Abstract
Background
The ability of echocardiographic dyssynchrony to predict response to cardiac resynchronization therapy (CRT) has been unclear.
Methods and Results
A prospective, longitudinal study was designed with pre-defined dyssynchrony indices and outcome variables to test the hypothesis that baseline dyssynchrony is associated with long term survival following CRT. We studied 229 consecutive Class III–IV heart failure patients, with ejection fraction (EF) ≤35%, and QRS duration ≥120ms for CRT. Dyssynchrony before CRT was defined as: tissue Doppler velocity opposing wall delay ≥ 65 ms, 12-site standard deviation (Yu Index) ≥32ms; speckle tracking radial strain anteroseptal to posterior wall delay ≥130ms; or pulsed Doppler interventricular mechanical delay (IVMD) ≥ 40 ms. Outcome was defined as freedom from death, heart transplant or left ventricular assist device (LVAD). Of 210 patients (89%) with dyssynchrony data available, there were 62 events: 47 deaths, 9 transplants, and 6 LVADs over 4 years. Event-free survival was associated with: Yu Index (p=0.003), speckle tracking radial strain (p=0.003), and IVMD (p=0.019). When adjusted for confounding baseline variables of ischemic etiology and QRS duration, Yu Index and radial strain dyssynchrony remained independently associated with outcome (p<0.05). Lack of radial dyssynchrony was particularly associated with unfavorable outcome in those with QRS duration 120–150 ms (p=0.002).
Conclusions
The absence of echocardiographic dyssynchrony was associated with significantly less favorable event-free survival following CRT. Patients with narrower QRS duration who lacked dyssynchrony had the least favorable long term outcome. These observations support the relationship of dyssynchrony to CRT response.
Keywords: Echocardiography, Heart Failure, Pacing Therapy
Cardiac resynchronization therapy (CRT) is an important therapy for patients with symptomatic heart failure, widened electrocardiographic QRS, and reduced ejection fraction (EF).1–3 Despite significant morbidity and mortality benefits from CRT, approximately one-third of patients do not appear to benefit from therapy. The prevailing concept is that correction of mechanical dyssynchrony is the principal mechanism for CRT and that patients who lack significant dyssynchrony despite having QRS widening may not respond. 4–8 Although a large body of literature supports echocardiographic dyssynchrony as a prognostic marker for patient response to CRT, the recent PROSPECT study (Predictors of Responders to Cardiac Resynchronization Therapy) failed at its attempt to define the optimal echocardiographic dyssynchrony approach.9,10 Furthermore, PROSPECT raised concerns about feasibility and reproducibly of echocardiographic methods.11,12 As a result, there is no consensus as to the predictive value of echocardiographic dyssynchrony and current clinical selection criteria for CRT include QRS widening as a surrogate for dyssynchrony. Acknowledged limitations of PROSPECT include confounding variables of different acquisition and analysis approaches, suboptimal end-points, and follow-up limited to 6 months after CRT.11,12 Accordingly, our objective was to test the hypothesis that echocardiographic dyssynchrony is associated with outcome following CRT. We used a prospective longitudinal study design with unified echocardiographic analysis and predefined important end-points of death, cardiac transplant or left ventricular assist device (LVAD) following CRT.
Methods
We prospectively studied a series of 229 consecutive heart failure patients referred for routine CRT indications. The protocol was approved by the Institutional Review Board on Biomedical Research and all patients gave informed consent consistent with this protocol. All patients had New York Heart Association functional class III or IV heart failure despite optimal pharmacological therapy, QRS duration ≥ 120ms and LV ejection fraction (EF) ≤ 35%. Optimal pharmacological therapy included angiotensin converting enzyme inhibitors or receptor blockers, β-blockers and spironolactone, as tolerated. A biventricular pacing system was implanted with a standard right ventricular apical lead and left ventricular lead positioned through the coronary sinus in an epicardial vein targeting posterolateral or lateral branches. In the event that a lead could not be placed transvenously because of anatomic constraints, epicardial LV leads were surgically implanted using a mini-thoracotomy in a small subset (<5%) of patients.
Echocardiography
All echocardiographic studies and off-line analyses were performed using a standard imaging system and software (VIVID 7, EchoPac BT08 GE-Vingmed, Horton, Norway). Briefly, routine digital 2- dimensional and tissue Doppler imaging (TDI) cine loops were obtained, including mid-LV short axis views at the level of the papillary muscle, apical views and pulsed-Doppler of the right ventricular and LV outflow. Mitral regurgitation was semi-quantitatively assessed by color Doppler jet area and vena contract width. 13 EF was calculated by biplane Simpson’s rule. 14 Intraventricular dyssynchrony was determined by TDI and speckle tracking as previously described in detail (Fig. 1). TDI regions of interest (7 × 15 mm) were placed in the basal and mid LV segments for the 3 standard views for 12-sites, adjusted for the most reproducible peak systolic velocities and time-to-peak calculated from the onset of the QRS.6,8,15 Longitudinal dyssynchrony was opposing wall delay (OWD) defined as the maximal difference in peak velocity at basal and mid segments in opposing walls for each view. The Yu index was calculated as the 12-site time-to-peak velocity standard deviation. 8,16 Significant longitudinal dyssynchrony by TDI was pre-defined as the maximal OWD in one view ≥ 65 ms,5,15,17 or Yu Index of ≥ 32 ms.6,8,16
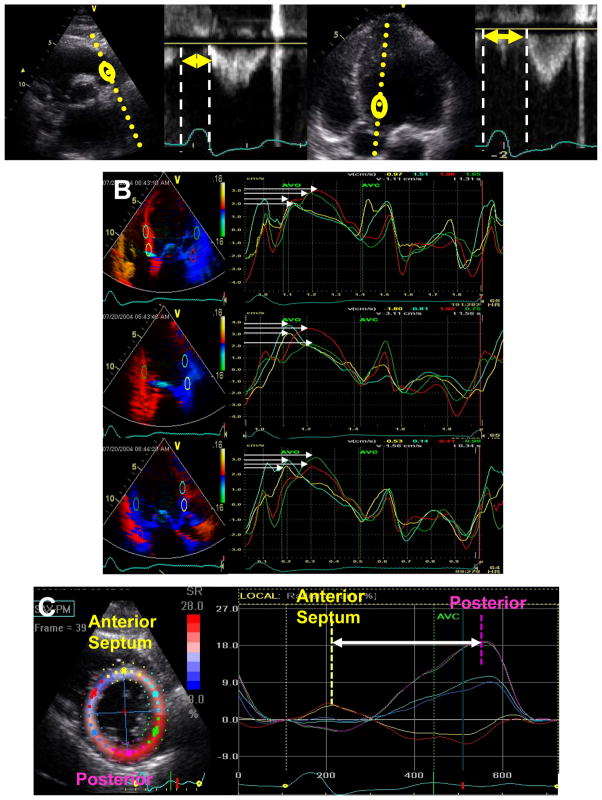
Figure 1.
Dyssynchrony indices included in this study. A: Interventricular mechanical delay calculated as difference between right ventricular ejection (arrow left panels) and left ventricular ejection (arrow right panels). B) Tissue Doppler longitudinal velocity from apical 4-chamber, 2-chamber and long-axis views with time to peak velocity from basal and mid ventricular sites. Opposing wall delay was the maximal difference in time to peak velocity (arrows) of opposing walls form each view, and the Yu index was the 12 site standard deviation. C) Speckle tracking radial strain from the mid-ventricular short axis view demonstrating the time difference between anteoseptum to posterior wall (arrow).
Speckle tracking of routine grayscale mid-LV short-axis images was performed as previously described to assess radial dyssynchrony. An end-diastolic circular region of interest was traced slightly within the endocardial cavity, using a point-and-click approach with special care taken to adjust tracking of all endocardial segments.7 A second larger concentric circle was then generated and manually adjusted near the epicardium. Time-to-peak segmental radial strain was determined from the highest peak positive strain value throughout the cardiac cycle, beginning slightly before the onset of the QRS complex to include very early mechanical activation. Radial dyssynchrony was determine as the time difference between the anteroseptal and posterior wall with ≥ 130ms predefined as significant.7,15
Interventricular dyssynchrony was calculated from routine pulsed-Doppler as previously described.6,18 LV pre-ejection delay was determined as the interval from the onset of the QRS to the onset of LV ejection velocity. Likewise, the right ventricular (RV) pre-ejection delay was determined as the interval from the onset of the QRS to the onset of the RV ejection velocity. Interventricular mechanical delay (IVMD) was determined as the difference between the RV and LV pre-ejection time with ≥ 40ms predefined as significant dyssynchrony. 18
Outcome and Subgroup Analysis
The principal outcome variable was the combined endpoint of mortality, cardiac transplant or ventricular assist device (LVAD) implantation. This combined end-point was pre-determined because only patients with end-stage heart failure with a limited anticipated survival would undergo transplant or LVAD implantation in our institution. The potential impact of dyssynchrony with respect to QRS width was prospectively evaluated using the pre-specified cut-off of QRS 120–150 ms versus QRS > 150 ms, as used in the CARE-HF trial for patient enrollment. 3
Statistical Analysis
Group data were presented as means ± standard deviation and were compared using the student t- test for unpaired data. Proportional differences were evaluated using Fisher’s exact test and Chi-square was used for non continuous variables. Kaplan-Meier survival curves were plotted to measure outcome and the log-rank test used to compare survival between patients with or without dyssynchrony. A Cox proportional hazard model was used to assess for any potential influence of covariates. To test the appropriateness of the proportional hazards assumption in the Cox model, we determined that differences in QRS width and incidence of ischemic etiology at baseline were potential confounding covariates. Adjustments were made with the influence of these explanatory variables assumed to be constant over time. We verified the proportional hazards assumption using graphical methods and the log-rank test and observed the proportional hazards assumption to be appropriate. Follow-up duration was truncated at 3 years for smaller sub-group samples. Receiver operator characteristic curve analysis was performed using a nonparametric estimate of the area under curve with its 95% confidence interval based on the method by Hanley et al.19 Data were reported as mean ± standard deviation throughout with a p value of < 0.05 considered as significant.
Results
Feasibility and Variability of Echocardiographic Dyssynchrony Analysis
Of the 229 consecutive patients referred for CRT with attempted echocardiographic studies, 19 (8%) had technically inadequate images for quantitative analysis due to poor windows and were excluded from all further analysis. Of these 210 patients, TDI analysis for the OWD and Yu index was feasible in 205 (90%), speckle tracking radial strain was feasible in 197 (86%). Routine pulsed Doppler for IVMD was added to the protocol after the first 27 patients, and was feasible in 180 of 202 (91%). From 20 randomly selected studies; intraobserver variability for determining routine pulsed Doppler dyssynchrony measures was 3 ± 3 ms (3 ± 4 %) and interobserver variability was 5 ± 5 ms (4 ± 5 %). Intraobserver variability for dyssynchrony by TDI using identical digital cineloops was 6 ± 5 ms (6 ± 7 %) and interobserver variability was 8 ± 7 ms (8 ± 7 %). Intraobserver variability for dyssynchrony by speckle tracking strain from the identical digital cineloops was 17 ± 10 ms (8 ± 7 %) and interobserver variability was 22 ± 14 ms (9 ± 7 %).
Dyssynchrony and Baseline Characteristics
The baseline characteristics of the 210 patients with echocardiographic data are shown in Table 1. The prevalence of TDI dyssynchrony by OWD ≥ 65ms and Yu Index ≥ 32ms was 78% and 72%, respectively. The 205 patients with and without dyssynchrony by TDI indices had similar age, proportion of female gender, EF, mitral regurgitation, and QRS duration. However, significant dyssynchrony was observed less often in those with ischemic disease compared with non-ischemic disease as follows: 53% vs. 71% for OWD ≥65ms and 51% vs. 73% for Yu Index ≥32ms (all p< 0.05). The 197 patients with and without significant radial dyssynchrony ≥ 130 ms by speckle tracking radial strain also had similar age, % of females, EF and mitral regurgitation. Patients with ischemic disease had less prevalent radial dyssynchrony than those with non-ischemic disease; 50% vs. 76%, respectively (p<0.05). QRS duration was greater in patients with significant radial dyssynchrony 161 ± 26ms compared with those without radial dyssynchrony 148 ± 23ms (p<0.05). The 180 patients with IVMD data available with and without dyssynchrony had similar age, % females, EF and mitral regurgitation. Once again, patients with ischemic disease had less prevalent dyssynchrony by IVMD ≥ 40 ms compared with those with nonischemic disease; 43% vs. 69%. Similar to radial dyssynchrony, significant IVMD dyssynchrony was associated with wider QRS duration 171 ± 27 ms vs. 147 ± 21 ms in those without significant IVMD (p<0.05).
Table 1.
Baseline Clinical Characteristics and Echocardiographic Dyssynchrony
| Dyssynchrony Index | Cut-Off | n | Age (years) | Female (%) | EF (%) | MR (degree) | QRS (ms) | Ischemic Etiology (%) |
|---|---|---|---|---|---|---|---|---|
| IVMD | ≥ 40 ms | 83 | 65±11 | 38 | 23±7 | 1.5±1.2 | 171±27 | 43 |
| < 40 ms | 97 | 67±11 | 23 | 24±6 | 1.4±1.3 | 147±21* | 69* | |
| TDI Opposing Wall Delay | ≥ 65 ms | 160 | 65±12 | 34 | 24±7 | 1.4±1.1 | 159±25 | 53 |
| < 65 ms | 45 | 67±11 | 18 | 25±6 | 1.4±1.2 | 159±30 | 71* | |
| TDI Opposing Wall Delay | ≥ 80 ms | 142 | 66±12 | 36 | 24±7 | 1.4±1.1 | 160±25 | 50 |
| < 80 ms | 63 | 66±11 | 18 | 24±6 | 1.4±1.2 | 157±29 | 73* | |
| TDI Yu Index | ≥ 32 ms | 148 | 65±12 | 35 | 24±7 | 1.3±1.1 | 161±25 | 51 |
| < 32 ms | 57 | 67±10 | 20 | 24±6 | 1.5±1.3 | 156±29 | 73* | |
| Radial Strain Anteroseptal to Posterior Wall Delay | ≥ 130 ms | 150 | 65±11 | 34 | 24±6 | 1.5±1.2 | 161±26 | 50 |
| < 130 ms | 47 | 66±13 | 18 | 24±7 | 1.3±1.1 | 148±23* | 76* |
IVMD = Interventricular mechanical delay, EF= ejection fraction TDI = tissue Doppler imaging, MR = mitral regurgitation; semi-quantitatively graded: 0 = none, 1 = mild, 2 = moderate, 3 = moderately severe, 4 = severe.
p<0.05 vs. patients with corresponding dyssynchrony variable. All data are presented as mean ± standard deviation.
Outcome Events and Dyssynchrony
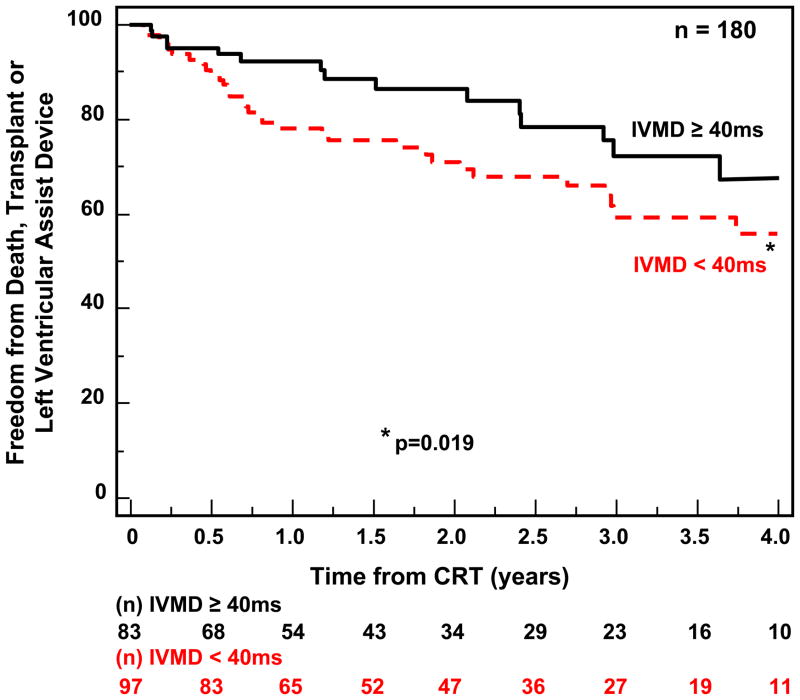
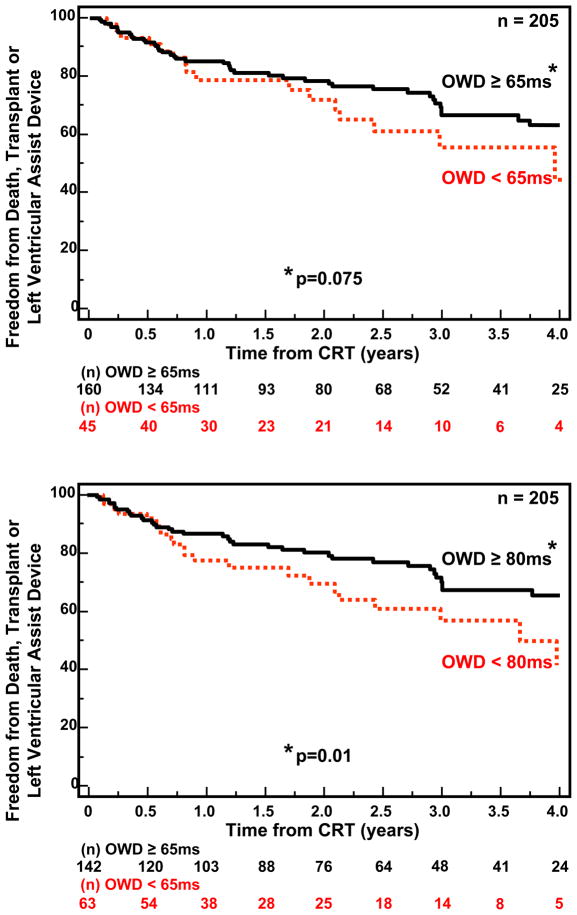
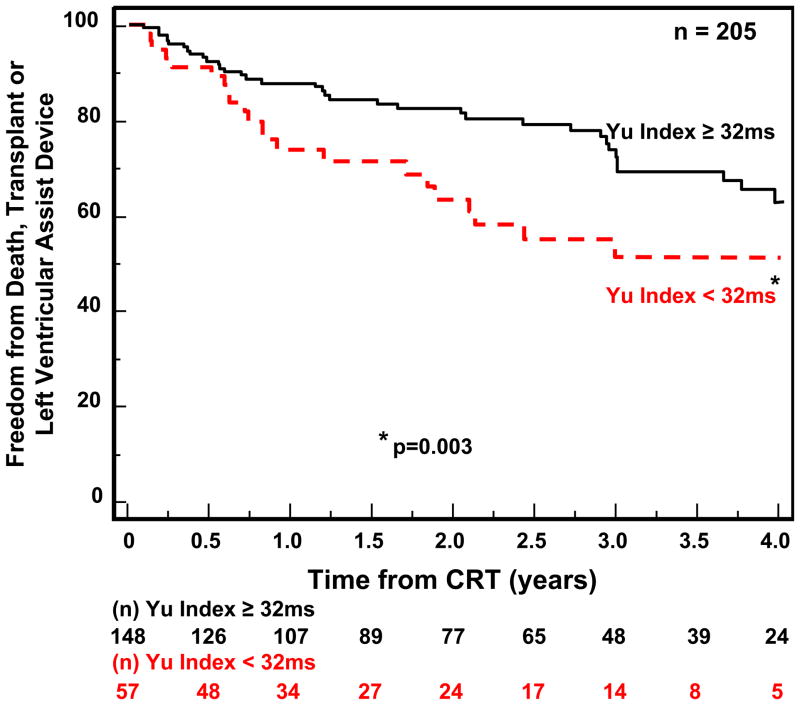
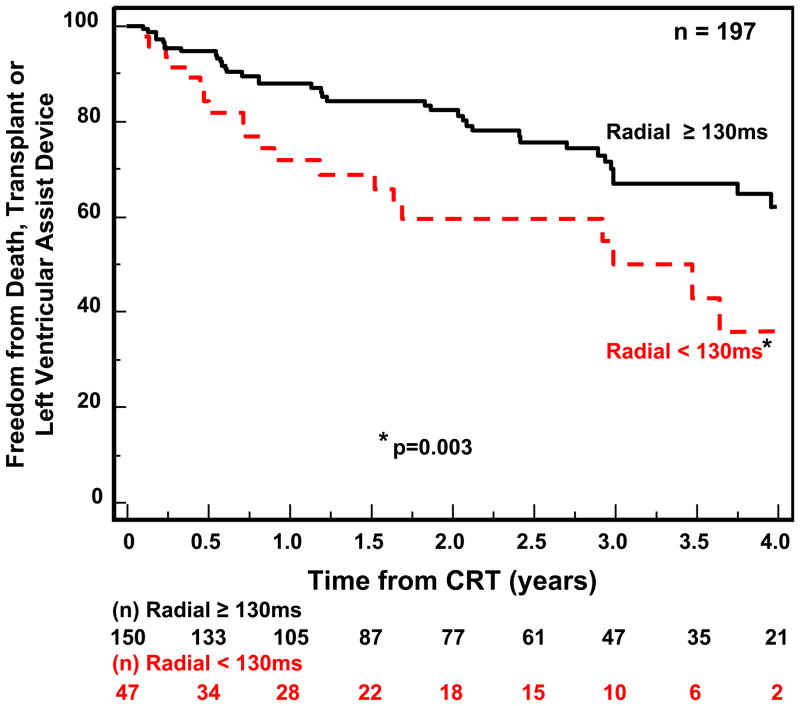
Follow-up for the 210 patients with available echocardiographic dyssynchrony data was for a period of 4 years. CRT implants occurred throughout this period in this single center experience, and the numbers reflect the variable follow-up duration, ranging from a minimum of 1 year to 4 years. During this period, 62 outcome events occurred including 47 deaths, 9 transplants and 6 LVAD implantations. Notably, one of these unfavorable clinical events occurred < 6 months after CRT implant in 30 patients. Patients with IVMD ≥ 40ms had a significantly more favorable outcome following CRT than those with IVMD < 40 ms (p=0.019) (Fig 2). OWD by TDI using our pre-defined cut-off of ≥ 65 ms had a trend for a better outcome, but this only came close to statistical significance (p= 0.075) (Fig. 3A) We then performed post hoc analysis with a OWD cut-off of ≥ 80 ms, which was associated with a statistically significant more favorable event-free survival after CRT (p=0.011) (Fig. 3B). Yu index as prospectively defined ≥ 32 ms was predictive of survival free from transplant or LVAD (p=0.003) (Fig 4). Radial dyssynchrony ≥ 130 ms by speckle tracking was highly associated with event-free survival following CRT (p=0.003) (Fig. 5). Importantly, when adjusted for the covariates of ischemic etiology and QRS duration using a Cox proportional hazard model, the Yu Index and radial strain remained independently predictive of event-free survival (p<0.05). The hazard ratio of radial dyssynchrony for predicting survival was 2.21, with a 95% confidence interval (CI) of 1.35 – 4.79, p = 0.003. When adjusted for QRS, the instantaneous relative risk was 0.55 with 95% CI of 0.32 – 0.944, p = 0.03. When adjusted for ischemic disease, the instantaneous relative risk was 0.53, with a 95% CI of 0.31 – 0.92, p = 0.03. The hazard ratio of the Yu Index for predicting survival was 2.13 with a 95% CI of 1.38 – 4.46, p = 0.003. When adjusted for QRS, the instantaneous relative risk was 0.51 with 95% CI of 0.30 – 0.85, p = 0.01 and when adjusted for ischemic disease, the instantaneous relative risk was 0.53 with a 95% CI of 0.31 – 0.90, p = 0.02. In contrast, IVMD and OWD were too closely associated with ischemic etiology to be independently predictive.
Figure 2.
Kaplan-Meier curves of probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT). Patients with significant interventricular mechanical delay (IVMD) had a more favorable outcome compared to those without significant IVMD.
Figure 3.
Kaplan-Meier curves of probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT). Patients with significant tissue Doppler opposing wall delay (OWD) were compared to those without significant OWD. The top panel utilized the pre-defined cut-off of 65 ms, which showed a trend for significance between groups. The bottom panel demonstrated that patients with significant OWD ≥ 80 ms had a more favorable outcome compared to those with OWD < 80 ms.
Figure 4.
Kaplan-Meier curves of probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT). Patients with significant dyssynchrony by the Yu Index had a more favorable outcome compared to those without significant Yu Index dyssynchrony.
Figure 5.
Kaplan-Meier curves of probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT). Patients with significant dyssynchrony by speckle tracking radial strain anteroseptal to posterior wall delay had a more favorable outcome than those without significant radial dyssynchrony.
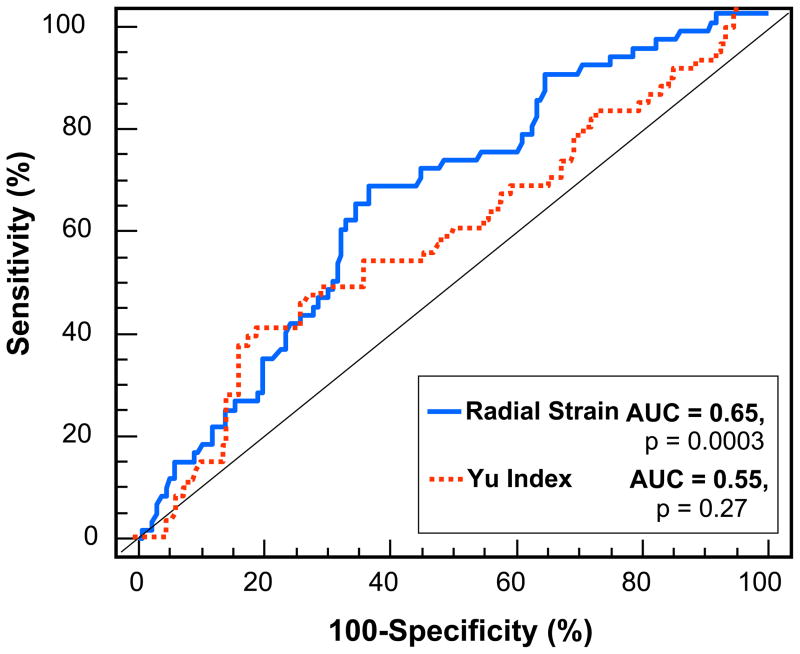
Using receiver operator characteristic curve analysis, the Yu Index with a cut-off of 32 ms was highly specific at 78%, but less sensitive at 40% for predicting death, transplant or LVAD. (Figure 6). Radial strain with a cut-off of 130 ms was similarly specific at 79% and less sensitive at 39% for predicting clinical outcome. Accordingly, excluding dyssynchrony by Yu Index or radial strain was specifically associated with death, transplant or LVAD following CRT. For comparison, the area under the curve for radial strain at 0.65 was greater than the Yu Index at 0.55.
Figure 6.
Receiver-operator characteristic curves for the associations of radial strain dyssynchrony and Yu index dyssynchrony with death, transplant, or ventricular assist device after cardiac resynchronization therapy with areas under the curve (AUC) shown.
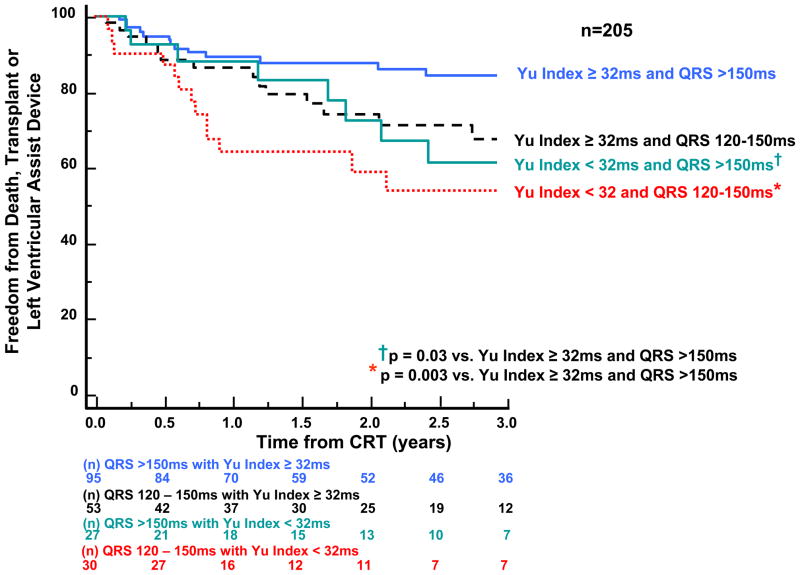
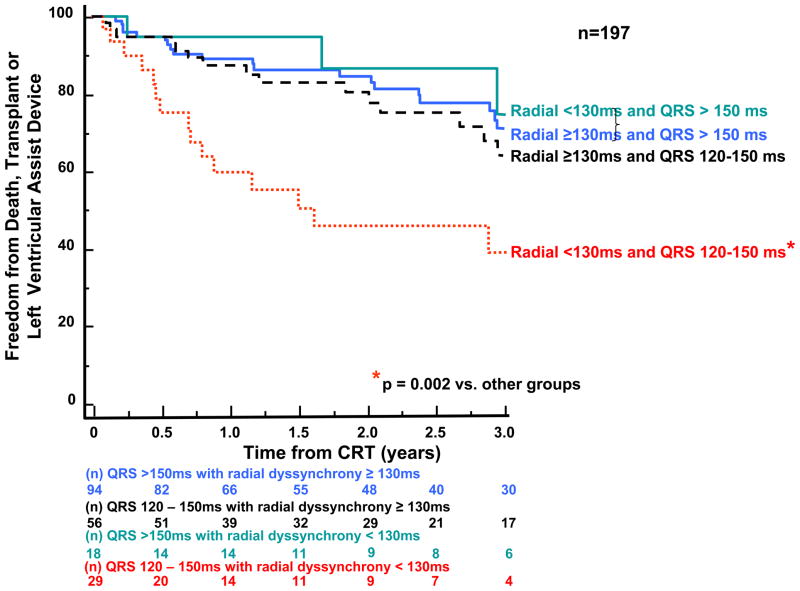
Subgroup analysis was performed to examine the potential relationship of QRS width and dyssynchrony to survival following CRT using a pre-defined QRS cut-off. Patients with narrower QRS width 120–150 ms (n = 122) were compared to those with QRS > 150 ms (n=136). Dyssynchrony by the Yu Index was associated with a more favorable outcome in either QRS duration group, with wider QRS patients with dyssynchrony having the best outcome, and narrower QRS patients without dyssynchrony having the poorest outcome. (Fig 7). With regard to radial dyssynchrony, patients with QRS width > 150 ms similarly favorable outcome, however, those with QRS width 120–150 ms who lacked radial dyssynchrony had a particularly poor prognosis (p=0.003 vs. all other groups). (Fig. 8).
Figure 7.
Kaplan-Meier curves of patients subdivided by baseline QRS duration and dyssynchrony by the Yu Index showing probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT). Significant dyssynchrony by the Yu Index before CRT was associated with a more favorable long-term outcome in patients with both wider and narrower QRS duration.
Figure 8.
Kaplan-Meier curves of patients subdivided by baseline QRS duration and dyssynchrony by the speckle tracking radial strain showing probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT). Patients with QRS duration 120–150 ms who lacked dyssynchrony by speckle tracking radial strain had a significantly less favorable outcome following CRT than the other patient subgroups.
Discussion
This study demonstrated the relationship between several echocardiographic dyssynchrony markers and the important outcome variable of long term survival following CRT. A large series of consecutive patients were included using a prospective longitudinal study design with pre-specified end-points. The echocardiographic approach revealed a relatively high yield (89%) in consecutive patients referred for CRT. Baseline clinical variables were balanced overall in those patients with and without dyssynchrony, however the lack of dyssynchrony was significantly more prevalent in those with ischemic disease. Radial dyssynchrony and IVMD were also related to baseline QRS width. As expected from this severely ill group of heart failure patients, there were a large number of the unfavorable events of death, heart transplant or LVAD. Using pre-defined cut-offs, baseline dyssynchrony before CRT by the Yu Index, speckle tracking radial strain anterioseptal to posterior wall delay, and IVMD were all associated with more favorable survival free from transplant or LVAD. The TDI OWD with the pre-defined cut-off of ≥ 65 ms had a trend for association with event-free survival (p=0.075), however, an OWD cut-off of ≥ 80 ms by post hoc analysis became significantly associated (p=0.011). Of note, the Kaplan-Meier curves for each of these TDI indices did not diverge until after the first 6 months after CRT, which was the follow-up interval of PROSPECT. Of the 191 patients with both Yu index and radial dyssynchrony data available, 128 (67%) were concordant for dyssynchrony using pre-specified cut-off values. When considering 8 additional patients with borderline dyssynchrony values ± 5 ms for Yu index and ± 10 ms for radial dyssynchrony, concordance increased to 71%. The exact reason for discordance is unknown. Perhaps TDI longitudinal velocity and speckle tracking radial strain assess different aspects of dyssynchrony and provide additional information. 15 When adjusted for covariates of ischemic etiology and QRS width, the Yu Index and radial strain remained independently predictive of outcome. Subgroup analysis demonstrated that patients with narrower QRS width 120–150 ms who lacked radial dyssynchrony had a particularly poor survival. These observations strongly support the association of echocardiographic dyssynchrony with long-term patient outcome after CRT.
Several previous studies have also shown the ability of echocardiographic mechanical dyssynchrony to predict response to CRT.4,5,7,8,15–17,20–25 Baseline dyssynchrony has been related to improvement in heart failure class, 6-minute walk distance, quality of life score, EF and reductions in end-systolic volumes. However, the multicenter PROSPECT study of predictors of response to cardiac resynchronization therapy failed to show conclusively that a single echocardiographic dyssynchrony measure was highly predictive. 9 Although there have been over a hundred published articles supporting the utility of echocardiographic dyssynchrony to predict CRT response, PROSPECT has had a particularly high impact on clinical opinion because of its prospective multicenter design. CRT guidelines continue to use QRS width as a surrogate for mechanical dyssynchrony. 26 Several acknowledged limitations of PROSPECT included a low yield of feasibility, high variability using three different echocardiographic systems and software, and three different echo core labs. 11,12 For example, the overall yield for routine measures in PROSPECT such as end-systolic volumes was approximately 67%, indicating poor image quality in a third of patients. Furthermore, TDI Yu Index was feasible in only 50% of attempted studies, in contrast to the present study with a yield of 89% of consecutive CRT patients. Importantly, the follow-up was limited to 6 months in PROSPECT which may likely have been too short a duration to demonstrate the relationship of dyssynchrony with patient outcome.
Specific echocardiographic measures of dyssynchrony predictive of outcome following CRT have been reported by several authors. Bax et al. demonstrated that a TDI OWD of ≥ 65 ms was associated with a lower incidence of heart failure hospitalizations and death in the first year following CRT. 5 This OWD cut off of 65 ms in our present study had a strong trend to significantly predicting survival free from transplant or LVAD, however, we did not include heart failure hospitalizations as and end-point. Pitzalis et al. used M-mode to show that an anteroseptal to posterior wall delay of ≥ 130 ms successfully predicted outcome. 23 We and others have had more success with speckle tracking radial strain to assess anteroseptal to posterior wall delay, with a favorable predictive value using the same 130 ms cut-off value. 7 Bank et al. showed that radial strain by speckle tracking was also predictive of response to CRT in a multicenter study. 27 Analyses from the CARE HF trial used a cut-off of 49 ms for IVMD to demonstrate its value to predict outcome following CRT. 28 We selected a pre-defined IVMD cut-off of ≥ 40 ms as originally described and also used in the PROSPECT trial. 9,18 More recently, Chailil et al. used cardiac magnetic resonance imaging to assess dyssynchrony and predict mortality following CRT. 29 Others have shown that mechanical dyssynchrony either independently or when combined with clinical markers is associated with a more favorable survival following CRT. 30 Furthermore, echocardiographic dyssynchrony has been shown to be a marker for mortality in heart failure patients with narrow QRS duration using TDI or after myocardial infarction using velocity vector imaging. 31 We observed that the absence of radial dyssynchrony in patients with QRS 120–150 ms to be associated with a particularly high probability of death, transplant or LVAD. Radial dyssynchrony was not associated with outcome in patients with QRS > 150 ms. However, we observed that the Yu Index was particularly associated with outcome in patients with QRS >150 ms. The exact reason for these results is unclear, but suggests that the TDI Yu index and speckle tracking radial strain may be assessing different aspects of LV dyssynchrony. 15 More recently, radial and transverse strain by speckle tracking was shown to be associated with ejection fraction response and survival after CRT in a separate multicenter study using different echocardiographic equipment and software.31 In summary, this present study demonstrates that baseline echocardiographic dyssynchrony is associated with a more favorable outcome following CRT, and that patients who lack dyssynchrony may be identified as being at comparatively higher risk for death, transplant or LVAD following CRT.
Limitations
The present study was not part of a randomized trial, and the relationship of echocardiographic dyssynchrony to survival in those who do not undergo CRT remains unknown. Although the absence of echocardiographic dyssynchrony appears to be a marker for a worse prognosis in patients following CRT, the potential influence of CRT on outcome in patients without dyssynchrony remains unknown. However, it is presently difficult to withhold CRT from patients who meet current CRT implantation criteria to elucidate this point. Another limitation was that echocardiographic dyssynchrony analysis could not be successfully performed on all consecutive CRT patients, and 10–11% of patients did not have adequate image quality for quantification. Furthermore, high quality images appear to especially important for speckle tracking analysis. 7,15,32 It is acknowledged that these echocardiographic methods are operator dependent, and require user experience. In particular, technical difficulties with speckle tracking strain occurred in regions of myocardial scar, and appeared to be more robust in patients with nonischemic cardiomyopathies. However, reproducible results may be achieved with a systematic core-lab approach, detailed in the methods. A limitation of the study design may be considered that all survival free from heart transplantation or LVAD was used as the primary end-point, rather than all cause mortality. However, heart transplantation and LVAD implantation in our institution are utilized only in heart failure patients with a very limited life span anticipated without these interventions. Another limitation was that markers of CRT response used in other clinical trials, such as heart failure hospitalizations, 6-minute walk distance, peak myocardial oxygen consumption, LV reverse remodeling or EF improvement were not part of the present study. It may be considered a limitation that ischemic etiology may influence response to CRT from scar burden or lead positioning and have confounding effects. 33,34 Analysis of scar burden and lead positioning was not part of the current study. We demonstrated that when adjusting specifically for ischemic etiology, the Yu index and speckle tracking radial strain remained independently associated with survival. Our subgroup analysis on heart failure etiology and QRS width provided additive information which may be of clinical impact. Recent data support the potential utility of echocardiographic dyssynchrony in patients with less wide QRS duration as an adjunct to clinical decision making. 22 It remains uncertain how these data will directly influence clinical practice, and ongoing further study is warranted.
Clinical Summary.
This study demonstrated the association of echocardiographic dyssynchrony with long term survival following cardiac resynchronization therapy (CRT). We studied 229 consecutive patients with routine CRT indications (symptomatic heart failure, reduced ejection fraction and widened QRS ≥ 120 ms) of which 210 (89%) had baseline echocardiographic dyssynchrony data available. Dyssynchrony was pre-specified as tissue Doppler longitudinal velocity opposing wall delay ≥ 65 ms, 12-site standard deviation (Yu Index) ≥ 32 ms, speckle tracking radial strain anteroseptal to posterior wall delay ≥130 ms, or pulsed Doppler interventricular mechanical delay ≥ 40 ms. Of 210 patients, there were 62 unfavorable events over 4 years after CRT: 47 deaths, 9 transplants, and 6 left ventricular assist device implantations. All echo dyssynchrony indices were significantly associated with a more favorable long term prognosis than patients without dyssynchrony, except tissue Doppler velocity opposing wall delay became significant at ≥ 80 ms. When adjusted for covariates of ischemic etiology and QRS width, the Yu Index and speckle tracking radial strain remained independently predictive of outcome. Subgroup analysis demonstrated that patients with narrower QRS width 120–150 ms who lacked radial dyssynchrony had a particularly poor survival. Although this study has identified the absence of echocardiographic dyssynchrony as a marker for a less favorable prognosis in patients who undergo CRT for routine indications, the potential influence of CRT on outcome in patients without dyssynchrony remains unknown. These observations strongly support the association of echocardiographic dyssynchrony with long-term patient outcome after CRT.
Acknowledgments
The authors are grateful to the entire University of Pittsburgh Medical Center Electrophysiology Laboratory faculty and staff for their continued support and cooperation.
Funding Sources: Dr. Gorcsan was supported in part by N.I.H. award K24 HL04503-01.
Abbreviations list
- CRT
cardiac resynchronization therapy
- EF
ejection fraction
- FT
filling time
- IVMD
interventricular mechanical delay
- LV
left ventricular
- LVAD
left ventricular assist device
- OWD
Opposing wall delay
- TDI
Tissue Doppler imaging
Footnotes
Disclosure: GE Medical Systems donated the software and technical support for this study.
References
- 1.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, Gorcsan J, 3rd, Hayes DL, Kass DA, Knuuti J, Leclercq C, Linde C, Mark DB, Monaghan MJ, Nihoyannopoulos P, Schalij MJ, Stellbrink C, Yu CM. Cardiac resynchronization therapy: Part 1--issues before device implantation. J Am Coll Cardiol. 2005;46:2153–67. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–40. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Gorcsan J, 3rd, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, Martin R, Steinberg JS, Sutton MS, Yu CM. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting--a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J., 3rd Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113:960–8. doi: 10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 8.Yu CM, Fung WH, Lin H, Zhang Q, Sanderson JE, Lau CP. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684–8. doi: 10.1016/s0002-9149(02)03404-5. [DOI] [PubMed] [Google Scholar]
- 9.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 10.Yu CM, Abraham WT, Bax J, Chung E, Fedewa M, Ghio S, Leclercq C, Leon AR, Merlino J, Nihoyannopoulos P, Notabartolo D, Sun JP, Tavazzi L. Predictors of response to cardiac resynchronization therapy (PROSPECT)--study design. Am Heart J. 2005;149:600–5. doi: 10.1016/j.ahj.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Bax JJ, Gorcsan J., 3rd Echocardiography and noninvasive imaging in cardiac resynchronization therapy: results of the PROSPECT (Predictors of Response to Cardiac Resynchronization Therapy) study in perspective. J Am Coll Cardiol. 2009;53:1933–43. doi: 10.1016/j.jacc.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 12.Yu CM, Bax JJ, Gorcsan J., 3rd Critical appraisal of methods to assess mechanical dyssynchrony. Curr Opin Cardiol. 2009;24:18–28. doi: 10.1097/hco.0b013e32831bc34e. [DOI] [PubMed] [Google Scholar]
- 13.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Gorcsan J, 3rd, Tanabe M, Bleeker GB, Suffoletto MS, Thomas NC, Saba S, Tops LF, Schalij MJ, Bax JJ. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J Am Coll Cardiol. 2007;50:1476–83. doi: 10.1016/j.jacc.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 16.Yu CM, Chau E, Sanderson JE, Fan K, Tang MO, Fung WH, Lin H, Kong SL, Lam YM, Hill MR, Lau CP. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002;105:438–45. doi: 10.1161/hc0402.102623. [DOI] [PubMed] [Google Scholar]
- 17.Gorcsan J, 3rd, Kanzaki H, Bazaz R, Dohi K, Schwartzman D. Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2004;93:1178–81. doi: 10.1016/j.amjcard.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 18.Cazeau S, Bordachar P, Jauvert G, Lazarus A, Alonso C, Vandrell MC, Mugica J, Ritter P. Echocardiographic modeling of cardiac dyssynchrony before and during multisite stimulation: a prospective study. Pacing Clin Electrophysiol. 2003;26:137–43. doi: 10.1046/j.1460-9592.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 20.Cannesson M, Tanabe M, Suffoletto MS, Schwartzman D, Gorcsan J., 3rd Velocity vector imaging to quantify ventricular dyssynchrony and predict response to cardiac resynchronization therapy. Am J Cardiol. 2006;98:949–53. doi: 10.1016/j.amjcard.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 21.Dohi K, Suffoletto MS, Schwartzman D, Ganz L, Pinsky MR, Gorcsan J., 3rd Utility of echocardiographic radial strain imaging to quantify left ventricular dyssynchrony and predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2005;96:112–6. doi: 10.1016/j.amjcard.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Oyenuga O, Hara H, Tanaka H, Kim HN, Adelstein EC, Saba S, Gorcsan J., 3rd Usefulness of echocardiographic dyssynchrony in patients with borderline QRS duration to assist with selection for cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2010;3:132–40. doi: 10.1016/j.jcmg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Pitzalis MV, Iacoviello M, Romito R, Guida P, De Tommasi E, Luzzi G, Anaclerio M, Forleo C, Rizzon P. Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J Am Coll Cardiol. 2005;45:65–9. doi: 10.1016/j.jacc.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 24.Sogaard P, Egeblad H, Kim WY, Jensen HK, Pedersen AK, Kristensen BO, Mortensen PT. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. J Am Coll Cardiol. 2002;40:723–30. doi: 10.1016/s0735-1097(02)02010-7. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Hara H, Saba S, Gorcsan J., 3rd Prediction of response to cardiac resynchronization therapy by speckle tracking echocardiography using different software approaches. J Am Soc Echocardiogr. 2009;22:677–84. doi: 10.1016/j.echo.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Riegel B, Tarkington LG, Yancy CW. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 27.Bank AJ, Kaufman CL, Kelly AS, Burns KV, Adler SW, Rector TS, Goldsmith SR, Olivari MT, Tang C, Nelson L, Metzig A. Results of the Prospective Minnesota Study of ECHO/TDI in Cardiac Resynchronization Therapy (PROMISE-CRT) study. J Card Fail. 2009;15:401–9. doi: 10.1016/j.cardfail.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Richardson M, Freemantle N, Calvert MJ, Cleland JG, Tavazzi L. Predictors and treatment response with cardiac resynchronization therapy in patients with heart failure characterized by dyssynchrony: a pre-defined analysis from the CARE-HF trial. Eur Heart J. 2007;28:1827–34. doi: 10.1093/eurheartj/ehm192. [DOI] [PubMed] [Google Scholar]
- 29.Chalil S, Stegemann B, Muhyaldeen S, Khadjooi K, Smith RE, Jordan PJ, Leyva F. Intraventricular dyssynchrony predicts mortality and morbidity after cardiac resynchronization therapy: a study using cardiovascular magnetic resonance tissue synchronization imaging. J Am Coll Cardiol. 2007;50:243–52. doi: 10.1016/j.jacc.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 30.Leyva F, Foley PW, Stegemann B, Ward JA, Ng LL, Frenneaux MP, Regoli F, Smith RE, Auricchio A. Development and validation of a clinical index to predict survival after cardiac resynchronisation therapy. Heart. 2009;95:1619–25. doi: 10.1136/hrt.2009.173880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho GY, Song JK, Park WJ, Han SW, Choi SH, Doo YC, Oh DJ, Lee Y. Mechanical dyssynchrony assessed by tissue Doppler imaging is a powerful predictor of mortality in congestive heart failure with normal QRS duration. J Am Coll Cardiol. 2005;46:2237–43. doi: 10.1016/j.jacc.2004.11.074. [DOI] [PubMed] [Google Scholar]
- 32.Delgado V, Ypenburg C, van Bommel RJ, Tops LF, Mollema SA, Marsan NA, Bleeker GB, Schalij MJ, Bax JJ. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol. 2008;51:1944–52. doi: 10.1016/j.jacc.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 33.Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153:105–12. doi: 10.1016/j.ahj.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, van der Wall EE, Schalij MJ, Bax JJ. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–76. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]