocarcinogenesis, and in the pathogenesis of the most frequent photodermatosis, polymorphic light eruption (Kligman et al., 1985; Norris, 1993; Setlow et al., 1993; Drobetsky et al., 1995; Gruijl et al., 1995). It might also help to understand the biological consequences resulting from exposure of human skin to artificial UVA radiation including high-intensity UVA radiation devices used increasingly for cosmetic and therapeutic reasons (Krutmann, 1999).
UVA radiation is weakly absorbed by most biomolecules but is oxidative in nature, generating reactive oxygen intermediates via a variety of chromophores. For UVA radiation-induced gene expression, this oxidative component involves the generation of singlet oxygen (Ryter and Tyrrell, 1998; Klotz et al., 2000). Singlet oxygen serves as the primary effector in UVA radiation-induced expression of intercellular adhesion molecule-1 (ICAM-1) in human epidermal keratinocytes by inducing a signal transduction pathway dependent on activation of transcription factor AP-2 (Grether-Beck et al., 1996). This signal transduction pathway can be completely blocked by pretreatment of cells with the singlet oxygen quencher and antioxidant vitamin E (Foote et al., 1974; Kaiser et al., 1990; Traber and Sies, 1996). Because of its lipophilic nature, vitamin E preferentially localizes within membranes. This indicated to us the possibility that the initial signal in UVA radiation-induced gene expression was generated at the level of the plasma membrane. The formation of second messenger ceramide from the cell membrane structural phospholipid sphingomyelin was previously shown to mediate increased gene expression in mammalian cells in response to a wide variety of stimuli (Ballou et al., 1996; Hannun, 1996; Spiegel et al., 1996). We therefore considered the possibility that ceramide formation might also be functionally involved in UVA radiation-induced AP-2 activation and increased gene transcription in human keratinocytes.
Results
Role of AP-2 in ceramide-induced ICAM-1 expression
In previous studies, UVA radiation has been shown to upregulate human keratinocyte ICAM-1 expression through activation of transcription factor AP-2 (Grether-Beck et al., 1996). In order to assess the role of ceramides in this system, human keratinocytes were stimulated with cell-permeable ceramides (data are only shown for C2-, but were identical for C6-ceramide stimulation), and subsequently analysed for AP-2 activation and ICAM-1 mRNA expression. Addition of exogenous ceramides increased the DNA binding activity of AP-2 in nuclear extracts that had been prepared from ceramide-stimulated keratinocytes (Figure 1A). Binding to the AP-2 oligonucleotide could be competed with specific competitor in 100-fold molar excess, but not with a consensus oligonucleotide containing an AP-1 site, as demonstrated previously (Grether-Beck et al., 1996; data not shown). The ceramide-induced activation pattern was biphasic with an early maximum after 1–2 h and a subsequent second peak after 24 h, and was associated with a biphasic upregulation of ICAM-1 mRNA (Figure 1B). In order to assess the functional relevance of AP-2 activation for ceramide-induced ICAM-1 expression, ICAM-1 promoter activation was studied in human keratinocytes that had been transiently transfected with various ICAM-1 promoter-based luciferase reporter gene constructs in the range of 6000–34 bp upstream of the transcription start site. Ceramide stimulation induced ICAM-1 promoter activation 3- to 4-fold if cells were transfected with the pIC6000 or pIC277 construct (Figure 1C). Deletion of the putative AP-2 binding site in the pIC277 construct (pIC277ΔAP-2) resulted in loss of the ceramide-induced ICAM-1 promoter activation. The pIC134 construct, which contains this AP-2 site but lacks an NFκB site, could still be activated. The pIC34 construct only contained the basal TATA box and was not activated by ceramides or UVA radiation (data not shown).
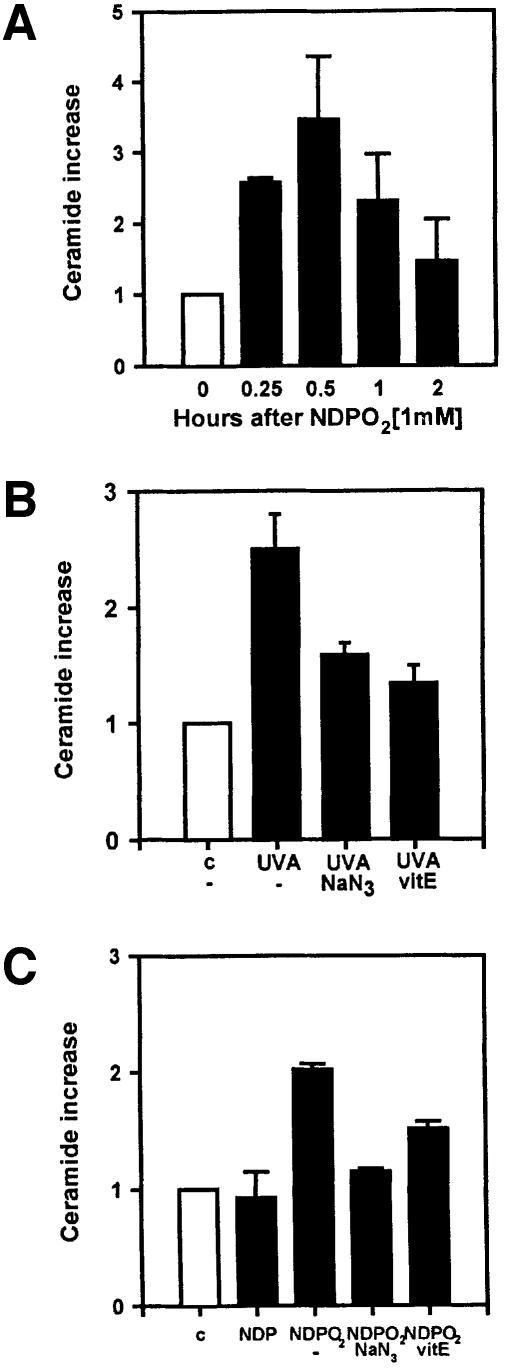
Fig. 1. Role of ceramide in UVA radiation-induced ICAM-1 expression in long-term cultured, normal human keratinocytes. (A) Time-dependent activation of transcription factor AP-2 after stimulation of cells with 10 µM C2-ceramide. Nuclear extracts were analysed by electrophoretic mobility shift assay using a radiolabelled AP-2 consensus site derived from the human ICAM-1 promoter. (B) Time-dependent ICAM-1 mRNA expression after stimulation of cells with 10 µM C2-ceramide (grey bar). ICAM-1 mRNA expression was assessed by differential RT–PCR based on the housekeeping gene β-actin. Unstimulated control (white bar) is set as 1. (C) C2-ceramide-induced reporter gene activity in transiently transfected human keratinocytes detected as relative specific luciferase activity (RLU/µg protein) (grey bars). Different deletion constructs based on the human ICAM-1 promoter linked to luciferase were assessed in triplicate. Unstimulated but transfected cells were set as 1 (white bar). (D) UVA-induced ceramide release was detected in human keratinocytes by sequential HPTLC in triplicate. Cells were sham irradiated (white bar) or irradiated with 30 J/cm2 UVA (black bars) and harvested at the indicated time points. Data were obtained as c.p.m./500 µg of protein (mean of three experiments) and are given as fold increase (control was set as 1). (E) Effect of l-cycloserine on UVA-induced ICAM-1 mRNA expression. UVA radiation-induced ICAM-1 mRNA expression was assessed by differential RT–PCR in cells that had been left untreated (black bar) or treated with 1 mM l-cycloserine (striped bar) for 3 days prior to, during and after UVA irradiation, or were additionally stimulated with 10 µM C2-ceramide (grey bar).
Role of ceramide in UVA radiation-induced ICAM-1 expression
Both ceramide-induced effects, i.e. the biphasic AP-2 activation and ICAM-1 mRNA induction as well as the abrogation of ICAM-1 reporter gene activation through deletion of the AP-2 binding site, were previously observed in an identical manner in human keratinocytes that had been exposed to UVA radiation (Grether-Beck et al., 1996). Therefore, the capacity of UVA radiation to cause ceramide generation in human keratinocytes was examined. Exposure to UVA radiation at doses that activate AP-2 and induce ICAM-1 mRNA expression resulted in a 3-fold increase in the level of ceramide in human keratinocytes (Figure 1D), detectable as early as 15 min post-irradiation with a maximum after 30 min.
To study the relevance of UVA radiation-induced ceramide generation for UVA radiation-induced ICAM-1 expression, keratinocytes were next treated with l-cyclo serine, which inhibits sphingomyelin synthesis by serving as a substrate analogue for serine-palmitoyl transferase. Pretreatment of cells with l-cycloserine completely suppressed UVA radiation-induced upregulation of ICAM-1 mRNA expression (Figure 1E). This inhibitory effect was specific, as it was overcome by addition of exogenous ceramide (Figure 1E), and the inhibitor, when used at identical concentrations, did not prevent interferon-γ-induced ICAM-1 mRNA expression in these cells (data not shown).
Role of singlet oxygen in UVA radiation-induced ceramide generation
UVA radiation-induced AP-2 activation and ICAM-1 mRNA expression were shown to be mediated through the generation of singlet oxygen (Grether-Beck et al., 1996). Both effects were abolished by the addition of singlet oxygen quenchers such as vitamin E or sodium azide, and were mimicked in unirradiated keratinocytes through stimulation with singlet oxygen, generated via thermal decomposition of the endoperoxide of the disodium salt 3,3′-(1,4-naphthylidene)dipropionate (NDPO2). Thus, we examined whether UVA radiation-induced ceramide formation was mediated through the generation of singlet oxygen. Like UVA irradiation, exposure to NDPO2 induced ceramide formation in human keratinocytes (Figure 2A). UVA radiation- and NDPO2-induced ceramide release followed an identical time course, which remained maximal up to 30 min after exposure and subsequently decreased to background levels (compare Figure 2A with 1D). Addition of the singlet oxygen quenchers sodium azide or vitamin E lowered both UVA radiation- and NDPO2-induced ceramide formation to background levels (Figure 2B and C). Inhibition of UVA radiation-induced ceramide formation by vitamin E was dose dependent over the range 10 (12% inhibition)–25 µM (87% inhibition, data not shown).
Fig. 2. Singlet oxygen-induced release of ceramide in long-term cultured, normal human keratinocytes. (A) Time-dependent release of ceramide after a 30 min exposure of cells to 1 mM NDPO2. Unstimulated control cells are set as 1 (white bar). Measurements were made in triplicate. Effect of sodium azide (50 mM, added during stimulation) and vitamin E (25 µM, added as α-tocopheryl succinate 24 h prior to stimulation) on UVA radiation- (B) and NDPO2 (1 mM)-induced (C) release of ceramide (black bars). Ceramide release was assessed 30 min after stimulation. Unstimulated controls are set as 1 (white bar).
Role of enzymes in UVA radiation- and singlet oxygen-induced ceramide formation
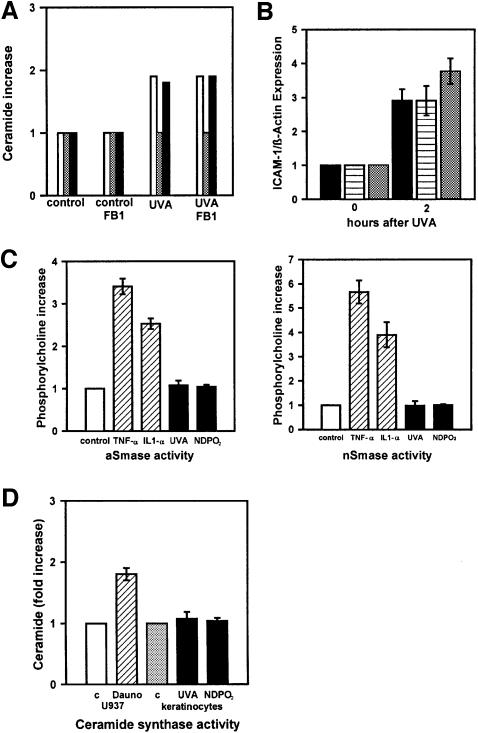
Ceramide can be generated by de novo synthesis (Bose et al., 1995; Ballou et al., 1996; Hannun, 1996; Spiegel et al., 1996). This pathway is initiated by condensation of serine and palmitoyl-CoA to form 3-ketodihydro sphingosine. After reduction to dihydrosphingosine and subsequent conversion to dihydroceramide by ceramide synthase, ceramide is formed by oxidation of dihydroceramide to introduce the trans-4,5 double bond. The rate-limiting enzyme of this reaction is ceramide synthase (sphinganine-acyltransferase), which can be specifically inhibited through addition of its natural inhibitor fumonisin B1 (Merrill et al., 1993). Addition of fumonisin B1 to keratinocytes during and after treatment of cells with UVA radiation or NDPO2 (data not shown) neither prevented ceramide formation (Figure 3A) nor upregulated ICAM-1 mRNA expression in human keratinocytes (Figure 3B). In addition, both stimuli did not increase ceramide synthase activity (Figure 3D).
Fig. 3. The role of ceramide synthase and sphingomyelinase activation in ceramide formation by long-term cultured, normal human keratinocytes. (A) UVA-induced ceramide (C24, white bar; C18, grey bar; C16, black bar) release was detected after lipid extraction by HPLC/mass spectroscopy in cells that had been incubated with 25 µM fumonisin B1 during and after irradiation (30 J/cm2 UVA). (B) Expression of ICAM-1 mRNA was assessed in cells that had been left untreated (black bar) or incubated with 25 µM fumonisin B1 without (striped bar) or with 10 µM C2-ceramide (grey bar) during and 30 min after irradiation (30 J/cm2 UVA). (C) Activation of acid and neutral sphingomyelinase was measured in cells 30 min after stimulation with cytokines [hatched bars; 1000 U/ml recombinant human (rh) TNF-α, 1000 U/ml rh IL-1α from R&D Systems], UVA radiation (30 J/cm2) or 1 mM NDPO2 (black bars). Unstimulated controls were set as 1 (white bars). (D) Activation of ceramide synthase was measured in vitro in microsomal membranes of U937 cells that had been left untreated (open bar) or stimulated with 1 µM daunorubicin for 24 h (hatched bar), and in keratinocytes that had been left untreated (grey bar) or stimulated with either 30 J/cm2 UVA or NDPO2 (black bars), and harvested after 30 min.
The other principal pathway for production of ceramide involved in signal transduction is the catabolic generation of ceramide by sphingomyelinase, which hydrolyses sphingomyelin to produce phosphorylcholine and ceramide (Ballou et al., 1996; Hannun, 1996; Spiegel et al., 1996; Krönke, 1997). Sphingomyelinase activities increase in response to a wide variety of external stimuli, including the pro-inflammatory cytokines tumour necrosis factor (TNF)-α and interleukin (IL)-1. We therefore assessed the effect of UVA irradiation or exposure to NDPO2 on sphingomyelinase activities in human keratinocytes. Stimulation of human keratinocytes with IL-1 and TNF-α increased the activities of acid as well as neutral sphingomyelinase (Figure 3C). In contrast, neither exposure to UVA radiation nor stimulation with NDPO2 enhanced sphingomyelinase activities above background levels (Figure 3C). Both stimuli, however, caused ceramide formation in the same experiments, indicating the existence of an alternative pathway.
Ceramide formation in sphingomyelin-containing liposomes
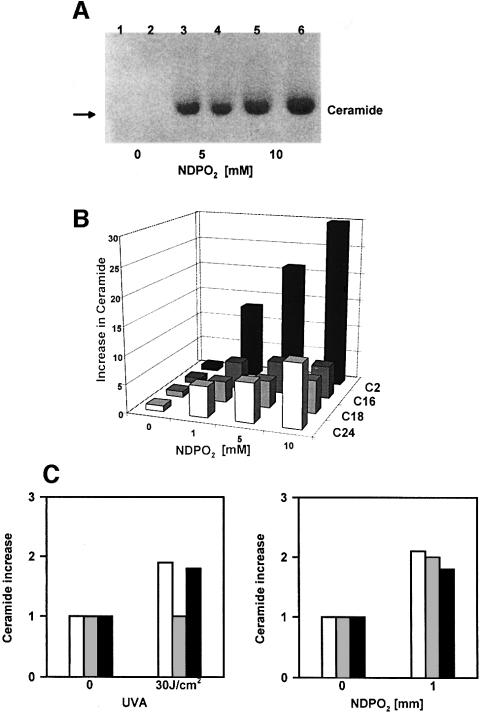
Since singlet oxygen directly reacts with plasmalogen lipids (Morand et al., 1988; Zoellner et al., 1988; Anderson and Thompson, 1992), we speculated that this mechanism might be non-enzymatic in nature. In order to test this hypothesis, liposomes composed exclusively of sphingomyelin from egg yolk, but containing no protein, were employed. Analysis of ceramide formation by high-performance thin-layer chromatography (HPTLC) demonstrated that in this enzyme-free system, exposure to NDPO2 or UVA radiation (data not shown) generated ceramide in a dose-dependent manner (Figure 4A). The liposomes used in our studies contained a mixture of several sphingomyelins with different free fatty acid chain residues. HPLC/mass spectroscopy demonstrated that treatment of liposomes with singlet oxygen generated a corresponding ceramide pattern with dose-dependent increases in C2-, C16-, C18- and C24-ceramides (Figure 4B). This ceramide pattern was identical to that observed in NDPO2-stimulated keratinocytes (Figure 4D), which do not contain C2-ceramide, and, except for C18-ceramide, also to that obtained in UVA-irradiated cells (Figure 4C).
Fig. 4. Singlet oxygen generates ceramide in sphingomyelin-containing liposomes. (A) HPTLC of NDPO2-induced ceramide formation in liposomes. Liposomes were treated with 0 (lanes 1 and 2), 5 (lanes 3 and 4) and 10 mM NDPO2 (lanes 5 and 6), and chloroform/methanol lipid extracts prepared immediately thereafter and analysed. Iodine vapour was used to stain ceramides. (B) Analysis of NDPO2-induced ceramide formation in liposomes by HPLC/mass spectroscopy. Liposomes were treated for 30 min with increasing doses (0–10 mM) of NDPO2 and lipid extracts prepared immediately thereafter. (C) Analysis of ceramide in long-term cultured human keratinocytes by HPLC/mass spectroscopy. Lipid extracts were prepared 30 min after stimulation of cells with 30 J/cm2 UVA or 1 mM NDPO2. C24, white bars; C18, grey bars; C16, black bars.
An important question is whether the amount of ceramide generated in liposomes corresponds to the amount of ceramide generated in intact cells. By using HPTLC we have found that sham-treated liposomes contain ∼10 mol% ceramide, which would thus be equivalent to basal sphingomyelin hydrolysis. The distribution of fatty acid residues in the liposomal sphingomyelin is 83.9% C16, 6.3% C18 and 4.2% C24 (see Materials and methods). Based on this information we have calculated that stimulation of liposomes with 1 mM NDPO2 generated 400 pmol of C16-, 90 pmol of C2-, 26 pmol of C18- and 23 pmol of C24-ceramide (Figure 5A). For C16-ceramide, this would correspond to a 4.8-fold increase after stimulation. In a similar manner, in unstimulated keratinocytes, the basal content of sphingomyelin was found to be ∼120 pmol/500 µg of protein. Similar amounts of both lipids were previously reported for P388 cells by Bose et al. (1995). After stimulation of cells with 1 mM NDPO2, there was a 3-fold increase for C16-ceramide (Figure 5B).
Fig. 5. Absolute amounts of ceramide and sphingomyelin in liposomes and human keratinocytes. (A) The absolute amounts of C2- (open circle), C16- (black circle), C18- (dark grey circle) and C24- (grey circle) ceramides were determined in picomoles in sphingomyelin-containing liposomes that had been treated with increasing concentrations of NDPO2 (0, 1, 5 and 10 mM) as described in Materials and methods. Data are given as the generation of ceramide (pmol)/sphingomyelin (µmol) versus increasing concentrations of NDPO2 (mM). (B) Time-dependent increase of absolute amounts of ceramide (grey bar) and sphingomyelin (black bar) in human keratinocytes. Cells were either left untreated or stimulated with 1 mM NDPO2, and subsequently the absolute amounts of ceramide and sphingomyelin determined as described in Materials and methods. All measurements were made in triplicate. Data are given in the form of a histogram of amount of lipid (pmol)/500 µg of protein versus time (h) after stimulation with 1 mM NDPO2.
Discussion
Ceramide as second messenger in UVA radiation-induced ICAM-1 expression
Ceramide mediates gene induction in the stress response of mammalian cells and is therefore likely to be capable of activating stress-inducible transcription factors (Ballou et al., 1996; Hannun, 1996; Spiegel et al., 1996). Transcription factor AP-2, which has a well documented role in gene regulation as it relates to differentiation and morphogenesis, was found to be involved in the transcriptional stress response of mammalian cells (Grether-Beck et al., 1996; Johnson, 1996; Kroeger and Abraham, 1996). In keeping with this concept we have observed in the present study that ceramide can activate AP-2. Addition of ceramide to keratinocytes induced ICAM-1 mRNA expression and activated transcription factor AP-2 in a biphasic manner. Ceramide stimulation activated the human ICAM-1 promoter and this activation was abrogated through deletion of the putative AP-2 binding site. These studies demonstrate (i) that ceramide has the capacity to activate transcription factors and (ii) that ceramide-induced AP-2 activation is functionally relevant because it mediates upregulation of ICAM-1 expression.
Previously, activation of trancription factor AP-2 was shown to be of functional relevance for ICAM-1 expression in human keratinocytes that had been exposed to physiologically relevant doses of UVA radiation (Grether-Beck et al., 1996). We now report that stimulation of keratinocytes with ceramide or exposure to UVA radiation induced ICAM-1 mRNA expression and AP-2 activation with identical kinetics, and for both stimuli ICAM-1 reporter gene activation was abrogated through deletion of the AP-2 binding site. Moreover, UVA irradiation of keratinocytes at dose levels that activate AP-2 and induce ICAM-1 mRNA expression generated ceramide in human keratinocytes. Maximal ceramide generation preceded the maximum of AP-2 activation and ICAM-1 mRNA steady-state levels in UVA-irradiated cells. Inhibition of ceramide generation in UVA-irradiated cells through sphingomyelin depletion rendered keratinocytes unresponsive to UVA radiation. Taken together, these studies demonstrate that ceramide is a second messenger in UVA radiation-induced AP-2 activation and thereby increases the transcriptional expression of the human ICAM-1 gene in irradiated keratinocytes. These studies extend the spectrum of intracellular stress responses mediated by ceramide to solar UVA radiation-induced gene expression in human skin cells.
Singlet oxygen as mediator of UVA radiation-induced ceramide generation
Singlet oxygen is an important biochemical intermediate in multiple biological processes, including UVA radiation-induced gene expression in human keratinocytes and fibroblasts (Tyrrell, 1996; Grether-Beck et al., 1996, 1997; Ryter and Tyrrell, 1998; Klotz et al., 2000). No direct methods exist to detect singlet oxygen in irradiated cells, and the role of singlet oxygen in gene induction has therefore been demonstrated through the use of singlet oxygen quenchers, and singlet oxygen-generating systems. In the present study, we have employed sodium azide and vitamin E as singlet oxygen quenchers, and both substances were found to suppress UVA-induced ceramide generation. In addition, UVA radiation-induced ceramide generation was mimicked in unirradiated cells that were stimulated with NDPO2 as a singlet oxygen-generating system. By using an identical experimental approach, singlet oxygen was previously found to mediate UVA radiation-induced AP-2 activation and induction of ICAM-1 expression (Grether-Beck et al., 1996), to induce apoptosis in human T-helper cells (Morita et al., 1997) and to generate mitochondrial DNA mutations in human dermal fibroblasts (Berneburg et al., 1999). This experimental approach can currently be viewed as state-of- the-art (Packer and Sies, 2000). Taken together, these experiments indicate that singlet oxygen (i) can generate ceramide and (ii) by virtue of this property mediates UVA radiation-induced ceramide generation in human keratinocytes. Based on the present findings, we propose that within the signal transduction cascade of UVA radiation-induced gene expression, ceramide generation occurs subsequently to singlet oxgen generation but prior to AP-2 activation. The membrane-localizing, lipophilic, singlet oxygen quencher vitamin E effectively inhibited UVA radiation as well as singlet oxygen-induced ceramide generation, AP-2 activation and induction of ICAM-1 expression (Grether-Beck et al., 1996). Also, the NDPO2 used in the present study is water soluble and generates singlet oxygen at the outer part of the cell membrane (Klotz et al., 1999; Pierlot et al., 2000). It is thus tempting to speculate that the initial step in intracellular signalling leading to UVA radiation-induced gene expression occurs at the level of the plasma membrane.
Non-enzymatic ceramide generation
In an attempt to define this initial signalling event in more detail we have studied the role of enzymes that previously were shown to mediate ceramide generation in response to extracellular stimuli and stress. One candidate enzyme is ceramide synthase, which mediates daunorubicin-induced ceramide generation and apoptosis in P388 and U937 cells (Bose et al., 1995). De novo synthesis of ceramide through this enzyme leads to prolonged ceramide elevations that occur only several hours after stimulation. This late response was thus unlikely to mediate the UVA radiation/singlet oxygen-induced ceramide formation in human keratinocytes that occurred within minutes of stimulation. Also, UVA radiation and singlet oxygen did not increase ceramide synthase activity (Figure 3D), and inhibition of the enzyme through addition of its natural specific inhibitor fumonisin B1 neither prevented ceramide formation (Figure 3A) nor induced ICAM-1 (Figure 3B) in irradiated keratinocytes. It should be noted that, under identical experimental conditions, fumonisin B1 was shown to be active because it inhibited ceramide generation in human keratinocytes that was due to de novo synthesis (data not shown). Taken together, these results support the concept that de novo synthesis of ceramide is not relevant for immediate signalling events initiated at the plasma membrane (Krönke, 1997).
Alternatively, it has been proposed that activation of sphingomyelinases is responsible for the release of ceramide as second messenger leading to transcription factor activation or the induction of apoptosis (Ballou et al., 1996; Hannun, 1996; Spiegel et al., 1996; Krönke, 1997). This concept, however, has recently been questioned by a number of studies in which ceramide generation was observed to occur independently of sphingomyelinase activation (Watts et al., 1997; Zumbansen and Stoffel, 1997; Boesen-de-Cock et al., 1998; Tomiuk et al., 1998; Chatterjee et al., 1999). In keeping with these observations, in the present study, neither UVA irradiation nor singlet oxygen generation increased sphingomyelinase activity. This is in contrast to cytokine stimulation of keratinocytes and to previous work employing UVB (280–315 nm) radiation, which does not generate singlet oxygen (Huang et al., 1997). Taken together, these observations point to a third pathway of ceramide generation, which does not require ceramide synthase or sphingomyelinase activation.
In the present study, this previously unknown pathway was found to be non-enzymatic in nature. Accordingly, UVA radiation and singlet oxygen generated ceramide in sphingomyelin-containing liposomes, which did not contain protein. The identity of the ceramides generated in this enzyme-free system was proven by HPLC/mass spectroscopy. The identification of a non-enzymatic pathway for ceramide generation might help to reconcile the discrepancies between ceramide generation and lack of enzyme activation that have been noted in previous reports (Zumbansen and Stoffel, 1996; Watts et al., 1997; Boesen-de-Cock et al., 1998; Tomiuk et al., 1998). When analysed for different free fatty acid chain residues, the patterns of ceramide generated by singlet oxygen stimulation and UVA irradiation in the enzyme-free liposome system and in intact human keratinocytes were essentially identical. Our data also indicate that the level of non-enzymatic hydrolysis of sphingomyelin contributes significantly to ceramide formation in human keratinocytes. We therefore propose that non-enzymatic triggering of ceramide generation is important for the generation of ceramide as signal transduction molecule and that it initiates UVA radiation-induced AP-2 activation and increases gene expression in human keratinocytes. It should be noted, however, that in keratinocytes, sphingomyelin content does not decrease, but increases up to 2.9-fold (Figure 5B). This is in contrast with liposomes and most likely reflects a reactive increase in sphingomyelin turnover, thus emphasizing the relevance of enzymatic mechanisms under conditions in which non-enzymatic ceramide formation occurs.
Non-enzymatic breakdown of sphingomyelin might first target the double bond of the sphingosine backbone. As a consequence, products would be generated containing a C14- or C15-carbon body. Oxidation would result in the formation of alcohol, aldehyde or carboxylate. When C14- and C15-alcohols, -aldehydes and -carboxylates were separated by HPTLC under conditions identical to those used for separation of sphingomyelin-containing liposomes, it was observed that the Rf values of ceramide and of C14- and C15-products differed markedly (data not shown). The observed increase in ceramide in NDPO2- or UVA-treated liposomes can thus not be attributed to the formation of C14- and C15-products, because ceramide and C14- and C15-products can be clearly discriminated under the separation conditions used in this study. Also, C14- or C15-products were not detected in UVA-irradiated as well as NDPO2-treated liposomes. It is thus unlikely that the double bond of the sphingosine backbone is the first target in the non-enzymatic hydrolysis of sphingomyelin. Singlet oxygen, however, can react directly with the plasmalogen ethyl ether linkage, leading to disruption of plasmalogen-containing liposomes (Morand et al., 1988; Anderson and Thompson, 1992). In an analogous manner, singlet oxygen might be capable of mediating the non-enzymatic hydrolysis of sphingomyelin leading to ceramide signalling. Further analysis of the underlying mechanism requires extensive photochemical examinations, which are beyond the scope of the present gene regulatory study.
Materials and methods
Cell culture
Long-term cultured, normal human keratinocytes prepared from neonatal foreskin were cultured under serum-free conditions as described (Grewe et al., 1995). U937 monoplastic leukaemia cells were grown in RPMI 1640 containing 10% fetal calf serum and 2 mM glutamine.
UV irradiation
For UV radiation, medium was replaced by phosphate-buffered saline (PBS), and cells were exposed to UVA radiation (30 J/cm2) using a Sellamed 24000 A irradiation device (Dr Sellmeier, Sellas GmbH Gevelsberg, Germany) as described previously (Morita et al., 1997).
Chemical treatments and singlet oxygen generation
All chemicals were purchased from Sigma except for C2- and C6-ceramide (Calbiochem-Novabiochem) and sodium azide (Merck); recombinant human IL-1α and TNF-α were obtained from R&D Systems. Vitamin E (α-tocopheryl succinate) was dissolved in ethanol and added to cell cultures 24 h before irradiation at a concentration of 25 µM. Sodium azide (50 mM in PBS) was present during irradiation of cells. Singlet oxygen was generated by thermal decomposition of NDPO2. This singlet oxygen-generating system was previously shown to be well suited for cell cultures because it is water soluble, non-toxic at concentrations up to 40 mM for a 1 h incubation and with a suitable singlet oxygen generator (Wlaschek et al., 1994; Grether-Beck et al., 1996; Morita et al., 1997; Berneburg et al., 1999; Packer and Sies, 2000). Accordingly, infrared emission of singlet oxygen was measured with a liquid nitrogen-cooled germanium photodiode detector (model EO-817L; North Coast Scientific, Santa Rosa, CA) and the rate of singlet oxygen generation was 3 µM/min (DiMascio and Sies, 1989). Control cells were stimulated with NDP, which had been generated by thermal decomposition from the same batch of NDPO2 used in these experiments. Daunorubicin hydrochloride was prepared as a 1 mM stock solution in sterile saline, frozen until time of use and used at an end concentration of 1 µM.
Transfection and reporter gene assay
Transfection was mediated by Polybrene (Aldrich). A commercially available luciferase reporter system (Promega) was used. Constructs containing various deletions of the ICAM-1 promoter were cloned as described (Stade et al., 1990; van de Stolpe et al., 1994). Transfection was performed on 2 × 105 cells in triplicate as described (Grether-Beck et al., 1996) using 5 µg of DNA. Transfection efficiency was monitored by co-transfecting cells with the simian virus 40 promoter and enhancer region–β-galactosidase control plasmid (Promega) using the β-galactosi dase assay system (Promega). Promoter activation was expressed as the mean ± SD of relative specific luciferase activity, which was based on protein content. Unstimulated cells were used as controls and were set equal to one.
Nuclear extracts and gel electrophoretic mobility shift assays
Nuclear extracts were prepared and gel electrophoretic mobility shift assays performed as described (Grether-Beck et al., 1996). The AP-2 consensus oligonucleotide (top strand, 5′-GACCCTCTCGGCCCGGGCACCCT-3′) was deduced from the ICAM-1 promoter.
RNA extraction and RT–PCR
Total RNA was isolated using an RNeasy Total RNA kit (Qiagen, Hilden, Germany). Expression of ICAM-1 mRNA was measured by differential RT–PCR as described (Ahrens et al., 1997) using a primer pair specific for ICAM-1 (5′-TGACCAGCCCAAGTTGTTGG-3′, 5′-ATCTCTCCTCACCAGCACCG-3′).
Quantification of ceramide
Cells were metabolically labelled with [3H]palmitic acid for 3 days. After stimulation, lipid extracts based on 500 µg of protein were prepared at the indicated time points. After lipid extraction and mild alkaline hydrolysis, the lower phase of the Folch extract was evaporated. Lipids were dissolved in 2:1 (v/v) chloroform/methanol and resolved by analytical sequential HPTLC using two successive runs with chloroform/methanol/H2O (100:42:6, v/v) and chloroform/methanol/acetic acid (94:1:5, v/v) using ceramides as standard. The lipid spots were stained with iodine vapour, scraped off the plates and quantified by liquid scintillation counting (Bonizzi et al., 1997).
Assessment of sphingomyelinase activity
To measure acid sphingomyelinase activity, protein extracts were prepared from stimulated cells. A protease inhibitor cocktail (Complete; Boehringer Mannheim, Germany) was added according to the manufacturer’s instructions in order to prevent their degradation. Protein (100 µg) was incubated for 1 h at 37°C in a buffer containing 250 mM sodium acetate pH 5.0, 1 mM EDTA and 0.2 mCi/ml [choline-methyl-14C]sphingomyelin. To measure neutral sphingomyelinase activity, 100 µg of protein were incubated for 2 h at 37°C in a buffer containing 20 mM HEPES pH 7.4, 1 mM MgCl2 and 0.2 mCi/ml [choline-methyl-14C]sphingomyelin. Released radioactive phosphocholine was extracted with 2:1 (v/v) chloroform/methanol and quantified by liquid scintillation (Bose et al., 1995; Mansat et al., 1997).
Assessment of ceramide synthase activity
Microsomal membranes were prepared as described by Liu et al. (1994). In brief, cells grown on 10 cm dishes were pelleted, washed once with cold PBS, and resuspended in 300 µl of homogenization buffer (25 mM HEPES pH 7.4), followed by the addition of proteinase inhibitor (Complete; Boehringer Mannheim, Germany). Cells were disrupted using a tissue homogenizer (Dounce). Lysates were centrifuged at 800 g for 5 min. The post-nuclear supernatant was centrifuged at 250 000 g for 30 min. The microsomal membrane pellet was resuspended in 1.0 ml of homogenization buffer. Membranes were freshly prepared each day. Assays of ceramide synthase were based on the protocol described by Harel and Futermann (1993). Microsomal membrane protein (75 µg) was incubated in a 1.0 ml reaction mixture containing 2 mM MgCl2, 20 mM HEPES pH 7.4, 20 µM defatted bovine serum albumin, varying concentrations (0.2–20 µM) of dihydrosphingosine, 70 µM unlabeled palmitoyl-coenzyme A and 3.6 µM (0.2 µCi) [1-14C]palmitoyl-coenzyme A. Dihydrosphingosine was dried under nitrogen from stock solution in 100% ethanol and dissolved by sonication in the reaction mixture prior to addition of microsomal membranes. The reaction was started through addition of palmitoyl-coenzyme A, incubated at 37°C for >1 h, and then stopped by extraction of lipids using 2 ml of 1:2 (v/v) chloroform/methanol. The lower phase was removed, concentrated under nitrogen and applied to a silica gel 60 TC plate (Merck, Darmstadt, Germany). Dihydroceramide was resolved from free radiolabelled fatty acid using a solvent system of chloroform/methanol/3.5 N ammonium hydroxide (85:15:1), identified by iodine vapour staining based on co-migration with ceramide standards, and quantified by liquid scintillation counting.
HPLC/mass spectroscopy
Liposomes were washed with PBS, sonicated 10 s before being extracted with 2:1 (v/v) chloroform/methanol and centrifuged at 1000 g. The lower phase was washed with chloroform/methanol/H2O (3:48:47) before being evaporated under nitrogen. The pellet was resuspended in chloroform/methanol. Cellular lipid extracts were prepared as for HPTLC analysis, except for radiolabelling. The evaporated extracts were dissolved in chloroform/methanol. Samples were analysed by HPLC (Chromcart column 125/2 Nucleosil 100-5 C18AB; Macherey Nagel, Germany). Mass spectrometry was carried out on a Quattro tandem mass spectrometer (Micromass, UK) with internal standards for C2-, C16-, C18- and C24-ceramide.
Liposomes
Liposomes were prepared by AGI Dermatics (Freeport, NY) using 99% pure sphingomyelin obtained from Avanti Polar Lipids (Alabaster, AL), which was purified from egg yolk by HPLC. According to the manufacturer, the lipid composition was 5.6% C2, 83.9% C16, 6.3% C18 and 4.2% C24 area percentage determined by FAME-GC/FID.
Determination and calculation of absolute amounts of lipids
For determination of absolute amounts of ceramide and sphingomyelin in liposomes and keratinocytes, lipid extracts were prepared and separated on 20 × 10 cm precoated Merck 60F254s silica gel plates (Merck, Darmstadt, Germany) using a CAMAG Automated Multiple Development (AMD) 2 device (CAMAG, Berlin, Germany). The AMD procedure consists of 26 repeated developments of the chromatogram using a stepwise elution gradient with methanol, dichloromethane and n-hexane. The combination of focusing effect and gradient elution results in extremely narrow bands and high resolution separation. Lipids were separated using a slightly modified application protocol for phospholipids developed by CAMAG (application note 5). Bands were visualized by post-chromatographic derivatization with manganese chloride dipping. Manganese chloride dipping solution was prepared as follows: 0.4 g of MnCl2⋅4H2O were dissolved in 60 ml of water under moderate heating, 60 ml of methanol and 4 ml of concentrated sulfuric acid were added after the solution had cooled down to room temperature. Plates were stained in a CAMAG Chromatogram Immersion Device III. Lipids were coloured as brown bands and detected by a CAMAG TLC Scanner II using CATS software III (CAMAG, Berlin, Germany) in absorption mode at 550 nm with a tungsten lamp, monochromator bandwidth 20 nm, slit width 0.45 × 6 mm. Quantification was performed using a second-order polynomial calibration curve (peak height) with four standard mixes in the range 50–1000 ng.
Basal hydrolysis of sphingomyelin in sphingomyelin-containing liposomes was determined by separation of several dilutions of sphingomyelin-containing liposomes using AMD2. Ceramide was detected as 10 mol% (correlation coefficient 0.9936). Mass spectroscopic analysis of liposomes was used to determine relative amounts of C2-, C16-, C18- and C24-ceramide in unstimulated and NDPO2-stimulated liposomes. The sum of the named ceramides in sphingomyelin-containing liposomes was set at 10 mol%. Absolute amounts of ceramide and sphingomyelin in keratinocytes were determined by separation of lipid extracts by AMD2.
Acknowledgments
Acknowledgements
We thank J.Degraeve and J.C.Van Heughen for HPLC/mass spectroscopy analysis and Lothar Jaenicke for advice on thermodynamics. This work was supported by a grant from the Deutsche Forschungsgemeinschaft, SFB 503, Projects B2 and B1. G.B. is supported by a grant from the EEC, J.P. is Research Director of the Belgian National Fund for Scientific Research (Brussels, Belgium) and H.S. is a fellow of the National Foundation for Cancer Research (NSCR), Bethesda, MD.
References
- Ahrens C. et al. (1997) Photocarcinogenesis and inhibition of intercellular adhesion molecule 1 expression in cells of DNA-repair defective individuals. Proc. Natl Acad. Sci. USA, 94, 6837–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V.C. and Thompson,D.H. (1992) Triggered release of hydrophilic agents from plasmalogen liposomes using visible light or acid. Biochim. Biophys. Acta, 1109, 33–42. [DOI] [PubMed] [Google Scholar]
- Ballou L.R., Laulederkind,S.J.F., Rosloniec,E.F. and Raghow,R. (1996) Ceramide signalling and the immune response. Biochim. Biophys. Acta, 1301, 273–287. [DOI] [PubMed] [Google Scholar]
- Bender K., Blattner,C., Knebel,A., Iordanov,M., Herrlich,P. and Rahmsdorf,H.J. (1997) UV-induced signal transduction. J. Photochem. Photobiol. B, 37, 1–17. [DOI] [PubMed] [Google Scholar]
- Berneburg M., Grether-Beck,S., Kürten,V., Ruzicka,T., Briviba,K., Sies,H. and Krutmann,J. (1999) Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion. J. Biol. Chem., 274, 15345–15349. [DOI] [PubMed] [Google Scholar]
- Boesen-de-Cock J.G.R., Tepper,A.D., de Vries,E., van Blitterswijk,W.J. and Borst,J. (1998) CD95 (Fas/Apo-1) induces ceramide formation and apoptosis in the absence of a functional acid sphingomyelinase. J. Biol. Chem., 272, 7560–7565. [DOI] [PubMed] [Google Scholar]
- Bonizzi G., Piette,J., Merville,M.P. and Bours,V. (1997) Distinct signal transduction pathways mediate nuclear factor κB by IL-1β in epithelial and lymphoid cells. J. Immunol., 159, 5264–5272. [PubMed] [Google Scholar]
- Bose R., Verheij,M., Haimovitz-Friedmann,A., Scotto,K., Fuks,Z. and Kolesnick,R. (1995) Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell, 82, 405–414. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Han,H., Rollins,S. and Cleveland,T. (1999) Molecular cloning, characterization and expression of a novel human neutral sphingomyelinase. J. Biol. Chem., 274, 37407–37412. [DOI] [PubMed] [Google Scholar]
- DiMascio P. and Sies,H. (1989) Quantification of singlet oxygen generated by thermolysis of 3,3-(1,4-naphthylidene) dipropionate. Monomol and dimol photoemission and the effects of 1,4-diazabicyclo (2.2.2) octane. J. Am. Chem. Soc., 11, 2909–2914. [Google Scholar]
- Drobetsky E.A., Turcotte,J. and Chateauneuf,A. (1995) A role of ultraviolet A in solar mutagenesis. Proc. Natl Acad. Sci. USA, 92, 2350–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote C.S., Ching,T.Y. and Geller,G.G. (1974) Chemistry of singlet oxygen. XVIII. Rates of reaction and quenching of α-tocopherol and singlet oxygen. Photochem. Photobiol., 20, 511–513. [DOI] [PubMed] [Google Scholar]
- Grether-Beck S., Olaizola-Horn,S., Schmitt,H., Grewe,M., Jahnke,A., Johnson,J.P., Sies,H. and Krutmann,J. (1996) Activation of transcription factor AP2 mediates UVA radiation and singlet oxygen-induced expression of the human intercellular adhesion molecule 1. Proc. Natl Acad. Sci. USA, 93, 14586–14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether-Beck S., Buettner,R. and Krutmann,J. (1997) Ultraviolet A radiation-induced expression of human genes: Molecular and photobiological mechanisms. Biol. Chem., 378, 1231–1236. [PubMed] [Google Scholar]
- Grewe M., Gyufko,K. and Krutmann,J. (1995) Interleukin-10 production by cultured human keratinocytes: regulation by ultraviolet B and ultraviolet A radiation. J. Invest. Dermatol., 104, 3–6. [DOI] [PubMed] [Google Scholar]
- Gruijl F.R. and Forbes,P.D. (1995) UV-induced skin cancer in a hairless mouse model. BioEssays, 17, 651–660. [DOI] [PubMed] [Google Scholar]
- Hannun Y.A. (1996) Functions of ceramide in coordinating cellular responses to stress. Science, 274, 1855–1859. [DOI] [PubMed] [Google Scholar]
- Harel R. and Futermann,A.H. (1993) Inhibition of sphingolipid synthesis affects axonal outgrowth in cultured hippocampal neurons. J. Biol. Chem., 268, 14476–14481. [PubMed] [Google Scholar]
- Huang C., Ma,W., Bowden,G.T. and Dong,Z. (1997) Direct evidence for an important role of sphingomyelinase in ultraviolet-induced activation of c-jun N-terminal kinase. J. Biol. Chem., 272, 27753–27757. [DOI] [PubMed] [Google Scholar]
- Johnson A.C. (1996) Activation of epidermal growth factor receptor gene transcription by phorbol 12-myristate 13-acetate is mediated by activator protein 2. J. Biol. Chem., 271, 3033–3038. [PubMed] [Google Scholar]
- Kaiser S., DiMascio,P., Murphy,M.E. and Sies,H. (1990) Physical and chemical scavenging of singlet molecular oxygen by tocopherols. Arch. Biochem. Biophys., 277, 101–108. [DOI] [PubMed] [Google Scholar]
- Kligman L.H., Akin,F.J. and Kligman,A.M. (1985) The contributions of UVA and UVB to connective tissue damage in hairless mice. J. Invest. Dermatol., 84, 272–276. [DOI] [PubMed] [Google Scholar]
- Klotz L.-O., Pellieux,C., Briviba,K., Pierlot,C., Aubry,J.-M. and Sies,H. (1999) Mitogen-activated protein kinase (p38-, JNK-, ERK-) activation pattern induced by extracellular and intracellular singlet oxygen and UVA. Eur. J. Biochem., 260, 917–922. [DOI] [PubMed] [Google Scholar]
- Klotz L.-O., Briviba,K. and Sies,H. (2000) Signaling by singlet oxygen in biological systems. In Sen,C., Sies,H. and Baeuerle,P. (eds), Antioxidant and Redox Regulation of Genes. Academic Press, San Diego, CA, pp. 3–20. [Google Scholar]
- Kroeger K.M. and Abraham,L.J. (1996) Identification of an AP-2 element in the –323 to –285 region of the TNF-α gene. Biochem. Mol. Biol. Int., 40, 43–51. [DOI] [PubMed] [Google Scholar]
- Krönke M. (1997) The mode of ceramide action: The alkyl protrusion model. Cytokine Growth Factor Rev., 8, 103–110. [DOI] [PubMed] [Google Scholar]
- Krutmann J. (1999) Therapeutic photomedicine: phototherapy. In Freedberg,I.M., Eisen,A.B., Wolff,K., Austen,K.F., Goldsmith,L.A., Katz,S.I. and Fitzpatrick,T.B. (eds), Fitzpatrick’s Dermatology in General Medicine, 5th edn. McGraw-Hill, New York, NY, pp. 2870–2879. [Google Scholar]
- Liu J., Mathias,S., Yang,Z. and Kolesnick,R.N. (1994) Renaturation and TNF-α stimulation of a 97 kDa ceramide-activated protein kinase. J. Biol. Chem., 269, 3047–3052. [PubMed] [Google Scholar]
- Mansat V., Bettaïeb,A., Levade,T., Laurent,G. and Jaffrézou,J.-P. (1997) Serine protease inhibitors block neutral sphingomyelinase activation, ceramide generation and apoptosis triggered by daunorubicin. FASEB J., 11, 695–702. [DOI] [PubMed] [Google Scholar]
- Merrill A.H. Jr, Van Echten,G., Wang,E. and Sandhoff,K. (1993) Fumonisin B1 inhibits sphingosine (sphinganine) N-acetyltransferase and de novo sphingolipid biosynthesis in cultured neurons in situ. J. Biol. Chem., 268, 27299–27306. [PubMed] [Google Scholar]
- Morand O.H., Zoellner,R.A. and Raetz,C.R. (1988) Disappearance of plasmalogens from membranes of animal cells subjected to photosensitized oxidation. J. Biol. Chem., 263, 11597–11606. [PubMed] [Google Scholar]
- Morita A. et al. (1997) Evidence that singlet oxygen-induced human T helper cell apoptosis is the basic mechanism of ultraviolet A radiation phototherapy. J. Exp. Med., 186, 1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris P.G. (1993) Advances in understanding the pathogenesis of photodermatosis. Curr. Opin. Dermatol., 1, 185–190. [Google Scholar]
- Packer L. and Sies,H. (2000) Singlet oxygen, UV-A and ozone. Methods Enzymol., 319, 1–622. [Google Scholar]
- Pierlot C., Aubry,J.-M., Briviba,K., Sies,H. and DiMascio,P. (2000) Naphthalene endoperoxides as generators of singlet oxygen in biological media. Methods Enzymol., 319, 3–20. [DOI] [PubMed] [Google Scholar]
- Ryter S.W. and Tyrrell,R.M. (1998) Singlet molecular oxygen (1O2): a possible effector of eukaryotic gene expression. Free Radic. Biol. Med., 24, 1520–1534. [DOI] [PubMed] [Google Scholar]
- Setlow R.B., Grist,E., Thompson,K. and Woodhead,A.D. (1993) Wavelengths effective in induction of malignant melanoma. Proc. Natl Acad. Sci. USA, 90, 6666–6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S., Foster,D. and Kolesnick,R. (1996) Signal transduction through lipid second messengers. Curr. Opin. Cell Biol., 8, 159–167. [DOI] [PubMed] [Google Scholar]
- Stade B.G., Messer,G., Riethmüller,G. and Johnson,J.P. (1990) Structural characteristics of the 5′ region of the human ICAM-1 gene. Immunobiology, 182, 79–87. [DOI] [PubMed] [Google Scholar]
- Tomiuk S., Hoffmann,K., Nix,M., Zumbansen,M. and Stoffel,W. (1998) Cloned mammalian neutral sphingomyelinase: functions in sphingolipid signaling? Proc. Natl Acad. Sci. USA, 95, 3638–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber M.G. and Sies,H. (1996) Vitamin E in humans: demand and delivery. Annu. Rev. Nutr., 16, 321–347. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. (1996) Activation of mammalian gene expression by the UV component of sunlight—from models to reality. BioEssays, 18, 139–148. [DOI] [PubMed] [Google Scholar]
- van de Stolpe A., Caldenhoven,E., Stade,B.G., Koendermann,L., Raaijmakers,A.M., Johnson,J.P. and van der Saag,P.T. (1994) 12-o-tetradecanoylphorbol-13-acetate and tumor necrosis factor α-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. J. Biol. Chem., 269, 6185–6192. [PubMed] [Google Scholar]
- Watts J.D., Gu,M., Polverino,A.J., Patterson,S.D. and Aebersold,R. (1997) Fas-induced apoptosis of T cells occurs independently of ceramide generation. Proc. Natl Acad. Sci. USA, 94, 7292–7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlaschek M., Heinen,G., Poswig,A., Schwarz,T., Krieg,T. and Scharfetter-Kochanek,K. (1994) UVA-induced autocrine stimulation of fibroblast-derived collagenase/MMP1 by interrelated loops of interleukin-1 and interleukin-6. Photochem. Photobiol., 59, 550–556. [DOI] [PubMed] [Google Scholar]
- Zoellner R.A., Morand,O.H. and Ratz,C.R. (1988) A possible role of plasmalogens in protecting animal cells against photosensitized killing. J. Biol. Chem., 263, 11590–11597. [PubMed] [Google Scholar]
- Zumbansen M. and Stoffel,W. (1997) Tumor necrosis factor α activates NF-κB in acid sphingomyelinase-deficient mouse embryonic fibroblasts. J. Biol. Chem., 272, 10904–10909. [DOI] [PubMed] [Google Scholar]