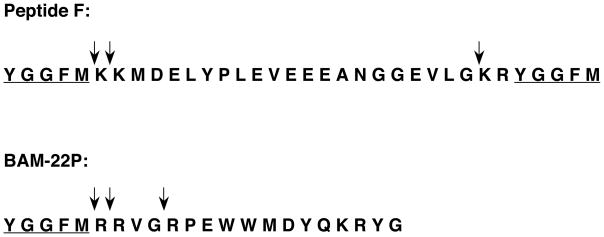Abstract
Neuropeptides are essential for cell-cell communication in the nervous and neuroendocrine systems. Production of active neuropeptides requires proteolytic processing of proneuropeptide precursors in secretory vesicles that produce, store, and release neuropeptides that regulate physiological functions. This review describes recent findings indicating the prominent role of cathepsin L in secretory vesicles for production of neuropeptides from their protein precursors. The role of cathepsin L in neuropeptide production was discovered using the strategy of activity-based probes for proenkephalin-cleaving activity for identification of the enzyme protein by mass spectrometry. The novel role of cathepsin L in secretory vesicles for neuropeptide production has been demonstrated in vivo by cathepsin L gene knockout studies, cathepsin L gene expression in neuroendocrine cells, and notably, cathepsin L localization in neuropeptide-containing secretory vesicles. Cathepsin L is involved in producing opioid neuropeptides consisting of enkephalin, β-endorphin, and dynorphin, as well as in generating the POMC-derived peptide hormones ACTH and α-MSH. In addition, NPY, CCK, and catestatin neuropeptides utilize cathepsin L for their biosynthesis. The neuropeptide-synthesizing functions of cathepsin L represent its unique activity in secretory vesicles, which contrasts with its role in lysosomes. Interesting evaluations of protease gene knockout studies in mice that lack cathepsin L compared to those lacking PC1/3 and PC2 (PC, prohormone convertase) indicate the key role of cathepsin L in neuropeptide production. Therefore, dual cathepsin L and prohormone convertase protease pathways participate in neuropeptide production. Significantly, the recent new findings indicate cathepsin L as a novel ‘proprotein convertase’ for production of neuropeptides that mediate cell-cell communication in health and disease.
Keywords: cathepsin L, neuropeptides, enkephalin, NPY, dynorphin, cholecystokinin, proteases, proprotein convertase, secretory vesicles, neurotransmission, endocrine, cell-cell communication
Introduction
Neuropeptides for cell-cell communication in the nervous and neuroendocrine systems
Neuropeptides in the nervous system are essential for activity-dependent neurotransmission of information among neurons. Many neuropeptide also function in peripheral systems for endocrine regulation of physiological functions. Moreover, the nervous and endocrine systems communicate with one another via these peptide neurotransmitters and hormones, collectively known as neuropeptides in this review. Knowledge of the biosynthetic mechanisms for the production of neuropeptides is critical for understanding cell-cell communication in neurotransmission and peptide hormone actions.
Production of neuropeptides requires proteolytic processing of their respective precursor proteins. This results in a multitude of distinct peptides with diverse physiological actions, such as enkephalin and opioid peptide regulation of analgesia (Law et al., 2000; Snyder and Pasternak, 2003), ACTH induction of steroid synthesis (Frohman, 1995), galanin involvement in cognition (Steiner et al., 2001), neuropeptide Y participation in regulating feeding behavior (Gehlert, 1999; Wieland et al., 2000), and numerous other functions (Table 1). The primary structures for proneuropeptides indicate that neuropeptides within the precursors are typically flanked at their NH2- and COOH-termini by pairs of basic residues, and sometimes by monobasic residues (Hook et al., 2008; Steiner, 1998; Seidah and Prat, 2002) (Figure 1). These multi-basic and monobasic residues provide sites of proteolytic processing that are cleaved to generate the active neuropeptides. Clearly, proteolytic pathways represent key steps for the biosynthesis of essential peptide neurotransmitters and hormones.
Table 1.
Neuropeptides in the Nervous and Endocrine Systems
| Neuropeptide |
Regulatory Function |
|---|---|
| enkephalin | analgesia |
| β-endorphin | analgesia |
| dynorphin | analgesia |
| ACTH | steroid production |
| α-MSH | skin pigmentation |
| insulin | glucose metabolism |
| glucagon | glucose metabolism |
| galanin | cognition |
| NPY | blood pressure (peripheral) and obesity (CNS) |
| cholecystokinin | anxiety, cognition, analgesia |
| somatostatin | growth regulation |
| vasopressin | water balance |
Peptide neurotransmitters and hormones are collectively termed neuropeptides. Examples of several neuropeptides and their primary regulatory functions are listed. Abbrevations are adrenocorticotropin hormone (ACTH), α-melanocyte stimulating hormone (α-MSH), and neuropeptide Y (NPY).
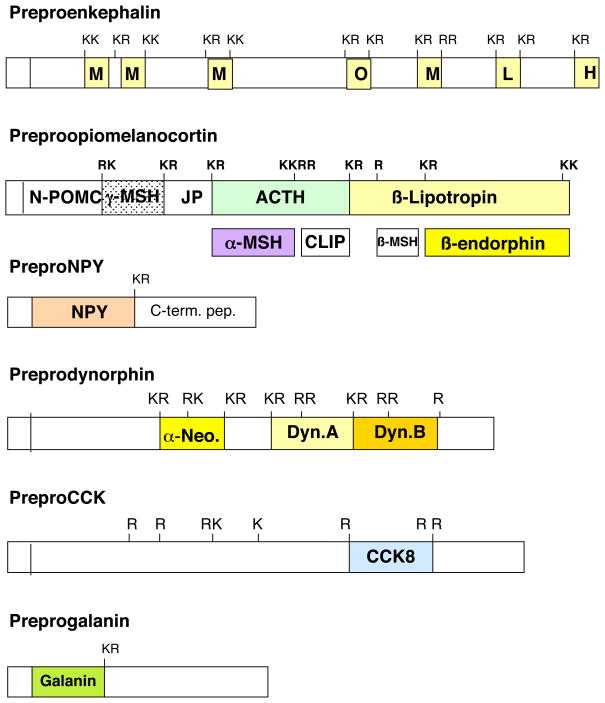
Figure 1. Structural Features of Proneuropeptides for Proteolytic Processing.
Prohormone precursor protein structures indicate that active peptide neurotransmitters and hormones are flanked by multi-basic residues that represent sites of proteolytic processing to generate active neuropeptides. The precursor proteins are shown for preproenkephalin, preproopiomelanocortin, preproNPY (NPY, neuropeptide Y), preprodynorphin, preproCCK (CCK, cholecystokinin), and preprogalanin. The NH2-terminal signal sequence is known to be cleaved by signal peptidases at the RER (rough endoplasmic reticulum) and the resultant prohormone undergoes trafficking to Golgi apparatus and packaging into secretory vesicles where prohormone processing occurs.
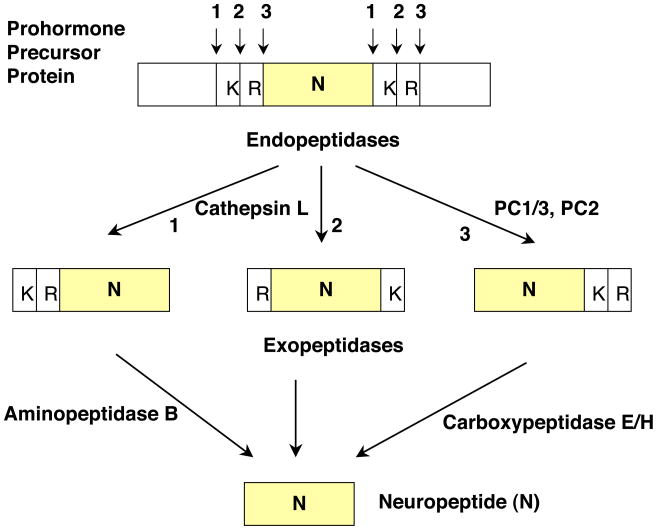
Proteolytic cleavage of proneuropeptides may occur at one of three positions at paired basic processing sites. These cleavages may consist of processing at the COOH- and NH2-termini of the dibasic residues, or between the dibasic residues (Figure 2). Resultant peptide intermediates require removal of basic residues from COOH- and/or NH2-termini by carboxypeptidase and aminopeptidase enzymes, respectively.
Figure 2. Cathepsin L and Proprotein Convertase Pathways for Neuropeptide Production.
Proneuropeptides typically contain active peptides flanked by paired basic residues. The dibasic processing sites undergo proteolytic cleavage at one of three sites (numbered 1, 2, and 3) which consist of cleavage at the NH2- or COOH-terminal sides of the dibasic residues, or between the dibasic residues. Peptide intermediates generated by cleavage at the NH2-terminal side of the dibasic site, or between the dibasic residues, will then require Arg/Lys aminopeptidase, represented by aminopeptidase B, to remove basic residues at the NH2-termini. Cleavage of proneuropeptides at the COOH-terminal side of dibasic residues then requires carboxypeptidase E to remove NH2-terminal basic residues.
Recent results indicate cathepsin L as a significant and novel proprotein convertase for neuropeptide production
Recent studies demonstrate a newly identified protease pathway mediated by secretory vesicle cathepsin L (Yasothornsrikul et al., 2003) and Arg/Lys aminopeptidase (Yasothornsrikul et al., 1998; Hwang et al., 2007b) for conversion of proneuropeptides into active neuropeptides. Cathepsin L cleaves dibasic residue sites at their NH2-termini and between the two basic residues (Yasothornsrikul et al., 1998). The peptide intermediates generated by cathepsin L subsequently require processing by Arg/Lys aminopeptidase and carboxypeptidase E to remove NH2- and COOH-terminal basic residues, respectively, for production of the final neuropeptide.
These findings complement ongoing studies in the field for proneuropeptide processing by the prohormone convertases, consisting of PC1/3 and PC2 as well as related PC enzymes (Steiner, 1998; Seidah and Prat, 2002; Hook et al., 2008). Subsequent to PC activity, the final neuropeptides are generated by carboxypeptidase E (Fricker, 1988; Hook et al., 1998).
The novel biological role of cathepsin L for proneuropeptide processing in regulated secretory vesicles is the focus of this review. The prominent role of cathepsin L in the production of active peptides contrasts with its previously known function as a lysosomal protease (Collette et al. 2004; Ishidoh and Kominami 2002). These recent results demonstrate cathepsin L as a novel ‘proprotein convertase’ for production of neuropeptides for cell-cell communication.
Strategy for Demonstration of Cathepsin L as a Proneuropeptide Processing Enzyme for Enkephalin Peptide Production
Identification of cathepsin L in proenkephalin processing by activity-based profiling and mass spectrometry
The biochemical strategy to elucidate the major proenkephalin-cleaving activity in neuropeptide-containing secretory vesicles was to identify the protease subclass for the activity and identify the responsible enzyme protein by activity-probe labeling followed by mass spectrometry (Yasothornsrikul et al., 2003). Model neuropeptide-containing secretory vesicles isolated from sympathoadrenal chromaffin cells of the sympathetic nervous system were utilized for purification of the proenkephalin cleaving activity. The activity was substantially inhibited by selective inhibitors of cysteine proteases (Yasothornsrikul et al., 1999, 2003).
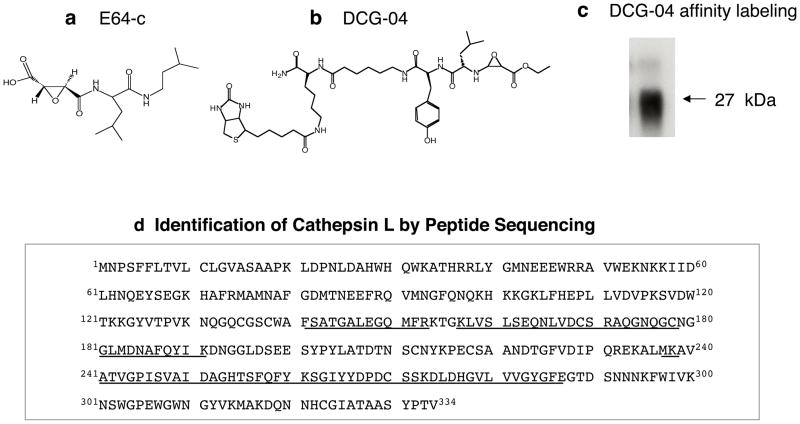
Chemical biology has developed sophisticated activity-based probes for identification of protease and enzyme families (Cravatt et al., 2008). Activity-based profiling of active cysteine proteases was instrumental for identification of the protease responsible for proenkephalin cleaving activity in chromaffin granules. The activity probe DCG-04, the biotinylated form of E64c that inhibits cysteine proteases (figure 3), was utilized for specific affinity labeling of the 27 kDa protease enzyme of the proenkephalin cleaving enzyme activity (Yasothornsrikul et al., 2003). Two-dimensional gels resolved DCG-04 labeled proteins of ~ 27 kDa, whose identification was indicated as cathepsin L by mass spectrometry of tryptic peptides (figure 3). These findings suggested a new biological function for cathepsin L in secretory vesicles for producing the enkephalin neuropeptide.
Figure 3. Identification of Proenkephalin-Cleaving Activity in Secretory Vesicles as Cathepsin L.
(a) E64-c cysteine protease inhibitor. The cysteine protease inhibitor E64-c was found to be a potent inhibitor of the proenkephalin (PE) – cleaving activity in secretory vesicles isolated from adrenal medullary chromaffin cells of the sympathetic nervous system.
(b) Structure of DCG-04, an activity-based probe for cysteine proteases. The modified cysteine protease inhibitor DCG-04, resulting from biotinylation of E64-c, was utilized for affinity-labeling of PE-cleaving activity in secretory vesicles.
(c) DCG-04 affinity labeling of cysteine protease activity in secretory vesicles. DCG-04 affinity labeling of purified PE-cleaving activity reveals a 27 kDa protein band. This band was subjected to peptide sequencing by tryptic digestion and tandem mass spectrometry.
(d) Identification of purified PE-cleaving activity as cathepsin L by mass spectrometry for peptide sequencing. Peptides derived from tryptic digests of DCG-04 affinity labeled 27 kDa proteins, sequenced by CID (MS/MS) mass spectrometry, are illustrated as the underlined amino acid sequences of bovine cathepsin L.
Cathepsin L localization in secretory vesicles that contain enkephalin and numerous neuropeptides
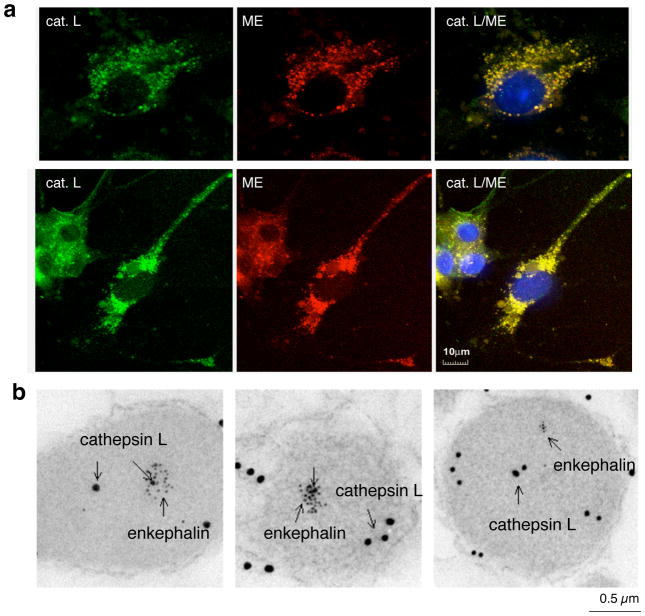
The biochemical identification of cathepsin L in secretory vesicles suggested the novel localization of cathepsin L in this organelle. Confirmation of the localization of cathepsin L within secretory vesicles (chromaffin granules) was demonstrated by immunofluorescence confocal microscopy and immunoelectron microscopy (figure 4) (Yasothornsrikul et al., 2003; Funkelstein et al., 2008a). Cathepsin L was also found to undergo cosecretion with enkephalin whose secretion is stimulated by activation of the regulated secretory pathway in these cells (Yasothornsrikul et al., 2003).
Figure 4. Localization of cathepsin L within enkephalin-containing secretory vesicles of neuronal-like chromaffin cells.
(a) Cathepsin L colocalization with (Met)enkephalin (ME) by immunofluorescence confocal microscopy. Immunofluorescence localization of cathepsin L (cat. L) was assessed by anti-cathepsin L detected with anti-rabbit IgG-Alexa 488 (green fluorescence), and ME was detected with anti-ME and anti-mouse IgG-Alexa 594 (red). Colocalization is illustrated by overlay of the images, illustrated by yellow fluorescence. Nuclei are illustrated by DAPI blue staining.
(b) Immunoelectron microscopy demonstrates colocalization of cathepsin L and (Met)enkephalin in secretory vesicles. Cathepsin L in secretory vesicles was indicated by anti-cathepsin L detected with 15 nm colloidal gold conjugated anti-rabbit IgG, and ME was detected with anti-ME and 6 nm colloidal gold conjugated to anti-mouse IgG. The presence of both 15 and 6 nm gold particles within these vesicles demonstrated the in vivo colocalization of cathepsin L and ME.
Cellular routing and trafficking of cathepsin L gene expression was demonstrated by coexpression of cathepsin L with proenkephalin in neuroendocrine PC12 cells (derived from rat adrenal medulla). Expression of cathepsin L resulted in its trafficking to secretory vesicles that contain enkephalin and neuropeptides (Hwang et al., 2007a). These findings indicated the novel location of cathepsin L in neuropeptide-containing secretory vesicles.
Cathepsin L gene knockout and expression result in regulation of enkephalin neuropeptide production from proenkephalin
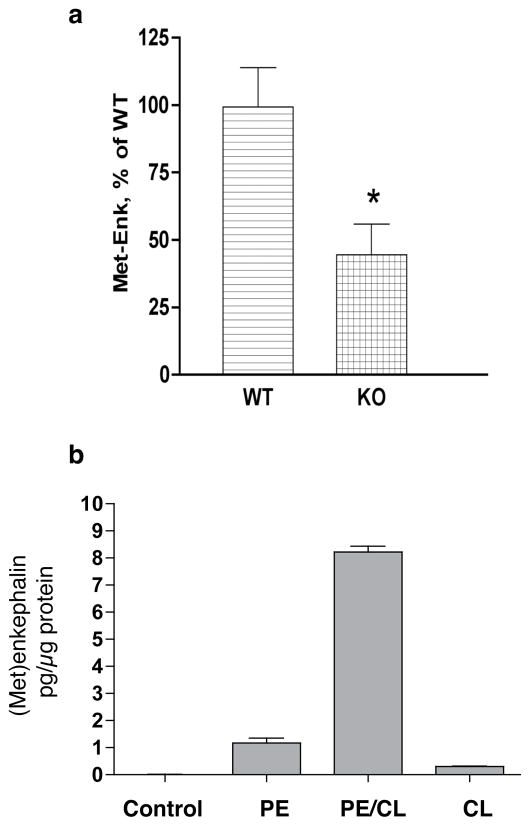
The in vivo role of cathepsin L in the production of enkephalin peptides was assessed in cathepsin L gene knockout mice. Brain levels of (Met)enkephalin (ME) were reduced by ~ 50% compared to wild-type control mice (figure 5) (Yasothornsikul et al., 2003). Enkephalin was measured by radioimmunoassay that specifically detected ME and not proenkephalin. Brains contained a higher ratio of proenkephalin/ME in brain, indicating retarded proenkephalin processing. Thus, the knockout results demonstrate the in vivo function of cathepsin L for enkephalin neuropeptide production.
Figure 5. Regulation of (Met)enkephalin in cathepsin L gene knockout mice.
(a) (Met)enkephalin (ME) levels in brains of knockout mice. ME levels in extracts of brain tissue from cathepsin L gene knockout mice (−/−) and wild-type control mice (+/+) were measured by RIA, shown as the mean + sem, with N = 10 for each group. A significant decrease (*) in enkephalin levels in knockout mice was observed (p < 0.013, two-tailed t-test).
(b) Elevated (Met)enkephalin production during cathepsin L expression. Elevation of cellular content of (Met)enkephalin in PC12 cells was observed after coexpression of cathepsin L (CL) and proenkephalin (PE). The radioimmunoassay (RIA) for (Met)enkephalin measures processed (Met)enkephalin since the RIA does not crossreact with PE. Controls included cells transfected with vector alone (no insert, control), PE alone, and cathepsin L (CL) alone. Experiments were conducted by transfection of triplicate wells of cells for each group, with RIA assay of (Met)enkephalin conducted in duplicate assays performed twice. Results are expressed as mean + standard error.
Studies of cathepsin L expression showed that cathepsin L participates in cellular processing of proenkephalin into (Met)enkephalin in the regulated secretory pathway of PC12 neuroendocrine cells (figure 5) (Hwang et al., 2007a). Cathepsin L generated high molecular weight PE-derived intermediates (of ~23, 18–19, 8–9, and 4.5 kDa) that were similar to those in vivo in chromaffin granules assessed by western blots (Hwang et al., 2007a). Such results demonstrated a cellular role for cathepsin L in the production of (Met)enkephalin in secretory vesicles.
Cleavage specificity of cathepsin L for dibasic processing sites of proneuropeptides
Cathepsin L cleaves at dibasic and monobasic processing sites that are cleaved within proneuropeptides. Production of (Met)enkephalin by cathepsin L was assessed with the enkephalin-containing peptide substrates BAM-22P and Peptide F, with identification of peptide products by MALDI-TOF mass spectrometry (Yasothornsrikul et al., 2003). Cathepsin generated (Met)enkephalin by cleaving BAM-22P at the dibasic ↓Arg-↓Arg and monobasic ↓Arg sites (figure 6). Peptide F was cleaved by cathepsin L at dibasic ↓Lys-↓Lys and ↓Lys-Arg sites (figure 6). Moreover, cathepsin L processing of full-length 35S-enkephalin precursor produced products of idential apparent molecular weight to those present in vivo in adrenal medulla (Hook et al., unpublished observations). Further cleavage studies with peptide-MCA substrates containing dibasic cleavage sites illustrated that cathepsin L cleaves at the COOH-terminal side of the dibasic sites, as well as at the N-terminal side of basic residues (Azaryan et al., 1994). Thus, cathepsin L generates peptide intermediates with basic residue extensions at NH2- and COOH-termini, which will then be removed by aminopeptidase B and carboxypeptidase E exopeptidases, respectively (figure 2). These exopeptidases have been characterized and shown to participate in neuropeptide biosynthesis in secretory vesicles (Hook et al., 2008; Fricker, 1988; Yasothornsrikul et al., 1998; Hwang et al., 2007b). The basic residue cleavage specificities of cathepsin L are appropriate for processing proprotein precursors into active neuropeptides.
Figure 6. Cathepsin L cleaves dibasic and monobasic processing sites of enkephalin-containing peptide substrates, BAM-22P and Peptide F.
(a) Cathepsin L cleavage of BAM-22P. BAM-22P was incubated with cathepsin L, and monoisotopic masses (MH+, in Daltons) of peptide products are illustrated for mass peaks. The primary sequence of BAM-22P (residues 1–22) is shown, and arrows indicate cathepsin L cleavage sites. The amino acid sequence of the (Met)enkephalin neuropeptide is underlined.
(b) Cathepsin L cleavage of Peptide F. Cathepsin L cleavage products of Peptide F were identified by MALDI-TOF mass spectrometry. Cathepsin L cleavage sites are illustrated by solid arrows above the Peptide F sequence.
Cathepsin L meets the criteria expected of a proneuropeptide processing enzyme for neuropeptide biosynthesis
Cathepsin L meets the key criteria expected of proteases for processing proneuropeptides into peptide neurotransmitters and hormones. These criteria are (1) the protease must be present in the organelle site where production of the active peptide occurs, primarily in secretory vesicles, (2) the protease must possess the appropriate substrate cleavage specificity to generate the active peptide, and (3) protease inhibition or gene knockdown should reduce production of the active peptide. Fulfillment of these criteria demonstrates the novel biological role of cathepsin L in the biosynthesis of enkephalin and numerous neuropeptides, as described in the next section.
Prominent Function of Cathepsin L for Production of Numerous Neuropeptides
The significant role of cathepsin L for processing proenkephalin into the active enkephalin neuropeptide raised the question of the possible function of cathepsin L for biosynthesis of other neuropeptides. Continued investigation of cathepsin L in secretory vesicles demonstrated its prominent role in the biosynthesis of numerous neuropeptides represented by neuropeptide Y (NPY), POMC-derived peptide hormones consisting of ACTH, β-endorphin, and α-MSH, as well as dynorphin, CCK, and catestatin neuropeptides (Funkelstein et al., 2008a, b; Minokadeh et al., 2010; Beinfeld et al., 2009; Biswas et al., 2009). Illustration of the biological function of cathepsin L for neuropeptide production has been demonstrated by protease gene knockout (summarized in Table 2), and protease gene expression combined with inhibitors and related approaches.
Table 2.
Reduced Levels of Neuropeptides in Cathepsin L Knockout Mice
| Neuropeptide |
Tissue |
Wild-Type |
Cathepsin L Knockout |
|---|---|---|---|
| Met-Enkephalin | Brain Cortex | 100% | 44% |
| NPY | Brain Cortex | 100% | 22% |
| CCK8 | Brain Cortex | 100% | 75% |
| Dynorphin A | Brain Cortex | 100% | 25% |
| Dynorphin B | Brain Cortex | 100% | 17% |
| α-neoendorphin | Brain Cortex | 100% | 10% |
| ACTH | Pituitary | 100% | 23% |
| β-Endorphin | Pituitary | 100% | 18% |
| α-MSH | Pituitary | 100% | 7% |
Results of tissue levels of several neuropeptides in brain cortex and pituitary in cathepsin L knockout mice compared to wild-type controls (100%) are illustrated (Yasothornsrikul et al., 2003; Funkelstein et al., 2008a, b; Beinfeld et al., 2009; Minokadeh et al., 2010). Substantial decreases in levels of these neuropeptides occur in cathepsin L knockout mice compared to control wild-type mice. These data indicate a role of cathepsin L in neuropeptide production.
Neuropeptide Y (NPY)
NPY in the brain functions as a peptide neurotransmitter in the regulation of feeding behavior (Wieland et al., 2000; Gehlert, 1999). In the peripheral sympathetic nervous system, NPY is released and regulates blood pressure (Walker et al., 1991). Thus, NPY has central and peripheral effects for regulating physiological functions. Novel findings show that cathepsin L participates as a key proteolytic enzyme for NPY production in secretory vesicles (Funkelstein et al., 2008a). Notably, NPY in cathepsin L knockout (KO) mice was substantially reduced in brain and adrenal medulla by 80% and 90%, respectively. Participation of cathepsin L in producing NPY predicts their colocalization in secretory vesicles, a primary site of NPY production. Indeed, cathepsin L is colocalized with NPY in brain cortical neurons and in chromaffin cells of adrenal medulla, demonstrated by immunofluorescence confocal microscopy and immunoelectron microscopy. Coexpression of proNPY with cathepsin L in neuroendocrine PC12 cells resulted in increased production of NPY. Furthermore, in vitro processing indicated cathepsin L processing of proNPY at paired basic residues. In contrast, PC2 gene knockout mice show no change in NPY brain levels (Miller et al., 2003b) but a peptidomic study showed a partial decrease in NPY (Zhang et al., 2010); the field has not yet reported studies of NPY in PC1/3 knockout mice. These studies of demonstrate an important role for cathepsin L in the production of NPY.
ACTH, β-endorphin, and α-MSH derived from the common POMC prohormone
Cathepsin L participates in the biosynthesis of the pituitary hormones ACTH, β-endorphin, and α-MSH that are derived from the common POMC (proopiomelanocortin) precursor by proteolytic processing (Funkelstein et al., 2008b). Each of these peptide hormones has distinct functions. ACTH stimulates glucocorticoid synthesis in adrenal cortex, β-endorphin is an endogenous opioid for analgesia, and α-MSH is involved in pigmentation of the skin. Notably, cathepsin L functions as a major proteolytic enzyme for production of POMC-derived peptide hormones in secretory vesicles.
Specifically, cathepsin L knockout (KO) mice showed major decreases in ACTH, β-endorphin, and α-MSH that were reduced to 23%, 18%, and 7%, respectively, of wild-type controls (100%) in pituitary. Increased levels of POMC in these knockout mice are consistent with cathepsin L processing of POMC. Cathepsin L is colocalized with β-endorphin and α-MSH in the intermediate pituitary, and with ACTH in the anterior pituitary. But cathepsin L was only partially colocalized with the lysosomal marker lamp-1 in pituitary. Expression of cathepsin L in pituitary AtT-20 cells resulted in increased ACTH and β-endorphin in the regulated secretory pathway. Furthermore, treatment of AtT-20 cells with CLIK-148, a specific inhibitor of cathepsin L, resulted in reduced production of ACTH and accumulation of POMC.
For comparison, PC2 knockout (KO) mice showed substantial reduction in α-MSH levels in pituitary and brain (Miller et al, 2003a). Since cathepsin L KO also results in a major reduction of α-MSH, these data indicate that cathepsin L and PC2 function jointly for α-MSH production. ACTH and β-endorphin levels were not altered in brains of PC2 KO mice compared to wild-type controls (Miller et al., 2003a), but these two peptides were elevated in pituitaries of PC2 KO mice suggesting that ACTH and β-endorphin serve as substrates for PC2. Interestingly, PC1/3 KO mice showed no changes in POMC-derived peptides (Pan et al., 2005).
These protease studies demonstrate a prominent role for cathepsin L, combined with PC2, in the production of ACTH, β-endorphin, and α-MSH peptide hormones in the regulated secretory pathway.
Dynorphin neuropeptides
Dynorphin opioid neuropeptides mediate neurotransmission for analgesia and behavioral functions (Akil et al., 1998; Wang et al., 2001; Shippenberg, 2007). Dynorphin A, dynorphin B, and α-neoendorphin are generated from prodynorphin by proteolytic processing. Recent studies demonstrate the significant role of the cysteine protease cathepsin L for producing dynorphins (Minokadeh et al., 2010). Cathepsin L KO mouse brains showed extensive decreases in dynorphin A, dynorphin B, and α-neoendorphin that were reduced by 75%, 83%, and 90%, respectively, compared to controls. Moreover, cathepsin L in brain cortical neurons was colocalized with dynorphins in secretory vesicles, the primary site of neuropeptide production. Cellular coexpression of cathepsin L with prodynorphin in PC12 cells resulted in increased production of dynorphins A and B. Comparative studies of PC1/3 and PC2 convertases showed that PC1/3 KO mouse brains had a modest decrease in dynorphin A, and PC2 knockout mice showed a minor decrease in α-neoendorphin. Overall, these results demonstrate a prominent role for cathepsin L, jointly with PC1/3 and PC2, for production of dynorphins in brain.
Cholecystokinin (CCK)
Cholecystokinin (CCK) is a peptide neurotransmitter whose production requires proteolytic processing of the proCCK precursor to generate active CCK8 neuropeptide in brain. Recent investigation demonstrates the significant role of the cysteine protease cathepsin L for CCK8 production (Beinfeld et al., 2009). In cathepsin L knockout (KO) mice, CCK8 levels were substantially reduced in brain cortex by an average of 75%. These amounts of decreased CCK8 in cathepsin L KO mice are greater than the modest decreases of CCK8 found in brain regions of PC1/3 or PC2 KO mice (Beinfeld et al., 2005; Cain et al., 2004; Rehfeld et al., 2008). Furthermore, brain cortex of PC1/3 KO mice shows no change in CCK8, and brain cortex of PC2 KO mice show an increase in CCK8. Therefore, data from the cathepsin L KO results provide strong evidence for cathepsin L as the major endoprotease responsible for CCK8 production in brain cortex.
Catestatin
The active catestatin peptide secreted from adrenal medulla of the sympathetic nervous system regulates blood pressure in stress (Mahata et al., 2010; Vaingankar et al., 2010). Catestatin is generated from the precursor chromogranin A (CgA) by proteolytic processing (Biswas et al., 2009). Notably, cathepsin L participates in catestatin formation. Endogenous cathepsin L colocalizes with CgA in the secretory vesicles of primary rat chromaffin cells. Transfection of PC12 cells with the cathepsin L cDNA resulted in its localization to secretory vesicles of PC12 cells. Cathepsin L cleaves CgA in vitro to catestatin-related peptides that show activity for inhibition of nicotine-induced catecholamine secretion from PC12 cells. These findings indicate that CgA can be utilized as a substrate for cathepsin L in the production of catestatin-like peptides. Thus far, PC1/3 KO mice show no change in CgA-derived peptides (Wardman et al., 2010); several peptides derived from CgA were reduced in PC2 KO mouse brains (Zhang et al., 2010). These protease investigations demonstrate a novel role for cathepsin L in the production of active catestatin peptide from CgA.
Novel Secretory Vesicle Function of Cathepsin L in Contrast to Lysosomal Function
These recent studies indicate the unique localization of cathepsin L in secretory vesicles for its biological function of neuropeptide production. As described in this review, cathepsin L is colocalized with the neuropeptides enkephalin, NPY, β-endorphin, ACTH, α-MSH, dynorphins, and CCK (Hook et al., 2008; Yasothornsrikul et al., 2003; Hwang et al., 2007a; Funkelstein et al., 2008a, b; Beinfeld et al., 2009; Biswas et al., 2009; Minokadeh et al., 2010).
The novel secretory vesicle function of cathepsin L contrasts with its known role in lysosomes for protein degradation. Cathepsin L was first identified as a degrading protease localized in rat liver lysosomes (Ansorge et al., 1977) and in lysosomes of other types of cells and species (Yokota et al., 1988, Ryvnyak et al., 2004). Yet, in addition to its lysosomal function, additional studies have indicated cathepsin L in secretory vesicles of rat pituitary GH4C1 (Waguri et al., 1995) and mouse NIH3T3 cell lines (Collette et al., 2004).
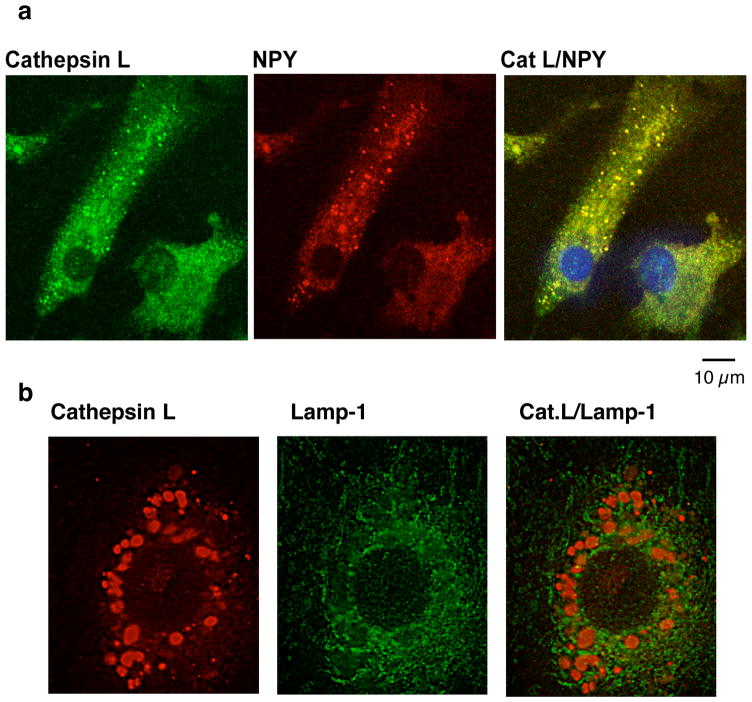
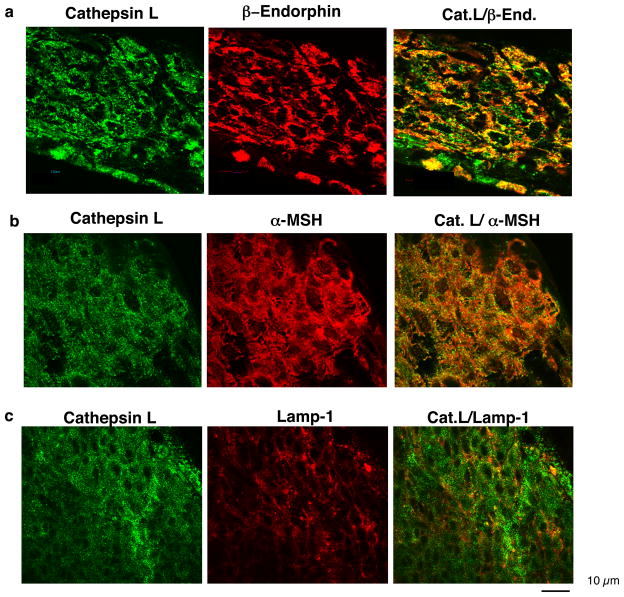
Comparison of the secretory vesicle localization of cathepsin L in secretory vesicles compared to lysosomes reveals differences in the relative portion of cathepsin L in these two organelles in different cell types. In bovine chromaffin cells of the sympathetic nervous system, cathepsin L is primarily colocalized with NPY and enkephalin (Yasothornsrikul et al., 2003, Funkelstein et al., 2008a), with no colocalization with the lysosomal marker lamp-1 (Fukuda, 1991) (figure 7). These data suggest a primary function of cathepsin L in secretory vesicles of chromaffin cells. But in mouse pituitary, while cathepsin L is colocalized with β-endorphin- and α-MSH in secretory vesicles, a portion of cellular cathepsin L is colocalized with the lysosomal marker lamp-1 (Funkelstein et al., 2008b) (figure 8). Also, in mouse pituitary AtT-20 cells, cathepsin L is partially colocalized with ACTH in secretory vesicles and with lamp-1 in lysosomes (Funkelstein et al., 2008b, Beinfeld et al., 2009). In the human pituitary, ACTH and beta-endorphin were also colocalized with cathepsin L (Hook et al., 2009).
Figure 7. In chromaffin cells of the sympathoadrenal nervous system, cathepsin L is primarily localized to neuropeptide-containing secretory vesicles, compared to lysosomes.
(a) Cathepsin L localization with NPY assessed by immunofluorescence confocal microscopy. Adrenal medullary chromaffin cells in primary culture were analyzed for colocalization of cathepsin L with NPY present in secretory vesicles assess by immunofluorescence confocal microscopy. Excellent colocalization of cathepsin L (green fluorescence) and NPY (red fluorescence) was observed in the merged image (yellow fluorescence), with the majority of cellular cathepsin L localized with NPY in secretory vesicles.
(b) Evaluation of cathepsin L with the lysosomal marker lamp-1 by immunofluorescence confocal microscopy. Cathepsin L localization in chromaffin cells (red fluorescence) was compared to that of lamp-1 (green fluorescence), a lysosomal marker. Results show that cathepsin L in chromaffin cells displays no colocalization with lamp-1. These data indicate that cathepsin L in chromaffin cells is primarily localized with NPY and other neuropeptides (Funkelstein et al., 2008a) in secretory vesicles.
Figure 8. In intermediate pituitary, cathepsin L is highly localized with β-endorphin and α-MSH peptide hormones, and is partially localized with the lysosomal marker lamp-1.
(a) Cathepsin L and β-endorphin colocalization. Colocalization of cathepsin L with β-endorphin in intermediate pituitary (mouse) was demonstrated by immunofluorescence confocal microscopy. Cathepsin L immunoreactivity (green fluorescence) showed excellent overlapping colocalization with β-endorphin (red fluorescence), shown by the yellow fluorescence of merged cathepsin L/β-endorphin fluorescent immunostaining. The majority of β-endorphin, contained in secretory vesicles, was colocalized with cathepsin L.
(b) Cathepsin L and α-MSH colocalization. The overlapping colocalization of cathepsin L with α-MSH peptide hormone in intermediate pituitary was illustrated by immunofluorescence confocal microscopy. The majority of cathepsin L (green fluorescence) and α-MSH (red fluorescence) were colocalized, shown by the merged areas of yellow fluorescence.
(c) Partial colocalization of cathepsin L and lamp-1 localization in mouse intermediate pituitary cells. The cellular distribution of cathepsin L in intermediate lobe pituitary cells was compared to that of the lysosomal marker lamp-1. Cathepsin L (green fluorescence) was partially colocalized with lamp-1 (red fluorescence). This subcellular distribution of cathepsin L is consistent with the localization of cathepsin L in secretory vesicles as well as in lysosomes.
In addition, cathepsin L has been found in the nucleus of mouse and human cells for proteolysis of the histone H3 in the regulation of gene expression (Hiwasa and Sakiyama, 1996, Duncan et al., 2008, Sullivan et al., 2009). Thus, cathepsin L has biological functions in secretory vesicles and the nucleus, that differ from its lysosomal functions.
Clearly, new findings indicate that cathepsin L localization in several organelles, with notable function in secretory vesicles for neuropeptide production as discussed in this review. These studies lead to the conclusion that cathepsin L not only functions in lysosomes for protein degradation, but cathepsin L also functions in secretory vesicles for neuropeptide production.
Combined Roles of Cathepsin L and Prohormone Convertases PC1/3 and PC2 for Neuropeptide Production
The biological role of cathepsin L for production of neuropeptides, combined with function of the subtilisin-like proprotein convertases, indicate dual protease pathways for neuropeptide production (figure 2). The proprotein convertase family consists of PC1/3, PC2, furin, PACE4, PC3, PC5/6, and PC7 for processing at basic residues (For reviews, see Seidah and Prat, 2002; Fugere and Day, 2005; Scamuffa et al., 2006; Hook et al., 2008). Several related proteases that cleave at non-basic residues have also been identified, consisting of the subtilisin/kexin-like isozyme-1 (SKI-1/SIP) and the neural apoptosis-regulated convertase-1 (PCSK9/NARC-1) (Seidah and Prat, 2002; Thomas, 2002; Fugere and Day, 2005; Scamuffa et al., 2006). These represent excellent reviews of the roles of proprotein convertases in neuropeptide biosynthesis. Due to the limitations in space of this article, readers are referred to these reviews on the PC enzymes.
Conclusion: Cathepsin L Represents a Distinct Protease Pathway, Combined with Proprotein Convertases, for Biosynthesis of Neuropeptides
Recent findings demonstrate the key biological role of cathepsin L in secretory vesicles for processing proneuropeptides into active peptide neuropeptides and hormones. The cathepsin L cysteine protease pathway participates with the prohormone convertase subtilisin-like protease pathway for neuropeptide biosynthesis (figure 2). The presence of the two distinct mechanistic protease classes for proneuropeptide processing indicates cellular insurance for selection of protease pathways for production of specific neuropeptides. It will be of interest to understand regulatory mechanisms for selection of both or either processing pathways for production of particular neuropeptides in different tissues. Indeed, endogenous protease inhibitors exist for each of these two distinct protease pathways. Cathepsin L in secretory vesicles is regulated by the endogenous inhibitors endopin 2 (a serpin inhibitor) (Hwang et al., 2005), kunitz protease inhibitor form of APP (KPI-APP (Hook et al., 1999), and cystatin C (Leonardi et al., 1996). The PC1/3 and PC2 enzymes are regulated by endogenous inhibitors consisting of proSAAS (Basak et al., 2001) and 7B2 (Fortenberry et al., 1999), respectively. The presence of different protease subclasses allows specific regulation by inhibitors.
Future knowledge of specific drug regulators for particular neuropeptides can lead to future translational research for small molecule regulation of cathepsin L and prohormone convertase pathways in the control of physiological functions. For example, regulation of opioid peptide production – enkephalin, β-endorphin, and dynorphin – may lead to new drugs for analgesia and pain relief. Specific small molecule control of hypothalamic NPY in the control of feeding behavior may lead to improvement in obese conditions. Regulation of hypothalamic CRF and pituitary ACTH production is important for the control of steroid biosynthesis in adrenal cortex for metabolic regulation. PC related proteases have been implicated in sterol and lipid metabolism (Seidah et al., 2006), tumor progression (Basi et al., 2005), atherosclerosis (Stawowy et al., 2005), and other physiological and disease conditions.
The newly identified biological function of cathepsin L for neuropeptide biosynthesis opens new avenues for understanding neuropeptide regulation and potentially for targeting this protease in drug development for control of neuropeptides in health and disease.
Acknowledgments
The authors appreciate collaboration of these studies with Dr. Thomas Reinheckel and Dr. Christoph Peters at the Albert-Ludwigs University, Freiburg, Germany. This work was supported by grants from the National Institutes of Health to V. Hook, and a NIH fellowship award to A. Minokadeh. Support for J. Zadina is from the VA and ONR. Contents do not represent the views of the VA or the US Government.
Support: The authors appreciate support of this research by grants to V. Hook from the National Institutes of Health
References
- Akil H, Owens C, Gutstein H, Taylor L, Currran E, Watson S. Endogenous opioids: overview and current issues. Drug Alcohol Depend. 1998;51:127–140. doi: 10.1016/s0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- Ansorge S, Kirschke H, Friedrich K. Conversion of proinsulin into insulin by cathepsins B and L from rat liver lysosomes. Acta Biol Med Ger. 1977;36:1723–1727. [PubMed] [Google Scholar]
- Azaryan AV, Hook VYH. Unique cleavage specificity of ‘prohormone thiol protease’ related to proenkephalin processing. FEBS Lett. 1994;341:197–202. doi: 10.1016/0014-5793(94)80456-7. [DOI] [PubMed] [Google Scholar]
- Basak A, Koch P, Dupelle M, Fricker LD, Devi LA, Chrétien M, Seidah NG. Inhibitory specificity and potency of proSAAS-derived peptides toward proprotein convertase 1. J Biol Chem. 2001;276:32720–32728. doi: 10.1074/jbc.M104064200. [DOI] [PubMed] [Google Scholar]
- Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Molec Carcinogenesis. 2005;44:151–161. doi: 10.1002/mc.20134. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Blum A, Vishnuvardhan D, Fanous S, Marchand JE. Cholecystokinin levels in prohormone convertase 2 knock-out mouse brain regions reveal a complex phenotype of region-specific alterations. J Biol Chem. 2005;280:38410–38415. doi: 10.1074/jbc.M500055200. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Funkelstein L, Foulon T, Cadel S, Kitagawa K, Toneff T, Reinheckel T, Peters C, Hook VYH. Cathepsin L plays a major role in cholecystokinin production in mouse brain and in pituitary AtT-20 cells: protease gene knockout and inhibitor studies. Peptides. 2009;30:1882–1991. doi: 10.1016/j.peptides.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas N, Rodriquez-Flores JL, Courel M, Gayen JR, Vaingankar SM, Mahata M, Torpey JW, Taupenot L, O’Connor DT, Mahata SK. Cathepsin L colocalizes with chromogranin A in chromaffin vesicles to generate active peptides. Endocrinology. 2009;150:3547–3557. doi: 10.1210/en.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain BM, Connolly K, Blum AC, Vishnuvardhan D, Marchand JE, Zhu X, Steiner DF, Beinfeld MC. Genetic inactivation of prohormone convertase (PC1) causes a reduction in cholecystokinin (CCK) levels in the hippocampus, amygdala, pons and medulla in mouse brain that correlates with the degree of colocdalization of PC1 and CCk mRNA in these structures in rat brain. J Neurochem. 2004;89:307–313. doi: 10.1046/j.1471-4159.2003.02295.x. [DOI] [PubMed] [Google Scholar]
- Collette J, Bocock JP, Ahn K, Chapman RL, Godbold G, Yeyeodu S, Erickson AH. Biosynthesis and alternate targeting of the lysosomal cysteine protease cathepsin L. Int Rev Cytol. 2004;241:1–51. doi: 10.1016/S0074-7696(04)41001-8. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Wright AR, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- Duncan EM, Muratore-Schroeder TL, Cook RG, Garcia BA, Shabanowitz J, Hunt DF, Allis CD. Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell. 2008;135:284–294. doi: 10.1016/j.cell.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortenberry Y, Liu J, Lindberg I. The role of the 7B2 CT peptide in the inhibition of prohormone convertase 2 in endocrine cell lines. J Neurochem. 1999;73:994–1003. doi: 10.1046/j.1471-4159.1999.0730994.x. [DOI] [PubMed] [Google Scholar]
- Fricker LD. Carboxypeptidase E. Annu Rev Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991;266:21327–21330. [PubMed] [Google Scholar]
- Frohman LA. Diseases of the anterior pituitary. In: Felig P, Baxter JD, Frohman LA, editors. Endocrinology and Metabolism. 3. McGraw-Hill, Inc. Health Professions Division; New York: 1995. pp. 293–297. [Google Scholar]
- Fugere M, Day R. Cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. Trends Pharmacol Sci. 2005;26:294–301. doi: 10.1016/j.tips.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkelstein L, Toneff T, Hwang SR, Reinheckel T, Peters C, Hook VYH. Cathepsin L participates in the production of neuropeptide Y in secretory vesicles, demonstrated by protease gene knockout and expression. J Neurochem. 2008a;106:384–391. doi: 10.1111/j.1471-4159.2008.05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkelstein L, Toneff T, Hwang SR, Beuschlein F, Lichtenauer UD, Reinheckel T, Peters C, Hook VYH. Major role of cathepsin L for producing the peptide hormones ACTH, β-endorphin, and α-MSH, illustrated by protease gene knockout and expression. J Biol Chem. 2008b;83:35652–35659. doi: 10.1074/jbc.M709010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33:329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- Hiwasa T, Sakiyama S. Nuclear localization of procathepsin L/MEP in ras-transformed mouse fibroblasts. Cancer Lett. 1996;99:87–91. doi: 10.1016/0304-3835(95)04041-2. [DOI] [PubMed] [Google Scholar]
- Hook VYH, Funkelstein L, Lu D, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu Rev Pharmacol Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V, Funkelstein L, Toneff T, Mosier C, Hwang SR. Human pituitary contains dual cathepsin L and prohormone convertase processing pathway components involved in converting POMC into the peptide hormones ACTH, alpha-MSH, and beta-endorphin. Endocrine. 2009;35:429–437. doi: 10.1007/s12020-009-9163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook VYH, Sei C, Yasothornsrikul S, Toneff T, Kang YH, Efthimiopoulos S, Robakis NK, Van Nostrand W. The kunitz protease inhibitor form of the amyloid precursor protein (KPI/APP) inhibits the proneuropeptide processing enzyme prohormone thiol protease (PTP). Colocalization of KPI/APP and PTP in secretory vesicles. J Biol Chem. 1999;274:3165–3172. doi: 10.1074/jbc.274.5.3165. [DOI] [PubMed] [Google Scholar]
- Hook VYH, Yasothornsrikul S. Carboxypeptidase and aminopeptidase proteases in pro-neuropeptide processing. In: Hook VYH, editor. Proteolytic and Cellular Mechanisms in Prohormone and Proprotein Processing. Landes Bioscience Publishers; Austin, Texas: 1998. pp. 121–140. [Google Scholar]
- Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, Hook VYH. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J Biol Chem. 2007a;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- Hwang SR, O’Neill A, Bark S, Foulon T, Hook VYH. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J Neurochem. 2007b;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- Hwang SR, Stoka V, Turk V, Hook VYH. The novel bovine serpin endopin 2C demonstrates selective inhibition of the cysteine protease cathepsin L compared to the serine protease elastase, in cross-class inhibition. Biochemistry. 2005;44:7757–7767. doi: 10.1021/bi050053z. [DOI] [PubMed] [Google Scholar]
- Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol Chem. 2002;383:1827–1831. doi: 10.1515/BC.2002.206. [DOI] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Leonardi A, Turk B, Turk V. Inhibition of cathepsins L and S by stefins and cystatins. Biol Chem Hoppe Seyler. 1996;377:319–321. [PubMed] [Google Scholar]
- Mahata SK, Mahata M, Fung MM, O’Connor DT. Catestatin: a multifunctional peptide from chromogranin A. Regul Pept. 2010 doi: 10.1016/j.regpep.2010.01.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld M, Hook VYH. Obliteration of α-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J Neurochem. 2003a;86:556–563. doi: 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- Miller R, Toneff T, Vishnuvardhan D, Beinfeld M, Hook VYH. Selective roles for the PC2 processing enzyme in the regulation of peptide neurotransmitter levels in brain and peripheral neuroendocrine tissues of PC2 deficient mice. Neuropeptides. 2003b;37:140–148. doi: 10.1016/s0143-4179(03)00027-1. [DOI] [PubMed] [Google Scholar]
- Minokadeh A, Funklestein L, Toneff T, Hwang SR, Reinheckel T, Peters C, Zadina J, Hook VYH. Cathepsin L participates in dynorphin neuropeptide production in brain cortex, illustrated by protease gene knockout and expression. Mol Cell Neurosci. 2010;43:98–107. doi: 10.1016/j.mcn.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Pan H, Nanno D, Che FY, Zhu X, Salton SR, Steiner DF, Fricker LD, Devi LA. Neuropeptide processing profiles in mice lacking prohormone convertase-1. Biochemistry. 2005;44:4939–4948. doi: 10.1021/bi047852m. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF, Bundgaard JR, Hannibal J, Zhu X, Norrbom C, Steiner DF, Friis-Hansen L. The cell-specific pattern of cholecystokinin peptides in endocrine cells versus neurons is governed by the expression of prohormone convertases 1/3, 2, and 5/6. Endocrinology. 2008;149:1600–1608. doi: 10.1210/en.2007-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvnyak VV, Ryvnyak EI, Tudos RV. Electron histochemical localization of cathepsin L in the liver. Bull Exp Biol Med. 2004;137:90–91. doi: 10.1023/b:bebm.0000024396.17895.77. [DOI] [PubMed] [Google Scholar]
- Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Khatib AM, Prat A. The proprotein convertases and their implication in sterol and/or lipid metabolism. Biol Chem. 2006;387:871–877. doi: 10.1515/BC.2006.110. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Prat A. Precursor convertases in the secretory pathway, cytosol and extracellular milieu. Essays Biochem. 2002;38:79–94. doi: 10.1042/bse0380079. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol & Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH, Pasternak GW. Historical review: opioid receptors. Trends Pharmacol Sci. 2003;24:198–205. doi: 10.1016/S0165-6147(03)00066-X. [DOI] [PubMed] [Google Scholar]
- Stawowy P, Fleck E. Proprotein convertases furin and PC5: targeting atherosclerosis and restenosis at multiple levels. J Molec Med. 2005;83:865–875. doi: 10.1007/s00109-005-0723-8. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Jureus A, Clifton DK, Luo M, Gutshall M, Ma SY, Mufson EJ, Crawley JN. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S, Tosetto M, Kevans D, Coss A, Wang L, O'Donoghue D, Hyland J, Sheahan K, Mulcahy H, O'Sullivan J. Localization of nuclear cathepsin L and its association with disease progression and poor outcome in colorectal cancer. Int J Cancer. 2009;125:54–61. doi: 10.1002/ijc.24275. [DOI] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nature Rev. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman JH, Zhang X, Gagnon S, Castro LM, Zhu X, Steiner DF, Day R, Fricker LD. Analysis of peptides in prohorone convertase 1/3 null mouse brain using quantitative peptidomics. J Neurochem. 2010:215–225. doi: 10.1111/j.1471-4159.2010.06760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaingankar SM, Li Y, Biswas N, Gayen J, Choksi S, Rao F, Ziegler MG, Mahata SK, O’Connor DT. Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses. J Hypertens. 2010 doi: 10.1097/HJH.0b013e328336ed3e. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguri S, Sato N, Watanabe T, Ishidoh K, Kominami E, Sato K, Uchiyama Y. Cysteine proteinases in GH4C1 cells, a rat pituitary tumor cell line, are secreted by the constitutive and regulated secretory pathways. Eur J Cell Biol. 1995;67:308–318. [PubMed] [Google Scholar]
- Walker P, Grouzmann E, Burnier M, Waeber B. The role of neuropeptide Y in cardiovascular regulation. TIPS. 1991;12:111–115. doi: 10.1016/0165-6147(91)90518-w. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gardell LW, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Hamilton BS, Krist B, Doods HN. The role of NPY in metabolic homeostasis: implications for obesity therapy. Expert Opin Investig Drugs. 2000;9:1327–1346. doi: 10.1517/13543784.9.6.1327. [DOI] [PubMed] [Google Scholar]
- Yasothornsrikul S, Aaron W, Toneff T, Hook VYH. Evidence for the proenkephalin processing enzyme prohormone thiol protease (PTP) as a multicatalytic cysteine protease complex: activation by glutathione localized to secretory vesicles. Biochemistry. 1999;38:7421–7430. doi: 10.1021/bi990239w. [DOI] [PubMed] [Google Scholar]
- Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook VYH. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasothornsrikul S, Toneff T, Hwang SR, Hook VYH. Arginine and lysine aminopeptidase activities in chromaffin granules of bovine adrenal medulla: relevance to prohormone processing. J Neurochem. 1998;70:153–163. doi: 10.1046/j.1471-4159.1998.70010153.x. [DOI] [PubMed] [Google Scholar]
- Yokota S, Nishimura Y, Kato K. Localization of cathepsin L in rat kidney revealed by immunoenzyme and immunogold techniques. Histochemistry. 1988;90:277–283. doi: 10.1007/BF00495971. [DOI] [PubMed] [Google Scholar]
- Zhang X, Panm H, Peng B, Steiner DF, Pintar JE, Fricker LD. Neuropeptidomic analysis establishes a major role for prohormone convertase-2 in neuropeptide biosynthesis. J Neurochem. 2010;112:168–1179. doi: 10.1111/j.1471-4159.2009.06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]