Abstract
Previous studies examining the relationship between hepatic iron deposition and histological severity in nonalcoholic fatty liver disease (NAFLD) have been inconclusive. The goal of this study was to examine the relationship between hepatic iron deposition and liver histology in 849 patients enrolled in the Nonalcoholic Steatohepatitis Clinical Research Network. Hepatic iron stains were performed in a central lab and scored for grade, cellular and parenchymal localization by a central pathology committee; the relationship between grade and pattern of iron deposition to clinical, laboratory and histological variables was examined using univariate and multivariate analyses. Stainable hepatic iron was present in 293 of 849 patients (34.5%) in one of three histological patterns: hepatocellular (HC) (63/849, {7.4%}), reticuloendothelial system cells (RES) (91/ 849, {10.7%}), or a mixed RES/HC pattern (139/849 {16.4%}). Patients with the RES iron staining pattern were more likely to have advanced fibrosis compared to those with HC iron (p=0.01). Patients with RES iron were also more likely to have advanced histologic features including: fibrosis (p=0.049), portal inflammation (p=0.002), hepatocellular ballooning (p=0.006) and definite NASH (p=0.007) compared to patients with HC or mixed iron patterns. Presence of RES iron (OR, 1.60, 95% CI, 1.10–2.33, p=0.015) was independently associated with advanced hepatic fibrosis on multiple regression analysis after adjustment for age, gender, diabetes status and BMI. Conclusion: The presence and pattern of hepatic iron deposition is associated with distinct histologic features among patients with NAFLD and may have implications for pathophysiology and therapy.
Keywords: NAFLD, Steatohepatitis, reticuloendothelial, hepcidin, reactive oxygen species
Increased deposition of iron within the liver may contribute to liver disease via the production of reactive oxygen species (ROS) which may lead to lipid peroxidation, and dysfunction of mitochondria and other organelles, cell injury and death (1). In addition to hemochromatosis, hepatic iron accumulation may occur in a variety of chronic liver diseases, including chronic hepatitis C, alcoholic and non-alcoholic fatty liver disease (NAFLD), cryptogenic cirrhosis and end stage liver disease (2–5). Hepatic iron deposition in the setting of chronic liver disease may be present in one of three different patterns: exclusively in hepatocytes (HC), exclusively in cells of the reticuloendothelial system (RES), or in a mixed pattern involving both HC and RES (Mixed) (2–5). In hereditary hemochromatosis types 1–3, iron preferentially accumulates in hepatocytes due to mutations in the HFE, HJV/HAMP or TFR2 genes respectively (2–5). By contrast, hepatic iron deposition in the setting of cirrhosis and secondary iron overload occurs primarily in RES cells usually beginning with sinusoidal lining cells in an azonal pattern (2–5). Iron deposition in alcoholic or non-alcoholic fatty liver disease or chronic hepatitis C infection may occur in any of these three patterns (2–5).
The contribution of hepatic iron accumulation to disease severity or progression in chronic liver diseases other than hemochromatosis remains unclear. A number of studies have assessed the relationship between hepatic iron loading and disease stage in chronic hepatitis C; the majority but not all of these studies support an association between advanced fibrosis and the presence of iron deposition in the nonparenchymal RES cells (i.e., sinusoidal, endothelial and portal tracts) (6, 7). By contrast, parenchymal iron deposition is a feature in alcoholic liver disease (ALD), although RES iron is more prevalent in the advanced stages of disease (8).
Nonalcoholic fatty liver disease is the most common liver disease in the USA and may be present in up to 30% of the general population (9). A subset of patients with NAFLD has nonalcoholic steatohepatitis (NASH), a more severe form of this disease associated with hepatocellular injury, inflammation and varying levels of fibrosis. A number of previous studies have investigated the role of iron stores in NAFLD by assessing the presence of stainable hepatic iron deposits, biochemical hepatic iron content (HIC) or both. However, the findings thus far have been conflicting, with some studies finding hepatic iron deposition to be associated with increased disease severity (10–12) and others not finding such an association (13–16). One previous study examining the distribution of iron in 157 patients with NASH-related cirrhosis, including 51 with HCC, demonstrated that patients with HCC were more likely to have mild to moderate RES cell iron deposits compared to patients without HCC (17). However, most prior studies examining the relationship between hepatic iron deposition and histologic features of NASH have had several limitations including small sample sizes, lack of uniform criteria for the diagnosis of NASH as well as a lack of a standardized liver histological scoring system for NASH and iron deposition. Most importantly, previous studies have not examined the relationship between each of the three distinct patterns of hepatic iron deposition and histological severity among patients with NASH.
The goal of the current study was to analyze the relationships among the pattern of hepatic iron deposition and liver histology in liver biopsy specimens from an unselected cohort of NAFLD patients prospectively enrolled in the National Institutes for Health-funded Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) from 8 participating centers in the USA.
MATERIALS AND METHODS
Participants were enrolled in the NASH CRN studies from October 2005 to February 2008 as per inclusion criteria described elsewhere (18,19). Briefly, NASH CRN study participants at least 18 years of age constituted the patient population for this study. Patients with known hemochromatosis (defined as Hepatic Iron Index ≥ 1.9 or removal of more than 4 g of iron by phlebotomy), C282Y homozygosity for the HFE gene or unexplained hepatic iron overload (≥3+ stainable iron on liver biopsy) were excluded from all NASH CRN studies. Demographic information such as age, gender, ethnicity, and race were obtained. A medical history was obtained in all subjects including menstrual history in women and presence of co-morbid conditions and medication usage. Total dietary consumption of iron, vitamin C, tea and coffee were determined from the Block 98 food frequency questionnaire; alcohol consumption was determined from the AUDIT-C questionnaires done during NASH CRN studies closest to the time of biopsy. A physical exam including body weight and height measures was performed in all subjects. Histologic evaluation was based on 849 liver biopsies with hepatic iron straining results which had been read centrally by the NASH CRN Pathology Committee. In addition, clinical and laboratory data obtained within 6 months of the liver biopsy were compared between iron stain positive and negative subjects if available (N=573).
Histological assessment
Histologic features of fatty liver disease and iron staining pattern were assessed by the Pathology Committee of the NASH CRN in a centralized consensus review format using criteria previously described (20). Pathologists were blinded to all clinical, laboratory and demographic information. Iron stains were performed by a central lab using Perls’ iron stain; iron stains were scored prospectively by a method agreed upon by the Pathology Committee. Only granular iron deposition was scored, based on agreement that only discernible hemosiderin granules represent significant iron deposition (3, 4). Hepatocellular iron was scored from 0 to 4 using the method of Rowe et al., with the modification that a 20x objective was used in place of the 25x objective (21). Non-hepatocellular iron (RES) was scored on a three point scale as none, mild or more than mild.
Statistical analysis
Baseline demographic, clinical, and laboratory characteristics were recorded as N (%), mean ± SD, or median (interquartile range [IQR]). Laboratory measures were not normally distributed and therefore were analyzed using the Wilcoxon rank sum or Kruskal Wallis test for continuous variables. Categorical variables including histological features like steatosis grade and location, fibrosis stage, and lobular inflammation grade were analyzed using either Fisher’s exact or chi-square tests. Multiple logistic regression analysis was used to examine the relationship between advanced fibrosis and the presence and grade of hepatocellular and RES iron. Stepwise conditional logistic regression was used to determine the effect of the following variables selected a priori on the presence of iron staining: ethnicity, history of GI bleeding or iron overload, menstrual history, alcohol consumption, tea and coffee consumption, dietary or supplemental iron and vitamin C, and controlling for age at biopsy, gender, presence of diabetes and BMI. All variables not independently associated with iron using a threshold p value of p≤0.20 were removed from the model. All analyses were performed using SAS (version 9, SAS Institute, Cary, NC) or STATA (version 9, College Station, TX, USA). Nominal, two-sided P-values were used and were considered to be statistically significant if P≤0.05; no adjustments for multiple comparisons were made.
RESULTS
Patient Characteristics
A subset of 849 subjects of the total number of 1,525 enrolled in the NASH CRN Database, PIVENS, and TONIC studies were included in this analysis of hepatic iron deposition. Reasons for exclusion of the remaining 676 subjects were: (1) age less than 18 years (n=368; because iron overload is rare in children in our cohort), (2) liver biopsy not available (n=167), (3) iron stain not performed on liver biopsy (n=141). A comparison of clinical and demographic data of subjects with positive hepatic iron staining to the entire cohort is shown in Table 1. Stainable hepatic iron was present in 293 of 849 patients (34.5%); the majority were men (57%, p<0.0001). There was no significant difference in age between subjects with and without stainable iron. Subjects with positive iron staining had a lower mean BMI than subjects without iron (33.2 vs 34.9, p=0.0002). Iron staining was more common in non-Hispanics (36%) compared to Hispanics (25%, p=0.04), otherwise no racial differences were identified between subjects with and without stainable iron.
Table 1.
Patient Characteristics
| Characteristic | All patients | Iron stain positive | P-value† |
|---|---|---|---|
| Total | 849 | 293 (35%) | |
| Male sex (No.) | 309 | 175 (57%) | <0.0001 |
| Age category: (years) | 0.34 | ||
| <40 | 198 (23%) | 60 (30%) | |
| 40<60 | 505 (60%) | 179 (35%) | |
| ≥60 | 146 (17%) | 54 (37%) | |
| Age mean (years) | 48.4 ± 11.7 | 49.0 ± 11.3 | 0.49 |
| BMI catagory: (kg/m2) | 0.004 | ||
| <25 | 34 (4%) | 18 (53%) | |
| 25<30 | 197 (23%) | 80 (41%) | |
| ≥30 | 616 (73%) | 194 (31%) | |
| BMI mean (kg/m2) | 34.3 ± 6.3 | 33.2 ± 6.0 | 0.0002 |
| Race: (No.) | 0.08 | ||
| White | 696 (82%) | 236 (34%) | |
| Black | 24 (3%) | 9 (38%) | |
| Asian | 43 (5%) | 23 (53%) | |
| AI or AN | 25 (3%) | 7 (28%) | |
| Other | 61(7%) | 18 (30%) | |
| Ethnicity: (No.) | 0.04 | ||
| Non-Hispanic | 750 (88%) | 268 (36%) | |
| Hispanic | 99 (12%) | 25 (25%) |
Values are N (%) or mean ± SD
P-values from Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables.
Laboratory data in subjects with and without stainable hepatic iron
Results of laboratory tests among subjects with and without stainable hepatic iron are shown in Table 2. Subjects with a liver biopsy showing a positive iron stain tended to have evidence of more active and advanced disease as shown by higher serum ALT (p=0.004), total bilirubin (p<0.0001) and prothrombin time (p=0.09) and lower platelet count (p<0.0001). In contrast, metabolic abnormalities including fasting insulin and glucose levels, HOMA-IR and lipid levels were slightly worse among subjects without stainable iron, but with the exception of total cholesterol (p=0.02) these were not statistically significant. HDL was higher in subjects without iron (p=0.004). As might be expected, patients with stainable hepatic iron had higher serum iron studies including iron, total iron binding capacity (TIBC), ferritin and percent transferrin-iron saturation (TS) (all p <0.0001).
TABLE 2.
Laboratory values in patients with nonalcoholic fatty liver with and without stainable iron.
| Characteristic | Iron stain negative (N=556) | Iron stain positive (N=293) | P-value† |
|---|---|---|---|
| Laboratory values’‡ | |||
| ALT (U/L) | 63 (42–91) | 75 (50–111) | 0.004 |
| AST (U/L) | 45 (32–68) | 48 (33–67) | 0.39 |
| AST/ALT | 0.74 (0.59–0.92) | 0.66 (0.51–0.84) | 0.0002 |
| Total bilirubin (mg/dL) | 0.6 (0.5–0.8) | 0.8 (0.6–1.0) | <0.0001 |
| Prothrombin time (sec) | 11.8 (10.3–12.7) | 12.0 (10.6–13.1) | 0.09 |
| Total cholesterol (mg/dL) | 200 (176–224) | 190 (166–222) | 0.02 |
| Triglycerides (mg/dL) | 159 (114–232) | 152 (105–214) | 0.26 |
| LDL (mg/dL) | 121(93–148) | 116 (90–141) | 0.10 |
| HDL (mg/dL) | 43 (36–51) | 39 (34–49) | 0.004 |
| Glucose (mg/dL) | 97 (86–110) | 95 (85–108) | 0.54 |
| Fasting insulin(μU/mL) | 18.8 (13–29) | 17.7 (11.8–26.0) | 0.10 |
| HOMA–IR | 4.6 (3.0–7.5) | 4.4 (2.5–6.5) | 0.10 |
| Platelets (K/cmm) | 255 (220–293) | 216 (179–261) | <0.0001 |
| Hemoglobin (g/dL) | 14.1 (13.4–15.0) | 15.1 (14.3–15.9) | <0.0001 |
| Serum iron studies | |||
| Iron (μg/dL) | 81 (61–103) | 103 (80–123) | <0.0001 |
| TIBC (μg/dL) | 380(337–424) | 336 (301–384) | <0.0001 |
| Ferritin (ng/mL) | 106 (62–192) | 328 (221–526) | <0.0001 |
| TS (iron/TIBC) | 0.21 (0.16–0.27) | 0.29 (0.23–0.39) | <0.0001 |
Values aremedian (IQR)
P-values from Wilcoxon rank-sum test.
Only laboratory values collected within 6 months of liver biopsy included, N=573.
Potential dietary and clinical factors affecting hepatic iron deposition
We examined the effect of factors potentially influencing body iron stores such as diet (i.e., iron consumption, vitamin C, coffee and tea), alcohol and other factors such as a history of GI bleeding, iron overload and menstruation (past 5 years). In a multivariate stepwise logistic regression analysis using these a priori selected variables and adjusting for age, gender, BMI, ethnicity and diabetes, male sex (Odds Ratio [OR] = 5.08, 95% confidence intervals [95% CI] = 3.67–7.02, p<0.0001), older age (OR = 1.02, 95%CI = 1.01–1.04, p=0.001) and lower BMI (OR = 0.967, 95% CI 0.941–0.991, p=0.009) were independently associated with the presence of hepatic iron. Among women, rare or no periods (last 5 years) was also strongly associated with iron deposition (OR = 1.57, 95%CI = 1.28–1.94, p<0.0001).
Relationship between patterns of hepatic iron staining and clinical and laboratory differences
Three distinct patterns of hepatic iron staining were observed as follows: iron was localized solely in hepatocytes (HC) in 63/849 subject biopsies (7.4%), solely in cells of the reticuloendothelial system (mainly Kupffer cells) (RES) in 91/849 biopsies (10.7%). A mixed pattern of HC/RES staining (mixed) was present in 139 of 849 biopsies (16.4%).
Clinical and laboratory values that were significantly different among the various iron staining groups and subjects without stainable hepatic iron are shown in Table 3. Subjects with RES iron had the highest serum ALT, AST, and HOMA-IR values among all groups. Subjects with HC iron generally had values similar to the no iron group such as for ALT, AST, total bilirubin and platelets. The mixed iron group tended to be intermediate or closer to the RES group than to the HC or no iron groups for most values. Subjects in the mixed iron group had the highest iron stores based on serum iron, TS and ferritin. This group also had a greater proportion of men (70%) compared to the other groups.
TABLE 3.
Comparison of clinical and laboratory values between subjects with different hepatic iron phenotypes
| Characteristic | No iron stain (N=556) | HC iron only (N=63) | RES iron only (N=91) | Mixed HC/RES iron (N=139) | P-value† |
|---|---|---|---|---|---|
| Male sex | 134 (24%) | 31 (49%) | 47 (52%) | 97 (70%) | 0.0001 |
| BMI (kg/m2) | 34.9 ± 6.4 | 32.7 ± 6.8 | 33.5 ± 5.7 | 33.2 ± 5.8 | 0.0015 |
| ALT (U/L) | 63 (42–91) | 62 (50–92) | 88 (58–128) | 70 (44–117) | 0.002 |
| AST(U/L) | 45 (32–68) | 40 (31–56) | 58 (39–77) | 45 (33–63) | 0.03 |
| AST/ALT | 0.74 (0.59–0.92) | 0.62 (0.53–0.90) | 0.64 (0.49–0.87) | 0.66 (0.52–0.79) | 0.003 |
| Total bilirubin (mg/dL | 0.6 (0.5–0.8) | 0.6 (0.5–0.9) | 0.7 (0.6–1.1) | 0.9 (0.6–1.1) | 0.0001 |
| HDL | 43 (36–51) | 41 (33–50) | 39 (33–49) | 40 (34–49) | 0.04 |
| Insulin (μU/mL) | 19 (13–29) | 16 (11–24) | 23 (12–32) | 18 (12–23) | 0.05 |
| HOMA–IR | 4.6 (3.0–7.5) | 3.7 (2.8–5.3) | 5.2 (2.8–8.9) | 4.2 (2.5–5.8) | 0.0001 |
| Platelets (K/cmm) | 255 (220–293) | 247 (198–286) | 215 (178–258) | 206 (174–258) | 0.0001 |
| Hemoglobin (g/dL) | 14.1 (13.4–15.0) | 15.1 (14.2–15.5) | 14.9 (14.2–15.6) | 15.5 (14.6–16.0) | 0.0001 |
| Serum iron (μg/dL) | 81 (61–103) | 91 (71–116) | 94 (76–114) | 107 (89–133) | 0.0001 |
| TIBC (μg/dL) | 380(337–424) | 348 (295–378) | 356 (324–406) | 320 (286–365) | 0.0001 |
| Ferritin (ng/mL) | 106 (62–192) | 218 (126–283) | 344 (227–497) | 378 (263–673) | 0.0001 |
| TS (iron/TIBC) | 0.21 (0.16–0.27) | 0.27(0.22–0.37) | 0.26 (0.21–0.32) | 0.33(0.27–0.43) | 0.0001 |
Values are median (IQR) except BMI: mean ± SD; and male sex: N (%).
P-values from Kruskal Wallis for continuous variables except male sex; chi-square test.
Only laboratory values collected within 6 months of liver biopsy were used in analyses, N=573.
Relationship between hepatic iron staining pattern and histologic severity
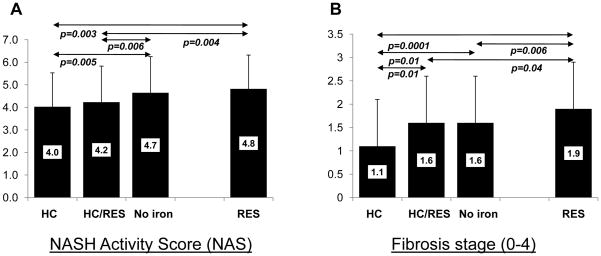
A comparison of histological grade and stage among the various hepatic iron staining groups is shown in Table 4. There were significant differences across all groups in the proportion of subjects in different categories of severity for portal inflammation, hepatocellular ballooning and NASH diagnosis, but not grade of steatosis, lobular inflammation or fibrosis stage. Mixed iron subjects had higher grades of HC and RES iron deposits than either the HC or RES groups, respectively. The NAFLD activity score (NAS) was significantly different across all group (Figure 1A, p=0.0007, Kruskal Wallis test) and was highest among subjects with RES iron staining (NAS=4.8), and lowest in the group with HC iron staining (NAS=4.0). The NAS was also significantly higher among subjects without iron compared to those with HC (p=0.005), or a mixed pattern (p=0.006). As shown in Figure 1B, the mean fibrosis score (0–4 scale) was also significantly different across all groups (p=0.0012, Kruskal Wallis test). The RES iron group had a significantly higher mean fibrosis stage (mean score 1.9) compared to each of the other groups including the no iron group (p=0.006). By contrast, the HC group had a significantly lower mean fibrosis stage than the other three groups (mean score 1.1).
Table 4.
Histologic differences between iron staining groups
| Histologic Features | No iron stain (N=556) | HC iron only (N=63) | RES iron only (N=91) | Mixed HC/RES iron (N=139) | P value† |
|---|---|---|---|---|---|
| Steatosis grade | p=0.170 | ||||
| 5–33% | 220 (40%) | 27 (43%) | 35 (38%) | 71 (51%) | |
| 34–66% | 190 (34%) | 24 (38%) | 29 (32%) | 35 (25%) | |
| >66% | 146 (26%) | 12 (19%) | 27 (30%) | 33 (24%) | |
| Fibrosis stage | p=0.092 | ||||
| None | 144 (26%) | 25 (40%) | 11 (12%) | 33 (24%) | |
| Mild to moderate, zone 3, perisinusoidal or portal/periportal only | 155 (28%) | 18 (28%) | 27 (30%) | 41 (29%) | |
| Zone 3 and periportal, any combination | 104 (19%) | 8 (13%) | 19 (21%) | 25 (18%) | |
| Bridging | 108 (19%) | 10 (16%) | 24 (26%) | 27 (19%) | |
| Cirrhosis | 45 (8%) | 2 (3%) | 10 (11%) | 13 (9%) | |
| Lobular inflammation | |||||
| 0 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1%) | p=0.110 |
| <2 under 20x | 275 (50%) | 36 (57%) | 37 (41%) | 73 (53%) | |
| 2–4 under 20x | 213 (38%) | 22 (35%) | 44 (48%) | 53 (38%) | |
| >4 under 20x | 68 (12%) | 5 (8%) | 10 (11%) | 11 (8%) | |
| Portal, chronic inflammation | |||||
| None | 81 (15%) | 9 (14%) | 10 (11%) | 23 (17%) | p=0.017 |
| Mild | 355 (64%) | 48 (76%) | 52 (57%) | 77 (55%) | |
| More than mild | 120 (21%) | 6 (10%) | 29 (32%) | 39 (28%) | |
| Hepatocellular ballooning | |||||
| None | 166 (30%) | 30 (48%) | 25 (28%) | 50 (36%) | p=0.005 |
| Mild | 136 (24%) | 18 (28%) | 22 (24%) | 42 (30%) | |
| More than mild | 254 (46%) | 15 (24%) | 44 (48%) | 47 (34%) | |
| NASH diagnosis | |||||
| No NASH | 106 (19%) | 19 (30%) | 12 (13%) | 33 (24%) | p=0.049 |
| Suspicious/borderline | 105 (19%) | 16 (25%) | 17 (19%) | 30 (21%) | |
| Definite | 345 (62%) | 28 (44%) | 62 (68%) | 76 (55%) | |
| Hepatocellular iron grade | |||||
| Absent or barely discernable, 40x | 556 (100%) | 0 (0%) | 91 (100%) | 0 (0%) | p=0.001 |
| Barely discernable granules, 20x | 0 (0%) | 52 (83%) | 0 (0%) | 80 (58%) | |
| Discrete granules resolved, 10x | 0 (0%) | 11 (17%) | 0 (0%) | 49 (35%) | |
| Discrete granules resolved, 4x | 0 (0%) | 0 (0%) | 0 (0%) | 10 (7%) | |
| RES iron grade | |||||
| None | 556 (100%) | 63 (100%) | 0 (0%) | 0 (0%) | P<0.001 |
| Mild | 0 (0%) | 0 (0%) | 74 (81%) | 79 (57%) | |
| More than mild | 0 (0%) | 0 (0%) | 17 (19%) | 60 (43%) | |
Values are N (%)
P-values from either Fisher’s exact or chi-square tests as appropriate.
FIGURE 1. Comparison of mean NAS and fibrosis scores among NAFLD subjects with different iron staining patterns.
A) The mean NASH Activity Score (NAS) and B) the mean histologic fibrosis score (scale 0–4) is shown for each study group. Significant differences between groups are indicated by the arrows (Wilcoxon rank sum test). Standard deviations are indicated by the error bars.
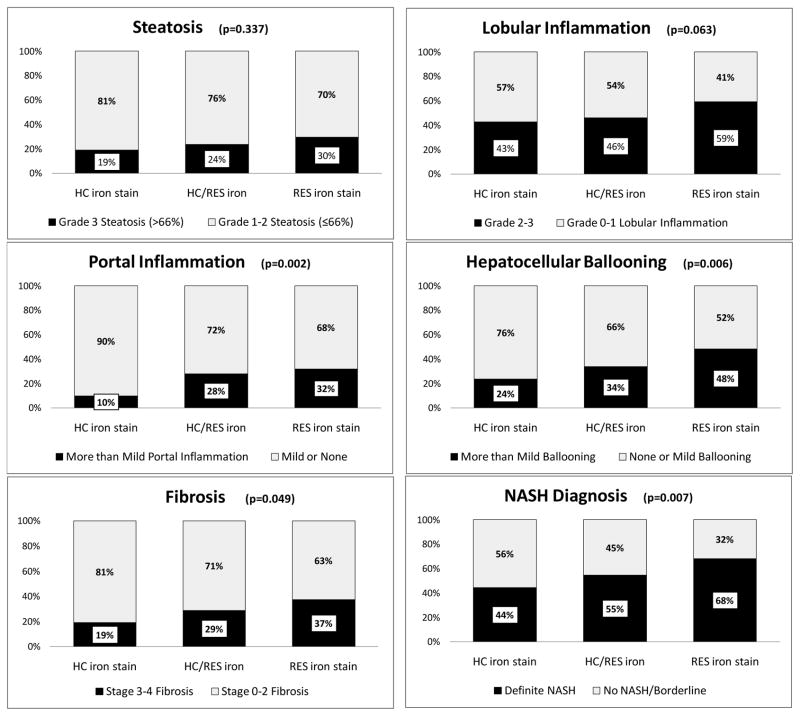
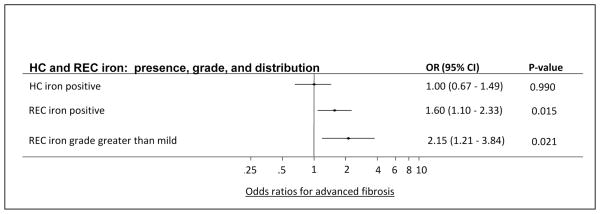
We also examined the relationship between hepatic iron pattern and advanced histologic features. The RES iron group had the highest proportion of subjects in the most severe category for each histological feature (Figure 2). By contrast, the HC iron group had the lowest proportion of subjects in the most severe categories for each histologic feature, and the mixed iron group had intermediate proportions of subjects in the most severe categories (Figure 2). The no iron group had similar proportions to the mixed iron group (data not shown). The differences in the proportion of subjects with advanced histological features across all iron groups was statistically significant for advanced fibrosis (p=0.049), portal inflammation (p=0.002), hepatocellular ballooning (p=0.006) and definite NASH (p=0.007). When compared to the HC iron group, the RES iron group had a significantly higher proportion of subjects with each advanced histological feature; stage 3–4 fibrosis (37% vs 19%, p=0.01), grade 2–3 lobular inflammation (59% vs 43%, p=0.04), grade 2 portal inflammation (32% vs 10%, p=0.001), grade 2 ballooning (48% vs 24%, p=0.002) and a definitive diagnosis of NASH (68% RES vs 44% HC, p=0.003). Subjects with HC iron alone (19%) were least likely to have advanced (stage 3–4) fibrosis compared to; no iron (27%), mixed iron (29%) or RES iron groups (37%). Advanced hepatic fibrosis was significantly more common in subjects with RES iron compared to HC iron (X2=5.96, p=0.01). A similar trend was observed in comparison with the no iron group (X2=3.69, p=0.055). On multiple regression analysis both the presence (OR, 1.60, 95% CI, 1.10–2.33, p=0.015) and grade (OR, 2.15, 95% CI, 1.21–3.84, p=0.021) of RES iron was independently associated with advanced fibrosis after adjusting for age at biopsy, gender, diabetes status, and BMI (Figure 3). Neither the presence nor grade of HC iron was associated with advanced fibrosis.
FIGURE 2. Relationship between histologic features and iron staining pattern among subjects with stainable iron.
The proportion of biopsies within each iron staining group scoring in the highest category of each histologic feature and definitive NASH diagnosis are shown relative to each other. The most severe category for each histologic feature is shown in black and the remaining categories are combined to add up to a total of 100%. P values were calculated using Fisher’s exact test as shown.
FIGURE 3. Multiple logistic regression analysis for independent risk of iron staining parameters on advanced fibrosis.
Multiple logistic regression analysis was used to model the independent risk for the presence and grade of HC and REC iron on the occurrence of advanced fibrosis (yes vs. no). Each of the predictors in the table was modeled individually after adjustment for age at biopsy, gender, diabetes status, and BMI.
DISCUSSION
We examined the relationship between pattern of hepatic iron distribution and clinical and histological findings in 849 unselected adult NAFLD patients from a total of 1525 subjects enrolled in the NASH CRN.
This study identified novel relationships between the pattern of hepatic iron deposition and histologic features of NAFLD. RES iron was associated with more severe disease as shown by the greater proportion of subjects with advanced histologic features, a higher mean NAS and fibrosis stage, and higher AST, ALT and total bilirubin values and lower platelet counts compared to the other study groups. By contrast, HC iron was associated with milder histologic features compared to the other groups, while the mixed iron group was intermediate. A similar relationship between iron distribution and disease severity has been observed in chronic HCV (6, 7) and alcoholic liver disease (8).
Previous studies have explored the relationship between hepatic iron deposition and disease severity in NAFLD; however, our study is unique in examining the relationship between histological severity and each of the three distinct patterns of hepatic iron deposition observed in NAFLD. Strengths of the present study include utilization of a centralized pathology committee review, multi-center design and a standardized histologic scoring system and the largest sample size to date exploring this issue. A recent study by Valenti et al found that “predominantly hepatocellular” iron was associated with an increased likelihood of fibrosis stage >1 in 587 Italian NAFLD patients, while “predominantly nonparenchymal” iron was not (12). However, a number of notable differences between the two study populations likely explain these seemingly discordant data (22). These include a higher proportion of subjects with stage 3–4 fibrosis (28% vs 14% Valenti et al) and our higher mean BMI and greater ethnic diversity. In addition, 60% of subjects in the present study had definitive NASH; Valenti et al did not report the proportion of patients with NASH.
The mechanism of differential iron deposition in liver cells among patients with NAFLD and NASH is unclear but is likely multifactorial; gender, age, menstruation and a lower BMI but not diet, alcohol or previous GI bleeding likely contribute to overall hepatic iron deposition. We also speculate that the degree and pattern of hepatic iron deposition in NAFLD may be related to the dual regulatory mechanism (iron stores and inflammation) of the key body iron regulator hepcidin. Hepcidin plays a central role in iron regulation by binding to and internalizing the cellular iron export protein ferroportin, thus downregulating iron efflux from the enterocyte, macrophage and hepatocyte (23, 24). Hepcidin is regulated in response to iron stores via the BMP/HJV/SMAD pathway (25) or the HFE/TFR1/TFR2 complex in response to plasma transferrin levels (26–28). Thus increased HC iron among patients with NAFLD may be due to increased iron absorption as a consequence of decreased hepcidin activity, possibly via mutations in hepcidin regulatory genes such as HFE, TFR1 or TFR2, HJV, FPN or the BMPs. We recently reported that over half of 126 NASH patients with hepatic iron staining carried common mutations in the HFE gene (29). Moreover, since hepcidin is expressed in adipose tissue, our observation that subjects with HC iron had a lower BMI, is consistent with the hypothesis that decreased serum hepcidin levels from less adipose mass results in increased iron absorption (30). Hepcidin expression is also induced during inflammation by activation of the transcription factor STAT3 by inflammatory cytokines interleukin-6 (IL-6) and interleukin-1 (IL-1) (31, 32) and has also recently been shown to be upregulated by ER stress (33). RES cell iron accumulation in NAFLD may be due to an increased systemic inflammatory state and/or other as yet undefined stimuli which increase hepatic necroinflammation and erythrocyte fragility resulting in increased iron uptake by Kupffer and other hepatic RES cells (34). Iron may then subsequently be retained within Kupffer cells and adjacent sinusoidal lining cells due to inflammatory mediated up-regulation of hepcidin expression. Upregulation of hepcidin via IL-6 is the mechanism responsible for “anemia of inflammation” often observed with chronic disease and associated with iron sequestration in Kupffer cells and other macrophages (35).
Our data are consistent with numerous studies suggesting that the consequences of iron overload in the liver are related to the role of iron in catalyzing the production of reactive oxygen species (ROS) which cause lipid peroxidation and stimulate a variety of proinflammatory, profibrogenic and cytotoxic pathways via induction of the red-ox sensitive transcription factor nuclear factor-κB (NF-κB) in Kupffer cells (the main component of RES; 36–41). Hepatic iron deposition also leads to activation of hepatic stellate cells (HSC) and deposition of extra cellular matrix components such as collagen type I and III (34, 42–46). A number of studies suggest this process may be mediated by iron-induced oxidative stress, particularly in Kupffer cells (1, 34, 42–44). Further support for this concept comes from a recent study by Otogawa et al, showing that iron depletion by phlebotomy in rabbit model of NAFLD was associated with significant reductions in Kupffer cell iron deposition, serum levels of lipid peroxidation and hydroxyproline (a marker of fibrosis), collagen and α-smooth muscle actin (a marker of HSC activation) deposition and decreased apoptosis (34). Thus, it is likely that the localized effects of iron, particularly in Kupffer cells and other RES cells may play a role in the progression of NASH.
A novel finding in this study is the inverse association between HC iron and phenotypic features of the metabolic syndrome (including lower BMI and HOMA-IR) as well as milder histologic findings among NAFLD patients. We speculate that these subjects may represent a novel form of NAFLD independent of the presence of metabolic syndrome, but rather related to the localized pathophysiology of iron such as direct cytotoxicity and ROS formation. It is also possible that in contrast to Kupffer cells, ROS may not be as pathogenic when present in HC, resulting in the milder phenotype of these patients. Consistent with our hypothesis described above that HC and RES iron deposition result from separate cellular processes that result in divergent hepcidin signaling, the presence of RES iron in the mixed patients likely appears after the establishment of HC iron, thus exacerbating the mild HC phenotype resulting in the intermediate disease severity of these patients.
Our study has practical clinical implications for the management of NASH. First, we found that hepatic iron deposition was common in this unselected population of patients with NAFLD. Furthermore, RES cell iron was found to be an independent predictor of advanced fibrosis and associated with histologic severity. Therefore, these data provide support for the implementation of clinical trials examining iron depletion as a treatment for NASH. Phlebotomy is safe, well tolerated and has been shown to lower serum ferritin and ALT levels and may improve insulin sensitivity as measured by the homeostasis model assessment for insulin resistance (HOMA-IR) in NAFLD subjects (47–50).
We recognize that the current study has limitations. We did not have data on hepatic hepcidin gene expression or serum hepcidin level and did not have information on HFE mutation status or biochemical hepatic iron measurement in our cohort. We also recognize that longitudinal follow-up studies will be required to definitively establish that RES cell iron causes more rapid disease progression and increased fibrosis in NAFLD.
In summary, our results have demonstrated novel relationships between the presence and pattern of hepatic iron staining and histologic severity in a large, systematic, unselected multicenter national cohort of patients with NAFLD. Further studies are warranted to define the mechanisms for hepatic iron deposition in NASH, the contribution of hepatic iron to disease severity and progression and the possible role of iron depletion as a treatment for this common disorder.
Acknowledgments
The authors would like to thank Pat Belt from the NASH CRN data Coordinating Center for assistance with data preparation and Jay H. Hoofnagle, MD of NIDDK for his careful review and contributions to the final manuscript.
Source of funding
The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD). KVK is also supported by NIH K24 Grant DK-02957.
Several clinical centers use support from General Clinical Research Centers or Clinical and Translational Science Awards in conduct of NASH CRN Studies (grants UL1RR024989, M01RR000750, M01RR00188, UL1RR02413101, M01RR000827, UL1RR02501401, M01RR000065, M01RR020359).
This work was supported in part by the Intramural Research Program of the National Cancer Institute.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- HC
hepatocellular
- RES
reticuloendothelial cell
- ROS
reactive oxygen species
- BMP
bone morphogenic protein
- HJV
hemojuvelin
- NF-κB
nuclear factor-κB
- TNF-α
tumor necrosis factor-α
- IL-6
interleukin-6
- TGF-β
transforming growth factor-β
- ECM
extra cellular matrix components
- SF
serum ferritin
- TS
transferrin saturation
Appendix
Members of the Nonalcoholic Steatohepatitis Clinical Research Network:
Clinical Centers
Baylor College of Medicine, Houston, TX: Stephanie Abrams, MD; Diana Arceo, MD, MS; Denise Espinosa; Leanel Angeli Fairly, RN
Case Western Reserve University Clinical Centers:
MetroHealth Medical Center, Cleveland, OH: Carol Hawkins, RN; Yao-Chang Liu, MD; Margaret Stager, MD
Cleveland Clinic Foundation, Cleveland, OH: Arthur McCullough, MD; Srinivasan Dasarathy, MD; Ruth Sargent, LPN
Seattle Children’s Hospital & Research Institute, WA: Melissa Coffey; Karen Murray, MD; Melissa Young
Children’s National Medical Center, Washington DC: Parvathi Mohan, MD; Kavita Nair
Duke University Medical Center, Durham, NC: Manal Abdelmalek, MD; Anna Mae Diehl, MD; Marcia Gottfried, MD (2004–2008); Cynthia Guy, MD; Paul Killenberg, MD (2004–2008); Samantha Kwan; Yi-Ping Pan; Dawn Piercy, FNP; Melissa Smith
Indiana University School of Medicine, Indianapolis, IN: Prajakta Bhimalli; Naga Chalasani, MD; Oscar W. Cummings, MD; Lydia Lee, Linda Ragozzino, Raj Vuppalanchi, MD
Riley Hospital for Children, Indianapolis, IN : Elizabeth Byam; Ann Klipsch, RN; Jean Molleston, MD; Girish Subbarao, MD
Johns Hopkins Hospital, Baltimore, MD: Kimberly Pfeifer; Ann Scheimann, MD; Michael Torbenson, MD
St. Louis University, St Louis, MO: Sarah Barlow, MD (2002–2007); Jose Derdoy, MD (2007-); Joyce Hoffmann; Debra King, RN; Andrea Morris; Joan Siegner, RN; Susan Stewart, RN; Brent A. Tetri, MD; Judy Thompson, RN
University of California San Diego, San Diego, CA: Cynthia Behling, MD, PhD; Lisa Clark, PhD, MPH; Janis Durelle; Tarek Hassanein, MD; Joel E. Lavine, MD, PhD; Susana Mendoza; Jeffrey B. Schwimmer, MD; Claude Sirlin, MD; Tanya Stein, MD; Zobeida Palomares
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Kiran Bambha, MD; Nathan M. Bass, MD, PhD; Linda D. Ferrell, MD; Danuta Filipowski, MD; Raphael Merriman, MD (2002–2007); Mark Pabst; Monique Rosenthal; Philip Rosenthal, MD; Tessa Steel (2006–2008)
University of Washington Medical Center, Seattle, WA: Matthew Yeh, MD, PhD
Virginia Commonwealth University, Richmond, VA: Sherry Boyett, RN; Melissa J. Contos, MD; Michael Fuchs, MD; Amy Jones; Velimir AC Luketic, MD; Bimalijit Sandhu, MD; Arun J. Sanyal, MD; Carol Sargeant, RN, MPH; Kimberly Selph; Melanie White, RN
Virginia Mason Medical Center Seattle, WA: Kris V. Kowdley, MD; Jody Mooney, MS; James Nelson, PhD; Sarah Ackermann; Cheryl Saunders, MPH; Vy Trinh; Chia Wang, MD
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David Kleiner, MD, PhD
National Institute of Child Health and Human Development, Bethesda, MD: Gilman D. Grave, MD; Terry TK Huang, PhD, MPH
National Institute of Diabetes, Digestive and Kidney Diseases, Bethesda, MD: Edward Doo, MD; James Everhart, MD, MPH; Jay Hoofnagle, MD; Patricia R. Robuck, PhD, MPH (Project Scientist); Leonard Seeff, MD
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Frederick L. Brancati, MD, MHS; Jeanne M. Clark, MD, MPH; Ryan Colvin, MPH; Michele Donithan, MHS; Mika Green, MA; Rosemary Hollick (2003–2005); Milana Isaacson; Wana Kim; Alison Lydecker, MPH (2006–2008), Pamela Mann, MPH; Laura Miriel; Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Laura Wilson, ScM; Katherine Yates, ScM
Reference List
- 1.Pietrangelo A. Metals, oxidative stress, and hepatic fibrogenesis. Semin Liver Dis. 1996;16:13–30. doi: 10.1055/s-2007-1007215. [DOI] [PubMed] [Google Scholar]
- 2.Piperno A. Classification and diagnosis of iron overload. Haematologica. 1998;83:447–455. [PubMed] [Google Scholar]
- 3.Brunt EM. Pathology of hepatic iron overload. Semin Liver Dis. 2005;25:392–401. doi: 10.1055/s-2005-923311. [DOI] [PubMed] [Google Scholar]
- 4.Deugnier Y, Turlin B. Pathology of hepatic iron overload. World J Gastroenterol. 2007;13:4755–4760. doi: 10.3748/wjg.v13.i35.4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batts KP. Iron overload syndromes and the liver. Mod Pathol. 2007;20:S31–S39. doi: 10.1038/modpathol.3800715. [DOI] [PubMed] [Google Scholar]
- 6.Price L, Kowdley KV. The role of iron in the pathophysiology and treatment of chronic hepatitis C. Can J Gastroenterol. 2009;23:822–828. doi: 10.1155/2009/290383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannou GN, Tung BY, Kowdley KV. Iron in hepatitis C: villain or innocent bystander? Semin Gastrointest Dis. 2002;13:95–108. [PubMed] [Google Scholar]
- 8.Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Hosoki Y, Saito H, et al. Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res. 2005;29:189S–193S. doi: 10.1097/01.alc.0000189274.00479.62. [DOI] [PubMed] [Google Scholar]
- 9.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 10.George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, et al. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–318. doi: 10.1016/s0016-5085(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 11.Sumida Y, Nakashima T, Yoh T, Furutani M, Hirohama A, Kakisaka Y, et al. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38:32–38. doi: 10.1016/s0168-8278(02)00331-8. [DOI] [PubMed] [Google Scholar]
- 12.Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, et al. HFE Genotype, Parenchymal Iron Accumulation, and Liver Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2010;138:905–912. doi: 10.1053/j.gastro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Bonkovsky HL, Jawaid Q, Tortorelli K, LeClair P, Cobb J, Lambrecht RW, et al. Non-alcoholic steatohepatitis and iron: increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatitis. J Hepatol. 1999;31:421–429. doi: 10.1016/s0168-8278(99)80032-4. [DOI] [PubMed] [Google Scholar]
- 14.Bugianesi E, Manzini P, D'Antico S, Vanni E, Longo F, Leone N, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–187. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 15.Chitturi S, Weltman M, Farrell GC, McDonald D, Kench J, Liddle C, et al. HFE mutations, hepatic iron, and fibrosis: ethnic-specific association of NASH with C282Y but not with fibrotic severity. Hepatology. 2002;36:142–149. doi: 10.1053/jhep.2002.33892. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, Gramlich T, Bacon BR, Matteoni CA, Boparai N, O'Neill R, et al. Hepatic iron and nonalcoholic fatty liver disease. Hepatology. 1999;30:847–850. doi: 10.1002/hep.510300407. [DOI] [PubMed] [Google Scholar]
- 17.Sorrentino P, D'Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2009;50:351–357. doi: 10.1016/j.jhep.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Wilson L, Kleiner DE, Cummings OW, Brunt EM, Unalp A. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J Hepatol. 2008;48:829–834. doi: 10.1016/j.jhep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Rowe JW, Wands JR, Mezey E, Waterbury LA, Wright JR, Tobin J, et al. Familial hemochromatosis: characteristics of the precirrhotic stage in a large kindred. Medicine (Baltimore) 1977;56:197–211. [PubMed] [Google Scholar]
- 22.Kowdley KV. The role of iron in nonalcoholic Fatty liver disease: the story continues. Gastroenterology. 2010;138:817–819. doi: 10.1053/j.gastro.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 23.De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18:2569–2578. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 25.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281:28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab. 2008;7:205–214. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson JE, Bhattacharya R, Lindor KD, Chalasani N, Raaka S, Heathcote EJ, et al. HFE C282Y mutations are associated with advanced hepatic fibrosis in Caucasians with nonalcoholic steatohepatitis. Hepatology. 2007;46:723–729. doi: 10.1002/hep.21742. [DOI] [PubMed] [Google Scholar]
- 30.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–796. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, et al. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otogawa K, Kinoshita K, Fujii H, Sakabe M, Shiga R, Nakatani K, et al. Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: implications for the pathogenesis of human nonalcoholic steatohepatitis. Am J Pathol. 2007;170:967–980. doi: 10.2353/ajpath.2007.060441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong S, She H, Sung CK, Tsukamoto H. Iron-dependent activation of NF-kappaB in Kupffer cells: a priming mechanism for alcoholic liver disease. Alcohol. 2003;30:107–113. doi: 10.1016/s0741-8329(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 37.Xiong S, She H, Takeuchi H, Han B, Engelhardt JF, Barton CH, et al. Signaling role of intracellular iron in NF-kappaB activation. J Biol Chem. 2003;278:17646–17654. doi: 10.1074/jbc.M210905200. [DOI] [PubMed] [Google Scholar]
- 38.Xiong S, She H, Tsukamoto H. Signaling role of iron in NF-kappa B activation in hepatic macrophages. Comp Hepatol. 2004;3 (Suppl 1):S36. doi: 10.1186/1476-5926-2-S1-S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.She H, Xiong S, Lin M, Zandi E, Giulivi C, Tsukamoto H. Iron activates NF-kappaB in Kupffer cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G719–26. doi: 10.1152/ajpgi.00108.2002. [DOI] [PubMed] [Google Scholar]
- 40.Lin M, Rippe RA, Niemela O, Brittenham G, Tsukamoto H. Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol. 1997;272:G1355–G1364. doi: 10.1152/ajpgi.1997.272.6.G1355. [DOI] [PubMed] [Google Scholar]
- 41.Videla LA, Fernández V, Tapia G, Varela P. Oxidative stress-mediated hepatotoxicity of iron and copper: role of Kupffer cells. Biometals. 2003;16:103–111. doi: 10.1023/a:1020707811707. [DOI] [PubMed] [Google Scholar]
- 42.Pietrangelo A, Gualdi R, Casalgrandi G, et al. Molecular and cellular aspects of iron-induced hepatic cirrhosis in rodents. J Clin Invest. 1995;95:1824–1831. doi: 10.1172/JCI117861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pietrangelo A, Montosi G, Garuti C, et al. Iron-induced oxidant stress in nonparenchymal liver cells: mitochondrial derangement and fibrosis in acutely iron-dosed gerbils and its prevention by silybin. J Bioenerg Biomembr. 2002;34:67–79. doi: 10.1023/a:1013874804911. [DOI] [PubMed] [Google Scholar]
- 44.Houglum K, Bedossa P, Chojkier M. TGF-beta and collagen-alpha 1 (I) gene expression are increased in hepatic acinar zone 1 of rats with iron overload. Am J Physiol. 1994;267:G908–G913. doi: 10.1152/ajpgi.1994.267.5.G908. [DOI] [PubMed] [Google Scholar]
- 45.Ramm GA, Crawford DHG, Powell LW, et al. Hepatic stellate cell activation in genetic hemochromatosis: lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol. 1997;26:584–592. doi: 10.1016/s0168-8278(97)80424-2. [DOI] [PubMed] [Google Scholar]
- 46.Sta°l P, Broome U, Scheynius A, et al. Kupffer cell iron overload induces intercellular adhesion molecule-1 expression on hepatocytes in genetic haemochromatosis. Hepatology. 1995;21:1308–1316. doi: 10.1002/hep.1840210514. [DOI] [PubMed] [Google Scholar]
- 47.Aigner E, Theurl I, Theurl M, Lederer D, Haufe H, Dietze O, et al. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87:1374–1383. doi: 10.1093/ajcn/87.5.1374. [DOI] [PubMed] [Google Scholar]
- 48.Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931–939. doi: 10.1053/gast.2002.32403. [DOI] [PubMed] [Google Scholar]
- 49.Valenti L, Fracanzani AL, Fargion S. Effect of iron depletion in patients with nonalcoholic fatty liver disease without carbohydrate intolerance. Gastroenterology. 2003;124:866–867. doi: 10.1053/gast.2003.50130. [DOI] [PubMed] [Google Scholar]
- 50.Valenti L, Fracanzani AL, Dongiovanni P, Bugianesi E, Marchesini G, Manzini P, et al. Iron depletion by phlebotomy improves insulin resistance in patients with nonalcoholic fatty liver disease and hyperferritinemia: evidence from a case-control study. Am J Gastroenterol. 2007;102:1251–1258. doi: 10.1111/j.1572-0241.2007.01192.x. [DOI] [PubMed] [Google Scholar]