Abstract
Cardiovascular diseases are the most common cause of death among the elderly in the Western world. Resveratrol (3,5,4´-trihydroxystilbene) is a plant-derived polyphenol that was shown to exert diverse anti-aging activity mimicking some of the molecular and functional effects of caloric restriction. This mini-review focuses on the molecular and cellular mechanisms activated by resveratrol in the vascular system, and explores the links between its anti-oxidative and anti-inflammatory effects, which could be exploited for the prevention or amelioration of vascular aging in the elderly.
Keywords: resveratrol, aging, vascular dysfunction
Introduction
Age-specific mortality rates from heart disease and stroke increase exponentially with age, which imposes a huge financial burden on the health care systems in the Western world. Therefore, there is an urgent need for effective therapeutic strategies that have the potential to promote cardiovascular health in the elderly, preventing or delaying the development of atherosclerotic vascular diseases. During the past decade dietary supplementation with the plant-derived polyphenol resveratrol (3,5,4´-trihydroxystilbene) has emerged as a promising approach to counteract aging-induced pro-atherogenic phenotypic changes in the vasculature. The first population-based studies demonstrated that Mediterranean diets, which are rich in resveratrol, are associated with significantly reduced risk of cardiovascular disease in humans1, 2. Subsequently resveratrol was shown to exert significant anti-aging action in invertebrates3, 4, mimicking many aspects of caloric restriction5–8. Importantly, resveratrol supplementation was also shown to exert anti-inflammatory and anti-oxidant effects in various mammalian models of aging and cardiovascular diseases5, 7. In this review, the potential mechanisms underlying the vasoprotective effects of resveratrol are considered and its use as a dietary supplement to promote cardiovascular health in the elderly is discussed.
Role of oxidative stress and inflammation in cardiovascular aging
Our current understanding of the pathogenesis of age-associated cardiovascular diseases is that age-related oxidative stress may promote vascular inflammation even in the absence of traditional risk factors associated with atherogenesis (e.g., hypertension or metabolic diseases; reviewed recently elsewhere9). It is well-established that age-associated low-grade inflammation accelerates the incidence of coronary artery disease and stroke in the elderly9. There is abundant experimental data suggesting that increased activity of NAD(P)H oxidases and mitochondrial overproduction of reactive oxygen species underlie age-related oxidative stress in the vasculature promoting inflammation and endothelial damage 10–14. Nitric oxide (NO) is a crucial factor for the health and function of endothelial cells. Consequences of increased oxidative stress in aging include functional inactivation of NO by high concentrations of O2.- resulting in significant vasomotor dysfunction (recently reviewed elsewhere15, increased apoptosis of endothelial cells16, 17, microvascular rarefaction and impaired mitochondrial biogenesis 18–20. The key role of endothelium-derived NO in protecting the cardiovascular system during aging is underscored by the findings that eNOS knockout mice exhibit a premature cardiac aging phenotype associated with early mortality21. The existing data also point to an important cross-talk between ROS production and inflammatory processes in the pathogenesis of cardiovascular aging9. On the one hand, ROS per se can act as signaling molecules activating pathways regulating inflammatory processes22, including endothelial activation and secretion of inflammatory mediators. Specifically, mitochondria-derived H2O2 is thought to contribute to the activation of NF-κB in the vasculature, resulting in a pro-inflammatory shift in endothelial gene expression profile22. Increased NF-κB binding in aging is likely responsible for the increased expression of iNOS10, 22, 23, which is a major source of vascular peroxynitrite production. On the other hand, inflammation itself promotes cellular oxidative stress (e.g. by TNFα-mediated activation of NAD(P)H oxidases)24. In that regard it is important to note that both in laboratory rodents and humans there is an age-related up-regulation of TNFα and that disruption of TNFα signaling confers vasoprotection in aging 24.
Anti-oxidative and anti-inflammatory effects of resveratrol in aging
Previous studies have established that resveratrol can exert significant cardiovascular protective effects in various models of myocardial injury25–27, hypertension26, 28, and type 2 diabetes7, 29–31. Recent studies provide clear evidence that resveratrol treatment can also confer vasoprotection in aged mice and rats7,22, attenuating ROS production, improving endothelial function, inhibiting inflammatory processes and decreasing the rate of endothelial apoptosis. The mechanisms underlying the cardiovascular protective action of resveratrol are likely multifaceted. Resveratrol was shown to up-regulate eNOS and increase NO bioavailability29, 32, 33, Resveratrol can also induce major cellular anti-oxidant enzymes (e.g. glutathione peroxidase, heme oxygenase, superoxide dismutase) in cardiac and vascular cells34–37, which result in a marked attenuation of oxidative stress. Resveratrol both down-regulates vascular and cardiac expression of TNFα and inhibits NADPH oxidases in the vasculature29, 31, 38. It is significant, that resveratrol was also shown to inhibit mitochondrial production of reactive oxygen species in the vasculature30. In addition, resveratrol both in vivo and at nutritionally relevant concentrations in vitro was shown to inhibit inflammatory processes, including NF-κB activation, inflammatory gene expression and attenuation of monocyte adhesiveness to endothelial cells7, 25, 39–47, all of which may contribute to its cardioprotective effects in aging. Recent studies showed that resveratrol, via an eNOS-dependent pathway, induces mitochondrial biogenesis both in cultured endothelial cells and in endothelia of mice with accelerated vascular aging20. Further studies are evidently needed to determine whether the aforementioned vasoprotective effects of resveratrol are manifested in the cardiovascular system of elderly humans as well. In that regard, studies on vessels isolated from non-human primates treated with resveratrol will also be highly informative.
The molecular targets of resveratrol, which mediate its diverse cellular effects, are the subject of ongoing investigations. On the basis of the structural similarity of resveratrol to diethylstilbestrol, resveratrol was characterized as a phytoestrogen48. Given the cardioprotective benefits attributed to estrogens at the time, this idea lead to a number of follow-up studies suggesting that some of the cardiovascular effects of high doses of resveratrol may indeed be modulated by activation of the estrogen receptor49. Yet, more recently the cardioprotective effects of estrogen replacement have become subjects of debate and there are also a number of studies extant, which suggest that the estrogen receptor is not the main cellular target of resveratrol in the vasculature. Since the original observation of Sinclair and co-workers4 a large body of evidence has been published linking the cellular action of resveratrol to regulation of a pathway dependent on SIRT1, a mammalian homolog of the Saccharomyces cerevisae silent information regulator 2 (Sir2) protein3, 5, 50–55. There is an ongoing debate whether resveratrol is a direct activator of SIRT1, which catalyzes NAD+-dependent protein deacetylation and is a critical regulator of transcription, genome stability, apoptosis and metabolism. Although recent studies suggest that in cell-free assays resveratrol may not activate SIRT1 directly 56, there is strong evidence that resveratrol and its metabolites both in vivo and ex vivo can promote SIRT1-dependent cellular responses, as demonstrated by resveratrol-induced decreases in acetylation of various known SIRT1 targets8. Furthermore, resveratrol has been shown to up-regulate protein expression of SIRT1 in multiple cell types, including endothelial cells20. In addition, overexpression of SIRT1 in endothelial cells (similar to many other cell types) can mimic many of the effects of resveratrol 57, whereas depletion of SIRT1 tends to attenuate resveratrol-induced cellular effects20, 30, 43, 57. There is also solid evidence that inhibition of NF-κB by resveratrol is mediated via SIRT158. SIRT1 is also needed for resveratrol-mediated induction of mitochondrial biogenesis and attenuation of mitochondrial oxidative stress in cardiovascular cells20, 30. In light of recent controversies regarding the interaction of resveratrol and SIRT1, further studies are needed to elucidate the role of SIRT1-regulated pathways in the vasoprotective action of resveratrol in aging and the cellular mechanisms responsible for resveratrol-induced up-regulation of SIRT1 in cardiovascular tissues. Future studies should also address the interaction of SIRT1-dependent pathways with alternative cellular targets of resveratrol (e.g. NQO259, 60, cyclooxygenase etc) in vascular endothelial and smooth muscle cells.
Nrf2 activation: a new target for resveratrol
There is increasing evidence that activation of NF-E2-related factor 2 (Nrf2) is a key mechanism by which resveratrol confers its cytoprotective effects in the cardiovascular system 27. Nrf2 is a basic leucine zipper transcription factor that regulates the coordinated expression of key antioxidant mechanisms in the cell by binding to the antioxidant response (ARE) elements in the promoter regions of target genes. The first evidence that resveratrol can activate Nrf2 came from studies on cultured PC12 cells61 and human lung epithelial cells62. Subsequent studies demonstrated that in cultured endothelial cells resveratrol also significantly increases transcriptional activity of Nrf2, which is associated with up-regulation of several Nrf2 target genes37. Many of these Nrf2 targets (e.g. NAD(P)H:quinone oxidoreductase 1, heme oxygenase-1) have been shown to promote endothelial health under conditions of increased oxidative stress34. Because Nrf2-driven pathways can be activated by concentrations of resveratrol readily achievable in vivo, future studies should elucidate whether Nrf2 activation contributes to the vasoprotective effects of resveratrol in aging. Recent evidence obtained using high fat diet-fed Nrf2−/− mice lend support to the hypothesis that Nrf2 activation plays an important role in the vasoprotective action of resveratrol37. Further, induction of the Nrf2 target HO-1 has also been implicated in the cardioprotective effects of resveratrol under conditions of experimentally-induced ischemia36. At present it is not well understood how pathways governed by Nrf2 and SIRT1 cross-talk. Further studies are warranted to test the possibility that SIRT1 acts as a permissive factor, modulating Nrf2-driven responses in the vasculature. Future studies also should test the possibility that Nrf2-dependent pathways regulate SIRT1 expression at the level of transcription.
Perspectives
Although significant progress has been achieved in elucidating the cellular mechanisms activated by resveratrol, the specific roles for pathways regulating mitochondrial function, cellular antioxidant defenses and mechanisms involved in macromolecular repair need to be elucidated further. There is reasonable consensus that oxidative stress plays a central role in the development of atherosclerosis and that redox-sensitive molecular pathways (e.g. NF-κB) promote vascular inflammation in aging. Yet, recent large randomized clinical trials have shown no significant benefit when anti-oxidants targeted to the cell membranes (such as vitamin E) were given to patients with a high-risk coronary arterial disease profile. At present it is unknown whether administration of mitochondria-targeted antioxidants would affect progression of cardiovascular diseases in elderly patients. In various experimental settings, including studies in aged laboratory rodents, resveratrol was shown to attenuate free radical production in multiple cellular compartments (i.e. both in the mitochondria and the cytosol). Thus further studies on the effects of resveratrol on aging-induced oxidative stress and inflammation and their role in cardiovascular pathology are warranted. Importantly, these studies should determine whether anti-oxidative and anti-inflammatory effects of resveratrol are manifested in primates. Finally, research efforts should persist to fully elucidate the effects of resveratrol on microvascular alterations in aging. Future studies should extend the results of earlier investigations63–65 assessing whether treatment with resveratrol can delay/prevent the age-associated decline in cerebral regional blood flow, the reduction in capillary and arteriolar density and angiogenesis, and the decline in spatial learning and memory in rodent and primate species and determine the roles of SIRT1 and Nrf2 in the microvascular protective effects of resveratrol treatment.
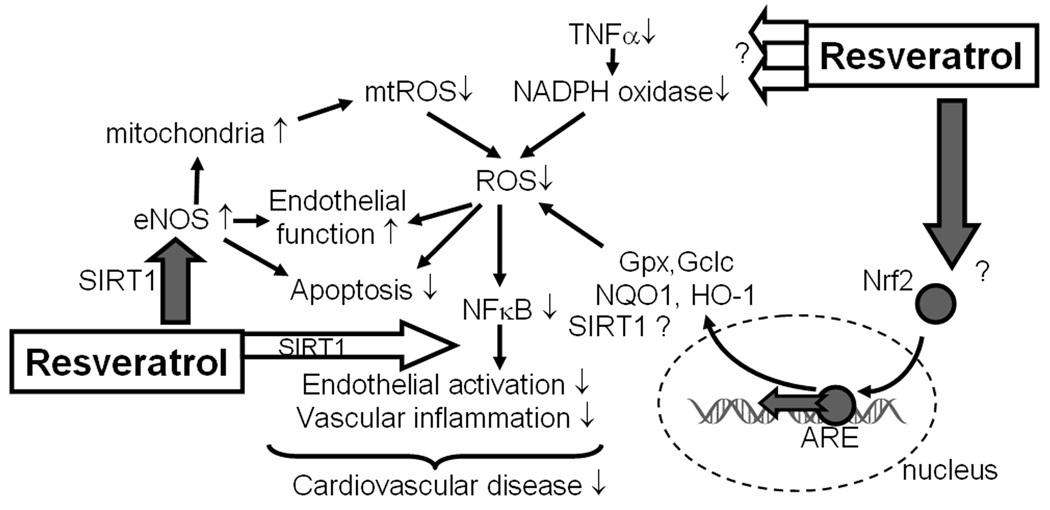
Figure 1.
Proposed scheme for the mechanisms by which resveratrol confers anti-oxidative and anti-inflammatory vasoprotective effects in aging. During aging increased NADPH oxidase- and mitochondria-derived ROS production enhances NF-κB activation, which promotes inflammatory cytokine and chemokine expression, endothelial activation and leukocyte adhesion. Increased oxidative stress also impairs endothelial vasomotor function and promotes endothelial apoptosis, which together with chronic low-grade vascular inflammation significantly increase the risk for the development of vascular diseases in the elderly. The model predicts that resveratrol, via up-regulating Nrf2-driven antioxidant enzymes and eNOS, down-regulating TNFα-activated NADPH oxidases, exerting mitochondrial protective effects and inhibiting NF-κB, significantly attenuates vascular oxidative stress and inflammation in aging. Empty block arrows: inhibition; filled block arrows: induction/activation.
Acknowledgments
This work was supported by grants from the American Federation for Aging Research (to AC), the University of Oklahoma College of Medicine Alumni Association (to AC) and the NIH (AT006526 and HL077256).
References
- 1.Keys A, Menotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MH, et al. The diet and 15-year death rate in the seven countries study. Am J Epidemiol. 1986;124:903–915. doi: 10.1093/oxfordjournals.aje.a114480. [DOI] [PubMed] [Google Scholar]
- 2.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 3.Wood J, Rogina GB, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 4.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 5.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. The EMBO journal. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-{kappa}B. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 11.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2003;285:H1015–H1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- 13.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circulation research. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol. 2007;293:H1344–H1350. doi: 10.1152/ajpheart.00413.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci. 2008;13:5056–5070. doi: 10.2741/3064. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann J, Haendeler J, Aicher A, Rossig L, Vasa M, Zeiher AM, Dimmeler S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89:709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 17.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- 18.Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 19.Addabbo F, Ratliff B, Park HC, Kuo MC, Ungvari Z, Csiszar A, Krasnikov B, Sodhi K, Zhang F, Nasjletti A, Goligorsky MS. The Krebs cycle and mitochondrial mass are early victims of endothelial dysfunction: proteomic approach. Am J Pathol. 2009;174:34–43. doi: 10.2353/ajpath.2009.080650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Mital S, Ojaimi C, Csiszar A, Kaley G, Hintze TH. Premature death and age-related cardiac dysfunction in male eNOS-knockout mice. J Mol Cell Cardiol. 2004;37:671–680. doi: 10.1016/j.yjmcc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 23.Cernadas MR, Sanchez de Miguel L, Garcia-Duran M, Gonzalez-Fernandez F, Millas I, Monton M, Rodrigo J, Rico L, Fernandez P, de Frutos T, Rodriguez-Feo JA, Guerra J, Caramelo C, Casado S, Lopez F. Expression of constitutive and inducible nitric oxide synthases in the vascular wall of young and aging rats. Circ Res. 1998;83:279–286. doi: 10.1161/01.res.83.3.279. [DOI] [PubMed] [Google Scholar]
- 24.Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvar Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. The American journal of pathology. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori R, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: role of nitric oxide. Am J Physiol Heart Circ Physiol. 2002;282:H1988–H1995. doi: 10.1152/ajpheart.01012.2001. [DOI] [PubMed] [Google Scholar]
- 26.Juric D, Wojciechowski P, Das DK, Netticadan T. Prevention of concentric hypertrophy and diastolic impairment in aortic-banded rats treated with resveratrol. American journal of physiology. 2007;292:H2138–H2143. doi: 10.1152/ajpheart.00852.2006. [DOI] [PubMed] [Google Scholar]
- 27.Gurusamy N, Ray D, Lekli I, Das DK. Red wine antioxidant resveratrol-modified cardiac stem cells regenerate infarcted myocardium. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari ZI, Zhang C. Resveratrol Improves Left Ventricular Diastolic Relaxation in Type 2 Diabetes by Inhibiting Oxidative/Nitrative Stress. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradamante S, Barenghi L, Piccinini F, Bertelli AA, De Jonge R, Beemster P, De Jong JW. Resveratrol provides late-phase cardioprotection by means of a nitric oxide- and adenosine-mediated mechanism. Eur J Pharmacol. 2003;465:115–123. doi: 10.1016/s0014-2999(03)01441-9. [DOI] [PubMed] [Google Scholar]
- 33.Taubert D, Berkels R. Upregulation and activation of eNOS by resveratrol. Circulation. 2003;107:e78–e79. doi: 10.1161/01.cir.0000060819.46705.ee. author reply e78-79. [DOI] [PubMed] [Google Scholar]
- 34.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. American journal of physiology. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 35.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med. 2007;43:720–729. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S, Fraga CG, Das DK. Cardioprotective effect of resveratrol via HO-1 expression involves p38 map kinase and PI-3-kinase signaling, but does not involve NFkappaB. Free radical research. 2006;40:1066–1075. doi: 10.1080/10715760600833085. [DOI] [PubMed] [Google Scholar]
- 37.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chow SE, Hshu YC, Wang JS, Chen JK. Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 2007;102:1520–1527. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 39.Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005;78:389–397. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 40.Shigematsu S, Ishida S, Hara M, Takahashi N, Yoshimatsu H, Sakata T, Korthuis RJ. Resveratrol, a red wine constituent polyphenol, prevents superoxide-dependent inflammatory responses induced by ischemia/reperfusion, platelet-activating factor, or oxidants. Free Radic Biol Med. 2003;34:810–817. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 41.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-{alpha}-induced activation of coronary arterial endothelial cells: role of NF-{kappa}B inhibition. Am J Physiol. 2006;291:H1694–H1699. doi: 10.1152/ajpheart.00340.2006. [DOI] [PubMed] [Google Scholar]
- 42.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 43.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 46.Ferrero ME, Bertelli AE, Fulgenzi A, Pellegatta F, Corsi MM, Bonfrate M, Ferrara F, De Caterina R, Giovannini L, Bertelli A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am J Clin Nutr. 1998;68:1208–1214. doi: 10.1093/ajcn/68.6.1208. [DOI] [PubMed] [Google Scholar]
- 47.Moon SO, Kim W, Sung MJ, Lee S, Kang KP, Kim DH, Lee SY, So JN, Park SK. Resveratrol suppresses tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells. Mol Pharmacol. 2006;70:112–119. doi: 10.1124/mol.106.022392. [DOI] [PubMed] [Google Scholar]
- 48.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 50.Danz ED, Skramsted J, Henry N, Bennett JA, Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46:1589–1597. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, Sunagawa K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 52.Gracia-Sancho J, Villarreal G, Jr, Zhang Y, Garcia-Cardena G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res. 2009;85:514–519. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 54.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pacholec M, Chrunyk BA, Cunningham D, Flynn D, Griffith DA, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsieh TC, Wang Z, Hamby CV, Wu JM. Inhibition of melanoma cell proliferation by resveratrol is correlated with upregulation of quinone reductase 2 and p53. Biochem Biophys Res Commun. 2005;334:223–230. doi: 10.1016/j.bbrc.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Hsieh TC, Zhang Z, Ma Y, Wu JM. Identification and purification of resveratrol targeting proteins using immobilized resveratrol affinity chromatography. Biochem Biophys Res Commun. 2004;323:743–749. doi: 10.1016/j.bbrc.2004.08.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochemical and biophysical research communications. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 62.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L478–L488. doi: 10.1152/ajplung.00361.2007. [DOI] [PubMed] [Google Scholar]
- 63.Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ranney A, Petro MS. Resveratrol protects spatial learning in middle-aged C57BL/6 mice from effects of ethanol. Behav Pharmacol. 2009;20:330–336. doi: 10.1097/FBP.0b013e32832f0193. [DOI] [PubMed] [Google Scholar]
- 65.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]