Abstract
Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) and inhibition of cyclooxygenase-2 (COX2) activity by non-steroidal anti-inflammatory drugs (NSAID) can both attenuate skin tumorigenesis. The present study examined the hypothesis that combining ligand activation of PPARβ/δ with inhibition of COX2 activity will increase the efficacy of chemoprevention of chemically-induced skin tumorigenesis over that observed with either approach alone. To test this hypothesis, wild-type and Pparβ/δ-null mice were initiated with 7, 12-dimethylbenz[a]anthracene (DMBA), topically treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) to promote tumorigenesis, and then immediately treated with topical application of the PPARβ/δ ligand GW0742, dietary administration of the COX2 inhibitor nimesulide, or both GW0742 and nimesulide. Ligand activation of PPARβ/δ with GW0742 caused a PPARβ/δ-dependent delay in the onset of tumor formation. Nimesulide also delayed the onset of tumor formation and caused inhibition of tumor multiplicity (46%) in wild-type mice but not in Pparβ/δ-null mice. Combining ligand activation of PPARβ/δ with dietary nimesulide resulted in a further decrease of tumor multiplicity (58%) in wild-type mice but not in Pparβ/δ-null mice. Biochemical and molecular analysis of skin and tumor samples demonstrate that these effects were due to modulation of terminal differentiation, attenuation of inflammatory signaling and induction of apoptosis, through both PPARβ/δ-dependent and PPARβ/δ-independent mechanisms. Increased levels and activity of PPARβ/δ by nimesulide was also observed. These studies support the hypothesis that combining ligand activation of PPARβ/δ with inhibition of COX2 activity increases the efficacy of preventing chemically-induced skin tumorigenesis as compared to either approach alone.
Keywords: peroxisome proliferator-activated receptor-β/δ, skin cancer, nonsteroidal anti-inflammatory drugs, chemoprevention, nuclear receptor
Introduction
Cyclooxygenase (COX) signaling pathways have important roles in modulating skin carcinogenesis. COX is the central enzyme in prostanoid biosynthesis that catalyzes the conversion of arachidonic acid to prostaglandin H2, which is then converted to biologically active lipids such as thromboxane (TXA2), prostaglandin E2 (PGE2) and prostacyclin (PGI2) by different enzymes (1). There are two isoforms of COX, COX1 and COX2. While COX1 is constitutively expressed, COX2 is induced by tumor promoters, growth factors and cytokines (2). Results from experimental animal models have established a causal relationship between COX2 and skin carcinogenesis. For example, genetic disruption of both COX1 and COX2 can prevent skin tumorigenesis (3) and non-steroidal anti-inflammatory drugs (NSAIDS) that inhibit COX activity inhibit both UV-induced and chemically-induced skin carcinogenesis (4–7). The proliferative effects of COX2 are due primarily to increased synthesis of prostaglandins (PGs), which directly influence cell growth after binding to specific cell surface receptors, including the prostaglandin E (EP), prostaglandin F (FP) and prostaglandin I (IP) class of receptors (8, 9). For example, pro-tumorigenic effect of PGE2 can be mediated by the EP2 receptor (10). While PGs can mediate their biological effects through specific prostaglandin receptors like EP, FP and IP, PGs might also modulate the activities of peroxisome proliferator-activated receptors (PPAR).
Three distinct isoforms, PPARα, PPARβ (also referred to as PPARδ or PPARβ/δ) and PPARγ exist with essential roles in the regulation of adipogenesis, lipid metabolism, cell proliferation/apoptosis, cell differentiation, inflammatory responses and carcinogenesis (11–16). PPARs regulate these pathways by modulation of gene expression through direct and indirect mechanisms. PPARβ/δ is found at very high levels in the nucleus of epithelium including intestine and in keratinocytes (17). In the absence of ligands, nuclear PPARβ/δ can also be co-immunoprecipitated with its heterodimerization partner RXRα, suggesting that PPARβ/δ has an important constitutive role in the epithelium (17). Thus, it is not surprising that important roles for PPARβ/δ have been observed in skin. For example, Pparβ/δ-null mice exhibit enhanced epidermal hyperplasia in response to phorbol ester treatment (18, 19) and exacerbated chemically-induced skin tumorigenesis in a two stage carcinogen bioassay as compared to wild-type mice (20), suggesting that PPARβ/δ inhibits epidermal cell proliferation in response to stimuli. Consistent with this idea, PPARβ/δ-dependent inhibition of skin tumorigenesis is found after topical application of the PPARβ/δ ligand GW0742 (21). The chemopreventive effects of ligand activation of PPARβ/δ are mediated in part by induction of unidentified target genes or non-transcriptional events that modulate terminal differentiation and inhibit cell proliferation and/or inhibition of pro-inflammatory signaling (reviewed in (11, 14, 15)).
Some reports suggest that NSAIDs attenuates carcinogenesis by inhibiting PPARβ/δ expression and/or activities although this view has yet to be experimentally confirmed and there are many inconsistencies with this hypothesis in the literature (reviewed in (14, 15)). For example, the hypothesis that NSAIDs inhibit cancer by decreasing PPARβ/δ expression/function is inconsistent with the observation that PPARβ/δ expression following exposure to NSAIDs is either unchanged or increased in human cancer cell lines (22). Further, inhibition of chemically induced skin tumorigenesis is found in both wild-type and Pparβ/δ-null mice following treatment with the COX1/COX2 inhibitor sulindac, suggesting that NSAIDs mediate chemoprevention of chemically-induced skin tumorigenesis through PPARβ/δ-independent mechanisms (6). This is consistent with a recent report showing that combining COX2 inhibition with ligand activation of PPARβ/δ resulted in increased efficacy in the inhibition of pre-existing skin tumor multiplicity (7). Collectively, these observations suggest that combining these two therapeutic approaches will increase the efficacy of chemoprevention as compared to either agent alone. Thus, the effect of combining COX2 inhibition and ligand activation of PPARβ/δ on chemoprevention of skin carcinogenesis was examined.
Materials and Methods
Two-stage chemical carcinogenesis bioassay
Female wild-type and Pparβ/δ-null mice on a C57BL/6 genetic background (19), 6~8 weeks of age, were initiated with 50 μg of 7,12-dimethylbenz[a]anthracene (DMBA; Sigma-Aldrich, St Louis, MO). One week after initiation, mice were treated topically with 5 μg of 12-O-tetradecanoylphorbol-13-acetate (TPA; NCI Chemical Carcinogen Reference Standard Repository), 3 days/week for forty-one weeks. Mice from both genotypes were randomly divided into one of the following four groups: 1) control diet and topical application of acetone, 2) control diet and topical application of GW0742 (5 μM), 3) nimesulide diet (400 mg/kg) and topical application of acetone, or 4) nimesulide diet (400 mg/kg) and topical application of GW0742 (5 μM). Since C57BL/6 mice weighing 20–30 grams typically consume approximately 4 grams of food per day (23), the estimated dose of nimesulide ranged from 50–80 mg/kg body weight per day. The concentrations of topical GW0742 and nimesulide in the diet were based on previous work showing inhibition of chemically-induced skin tumorigenesis by GW0742 or nimesulide in related models (7, 21). After forty-two weeks, mice were euthanized by overexposure to carbon dioxide. Tumor samples were either fixed or snap frozen in liquid nitrogen for future analysis. Fixed tumor samples were embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE) and scored for benign or malignant pathology by two independent pathologists.
Short-term bioassay
Female wild-type and Pparβ/δ-null mice were acclimated to either a control or nimesulide diet (400 mg/kg) for one week and then treated topically with acetone or TPA dissolved in acetone (5 μg) followed one hour later by topical application of either acetone or GW0742 (5 μM) every other day for a total of three applications. Mice were fed either the control or nimesulide diet during this period of topical GW0742 treatment. Mice were euthanized 6 hours after the last acetone or GW0742 treatment and skin samples were obtained for RNA and protein isolation.
Keratinocyte culture
Primary mouse keratinocytes were isolated from 2-day postnatal wild-type and Pparβ/δ-null mice as described previously (24). Keratinocytes were cultured in low calcium (0.05 mM) Eagle’s minimal essential medium with 8% chelexed fetal bovine serum at 37°C and 5% carbon dioxide.
Caspase 3/7 activity assay
Skin samples were ground to a fine powder in liquid nitrogen and then homogenized in buffer containing 10 mM Tris (pH 7.5), 100 mM NaCl, 1 mM EDTA, 0.01% Triton-X100. For in vitro analysis of caspase 3/7 activity, primary keratinocytes were cultured as described above for two days before treatment with either DMSO, 1μM GW0742, 500 μM nimesulide, or the combination of 1μM GW0742 and 500 μM nimesulide for 24 hours. Cells were then trypsinized and lysed in the Tris buffer described above for 30 min on ice. Homogenates were centrifuged at 16,000 × g, and the supernatant was used for analysis. Caspase 3/7 activity was measured using a luminescent assay (Promega, Madison, WI).
Western blot analysis
Primary keratinocytes were cultured as described above for two days before treatment with either DMSO, 1μM GW0742, 500 μM nimesulide or the combination of 1μM GW0742 and 500 μM nimesulide for 24 hours. Cells were then trypsinized and then lysed in buffer containing protease inhibitors. Samples were sonicated to facilitate cell lysis before centrifugation at 16,000 × g at 4 °C for 30 min and the supernatant was used for western blot analysis. Protein from skin samples was isolated similarly with the same buffer. Separation of proteins by electrophoresis, transfer to membranes and blocking was performed as previously described (25). After incubation overnight at 4 °C with the primary antibody, membranes were incubated with biotinylated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for one hour at room temperature followed by incubation with 125I-labeled streptavidin. Membranes were exposed to plates and the level of radioactivity quantified with filmless autoradiographic analysis. Hybridization signals for specific proteins were normalized to the signal for the loading control lactate dehydrogenase (LDH) or ACTIN. The following primary antibodies were used: anti-PARP (Cell Signaling Technology, Danvers, MA), anti-K1 (Covance, Berkeley, CA), anti-K10 (Covance, Berkeley, CA), anti-PPARβ/δ (17), anti-ACTIN (Rockland, Gilbertsville, PA) and anti-LDH (Rockland, Gilbertsville, PA). The ratio of cleaved PARP to uncleaved PARP was calculated using Optiquant software.
RNA isolation and quantitative real-time PCR (qPCR) analysis
Total RNA was isolated from skin and tumor samples using TRIZOL reagent (Invitrogen, Carlsbad, CA). Reverse transcription and qPCR was performed as previously described (25). Primers for keratin 1 (K1), keratin 10 (K10), angiopoetin-like protein 4 (Angptl4), interleukin 6 (Il6) and tumor necrosis factor-α (Tnfα) have been previously described (7, 21, 26, 27). The relative level of mRNA was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (Gapdh) or 18s RNA levels.
Statistical analysis
The significance of tumor incidence between each treatment and genotype was determined by Chi-square test for trend analysis (Prism 5.0, GraphPad Software, Inc., La Jolla, CA). Fisher’s exact test was used to determine the significance of the incidence of mice with keratoacanthomas and/or squamous cell carcinomas (SCC). For all other analysis, a one-tailed student t-test was used.
Results
Ligand activation of PPARβ/δ and inhibition of COX2 enhances chemoprevention of chemically-induced skin tumorigenesis
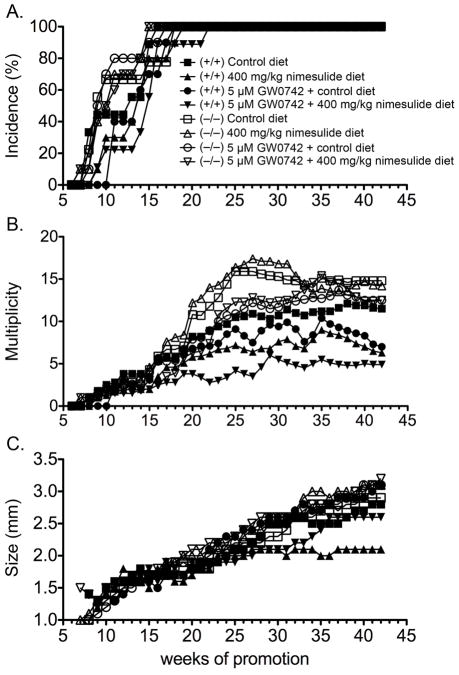
Combining ligand activation of PPARβ/δ with COX2 inhibition results in a modest decrease of multiplicity of pre-existing tumors in a chemotherapeutic model (7). Since later stage tumors can be resistant to therapies designed to regress tumor growth, the effect of combining ligand activation of PPARβ/δ with COX2 inhibition was examined in a chemoprevention model. Marked changes were observed in both genotypes (Fig. 1, Supplemental Fig. 2). The onset of papilloma formation was sooner and the incidence of papilloma was greater in control Pparβ/δ-null mice compared to control wild-type mice prior to week 16 of the two-stage bioassay (P ≤ 0.05; Fig. 1A), consistent with previous studies (7, 20, 21). Topical application of the PPARβ/δ ligand GW0742, or dietary nimesulide, caused a delay in the onset of tumor formation (P ≤ 0.05; Fig. 1A). These effects were not found in Pparβ/δ-null mice. Compared to control, combining ligand activation of PPARβ/δ with inhibition of COX2 activity caused a delay in the onset of tumor formation in wild-type mice, and this effect was not found in Pparβ/δ-null mice (Fig. 1A). In response to either GW0742 or nimesulide, the percentage of wild-type mice with skin tumors from week 11 to 16 was lower but not statistically different compared to control wild-type mice (Fig. 1A). However, in response to both GW0742 and nimesulide, the percentage of wild-type mice with skin tumors from week 11 to 16 was decreased as compared to control wild-type mice (P ≤ 0.05; Fig. 1A). These effects of GW0742, nimesulide or the combination of GW0742 and nimesulide were not found in Pparβ/δ-null mice (Fig. 1A). Skin tumor multiplicity was significantly greater (29–30%) in control Pparβ/δ-null mice as compared to control wild-type mice from week 20 until week 42 of the two-stage bioassay (P ≤ 0.05; Fig. 1B). Ligand activation of PPARβ/δ with GW0742 resulted in decreased (20–40%) tumor multiplicity in wild-type mice during week 37 to week 42 of the bioassay and this effect was not found in Pparβ/δ-null mice (P ≤ 0.05; Fig. 1B). Interestingly, skin tumor multiplicity was lower (24–27%) in Pparβ/δ-null mice in response to topical GW0742 from week 20 to week 30 of the bioassay as compared to control Pparβ/δ-null mice (P ≤ 0.05; Fig. 1B). Dietary nimesulide caused a decrease (30–46%) in tumor multiplicity in wild-type mice during week 24 to week 42 of the bioassay, and this effect was not found in Pparβ/δ-null mice (P ≤ 0.05; Fig. 1B). The combination of topical application of GW0742 and dietary nimesulide resulted in a marked decrease (57–69%) of tumor multiplicity from week 22 onward in wild-type mice and the effect was greater compared to either GW0742 or nimesulide treatment alone from week 21 to week 40 (P ≤ 0.05; Fig. 1B). In Pparβ/δ-null mice, the combination of GW0742 with nimesulide caused a decrease (27–42%) in tumor multiplicity from week 20 to week 30 (P ≤ 0.05; Fig. 1B).
Figure 1.
Chemoprevention of chemically-induced skin tumorigenesis by combining ligand activation of PPARβ/δ and inhibition of COX2. Wild-type (+/+) and Pparβ/δ-null (−/−) mice were treated with topical GW0742 (5 μM), dietary nimesulide (400 mg/kg) or the combination of GW0742 and nimesulide during a forty-two week two-stage bioassay (initiation with DMBA and promotion with TPA) as described in Methods. A, The incidence and onset of skin tumor formation. B, Skin tumor multiplicity. C, The average tumor size per mouse. Values represent the mean.
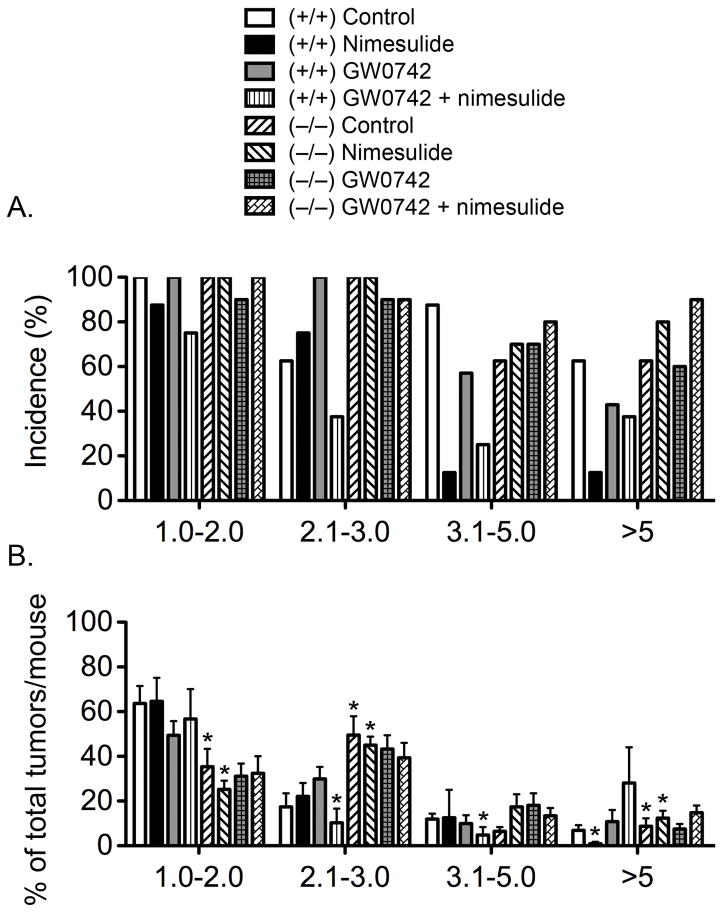
Average tumor size was greater in the Pparβ/δ-null mice compared to wild-type mice, but this difference was not statistically significant (Fig. 1C). Topical GW0742 or the combined treatment of topical GW0742 and dietary nimesulide did not cause a significant decrease of average tumor size in either genotype (Fig. 1C). Dietary nimesulide caused a decrease in average tumor size in wild-type mice and this effect was not observed in Pparβ/δ-null mice (P ≤ 0.05; Fig. 1C). Closer examination of the distribution of the tumor size also revealed some striking differences (Fig. 2). The percentage of control Pparβ/δ-null mice with tumors in the 2–3 mm was greater than control wild-type mice (Fig. 2A). Additionally, the average percentage of total tumors per mouse in the 1–2 mm range was greater in control wild-type mice as compared to control Pparβ/δ-null mice, and this difference was consistent with a greater percentage of total tumors per mouse in the 2–3 mm and greater than 5 mm size ranges in control Pparβ/δ-null mice as compared to control wild-type mice (Fig. 2B). In wild-type mice fed nimesulide, the percentage of mice with tumors in the 3–5 mm size range, and the percentage of mice with tumors greater than 5 mm in size, was significantly less as compared to control wild-type mice (Fig. 2A). Similarly, the average percentage of total tumors per mouse greater than 5 mm was lower in wild-type mice fed nimesulide as compared to control wild-type mice (Fig. 2B). The average percentage of total tumors per mouse in the 1–2, 2–3 and 3–5 mm range was similar in wild-type mice fed nimesulide as compared to control wild-type mice (Fig. 2B). Dietary nimesulide had no effect on the distribution of tumors with different sizes in Pparβ/δ-null mice as compared to control Pparβ/δ-null mice (Figs. 2A, 2B). However, compared to wild-type mice fed nimesulide, the average percentage of total tumors per mouse in the 1–2 mm size range was lower in Pparβ/δ-null mice fed nimesulide (Fig. 2B). This difference was due to the increase in the average percentage of total tumors per mouse in the 2–3 mm and greater than 5 mm size ranges in Pparβ/δ-null mice fed nimesulide as compared to similarly treated wild-type mice (Fig. 2B). In wild-type mice treated with GW0742, the percentage of mice with tumors in the 2–3 mm size range was greater, while the percentage of mice with tumors in the 3–5 mm and greater than 5 mm size ranges was less as compared to control wild-type mice (Fig. 2A). This effect was not found in GW0742-treated Pparβ/δ-null mice (Fig. 2A). GW0742 had no effect on the average size distribution of total tumors per mouse in either genotype (Fig. 2B). The percentage of wild-type mice treated with both topical GW0742 and dietary nimesulide with tumors in all size ranges was markedly lower as compared to control wild-type mice and this effect was not found in similarly treated Pparβ/δ-null mice (Fig. 2A). The average percentage of total tumors per mouse in the 2–3 mm range and 3–5 mm ranges was lower in wild-type mice treated with both topical GW0742 and dietary nimesulide as compared to control wild-type mice; these effects were not found in similarly treated Pparβ/δ-null mice (Fig. 2B).
Figure 2.
Skin tumor size following ligand activation of PPARβ/δ and inhibition of COX2. Wild-type (+/+) and Pparβ/δ-null (−/−) mice were treated with topical GW0742 (5 μM), dietary nimesulide (400 mg/kg) or the combination of GW0742 and nimesulide during a forty-two week two-stage bioassay (initiation with DMBAand promotion with TPA) as described in Methods. A, Incidence of mice with different tumor sizes. These values represent the percentage of mice within a given group that exhibited skin tumors with the indicated size range. B, The distribution of average tumor size for each treatment group. Mice within each treatment were used to calculate the percentage of tumors of that particular size range for each treatment group. *Significantly different than control wild-type, P ≤ 0.05.
The majority of representative skin lesions examined in all groups were squamous cell papillomas (data not shown). Skin lesions macroscopically suspected of being SCC were examined for histopathology. Skin lesions macroscopically suspected of being SCC were not observed in wild-type mice treated with nimesulide. For control, nimesulide-treated, GW0742-treated and nimesulide+GW0742 treated wild-type mice, 2/8, 0/7, 3/10 and 2/10 mice, respectively, had lesions macroscopically suspected of being SCC. For control, nimesulide-treated, GW0742-treated and nimesulide+GW0742 treated Pparβ/δ-null mice, 5/8, 3/10, 4/10 and 5/10 mice, respectively, had lesions macroscopically suspected of being SCC. Histopathological analysis revealed that these lesions were typically either keratoacanthomas or SCC. A higher incidence of keratoacanthoma was observed in control Pparβ/δ-null mice (3/8) compared to control wild-type mice (1/8; Supplemental Fig. 3A). No keratoacanthomas were found in wild-type mice fed dietary nimesulide, but neither GW0742, nimesulide or the combined treatment caused any statistically significant changes in the incidence of keratoacanthoma in either genotype (Supplemental Fig. 3A). The average number of keratoacanthomas per mouse was comparable between both genotypes, although no keratoacanthomas were noted in wild-type mice fed nimesulide (Supplemental Fig. 3B). While 25% of control wild-type mice (2/8) had SCC, no SCC were found in wild-type mice treated with dietary nimesulide or topical GW0742 and only 10% of wild-type mice treated with both dietary nimesulide and topical GW0742 (1/10) had SCC (Supplemental Fig. 3C). SCC were found in 25% of control Pparβ/δ-null mice (2/8), 20% of nimesulide-treated Pparβ/δ-null mice (2/10), none of GW0742-treated and 40% of nimesulide and GW0742-treated Pparβ/δ-null mice (4/10) (Supplemental Fig. 3C). None of these differences achieved statistical significance. The average number of SCC per mouse was comparable between both genotypes, although no SCC were observed in nimesulide-treated or GW0742-treated wild-type mice or GW0742-treated Pparβ/δ-null mice (Supplemental Fig. 3D). One hemangioma was observed in one GW0742-treated Pparβ/δ-null mouse, and one malignant basal cell tumor was found in one nimesulide and GW0742-treated Pparβ/δ-null mouse (data not shown). Interestingly, polymorphonuclear neutrophil infiltrates were more commonly observed in Pparβ/δ-null mouse skin lesions as compared to wild-type mouse lesions (Supplemental Fig. 4), consistent with past results (19). Additionally, polymorphonuclear neutrophil infiltrates were less common in skin lesions from wild-type mice treated with either nimesulide, GW0742 or the combined treatment, but were more commonly found in similarly treated Pparβ/δ-null mice.
Effect of GW0742 and nimesulide on terminal differentiation markers
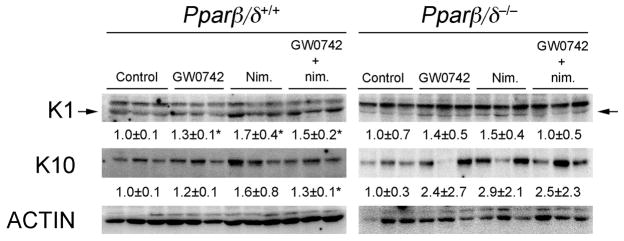
Ligand activation of PPARβ/δ or inhibition of COX activity can both induce terminal differentiation in primary keratinocytes and skin (3, 28–31). To determine if the enhanced efficacy of inhibiting chemically-induced skin tumorigenesis by combining GW0742 with nimesulide was due in part to modulation of terminal differentiation, expression of differentiation markers was examined. Dietary nimesulide, topical GW0742, and topical GW0742 in combination with dietary nimesulide increased expression of KERATIN 1 (K1) protein in wild-type mouse skin as compared to control, and this effect was not found in Pparβ/δ-null mouse skin (Fig. 3). Dietary nimesulide or topical GW0742 did not alter expression of KERATIN 10 (K10) protein in mouse skin from either genotype (Fig. 3). However, topical GW0742 in combination with dietary nimesulide increased expression of K10 protein in wild-type mouse skin as compared to control, and this effect was not found in Pparβ/δ-null mice (Fig. 3)
Figure 3.
Expression of differentiation markers in skin following ligand activation of PPARβ/δ and inhibition of COX2. Protein was isolated from wild-type (Pparβ/δ+/+) and Pparβ/δ-null (Pparβ/δ−/−) mouse skin following treatment with GW0742, nimesulide or both GW0742 and nimesulide and western blots performed as described in Methods. Hybridization signals for KERATIN 1 (K1) and KERATIN 10 (K10) were normalized to ACTIN. Values represent the mean ± SEM. *Significantly different from control P ≤ 0.05.
Effect of GW0742 and nimesulide on the inflammatory response
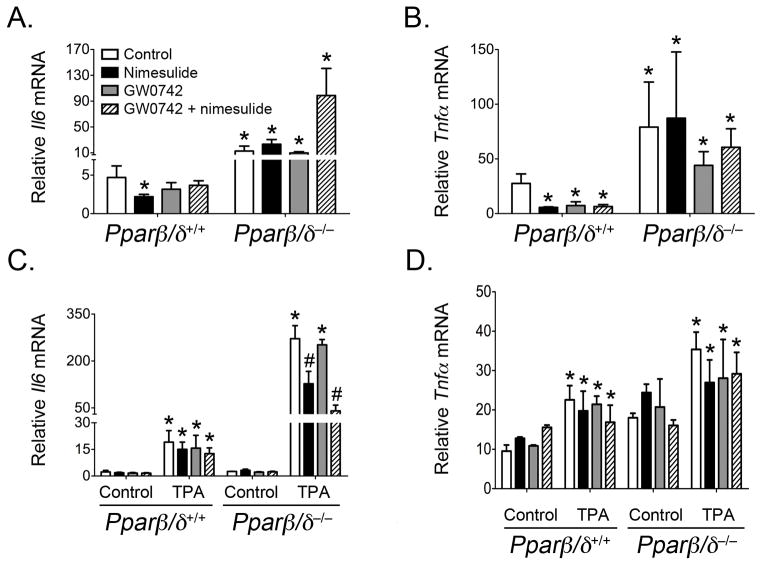
Inflammation can influence different stages of tumorigenesis. Secretion of pro-inflammatory signaling molecules by immune and somatic cells such as tumor necrosis factor-α (TNFα) and interleukin 6 (IL6) can act on cancer cells and promote tumor growth and malignant conversion (reviewed in (32)). The NSAID nimesulide is known to attenuate inflammation by inhibiting COX2 activity and the subsequent production of arachidonic acid metabolites. In addition, ligand activation of PPARβ/δ is also known to have anti-inflammatory activities in rodent and human models (reviewed in (14, 15, 33)). To determine if attenuation of inflammation could in part underlie the observed inhibition of chemically-induced skin tumorigenesis, expression of the mRNA encoding two important pro-inflammatory cytokines, TNFα and IL6, was examined in both the tumor samples and mouse skin. Tumors from Pparβ/δ-null mice from all treatment groups had a higher level of Il6 mRNA (2 to 20-fold) and Tnfα mRNA (2 to 3-fold) compared to that of similarly treated wild-type mice (Figs. 4A, 4B). Dietary nimesulide caused a significant decrease of both Il6 mRNA (53% lower) and Tnfα mRNA (79% lower) in tumors from wild-type mice but not in tumors from Pparβ/δ-null mice. Tumors from wild-type mice treated only with topical GW0742 or the combination of topical GW0742 and dietary nimesulide exhibited a decrease in mRNA encoding Il6 and Tnfα but this change was only statistically significant for Tnfα mRNA (73–77% lower; Fig. 4B). No change in expression of Il6 or Tnfα mRNA was found in tumors from Pparβ/δ-null mice treated with topical GW0742 or the combination of topical GW0742 and dietary nimesulide (Fig. 4A, 4B). These data are consistent with the presence of polymorphonuclear neutrophil infiltrates found more commonly in Pparβ/δ-null mice as compared to wild-type mice (Supplemental Fig. 4).
Figure 4.
Effect of ligand activation of PPARβ/δ and/or inhibition of COX2 on Il6 and Tnfα mRNA in skin tumors and skin. Total RNA was isolated from tumor samples or skin samples from wild-type (Pparβ/δ+/+) and Pparβ/δ-null (Pparβ/δ−/−) mice following treatment with GW0742, nimesulide or both GW0742 and nimesulide as described in Methods. The mRNA encoding Il6 (A,C) or Tnfα (B,D) was quantified from skin tumors (A,B) or mouse skin following acute TPA treatment (C,D) using qPCR and normalized to 18s mRNA. Values represent the mean ± SEM. *Significantly different from control P ≤ 0.05.
A short-term bioassay was also performed using wild-type and Pparβ/δ-null mice that were acclimated to either a control or nimesulide diet for one week and then treated with or without TPA followed one hour later with either acetone (vehicle control) or GW0742. The rationale for this approach is that TPA is known to increase inflammatory signaling that could influence tumor promotion. Expression of Il6 mRNA was similar in both control wild-type and control Pparβ/δ-null mouse skin (Fig. 4C). Expression of Il6 mRNA was increased in both wild-type and Pparβ/δ-null mouse skin in response to TPA treatment but was markedly higher (8-fold versus 106-fold, respectively)in Pparβ/δ-null mouse skin compared to wild-type mouse skin (Fig. 4C). Expression of Il6 mRNA was not influenced by GW0742, nimesulide or GW0742 and nimesulide treatment in either control or TPA-treated wild-type mouse skin or control Pparβ/δ-null mouse skin (Fig. 4C). Dietary nimesulide in Pparβ/δ-null mice resulted in lower Il6 mRNA (63% lower) following topical TPA, and a similar effect was also found in Pparβ/δ-null mice that were treated with GW0742 and nimesulide (84% lower) following topical TPA treatment (Fig. 4C). Expression of Tnfα mRNA was similar in both control wild-type and control Pparβ/δ-null mouse skin (Fig. 4D). Expression of Tnfα mRNA was increased in both wild-type and Pparβ/δ-null mouse skin following TPA treatment and this effect was greater in Pparβ/δ-null mouse skin compared to wild-type mouse skin (Fig. 4D). Expression of Tnfα mRNA was not influenced by GW0742, nimesulide or GW0742 and nimesulide treatment in either control or TPA-treated mouse skin from either genotype (Fig. 4D). Whereas the relative change in expression of Tnfα mRNA in response to TPA was approximately two-fold in all groups of both genotypes, the relative fold change in expression of Tnfα mRNA was less (1.1-fold) in TPA-treated wild-type mouse skin treated with dietary nimesulide and topical GW0742 compared to similarly treated control wild-type mouse skin, and this effect was not observed in Pparβ/δ-null mice (Fig. 4D).
Effect of GW0742 and nimesulide on apoptosis
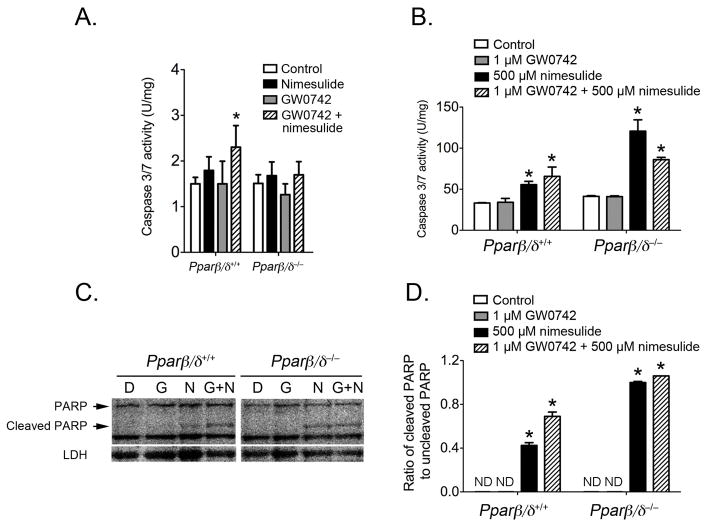
Since combining ligand activation of PPARβ/δ by GW0742 with inhibition of COX2 activity by dietary nimesulide caused the most marked effect on tumor multiplicity, the effect of these treatments on apoptosis was examined. There is compelling evidence that one mechanism by which nimesulide inhibits tumorigenesis is through the induction of apoptosis (reviewed in (34)). In contrast, the effect of ligand activation of PPARβ/δ on apoptotic signaling remains uncertain. This is due to conflicting studies suggesting that ligand activation of PPARβ/δ causes pro-apoptotic signaling, anti-apoptotic signaling or has no effect on apoptosis (reviewed in (14, 15)). Caspase 3/7 activity and poly (ADP-ribose) polymerase (PARP) cleavage were measured to determine if ligand activation of PPARβ/δ with GW0742 and/or inhibition of COX2 by nimesulide modulate apoptosis in mouse skin and keratinocytes. In control wild-type and Pparβ/δ-null mouse skin, dietary nimesulide or topical GW0742 did not modulate caspase 3/7 activity compared to control in either genotype (Fig. 5A). However, the combined treatment of GW0742 with nimesulide caused an increase in caspase 3/7 activity in wild-type mouse skin, and this effect was not seen in Pparβ/δ-null mouse skin. Since different cell types can influence apoptosis, the effect of GW0742 and nimesulide was examined in primary keratinocytes from wild-type and Pparβ/δ-null mice. Consistent with results obtained from analysis of whole skin, GW0742 did not alter caspase 3/7 activity or PARP cleavage in either wild-type and Pparβ/δ-null primary keratinocytes (Figs. 5B–D). In contrast, culturing primary keratinocytes with nimesulide increased apoptotic signaling in primary keratinocytes in both genotypes, as evidenced by an increase of caspase 3/7 activity and PARP cleavage (Figs. 5B–D). Co-treatment of wild-type primary keratinocytes with nimesulide and GW0742 led to enhanced caspase 3/7 activity and PARP cleavage as compared to that observed with either compound alone, but this increase was not found in Pparβ/δ-null keratinocytes (Figs. 5B–D).
Figure 5.
Effect of ligand activation of PPARβ/δ and/or inhibition of COX2 on apoptotic signaling. Total cytosolic protein was isolated from wild-type (Pparβ/δ+/+) and Pparβ/δ-null (Pparβ/δ−/−) mouse skin or primary keratinocytes following treatment with GW0742, nimesulide or both GW0742 and nimesulide as described in Methods. Caspase 3/7 activity in mouse skin (A) or primary keratinocytes (B) was normalized to total protein concentration. C, Representative western blot showing the full length and cleaved PARP protein in response to control (DMSO-D), GW0742 (G), nimesulide (N) or the combination of both GW0742 and nimesulide (G+N). D, Quantification of the ratio of cleaved PARP to full length PARP from western blots. ND = not detected. Values represent the mean ± SEM. *Significantly different from control P ≤ 0.05.
Effect of GW0742 and nimesulide on expression and function of PPARβ/δ
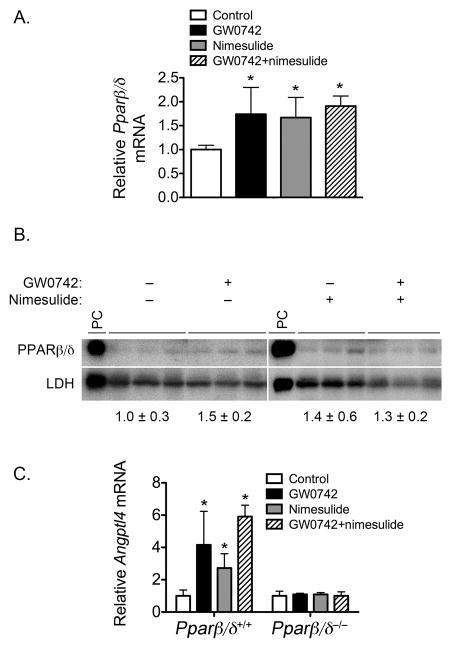
Nimesulide is not known to activate PPARβ/δ by acting as an agonist. One mechanism that may explain some of the modest PPARβ/δ-dependent changes resulting from nimesulide treatment is increased expression and function of PPARβ/δ. Indeed, increased expression of PPARβ/δ has been observed following exposure to NSAIDs including nimesulide (22). Thus, expression and function of PPARβ/δ was examined. Interestingly, dietary nimesulide, topical GW0742 and the combined treatment of GW0742 and nimesulide all increased expression of Pparβ/δ mRNA in wild-type mouse skin (Fig. 6A). This increase in expression was also found at the protein level, but the changes were not statistically significant (Fig. 6B). However, examination of expression of the PPARβ/δ target gene Angptl4 demonstrated that dietary nimesulide, topical GW0742 and the combined treatment of GW0742 and nimesulide all increased expression of Angptl4 mRNA in wild-type mouse skin, and this effect was not found in similarly treated Pparβ/δ-null mouse skin (Fig. 6C).
Figure 6.
Effect of ligand activation of PPARβ/δ and/or inhibition of COX2 on expression and activity of PPARβ/δ. Protein or mRNA was isolated from wild-type (Pparβ/δ+/+) or Pparβ/δ-null (Pparβ/δ−/−) mouse skin following treatment with GW0742, nimesulide or both GW0742 and nimesulide as described in Methods. Expression of Pparβ/δ mRNA (A) or PPARβ/δ protein (B) was quantified by qPCR or western blotting, respectively. C, Expression of the PPARβ/δ target Angptl4 mRNA. *Significantly different than control, P ≤ 0.05.
Discussion
Consistent with past studies (20, 21), chemically-induced skin tumorigenesis was exacerbated in Pparβ/δ-null mice as compared to wild-type mice as assessed by differences in the onset tumor formation, the incidence of keratoacanthomas, and tumor multiplicity. Further, ligand activation of PPARβ/δ inhibited chemically-induced skin tumorigenesis through PPARβ/δ-dependent mechanisms similar to results from past studies (7, 21). Dietary nimesulide was also effective for chemoprevention as shown by decreased tumor multiplicity and a decrease in tumor size distribution. As compared to dietary nimesulide or topical GW0742, the combination of dietary nimesulide and topical GW0742 enhanced the chemopreventive activity of either agent alone, most notably by the prolonged marked decrease in tumor multiplicity. Interestingly, the effect of GW0742, nimesulide and the combined treatment of nimesulide and GW0742 appear to be due in part to modulation of PPARβ/δ-dependent and PPARβ/δ-independent mechanisms that influence differentiation, inflammation and apoptosis.
PPARβ/δ-dependent chemoprevention of chemically-induced skin tumorigenesis by GW0742 is likely due in part to enhanced terminal differentiation, as observed in the present study and previous reports (7, 21, 29–31). However, reduced expression of Tnfα mRNA was also observed in skin tumors from GW0742-treated wild-type mice, but not in similarly treated Pparβ/δ-null mice. Since activating PPARβ/δ is known to inhibit inflammatory signaling (reviewed in (33)), it is possible that inhibition of inflammatory signaling by PPARβ/δ also contributes to the mechanisms underlying the chemopreventive effects of GW0742 in this model. This is consistent with the reduced accumulation of infiltrating polymorphic neutrophils in GW0742-treated skin tumors. The mechanism underlying GW0742-dependent inhibition of skin tumor multiplicity in both wild-type and Pparβ/δ-null mice is uncertain, but this has been found previously (21). One possible mechanism is that GW0742 inhibits myeloperoxidase activity through direct enzyme inhibition (29). Since myeloperoxidase is found in neutrophils that accumulate during tumor promotion with TPA, it is possible that GW0742 inhibits the activity of infiltrating neutrophils. Additional studies are needed to determine how GW0742 inhibits chemically-induced skin tumorigenesis through PPARβ/δ-independent mechanisms.
It is of interest to note that the chemopreventive effect of nimesulide was also dependent on PPARβ/δ. Indeed, delayed onset of skin tumorigenesis, reduced tumor multiplicity and larger proportion of smaller versus larger tumors were all observed in wild-type mice fed the nimesulide diet, and these effects were diminished in similarly treated Pparβ/δ-null mice. One possible mechanism that may underlie this effect is the observed increase in PPARβ/δ function resulting from nimesulide treatment. Similar increases in PPARβ/δ expression and function have also been observed in colon cancer cell lines, and these changes were also associated with inhibition of cell growth by nimesulide (22). The increase in PPARβ/δ expression by nimesulide could lead to enhanced terminal differentiation or anti-inflammatory activities. This is consistent with the observed increase in K1 expression and the inhibition of Il6 and Tnfα mRNA in skin tumors found in wild-type mice treated with dietary nimesulide but not in similarly treated Pparβ/δ-null mice. While dietary nimesulide at the concentration used in the present study is known to inhibit COX2 activity in mouse skin (7), expression of COX2 is also known to be higher in phorbol ester treated Pparβ/δ-null mouse skin as compared to control (35). Further, inhibition of COX2 activity is found in wild-type mouse skin and this effect is diminished in Pparβ/δ-null mouse skin (7). Thus, the observed PPARβ/δ-dependent chemoprevention by nimesulide could be due to differences in stoichiometry between nimesulide and COX2. Further studies are needed to examine this possibility.
The efficacy of chemoprevention of chemically-induced skin tumorigenesis was greatest when nimesulide was combined with GW0742. This was most evident by the prolonged inhibition of tumor multiplicity. Interestingly, this effect was due to both PPARβ/δ-dependent and PPARβ/δ-independent mechanisms. Inhibition of tumor multiplicity was observed in both wild-type and Pparβ/δ-null mice from week 20 to week 32, after which this was found in wild-type but not Pparβ/δ-null mice. This is of interest because dietary nimesulide was only effective for inhibiting tumor multiplicity in wild-type mice but not Pparβ/δ-null mice, while GW0742 was effective in both genotypes during this timeframe. Combining inhibition of COX2 activity with inhibition of myeloperoxidase activity could result in synergistic or additive effects that contribute to the observed enhanced chemoprevention by both nimesulide and GW0742. However, results from the present study also show that nimesulide effectively increases apoptotic signaling in mouse keratinocytes in both genotypes. This suggests that the observed PPARβ/δ-independent inhibition of tumor multiplicity resulting from the combination of nimesulide and GW0742 could be influenced in part by increased apoptotic signaling. Why the observed chemoprevention becomes dependent on PPARβ/δ during the later stages of the bioassay is uncertain but could be due to the combined effects on differentiation and anti-inflammatory activities that become more dominant during this period. Because of the striking enhanced chemoprevention of chemically-induced skin tumorigenesis by combining ligand activation of PPARβ/δ with inhibition of COX2 activity, as compared to either agent alone, it will be of great interest to determine whether this approach can be used for UV-induced skin tumorigenesis, a more predominant etiological risk factor for skin cancer in humans. Alternatively, whether inhibiting EP receptor activity and activating PPARβ/δ will provide a safer approach, due to known issues associated with COX2 inhibitors, should be of good interest based on these original studies. Combining inhibition of COX2 signaling with ligand activation of PPARβ/δ could provide a new approach for chemoprevention of skin tumorigenesis.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Drs. Andrew Billin and Timothy Willson for providing the GW0742 used for these studies.
Financial support: This work supported in part by National Institutes of Health [CA124533, CA126826, CA141029, CA140369 to J.M.P.]
Abbreviation List
- ANGPTL4
angiopoetin-like protein 4
- COX
cyclooxygenase
- DMBA
7, 12-dimethylbenz[a]anthracene
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IL6
interleukin 6
- K1
keratin 1
- K10
keratin 10
- LDH
lactate dehydrogenase
- NSAID
non-steroidal anti-inflammatory drugs
- PPAR
peroxisome proliferator-activated receptor
- PPRE
peroxisome proliferator response elements
- PGs
prostaglandins
- EP
prostaglandin E receptor
- FP
prostaglandin F receptor
- IP
prostaglandin I receptor
- RXRα
retinoid X receptor-α
- SCC
squamous cell carcinomas
- TNFα
tumor necrosis factor-α
- TPA
12-O-tetradecanoylphorbol-13-acetate
Footnotes
Conflicts of Interests: None
References
- 1.Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259:315–24. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hla T, Ristimaki A, Appleby S, Barriocanal JG. Cyclooxygenase gene expression in inflammation and angiogenesis. Ann N Y Acad Sci. 1993;696:197–204. doi: 10.1111/j.1749-6632.1993.tb17152.x. [DOI] [PubMed] [Google Scholar]
- 3.Tiano HF, Loftin CD, Akunda J, et al. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–401. [PubMed] [Google Scholar]
- 4.Fischer SM, Conti CJ, Viner J, Aldaz CM, Lubet RA. Celecoxib and difluoromethylornithine in combination have strong therapeutic activity against UV-induced skin tumors in mice. Carcinogenesis. 2003;24:945–52. doi: 10.1093/carcin/bgg046. [DOI] [PubMed] [Google Scholar]
- 5.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–44. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 6.Kim DJ, Prabhu KS, Gonzalez FJ, Peters JM. Inhibition of chemically-induced skin carcinogenicity by sulindac is independent of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) Carcinogenesis. 2006;27:1105–12. doi: 10.1093/carcin/bgi346. [DOI] [PubMed] [Google Scholar]
- 7.Bility MT, Zhu B, Kang BH, Gonzalez FJ, Peters JM. Ligand activation of peroxisome proliferator-activated receptor-β/δ and inhibition of cyclooxygenase-2 enhances inhibition of skin tumorigenesis. Toxicol Sci. 2010;113:27–36. doi: 10.1093/toxsci/kfp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacology & therapeutics. 2004;103:147–66. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Tober KL, Thomas-Ahner JM, Kusewitt DF, Oberyszyn TM. Effects of UVB on E prostanoid receptor expression in murine skin. J Invest Dermatol. 2007;127:214–21. doi: 10.1038/sj.jid.5700502. [DOI] [PubMed] [Google Scholar]
- 10.Sung YM, He G, Fischer SM. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 2005;65:9304–11. doi: 10.1158/0008-5472.CAN-05-1015. [DOI] [PubMed] [Google Scholar]
- 11.Burdick AD, Kim DJ, Peraza MA, Gonzalez FJ, Peters JM. The role of peroxisome proliferator-activated receptor-β/δ in epithelial cell growth and differentiation. Cell Signal. 2006;18:9–20. doi: 10.1016/j.cellsig.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–7. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 13.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR) Toxicol Sci. 2006;90:269–95. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- 14.Peters JM, Gonzalez FJ. Sorting out the functional role(s) of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in cell proliferation and cancer. Biochim Biophys Acta. 2009;1796:230–41. doi: 10.1016/j.bbcan.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters JM, Hollingshead HE, Gonzalez FJ. Role of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) in gastrointestinal tract function and disease. Clin Sci (Lond) 2008;115:107–27. doi: 10.1042/CS20080022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lalloyer F, Staels B. Fibrates, glitazones, and peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2010;30:894–9. doi: 10.1161/ATVBAHA.108.179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girroir EE, Hollingshead HE, He P, Zhu B, Perdew GH, Peters JM. Quantitative expression patterns of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) protein in mice. Biochem Biophys Res Commun. 2008;371:456–61. doi: 10.1016/j.bbrc.2008.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalik L, Desvergne B, Tan NS, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)α and PPARβ mutant mice. J Cell Biol. 2001;154:799–814. doi: 10.1083/jcb.200011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters JM, Lee SST, Li W, et al. Growth, adipose, brain and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor β/δ. Molecular and Cellular Biology. 2000;20:5119–28. doi: 10.1128/mcb.20.14.5119-5128.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DJ, Akiyama TE, Harman FS, et al. Peroxisome proliferator-activated receptor β (δ)-dependent regulation of ubiquitin C expression contributes to attenuation of skin carcinogenesis. J Biol Chem. 2004;279:23719–27. doi: 10.1074/jbc.M312063200. [DOI] [PubMed] [Google Scholar]
- 21.Bility MT, Devlin-Durante MK, Blazanin N, et al. Ligand activation of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits chemically-induced skin tumorigenesis. Carcinogenesis. 2008;29:2406–14. doi: 10.1093/carcin/bgn219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foreman JE, Sorg JM, McGinnis KS, et al. Regulation of peroxisome proliferator-activated receptor-β/δ by the APC/β-CATENIN pathway and nonsteroidal antiinflammatory drugs. Mol Carcinog. 2009;48:942–52. doi: 10.1002/mc.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–43. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dlugosz AA, Glick AB, Tennenbaum T, Weinberg WC, Yuspa SH. Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol. 1995;254:3–20. doi: 10.1016/0076-6879(95)54003-2. [DOI] [PubMed] [Google Scholar]
- 25.Palkar PS, Borland MG, Naruhn S, et al. Cellular and Pharmacological Selectivity of the PPARβ/δ Antagonist GSK3787. Mol Pharmacol. 2010;78:419–30. doi: 10.1124/mol.110.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray IA, Krishnegowda G, Dinatale BC, et al. Development of a selective modulator of aryl hydrocarbon (Ah) receptor activity that exhibit anti-inflammatory properties. Chem Res Toxicol. 2010;23:955–66. doi: 10.1021/tx100045h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan W, Palkar PS, Murray IA, et al. Ligand activation of peroxisome proliferator-activated receptor β/δ (PPARβ/δ) attenuates carbon tetrachloride hepatotoxicity by downregulating proinflammatory gene expression. Toxicol Sci. 2008;105:418–28. doi: 10.1093/toxsci/kfn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akunda JK, Lao HC, Lee CA, Sessoms AR, Slade RM, Langenbach R. Genetic deficiency or pharmacological inhibition of cyclooxygenase-1 or -2 induces mouse keratinocyte differentiation in vitro and in vivo. Faseb J. 2004;18:185–7. doi: 10.1096/fj.02-1192fje. [DOI] [PubMed] [Google Scholar]
- 29.Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM. PPARβ/δ selectively induces differentiation and inhibits cell proliferation. Cell Death Differ. 2006;13:53–60. doi: 10.1038/sj.cdd.4401713. [DOI] [PubMed] [Google Scholar]
- 30.Schmuth M, Haqq CM, Cairns WJ, et al. Peroxisome proliferator-activated receptor (PPAR)-β/δ stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol. 2004;122:971–83. doi: 10.1111/j.0022-202X.2004.22412.x. [DOI] [PubMed] [Google Scholar]
- 31.Westergaard M, Henningsen J, Svendsen ML, et al. Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. J Invest Dermatol. 2001;116:702–12. doi: 10.1046/j.1523-1747.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- 32.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilgore KS, Billin AN. PPARβ/δ ligands as modulators of the inflammatory response. Curr Opin Investig Drugs. 2008;9:463–9. [PubMed] [Google Scholar]
- 34.Renard JF, Julemont F, de Leval X, Pirotte B. The use of nimesulide and its analogues in cancer chemoprevention. Anticancer Agents Med Chem. 2006;6:233–7. doi: 10.2174/187152006776930855. [DOI] [PubMed] [Google Scholar]
- 35.Kim DJ, Murray IA, Burns AM, Gonzalez FJ, Perdew GH, Peters JM. Peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) inhibits epidermal cell proliferation by down-regulation of kinase activity. J Biol Chem. 2005;280:9519–27. doi: 10.1074/jbc.M413808200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.