Abstract
Measurement of impedance is becoming increasingly available in the clinical setting as a tool for assessing hemodynamics and volume status in patients with heart failure. The 2 major categories of impedance assessment are the band electrode method and the implanted device lead method. The exact sources of the impedance signal are complex and can be influenced by physiologic effects such as blood volume, fluid, and positioning. This article provides a critical review of our current understanding and promises of impedance measurements, the techniques that have evolved, as well as the evidence and limitations regarding their clinical applications in the setting of heart failure management.
Beginning in the 1940s, there has been recognition that changes in impedance are related to pulsatile blood volume,1 and the assessment of impedance has been explored in aerospace applications as measures of cardiac output and stroke volume to monitor in-flight physiology.2,3 Over the past decades, refinement in impedance techniques has led to the development of diagnostic and prognostic tools in cardiovascular medicine.4,5 With commercial development of diagnostic devices and add-on functionalities in implanted devices that measure impedance, there is increasing interest in the clinical applications of impedance measurements in the management of heart failure. This article reviews our current understanding and promises of impedance measurements, the techniques that have evolved, and the evidence and limitations regarding their clinical applications, with the focus on heart failure management.
What does impedance measure?
Impedance is a measure of the degree a substance resists the flow of electrical current of a given voltage. The symbol Z denotes impedance and is measured in ohms. In simple terms, impedance measures the effective “resistance” to current flow through the body by applying a small alternating current. Just as when lightning strikes the ocean, it readily creates moving charges in the ionized salt water, so do the body’s fluid and tissues act as conductors of electrical current. From Ohm’s law, when electrical current is passed through human tissue, the voltage difference between 2 points on the body is proportional to the impedance.6 A high-frequency signal is therefore necessary for the current to penetrate cell membranes for aggregate tissue impedance, whereas a low frequency input only characterizes extracellular impedance.7
In humans, a low current level is often applied to prevent tissue damage.8 Compared to the high resistivity of thoracic tissue (ρ = 200–5,000 Ω cm), blood and fluid (ρ = 65–150 Ωcm) provide much lower resistance to current.9 Thus, regions of the body with higher blood or fluid content will present with lower impedance, whereas regions with more solid tissue will show higher impedance. This physical basis has been exploited to assess hemodynamic measurements and changes in fluid accumulation in the setting of congestion. Although less mentioned, there are many determinants of impedance signals that may affect the results of the measurement (see Table I for a summary of factors affecting impedance signals).
Table I.
Factors affecting impedance measurements
| Factor | Method affected (ICG or fluid) | Summary of mechanism |
|---|---|---|
| Blood volume10,11 | ICG and fluid | Causes approximately 60% of the total impedance change during the cardiac cycle. For fluid status, hypervolemia is associated with fluid overload. |
| Aortic volume change10,11 | ICG | Aortic expansion is attributed to approximately 30% of the impedance change during ventricular ejection. |
| Blood velocity10,11 | ICG | Approximately 10% of the impedance signal. Shear stress from blood flow across vessel walls affects the blood resistivity, which can be significant for post-CABG surgery. |
| Valvular regurgitation12–14 | ICG | Affects the flow of electrical current through the aorta, which can give widely varying CO intrapatient readings. |
| Sensor placement15,16 | ICG and fluid | Conflicting results for the accuracy of whole-body impedance measurements with electrodes placed at the extremities. Pacer leads at different positions may have different sensitivity to fluid overload. |
| Algorithm2,17 | ICG and fluid | Early ICG algorithms are inaccurate, later versions have better correlation to catheterization. Fluid monitor affected by impedance threshold causing interpatient variation. |
| Atrial fibrillation18,19 | ICG | Greater deviation N15% from TD for CO measurements from decreased impedance. Unknown if any correlation exists between atrial fibrillation and fluid overload episodes. |
| Body dimensions20 | ICG | Extreme dimensions show poor correlation with TD and Fick principle for CO. Excessive fat influences total resistivity and sensitivity of the impedance signal. |
| Body posture21 | ICG and fluid | Changes in posture can shift fluid distribution, which causes deviations in impedance over time. |
ICG, Impedance cardiography; CABG, coronary artery bypass graft; CO, cardiac output.
The exact source of the impedance signal in humans (sometimes referred to as “bioimpedance” or “bioelectrical impedance”) is not entirely understood, but several models have been proposed. One early model assumes that the lung impedance increases as newly oxygenated blood leaves the lungs and travels toward the atria during ventricular relaxation.2–4,22 The resulting change in lung volume gives the stroke volume, which is directly proportional to the increase in impedance. The maximum first time derivative of the impedance signal is used to calculate the impedance change. However, this model is oversimplified by assuming that the thorax is a cylindrical model and pulmonary flow has a fixed volume. The more widely accepted model is that the current flows through the path of least resistance, which is mostly through the aorta (which carries the largest volume of blood in the thorax).9,23 This theory is based on the beat-by-beat expansion of the aorta caused by the stroke volume. In actuality, both models may apply to the generation of the impedance signal in the body. Therefore, what is important to appreciate in the clinical setting is that these impedance signals likely represent an integrated measurement of underlying physiologic alterations as opposed to a specific function or process.
Factors affecting impedance signals
Several factors may affect the impedance measured in patients with heart failure, either at a single time point or with changes (summarized in Table I). Clearly, electrode placement, movement, skin moisture, blood composition (hemoglobin levels and specific resistivity of blood), and body composition (including body habitus, lung tissue, chest wall fat, air), and even environmental radio-frequency “noise” can affect the conductivity and vectors of the signals.24,25 Fluid shifts after changes in body position and posture over time may cause these variations in the impedance signal.21 As the impedance signal for cardiac applications must travel through the aorta to provide an accurate assessment, therefore aortic valve defects and overall aortic compliance may also affect the accuracy of measurements.
Methods of impedance measurement
Currently, the 2 main approaches to measure impedance in cardiac applications are the band electrode method and the implanted device–based method (Table II). Both methods measure “impedance,” but what their absolute values are and what they are measuring may differ significantly. Other configurations of electrode placements exist (including multiple vector analyses), and algorithms may vary widely among devices despite using the same nomenclature for the variables measured.
Table II.
Comparison for impedance measurement techniques for cardiac applications
| Device | Advantages | Limitations | Assumptions |
|---|---|---|---|
| Band electrode method | Noninvasive, low-cost, simple to use, continuous time-dependent monitoring Can give assessment of relative cardiac output changes in response to therapy |
Some questionable accuracy and consistency of cardiac output measurements for late-stage, chronic heart failure, and patients in unstable conditions Unresolved discrepancies in measurement (pulmonary edema, body size, irregular blood flow, blood volume) |
No valve defects No respiratory diseases No renal failure, no implanted pacing device |
| Implanted device-based method | Noninvasive (external), early detection of symptoms before hospitalization Continuous monitoring Sensitive to fluid accumulation, localized to thorax |
Invasive (implant), some unresolved impedance sources (blood volume, lung resistivity) No cardiac output, SVR, or filling pressure monitor No detection of peripheral edema or hypertension |
No valve defects No acute pulmonary processes (eg, pneumonia) |
Band electrode method
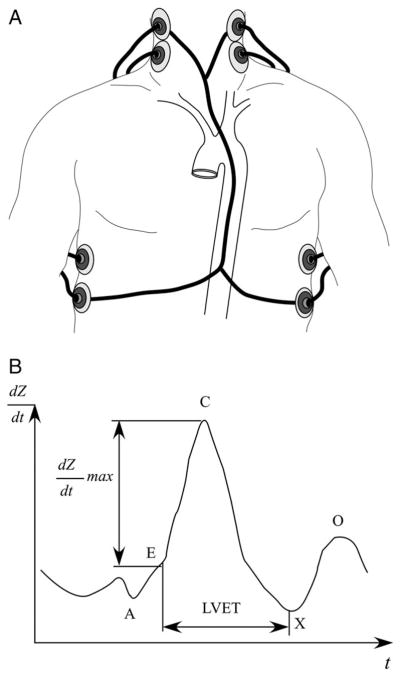
The most common band electrode method uses external band electrodes placed on the body with 2 pairs of electrodes between the neck and thorax.26–28 Four electrodes are needed to cancel the unwanted impedance signal caused by external contacts to the skin (Figure 1, A). A high-frequency, low-amplitude current (50–100 kHz, 1–4 mA rms) is applied between the neck and thorax of the first pair of contacts. The impedance signal shown in Figure 1, B, is obtained from the potential difference measured between the second pair. Other electrode configurations are required for different machines using different algorithms. The impact of such currents on defibrillator or pacemaker settings has not been reported in the published literature. Although there are very few contraindications for this test, the use of the band-electrode method can be limited by the need for the patient to be on location for the test, and sequential measurements may be confounded by several factors including variable electrode placement sites. Some newer algorithms used the phenomenon that changes in fluid volume drive changes in the frequency of propagating waves rather than changes in the amplitude of the signal. Hence, detection of such phase shift of currents (so-called bioreactance) may allow less variability and therefore more reliable hemodynamic assessment such as cardiac output.29
Figure 1.
Band electrode impedance measurement. A, Schematic representation of the band electrode technique. B, Time derivative impedance dZ/dt plotted against time t. Point A marks the fourth heart sound of atrial contraction, point B signals the first heart sound before ventricular isovolumetric contraction and rapid ejection, point C is the maximum dZ/dt, point X is the second heart sound of the closing aortic valve, and point O marks the diastolic filling. Left ventricular ejection time (LVET) is the time between points B and X.
Implanted device–based method
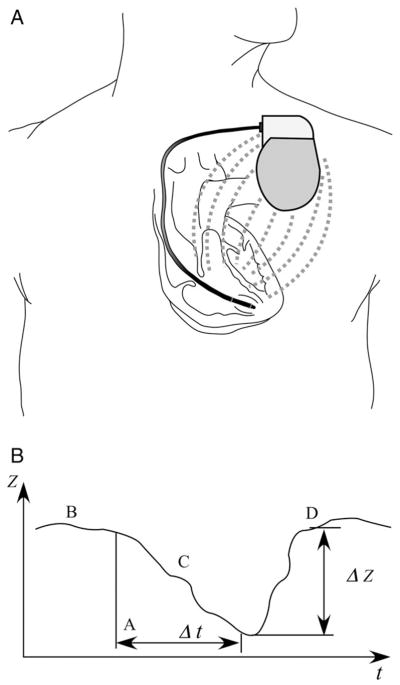
Impedance has long been used to check for lead integrity in pacemaker or defibrillator devices. With the current generated from the pacing wire, current travels across the thoracic organs toward the can of the device (Figure 2, A). Hence, changes in impedance can be determined across 2 relatively fixed points, thereby minimizing distortion or variations in electrode placement. The impedance signal is shown in Figure 2, B, with the time axis on the magnitude of days to weeks, rather than confined to the length of the cardiac cycle as with the band electrode method. This facilitates the detection of changes in impedance trends over time in a particular individual rather than a spot measurement. Hence, the primary purpose of implanted device–based methodology is to monitor clinical status over time in chronically ill patients as an ancillary functionality of the implanted device.30 As expected, the biggest limitation is the requirement of an implanted device capable of measuring intrathoracic impedance, which may apply to only a relatively small subset of patients at present. Similar to the band-electrode method, different analytic algorithms and positioning of the leads and the device may also produce slight interindividual variations in absolute impedance values.
Figure 2.
Implanted electrode impedance measurement. A, Schematic representation of the implanted electrode technique. B, Plot of the impedance Z for device-based fluid monitoring for a hypothetical episode of fluid overload. Point B is the baseline impedance in the absence of fluid overload, point C marks the steady decline in impedance with accumulating pulmonary fluid over several days or weeks given by Δt, and point D follows the restoration of baseline impedance ΔZ with applied diuretic therapy.
Current applications of impedance measurement in heart failure
Determining hemodynamics
To assess cardiac function, the stroke volume and cardiac output calculations rely on several factors inherent in the cardiac cycle. Figure 2, A, shows a hypothetical schematic of the impedance signal. Events in the cardiac cycle are correlated to their position on the impedance signal.31,32 Modern algorithms have used several measured variables including body dimensions, left ventricular ejection time, the measured base impedance, and the first time derivative of impedance when using the band electrode method.22,26 Stroke volume can be calculated by measuring the changes in the size and volume of the aorta during systole, whereas the product of stroke volume and heart rate can derive the estimated cardiac output, and the cardiac power output (CPO) can derive the product of cardiac output and mean arterial pressure. Other hemodynamic variables that have been introduced include estimates of arterial compliance and a wide variety of contractility indices. However, wide variations in cardiac cycles (as in the case of atrial fibrillation) may potentially affect the consistency of these hemodynamic measurements. Several reports have also provided reliable correlations in the continuous cardiac output assessment using bioreactance techniques compared to that derived from standard invasive measurements.33,34
The premise of monitoring hemodynamics is to provide additional information that is incremental to the determination of the patient’s clinical condition. For example, patients with higher exercise CPO may have a better survival than those with low exercise CPO, <1.96 W.35 In addition, a high CPO with moderately high systemic vascular resistance (SVR) can be associated with acute hypertension, whereas a low CPO with high SVR is characteristic of pulmonary edema.36 Retrospective studies into the first derivative of the impedance signal suggest that an abnormal impedance increase in early diastole (O wave) may be linked to severe heart failure, myocarditis, or valvular heart disease.31,37,38 The O wave appears most commonly in patients with increased diastolic flow velocity and may be used as an indicator of late-stage heart failure. However, few studies relate changes in the impedance signal using the band-electrode method to predict discrete physiologic events.
Assessing volume status
Depending upon tissue composition, the body’s impedance can be lower in areas of higher fluid, as fluid provides less resistance to current flow than tissue or air.39 This principle is used as a diagnostic tool for detecting subclinical signs and symptoms of congestive heart failure.40–42 Build-up of lung fluid results in increased capillary hydrostatic pressure and leads to backward failure with fluid accumulation of the interstitial lung tissue.39 Therefore, at the onset of edema, the impedance signal decreases and can restore to baseline after diuretic therapy. It is important to recognize that estimates of thoracic fluid content is dependent on the placement of the electrodes that span across the thoracic region and may not necessarily correlate with invasive hemodynamic measurements (although their changes are likely to be concordant).43 The implanted device–based method measures fluid in the extracellular space in the lungs, which includes both the extravascular fluid of the interstitium and the intravascular plasma volume.44,45 In contrast, the band electrode method uses external electrodes that subtract the external impedance to measure only the internal thoracic impedance.15 Such data have been proposed to detect pulmonary edema as an aid in the diagnosis of heart failure or as an early guide to therapy.16 However, these findings may not be specific owing to the potential differences between total-body versus compartmental fluid accumulation in the setting of congestion. In a patient with significant peripheral edema caused by right heart failure, there may be lack of reduction in intrathoracic impedance signals despite the increase in total body volume by clinical assessment. By the same token, the lack of peripheral edema may still produce substantial alteration in impedance signals when compartmental fluid accumulation is evident.
Predicting future risks
The ability of impedance data to predict future heart failure events (and the possibility of intervention to prevent such events) is the ultimate justification for its clinical use. In the Prospective Evaluation and Identification of Decompensation by ICG test (PREDICT) study, 212 patients with chronic heart failure stabilized after recent heart failure admission were followed every 2 weeks using clinical assessments. Collection of impedance data using the band electrode method was blinded from usual clinical care. Subjects’ self-assessment of heart failure severity symptom burden, systolic blood pressure, and impedance-derived variables were predictive of future decompensation risks in short-term follow-up within 14 days.46 However, neither clinical nor impedance cardiography variables measured at the start of the study were predictive of long-term events as many factors can affect such dynamic risks over time.46 These results were derived in a post hoc manner in an observational series and should be interpreted with great caution, as addition of the impedance data to the assessment of clinical data was neither clearly additive nor predictive. Associations between high-risk variables from the band-electrode technique have also been associated with higher natriuretic peptide levels and more adverse hemodynamics.47 It should be recognized that these are merely surrogates of adverse outcomes in heart failure, and data demonstrating incremental clinical benefit are still lacking.
Similar risk prediction models have been constructed from data derived from implanted device–based methods, where fluid index based on relative changes in impedance trends is used with threshold determination for detection of underlying physiologic alterations. Several observational series have indicated that using an arbitrary threshold allows a relatively consistent accuracy in the prediction of subsequent heart failure hospitalization in the range of 60% to 70% and may vary with different “cut-off” values.48–50 In a small series of patients, the time lapse from the onset of lowering impedance trends to hospitalization averaged 12 to 15 days, thereby potentially providing ample opportunities to prescribe appropriate interventions.50 Changes in impedance trends derived from implanted device–based methods correlated with plasma natriuretic peptide levels.51 Preliminary data from the Program to Assess and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure (PARTNERS-HF) study presented at the recent Heart Failure Society of American Annual Scientific Sessions also indicated that changes in impedance trends across a set threshold of 100-Ω days were 3.5 times more likely to have a subsequent heart failure event.52
Limitations and challenges in clinical applications
There is extensive published literature regarding the clinical applications of impedance assessment in the setting of heart failure. However, several challenges continue to hinder the broad adoption of impedance measurements as a tool for managing patients with heart failure.
Lack of a comparative “gold standard”
The lack of a universally accepted “gold standard” of clinical monitoring and objective assessment in the disease severity of heart failure has limited the evaluation of the applicability of impedance techniques in clinical practice. Although impedance techniques may use the same nomenclature to describe the hemodynamic profiles, the accuracy and reliability of each variable may not be consistent among different devices, making it difficult to generalize the findings from individual studies. Also, the large majority of data available in support of impedance measurements are designed as cross-sectional correlative analyses.
To consider impedance as a viable method to assess fluid and hemodynamics, the accuracy and reproducibility of impedance measurements to standard cardiac output and filling pressure methods must be evaluated (even with the inherent variability of direct hemodynamic measurement methods). Table III presents various published studies validating data yielded from impedance measurement to standard invasive methodologies (the presumed “gold standard”). The majority of these positive comparison studies include patients with relatively stable hemodynamic status (eg, no pulmonary distress, no excessive thoracic fluid, and no mitral/tricuspid valve disorders) or in relatively early stages of heart failure.18,22,53–57 In contrast, other studies have also shown relatively poor correlation with thermodilution and pulmonary capillary wedge pressure measurements.60–62
Table III.
Selected major impedance comparison studies of band electrode cardiac output measurement
| Study setting | Eligibility criteria and patient group | Sample size | Method | R | Results and conclusions |
|---|---|---|---|---|---|
| Heart failure ICU18 | Stable clinical condition: no respiratory distress, no increase in drug therapy | 33 | TD vs ICG | 0.89 | ICG gave good correlation with TD, but 21% of interpatient CO measurements differed by >15%. |
| Heart failure catheterization laboratory53 | Ischemic and nonischemic cardiomyopathy | 59 | TD vs ICG Fick vs ICG (n = 28) |
0.76 0.73 |
ICG correlates with TD and Fick for CO and CI, but ICG gives no correlation between thoracic fluid and PA wedge pressure. 62% sensitivity, 79% specificity to CO by TD. |
| Catheterization laboratory54 | Pulmonary artery hypertension, clinically stable | 39 | TD vs ICG Fick vs ICG |
0.8 0.84 |
Similar 3-way correlation of ICG with TD and Fick. 1.01 L/min precision, −0.43 L/min bias to CO by TD. |
| ICU university hospital14 | NYHA stage IV, LVEF <30%, ischemic and dilated cardiomyopathy, transplant evaluation, no pacemaker, no renal failure | 44 | TD vs ICG | 0.51 | Only 31% of CO measurements were within ±0.5 L/min to TD, large interpatient variation from 0.2% to 133% difference. Caused by larger body size, more dyspnea, mitral/tricuspid valve regurgitation, not dependable for severe heart failure. |
| Heart hospital55 | Ischemic cardiomyopathy, no pulmonary disease, no hypertension, no NYHA class IV (n = 25) | 25 | TD vs ICG | 0.89 | Similar high correlation to CO at rest and during exercise, hemodynamic changes in high-intensity exercise may reduce precision. Low 0.9% interpatient variation. |
| Hospital ICU56 | Acute heart failure | 31 | TD vs ICG | 0.85 | Similar good correlation to CI at baseline and follow-up with vasodilation therapy. High correlation for whole-body impedance, 1.4 L/min precision. |
| Heart surgery ICU10 | Within 24 h of post-CABG surgery | 53 | TD vs ICG | 0.81 | ICG agrees with TD for CO, better intrapatient correlation than TD. |
| Hospital ICU57 | Post-CABG or valve surgery | 20 | TD vs ICG | 0.93 | All ICG data correlate to TD within ±20%, 0.4 L/(min m2) precision. |
| ICU university hospital58 | Post-CABG or mitral valve surgery, no aortic valve defect | 34 | TD vs ICG | 0.34 | Good agreement preoperative CI measurement (R = 0.88), poor correlation for postoperative (R = 0.34). Large interpatient variation in bias (0.02–0.21 L/[min m2]) and precision (1.06–1.52 L/[min m2]). Variations caused by low flow, low mean arterial pressure, increased fluid and SVR. |
| Hospital ICU59 | Post-CABG | 50 | TD vs ICG | 0.49 | Very high −0.33 L/min bias and 6.2 L/min precision. Better agreement with normal body dimensions and without mechanical ventilation (R = 0.65), worse correlation with deviation from normal anatomy and poor timing with ECG. |
ICU, Intensive care unit; CABG, coronary artery bypass graft; TD, thermodilution; CO, cardiac output; CI, cardiac index; NYHA, New York Heart Association; LVEF, left ventricular ejection fraction; PA, pulmonary artery.
Improvements to the existing model17 and 3-dimensional finite difference calculations have been developed to analyze the physiologic sources of the impedance signal.10,11,63–65 Newer algorithms and devices have also been developed that provide better correlation with invasive pulmonary artery catheterization methods for patients with advanced heart failure.18,22 This, however, also posed some challenges when using the same terminology despite the use of different algorithms.
Lack of specificity in impedance signal
In both hemodynamics and fluid status analysis methods, the signal is convoluted with many different sources inherent in the physiology. For instance, the cardiac output calculations assume that the major sources of impedance change originate from aortic expansion in systole or blood return from the lungs (Table IV).9,66 However, these structures may only contribute a proportion of the total impedance change, where a proportion may be caused by changes in blood volume.10,11,63 Combined with several other sources of impedance, such as lead placement, body position, tissue composition, and fluid, it may be difficult to completely isolate each individual contribution in experimental trials.20,21,67 More advanced algorithms which use post-processing of the impedance signal may improve the accuracy of band electrode cardiac output measurements.68–70 These new adjustments also allow more accurate estimates of SVR and other parameters.
Table IV.
Selected major impedance comparison studies of fluid measurement
| Study setting | Eligibility criteria and patient group | Sample size | Method | R | Results and conclusions |
|---|---|---|---|---|---|
| Hospital ICU50 | NYHA class III-IV, implanted pacers | 17 | PCWP vs ICG (implant) | −0.61 | 14% average decrease in impedance over 2 wk inversely correlates to increase in PCWP, impedance, and PCWP restored to baseline after intravenous therapy |
| University hospital15 | Coronary disease, valvular disease, hypertension, pulmonary edema, control group without edema | 60 | CX-ray and PE vs ICG (external) | NR | 15% decrease in impedance from baseline 1 h before clinical symptoms, 22% greater decrease than control group inversely correlates to crepitation rales, impedance returned to baseline after edema resolution |
| Teaching hospital16 | Suspected heart failure, dyspnea (n = 131) | 131 | CX-ray vs ICG (external) | NR | Lower impedance in cardiomegaly (25%) and pulmonary edema (26%) compared to normals, all groups with same average baseline impedance, no difference between cardiomegaly and edema |
PCWP, Pulmonary capillary wedge pressure; CX-ray, chest X-ray; PE, physical exam; NR, not reported.
Lack of reliable therapeutic responses and infrastructure
Only a small number of studies have evaluated the outcomes of intervention to impedance measurements. A small observational series of patients with acute decompensated heart failure found that band electrode–derived impedance data avoided invasive catheterization in 10 of 14 patients and improved outcome in 6 of the 10 patients using impedance measurements (Table V).71 Many studies tracked impedance data with therapeutic responses, but few examined how and whether the measured impedance changed or influenced therapy decisions. Three case studies showed that impedance-derived hemodynamic data were consistent for patients with acute heart failure before and after therapy and aided in determining dosage.76 Case-controlled comparisons between impedance-enhanced implanted devices versus those without impedance data showed favorable trends toward less hospitalizations, but the event rates were small.77
Table V.
Selected outcome studies with impedance measurement in heart failure
| Study | Patient target group | Objective | Results |
|---|---|---|---|
| ED-IMPACT72 | Emergency department, age >65 y, dyspnea, heart failure, pulmonary disease | Determine changes in diagnosis and therapy with ICG hemodynamic data compared with the ED physician diagnosis | ICG data changed diagnosis in 5.4%, medication in 23.6%, and dosage in 25% of patients. |
| ESCAPE BIG substudy73 | NYHA class IV, LVEF<30%, symptoms for congestion, prior hospitalization, systolic BP <125 mm Hg, and stable enough to not require catheterization | Evaluate changes in therapy with and without hemodynamic data from ICG and catheterization, determine deaths and days needed for hospitalization as a result of therapy decisions | No significant correlation between ICG measurement and hemodynamics measured from catheterization. |
| PREDICT74 | Chronic heart failure, prior heart failure hospitalization, NYHA class II-IV | Analyze ICG data to determine low, average, or high risk for heart failure symptom, and to predict death and hospitalization | High risk for heart failure event within 14 d for patients with low stroke index <34 mL/m2 and high thoracic fluid >32 k Ω−1. |
| MIDHeFT50 | Critically ill chronic heart failure requiring implanted investigational pacemaker | Determine timeframe for automated early detection of fluid, outcome of early hospitalization and therapy | Algorithm calculates impedance threshold to predict 12 of 14 hospitalizations, predicts fluid overload on average 18 d early. |
| European InSync Sentry Observational study49 | Subjects with chronic heart failure with implanted CRT devices and audible alerts | To evaluate the utility of intrathoracic impedance monitoring for detecting heart failure deterioration in patients with an implanted cardiac resynchronization/defibrillation device | Adjusted for multiple events per patient, the alert detected clinical HF deterioration with 60% sensitivity and with a positive predictive value of 60%. |
| PARTNERS-HF52 | Subjects with chronic heart failure with impedance-enabled CRT implanted devices | To determine the value of intrathoracic impedance and other diagnostic data to evaluate cardiovascular and heart failure–related adverse events and health care utilization | Patients with a fluid index crossing the predefined threshold in the 21-d evaluation period were twice as likely to have subsequent heart failure event (at 100-Ω d cut-off, 3.5 times higher risk). |
| Italian OptiVol-CRT Clinical Service Observational Group75 | Subjects with chronic heart failure with impedance-enabled implanted CRT devices | To determine the association between device-determined diagnostic indices and heart failure hospitalization | Threshold crossing (>60-Ω d cut-off) resulted in 36% increased probability of heart failure hospitalizations. |
ED-IMPACT, Emergent Dyspnea Impedance cardiography-aided Assessment Changes Therapy; ESCAPE BIG, Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness Bioimpedance cardiography substudy; PREDICT, Prospective Evaluation and Identification of Decompensation by ICG Test; MIDHeFT, Medtronic Impedance Diagnostics in Heart Failure Patients; EU Registry, European Observational InSync Sentry Study; PARTNERS-HF, Program to Assess and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure; BP, blood pressure; CRT, cardiac resynchronization therapy; HF, heart failure; ED, emergency department.
The ability of implanted devices to assess trends in changes of impedance allows a new dimension of data integration and generation of clinically relevant parameters to monitor for the purpose of risk prediction. In some cases, dynamic built-in “alerts” may provide early warning of impending deterioration of clinical status beyond the scheduled interrogations, allowing prompt attention by the patient and/or health care provider to actively pursue risk reduction interventions. However, conducting clinical trials of management strategies are exceedingly challenging, as the interventions are often difficult to be double blinded, and many unforeseeable factors other than the designated strategy may ultimately influence the outcomes tested regardless of the interventions.
A large issue looms as to how the data derived will be delivered from the patient to the health care provider, and how can the clinical decisions made based on these new measurements improve the care and reduce morbidity and mortality. Should treatment guided by these new measurements be proven to make a difference in outcomes, another big hurdle will involve the redesign of the process of care to cater for such diagnostic information to be available to the health care provider in an efficient and seamless manner.78 However, more data may not provide correspondingly better understanding of the condition. Furthermore, the assumption is primarily based on the fact that the trajectory of disease progression (based on data derived from clinical or impedance assessment) can be altered via careful monitoring of hemodynamic or volume status. As observed in the case of the PREDICT study, short-term risk prediction does not translate into long-term prognosis.46 It is important to point out that despite decades of available hemodynamic data (derived primarily from the pulmonary arterial catheter) and the availability of diuretic therapy and vasoactive drugs to alter hemodynamics, we have yet to answer the fundamental question of how to best respond to a hemodynamic profile. Even with the most accurate measurements, we continue to struggle with what is the best treatment strategy to achieve optimal results. Nevertheless, using impedance data, preliminary observational series have shown a reduction in hospital admission rates compared to the national benchmark and projected total annual reduced costs for the management of heart failure.79 Large-scale randomized controlled trials are currently underway aiming to address these issues. Different configurations of measuring impedance using different lead positions and algorithms to estimate different hemodynamic or clinical parameters are also under investigation.
“Next-generation” devices will likely go beyond intra-cardiac implantations80 and will incorporate with existing home monitoring infrastructures used for home care or remote telemonitoring (especially those with external applications). The exact measurements and algorithms may vary, which may affect their diagnostic accuracies. Some of these devices are currently undergoing early phase preclinical and clinical evaluations, with a relatively broad clinical appeal because of their noninvasiveness and wide applicability.
Conclusions
The concept of measuring an altered physiologic surrogate such as impedance has evolved over the past 40 years and continues to be an attractive means to change the way we conceptualize and approach subclinical vulnerabilities that often lead to alterations in clinical status. However, uniformity and consensus in the measurement of “impedance” is lacking, and the treatment responses for abnormal impedance values remain heterogeneous and poorly defined. Early studies provide only associations in small sample sizes without any meaningful outcome measures to justify their incremental benefit. These factors may explain why despite their potential utility in patients with heart failure, clinical adoption of the concept of impedance measurements remains a challenge. Hence, there is a need for more careful and collaborative research efforts as well as practical experience to move the field forward.
Acknowledgments
Dr. Tang is supported in part by the National Institutes of Health, National Center for Research Resources, CTSA 1UL1RR024989, Cleveland, OH.
Footnotes
Disclosures
Dr. Tang served as a consultant for Medtronic (Minneapolis, MN), Inc, and Boston Scientific (Natick, MA), Inc.
References
- 1.Nyboer J, Bango S, Barnett A, et al. Radiocardiograms: Electrical impedance changes of the heart in relation to electrocardiograms and heart sounds. J Clin Invest. 1940;19:773. [Google Scholar]
- 2.Kubicek WG, Karnegis JN, Patterson RP, et al. Development and evaluation of an impedance cardiac output system. Aerosp Med. 1966;37:1208–12. [PubMed] [Google Scholar]
- 3.Patterson RP, Kubicek WG, Kinnen E, et al. Development of an electrical impedance plethysmography system to monitor cardiac output. Proc of the First Ann Rocky Mountain Bioengineering Symposium; 1964. pp. 56–71. [Google Scholar]
- 4.Kubicek WG, Patterson RP, Lillehei RC. Impedance cardiography as a non-invasive method to monitor cardiac function and other parameters of the cardiovascular system. Ann NY Acad Sci. 1970;170:724–32. [Google Scholar]
- 5.Penney BC. Theory and cardiac applications of electrical impedance measurements. Crit Rev Biomed Eng. 1986;13:227–81. [PubMed] [Google Scholar]
- 6.Dorf RC, Svoboda JA. Introduction to electric circuits. 6. New York: Wiley; 2003. [Google Scholar]
- 7.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—Part I. Review of principles and methods. Clin Nutr. 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Thomasset A. Bio-electrical properties of tissue impedance measurements. Lyon Med. 1962;207:107–18. [PubMed] [Google Scholar]
- 9.Summers RL, Shoemaker WC, Peacock WF, et al. Bench to bedside: electrophysiologic and clinical principles of noninvasive hemodynamic monitoring using impedance cardiography. Acad Emerg Med. 2003;10:669–80. doi: 10.1111/j.1553-2712.2003.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Patterson RP. Effect of blood resistivity changes on impedance cardiography determined by 3-D finite difference models of human thorax. 14th Int Conf IEEE-EMBS; 1992. pp. 1736–7. [Google Scholar]
- 11.Wang L, Patterson RP. Multiple sources of the impedance cardiogram based on 3-D finite difference human thorax models. IEEE Trans Biomed Eng. 1995;42:141–8. doi: 10.1109/10.341826. [DOI] [PubMed] [Google Scholar]
- 12.Campos PC, D’Cruz I. Functional mitral regurgitation in decompen-sated heart failure: combined bio-impedance and 2D echocardiography follow-up monitoring. Echocardiography. 2004;21:337–9. doi: 10.1111/j.0742-2822.2004.03118.x. [DOI] [PubMed] [Google Scholar]
- 13.Boerboom LE, Kinney TE, Olinger GN, et al. Validity of cardiac output measurement by the thermodilution method in the presence of acute tricuspid regurgitation. J Thorac Cardiovasc Surg. 1993;106:636–42. [PubMed] [Google Scholar]
- 14.Woo MA, Hamilton M, Stevenson LW, et al. Comparison of thermodilution and transthoracic electrical bioimpedance cardiac outputs. Heart Lung. 1991;20:357–62. [PubMed] [Google Scholar]
- 15.Charach G, Rabinovich P, Grosskopf I, et al. Transthoracic monitoring of the impedance of the right lung in patients with cardiogenic pulmonary edema. Crit Care Med. 2001;29:1137–44. doi: 10.1097/00003246-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Peacock WFI, Albert NM, Kies P, et al. Bioimpedance monitoring: better than chest x-ray for predicting abnormal pulmonary fluid? Congest Heart Fail. 2000;6:86–9. doi: 10.1111/j.1527-5299.2000.80141.x. [DOI] [PubMed] [Google Scholar]
- 17.Raaijmakers E, Faes TJC, Goovaerts HG, et al. The inaccuracy of Kubicek’s one-cylinder model in thoracic impedance cardiography. IEEE Trans Biomed Eng. 1997;44:70–6. doi: 10.1109/10.553714. [DOI] [PubMed] [Google Scholar]
- 18.Albert NM, Hail MD, Li J, et al. Equivalence of the bioimpedance and thermodilution methods in measuring cardiac output in hospitalized patients with advanced, decompensated chronic heart failure. Am J Crit Care. 2004;13:469–79. [PubMed] [Google Scholar]
- 19.Schmidt B, Asbach S, Schweika O, et al. Atrial fibrillation reduces the atrial impedance amplitude during cardiac cycle: a novel detection algorithm to improve recognition of atrial fibrillation in pacemaker patients. Europace. 2007;9:812–6. doi: 10.1093/europace/eum106. [DOI] [PubMed] [Google Scholar]
- 20.Kauppinen PK, Hyttinen JA, Malmivuo JA. Effects of fat resistivity changes on measurement sensitivity of impedance cardiography determined by a 3D finite element model of the visible human man. 18th Ann Int Conf IEEE-EMBS; 1996. pp. 1936–7. [Google Scholar]
- 21.Lozano-Nieto A, Turner AA. Effects of orthostatic fluid shifts on bioelectrical impedance measurements. Biomed Instrum Technol. 2001;35:249–58. [PubMed] [Google Scholar]
- 22.Van De Water JM, Miller TW, Vogel RL, et al. Impedance cardiography: the next vital sign technology? Chest. 2003;123:2028–33. doi: 10.1378/chest.123.6.2028. [DOI] [PubMed] [Google Scholar]
- 23.Yancy C, Abraham WT. Noninvasive hemodynamic monitoring in heart failure: utilization of impedance cardiography. Congest Heart Fail. 2003;9:241–50. doi: 10.1111/j.1751-7133.2003.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 24.Barry BN, Mallick A, Bodenham AR, et al. Lack of agreement between bioimpedance and continuous thermodilution measurement of cardiac output in intensive care unit patients. Crit Care (Lond) 1997;1:71–4. doi: 10.1186/cc106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imhoff M, Lehner JH, Lohlein D. Noninvasive whole-body electrical bioimpedance cardiac output and invasive thermodilution cardiac output in high-risk surgical patients. Crit Care Med. 2000;28:2812–8. doi: 10.1097/00003246-200008000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Patterson RP, Kubicek WG, Kinnen E, et al. Fundamentals of impedance cardiography. IEEE Eng Med Biol. 1989;8:35–8. doi: 10.1109/51.32403. [DOI] [PubMed] [Google Scholar]
- 27.Patterson RP, Wang L, Raza SB. Impedance cardiography using band and regional electrodes in supine, sitting, and during exercise. IEEE Trans Biomed Eng. 1991;38:393–400. doi: 10.1109/10.81557. [DOI] [PubMed] [Google Scholar]
- 28.Patterson R, Wang L, McVeigh G, et al. Impedance cardiography: the failure of sternal electrodes to predict changes in stroke volume. Biol Psychol. 1993;36:33–41. doi: 10.1016/0301-0511(93)90078-m. [DOI] [PubMed] [Google Scholar]
- 29.Keren H, Burkhoff D, Squara P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am J Physiol Heart Circ Physiol. 2007;293:H583–H589. doi: 10.1152/ajpheart.00195.2007. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Yu CM, Chau E, et al. Prediction of CHF Hospitalization by ambulatory intrathoracic impedance measurement in CHF patients is feasible using pacemaker or ICD lead systems. PACE. 2003;26:959. [Google Scholar]
- 31.Lababidi Z, Ehmke DA, Durnin RE, et al. The first derivative thoracic impedance cardiogram. Circulation. 1970;41:651–8. doi: 10.1161/01.cir.41.4.651. [DOI] [PubMed] [Google Scholar]
- 32.Woltjer HH, Bogaard HJ, de Vries PM. The technique of impedance cardiography. Eur Heart J. 1997;18:1396–403. doi: 10.1093/oxfordjournals.eurheartj.a015464. [DOI] [PubMed] [Google Scholar]
- 33.Raval NY, Squara P, Cleman M, et al. Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comput. 2008;22:113–9. doi: 10.1007/s10877-008-9112-5. [DOI] [PubMed] [Google Scholar]
- 34.Squara P, Denjean D, Estagnasie P, et al. Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med. 2007;33:1191–4. doi: 10.1007/s00134-007-0640-0. [DOI] [PubMed] [Google Scholar]
- 35.Williams SG, Cooke GA, Wright DJ, et al. Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. Eur Heart J. 2001;22:1496–503. doi: 10.1053/euhj.2000.2547. [DOI] [PubMed] [Google Scholar]
- 36.Cotter G, Moshkovitz Y, Kaluski E, et al. The role of cardiac power and systemic vascular resistance in the pathophysiology and diagnosis of patients with acute congestive heart failure. Eur J Heart Fail. 2003;5:443–51. doi: 10.1016/s1388-9842(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 37.Ramos MU. An abnormal early diastolic impedance waveform: a predictor of poor prognosis in the cardiac patient? Am Heart J. 1977;94:274–81. doi: 10.1016/s0002-8703(77)80468-7. [DOI] [PubMed] [Google Scholar]
- 38.Hubbard WN, Fish DR, McBrien DJ. The use of impedance cardiography in heart failure. Int J Cardiol. 1986;12:71–9. doi: 10.1016/0167-5273(86)90100-2. [DOI] [PubMed] [Google Scholar]
- 39.Lange NR, Schuster DP. The measurement of lung water. Crit Care (Lond) 1999;3:R19–24. doi: 10.1186/cc342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cody RJ, Covit AB, Schaer GL, et al. Sodium and water balance in chronic congestive heart failure. J Clin Invest. 1986;77:1441–52. doi: 10.1172/JCI112456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lucas C, Johnson W, Hamilton MA, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140:840–7. doi: 10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 42.Drazner MH, Hamilton MA, Fonarow G, et al. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18:1126–32. doi: 10.1016/s1053-2498(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 43.Stahl C, Beierlein W, Walker T, et al. Intracardiac impedance monitors hemodynamic deterioration in a chronic heart failure pig model. J Cardiovasc Electrophysiol. 2007 doi: 10.1111/j.1540-8167.2007.00868.x. [DOI] [PubMed] [Google Scholar]
- 44.Androne AS, Hryniewicz K, Hudaihed A, et al. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol. 2004;93:1254–9. doi: 10.1016/j.amjcard.2004.01.070. [DOI] [PubMed] [Google Scholar]
- 45.Kalra PR, Anagnostopoulos C, Bolger AP, et al. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol. 2002;39:1901–8. doi: 10.1016/s0735-1097(02)01903-4. [DOI] [PubMed] [Google Scholar]
- 46.Packer M, Abraham WT, Mehra MR, et al. Utility of impedance cardiography for the identification of short-term risk of clinical decompensation in stable patients with chronic heart failure. J Am Coll Cardiol. 2006;47:2245–52. doi: 10.1016/j.jacc.2005.12.071. [DOI] [PubMed] [Google Scholar]
- 47.Velazquez-Cecena JL, Sharma S, Nagajothi N, et al. Left ventricular end diastolic pressure and serum brain natriuretic peptide levels in patients with abnormal impedance cardiography parameters. Arch Med Res. 2008;39:408–11. doi: 10.1016/j.arcmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Ypenburg C, Bax JJ, van der Wall EE, et al. Intrathoracic impedance monitoring to predict decompensated heart failure. Am J Cardiol. 2007;99:554–7. doi: 10.1016/j.amjcard.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 49.Vollmann D, Nagele H, Schauerte P, et al. Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur Heart J. 2007;28:1835–40. doi: 10.1093/eurheartj/ehl506. [DOI] [PubMed] [Google Scholar]
- 50.Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–8. doi: 10.1161/CIRCULATIONAHA.104.492207. [DOI] [PubMed] [Google Scholar]
- 51.Luthje L, Vollmann D, Drescher T, et al. Intrathoracic impedance monitoring to detect chronic heart failure deterioration: relationship to changes in NT-proBNP. Eur J Heart Fail. 2007;9:716–22. doi: 10.1016/j.ejheart.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Whellan D. Program to Assess and Review Trending Information and Evaluate Correlation to Symptoms in Patients with Heart Failure (PARTNERS-HF). Late breaking clinical trials presentation, Heart Failure Society of America 2008 Annual Scientific Meeting; September 24, 2008; Toronto, Canada. 2008. [Google Scholar]
- 53.Drazner MH, Thompson B, Rosenberg PB, et al. Comparison of impedance cardiography with invasive hemodynamic measurements in patients with heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2002;89:993–5. doi: 10.1016/s0002-9149(02)02257-9. [DOI] [PubMed] [Google Scholar]
- 54.Yung GL, Fedullo PF, Kinninger K, et al. Comparison of impedance cardiography to direct Fick and thermodilution cardiac output determination in pulmonary arterial hypertension. Congest Heart Fail. 2004;10(2 Suppl 2):7–10. doi: 10.1111/j.1527-5299.2004.03406.x. [DOI] [PubMed] [Google Scholar]
- 55.Belardinelli R, Ciampani N, Costantini C, et al. Comparison of impedance cardiography with thermodilution and direct Fick methods for noninvasive measurement of stroke volume and cardiac output during incremental exercise in patients with ischemic cardiomyopathy. Am J Cardiol. 1996;77:1293–301. doi: 10.1016/s0002-9149(97)89153-9. [DOI] [PubMed] [Google Scholar]
- 56.Cotter G, Moshkovitz Y, Kaluski E, et al. Accurate, noninvasive continuous monitoring of cardiac output by whole-body electrical bioimpedance. Chest. 2004;125:1431–40. doi: 10.1378/chest.125.4.1431. [DOI] [PubMed] [Google Scholar]
- 57.Sageman WS, Riffenburgh RH, Spiess BD. Equivalence of bioimpedance and thermodilution in measuring cardiac index after cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:8–14. doi: 10.1053/jcan.2002.29635. [DOI] [PubMed] [Google Scholar]
- 58.Doering L, Lum E, Dracup K, et al. Predictors of between-method differences in cardiac output measurement using thoracic electrical bioimpedance and thermodilution. Crit Care Med. 1995;23:1667–73. doi: 10.1097/00003246-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Sageman WS, Amundson DE. Thoracic electrical bioimpedance measurement of cardiac output in postaortocoronary bypass patients. Crit Care Med. 1993;21:1139–42. doi: 10.1097/00003246-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Donovan KD, Dobb GJ, Woods WP, et al. Comparison of transthoracic electrical impedance and thermodilution methods for measuring cardiac output. Crit Care Med. 1986;14:1038–44. doi: 10.1097/00003246-198612000-00009. [DOI] [PubMed] [Google Scholar]
- 61.Bache RJ, Harley A, Greenfield JCJ. Evaluation of thoracic impedance plethysmography as an indicator of stroke volume in man. Am J Med Sci. 1969;258:100–13. doi: 10.1097/00000441-196908000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Handelsman H. Measuring cardiac output by electrical bioimpedance. Rockville, MD: Agency for Health Care Policy and Research, U.S. Department of Health and Human Services; 1992. Health and Technology Assessment Reports 1001. AHCPR publication 92–0073. [Google Scholar]
- 63.Kim DW, Baker LE, Pearce JA, et al. Origins of the impedance change in impedance cardiography by a three-dimensional finite element model. IEEE Trans Biomed Eng. 1988;35:993–1000. doi: 10.1109/10.8683. [DOI] [PubMed] [Google Scholar]
- 64.Peters DJ, Rhyne TL. A 3-dimensional FEM model of the human thoracic cavity for simulation of impedance cardiography. Proc Comput Cardiol. 1989:111–4. [Google Scholar]
- 65.Kauppinen PK, Hyttinen JA, Malmivuo JA. Lead field theoretical approach to impedance cardiography using 3-D finite difference element modeling. 19th Int. Conf. IEEE-EMBS; 1997. pp. 2068–71. [Google Scholar]
- 66.Visser KR. Electric properties of flowing blood and impedance cardiography. Ann Biomed Eng. 1989;17:463–73. doi: 10.1007/BF02368066. [DOI] [PubMed] [Google Scholar]
- 67.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—Part II. Utilization in clinical practice. Clin Nutr. 2004;23:1430–53. doi: 10.1016/j.clnu.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Hurwitz BE, Shyu LY, Reddy SP, et al. Coherent ensemble averaging techniques for impedance cardiography. Proc of 3rd Ann IEEE Symp on CBMS; 1990. pp. 228–35. [Google Scholar]
- 69.Wang X, Sun HH, Van de Water JM. An advanced signal processing technique for impedance cardiography. IEEE Trans Biomed Eng. 1995;42:224–30. doi: 10.1109/10.341836. [DOI] [PubMed] [Google Scholar]
- 70.Barros AK, Ohnishi N. MSE behavior of biomedical event-related filters. IEEE Trans Biomed Eng. 1997;44:848–55. doi: 10.1109/10.623054. [DOI] [PubMed] [Google Scholar]
- 71.Silver MA, Cianci P, Brennan S, et al. Evaluation of impedance cardiography as an alternative to pulmonary artery catheterization in critically ill patients. Congest Heart Fail. 2004;10(2 Suppl 2):17–21. doi: 10.1111/j.1527-5299.2004.03410.x. [DOI] [PubMed] [Google Scholar]
- 72.Peacock WF, Summers R, Emerman C. Emergent Dyspnea IMPedance cardiography-aided Assessment Changes Therapy: The ED-IMPACT Trial. Ann Emerg Med. 2003;42:S82. doi: 10.1197/j.aem.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 73.Yancy C, Rogers J, Pauly D, et al. Diagnostic implications of impedance cardiography in the setting of severe acute decompensated heart failure: results of the bioimpedance cardiography (BIG) substudy in the ESCAPE trial. Circulation. 2005;112:II-639–II-40. [Abstract] [Google Scholar]
- 74.Abraham WT. Intrathoracic impedance monitoring for early detection of impending heart failure decompensation. Congest Heart Fail. 2007;13:113–5. doi: 10.1111/j.1527-5299.2007.06255.x. [DOI] [PubMed] [Google Scholar]
- 75.Perego GB, Landolina M, Vergara G, et al. Implantable CRT device diagnostics identify patients with increased risk for heart failure hospitalization. J Interv Card Electrophysiol. 2008 doi: 10.1007/s10840-008-9303-5. Online access at: DOI 10.1007/s10840-008-09303-5 on September 23, 2008. [DOI] [PubMed] [Google Scholar]
- 76.Summers RL, Parrott CW, Quale C, et al. Use of noninvasive hemodynamics to aid decision making in the initiation and titration of neurohormonal agents. Congest Heart Fail. 2004;10(2 Suppl 2):28–31. doi: 10.1111/j.1527-5299.2004.03552.x. [DOI] [PubMed] [Google Scholar]
- 77.Maines M, Catanzariti D, Cemin C, et al. Usefulness of intrathoracic fluids accumulation monitoring with an implantable biventricular defibrillator in reducing hospitalizations in patients with heart failure: a case-control study. J Interv Card Electrophysiol. 2007;19:201–7. doi: 10.1007/s10840-007-9155-4. [DOI] [PubMed] [Google Scholar]
- 78.Tang WH. Collaboration among general cardiologists, heart failure specialists, and electrophysiologists: what are the barriers? Am J Cardiol. 2007;99:41G–4G. doi: 10.1016/j.amjcard.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 79.Dimmick SL, Burgiss SG, Robbins S, et al. Outcomes of an integrated telehealth network demonstration project. Telemed J E Health. 2003;9:13–23. doi: 10.1089/153056203763317611. [DOI] [PubMed] [Google Scholar]
- 80.Freimark D, Arad M, Sokolover R, et al. Monitoring lung fluid content in CHF patients under intravenous diuretics treatment using bio-impedance measurements. Physiol Meas. 2007;28:S269–S277. doi: 10.1088/0967-3334/28/7/S20. [DOI] [PubMed] [Google Scholar]