Abstract
A large number of plant and bacterial toxins with enzymatic activity on intracellular targets are now known. These toxins enter cells by first binding to cell surface receptors, then they are endocytosed and finally they become translocated into the cytosol from an intracellular compartment. In the case of the plant toxin ricin and the bacterial toxin Shiga toxin, this happens after retrograde transport through the Golgi apparatus and to the endoplasmic reticulum. The toxins are powerful tools to reveal new pathways in intracellular transport. Furthermore, knowledge about their action on cells can be used to combat infectious diseases where such toxins are involved, and a whole new field of research takes advantage of their ability to enter the cytosol for therapeutic purposes in connection with a variety of diseases. This review deals with the mechanisms of entry of ricin and Shiga toxin, and the attempts to use such toxins in medicine are discussed.
Keywords: endocytosis/endoplasmic reticulum/Golgi apparatus/ricin/Shiga toxin
Introduction
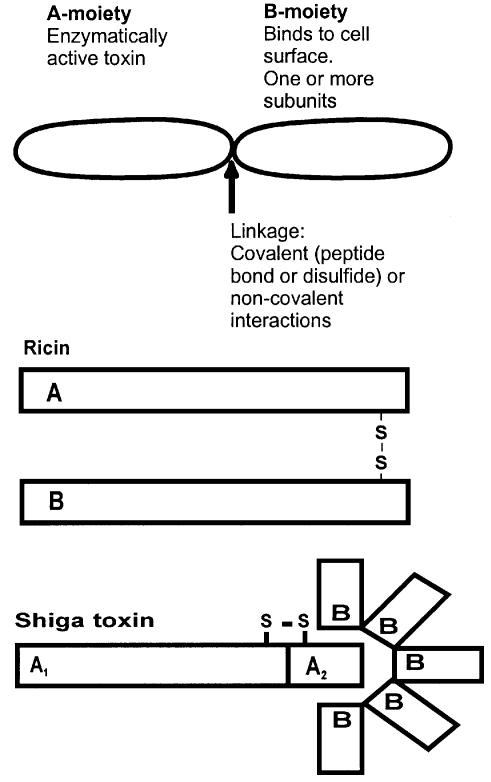
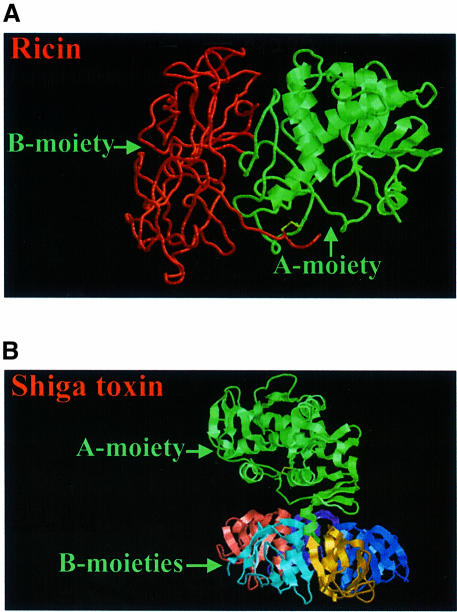
The plant toxin ricin and the bacterial Shiga toxin are members of a family of protein toxins that are highly toxic to a large number of eukaryotic cells (Sandvig and van Deurs, 1996, 1999; Acheson and Keusch, 1999; Olsnes et al., 1999). Although such toxins are found in different plants and bacteria, they have several common characteristics. They have one moiety responsible for binding to the cell surface, whereas another moiety enters the cytosol and inactivates protein synthesis enzymatically (Figure 1). The crystallographic structures of ricin (Rutenber et al., 1991) and Shiga toxin (Fraser et al., 1994) with their respective binding (B) and enzymatically active (A) moieties are shown in Figure 2. The cytosolic target of ricin and Shiga toxin is the 28S RNA of the 60S ribosomal subunit (Endo et al., 1987). Reduction of the disulfide bond connecting the A- and B-moieties of ricin (yellow area in Figure 2A) is required for optimal enzymatic activity (Sandvig and van Deurs, 1996). In the case of Shiga toxin, proteolytic cleavage of the A-moiety in a loop formed by the internal disulfide bond (yellow area in Figure 2B) and reduction of this disulfide bond facilitate rapid intoxication (Sandvig and van Deurs, 1996). In most cells, the processing of the Shiga toxin A-moiety is performed by the enzyme furin (Garred et al., 1995), located in the Golgi apparatus and in endosomes. This enzyme recognizes an amino acid sequence found not only in the loop of the Shiga toxin A-moiety, but also in a number of other bacterial toxins where proteolytic cleavage is important for activation of the toxin (Gordon and Leppla, 1994). Similar two-moiety structures to those of ricin and Shiga toxin are found in bacterial toxins such as diphtheria toxin and Pseudomonas exotoxin A (Wick et al., 1990; Sandvig and Olsnes, 1991; Olsnes et al., 1999; Pizza et al., 1999). In all cases, these toxins become endocytosed after binding to the cell surface. After transport to different intracellular destinations, they cross the membrane and exert their toxic effect in the cytosol. Whereas ricin and Shiga toxin attack ribosomes, diphtheria toxin and Pseudomonas exotoxin A inactivate elongation factor 2 (EF2) and thereby inhibit protein synthesis. Both ricin and Shiga toxin are transported in a retrograde manner to the endoplasmic reticulum (ER) before being translocated to the cytosol (Sandvig and van Deurs, 1996, 1999; Rapak et al., 1997; Arab and Lingwood, 1998; Lingwood et al., 1998; Girod et al., 1999; White et al., 1999). Ricin and the other members of this toxin family are very efficient at cell killing. For instance, one molecule of ricin can inactivate ∼2000 ribosomes/min. Since the toxins are also quite stable, one or a few molecules in the cytosol are sufficient to kill a cell (Olsnes and Sandvig, 1988). In addition to having a direct effect on protein synthesis, protein toxins can also induce DNA cleavage and cause apoptosis-like changes in cells (Sandvig and van Deurs, 1996), and new studies indicate that in the case of Shiga toxin this process may be regulated by proteins of the Bcl-2 family (Jones et al., 2000; Suzuki et al., 2000).
Fig. 1. Schematic structure of protein toxins. The top drawing applies to a number of different toxins such as the bacterial toxins Pseudomonas exotoxin A (Wick et al., 1990; Pizza et al., 1999), diphtheria toxin (Sandvig and Olsnes, 1991; Olsnes et al., 1999; Pizza et al., 1999), cholera toxin (Holmgren, 1981; Fishman and Orlandi, 1994; Pizza et al., 1999) and Shiga toxin (Sandvig and van Deurs, 1996, 1999; Acheson and Keusch, 1999), as well as the plant toxins ricin (Sandvig and van Deurs, 1996, 1999; Olsnes et al., 1999), abrin, modeccin, volkensin and viscumin (Sandvig and van Deurs, 1996, 1999; Olsnes et al., 1999). In addition, ricin with its disulfide bond between the A- and B-moieties, and Shiga toxin with five subunits in the B-moiety are shown.
Fig. 2. Crystallographic structures of ricin (A) and Shiga toxin (B). The enzymatically active subunits are in green, whereas the binding moiety of ricin is in red, and the five small binding subunits of Shiga toxin are multicoloured. The disulfide bond (and the neighbouring carbon atoms) connecting the two chains of ricin, and the internal disulfide bond in the A-moiety of Shiga toxin are yellow. The structures have been obtained from the PDB protein data bank (ricin: 1DMO; Shiga toxin:2AA1), and are based on work published by Rutenber et al. (1991) and Fraser et al. (1994).
The protein toxins have proven valuable in studies of endocytic processes and intracellular transport in general. As described below, ricin has been used to study different types of endocytosis (Llorente et al., 1998a; Rodal et al., 1999; Sandvig and van Deurs, 1999), and the toxin has been useful in studies of membrane transport to various intracellular destinations such as the Golgi apparatus and the ER, as well as to late endosomes/lysosomes and across polarized epithelial cell layers (Sandvig and van Deurs, 1996, 1999; Rapak et al., 1997; Llorente et al., 1998a,b). Investigations of Shiga toxin have revealed novel apects of glycolipid transport. Results obtained with this toxin show that it is not only protein sequences that are of importance for intracellular sorting, but also the glycosphingolipid composition (Sandvig et al., 1994; Sandvig and van Deurs, 1996; Arab and Lingwood, 1998). In fact, studies of Shiga toxin were the first to reveal that a molecule can be transported from the cell surface through the Golgi apparatus and to the ER (Sandvig et al., 1992). The toxins can also be used to study translocation to the cytosol, since the activity of even a few toxin molecules can be monitored as an inhibition of protein synthesis. Thus, ricin and Shiga toxin can be used to investigate transport from the ER, and other toxins, such as diphtheria toxin, can be used to study transport from endosomes or across the plasma membrane (Sandvig and Olsnes, 1991; Olsnes et al., 1999; Pizza et al., 1999).
A number of infectious diseases are associated with secretion of toxins (e.g. diphtheria and dysentery), and the extreme toxicity of the toxin molecules contributes greatly to the severity of such diseases. Although in many cases infectious diseases are under reasonable control today, Shiga toxin, which is secreted by Shigella dysenteriae, and the almost identical Shiga-like toxin 1 and related Shiga-like toxin 2 secreted by Escherichia coli and other bacteria are responsible for widespread disease and for the deaths of a large number of people on a worldwide basis (Takeda et al., 1993; Kaper, 1998; Bower, 1999; Uchida et al., 1999; Kitov et al., 2000; Paton et al., 2000). Shiga-like toxins have received considerable attention during the last decade. They have become an increasing threat to human health also in developed countries where they are responsible for the so-called ‘hamburger’ disease. Bacteria secreting Shiga-like toxins can contaminate different types of food, including milk, apple juice and vegetables (Kaper, 1998; Bower, 1999; Uchida et al., 1999). Infections with these bacteria may lead to haemolytic uraemic syndrome and kidney failure, particularly in children (Bower, 1999; Uchida et al., 1999). Clarification of the mechanism of action of Shiga toxin and other bacterial toxins in different cell types is therefore warranted in order to control the diseases (Kitov et al., 2000; Paton et al., 2000). Knowledge of toxin–receptor interactions at the molecular level provides us with tools to treat such infectious diseases. Recent publications report the development of bacteria with a Shiga toxin receptor mimic that binds and thereby neutralizes Shiga toxin (Paton et al., 2000), and the production of a pentamer of trisaccharides that efficiently binds Shiga toxin (Kitov et al., 2000).
Medical research is now also taking advantage of the unique properties of ricin and Shiga toxin as well as other protein toxins in order to develop novel therapeutics for other diseases. Ricin and other toxins that intoxicate different cell types rather non-specifically can be targeted to specific cells by coupling the enzymatically active part of the molecule to other ligands or to antibodies directed against, for instance, cancer cells (Frankel et al., 1996; Laske et al., 1997; Kreitman, 1999; Kitov et al., 2000; Paton et al., 2000; and see below). Furthermore, as discussed herein, the toxins (or parts of the toxins) can be used as vectors to bring other molecules into cells, an ability of toxins that is of great importance in cell biology as well as medicine.
Endocytosis of ricin and Shiga toxin
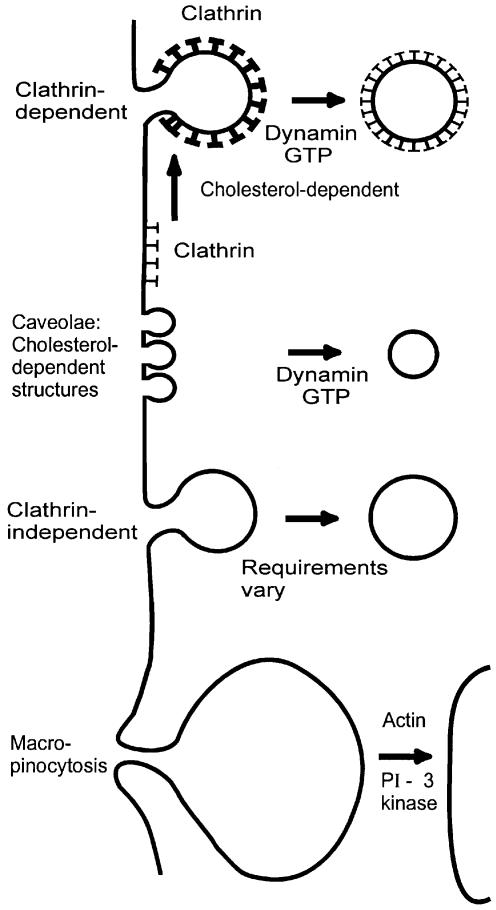
Since the plant toxin ricin binds to both glycolipids and glycoproteins with terminal galactose all over the cell surface and is therefore localized to all types of membrane invaginations (Sandvig and van Deurs, 1996), the toxin is presumably internalized by all endocytic mechanisms operating in a given cell (Sandvig and van Deurs, 1996, 1999) (Figure 3). Ricin has been localized in clathrin-coated pits, but is still endocytosed when this pathway is blocked (Sandvig and van Deurs, 1996). It was actually by using ricin that some of the early evidence for a clathrin-independent endocytic mechanism was obtained (Moya et al., 1985; Sandvig et al., 1987).
Fig. 3. Structures proposed to be involved in endocytosis.
It is now clear that clathrin-independent endocytosis can also be different from uptake by caveolae and macropinocytosis (Sandvig and van Deurs, 1999). For instance, clathrin-independent endocytosis occurs on the apical side of polarized MDCK cells (Eker et al., 1994; Holm et al., 1995; Llorente et al., 1996, 2000), whereas all of the morphologically identifiable, deeply invaginated caveolae in this cell type are localized in the basolateral domain (Vogel et al., 1998). Furthermore, clathrin-independent endocytosis of ricin occurs even when uptake from caveolae and clathrin-dependent endocytosis are inhibited by extraction of membrane cholesterol (Rodal et al., 1999). Removal of cholesterol leads to the disappearance of caveolae (Rothberg et al., 1990) and inhibits formation of invaginated clathrin-coated pits (Rodal et al., 1999; Subtil et al., 1999).
Clathrin-independent endocytosis seems to comprise more than one mechanism (Herskovits et al., 1993; van der Bliek et al., 1993; Artalejo et al., 1995; Damke et al., 1995; Sandvig and van Deurs, 1996; Llorente et al., 2000), and clearly clathrin-independent endocytosis of ricin in a polarized cell can be differentially regulated on the apical and basolateral poles (Sandvig and van Deurs, 1996; Llorente et al., 2000). So far, it is not known whether the endocytic machinery is the same but subjected to different regulation. The molecular mechanisms behind clathrin-independent endocytosis have not yet been clarified, but this type of endocytosis is involved in the uptake of physiological molecules as well as infectious agents. Similarly to ricin, Moloney murine leukaemia virus (Lee and Anderson, 1999), angiotensin (Zhang et al., 1996), muscarinic receptors (Pals-Rylaarsdam et al., 1997) and D2 dopamine receptors (Vickery and Zastrow, 1999) are endocytosed upon expression of mutant dynamin, which blocks entry both from clathrin-coated pits (Herskovits et al., 1993; van der Bliek et al., 1993; Damke et al., 1995) and from caveolae (Henley et al., 1998; Oh et al., 1998).
In contrast to ricin, Shiga toxin is endocytosed preferentially by the clathrin-coated pathway, although it is bound to a glycolipid receptor (globotriasylceramide; Gb3) (Sandvig and van Deurs, 1996). The aggregation of the toxin–Gb3 complex in these pits is mediated by the toxin by a so far unknown mechanism (Sandvig and van Deurs, 1996).
A commonly asked question is: how much do the different endocytic pathways contribute to membrane uptake? Studies of toxin uptake may help to answer this question. Although the area of the plasma membrane occupied by clathrin-coated pits and caveolae differs greatly from one cell type to another (van Deurs et al., 1989), and interfering with one type of endocytosis might actually up-regulate another mechanism (Damke et al., 1995; Llorente et al., 1998a), ricin endocytosis is reduced to ∼50% under several sets of conditions that interfere with the clathrin-dependent pathway (Sandvig and van Deurs, 1996, 1999). This suggests that ∼50% of membrane uptake may also occur by a clathrin-independent endocytosis under normal conditions.
Transport of toxins to the Golgi apparatus
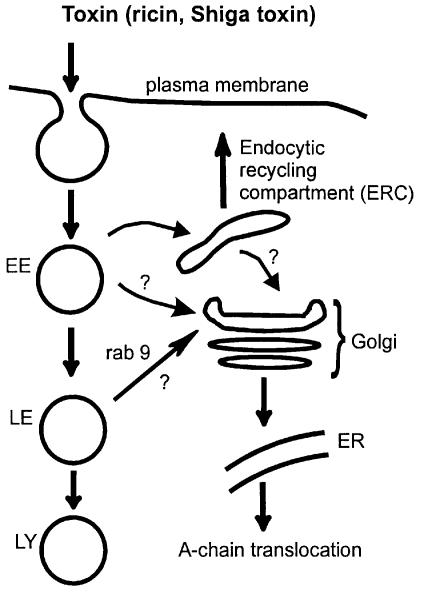
As described above (and shown in Figure 4), ricin and Shiga toxin have to pass through the Golgi apparatus on their way to the cytosol. A relatively small fraction of the endocytosed toxin molecules are transported to the Golgi apparatus (Sandvig and van Deurs, 1996; van Deurs et al., 1988) (Figure 4). However, Golgi modification (sulfation), cell fractionation or microscopy can be used to monitor this transport (Sandvig and van Deurs, 1996; Rapak et al., 1997; Llorente et al., 1998a,b). Toxins were actually the first molecules demonstrated to go from endosomes to the Golgi apparatus (Gonatas et al., 1980; Sandvig and van Deurs, 1996). Importantly, such studies reveal that it is not only transport from the Golgi apparatus to the plasma membrane that is under regulation (Pimplikar and Simons, 1993; Keller and Simons, 1997). Transport from endosomes to the Golgi apparatus is also regulated, and it can even be differentially regulated depending on whether the toxins are entering from the apical or the basolateral pole of a cell (Sandvig and van Deurs, 1996; Llorente et al., 1998b), implying that they may reach the Golgi complex from different endocytic compartments. Interest ingly, transport of ricin and Shiga toxin to the Golgi apparatus is not dependent on low endosomal pH (Melby et al., 1991; Sandvig and van Deurs, 1996; Schapiro et al., 1998), indicating that this transport step occurs independently of the low pH-dependent formation of carrier vesicles reported to operate between early and late endosomes in some cells (Clague et al., 1994).
Fig. 4. Some intracellular transport routes followed by protein toxins. Question marks indicate routes for toxin transport currently under debate (see text). EE, early endosomes; LE, late endosomes; LY, lysosomes; ER, endoplasmic reticulum.
What is the route that ricin and Shiga toxin use on their way to the Golgi apparatus? The routing of these two toxins to the Golgi apparatus could actually differ. Ricin could follow membrane bulk transport and thereby use more than one pathway, whereas aggregation of Shiga toxin in specialized membrane domains might be required not only for uptake from the cell surface (Sandvig and van Deurs, 1996), but also for transport from endosomes. It was suggested recently that Shiga toxin goes to the Golgi apparatus via the perinuclear endocytic recycling compartment (ERC), and that the transport of Shiga toxin from the ERC to the Golgi apparatus occurs via clathrin-coated structures containing AP1 (Mallard et al., 1998) (Figure 4).
It might be difficult to follow toxin transport using only microscopy since failure to visualize a certain ligand in a given compartment could be due to rapid transport out of this compartment. Thus, to exclude trafficking in a certain direction, one may have to interfere with the transport machinery responsible for trafficking at this location. Along these lines, we have recently generated HeLa cells with inducible expression of a dominant-negative mutant of Rab9 (Goda and Pfeffer, 1988; Lombardi et al., 1993) that inhibits late endosome-to-Golgi transport of the mannose-6-phosphate receptor. Interestingly, ricin transport to the Golgi apparatus, measured as sulfation of modified ricin, was not inhibited by mutant Rab9 (T.-G.Iversen, A.Llorente, P.Nicoziani, B.van Deurs and K.Sandvig, in preparation), implying that ricin can enter the Golgi apparatus by mechanisms different from that of the mannose-6-phosphate receptor. Much work remains before toxin transport to the Golgi apparatus is completely understood, but it is most likely that such studies will provide us with information relevant for transport of molecules that are physiologically important.
Even though the routing of toxins to the Golgi apparatus is not clarified, the toxins can be used to investigate the Golgi apparatus itself. For example, fluorescent derivatives of the non-toxic B-fragment of Shiga toxin were used recently to study the pH of both the Golgi apparatus and the ER (Kim et al., 1998; Schapiro and Grinstein, 2000).
Retrograde transport of toxin through the Golgi apparatus and translocation to the cytosol
A pathway leading all the way from the cell surface to the Golgi apparatus and the ER was first detected by studying Shiga toxin transport (Sandvig et al., 1992). Later on, similar transport was also shown for ricin (Rapak et al., 1997) and cholera toxin (Majoul et al., 1996; Sandvig et al., 1996). How are toxins transported retrogradely through the Golgi apparatus and to the ER? Although the mechanisms of transport within the Golgi apparatus and retrieval of membrane proteins containing the sequence KDEL from Golgi to the ER via COPI-coated vesicles have been studied extensively (Warren and Malhotra, 1998; Pelham, 2000), a number of basic questions are still not answered. For instance, how many mechanisms and pathways can be used by molecules going from the trans-Golgi network (TGN) to the ER? Investigations of toxins have revealed that more than one mechanism exists (Jackson et al., 1999; Johannes and Goud, 2000), and the various toxins seem to use different mechanisms.
Both cholera toxin (Lencer et al., 1995; Majoul et al., 1996) and Pseudomonas exotoxin A (Seetharam et al., 1991; Kreitman and Pastan, 1995; Jackson et al., 1999) have a KDEL sequence that can facilitate retrograde transport in general (Lewis and Pelham, 1992; Tang et al., 1992), and that seems to be important for efficient retrograde transport of these toxins, whereas neither ricin (Lamb et al., 1985) nor Shiga toxin (Seidah et al., 1986; Kozlov et al., 1987; Strockbine et al., 1988) has such a sequence. It was recently determined that expression of lysozyme–KDEL, which leads to a redistribution of the KDEL receptor from the Golgi complex to the ER, interferes with the intoxication with Pseudomonas exotoxin A, but has no effect on the toxicity of Shiga-like toxin-1 (Jackson et al., 1999), which is almost identical to Shiga toxin (Takeda et al., 1993). In fact, a Rab6-dependent, COPI-independent retrograde transport route used by the Shiga B-chain has been described recently (Girod et al., 1999; White et al., 1999). Studies of toxin transport might thus be useful to clarify the unresolved question of retrograde transport within the Golgi complex.
Since the enzymatically active part of the toxins is the A-moiety (Figures 1 and 2), it is clear that this part has to reach the cytosol. Until recently, it was assumed that the B-moiety remained in the ER. Interestingly, it was reported that Shiga B-chain has the ability to induce DNA cleavage and apoptosis in fibroblasts upon regulated expression in these cells, whereas the A-chain is unable to induce degradation of DNA, suggesting that Shiga B-chain translocation to the cytosol might occur and produce additional effects (Nakagawa et al., 1999).
What is the mechanism involved in toxin translocation from the ER to the cytosol? Studies of cells with mutations in the transporter associated with antigen processing (TAP) suggested that this system is not involved in toxin translocation (Sandvig and van Deurs, 1996). During recent years, the ability of the heterotrimeric protein complex Sec61p (known to be required for protein translocation from the cytosol to the ER; Matlack et al., 1998) to translocate proteins from the ER to the cytosol, even after glycosylation, has been well characterized (Suzuki et al., 1998; Cacan and Verbert, 1999). This protein complex might be involved in toxin translocation. In fact, ricin A-chain seems to interact with this complex since it can be co-immunoprecipitated with Sec61α (Wesche et al., 1999). Furthermore, a ricin A-chain mutant that was translocated into the yeast ER after synthesis in the same cell seemed to re-enter the cytosol by the Sec61p translocon (Simpson et al., 1999). In addition to being an ER component, Sec61 has recently been found in the ERGIC (ER–Golgi intermediary compartment), and the possibility exists that toxins might be translocated from this compartment, for instance after reduction/interaction with ER proteins such as disulfide isomerase/chaperones. It has actually been suggested that misfolded major histocompatibility complex (MHC) class I proteins might be transported to the cytosol from this destination (Greenfield and High, 1999). The toxins described here clearly exploit the cell machinery to gain access to their cytosolic target.
So far, little is known about the molecular details of toxin transport across the membrane, but unfolding of the A-moiety and exposure of a hydrophobic stretch might be required for transport to the cytosol (Lord and Roberts, 1998). Mutations in this area inhibit translocation without reducing the catalytic activity of the toxin (Lord and Roberts, 1998).
As illustrated above, it is amazing what studies of toxins can reveal about transport processes in cells. An important question is to what extent these pathways are used by other types of molecules. For instance, do proteins other than toxins take advantage of retrograde transport from the cell surface to the ER? The answer seems to be yes. Recent studies revealed retrograde transport of CD19, a molecule with sequence similarity to the Shiga B-chain (Khine et al., 1998). CD19 actually binds to Gb3, the Shiga toxin receptor, and retrograde transport of CD19 seems to be important for induction of apoptosis in B cells (Khine et al., 1998). Furthermore, a number of growth factors are transported to the nucleus (Pederson, 1998). However, it is still not known whether retrograde transport through the Golgi apparatus is involved in such transport.
Toxins as tools in medicine
It is an old idea that protein toxins might be targeted to certain cell types such as cancer cells and serve as ‘magic bullets’ (Figure 5A). This was proposed originally by Paul Ehrlich (1854–1915) (Ehrlich, 1957). Preparation of toxin conjugates has been facilitated by new technologies and the production of monoclonal antibodies (Frankel et al., 1996; Laske et al., 1997; Kreitman, 1999). Today, a large number of laboratories are working with toxin conjugates, and immunotoxins and toxins conjugated to other ligands are being tested clinically with promising results on both solid tumours and haematological malignancies (Frankel et al., 1996, 2000a,b; Laske et al., 1997; Kreitman, 1999). Interestingly, compounds known to sensitize cells to some of the toxins may also potentiate the action of immunotoxins (Sandvig and van Deurs, 1996). An example is found in a recent article by van Horssen et al. (1999), who report that the action of a recombinant ricin A-chain conjugate is potentiated by chloroquine, and propose that this could also be applied in vivo. In the case of Shiga toxin, which binds to the glycosphingolipid Gb3, it has been suggested that the toxin might be used even in its native form, since some tumour cells have an increased number of binding sites for the toxin (Lingwood et al., 1998; LaCasse et al., 1999).
Fig. 5. Model of modified toxin molecules entering cells. (A) Cell-specific targeting of toxin with translocation from the ER. Binding occurs either via antibodies directed against a certain cell type, or by using ligands that bind to a given cell. (B) Toxins transported to the Golgi/ER as vehicles to mediate MHC class I presentation of peptides. End, endosome; A, A-moiety (modified) of toxin (see Figure 1); B, B-moiety of toxin (see Figure 1).
The ability of protein toxins to enter the cytosol can also be exploited to bring other proteins or peptides into the cytosol. Toxin vehicles can be useful for both experimental and therapeutic purposes, and since cells differ in their number of receptors for the various protein toxins (Sandvig and van Deurs, 1996; LaCasse et al., 1999), the availability of more than one type of translocation system may be necessary to obtain transport into a given cell type. Furthermore, the efficiency of membrane translocation for a protein or peptide is likely to vary depending on the toxin used, since the mechanisms of transport for the various toxins differ. These systems might be used to deliver molecules of a wide range, both larger proteins with enzymatic activity on their own and peptides that can be used to create immunity against virus and tumour cells. Normally, peptides released in the cytosol by proteasomal degradation are transported into the ER by the specialized transporter TAP, are bound by MHC class I receptors and are transported to the cell surface where the complex is recognized by cytotoxic T cells (Williams et al., 1996). The idea is to use toxins to get a peptide into the cytosol, where it would be released and subsequently presented by MHC class I molecules (Figure 5B). There are now several examples of this idea actually working. In one case, E.coli heat-labile toxin was demonstrated to deliver both antiviral and antigenic peptides (Marcello et al., 1994; Loregian et al., 1999). Also, a modified form of Pseudomonas exotoxin A was used to deliver epitopes to class I MHC molecules (Donnelly et al., 1993). Anthrax toxin-carrying epitopes can induce protective antiviral immunity (Doling et al., 1999), and Shiga-like toxin I (Noakes et al., 1999) can deliver antigenic peptides into cells. A peptide derived from influenza virus matrix protein was added at the DNA level to both the N- and C-terminal ends of a catalytically inactive A-fragment of Shiga toxin. The modified toxin was able to sensitize cells to lysis by cytotoxic T lymphocytes in both cases (Noakes et al., 1999). This might involve translocation of the epitope into the cytosol and subsequent delivery into the ER where binding to MHC class I molecules could take place (Noakes et al., 1999). Even peptides coupled to Shiga toxin B-fragment (Lee et al., 1998) can be presented by MHC class I molecules, but it is not yet known whether epitopes coupled to the B-subunit of the toxin might be released from the B-chain within the ER, or if release occurs after translocation of the B-chain to the cytosol. Altogether, experiments with toxins as tools in creating an immune response are promising.
Concluding comments
Although protein toxins may originally have been regarded only as disease-causing agents, the technology available today provides us with tools to use these toxins to fight disease. They can be used as constituents of immunotoxins or other targeted (cell-specific) chimeric molecules. Furthermore, they can be used as vehicles to bring proteins and peptides into cells, with the possibility of creating immune responses to virus or cancer cells. Clearly, further studies are required in order to clarify the potential of these agents in medicine. Importantly, basic information about transport pathways in cells can be obtained from studies of toxins, and investigations of the roles of the various domains of the protein toxins, as well as increased knowledge about their translocation to the cytosol, will increase their importance as general tools in cell biology.
Acknowledgments
Acknowledgements
The work performed by the authors of the present article has been supported by the Norwegian and Danish Cancer Societies, the Norwegian Research Council for Science and the Humanities, The Danish Medical Research Council, The Novo Nordisk Foundation, the Jahre Foundation, an HFSP grant (RG404/96 M) and a Jeanette and Søren Bothners legacy.
References
- Acheson D.W.K. and Keusch,G.T. (1999) The family of Shiga toxins. In Alouf,J.E. and Freer,J.H. (eds), The Comprehensive Sourcebook of Bacterial Protein Toxins. 2nd edn. Academic Press, London, UK, pp. 229–242. [Google Scholar]
- Arab S. and Lingwood,C.A. (1998) Intracellular targeting of the endoplasmic reticulum/nuclear envelope by retrograde transport may determine cell hypersensitivity to verotoxin via globotriasyl ceramide fatty acid isoform traffic. J. Cell. Physiol., 177, 646–660. [DOI] [PubMed] [Google Scholar]
- Artalejo C.R., Henley,J.R., McNiven,M.A. and Palfrey,C.H. (1995) Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP and dynamin but not clathrin. Proc. Natl Acad. Sci. USA, 92, 8328–8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J.R. (1999) Foodborne diseases: Shiga toxin producing E.coli (STEC). Pediatr. Infect. Dis. J., 18, 909–910. [DOI] [PubMed] [Google Scholar]
- Cacan R. and Verbert,A. (1999) Free and N-linked oligomannosides as markers of the quality control of newly synthesized glycoproteins. Biochem. Biophys. Res. Commun., 258, 1–5. [DOI] [PubMed] [Google Scholar]
- Clague M.J., Urbe,S., Aniento,F. and Gruenberg,J. (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem., 269, 21–24. [PubMed] [Google Scholar]
- Damke H., Baba,T., van der Bliek,A.M. and Schmid,S.L. (1995) Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J. Cell Biol., 131, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doling A.M., Ballard,J.D., Shen,H., Krishna,K.M., Ahmed,R., Collier,R.J. and Starnbach,M.N. (1999) Cytotoxic T-lymphocyte epitopes fused to anthrax toxin induce protective antiviral immunity. Infect. Immun., 67, 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J.J. et al. (1993) Targeted delivery of peptide epitopes to class I major histocompatibility molecules modified by Pseudomonas exotoxin. Proc. Natl Acad. Sci. USA, 90, 3530–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. (1957) The Collected Papers of Paul Ehrlich. Himmelweit,F., Marquardt,M. and Dale,S.S. (eds), Pergamon Press, Oxford, UK, pp. 596–618. [Google Scholar]
- Eker P., Holm,P.K., van Deurs,B. and Sandvig,K. (1994) Selective regulation of apical endocytosis in polarized MDCK cells by mastoparan and cAMP. J. Biol. Chem., 269, 18607–18615. [PubMed] [Google Scholar]
- Endo Y., Mitsui,K., Motizuki,M. and Tsurugi,K. (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in the 28S ribosomal RNA caused by the toxins. J. Biol. Chem., 262, 5908–5912. [PubMed] [Google Scholar]
- Fishman P.H. and Orlandi,P.A. (1994) Mechanism of the interaction of cholera toxin and Escherichia coli heat-labile enterotoxin with cells. Trends Glycosci. Glycotechnol., 6, 387–406. [Google Scholar]
- Frankel A.E., Fitzgerald,D., Siegall,C. and Press,O.W. (1996) Advances in immunotoxin biology and therapy: a summary of the Fourth International Symposium on Immunotoxins. Cancer Res., 56, 926–932. [PubMed] [Google Scholar]
- Frankel A.E., Kreitman,R.J. and Sausville,E.A. (2000a) Targeted toxins. Clin. Cancer Res., 6, 326–334. [PubMed] [Google Scholar]
- Frankel A.E. et al. (2000b) Diphtheria toxin fused to human interleukin-3 is toxic to blasts from patients with myeloid leukemias. Leukemia, 14, 576–585. [DOI] [PubMed] [Google Scholar]
- Fraser M.E., Chernaia,M.M., Kozlov,Y.V. and James,M.N. (1994) Crystal structure of the holotoxin from Shigella dysenteriae at 2.5 Å resolution. Nature Struct. Biol., 1, 59–64. [DOI] [PubMed] [Google Scholar]
- Garred Ø., van Deurs,B. and Sandvig,K. (1995) Furin-induced cleavage and activation of Shiga toxin. J. Biol. Chem., 270, 10817–10821. [DOI] [PubMed] [Google Scholar]
- Girod A., Storrie,B., Simpson,J.C., Johannes,L., Goud,B., Roberts,L.M., Lord,J.M., Nilsson,T. and Pepperkok,R. (1999) Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nature Cell Biol., 1, 423–430. [DOI] [PubMed] [Google Scholar]
- Goda Y. and Pfeffer,S.R. (1988) Selective recycling of the mannose-6-phosphate/IGF-II receptor to the trans-Golgi network in vitro. Cell, 55, 309–320. [DOI] [PubMed] [Google Scholar]
- Gonatas J., Stieber,A., Olsnes,S. and Gonatas,N.K. (1980) Pathways involved in fluid phase and adsorptive endocytosis in neuroblastoma. J. Cell Biol., 87, 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V.M. and Leppla,S.H. (1994) Proteolytic activation of bacterial toxins: role of bacterial and host cell proteases. Infect. Immun., 62, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield J.J.A. and High,S. (1999) The sec61 complex is located in both the ER and the ER–Golgi intermediate compartment. J. Cell Sci., 112, 1477–1486. [DOI] [PubMed] [Google Scholar]
- Henley J.R., Krueger,E.W.A., Oswald,B.J. and NcNiven,M.A. (1998) Dynamin-mediated internalization of caveolae. J. Cell Biol., 141, 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits J.S., Burgess,C.C., Obar,R.A. and Vallee,R.B. (1993) Effects of mutant rat dynamin on endocytosis. J. Cell Biol., 122, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm P.K., Eker,P., Sandvig,K. and van Deurs,B. (1995) Phorbol myristate acetate selectively stimulates apical endocytosis via protein kinase C in polarized MDCK cells. Exp. Cell Res., 217, 157–168. [DOI] [PubMed] [Google Scholar]
- Holmgren J. (1981) Actions of cholera toxin and the prevention and treatment of cholera. Nature, 292, 413–417. [DOI] [PubMed] [Google Scholar]
- Jackson M.E., Simpson,J.C., Girod,A., Pepperkok,R., Roberts,L.M. and Lord,J.M. (1999) The KDEL retrieval system is exploited by Pseudomonas exotoxin A, but not by Shiga-like toxin-1, during retrograde transport from the Golgi complex to the endoplasmic reticulum. J. Cell Sci., 112, 467–475. [DOI] [PubMed] [Google Scholar]
- Johannes L. and Goud,B. (2000) Facing inward from compartment shores: how many pathways were we looking for? Traffic, 1, 119–123. [DOI] [PubMed] [Google Scholar]
- Jones N.L., Islur,A., Haq,R., Mascarenhas,M., Karmali,M.A., Perdue,M.H., Zanke,B.W. and Sherman,P.M. (2000) Escherichia coli Shiga toxins induce apoptosis in epithelial cells that is regulated by the Bcl-2 family. Am. J. Physiol., 278, G811–G819. [DOI] [PubMed] [Google Scholar]
- Kaper J.B. (1998) Enterohemorrhagic Escherichia coli. Curr. Opin. Microbiol., 1, 103–108. [DOI] [PubMed] [Google Scholar]
- Keller P. and Simons,K. (1997) Post-Golgi biosynthetic trafficking. J. Cell Sci., 110, 3001–3009. [DOI] [PubMed] [Google Scholar]
- Khine A.A., Firtel,M. and Lingwood,C.A. (1998) CD77-dependent retrograde transport of CD19 to the nuclear membrane: functional relationship between CD77 and CD19 during germinal center B-cell apoptosis. J. Cell. Physiol., 176, 281–292. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Johannes,L., Goud,B., Antony,C., Lingwood,C.A., Daneman,R. and Grinstein,S. (1998) Noninvasive measurement of the pH of the endoplasmic reticulum at rest and during calcium release. Proc. Natl Acad. Sci. USA, 95, 2997–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitov P.I., Sadowska,J.M., Mulvey,G., Armstrong,G.D., Ling,H., Pannu,N.S., Read,R.J. and Bundle,D.R. (2000) Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature, 403, 669–672. [DOI] [PubMed] [Google Scholar]
- Kozlov Y.V., Kabishev,A.A., Fedchenko,V.I. and Bayev,A.A. (1987) Cloning and sequencing of Shiga toxin structural genes. Proc. Natl Acad. Sci. USSR, 295, 740–744. [Google Scholar]
- Kreitman R.J. (1999) Immunotoxins in cancer therapy. Curr. Opin. Immunol., 11, 570–578. [DOI] [PubMed] [Google Scholar]
- Kreitman R.J. and Pastan,I. (1995) Importance of the glutamate residue of KDEL in increasing the cytotoxicity of Pseudomonas exotoxin derivatives and for increased binding to the KDEL receptor. Biochem. J., 307, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCasse E.C. et al. (1999) Shiga-like toxin-1 receptor on human breast cancer, lymphoma and myeloma and absence from CD34+ hematopoietic stem cells: implications for ex vivo tumor purging and autologous stem cell transplantation. Blood, 94, 2901–2910. [PubMed] [Google Scholar]
- Lamb F.I., Roberts,L.M. and Lord,J.M. (1985) Nucleotide sequence of cloned cRNA coding for preproricin. Eur. J. Biochem., 148, 265–270. [DOI] [PubMed] [Google Scholar]
- Laske D.W., Youle,R.J. and Oldfield,E.H. (1997) Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nature Med., 3, 1362–1368. [DOI] [PubMed] [Google Scholar]
- Lee R.-S., Tartour,E., van der Bruggen,P., Vantomme,V., Joyeux,I., Goud,B., Fridman,W.H. and Johannes,L. (1998) Major histo compatibility complex class I presentation of exogenous tumor antigen fused to the B-fragment of Shiga toxin. Eur. J. Immunol., 28, 2726–2737. [DOI] [PubMed] [Google Scholar]
- Lee S.Z.Y. and Anderson,W.F. (1999) Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway. J. Virol., 73, 5994–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer W.I., Constable,C., Moe,S., Jobling,M.G., Webb,H.M., Ruston,S., Madara,J.L., Hirst,T.R. and Holmes,R.K. (1995) Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J. Cell Biol., 131, 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M.J. and Pelham,R.B. (1992) Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell, 68, 353–364. [DOI] [PubMed] [Google Scholar]
- Lingwood C.A., Khine,A.A. and Arab,S. (1998) Globotriasyl ceramide (Gb3) expression in human tumour cells: intracellular trafficking defines a new retrograde transport pathway from the cell surface to the nucleus, which correlates with sensitivity to verotoxin. Acta Biochim. Pol., 45, 351–359. [PubMed] [Google Scholar]
- Llorente A., Garred,Ø., Holm,P.K., Eker,P., Jacobsen,J., van Deurs,B. and Sandvig,K. (1996) Effect of calmodulin antagonists on endocytosis and intracellular transport of ricin in polarized MDCK cells. Exp. Cell Res., 227, 298–308. [DOI] [PubMed] [Google Scholar]
- Llorente A., Rapak,A., Schmid,S.L., van Deurs,B. and Sandvig,K. (1998a) Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus. J. Cell Biol., 140, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente A., van Deurs,B. and Sandvig,K. (1998b) Transport of apically but not basolaterally internalized ricin to the Golgi apparatus is stimulated by 8-Br-cAMP in MDCK cells. FEBS Lett., 431, 200–204. [DOI] [PubMed] [Google Scholar]
- Llorente A., van Deurs,B., Garred,Ø., Eker,P. and Sandvig,K. (2000) Apical endocytosis of ricin in MDCK cells is regulated by the cyclooxygenase pathway. J. Cell Sci., 113, 1213–1221. [DOI] [PubMed] [Google Scholar]
- Lombardi D., Soldati,T., Riederer,M.A., Goda,Y., Zerial,M. and Pfeffer,S.R. (1993) Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J., 12, 677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J.M. and Roberts,L.M. (1998) Toxin entry: retrograde transport through the secretory pathway. J. Cell Biol., 140, 733–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loregian A., Papini,E., Satin,B., Marsden,B. and Hirst,T.R. (1999) Intranuclear delivery of an antiviral peptide mediated by the B subunit of Escherichia coli heat-labile enterotoxin. Proc. Natl Acad. Sci. USA, 96, 5221–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul I.V., Bastiaens,P.I.H. and Söling,H.-D. (1996) Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in Vero cells. J. Cell Biol., 133, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard F., Antony,C., Tenza,D., Salamero,J., Goud,B. and Johannes,L. (1998) Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol., 143, 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcello A., Loregian,A., Cross,A., Marsden,H., Hirst,T.R. and Palù,G. (1994) Specific inhibition of herpes virus replication by receptor-mediated entry of an antiviral peptide linked to Escherichia coli enterotoxin B subunit. Proc. Natl Acad. Sci. USA, 91, 8994–8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack K.E.S., Mothes,W. and Rapoport,T.A. (1998) Protein translocation: tunnel vision. Cell, 92, 381–390. [DOI] [PubMed] [Google Scholar]
- Melby E.L., Prydz,K., Olsnes,S. and Sandvig,K. (1991) Effect of monensin on ricin and fluid phase transport in polarized MDCK cells. J. Cell. Biochem., 47, 1–10. [DOI] [PubMed] [Google Scholar]
- Moya M., Dautry-Varsat,A., Goud,B., Louvard,D. and Boquet,P. (1985) Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin. J. Cell Biol., 101, 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa I., Nakata,M., Kawabata,S. and Hamada,S. (1999) Regulated expression of Shiga toxin B gene induces apoptosis in mammalian fibroblastic cells. Mol. Microbiol., 33, 1190–1199. [DOI] [PubMed] [Google Scholar]
- Noakes K.L., Teisserenc,H.T., Lord,J.M., Dunbar,P.R., Cerundolo,V. and Roberts,L.M. (1999) Exploiting retrograde transport of Shiga-like toxin 1 for delivery of exogenous antigens into MHC class I presentation pathway. FEBS Lett., 453, 95–99. [DOI] [PubMed] [Google Scholar]
- Oh P., McIntosh,D.P. and Schnitzer,J.E. (1998) Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol., 141, 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S. and Sandvig,K. (1988). How protein toxins enter and kill cells. In Frankel,A.E. (ed.), Immunotoxins. Kluwer Academic Publishers, Boston, MA, pp. 39–73. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Wesche,J. and Falnes,P.O. (1999) Binding, uptake, routing and translocation of toxins with intracellular sites of action. In Alouf,J.E. and Freer,J.H. (eds), The Comprehensive Sourcebook of Bacterial Protein Toxins. 2nd edn. Academic Press, London, UK, pp. 73–93. [Google Scholar]
- Pals-Rylaarsdam R., Gurevich,V.V., Lee,K.B., Ptasienski,J.A., Benovic,J.L. and Hosey,M.M. (1997) Internalization of the m2 muscarinic acetylcholine receptor. Arrestin-independent and -dependent pathways. J. Biol. Chem., 272, 23682–23689. [DOI] [PubMed] [Google Scholar]
- Paton A.W., Morona,R. and Paton,J.C. (2000) A new biological agent for treatment of Shiga toxigenic Escherichia coli infections and dysentery in humans. Nature Med., 6, 265–270. [DOI] [PubMed] [Google Scholar]
- Pederson T. (1998) Growth factors in the nucleolus? J. Cell Biol., 143, 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. (2000) Getting stuck in the Golgi. Traffic, 1, 191–192. [DOI] [PubMed] [Google Scholar]
- Pimplikar S.W. and Simons,K. (1993) Regulation of apical transport in epithelial cells by a Gs class of heterotrimeric G protein. Nature, 362, 456–458. [DOI] [PubMed] [Google Scholar]
- Pizza M., Masignani,V. and Rappuoli,R. (1999) Molecular, functional and evolutionary aspects of ADP-ribosylating toxins. In Alouf,J.E. and Freer,J.H. (eds), The Comprehensive Sourcebook of Bacterial Protein Toxins. 2nd edn. Academic Press, London, UK, pp. 45–72. [Google Scholar]
- Rapak A., Falnes,P.O. and Olsnes,S. (1997) Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc. Natl Acad. Sci. USA, 94, 3783–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodal S.K., Skretting,G., Garred,Ø., Vilhardt,F., van Deurs,B. and Sandvig,K. (1999) Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell, 10, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K.G., Ying,Y.S., Kamen,B.A. and Anderson,R.G. (1990) Cholesterol controls the clustering of glycosphingolipid-anchored membrane receptor for 5-methyltetrahydrofolate. J. Cell Biol., 111, 2931–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenber E., Katzin,B.J., Ernst,S., Collins,E.J., Mlsna,D., Ready,M.P. and Robertus,J.D. (1991) Crystallographic refinement of ricin to 2.5 Å. Proteins, 10, 240–250. [DOI] [PubMed] [Google Scholar]
- Sandvig K. and Olsnes,S. (1991) Membrane translocation of diphtheria toxin. In Alouf,J.E. and Freer,J.H. (eds), Sourcebook of Bacterial Protein Toxins. Academic Press, London, UK, pp. 57–73. [Google Scholar]
- Sandvig K. and van Deurs,B. (1996) Endocytosis, intracellular transport and cytotoxic action of Shiga toxin and ricin. Physiol. Rev., 76, 949–966. [DOI] [PubMed] [Google Scholar]
- Sandvig K. and van Deurs,B. (1999) Endocytosis and intracellular transport of ricin: recent discoveries. FEBS Lett., 452, 67–70. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes,S., Petersen,O.W. and van Deurs,B. (1987) Acidification of the cytosol inhibits endocytosis from coated pits. J. Cell Biol., 105, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Garred,Ø., Prydz,K., Kozlov,J.V., Hansen,S.H. and van Deurs,B. (1992) Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature, 358, 510–511. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Ryd,M., Garred,Ø., Schweda,E., Holm,P.K. and van Deurs,B. (1994) Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J. Cell Biol., 126, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Garred,Ø. and van Deurs,B. (1996) Regulated transport of cholera toxin to the endoplasmic reticulum: correlation with cAMP production. Proc. Natl Acad. Sci. USA, 93, 12339–12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro F.B. and Grinstein,S. (2000) Determinants of the pH of the Golgi complex. J. Biol. Chem., 275, 21025–21032. [DOI] [PubMed] [Google Scholar]
- Schapiro F.B., Lingwood,C., Furuya,W. and Grinstein,S. (1998) pH-independent retrograde targeting of glycolipids to the Golgi complex. Am. J. Physiol., 274, C319–C332. [DOI] [PubMed] [Google Scholar]
- Seetharam S., Chaudhary,V.K., Fitzgerald,D. and Pastan,I. (1991) Increased cytotoxic activity of Pseudomonas exotoxin and two chimeric toxins ending in KDEL. J. Biol. Chem., 266, 17376–17381. [PubMed] [Google Scholar]
- Seidah N.G., Donohue-Rolfe,A., Lazure,C., AuClair,F., Keusch,G.T. and Chretien,M. (1986) Complete amino acid sequence of Shigella toxin B-chain. A novel polypeptide containing 69 amino acids and one disulfide bridge. J. Biol. Chem., 261, 13928–13931. [PubMed] [Google Scholar]
- Simpson J.C., Roberts,L.M., Römisch,K., Davey,J., Wolf,D.H. and Lord,J.M. (1999) Ricin A chain utilises the endoplasmic reticulum-associated protein degradation pathway to enter the cytosol of yeast. FEBS Lett., 459, 80–84. [DOI] [PubMed] [Google Scholar]
- Strockbine N.A., Jackson,M.P., Sung,L.M., Holmes,R.K. and O’Brien,A. (1988) Cloning and sequencing of the genes for Shiga toxin from Shigella dysenteriae type 1. J. Bacteriol., 170, 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A., Gaidarov,I., Kobylarz,K., Lampson,M.A., Keen,J.H. and McGraw,T.E. (1999) Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl Acad. Sci. USA, 96, 6775–6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Doi,H., Matsuzawa,F., Aikawa,S., Takiguchi,K., Kawano,H., Hayashida,M. and Ohno,S. (2000) Bcl-2 antiapoptotic protein mediates verotoxin II-induced cell death: possible association between bcl-2 and tissue failure by E.coli O157:H7. Genes Dev. 14, 1734–1740. [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Yan,Q. and Lennardz,W.J. (1998) Complex, two-way traffic of molecules across the membrane of the endoplasmic reticulum. J. Biol. Chem., 273, 10083–10086. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Kurazono,H. and Yamasaki,S. (1993) Vero toxins (Shiga-like toxins) produced by enterohemorrhagic Escherichia coli (verocytotoxin-producing E.coli). Microbiol. Immunol., 37, 591–599. [DOI] [PubMed] [Google Scholar]
- Tang B.L., Wong,S.H., Low,S.H. and Hong,W. (1992) Retention of a type II surface membrane protein in the endoplasmic reticulum by the Lys-Asp-Glu-Leu sequence. J. Biol. Chem., 267, 7072–7076. [PubMed] [Google Scholar]
- Uchida H., Kiyokawa,N., Horie,H., Fujimoto,J. and Takeda,Y. (1999) The detection of Shiga toxin in the kidney of a patient with hemolytic uremic syndrome. Pediatr. Res., 45, 133–137. [DOI] [PubMed] [Google Scholar]
- van der Bliek A.M., Redelmeier,T.E., Damke,H., Tisdale,E.J., Meyerowitz,E.M. and Schmid,S.L. (1993) Mutations in human dynamin block an intermediate stage in coated vesicle formation. J. Cell Biol., 122, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B., Sandvig,K., Petersen,O.W., Olsnes,S., Simons,K. and Griffiths,G. (1988) Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J. Cell Biol., 106, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B., Petersen,O.W., Olsnes,S. and Sandvig,K. (1989) The ways of endocytosis. Int. Rev. Cytol., 117, 131–177. [DOI] [PubMed] [Google Scholar]
- van Horssen P.J., van Oosterhout,Y.V.J.M., Evers,S., Backus,H.H.J., van Oijen,M.G.C.T., Bongaerts,R., de Witte,T. and Preijers,F.W.M.B. (1999) Influence of cytotoxicity enhancers in combination with human serum on the activity of CD22-recombinant ricin A against B cell lines, chronic and acute lymphocytic leukemia cells. Leukemia, 13, 241–249. [DOI] [PubMed] [Google Scholar]
- Vickery R.G. and Zastrow,M. (1999) Distinct dynamin-dependent and -independent mechanisms target structurally homologous dopamine receptors to different endocytic compartments. J. Cell Biol., 144, 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel U., Sandvig,K. and van Deurs,B. (1998) Expression of caveolin-1 and polarized formation of caveolae in Caco-2 and MDCK II cells. J. Cell Sci., 111, 825–832. [DOI] [PubMed] [Google Scholar]
- Warren G. and Malhotra,V. (1998) The organisation of the Golgi apparatus. Curr. Opin. Cell Biol., 10, 493–498. [DOI] [PubMed] [Google Scholar]
- Wesche J., Rapak,A. and Olsnes,S. (1999) Dependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosol. J. Biol. Chem., 274, 34443–34449. [DOI] [PubMed] [Google Scholar]
- White J. et al. (1999) Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol., 147, 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick M.J., Hamood,A.N. and Iglewski,B.H. (1990) Analysis of the structure–function relationship of Pseudomonas aeruginosa exotoxin A. Mol. Microbiol., 4, 527–535. [DOI] [PubMed] [Google Scholar]
- Williams D.B., Vassilakos,A. and Woong-Kyung,S. (1996) Peptide presentation by MHC class I molecules. Trends Cell Biol., 6, 267–273. [DOI] [PubMed] [Google Scholar]
- Zhang J., Ferguson,S.S.G., Barak,L.S., Ménard,L. and Caron,M.G. (1996) Dynamin and β-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. J. Biol. Chem., 271, 18302–18305. [DOI] [PubMed] [Google Scholar]