Abstract
Purpose
Cumulative sensory neurotoxicity (sNT) is the dose-limiting toxicity of oxaliplatin, which commonly leads to early discontinuation of oxaliplatin-based therapy in the palliative and adjuvant settings. In a nonrandomized, retrospective study, intravenous (IV) calcium/magnesium (Ca/Mg) was associated with reduced oxaliplatin-induced sNT.
Methods
Patients with colon cancer undergoing adjuvant therapy with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) were randomly assigned to Ca/Mg (1g calcium gluconate plus 1g magnesium sulfate pre- and post-oxaliplatin) or placebo, in a double-blinded manner. The primary end point was the percentage of patients with grade 2 or greater sNT at any time during or after oxaliplatin-based therapy by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; version 3) criteria. An oxaliplatin-specific sNT scale and patient questionnaires were also used to assess sNT. After 104 of 300 planned patients were enrolled, the study was closed. This was due to preliminary reports from another trial that suggested that Ca/Mg decreased treatment efficacy; these data were subsequently found to be incorrect.
Results
Overall, 102 patients were available for analysis. Ca/Mg decreased the incidence of chronic, cumulative, grade 2 or greater sNT, as measured by NCI CTCAE (P = .038) and also by the oxaliplatin-specific sNT scale (P = .018). In addition, acute muscle spasms associated with oxaliplatin were significantly reduced (P = .01) No effect on acute, cold-induced sNT was found. No substantial differences in adverse effects were noted between Ca/Mg and placebo.
Conclusion
Despite early termination and decreased statistical power, this study supports IV Ca/Mg as an effective neuroprotectant against oxaliplatin-induced cumulative sNT in adjuvant colon cancer.
INTRODUCTION
Oxaliplatin is an integral component of adjuvant and palliative therapy of colorectal cancer (CRC), commonly used with infusional fluorouracil (FU) and leucovorin (LV) to form the FOLFOX regimen.1 In a pivotal phase III trial, MOSAIC (Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer), FOLFOX was superior to FU/LV in patients with resected stage III colon cancer for disease-free and overall survivals.2 The most relevant toxicity of oxaliplatin is sensory neurotoxicity, manifested in two distinct forms.3 First, it manifests as a unique acute, mainly cold-triggered, sensory neuropathy affecting most patients, transient in nature and usually not lasting more than 7 days after oxaliplatin administration. Additionally, laryngopharyngeal dysesthesia, jaw spasms, and muscle cramps—sometimes described as stiffness in the hands or feet or the inability to release a grip, similar to neuromyotonia—have been included in the acute symptom complex.4,5 Second, oxaliplatin is associated with a chronic sensory neurotoxicity that includes numbness and tingling, affects hands and feet, and is correlated with the cumulative dose of oxaliplatin administered over time. This chronic neurotoxicity may also include burning/shooting pain symptoms, which mimics the sensory neurotoxicity observed with cisplatin. Although this cumulative, chronic neurotoxicity is often reversible, it is commonly the dose-limiting toxicity of oxaliplatin.
It has been speculated that the acute phenomena are a result of hyperexcitability of neurons as a result of functional impairment of voltage-gated ion channels in nerve membranes and the neuromuscular junction as a result of chelation of calcium by oxalate.6 In support of this hypothesis, increases in the concentration of extracellular calcium have facilitated sodium channel closing and, thus, potentially might decrease the observed oxaliplatin-induced hyperexcitability of peripheral neurons.7,8 Furthermore, magnesium supplementation has long been established to prevent hypomagnesemia associated with cisplatin.9 Although the acute hyperexcitability syndrome appears to be closely linked to functional alterations of voltage-gated ion channels, the pathogenesis of the cumulative, chronic neurotoxicity leading to structural changes in peripheral neurons and dorsal root ganglia (DRG) is largely unknown. One hypothesis suggested that repeated episodes of acute hyperexcitability could eventually lead to structural damage of neurons. These considerations provided the background for clinical investigations to test the use of high intravenous doses of calcium and magnesium salts to prevent the acute and chronic sensory effects associated with oxaliplatin.
In a nonrandomized, retrospective analysis, 161 patients with advanced CRC were treated with three different oxaliplatin-based regimens.10 Ninety-six patients of this series received calcium gluconate 1 g and magnesium sulfate 1 g (ie, Ca/Mg) before and after oxaliplatin; the remaining 65 patients served as control group. The median administered cumulative oxaliplatin dose was 910 mg/m2 in the Ca/Mg group but was only 650 mg/m2 in the control group. Only 4% of patients receiving Ca/Mg, compared with 31% patients of the control group, stopped chemotherapy as a result of neurotoxicity (P < .001). Laryngopharyngeal dysesthesias affected 9% of the control group but were absent in patients receiving Ca/Mg. Likewise, grade 3 neurotoxicity was less frequently observed in the Ca/Mg group (8% v 20%, P = .003), and more patients with Ca/Mg remained on chemotherapy after 9 months (15% v 9%). In this retrospective analysis, the antitumor efficacy of treatment did not appear to be affected by Ca/Mg. In contrast, patients were able to stay on therapy longer, thus potentially obtaining prolonged benefit from oxaliplatin-based therapy.
On the basis of these intriguing data, the current trial was developed to determine whether Ca/Mg infusions would prevent and/or ameliorate chronic, cumulative neurotoxicity associated with oxaliplatin; increase the cumulative oxaliplatin doses that could be delivered without chronic neurotoxicity; ameliorate the acute neuropathy associated with oxaliplatin; and cause any adverse events.
PATIENTS AND METHODS
Patients considered for this clinical trial had to be adults with stage II or stage III adenocarcinoma of the colon who, after curative-intent resection, were scheduled to receive 6 months of oxaliplatin-based adjuvant FOLFOX chemotherapy involving 85 mg/m2 oxaliplatin every 2 weeks, either as FOLFOX4 or modified FOLFOX6.2,11 Patients were allowed to receive bevacizumab or cetuximab in combination with FOLFOX. Participants needed to have adequate hematologic parameters to allow chemotherapy and had to have serum total bilirubin, creatinine, and calcium concentrations ≤ 1.5× upper normal limit (UNL). Women of childbearing potential needed to have a negative pregnancy test.
Patients were not allowed on trial if they had a pre-existing peripheral neuropathy of any grade; had hypercalcemia; had received prior treatment with neurotoxic chemotherapy, such as oxaliplatin, cisplatin, a taxane, or a vinca alkaloid; were taking digoxin; had a history of heart block; were receiving treatment with anticonvulsants; had other medical conditions which, in the opinion of the treating physician, would make the protocol unreasonably hazardous for the patient; had a family history of a genetic/familial neuropathy; or were not considered to be able to comply with the protocol. The protocol was approved per US federal guidelines, and patients needed to provide appropriate informed written consent.
At study entry and before each 2-week cycle of chemotherapy, patients underwent a history and physical exam along with laboratory tests (ie, serum Ca, Mg, sodium, potassium, creatinine, AST, total bilirubin, and alkaline phosphatase). At the same times, neuropathy assessments were obtained by three separate means. The primary neuropathy assessment was measured by the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE; version 3.0). Standardized questions regarding neurotoxic symptoms and examples of answers (Appendix Table A1, online only) were used to allow a more accurate classification of patient-reported symptoms as grade 1, 2, 3, or 4. The second neuropathy assessment method used an oxaliplatin-specific scale to generate an investigator-assigned score that focused on the reversibility of neurotoxicity symptoms between treatment cycles.12 The third means of collecting neuropathy data consisted of asking patients to rate, on a 0 to 10 numerical analog scale, both of the following: “how much of a problem has numbness in fingers and toes been for the last 2 weeks”; and “how much of a problem has tingling in fingers and toes been for the last 2 weeks.” These North Central Cancer Treatment Group (NCCTG) –developed patient-reported outcome (PRO) questions were able to detect the onset of clinically meaningful peripheral neuropathy, on average, 2 to 3 months earlier than the NCI CTCAE measure.13
In addition, patients completed daily questionnaires before each dose of FOLFOX and for 5 additional days after the initiation of each cycle of FOLFOX to provide data regarding the acute, transient neuropathy observed with oxaliplatin therapy.3 With this questionnaire, questions answered on a 0 to 10 numerical analog scale, which related to the previous 24 hours, addressed the following: sensitivity touching cold items, discomfort swallowing cold items, throat discomfort, and muscle cramps.
At study entry and before each 2-week cycle of chemotherapy, patients had adverse event monitoring. This included PRO variables evaluated by questionnaires for diarrhea, abdominal cramping, constipation, dyspnea, and dysphagia, which all used a numerical analog scale ranging from 0 to 10.
Patients were randomly allocated to receive either intravenous calcium gluconate plus magnesium sulfate, 1 g of each agent in 100 mL D5W over 30 minutes, immediately before and after each dose of oxaliplatin, or an identical-appearing placebo. Patients and all clinical study personnel who interacted with them were blinded to the treatment arm. Patients were stratified by age (younger than 65 years v older), sex, and whether they were to receive FOLFOX4 versus modified FOLFOX6.
If the patient developed any clinically significant adverse event attributed to Ca/Mg (or placebo), the Ca/Mg (or placebo) was stopped. The patient then continued to be observed according to protocol criteria. The Ca/Mg (or placebo) was held if a serum calcium was greater than UNL or if a serum creatinine was greater than 1.5 × UNL. Serum Ca and serum creatinine levels were required to be lower than these limits before resuming Ca/Mg.
Oxaliplatin dose-modification guidelines per oxaliplatin package insert were provided and supported a dose reduction of oxaliplatin to 65 mg/m2 for patients who experienced persistent grade 2 sensory neurotoxicity that did not resolve within 2 weeks; patients with persistent grade 3 sensory neurotoxicity were to discontinue oxaliplatin but continue FU/LV. Guidelines also indicated an oxaliplatin dose reduction to 65 mg/m2 and a 20% FU reduction in patients who recovered from grades 3 to 4 gastrointestinal toxicity, grade 4 neutropenia, or grades 3 to 4 thrombocytopenia. The next dose was to be delayed until neutrophils were ≥ 1.5 × 109/L and platelets were ≥ 75 × 109/L.
A two-arm, randomized, placebo-controlled, double-blind, phase III design was employed. The primary end point was the percentage of patients with grade 2 or greater chronic sensory neurotoxicity during or at the end of therapy. The differences in proportions between the two treatment arms were tested by using the χ2 test in an intent-to-treat fashion. Patients with missing values for the primary end point were considered to not have grade 2or greater chronic sensory neurotoxicity. In the MOSAIC trial, 32% and 12% of patients receiving FOLFOX experienced grade 2 and grade 3 sensory neurotoxicity, respectively.2 For the original design of 150 patients per arm, the χ2 test had 80% power to detect a 15% difference (25% v 40%) in incidence of grade 2 or greater sensory neurotoxicity. Because the study was stopped early, with 50 patients per arm, there was 80% power to detect a 27% difference (13% v 40%) in the incidence rate of grade 2 or greater sensory neurotoxicity.
Secondary end points were numerous and included the percentage of patients with acute neuropathic events, which was compared between groups by using the same approach as the primary analysis. The various alternative continuous measures of sensory neurotoxicity were compared at specific time points and over time through the use of area under the curve summary statistics between groups via Wilcoxon rank sum testing, whereas the time to onset of grade 2 or greater sensory neurotoxicity was compared between groups by using Kaplan-Meier survival curves and log-rank testing. Descriptive summary statistics for the neuropathy-related symptom scores on the 0 to 10 scale included the mean at each time point per group and associated 95% CI. Spline smoothing was applied to estimate the trends over time.
RESULTS
Baseline Characteristics
This study opened for accrual in January 2006, and it was closed in June 2007. At the time of study closure, 104 patients had been randomly assigned from 20 NCCTG member sites, and 102 patients had started study medication (n = 50, Ca/Mg; n = 52, placebo).
The study accrual was stopped short of the original accrual goal because of an unsubstantiated data monitoring committee (DMC) report of another clinical trial that was evaluating the use of Ca/Mg with palliative FOLFOX treatment in advanced CRC.14 This trial, named CONcePT (ie, Combined Oxaliplatin Neurotoxicity Prevention Trial), used a 2 × 2 study design to study two strategies to decrease oxaliplatin-induced neurotoxicity by using Ca/Mg and also by using different chemotherapy scheduling options (intermittent v continuous use of oxaliplatin). In mid-2007, an interim analysis of study data was presented to the CONcePT DMC. These data were interpreted as demonstrating a lower response rate in the group getting Ca/Mg versus the placebo group,15 leading to an early closure of this study. Given this new information, which had not been audited, new patient entry on the current clinical trial was terminated, and Ca/Mg/placebo infusions were stopped in all patients who were still on therapy. A subsequent independent, blinded radiologic review of radiologic scans from the CONcePT trial, however, demonstrated that the antitumor response rate was actually numerically lower in the group receiving the placebo versus the group receiving Ca/Mg.14
The present analysis is based on the 102 patients who initiated study medication. Data cutoff was done after 127 days (ie, 4 months plus 1 week) because of premature study closure and because of the protocol modification mandating that the Ca/Mg therapy be discontinued.
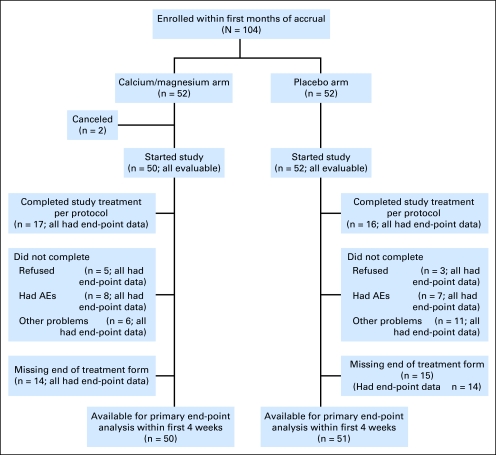
Patient study flow is illustrated in a consort diagram (Fig 1). Baseline patient characteristics, detailed in Table 1, were equivalent in the two treatment groups.
Fig 1.
CONSORT flow diagram. AE, adverse event.
Table 1.
Patient Demographic and Clinical Characteristics
| Variable | Ca/Mg (n = 50) |
Placebo (n = 52) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .78 | ||||
| < 65 | 33 | 66 | 33 | 63 | |
| Sex | .83 | ||||
| Male | 27 | 54 | 27 | 52 | |
| Ethnicity | .57 | ||||
| White | 48 | 96 | 50 | 96 | |
| Black or African American | 1 | 2 | 1 | 2 | |
| American Indian or Alaska Native | 0 | 0 | 1 | 2 | |
| Not reported: patient refused or not available | 1 | 2 | 0 | 0 | |
| Regimen | .96 | ||||
| FOLFOX4 | 3 | 6 | 3 | 6 | |
| Modified FOLFOX6 | 47 | 94 | 49 | 94 | |
Abbreviations: Ca, calcium; Mg, magnesium; FOLFOX4, 2-hour infusion of leucovorin (200 mg/m2/d) followed by a fluorouracil bolus (400 mg/m2/d) and 22-hour infusion (600 mg/m2/d) for 2 consecutive days every 2 weeks, together with oxaliplatin 85 mg/m2 as a 2-hour infusion on day 1; FOLFOX6, 2-hour infusion of leucovorin (400 mg/m2) followed by a fluorouracil bolus (400 mg/m2) and 46-hour infusion (2,400 mg/m2) every 2 weeks, together with oxaliplatin 85 mg/m2 as a 2-hour infusion on day 1.
Neuropathy Data
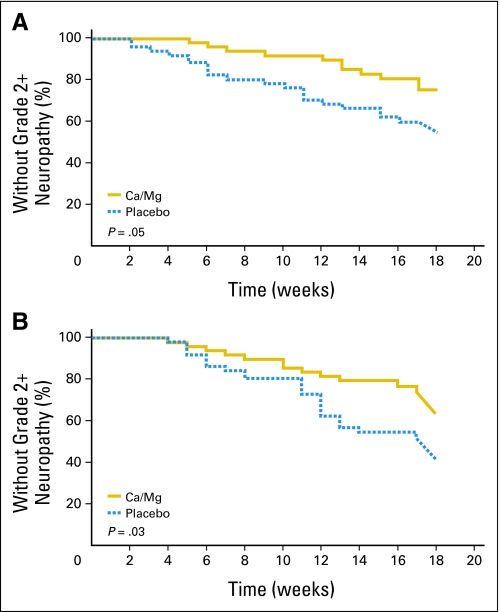
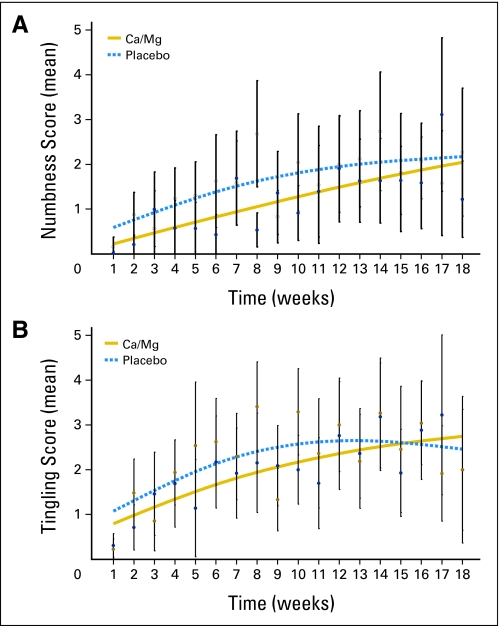
The use of Ca/Mg reduced the incidence of grade 2 or greater sensory neurotoxicity significantly (the primary study end point) with both the NCI CTCAE classification (22% v 41%; P = .038) and the oxaliplatin-specific scale (28% v 51%,; P = .018). In addition, the onset of grade 2 or greater sensory neurotoxicity was significantly delayed in patients who received Ca/Mg. Data regarding the time to grade 2 sensory neurotoxicity per CTCAE and the time to grade 2 sensory neurotoxicity per the oxaliplatin-specific scale are illustrated in Table 2 and Figure 2. Data from the third means of evaluating neuropathic symptoms used in this trial, related to PRO numbness and tingling symptoms, are illustrated in Figure 3 . All three measures show similar patterns, with substantially less sensory neurotoxicity in the patients who received Ca/Mg.
Table 2.
Primary End Point Data Regarding Sensory Neuropathy
| Neurotoxicity Measure and Grade | Placebo(n = 52) |
Ca/Mg(n = 50) |
χ2P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| CTCAE | |||||
| 0-1 | 30 | 50 | 39 | 78 | .038 |
| ≥ 2 | 21 | 41 | 11 | 22 | |
| Oxaliplatin-specific scale | |||||
| 0-1 | 25 | 49 | 36 | 72 | .018 |
| ≥ 2 | 26 | 51 | 14 | 28 | |
Abbreviation: Ca, calcium; Mg, magnesium; CTCAE, Common Terminology Criteria for Adverse Events.
Fig 2.
Time to grade 2 or worse sensory neuropathy as measured by (A) Common Toxicity Criteria for Adverse Events or by (B) an oxaliplatin-specific scale. Ca, calcium; Mg, magnesium.
Fig 3.
Patient-reported outcomes regarding questions about (A) numbness in fingers/toes (P = .021) and (B) tingling in fingers/toes (P = .662). Open circles represent mean values for the placebo group; filled circles represent mean values for the calcium/magnesium (Ca/Mg) group. Vertical lines represent 95% CIs about the means.
One of the goals of this trial was to compare the rates of FOLFOX dose reductions or discontinuation as a result of neuropathy in the two arms. However, because of the early closure of this trial, no reliable data were available to address this issue.
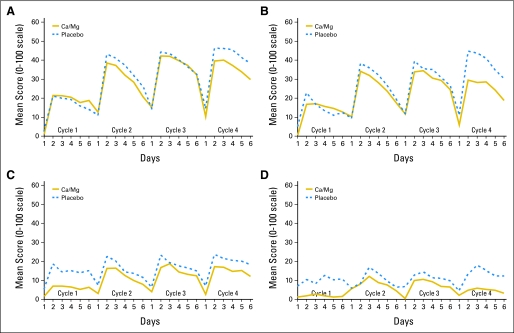
The acute oxaliplatin-related neuropathic effects were evaluated regarding sensitivities to touching cold items, discomfort swallowing cold liquids, throat discomfort, and muscle cramps. In a sequential analysis over several treatment cycles, it is apparent that there are differences in some of these symptoms in the first cycle, compared with subsequent cycles, of therapy (Table 3 and Fig 4). However, there were no evident differences in these symptoms between cycles 2 to 4 of therapy. Ca/Mg did not appear to decrease cold sensitivity toxicities. Throat discomfort and muscle cramps, although still present, had a less apparent difference in cycle 1 versus subsequent cycles. With regard to there being a decrease in symptoms in those receiving Ca/Mg, this was primarily seen with muscle cramps, especially with the first cycle.
Table 3.
Acute Neurotoxicity Data
| Symptom and Cycle | Placebo AUC |
Ca/Mg AUC |
P* |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Between Treatment Arms | Between Cycle 1 and Cycles 2-4 for Placebo | Between Cycle 1 and Cycles 2-4 for Ca/Mg | |
| Sensitivities to touching cold items | |||||||
| Cycle 1 | 75 | 107 | 78 | 97 | .64 | < .001 | < .001 |
| Cycles 2-4 | 155 | 115 | 149 | 109 | .85 | ||
| Discomfort swallowing cold liquids | |||||||
| Cycle 1 | 65 | 94 | 59 | 94 | .58 | < .001 | < .001 |
| Cycles 2-4 | 133 | 109 | 115 | 108 | .27 | ||
| Throat discomfort | |||||||
| Cycle 1 | 65 | 103 | 26 | 39 | .12 | .17 | .004 |
| Cycles 2-4 | 72 | 93 | 60 | 76 | .44 | ||
| Muscle cramps | |||||||
| Cycle 1 | 46 | 101 | 8 | 20 | .01 | .09 | .007 |
| Cycles 2-4 | 49 | 70 | 29 | 60 | .05 | ||
NOTE. Area under the curve: lower numbers are better.
Abbreviations: AUC, area under the curve; Ca, calcium; Mg, magnesium; SD, standard deviation.
Rank sum test.
Fig 4.
Acute symptoms regarding (A) sensitivities to touching cold items, (B) discomfort swallowing cold liquids, (C) throat discomfort, and (D) muscle cramps. Ca, calcium; Mg, magnesium.
In evaluating whether acute symptoms with the first cycle predicted for acute symptoms with subsequent cycles, Spearman rank correlations were between 0.41 and 0.54, demonstrating that patients with high scores on cycle 1 were moderately more likely to also have high values in cycle 2. The degree of acute symptoms with the first cycle, however, did not predict for the development of chronic, cumulative neurotoxicity.
Evaluation of Toxicity of Intravenous Calcium/Magnesium
There were no toxicity differences between the two study arms in any of the elicited toxicities that were described in the Methods section. Hypercalcemia occurred in none and in one (2%) of the patients in the Ca/Mg and placebo groups, respectively, and hypermagnesemia occurred in seven (14%) and eight (16%) patients, respectively. One patient in the Ca/Mg arm did have a lab value illustrating grade 3 hypermagnesemia 2 weeks after the fourth cycle of treatment but none during three prior cycles. He stopped the Ca/Mg treatment then. In retrospect, this was considered to likely be a lab error.
DISCUSSION
The data from this prospective, randomized, double-blinded, placebo-controlled trial support the prestudy hypothesis that Ca/Mg infusions decrease oxaliplatin-mediated chronic, cumulative sensory neurotoxicity.
However, it is clear that there still is not general agreement among oncologists as to whether or not Ca/Mg therapy clearly decreases oxaliplatin-induced neuropathy. The above-noted DMC report regarding the CONcePT trial, which involved patients with metastatic disease, is a cause of this confusion. This report15 (subsequently found to be unsubstantiated on independent radiology review14) initially purported that the Ca/Mg infusion arm was associated with a decreased response rate. The final results of this prematurely aborted trial were unable to demonstrate a substantial decrease in neuropathy by CTC criteria in the patients who received Ca/Mg versus those receiving placebo, although patients receiving Ca/Mg reported decreased severity of cumulative sensory neurotoxicity by PRO assessment.14 These results might well be explained by hindrance of the study with low patient numbers and early study closure.
With regard to the current NCCTG trial, results are affected by a lower-than-planned number of patients. In addition, when the study was stopped in view of the initial results of the CONcePT DMC report, the patients who were receiving Ca/Mg were instructed to stop receiving such. Thus, intermediate or long-term data with regard to the potential benefit of Ca/Mg are lacking from this current trial.
In response to the DMC report of the CONcePT trial, preliminary data of a French study entitled NEUROXA (Neurotoxicity of Oxaliplatin) were released.16 This study included 144 patients who were randomly assigned, in a double-blind manner, to receive Ca/Mg versus placebo. Early, still-blinded analysis, of this trial noted substantially less neurotoxicity in one group versus the other (5% v 24% grade 3 neurotoxicity according to NCI CTCAE; P < .001). The blind for this study, however, has not yet been broken. Thus, although it could be surmised that the lower neuropathy was found in the Ca/Mg arm, this has yet to be clarified. Additionally, this study included patients in both the adjuvant and palliative care settings. In contrast, the current trial only studied patients receiving curative-intent therapy with a predefined number of planned oxaliplatin-based treatment cycles. In addition, the NEUROXA study did not utilize the comprehensive ways to assess neurotoxicity that were utilized in the current trial, with the enhanced NCI-CTCAE scale, the oxaliplatin-specific scale, and the patient-reported outcomes measures.
With regard to the acute, transient symptoms associated with oxaliplatin, data from this trial did not support that Ca/Mg decreased cold sensitivity, but there was a suggestion that it decreased muscle cramping. This trial did demonstrate that patients were less troubled by cold sensitivities, throat discomfort, and muscle cramps in the first cycle, compared with subsequent cycles.
The question about the safety of Ca/Mg infusions deserves discussion. Given the corrected results of the CONcePT trial data DMC report, demonstrating no evidence of inferior antitumor activity with the Ca/Mg study arm,14 and reported results from the French NEUROXA study that demonstrated equivalent antitumor activity in patients receiving Ca/Mg and those on a placebo16; physicians should be comfortable that Ca/Mg therapy does not interfere with the antitumor activity of FOLFOX.
Given the equipoise regarding the utility of Ca/Mg infusions for preventing oxaliplatin-induced neuropathy and to provide additional data regarding this issue, it can be rationally argued that the data from the present trial should be confirmed. To this end, the NCCTG has initiated patient entry on a subsequent randomized, double-blind trial, whereby a similar group of patients is being randomly assigned to receive identical doses of Ca/Mg before and after FOLFOX versus placebos on the same schedule versus a dose of Ca/Mg before FOLFOX with a dose of placebo after FOLFOX treatment. This will hopefully settle whether this therapy is worthwhile and whether both doses are needed.
Appendix
Additional participating institutions.
Upstate Carolina CCOP, Spartanburg, SC (James D. Bearden, III, MD); Medcenter One Health Systems, Bismarck, ND (Edward J. Wos, DO); Michigan Cancer Research Consortium, Ann Arbor, MI (Philip J. Stella, MD); Carle Cancer Center CCOP, Urbana, IL (Kendrith M. Rowland, Jr, MD); Iowa Oncology Research Association CCOP, Des Moines, IA (Roscoe F. Morton, MD); Montana Cancer Consortium, Billings, MT (Benjamin T. Marchello, MD); Heartland Cancer Research CCOP, St Louis, MO (Alan P. Lyss, MD); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN (Robin Zon, MD); Cancer Care Associates, Tulsa, OK (Mark R. Olsen, MD); St Vincent Regional Cancer Center CCOP, Green Bay, WI (Anthony J. Jaslowski, MD); Sioux Community Cancer Consortium, Sioux Falls, SD (Loren K. Tschetter, MD); Toledo Community Hospital Oncology Program CCOP, Toledo, OH (Paul L. Schaefer, MD); Medical College of Georgia, Augusta, GA (Anand P. Jillella, MD).
Table A1.
Neurotoxicity Evaluation
| Question | Sample Answers for Each Toxicity Grade and NCI CTCAE Definition |
|||
|---|---|---|---|---|
| I: Loss of Deep Tendon Reflexes or Paresthesia, Including Tingling, but Not Interfering With Function | II: Objective Sensory Alteration or Paresthesia, Including Tingling, Interfering With Function, but Not With Activities of Daily Living | III: Sensory Alteration or Paresthesia Interfering With Activities of Daily Living | IV: Permanent Sensory Losses That Are Disabling | |
| Do you have problems tying your shoe laces, buttoning your shirts, fastening buckles or pulling up zippers? | “No, I might feel some tingling in my hands, but I have no problems tying laces, buttoning shirts, fastening buckles or pulling up zippers” | “It is a bit harder than before, but I can still tie laces, button shirts, fasten buckles or pull up zippers” | “I have severe difficulties tying shoe laces, buttoning shirts, fastening buckles or pulling up zippers” or “I cannot tie laces, button shirts, fasten buckles or pull up zippers anymore” | “I haven't been able to tie laces, button shirts, fasten buckles or pull up zippers for weeks” |
| Do you have problems writing? | “No, I might feel some tingling in my hands, but I have no problems writing” | “It is a bit harder than before, but I can still write” | “I have severe difficulties writing” or “I cannot write anymore” | “I haven't been able to write for weeks” |
| Do you have problems putting on your jewelry or your watch? | “No, I might feel some tingling in my hands, but I have no problems putting on my jewelry or my watch” | “It is a bit harder than before, but I can still put on my jewelry or my watch” | “I have severe difficulties putting on my jewelry or my watch” or “I cannot put on my jewelry or my watch anymore” | “I haven't been able to put on my jewelry or my watch for weeks” |
| Do your have problems walking? | “No, I might feel some tingling in my feet, but I have no problems walking” | “It is a bit harder than before, but I can still walk” | “I have severe difficulties walking” or “I cannot walk anymore” | “I haven't been able to walk for weeks |
NOTE. Adverse events definitions per NCI CTCAE version 3.
Abbreviation: NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
Footnotes
Supported in part by Public Health Service grants No. CA-25224, CA-37404, CA-35113, CA-63849, CA-35269, CA-35103, CA-63848, CA-35195, CA-35101, CA-35119,and CA-35415.
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00316914.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Axel Grothey, sanofi-aventis; Steven R. Alberts, Bristol-Meyers Squibb; Charles L. Loprinzi, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Axel Grothey, Daniel A. Nikcevich, Jeff A. Sloan, Paul J. Novotny, Charles L. Loprinzi
Financial support: John W. Kugler, Todor Dentchev, Donald B. Wender, Charles L. Loprinzi
Administrative support: John W. Kugler, Todor Dentchev, Donald B. Wender, Charles L. Loprinzi
Provision of study materials or patients: Axel Grothey, Daniel A. Nikcevich, John W. Kugler, Peter T. Silberstein, Todor Dentchev, Donald B. Wender, Umesh Chitaley, Steven R. Alberts
Collection and assembly of data: Axel Grothey, Jeff A. Sloan, Todor Dentchev, Paul J. Novotny, Charles L. Loprinzi
Data analysis and interpretation: Axel Grothey, Jeff A. Sloan, Todor Dentchev, Paul J. Novotny, Charles L. Loprinzi
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 2.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 3.Grothey A. Oxaliplatin-safety profile: Neurotoxicity. Semin Oncol. 2003;30(4 suppl 15):5–13. doi: 10.1016/s0093-7754(03)00399-3. [DOI] [PubMed] [Google Scholar]
- 4.Lehky TJ, Leonard GD, Wilson RH, et al. Oxaliplatin-induced neurotoxicity: Acute hyperexcitability and chronic neuropathy. Muscle Nerve. 2004;29:387–392. doi: 10.1002/mus.10559. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RH, Lehky T, Thomas RR, et al. Acute oxaliplatin-induced peripheral nerve hyperexcitability. J Clin Oncol. 2002;20:1767–1774. doi: 10.1200/JCO.2002.07.056. [DOI] [PubMed] [Google Scholar]
- 6.Gamelin L, Grolleau F, Boisdron-Celle M, et al. Acute neurotoxicity of oxaliplatin: Effects on neuronal voltage-gated soidum channels. Proc Am Assoc Cancer Res. 2001;42:935. doi: 10.1152/jn.2001.85.5.2293. abstr 5020. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong CM, Cota G. Calcium block of Na+ channels and its effect on closing rate. Proc Natl Acad Sci U S A. 1999;96:4154–4157. doi: 10.1073/pnas.96.7.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adelsberger H, Quasthoff S, Grosskreutz J, et al. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. Eur J Pharmacol. 2000;406:25–32. doi: 10.1016/s0014-2999(00)00667-1. [DOI] [PubMed] [Google Scholar]
- 9.Lajer H, Daugaard G. Cisplatin and hypomagnesemia. Cancer Treat Rev. 1999;25:47–58. doi: 10.1053/ctrv.1999.0097. [DOI] [PubMed] [Google Scholar]
- 10.Gamelin L, Boisdron-Celle M, Delva R, et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: A retrospective study of 161 patients receiving oxaliplatin combined with 5-fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. 2004;10:4055–4061. doi: 10.1158/1078-0432.CCR-03-0666. [DOI] [PubMed] [Google Scholar]
- 11.Wolmark N, Yothers G, O'Connell MJ, et al. A phase III trial comparing mFOLFOX6 to mFOLFOX6 plus bevacizumab in stage II or III carcinoma of the colon: Results of NSABP Protocol C-08. J Clin Oncol. 2009;27(suppl 15S):6s. abstr LBA4. [Google Scholar]
- 12.Lévi FA, Zidani R, Vannetzel JM, et al. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: A randomized multi-institutional trial. J Natl Cancer Inst. 1994;86:1608–1617. doi: 10.1093/jnci/86.21.1608. [DOI] [PubMed] [Google Scholar]
- 13.Sloan JA, Berk L, Roscoe J, et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute–sponsored clinical trials networks. J Clin Oncol. 2007;25:5070–5077. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 14.Grothey A, Hart LL, Rowland KM, et al. Intermittent oxaliplatin administration and time-to-treatment-failure in metastatic colorectal cancer: Final results of the phase III CONCEPT trial. J Clin Oncol. 2008;26(suppl 15S):180s. abstr 4010. [Google Scholar]
- 15.Hochster HS, Grothey A, Childs BH. Use of calcium and magnesium salts to reduce oxaliplatin-related neurotoxicity. J Clin Oncol. 2007;25:4028–4029. doi: 10.1200/JCO.2007.13.5251. [DOI] [PubMed] [Google Scholar]
- 16.Gamelin L, Boisdron-Celle M, Morel A, et al. Oxaliplatin-related neurotoxicity: Interest of calcium-magnesium infusion and no impact on its efficacy. J Clin Oncol. 2008;26:1188–1189. doi: 10.1200/JCO.2007.15.3767. author reply 1189–1190. [DOI] [PubMed] [Google Scholar]