Abstract
BACKGROUND AND PURPOSE
Cystine-knot miniproteins are characterized by a similar molecular structure. Some cystine-knot miniproteins display therapeutically useful biological activities, as antithrombotic agents or tumour growth inhibitors. A critical event in the progression of tumours is the formation of new blood vessels. The aim of this work was to test two tomato cystine-knot miniproteins for their effects on endothelial cell proliferation and angiogenesis in vitro.
EXPERIMENTAL APPROACH
Two tomato cystine-knot miniproteins (TCMPs) were expressed and purified either as recombinant or as native proteins from tomato fruits. The Matrigel assay was used to investigate the effects of TCMPs on in vitro angiogenesis. Viability and proliferation of endothelial cells were tested. Extracellular signal-regulated kinase (ERK)1/2 phosphorylation was assayed in either HUVEC or A431 epidermal growth factor receptor (EGFR)-overexpressing cells treated with TCMPs. EGFR phosphorylation was tested in A431 cells.
KEY RESULTS
Both recombinant and native TCMPs inhibited in vitro angiogenesis of HUVEC cells at concentrations of 15–100 nM. The anti-angiogenic effect of TCMPs was associated with the inhibition of ERK phosphorylation. The two miniproteins did not alter the viability and proliferation of the endothelial cells.
CONCLUSIONS AND IMPLICATIONS
The anti-angiogenetic properties of TCMPs are of potential pharmacological interest because they are common and natural components of the human diet, they possess low toxicity, they are active at submicromolar concentrations, they share a common molecular structure that can be used as a molecular platform for the design of molecules with enhanced biological activity.
Keywords: cystine-knot miniproteins, angiogenesis, MAPK pathway, tomato fruit
Introduction
Tomato was introduced into the human diet approximately 500 years ago (Mattioli, 1568) and during the last two centuries has become one of the most important horticultural crops due to the worldwide consumption of its fruit. Several studies have indicated that regular consumption of tomato fruit is consistently associated with lower risk of several types of cancer and vascular diseases (Hwang and Bowen, 2002; Etminan et al., 2004). The active principles of tomato fruit have eagerly been searched, and yet their identification is incomplete. Beta-carotene and lycopene have been indicated as putative active principles; however, evidence for their role as the main cardioprotective and/or anticancer principle is either negative or not conclusive (Canene-Adams et al., 2007). In fact, evidence suggests that lycopene by itself might have some antiprostate cancer activity, but tomato fruit has anticancer effects superior to those of pure carotenoid(s) (Canene-Adams et al., 2007). Other bioactive compounds present in tomato fruit might contribute to the anti-cancer and anti-inflammatory properties of tomato fruit. Peptides and proteins present in tomato fruit have received little attention even though such compounds might have biological activity in human cells.
Cystine-knot miniproteins are members of a large family of cysteine-rich proteins widespread in eukaryotes. Cystine-knot miniproteins typically consist of less than 50 amino acids and they have a unique three-dimensional structure characterized by three intra-molecular disulphide bonds forming a cystine-knot and a small tripled stranded β-sheet (Craik, 2001). The cystine-knot acts as a structural scaffold for the whole protein or for one of its domains conferring to the cystine-knot miniproteins a compact and remarkably stable structure against extreme pH, chemical and thermal denaturation, and proteolytic attack (Ireland et al., 2006; Werle et al., 2007). Naturally-occurring cystine-knot miniproteins, despite their similar molecular structure, feature a multitude of different biological functions. In animals, cystine-knot miniproteins include peptides that function as extracellular ligands and regulate numerous cellular functions such as cell growth and development (Vitt et al., 2001). In plants, cystine-knot miniproteins are often involved in resistance to pathogens with the function of protease inhibitors and/or as elicitors of defence responses against pathogens (Norton and Pallaghy, 1998; Quilis et al., 2007). A cystine-knot protease inhibitor was first discovered in 1980 in potato and it was shown that this miniprotein acts as a carboxypeptidase inhibitor (Rees and Lipscomb, 1980). The potato metallocarboxypeptidase inhibitor (PCI) is a cystine-knot miniprotein of 39 amino acids. Other putative cystine-knot metallocarboxypeptidase inhibitors with homologous sequences and a conserved cysteine-based scaffold have been identified in potato and tomato.
Cystine-knot miniproteins display a broad spectrum of therapeutically useful natural biological activities and several members of this family are marketed as therapeutics or are in clinical development (Gustafson et al., 2004; Krause et al., 2007). Potato PCI inhibits mammalian pancreatic carboxypeptidase and can act as antithrombotic agent (Wang et al., 2006). It has been shown that PCI also functions as an inhibitor of pancreatic adenocarcinoma cell growth, acting as an antagonist of the human epidermal growth factor (EGF) by binding to the EGF receptor (EGFR) (Blanco-Aparicio et al., 1998; Sitja-Arnau et al., 2005).
Angiogenesis is the formation of new blood vessels from pre-existing vessels to form capillary networks. It is a fundamental event of physiological processes, like organogenesis, wound healing and muscle growth (Carmeliet, 2005). Angiogenesis also takes place in pathological situations and promotes tumour growth and progression, metastasis as well as diabetic retinopathy and psoriasis (Folkman, 1995). Angiogenesis involves a complex balance of positive and negative regulators. Vascular endothelial growth factor (VEGF-A) is one of the most important pro-angiogenic factors involved in angiogenesis and acts through the activation of VEGF receptors (VEGFR). Stimulation of tumour and physiological angiogenesis is also regulated by EGFR activation (Amin et al., 2006). For instance, VEGF-A can induce other pro-angiogenic factors, such as heparin-binding EGF (HB-EGF), which promotes angiogenesis in endothelial cells via EGFR activation (Mehta and Besner, 2007). Furthermore, the angiotensin II-mediated cross-activation of EGFR is responsible for neo-angiogenesis in endothelial cells and for the expression of VEGF-A (Fujiyama et al., 2001).
Here we show that two cystine-knot miniproteins of tomato, named TCMP-1 and TCMP-2, identified almost 20 years ago (Pear et al., 1989; Martineau et al., 1991), have an anti-angiogenic effect on endothelial cells in vitro. TCMP-1 amino acid sequence is 71% identical to the amino acid sequence of the cystine-knot carboxypeptidase inhibitor of potato PCI. TCMP-1 amino acid sequence is 32% identical to TCMP-2. Both recombinant TCMP-1 and TCMP-2 and native TCMPs purified from mature tomato fruit inhibited angiogenesis in vitro at concentrations in the nanomolar range. The miniproteins do not exert toxic effects on endothelial cell proliferation and viability. Moreover, TCMP miniproteins antagonized EGF-induced extracellular signal-regulated kinase (ERK) activation.
Methods
Plant materials
UC82 tomato plants were grown in a greenhouse under a 10/14 h light/dark cycle at 24°C and 18°C respectively. UC82 is a commercially available tomato cultivar used by the processing industry.
Northern blot analysis
Total RNA was isolated with Trizol (Invitrogen Ltd., Paisley, UK) and 20 µg of total RNA were separated on 1% agarose-formaldehyde denaturing gels. The gels were blotted overnight on Hybond N+ membrane (Amersham Biosciences, GE Healthcare Europe GmbH, Munich, Germany) in 10× SSC. The DNA probes were labelled with (α-32P)-dCTP using ‘Ready to go DNA labelling beads (-dCTP)’ (Amersham Biosciences, GE Healthcare Europe GmbH). Unincorporated nucleotides were removed with Probe G-50 microcolumns (Amersham Biosciences, GE Healthcare). The membranes were hybridized overnight at 42°C in ULTRAhyb buffer (Applied Biosystem/Ambion, Austin, TX, USA). Then 106 cpm·mL−1 of labelled probe was added to the hybridization buffer. The membranes were washed twice in 2× SSC/0.1% sodium dodecyl sulfate (SDS) for 5 min and twice in 0.1× SSC/0.1% SDS for 15 min at 42°C. Autoradiography was then performed using Kodak X-AR5 film (Carestream Health Inc., Rochester, NY, USA). For TCMP-1, TCMP-2 and actin mRNAs analysis, the DNA probes were obtained by PCR using the following forward (F) and reverse (R) primers: for TCMP-1 (F 5′-ATGGCACAAAAATTTACTATCCTTTTCACC-3′ and R 5′-GATTACATATCACACCCTAATGACATAATT-3′), for TCMP-2 (F 5′-TGAAGCTACTTCCCACAAATATTTTG-3′ and R 5′-TCCCTTTATTCATATTCTTCACACC-3′) and for actin (F 5′-CCCGTTCAGCAGTGGTGGT-3′ and R 5′-TACGAGGGTTATGCTTTGCC-3′).
Cloning and expression of recombinant TCMP-1 and TCMP-2
The DNA sequences corresponding to the coding regions of the mature TCMP-1 and TCMP-2 protein were amplified by PCR using cDNAs as a template. The upstream and downstream primers were designed in order to introduce at the 5′-terminal a restriction site for NdeI and a His6-tag sequence and at the 3′-terminal end two stop codons and a restriction site for BamHI. The primers sequences are listed as follows: TCMP-1 forward: 5′GGGAATTCCATATGCATCATCATCATCATCACCAGCAATATGATCCAGTTTGTCACAAACCT-3′; TCMP-1 reverse: 5′-CGGGATCCTTATTAAACATAGGGCCCACATGTCCCCGCGAA-3′; TCMP-2 forward: 5′-TCCTGTAACCATATGCATCATCATCATCATCACACAAATATTTTGGGACTTTGTAACGAACCT-3′; TCMP-2 reverse: 5′-TGGGATCCTTATTAAGGCAACAGGTTGCATGTACGGTATGT-3′ (sequences for restriction sites are reported in italics, the His6-tag sequence is in bold and sequences for stop codons are underlined). PCR products were subcloned using pGEM®-T Easy Vector System (Promega GmbH, Mannheim, Germany) and checked by sequencing. The PCR products were double-digested with NdeI-BamHI and the resulting fragments were ligated into pET12B vector (Novagen, Merck Chemicals Ltd., Nottingham, UK). The recombinant plasmids were named pET12-T1 (for TCMP-1) and pET12-T2 (for TCMP-2). Origami B and BL21 pLysS (Novagen, Merck Chemicals Ltd, Nottingham, UK) Escherichia coli (DE3) competent cells were transformed with pET12-T1 and pET12-T2 plasmids respectively. For the TCMP-1, the expression of recombinant protein was induced by 0.7 mM of isopropyl-β-D-thiogalactopyranoside (IPTG) at 24°C for 5 h. For the TCMP-2, the expression of the recombinant protein was induced by 0.4 mM IPTG at 37°C for 5 h.
Purification of recombinant TCMP-1 and TCMP-2
For the isolation of TCMP-1 and TCMP-2 recombinant proteins, cell cultures were centrifuged at 10 000× g for 10 min at 4°C. The cell pellets were resuspended in lysis buffer (10 mM Tris-HCl pH 7.5, 50 mM NaCl and 5% glycerol). Cell suspensions were lysed by lysozyme (0.1 mg·mL−1), by repeated freeze thawing (three times) and by mild sonication. DNase (20 µg·mL−1) was also added to cell suspensions in order to decrease the viscosity of the samples. The insoluble fraction, containing aggregated target protein (inclusion bodies) was recovered by centrifugation at 16 000× g for 20 min at 4°C. The pellets were washed three times with wash buffer (20 mM Tris-HCl pH 8.0, 50 mM NaCl, 60 mM 2-mercaptoethanol, 5 mM EDTA, 2% Triton). Inclusion bodies were resuspended in the solubilization buffer (20 mM Tris-HCl pH 8.0, 0.5 M NaCl, 6 M guanidine hydrochloride, 50 mM 2-mercaptoethanol, and 10 mM imidazole). The recombinant proteins (His-tagged) were affinity purified using Ni2+-loaded HiTrap Chelating column (Amersham Biosciences, GE Healthcare Europe GmbH). The miniproteins were refolded on a column with a decreasing gradient (0.1 mL·min−1) of guanidine hydrochloride, from 6 to 0 M, and 2-mercaptoethanol, from 50 mM to 5 mM. Refolded TCMPs were eluted with an increasing gradient (0.5 mL·min−1) of imidazole, from 10 mM to 0.8 M, and the eluted fractions were loaded on a polyacrylamide gel and stained. Fractions containing the miniproteins of interest were pooled, dialysed and concentrated by Amicon stirred cells with a 1 kDa cut-off, and this material was used in all subsequent experiments (Ultrafiltration Membranes, Millipore, Bedford, MA, USA). Purified protein concentrations were determined by the Bradford method (Bradford, 1976), using bovine serum albumin (BSA) as standard. In order to verify the refolding procedure applied to the recombinant proteins, reverse phase HPLC analysis was carried out after purification. The samples were separated by RP-HPLC performed by a Beckman System Gold chromatographer (Beckman Coulter, Fullerton, CA, USA), on an Altech Alltima C4-WP column (4.6 mm × 250 mm, particle size 5 µm) (Delta Technical Products, Des Plaines, IL, USA). Absorbance was monitored at 214 nm by a Beckman 168 Diode Array Detector. Proteins were loaded in 0.1% trifluoroacetic acid–20% acetonitrile (ACN) and eluted by a linear ACN gradient from 20 to 60% in 40 min. The flow rate was 1 mL·min−1. The elution profile of TCMP-2 show a prevalent peak that differs from that obtained after overnight treatment with either 50 mM DTT or 50 mM DTT and iodoacetamide (Supporting Information Figure S1), indicating the presence of a single oxidized form of TCMP.
Purification of TCMP from tomato
Ripe tomatoes were peeled and, after seed removal, homogenized with 1 mL·g−1 of extraction buffer (30 mM Tris-HCl pH 8.2, 50 mM KCl, 1 mM EDTA, 5 mM 2-mercaptoethanol, 0.19% EGTA, 0.5% Tween-20, 0.1% PVPP and protease inhibitors cocktail). The homogenate was centrifuged at 12000× g for 20 min at 4°C and the supernatant was collected. TCMP was affinity purified from total extract using either IgG antibodies or carboxypeptidase A (CPA), which were previously immobilized on columns adopting AminoLink Plus Immobilization Kit (Thermo Scientific-Pierce Protein Research Products, Rockford, IL, USA).
Carboxypeptidase inhibition assay
Inhibition of carboxypeptidase activity was assayed using 28 nM bovine CPA and 1 mM hippuryl-L-phenylalanine (Sigma-Aldrich, St. Louis, MO, USA) as substrates. Assays were performed as described previously (Hass and Ryan, 1982), monitoring the substrate hydrolysis at 254 nm in 20 mM Tris-HCl buffer (pH 7.5) containing 0.5 M NaCl. BSA was used as negative control.
Human cell culture
Human umbilical vein endothelial cells (HUVECs) (Clonetics, Lonza, Basel, Switzerland) were plated on a 25 cm2 flask coated with bovine fibronectin (Sigma-Aldrich). Cells were maintained on endothelial growth media (EGM-2) supplemented with EGM-2 Single Quots (Clonetics, Lonza, Basel, Switzerland) at 37°C and 5% CO2 in a humidified atmosphere. The human vulvar epidermoid A431 cells were plated on a 25 cm2 flask. Cells were maintained on RPMI 1640 medium in the presence of 10% foetal bovine serum and 50 µg·mL−1 gentamicin (Sigma-Aldrich) at 37°C and 5% CO2 in a humidified atmosphere. For all applications, HUVEC and A431 cells were used from second to fourth passages.
In vitro capillary formation of HUVEC plated on Matrigel
The assessment of in vitro capillary formation was performed using a Matrigel basement membrane matrix (BD Biosciences, San Jose, CA, USA). The wells of 24-well plates were coated with Matrigel under sterile conditions. After the Matrigel suspension (300 µL per well) had gelled for 1 h at 37°C, 4 × 104 HUVEC were plated in each well and incubated for 24 h at 37°C and 5% CO2 in a humidified atmosphere in 0.7 mL of EGM-2 complete medium with or without TCMPs (100 and 200 nM) or AG1478 (tyrphostin, N-(3-chlorophenyl)-6,7-dimethoxy-4-quinazolinamine) (500 nM), an inhibitor of EGF receptor kinase. Cells were photographed using a phase contrast microscope (Olympus CK2) (Olympus Corporation, Tokyo, Japan) coupled with D-100 Nikon digital camera (Nikon Corporation, Tokyo Japan) after 3, 6 and 24 h after cell plating. Tube formation was quantified by measuring the length of tube-like structures with the image-processing software ImageJ (http://rsb.info.nih.gov/ij/). For each condition, three to five wells were plated. For each well, tube length measurement was carried out, analysing three randomly-chosen fields (2.00 × 1.33 mm; magnification 40×).
Cell viability and cell proliferation assays
Cell viability assay was performed using a WST-8 (MTT) cell proliferation assay kit (Cayman Chemical Company, Ann Arbor, MI, USA), AnnexinV-FITC apoptosis detection kit (Bender MedSystem, Vienna, Austria) and trypan blue test.
For the MTT assay, HUVECs were either treated or not with experimental compounds (200 nM). Cells were seeded, at a density of 1 × 104 cells per well, in a 96 well plate. After 24 and 48 h of growth, cell viability was evaluated. The assay was performed according to manufacturer's instructions.
The AnnexinV-FITC assay was also performed in triplicate and according to the manufacturer's recommendations. HUVECs were seeded at a density of 3 × 104 cells per well, in a 6-well plate. After 48 h of growth, cells were stained with annexinV, that binds phosphatidylserine, and then with propidium iodide. Stained cells, at least 10 000 events, were acquired on a FACSCalibur™ flow cytometer (BD Biosciences) and analysed by FlowJo software (TreeStar, Ashland, OR, USA).
For the trypan blue test, HUVECs were seeded, at a density of 48 × 103 cells per well, in 6-well plates. EGM-2 Single Quots and recombinant peptides were added to the endothelial growth medium. After 48 h of cultivation, cells were treated with trypsin, collected and resuspended in 0.5 mL of serum-free culture medium. The 0.1 mL of 0.4% trypan blue (Sigma-Aldrich) was added to the cell suspension and mixed thoroughly. After a 5 min incubation at room temperature, trypan blue/cell mixture was applied to a haemocytometer and placed under an Olympus CK2 microscope (100×). The unstained (viable) and blue stained (non-viable) cells were counted.
Cell proliferation was measured by counting the cell numbers and by 5-ethynyl-2′-deoxyuridine (EdU; Click-iT™ EdU Alexa Fluor Cell Proliferation Assay kit, Invitrogen Ltd., Paisley, UK). HUVECs were seeded, at a density of 3 × 104 cells per well, in a 6-well plate coated with bovine fibronectin in culture medium containing the recombinant TCMP-2 (170 nM). After 24, 48, 72 and 96 h of cultivation, cells were counted in Neubauer chamber using optical microscope Olympus CK2 (100×).
For the EdU assay, HUVEC were seeded, at a density of 3 × 104 cells per well, in a 6-well plate coated with bovine fibronectin in culture medium with or without the recombinant TCMP-2 (200 nM) and 10 µM EdU. After 48 h, cells were detached and washed once with phosphate buffer before being fixed and permeabilized according to the manufacturer's protocol. Stained cells, at least 10 000 events, were acquired on a FACSCalibur™ flow cytometer (BD Biosciences) and analysed with the use of FlowJo software (TreeStar).
Cell migration assay
Migration assay was performed by modifying the method described by van Horssen et al. (2006). Briefly, HUVEC were cultured until confluence in EGM-2 medium on fibronectin-coated glass cover slips. Cover slips were then transferred into a fibronectin-treated 6-well plate and incubated in EGM-2 either in the presence or absence of AG1478 500 nM or TCMP-2 100 nM. After 12 h, migration of the cells was monitored by an inverted microscope (Olympus CK2) and images were recorded with D-100 Nikon digital camera. For each condition, three wells were analysed. In each well, cells were counted by analysing four randomly-chosen fields (magnification 40×).
SDS- polyacrylamide gel (PAGE) and Western blot assays
For TCMPs analyses, protein samples were loaded on 15% Tris-Tricine SDS-PAGE and transferred to an Immobilon PVDF membrane (Millipore). Membranes were incubated with primary polyclonal antibodies produced in rabbit (PRIMM s.r.l., Milano, Italy), using as an antigen either the purified recombinant TCMP-1 or a mixture of 17 and 20 amino acid-long synthetic peptides corresponding to the N-terminal (QQYDPVCHKPCSTQDDC) and C-terminal (SGGTFCQACWRFAGTCGPYV) ends of TCMP-1. The binding of secondary anti-rabbit antibodies was detected using the chromogenic substrate nitro-blue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (BCIP).
For signalling pathway analyses, HUVEC and A431 cells overexpressing EGFR (Graness et al., 2000) were treated with different concentrations of TCMPs alone or in combination with 5 ng·mL−1 EGF (PeproTech, London, UK) or with the specific EGFR inhibitor AG1478 (Calbiochem, Gibbstown, NJ, USA) (500 nM). Cells samples were loaded onto a 7.5 or 10% Tris-glycine SDS-PAGE and proteins transferred to a PVDF membrane.
The level of total and phosphorylated EGFR (ErbB1) was detected in A431 cells by using EGFR and phospho-EGFR Tyr1173 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) respectively. The level of total and phosphorylated ERK1/2 was detected using ERK1/2 and phospho-ERK1/2 antibodies (Cell Signalling Technology, Danvers, MA, USA) respectively. Blots were developed using chemiluminescent substrate Lite A Blot Plus (Euroclone S.p.A., Siziano, Italy) using horseradish peroxidase (HRP)-conjugated secondary antibodies.
Receptor and drug nomenclature conforms to BJP's Guide to Receptor and Channels (Alexander et al., 2008).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4.02 software. When multiple variables were analysed the one-way anova was applied followed by Bonferroni's test for pairwise comparison as post hoc analysis. Two-way anova for repeated measures followed by Bonferroni's test was applied when the effects of treatments at different time points were compared (as for data shown in Figure 3C). Student's t-test for unpaired data was applied when two independent variables were compared as is the case of data shown in Figures 3B, D, 4 and 5, and a P value < 0.05 was accepted as statistically significant. Data are presented in the figures as mean and standard deviation.
Figure 3.
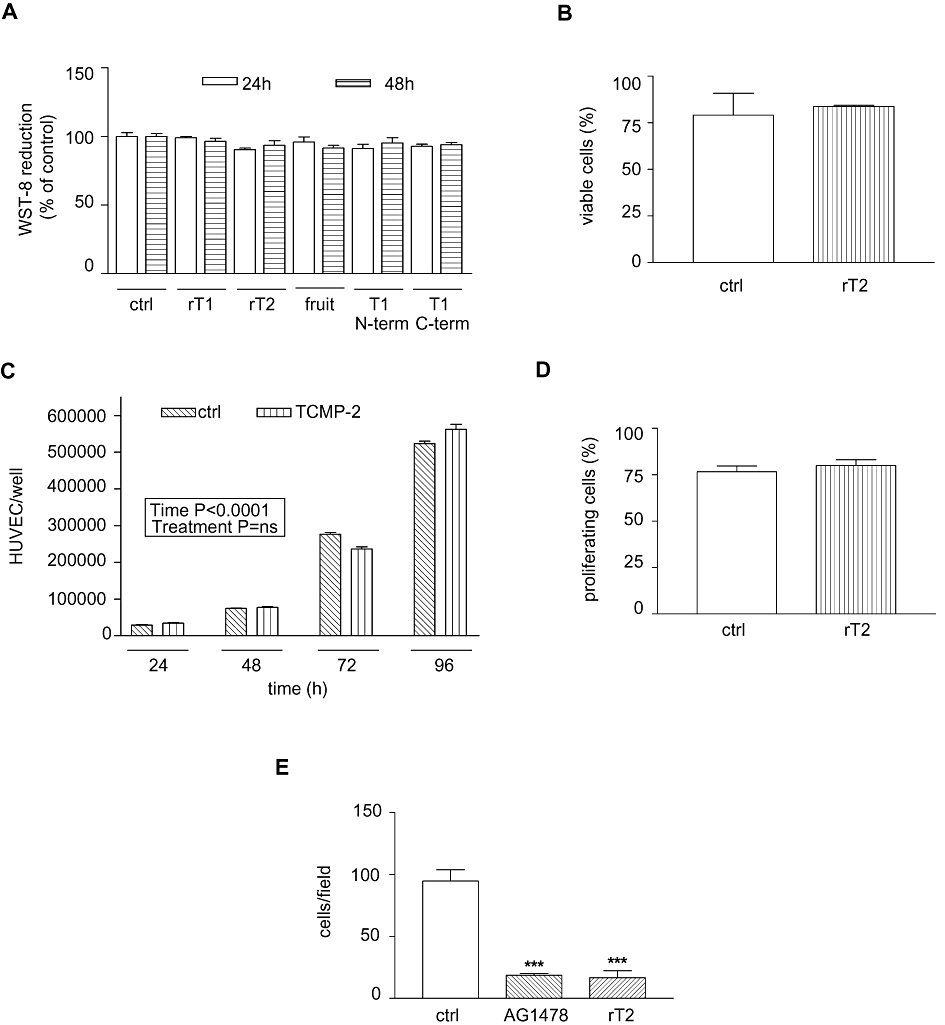
Effects of tomato cystine-knot miniproteins on HUVEC viability and proliferation. (A) WST-8 (MTT) cell proliferation assay. Number of live cells measured after 24 and 48 h of treatment is shown (percentage of control value, 3 independent experiments). Statistical analysis was performed using one-way anova and Bonferroni's tests. (B) Annexin V-propidium iodide assay. The percentage of viable cells after 48 h of treatment with or without 200 nM TCMP-2 is presented (n= 3). Student's t-test was applied. (C) Number of HUVEC per well counted 24, 48, 72, 96 h after treatment with or without 170 nM TCMP-2 (n= 3). The two-way anova for repeated measures was applied. (D) EdU proliferation assay. The percentage of proliferating cells after 48 h of treatment with or without 200 nM TCMP-2 is presented (n= 3). Student's t test was applied. (E) Quantitative analysis of cell migration 12 h after treatment with 100 nM TCMP-2, AG1478 500 nM or no added compounds (n= 3–4 independent experiments, three replicates). The one-way anova and Bonferroni's tests were applied. Data presented in all panels as means ± SD. ***P < 0.001 versus controls. Ctrl, control; rT1, purified recombinant TCMP-1; rT2, purified recombinant TCMP-2; fruit, TCMP miniproteins purified from tomato fruit; T1 N-term and T1 C-term indicate the treatment with peptides corresponding to the N-terminal (17 amino acids in length) or the C-terminal (20 amino acids in length) regions of TCMP-1 respectively.
Figure 4.
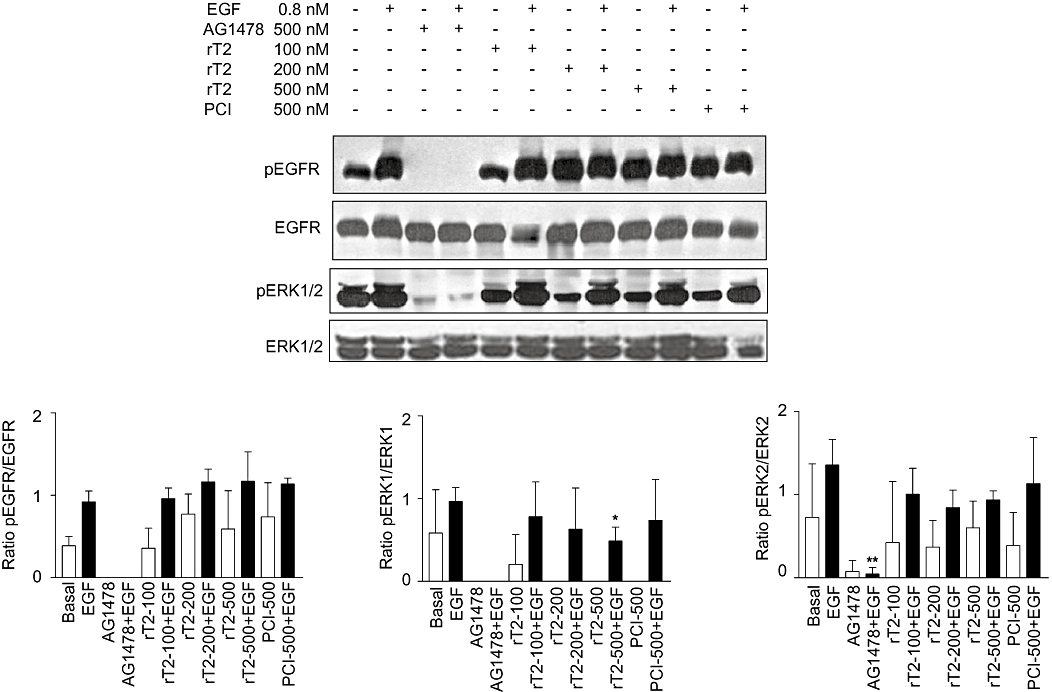
Western blot analysis of p-epidermal growth factor receptor (p-EGFR) and p-extracellular signal-regulated kinase (ERK)1/2 performed on A431 cells treated with TCMPs. EGFR phosphorylation (upper panel); ERK1/2 phophorylation (lower panel). Densitometric analysis performed on Western blots was reported in the graphs. The values represent the ratio between the densities of phosphorylated form and total protein. Data presented as means ± SD (n= 3). Student's t test was applied. *P < 0.05 versus EGF-treated cells. rT2, purified recombinant TCMP-2; PCI, potato carboxypeptidase inhibitor.
Figure 5.
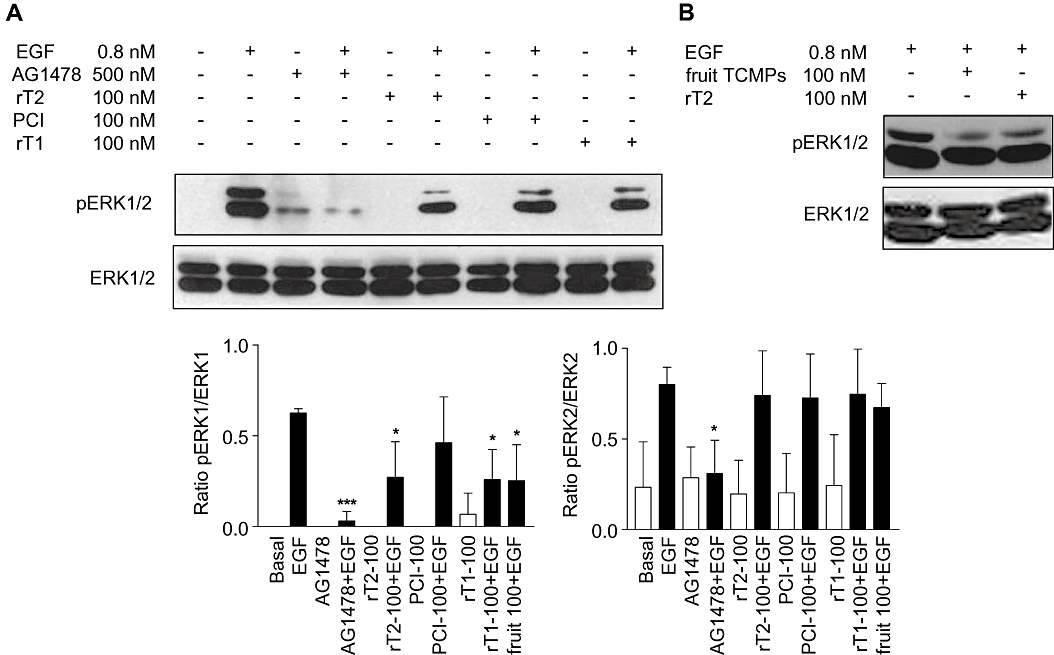
Western blot analysis of p-ERK1/2 (ERK1/2 phosporylation) performed on HUVEC treated with TCMPs. (A) HUVEC treated with recombinant TCMPs. (B) HUVEC treated with TCMP purified from tomato fruit. Densitometric analysis performed on Western blots was reported in the graphs. The values represent the ratio between the densities of phosphorylated form and total protein. Data presented in all panels as mean ± SD (n= 3). Student's t test was applied. *P < 0.05 versus EGF-treated cells; ***P < 0.001. rT2, purified recombinant TCMP-2; PCI, potato carboxypeptidase inhibitor; rT1, purified recombinant TCMP-1, fruit TCMP, TCMP miniproteins purified from tomato fruit; ERK, extracellular signal-regulated kinase.
Results
Expression of recombinant tomato cystine-knot miniproteins TCMP-1 and TCMP-2
In order to test the biological activity of two tomato cystine-knot miniproteins (TCMP-1 i.d. CAA41973; TCMP-2 i.d. AAA34129), they have been expressed and purified either as recombinant proteins using a bacterial expression system or purified as native proteins from tomato fruit.
TCMP-1 is a miniprotein consisting, in its mature form, of 37 amino acids. TCMP-1 is homologous (71% identity) to a cystine-knot carboxypepdidase inhibitor of potato (PCI i.d. AAC95130) (Hass and Hermodson, 1981; Figure 1A, upper). The TCMP-1 precursor has an additional N-terminal sequence 32 amino acids long and an extension of 8 amino acids at the C-terminus (Martineau et al., 1991). The hydrophobic N-terminal region represents a signal sequence for the transport across the rough endoplasmic reticulum (Martineau et al., 1991). In the mature region of TCMP-1 and potato PCI, the number and the spacing of the 6 cysteine residues is highly conserved suggesting that the two miniproteins share a similar structure (Figure 1A, upper). TCMP-1 gene is expressed in flowers and during early phases of fruit development (Figure 1B). The highest level of TCMP-1 protein is detected 4–5 days after flower anthesis (Figure 1B).
Figure 1.
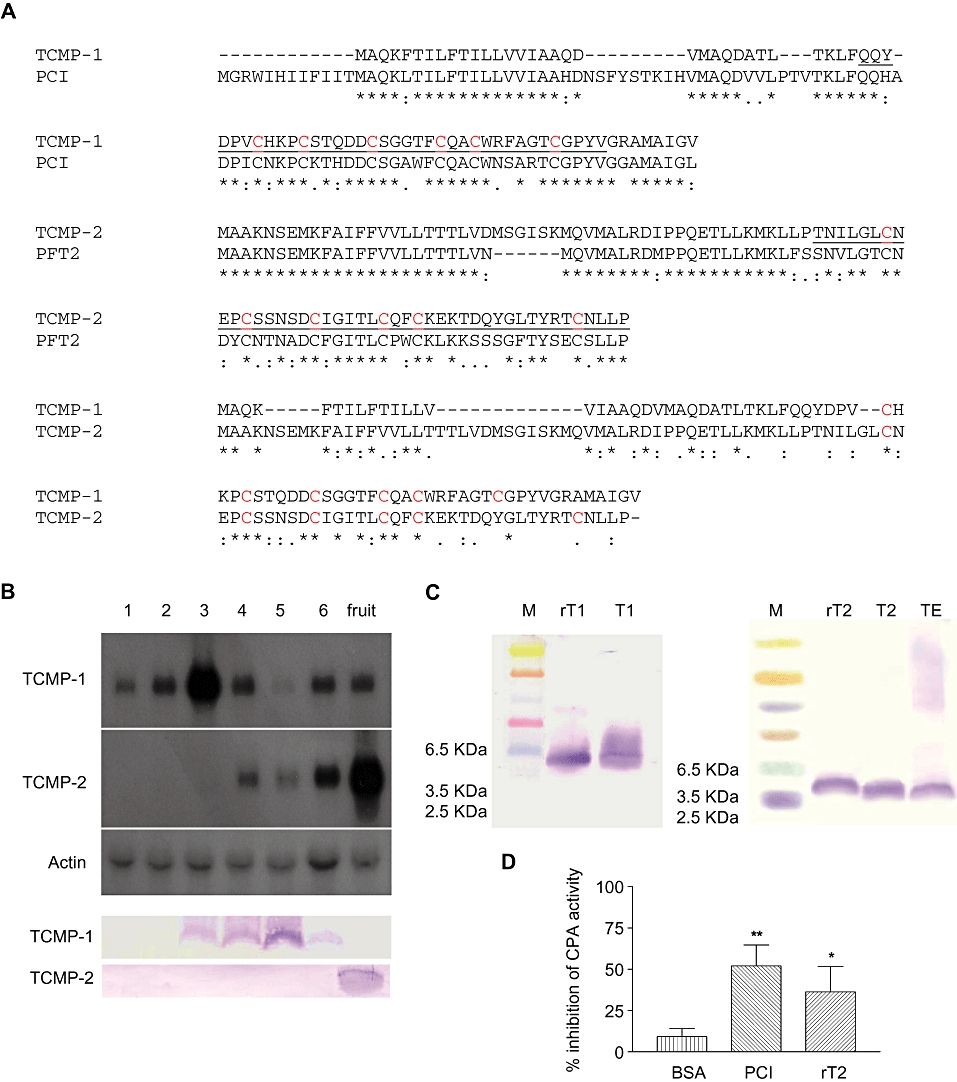
(A) Pairwise alignments of the deduced amino acid sequences of tomato TCMP-1 miniprotein and the potato metallocarboxypeptidase inhibitor PCI (i.d. AAC95130) (upper), tomato TCMP-2 miniprotein and metallocarboxypeptidase inhibitor PFT2 (i.d. BAA21494) (middle) and TCMP-1 and TCMP-2 miniproteins (lower). Conserved cysteine residues are reported in red. (*) Residues identical in all sequences; (:) for conserved substitutions; (.) for semi-conserved substitutions. The sequences of the recombinant proteins are underlined. (B) Northern blot analysis of the TCMPs mRNA at different flower/fruit development stages. The filter was also hybridized with an actin probe (upper). Western blot analysis of the TCMP miniproteins at different flower/fruit development stages (lower). 1: Flower buds 0.5–0.6 cm long; 2: flower buds 0.7–0.9 cm long; 3: flower buds 1.0–1.1 cm long; 4: anthesis and 1 day post anthesis; 5: 4–5 days post anthesis; 6: ovary 0.5–1 cm long; fruit: tomato red fruit. (C) Western blot analysis of recombinant TCMP miniproteins. rT1: purified recombinant TCMP-1; T1: flower bud extract; rT2: purified recombinant TCMP-2; T2: TCMP miniproteins purified from tomato fruit; TE: tomato fruit crude extract. (D) Inhibitory effect of TCMP-2 on bovine carboxypeptidase A activity. PCI: potato carboxypeptidase inhibitor, rT2: 200 nM recombinant TCMP-2; Data are presented as means ± SD (n= 3–6). Statistical analysis was performed by one-way anova and Bonferroni's multiple comparison as post hoc test **P < 0.01, *P < 0.05 versus bovine serum albumin (BSA).
The second tomato cystine-knot miniprotein analysed (TCMP-2) is fruit-specific (Pear et al., 1989). TCMP-2 2A11 gene is expressed in flowers after anthesis and in fruits; the maximum level of TCMP-2 miniprotein is detected at fruit maturity (Pear et al., 1989; Figure 1B). The putative TCMP-2 miniprotein – 96 amino acids in length – contains a predicted N-terminal signal peptide (Pear et al., 1989). The apparent molecular mass of TCMP-2 in tomato fruit crude extract is around 3–4 kDa, this finding is consistent with the hypothesis of the presence of a signal peptide (Figure 1C).
TCMP-2 is highly similar to members of a family of metallocarboxypeptidase inhibitors expressed in potato tuber. For instance, it shows 62% amino acid identity with a GM-7 type metallocarboxypeptidase inhibitor (PFT2 gene i.d. BAA21494) (Molnar et al., 2001), a member of the GM-7 gene family (Figure 1A, middle). TCMP-2 is 32% identical to TCMP-1 (Figure 1A, lower).
The mature 37 amino acid-long TCMP-1 miniprotein and 44 amino acid-long C-terminal portion of TCMP-2 miniprotein, containing the cystine-knot motif, were expressed in Escherichia coli as His-tag fusion proteins (Figure 1A). The apparent molecular masses of the recombinant miniproteins on SDS-PAGE were compatible with the mobility expected on the basis of the electrophoretic migration of native mature miniproteins and taking into consideration the His-tail added at the amino terminal end (Figure 1C). TCMP was also extracted from mature tomato fruits and affinity purified against either polyclonal antibody raised against TCMP-1 recombinant protein (Figure 1C) or carboxypeptidase A (Hass and Ryan, 1980). The purified miniproteins showed identical electrophoretic mobility irrespectively of the purification method (data not shown).
Potato carboxypeptidase inhibitor (PCI) competitively inhibits several metallocarboxypeptidases with a Ki in the nanomolar range (Hass and Derr, 1979). A similar inhibitory activity towards metallocarboxypeptidase A (CPA) has been shown with tomato TCMP-1 extracted from wound-induced leaves (Diez-Diaz et al., 2004). Potato GM-7 type miniproteins have been annotated as metallocarboxypeptidase inhibitors on the basis of their sequence similarity with PCI and TCMP-1; nevertheless, their inhibitory activity has not been reported. We observed that the recombinant TCMP-2 miniprotein possesses inhibitory activity against metallocarboxypeptidase A (Figure 1D).
Inhibition of angiogenesis by TCMPs in vitro
Potato PCI is an antagonist analogue of EGF able to inhibit the growth of pancreatic adenocarcinoma cells (Blanco-Aparicio et al., 1998). On the basis of the structural homology between TCMP miniproteins and potato PCI, current knowledge suggests that these miniproteins may display a spectrum of biological effects on human cells related to their predictable activity as an EGF antagonist. EGF and its receptor (EGFR) are involved in many aspects of the development of carcinoma including cell growth and vascularization. EGFR antagonists and blockers can inhibit neo-vascularization during tumour progression (Michaelis et al., 2003). To test the hypothesis that TCMP miniproteins can act as anti-angiogenic compounds, we investigated the effects of TCMP miniproteins, both natural and recombinants, on in vitro angiogenesis using Matrigel matrix. The EGFR inhibitor AG1478 was tested as a positive control. To exclude side effects on capillary tube formation caused by bacterial lipopolysaccharides (LPS), recombinant protein preparations were treated with 10 µg·mL−1 polymyxin B, an antibiotic that binds to and neutralizes LPS (Cardoso et al., 2007). The selective EGF receptor blocker AG1478 exhibited a strong inhibitory effect on tube formation at 500 nM, indicating that EGF signalling pathway activation is a crucial event in capillary network formation. HUVEC cells treated with nanomolar concentrations of TCMP miniproteins were not able to form a complete vascular network within 24 h as compared with control (Figure 2A, B). The inhibitory activity of TCMP miniproteins was already detectable 3 h after cell seeding (Figure 2C). Quantitative analysis of vascular network length was carried out to characterize the anti-angiogenic effect of TCMP miniproteins (Figure 2B). We found a dose-dependent significant reduction of tube length in the presence of TCMP miniproteins at all the concentrations tested (20, 50 and 100 nM). At the highest concentration tested (100 nM), the vascular network length was reduced by 64% (TCMP-1) and 86% (TCMP-2), respectively, as compared with that measured in untreated HUVECs. Native TCMP miniprotein, extracted from mature tomato fruit, inhibited tube formation as well, and the effect was comparable with that of the recombinant miniproteins (Figure 2A, B). The formation of a vascular network was not affected when HUVEC were treated with synthetic peptides corresponding to either the N-terminal (17 amino acids in length) or the C-terminal (20 amino acids in length) regions of TCMP-1 (Figure 2B), suggesting that these two TCMP-1 peptidic fragments do not possess anti-angiogenic activity. To investigate whether TCMP proteins could have a biphasic effect on angiogenesis, that is, are pro-angiogenic at low concentrations and anti-angiogenic at higher concentrations, we tested the effects of TCMP-2 at concentrations lower than 20 nM (5, 10 and 15 nM) (Figure 2C). The lowest inhibitory concentration was 15 nM; TCMP-2 at 5 and 10 nM did not alter the formation of the capillary network (Figure 2C).
Figure 2.
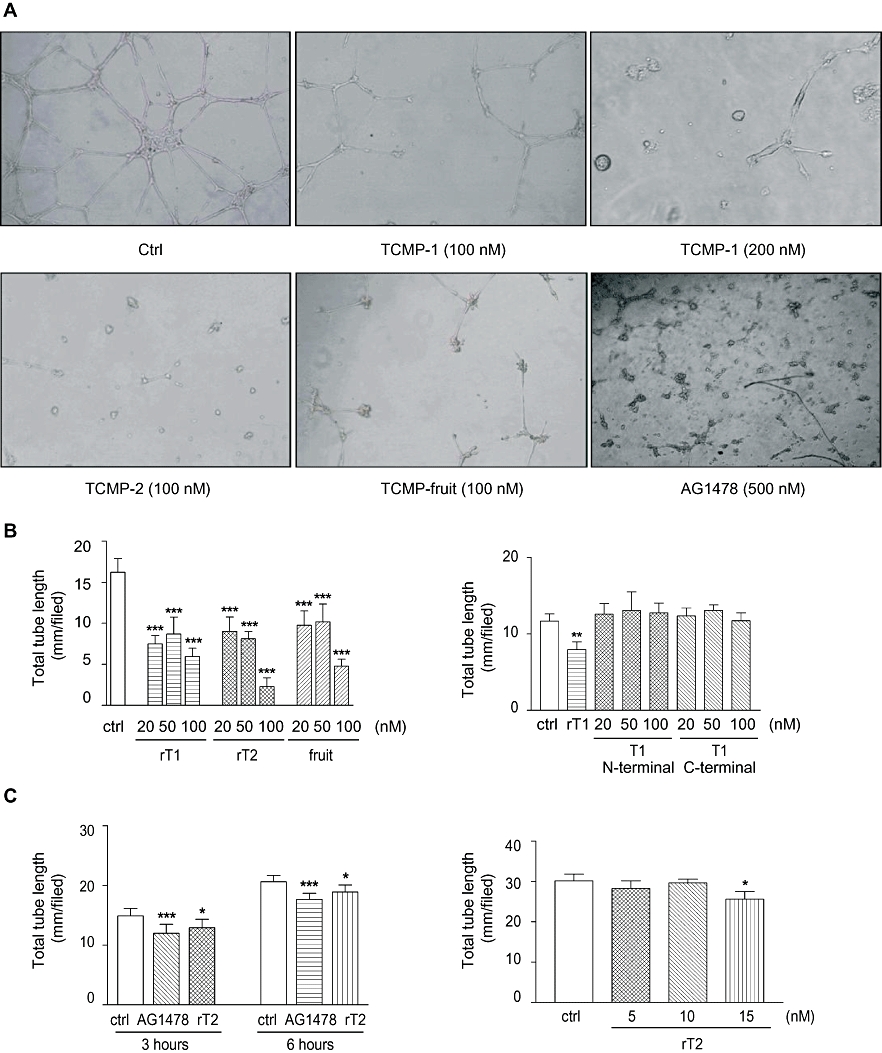
Effects of tomato cystine-knot miniproteins on angiogenesis in vitro. (A) HUVEC were grown in 24-well plates on growth factor reduced Matrigel and treated with either recombinant TCMPs or with TCMPs purified from mature tomato fruits. Endothelial cells were observed 24 h after treatment and results recorded digitally. (B) Quantitative analysis of tube length formation 24 h after treatments with recombinant TCMP-1, recombinant TCMP-2 and TCMPs purified from fruit (left panel) or with the peptides corresponding to the N-terminal or the C-terminal regions of TCMP-1 (right panel). (C) Quantitative analysis of tube length formation 3 and 6 h after treatment (100 nM TCMP-2) (left panel) and quantitative analysis of tube length formation in the presence of 5, 10 and 15 nM TCMP-2 (right panel). Statistical analysis was performed by one-way anova and Bonferroni's multiple comparison as post hoc test. For each variable shown in panels B and D, individual data were entered as replicates of independent experiments performed using a different cell preparation and different protein batches (n= 3). In each experiment 3–5 wells for any tested conditions were assayed. Data shown in (C) were from different experiments performed using one batch of miniproteins (n= 4–8). Data presented in all panels as means ± SD. ***P < 0.001, **P < 0.01, *P < 0.05 versus controls. Ctrl: control; rT1: purified recombinant TCMP-1; rT2: purified recombinant TCMP-2; fruit: TCMP miniproteins purified from tomato fruit; T1 N-terminal and T1 C-terminal indicate the treatment with peptides corresponding to the N-terminal (17 amino acids in length) or the C-terminal (20 amino acids in length) regions of TCMP-1 respectively.
These results indicate that both recombinant and native TCMP miniproteins exibit anti-angiogenic effects at concentrations in the nanomolar range.
Effects of TCMP miniproteins on the viability and proliferation of endothelial cells
Angiogenesis is a multi step process that involves a phase of active proliferation of endothelial cells. In order to test the effects of TCMP miniproteins on the activity of endothelial cells, we treated HUVEC with TCMP miniproteins at a concentration of 200 nM and measured cell viability after 24 and 48 h of culture by MTT assay (WST-8). The recombinant TCMP-1 miniprotein did not have a toxic effect on HUVEC (Figure 3A). A limited reduction in viable cell number (5%, P < 0.05) was detected with recombinant TCMP-2 after 24 h of treatment and with TCMP purified from mature tomato fruit after 48 h of treatment. The two synthetic peptides, corresponding to the first 17 N-terminal amino acids and to the last 20 C-terminal amino acids of TCMP-1, respectively, displayed a similar modest effect on HUVEC viability. The absence of deleterious effects of 200 nM TCMPs on HUVEC viability was further confirmed by Annexin V and propidium iodide test and by the trypan blue exclusion method (Figure 3B and data not shown respectively).
The growth of HUVEC cells was followed up to 96 h in the presence of TCMP-2 at a concentration of 170 nM. This treatment did not affect cell proliferation (Figure 3C).
Cell proliferation was also assayed by the incorporation of EdU, a nucleoside analogue of thymidine, into DNA during active DNA synthesis. Detection is based on a click reaction (Breinbauer and Köhn, 2003; Wang et al., 2003), a copper-catalysed covalent reaction between an azide and an alkyne. The EdU contains the alkyne, while the dye contains the azide. Standard flow cytometry methods are used for determining the percentage of cells in S-phase. This analysis demonstrated that the treatment with 200 nM TCMP-2 for 48 h did not affect the proliferation capability of HUVEC cells (Figure 3D).
On the whole, these data indicate that the anti-angiogenic effect displayed by TCMPs does not depend on the inhibition of either cell proliferation or viability.
Effect of TCMP on cell migration
In the angiogenic process, one of the key events is the migration of endothelial cells towards angiogenic stimuli. To investigate the effects of TCMPs on cell migration we applied a modified barrier-migration assay described by van Horssen et al. (2006). The number of HUVEC cells that moved outside the cover slips within 12 h was significantly reduced for samples treated with either 500 nM AG1478 or 100 nM recombinant TCMP-2 (Figure 3E and Supporting Information Figure S2).
These results suggest that the efficacy of TCMPs in inhibiting angiogenesis is due to a reduction in the invasive potential of cells.
Effect of TCMP on EGFR signalling pathway in A431 cells
EGFR signal transduction cascade is implicated in angiogenesis: oncogenic EGFR contributes to angiogenic phenotype of cancer cells (Al-Nedawi et al., 2009). The cystine-knot PCI at a concentration of approximately 10 µM was reported to interfere with EGFR activation through inhibition of receptor transphosphorylation induced by EGF (Sitja-Arnau et al., 2005). To investigate the mechanism underlying the anti-angiogenic effect displayed by TCMPs, we monitored the phosphorylation state of EGFR in the presence of nanomolar concentrations of TCMP using vulvar carcinoma A431 cells overexpressing EGFR and displayng an activated EGFR signalling pathway (Graness et al., 2000). One fundamental signalling pathway downstream of EGFR activation is mitogen-activated protein kinase (MAPK) cascade. Therefore we examined the phosphorylation state of ERK1/2, one of the main effector proteins downstream of EGFR, to determine whether TCMP affects the MAPK pathway.
A431 cells were treated with nanomolar concentrations of PCI, recombinant TCMP-2 and AG1478 alone and in combination with EGF to test their activity on basal and stimulated EGFR phosphorylation (Figure 4). The EGFR specific inhibitor AG1478 at a concentration of 500 nM completely blocked basal and EGF-induced receptor phosphorylation. PCI at a concentration of 500 nM did not block the autophosphorylation of EGFR. Similarly, recombinant TCMP-2 (100–500 nM) did not curtail EGFR activation at the concentrations tested.
Both PCI and TCMP-2 abolished basal phosphorylation of ERK1, with a limited effect when they were applied in combination with EGF: only at 500 nM TCMP-2, a statistically significant attenuation in ERK1 phosporylation was detected (Figure 4). None of the tested compounds, except A1478, altered basal and stimulated phosphorylation of ERK2 (Figure 4).
These data suggest that TCMP-2 and PCI at concentrations in the nanomolar range interfere with the MAPK signalling pathway affecting ERK1 activation and this is unrelated to the phosphorylation of EGFR (Tyr 1173).
TCMP miniproteins inhibit ERK1/2 phosphorylation in HUVEC
To test whether TCMP miniproteins can interfere with MAPK pathway in endothelial cells, HUVEC cells were treated with nanomolar concentrations of recombinant TCMPs and the phosphorylation level of ERK1/2 was examined with or without EGF. In HUVEC no or little phosphorylation of ERK1/2 was detectable under resting conditions. The recombinant TCMP miniproteins at a concentration of 100 nM blunted EGF-induced ERK1 phosphorylation (Figure 5A). TCMP extracted from mature fruits showed an inhibitory effect on ERK1 phosphorylation similar to that observed with recombinant TCMP-1 and TCMP-2 (Figure 5B). Consistent with the effects of TCMPs in A431 cells (Figure 4), ERK2 phosphorylation was not altered by miniproteins in HUVEC (Figure 5). On the whole, these data suggest that the biological effects exerted by TCMPs on HUVEC are associated with an alteration of the EGF-activated MAPK signalling pathway.
Discussion
The results from the present study show that two cysteine-rich miniproteins derived from either tomato flowers or tomato fruit are endowed with anti-angiogenic activity that is detectable in the nanomolar concentration range. We have addressed the anti-angiogenic activity by testing their effects on angiogenesis in vitro using human-derived endothelial cells. The biological action exhibited by TCMPs extracted from mature tomato fruit was further verified by testing the anti-angiogenic ability of the mature TCMP-1 and TCMP-2 expressed as recombinant proteins in a bacterial expression system. For the anti-angiogenic activity to be present, the integrity of the mature part of the miniproteins was required; neither the N-terminal nor the C-terminal part of TCMP-1 possess biological activity per se, a finding consistent with the hypothesis that the presence of intra-molecular disulphide bonds might be important for the biological function of cystine-knot miniproteins.
The formation of new blood vessels is a multi-step process that includes a phase of endothelial cell proliferation preceding tube formation. The anti-angiogenic activity of TCMPs was not accompanied by any relevant effect on cell proliferation and viability. This implies that the inhibitory effect on angiogenesis is not the consequence of a decreased proliferation capacity, or merely loss of vitality of endothelial cells. The viability of HUVEC cells even in the presence of TCMP concentrations higher than those necessary for inhibiting angiogenesis, indicates a very low cellular toxicity of these miniproteins.
The establishment of new blood vessel is under the control of several paracrine signals whose action is mediated by tyrosine kinase receptors. Regulatory signals include pro-angiogenic factors, namely vascular endothelial cell growth factor (VEGF-A), fibroblast growth factor-2 (FGF-2), EGF and anti-angiogenic factors. TCMP-1 is highly homologous to a cystine-knot miniproteins of potato (PCI) that acts as EGFR antagonist inhibiting the transphosphorylation and internalization of EGFR at micromolar concentrations (Sitja-Arnau et al., 2005). The hypothesis that TCMP anti-angiogenic action could be linked to inhibition of EGFR transphosphorylation is not supported by our experimental data. Nanomolar concentrations of TCMP did not inhibit basal and EGF-stimulated EGFR phosphorylation in A431 cell line. However, in both HUVEC and A431 lines threonine phosphorylation of ERK1 was inhibited. Phosphorylation of ERK1/2 occurs after the engagement of EGFR in human endothelial cells (Mehta and Besner, 2007) but also activation of signal transduction pathway following either FGF or VEGF-A binding involves the phosphorylation of ERK1/2, which is required for tube formation (Slevin et al., 2000).
The inhibitory effect observed on angiogenesis is consistent with inhibition of ERK1 phosphorylation. Based on the available evidence, TCMPs should act via binding to EGFRs, although a direct or indirect action on MAP kinase signalling triggered by either FGF or VEGF-A receptors cannot be excluded. Research on TCMP binding to EGFRs and on the downstream signalling pathways involved needs to be further extended. Presently, we can only infer that inhibition via ERK of downstream signalling is implicated in the anti-angiogenic effects of miniproteins and occurs independently of the phosphorylation state of EGFR in A431 cells (Mehta and Besner, 2007).
TCMP-1 and PCI are known to be inhibitors of metallocarboxypeptidases. We have shown that TCMP-2 also possesses such biochemical activity. The formation of new vessels requires the degradation of the basement membrane and the removal of obstructing matrix proteins to allow the formation of the vessel lumen by the intervention of matrix metalloproteinases (MMPs). Furthermore, members of a family of membrane-anchored metalloproteases, known as ‘a disintegrin and metalloprotease’ (ADAM) proteins, which are key components in protein ectodomain shedding, have been implicated in angiogenesis (Blobel, 2005). ADAM15 has been found to have a role in pathological neovascularization in a mouse model for retinopathy of prematurity, even though it is not required for developmental angiogenesis or adult homeostasis (Horiuchi et al., 2003). It will be interesting to test whether the metalloendopeptidase inhibitor activity of TCMP proteins might contribute to their anti-angiogenic properties in vivo.
In summary, we have demonstrated that native and recombinant TCMPs are active as anti-angiogenic compounds in vitro at nanomolar concentrations. Furthermore, as TCMP-2 is derived from edible fruits this compound should be endowed with low toxicity when tested in animal models of disease and in humans. Neovascularization of tumours could represent a target for therapeutic intervention with tomato miniproteins, and their anti-angiogenic activity could also be used to prevent atherosclerosis and thrombosis. Miniproteins from tomato displaying anti-angiogenic properties in vitro may be of potential pharmacological interest and, in the case of TMCP-2 are a common component of human diet. To ascertain whether dietary consumption of tomato-derived foods (e.g. tomato sauce) is compatible with biological activity of cystine-knot miniproteins in vivo as we found in vitro requires further and specific experimental evaluation.
Acknowledgments
We thank Dr. Gianni Zoccatelli for the HPLC analysis of TCMP. This study was supported by research grants from the Italian Ministry of University and Research and the University of Verona (to PM) and by a fellowship from the Consorzio per gli Studi Universitari di Verona.
Glossary
Abbreviations
- ADAM
a disintegrin and metalloprotease
- CPA
bovine carboxypeptidase A
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- HUVEC
human umbilical vein endothelial cell
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PCI
potato metallocarboxypeptidase inhibitor
- TCMP
tomato cystine-knot miniprotein
Conflicts of interest
The authors declare no conflict of interest.
Supplementary material
Additional Supporting Information may be found in the online version of this article:
Supporting Information: Teaching Materials; Figs 1–5 as PowerPoint slide.
Figure S1 RP-HPLC analysis of TCMP. Elution profile of purified recombinant TCMP-2. Green line: recombinant TCMP-2; blue line: recombinant TCMP-2 after an overnight treatment with 50 mM DTT; red line: recombinant TCMP-2 after an overnight treatment with 50 mM DTT and iodoacetamide (see the Methods for details).
Figure S2 Cell migration assay. Pictures showing HUVEC migration monitored after 12 h by an inverted microscope, either in the presence or in the absence of AG1478 (500 nM) or TCMP-2 (100 nM). Ctrl: control after 12 h; AG1478: AG1478-treated cells after 12 h; rT2: recombinant TCMP-2-treated cells after 12 h.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn) 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- Blanco-Aparicio C, Molina MA, Fernandez-Salas E, Frazier ML, Mas JM, Querol E, et al. Potato carboxypeptidase inhibitor, a T-knot protein, is an epidermal growth factor antagonist that inhibits tumor cell growth. J Biol Chem. 1998;273:12370–12377. doi: 10.1074/jbc.273.20.12370. [DOI] [PubMed] [Google Scholar]
- Blobel CP. Adams: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities 434 utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breinbauer R, Köhn M. Azide-alkyne coupling: a powerful reaction for bioconjugate chemistry. Chembiochem. 2003;4:1147–1149. doi: 10.1002/cbic.200300705. [DOI] [PubMed] [Google Scholar]
- Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67:836–843. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- Cardoso LS, Araujo MI, Goes AM, Pacifico LG, Oliveira RR, Oliveira SC. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb Cell Fact. 2007;6:1. doi: 10.1186/1475-2859-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Craik DJ. Plant cyclotides: circular, knotted peptide toxins. Toxicon. 2001;39:1809–1813. doi: 10.1016/s0041-0101(01)00129-5. [DOI] [PubMed] [Google Scholar]
- Diez-Diaz M, Conejero V, Rodrigo I, Pearce G, Ryan CA. Isolation and characterization of wound-inducible carboxypeptidase inhibitor from tomato leaves. Phytochemistry. 2004;65:1919–1924. doi: 10.1016/j.phytochem.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Etminan M, Takkouche B, Caamano-Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2004;13:340–345. [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Fujiyama S, Matsubara H, Nozawa Y, Maruyama K, Mori Y, Tsutsumi Y, et al. Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ Res. 2001;88:22–29. doi: 10.1161/01.res.88.1.22. [DOI] [PubMed] [Google Scholar]
- Graness A, Hanke S, Boehmer FD, Presek P, Liebmann C. Protein-tyrosine-phosphatase-mediated epidermal growth factor (EGF) receptor transinactivation and EGF receptor-independent stimulation of mitogen-activated protein kinase by bradykinin in A431 cells. Biochem J. 2000;347:441–447. doi: 10.1042/0264-6021:3470441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson KR, McKee TC, Bokesch HR. Anti-HIV cyclotides. Curr Protein Pept Sci. 2004;5:331–340. doi: 10.2174/1389203043379468. [DOI] [PubMed] [Google Scholar]
- Hass GM, Derr JE. Distribution of carboxypeptidase isoinhibitors in the potato plant. Plant Physiol. 1979;64:1029–1031. doi: 10.1104/pp.64.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass GM, Hermodson MA. Amino acid sequence of a carboxypeptidase inhibitor from tomato fruit. Biochemistry. 1981;20:2256–2260. doi: 10.1021/bi00511a029. [DOI] [PubMed] [Google Scholar]
- Hass GM, Ryan CA. Cleavage of the carboxypeptidase inhibitor from potatoes by carboxypeptidase A. Biochem Biophys Res Commun. 1980;97:1481–1486. doi: 10.1016/s0006-291x(80)80032-5. [DOI] [PubMed] [Google Scholar]
- Hass GM, Ryan CA. Carboxypeptidase inhibitor from potatoes. In: Lorand L, editor. Methods in Enzymology, Proteolytic Enzymes Part C. 3rd edn. Vol. 80. New York: Academic Press; 1982. pp. 778–791. [Google Scholar]
- Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie TA, et al. Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol. 2003;23:5614–5624. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen R, Galjart N, Rens JA, Eggermont AM, ten Hagen TL. Differential effects of matrix and growth factors on endothelial and fibroblast motility: application of a modified cell migration assay. J Cell Biochem. 2006;99:1536–1552. doi: 10.1002/jcb.20994. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Bowen PE. Can the consumption of tomatoes or lycopene reduce cancer risk? Integr Cancer Ther. 2002;1:121–132. doi: 10.1177/153473540200100203. [DOI] [PubMed] [Google Scholar]
- Ireland DC, Colgrave ML, Craik DJ. A novel suite of cyclotides from Viola odorata: sequence variation and the implications for structure, function and stability. Biochemical J. 2006;400:1–12. doi: 10.1042/BJ20060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause S, Schmoldt HU, Wentzel A, Ballmaier M, Friedrich K, Kolmar H. Grafting of thrombopoietin-mimetic peptides into cystine knot miniproteins yields high-affinity thrombopoietin antagonists and agonists. FEBS J. 2007;274:86–95. doi: 10.1111/j.1742-4658.2006.05567.x. [DOI] [PubMed] [Google Scholar]
- Martineau B, McBride KE, Houck CM. Regulation of metallocarboxypeptidase inhibitor gene-expression in tomato. Mol Gen Genet. 1991;228:281–286. doi: 10.1007/BF00282477. [DOI] [PubMed] [Google Scholar]
- Mattioli PA. I discorsi nelli sei libri di Pedacio Dioscoride Anarzabeo della materia medicinale. Venezia, Italy: Vincenzo Valgrisi; 1568. [Google Scholar]
- Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signalling pathways. Growth Factors. 2007;25:253–263. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Fisslthaler B, Medhora M, Harder D, Fleming I, Busse R. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor. FASEB J. 2003;17:770–772. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- Molnar A, Lovas A, Banfalvi Z, Lakatos L, Polgar Z, Horvath S. Tissue-specific signal(s) activate the promoter of a metallocarboxypeptidase inhibitor gene family in potato tuber and berry. Plant Mol Biol. 2001;46:301–311. doi: 10.1023/a:1010649503229. [DOI] [PubMed] [Google Scholar]
- Norton RS, Pallaghy PK. The cystine knot structure of ion channel toxins and related polypeptides. Toxicon. 1998;36:1573–1583. doi: 10.1016/s0041-0101(98)00149-4. [DOI] [PubMed] [Google Scholar]
- Pear JR, Ridge N, Rasmussen R, Rose RE, Houck CM. Isolation and characterisation of a fruit-specific cDNA and the corresponding genomic clone from tomato. Plant Mol Biol. 1989;13:639–651. doi: 10.1007/BF00016019. [DOI] [PubMed] [Google Scholar]
- Quilis J, Meynard D, Vila L, Aviles FX, Guiderdoni E, San Segundo B. A potato carboxypeptidase inhibitor gene provides pathogen resistance in transgenic rice. Plant Biotechnol J. 2007;5:537–553. doi: 10.1111/j.1467-7652.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- Rees DC, Lipscomb WN. Structure of potato inhibitor complex of carboxypeptidase A at 5.5-A resolution. Proc Natl Acad Sci U S A. 1980;77:277–280. doi: 10.1073/pnas.77.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitja-Arnau M, Molina MA, Blanco-Aparicio C, Ferrer-Soler L, Lorenzo J, Aviles FX, et al. Mechanism of action of potato carboxypeptidase inhibitor (PCI) as an EGF blocker. Cancer Lett. 2005;226:169–184. doi: 10.1016/j.canlet.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Slowik A, Rubio F, Szczudlik A, Gaffney J. Activation of MAP kinase (ERK-1/ERK-2), tyrosine kinase and VEGF in the human brain following acute ischaemic stroke. Neuroreport. 2000;11:2759–2764. doi: 10.1097/00001756-200008210-00030. [DOI] [PubMed] [Google Scholar]
- Vitt UA, Hsu SY, Hsueh AJ. Evolution and classification of cystine knot-containing hormones and related extracellular signalling molecules. Mol Endocrinol. 2001;15:681–694. doi: 10.1210/mend.15.5.0639. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- Wang X, Smith PL, Hsu MY, Ogletree ML, Schumacher WA. Murine model of ferric chloride-induced vena cava thrombosis: evidence for effect of potato carboxypeptidase inhibitor. J Thromb Haemost. 2006;4:403–410. doi: 10.1111/j.1538-7836.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- Werle M, Kafedjiiski K, Kolmar H, Bernkop-Schnurch A. Evaluation and improvement of the properties of the novel cystine-knot microprotein McoEeTI for oral administration. Int J Pharm. 2007;332:72–79. doi: 10.1016/j.ijpharm.2006.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


