Evasion of host antiviral mechanisms
Many cellular messenger RNAs and viral RNAs are methylated at the 2′-O position of the 5′ guanosine cap. The role of this modification in virus infection has been unclear. Michael Diamond and colleagues now show that this form of methylation enables several unrelated viruses to evade innate host antiviral responses through escape from suppression by interferon-stimulated genes. This suggests an evolutionary explanation for 2′-O methylation of cellular mRNA: it may distinguish self from non-self RNA under conditions of infection. Novel classes of pharmacological agents that specifically inhibit cytoplasmic viral 2′-O methyltransferases may be expected to have broad-spectrum antiviral activity.
Supplementary information
The online version of this article (doi:10.1038/nature09489) contains supplementary material, which is available to authorized users.
Subject terms: West nile virus, Viral pathogenesis, Viral immune evasion
Many cellular and virus messenger RNAs are methylated at the 2′-O positions of the 5′ guanosine cap. The role of 2′-O methylation in virus infection has been unclear. These authors show that this form of methylation enables several unrelated viruses to evade the antiviral effects of genes stimulated by type I interferon.
Supplementary information
The online version of this article (doi:10.1038/nature09489) contains supplementary material, which is available to authorized users.
Abstract
Cellular messenger RNA (mRNA) of higher eukaryotes and many viral RNAs are methylated at the N-7 and 2′-O positions of the 5′ guanosine cap by specific nuclear and cytoplasmic methyltransferases (MTases), respectively. Whereas N-7 methylation is essential for RNA translation and stability1, the function of 2′-O methylation has remained uncertain since its discovery 35 years ago2,3,4. Here we show that a West Nile virus (WNV) mutant (E218A) that lacks 2′-O MTase activity was attenuated in wild-type primary cells and mice but was pathogenic in the absence of type I interferon (IFN) signalling. 2′-O methylation of viral RNA did not affect IFN induction in WNV-infected fibroblasts but instead modulated the antiviral effects of IFN-induced proteins with tetratricopeptide repeats (IFIT), which are interferon-stimulated genes (ISGs) implicated in regulation of protein translation. Poxvirus and coronavirus mutants that lacked 2′-O MTase activity similarly showed enhanced sensitivity to the antiviral actions of IFN and, specifically, IFIT proteins. Our results demonstrate that the 2′-O methylation of the 5′ cap of viral RNA functions to subvert innate host antiviral responses through escape of IFIT-mediated suppression, and suggest an evolutionary explanation for 2′-O methylation of cellular mRNA: to distinguish self from non-self RNA. Differential methylation of cytoplasmic RNA probably serves as an example for pattern recognition and restriction of propagation of foreign viral RNA in host cells.
Supplementary information
The online version of this article (doi:10.1038/nature09489) contains supplementary material, which is available to authorized users.
Main
Most eukaryotic mRNA contains a 5′ Cap 0 (7mGpppN) structure with a methyl group at the N-7 position. In higher eukaryotes, methylation of cellular mRNA occurs additionally at the 2′-O site of the penultimate (7mGpppNm, Cap 1) and antepenultimate (7mGpppNmNm, Cap 2) 5′ nucleotides in the nucleus and cytoplasm, respectively3,5. Many viral mRNAs also contain Cap 1 and 2 structures, but cap acquisition occurs distinctly among virus families2,6. RNA and DNA viruses that replicate in the cytoplasm cannot use the host nuclear capping machinery, and thus have evolved MTases to facilitate N-7 and 2′-O capping or mechanisms to ‘snatch’ the cap from host cell mRNA1. It remains unclear how 2′-O methylation contributes to viral infection or cellular mRNA homeostasis2,3.
Flavivirus is a genus of positive-strand RNA viruses with a 5′ Cap 1 structure that is generated by an MTase in the NS5 protein7. Whereas mutations abrogating the N-7 MTase activity abort WNV infection, an E218A substitution that completely abolished the 2′-O but not N-7 MTase activity (Supplementary Fig. 1) did not affect replication in permissive BHK cells8. Although C57BL/6 mice infected subcutaneously with the parental WNV wild-type (WNV-WT) strain had an approximately 40% mortality rate, recipients of WNV-E218A showed 0% mortality, even at high challenge doses (Fig. 1a, P < 0.05, n = 10) or after direct intracranial infection (Fig. 1c). Levels of WNV-E218A after subcutaneous inoculation were markedly decreased in the spleen, serum or brain compared with infection by WNV-WT (Fig. 1b).
Figure 1. WNV-E218A is attenuated in wild-type mice and cells but is virulent in Ifnar1−/− mice and cells.
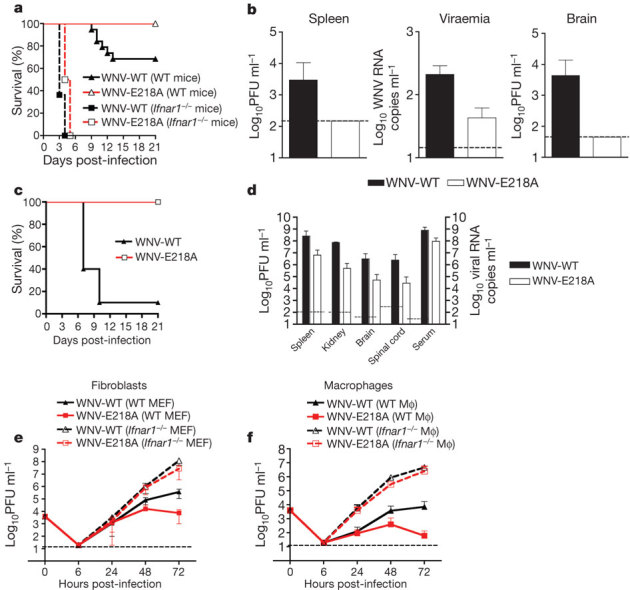
a, Survival curves of wild-type and Ifnar1−/− C57BL/6 mice after subcutaneous infection with WNV-WT or WNV-E218A. b, Virus replication in wild-type mice in blood (day 4), spleen (day 4) or brain (day 8) after subcutaneous infection with WNV-WT or WNV-E218A. c, Survival curves of wild-type mice after intracranial infection with WNV-WT (101) or WNV-E218A (105 plaque-forming units (PFU)). d, Viral burden in the serum, spleen, kidney, spinal cord and brain from Ifnar1−/− mice at day 3 after infection. e, f, Replication of WNV-WT and WNV-E218A in wild-type or Ifnar1−/− MEFs (e) or Mφ (f). Results are the average of three experiments performed in triplicate. Error bars, s.d.; dashed line, limit of sensitivity of the assay.
Because dissemination of WNV-E218A was aborted in vivo, we assessed whether 2′-O methylation restricted the protective IFN-induced immune response. Mice lacking type I IFN signalling (Ifnar1−/−) that were infected with WNV-WT showed 100% mortality and a mean time to death of 3.5 days, as seen previously9 (Fig. 1a). Remarkably, Ifnar1−/− mice infected with the WNV-E218A exhibited a similar phenotype with only a slightly delayed mean time to death of 4.5 days. Ifnar1−/− mice infected with WNV-E218A at day 3 sustained tissue titres that approached those of WNV-WT (Fig. 1d). Thus 2′-O methylation of WNV RNA is required for virulence in vivo, and its absence renders the virus sensitive to the IFN response.
Analysis of viral growth in primary mouse embryonic fibroblasts (MEFs) and macrophages (Mφ), which both produce and respond to type I IFN after WNV infection10, confirmed attenuation of WNV-E218A in wild-type cells (50- and 151-fold lower at 72 h, P < 0.05, n = 3 in MEF and Mφ, respectively) and restored growth in Ifnar1−/− cells (Fig. 1e, f). Replication of WNV-E218A was also rescued in Irf3−/−, Irf3−/− × Irf7−/− or IPS-1−/− cells that had altered or abolished IFN-α/β responses11 (Supplementary Figs 2a–c and 3a–d, respectively), but not in Irf7−/− or Tlr3−/− cells, which have normal IFN-β or IFN-α and -β responses after WNV infection, respectively10,12 (Supplementary Fig. 2d, e). These experiments confirmed that rescue of WNV-E218A in primary cells requires attenuation of the IFN response.
Because 2′-O methylation rendered WNV-WT less susceptible to the IFN response than WNV-E218A, we hypothesized that it might directly limit IFN induction by affecting the avidity of viral RNA for the host sensor, RIG-I. However, direct binding assays with recombinant RIG-I and 2′-O unmethylated or methylated WNV RNA (5′ untranslated region) showed no change in binding (Supplementary Fig. 4). It remained possible that 2′-O methylation of WNV RNA affected other proteins required for transcriptional activation of the IFN-β gene. To evaluate this idea, Ifnar1−/− MEFs, which produce IFN-β without responding to it, were infected at a high multiplicity of infection (MOI) and IFN-β mRNA was measured. Notably, both WNV-WT and WNV-E218A stimulated IFN-β transcription equivalently after infection (Fig. 2a). Thus a lack of 2′-O methylation does not affect pathogen sensing or IFN induction. To address whether 2′-O methylation of viral RNA serves to antagonize or evade IFN effector functions, IPS-1−/− MEFs, which do not produce type I IFN after WNV infection but can respond to it11, were exposed to IFN-β to induce ISGs, and then infected. WNV-E218A displayed increased sensitivity to IFN-β pretreatment compared with WNV-WT (2,400,000- and 20,000-fold inhibition with 500 international units ml−1 of IFN-β, respectively) (Fig. 2b).
Figure 2. 2′-O methylation of viral RNA alters the sensitivity of WNV to the antiviral effects of IFN.

a, IFN-β gene induction in Ifnar1−/− MEF after WNV-WT or WNV-E218A infection. Results are representative of three independent experiments performed in duplicate. b, Viral replication in IPS-1−/− MEF after IFN-β pretreatment. The data are the average of two independent experiments performed in triplicate, and the asterisks indicate differences that are statistically significant (***P < 0.0001; **P < 0.005; *P < 0.05). Error bars, s.d. IU, international units.
IFN induces hundreds of ISGs, some of which may have antiviral effector functions13. Among these, Ifit family members (for example, Ifit1 and Ifit2 (also known as ISG56 and ISG54, respectively)) are induced after WNV infection14, reduced in Irf3−/− and Ifnar1−/− cells (ref. 15 and Supplementary Fig. 5) and inhibit replication of some viruses16,17,18 in part, by interacting with eIF3 and limiting translation of viral mRNA19,20. To assess whether differential 2′-O methylation of viral RNA might affect suppression by IFIT-1 and/or IFIT-2, we evaluated infection in 3T3 MEFs expressing a murine Ifit1 or Ifit2 transgene. As observed in primary cells, WNV-E218A replication in control 3T3 cells was reduced (∼5- to 60-fold decrease at 24–72 h, P < 0.05, n = 3) compared with WNV-WT, confirming that 2′-O methylation is required for optimal infectivity (Fig. 3a). Transgenic expression of IFIT-2 reduced infection of WNV-WT (∼56- to 100-fold decrease at 24–72 h, P < 0.0005, n = 3) (Fig. 3b) compared with replication in 3T3–green fluorescent protein (GFP) cells. In comparison, expression of IFIT-2 virtually abolished replication of WNV-E218A (up to 2,700-fold decrease at 72 h, P < 0.0005, n = 3) (Fig. 3b). Expression of IFIT-1 in 3T3 cells had minimal inhibitory effects on WNV infection (Fig. 3c). To confirm the linkage between IFIT-2 expression and restriction of infection, short interfering RNA (siRNA) knockdown experiments were performed. Transfection of a sequence-specific siRNA that reduced protein expression of IFIT-2 enhanced replication of WNV-E218A (P < 0.01, n = 3) (Fig. 3d). These experiments demonstrate that mouse IFIT-2 is an antiviral effector of IFN actions, whose inhibitory activity is minimized by 2′-O methylation of viral RNA.
Figure 3. WNV-E218A is more sensitive to the antiviral actions of Ifit genes.
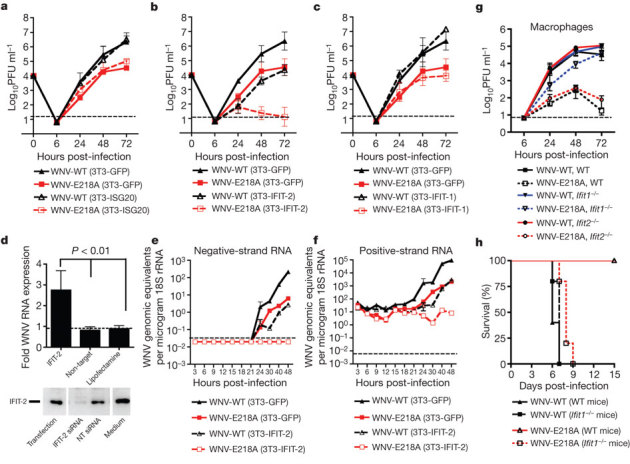
a–c, Viral replication of WNV-WT or WNV-E218A in 3T3 MEFs transgenically expressing GFP (a–c), ISG20 (a), IFIT-2 (b) or IFIT-1 (c). The data are the average of three experiments performed in duplicate. d, siRNA knockdown of IFIT-2 enhances replication of WNV-E218A. 3T3 cells were transfected with a non-target (NT) or IFIT-2 siRNA and then infected with WNV-E218A. One day post-infection cells were collected and (top) viral RNA was assayed by quantitative reverse transcriptase PCR. The data are the average of three experiments performed in duplicate. Bottom, knockdown of IFIT-2 protein was confirmed by western blot. e, f, Murine IFIT-2 expression prevents accumulation of negative- and positive-strand viral RNA in WNV-E218A-infected cells. g, Replication of WNV-E218A is attenuated in wild-type and Ifit2−/− Mφ but restored in Ifit1−/− cells. h, Survival curves of wild-type or Ifit1−/− mice after intracranial challenge with 105 plaque-forming units of WNV-WT or WNV-E218A. Error bars, s.d.; dashed line, limit of sensitivity of the assay.
Although IFIT family orthologues exist over a broad evolutionary time-frame21, humans have a distinct complement of Ifit genes (Ifit1 (ISG56), Ifit2 (ISG54), Ifit3 (ISG60) and Ifit5 (ISG58)). Transient transgenic expression of human IFIT-5 but not IFIT-1, IFIT-2 or IFIT-3 in human 293T cells inhibited infection of WNV-E218A (P = 0.003, n = 3) (Supplementary Fig. 6), which suggests a species-specificity of Ifit genes in restricting WNV lacking 2′-O methylated RNA.
We assessed the stage of the WNV life cycle that was restricted by mouse IFIT-2. Using strand-specific quantitative reverse transcriptase PCR to quantify genomic (positive strand) and replicative intermediate (negative strand) viral RNA, we found that in control 3T3 cells each increased by 18 h after infection (Fig. 3e, f), whereas the expression of mouse IFIT-2 delayed production of both by approximately 15 h in the context of WNV-WT infection. In comparison, increases in negative and positive strand RNA were abolished in IFIT-2 transgenic cells infected with WNV-E218A. The levels of WNV-E218A positive-strand RNA remained essentially constant over the time course, suggesting that the lack of 2′-O methylation did not affect viral RNA stability. Thus mouse IFIT-2 blocks infection of the E218A mutant in fibroblasts at or before negative-strand synthesis.
As other virus families encode 2′-O MTases, we sought to determine if 2′-O-methylation-dependent evasion of IFIT proteins functions as a more general immune escape mechanism. We obtained a vaccinia virus (VACV) mutant (J3-K175R) that lacked 2′-O MTase activity, replicated normally in BSC40 cells22 but was attenuated in wild-type Mφ (approximately six- to eightfold reduction at 24–72 h) and fully rescued in Ifnar1−/− Mφ (Fig. 4a). Growth curves with VACV-WT and VACV-J3-K175R in 3T3 cells expressing GFP or ISG20 confirmed an essential role of 2′-O methylation in poxvirus infection (approximately three- to fivefold reduction at 24–72 h, P < 0.005, n = 3) (Fig. 4b). Transgenic expression of IFIT-2, however, did not affect replication of VACV-WT (P > 0.5, n = 3), which suggests that IFIT-2 lacks activity against VACV-WT or that the virus efficiently antagonizes its antiviral effect. Expression of mouse IFIT-2 but not IFIT-1 further reduced infection of VACV-J3-K175R (6- to 25-fold decrease, P < 0.01, n = 3) (Fig. 4c, d). Consistent with these findings, wild-type C57BL/6 mice were resistant to lethal challenge with VACV-J3-K175R (0% lethality, n = 6) but sensitive to infection with VACV-WT (100% lethality, n = 13). In contrast, in Ifnar1−/− mice, VACV-J3-K175R was virulent as all animals succumbed to infection with similar kinetics compared with those infected with VACV-WT (Supplementary Fig. 7).
Figure 4. Poxvirus and coronavirus mutants lacking 2′-O methylation are more sensitive to the antiviral effects of murine IFIT-2.

a–d, Studies with VACV. a, Viral replication of VACV-WT or VACV-J3-K175R in wild-type or Ifnar1−/− Mφ (a) or 3T3 MEF expressing GFP (b–d), ISG20 (b), Ifit2 (c) or Ifit1 (d). e, Viral replication of MHV-WT or MHV-D130A in 3T3 cells expressing GFP or IFIT-2. f, Viral replication of EMCV in 3T3 cells expressing GFP or IFIT-2. Error bars, s.d.; dashed line, limit of sensitivity of the assay.
We examined the replication of a wild type and 2′-O MTase mutant (D130A in the nsp16 protein)23 of mouse hepatitis virus (MHV). MHV-D130A was more sensitive to the effects of IFN-β pretreatment (Supplementary Fig. 8), attenuated in control 3T3 cells (approximately 6- to 15-fold reduction at 9–24 h, P < 0.05, n = 3) (Fig. 4e), and sensitive to transgenic expression of mouse IFIT-2 (approximately 8- to 234-fold reduction, P < 0.05, n = 3) compared with MHV-WT (approximately two- to fivefold decrease at 9–24 h, P < 0.05, n = 3). Thus, analogous to flaviviruses and poxviruses, the 2′-O methylation of coronavirus RNA supports evasion from the antiviral effects of IFIT-2. In contrast, transgenic expression of IFIT-2 did not affect replication of a picornavirus, which lacks a 5′ cap structure (Fig. 4f).
To confirm the role of IFIT proteins in restricting viruses lacking 2′-O methylation, growth curves were performed in wild-type, Ifit1−/− or Ifit2−/− Mφ. Surprisingly, the infectivity of WNV-E218A was almost completely rescued in IFIT-1−/− Mφ (2,300-fold increase in titre at 72 h, P < 0.04) but not in Ifit2−/− Mφ (Fig. 3g), and the virulence of WNV-E218A was almost entirely restored in Ifit1−/− mice (Fig. 3h). Thus, in primary Mφ and in mice, IFIT-1 plays a dominant role in restricting infection of WNV lacking 2′-O methylation.
We demonstrate that among unrelated RNA and DNA viruses that replicate in the cytoplasm and contain 5′ cap structures, 2′-O methylation of viral RNA enhances virulence through evasion of intrinsic cellular defence mechanisms. 2′-O methylation of cellular RNA may have evolved as a means of distinguishing self from non-self RNA by the host during virus infection. Induction of Ifit family genes, several of which attenuate translation19,20,24, could preferentially recognize viral mRNA lacking 2′-O methylation and selectively restrict propagation. Plants, which lack an IFN response network or Ifit family member orthologues, and their viruses, accordingly lack 2′-O-methylation of mRNA. Given that host 2′-O methylation of cellular mRNA largely occurs in the nucleus, pharmacological strategies that disrupt cytoplasmic 2′-O MTase activity could represent a novel class of therapy against several globally relevant pathogenic viruses that replicate exclusively in the cytoplasm.
Methods Summary
Viruses
WNV-WT and WNV-E218A were propagated in BHK21 cells as described8. VACV-WT and VACV-J3-K175R22 (a gift from R. Condit) and encephalomyocarditis virus (EMCV) (strain K) were propagated in HeLa and L929 cells, respectively. Generation of MHV-WT (strain A59) and MHV-D130A recombinant coronaviruses has been described25.
Mouse experiments
C57BL/6 wild-type and immunodeficient (Ifnar1−/−, Ifit1−/−, Ifit2−/−, Irf3−/−, Irf7−/−, Irf3−/− × Irf7−/− and IPS-1−/−) mice were bred at Washington University. Infection experiments were performed with approval of the Washington University and St Louis University Animal Studies Committees. Viral titres in blood and organs were quantified as previously described11.
Cell culture and viral infection
Bone-marrow-derived Mφ and MEF were generated as described11. 3T3 fibroblasts expressing GFP or ISG were previously described18. Cells were infected with WNV, VACV, MHV or EMCV at MOIs of 0.01, 1, 1 and 0.001, respectively. Lysates or supernatants were titred by plaque assay on BHK21-15 cells for WNV and EMCV, BSC-1 cells for VACV and L929 cells for MHV.
Quantification of IFN-β mRNA
Ifnar1−/− MEFs were infected at an MOI of 10 with WNV-WT or WNV-E218A. Total RNA was isolated, treated with DNase (Qiagen), and IFN-β mRNAs were amplified by quantitative reverse transcriptase PCR as described previously11.
IFN-β pretreatment experiment
IPS-1−/− MEFs were pretreated with increasing doses of mouse IFN-β (PBL Laboratories) for 24 h and then infected with WNV or MHV at an MOI of 0.1. Supernatants were collected at 48 or 12 h after infection, respectively, and titred by plaque assay.
Strand-specific real-time reverse transcriptase PCR
Quantification of positive- and negative-strand WNV RNA was performed using a T7-tagged primer strategy9. Fibroblasts expressing GFP or mouse IFIT-2 were infected with WNV-WT or WNV-E218A at an MOI of 1 and total RNA was collected at indicated time points.
Supplementary information
This file contains Supplementary Figures 1-8 with legends, Supplementary Methods and additional references. (PDF 2375 kb)
Acknowledgements
This work was supported by National Institutes of Health grants U54 AI081680, U19 AI083019 and R01 AI074973 (to M.G. and M.S.D.), R01 AI56540 (to S.S.), U54 AI057160 (to R.M.B.) and U54 AI057158 (to P.Y.S.), R01 CA068782 (to G.C.S.) the Swiss National Science Foundation, 3100A0-118425/1, and the Novartis Foundation (to V.T. and R.Z.). We thank H. Virgin and B. Moss for reading the manuscript.
PowerPoint slides
Author Contributions
S.D., K.J.S., R.M.B., T.C.P., M.G., P.-Y.S. and M.S.D. designed the experiments. S.D., K.J.S., J.S., J.L., T.-Y.L. and H.D. performed the experiments. S.D., K.J.S., J.S., R.M.B., M.G., P.-Y.S. and M.S.D. analysed the data. S.Y., V.F., G.C.S., W.B.K., R.Z. and V.T. provided key reagents and expertise. S.D., K.J.S., R.M.B, T.C.P., M.G., P.-Y.S. and M.S.D. wrote and edited the manuscript.
Competing interests
The authors declare no competing financial interests.
Footnotes
Stephane Daffis and Kristy J. Szretter: These authors contributed equally to this work.
References
- 1.Furuichi Y, Shatkin AJ. Viral and cellular mRNA capping: past and prospects. Adv. Virus Res. 2000;55:135–184. doi: 10.1016/S0065-3527(00)55003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei CM, Moss B. Methylated nucleotides block 5′-terminus of vaccinia virus messenger RNA. Proc. Natl Acad. Sci. USA. 1975;72:318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell. 1975;4:379–386. doi: 10.1016/0092-8674(75)90158-0. [DOI] [PubMed] [Google Scholar]
- 4.Muthukrishnan S, Moss B, Cooper JA, Maxwell ES. Influence of 5′-terminal cap structure on the initiation of translation of vaccinia virus mRNA. J. Biol. Chem. 1978;253:1710–1715. [PubMed] [Google Scholar]
- 5.Langberg SR, Moss B. Post-transcriptional modifications of mRNA. Purification and characterization of cap I and cap II RNA (nucleoside-2′-)-methyltransferases from HeLa cells. J. Biol. Chem. 1981;256:10054–10060. [PubMed] [Google Scholar]
- 6.Fechter P, Brownlee GG. Recognition of mRNA cap structures by viral and cellular proteins. J. Gen. Virol. 2005;86:1239–1249. doi: 10.1099/vir.0.80755-0. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Zhang B, Shi PY. Flavivirus methyltransferase: a novel antiviral target. Antiviral Res. 2008;80:1–10. doi: 10.1016/j.antiviral.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, et al. Structure and function of flavivirus NS5 methyltransferase. J. Virol. 2007;81:3891–3903. doi: 10.1128/JVI.02704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel MA, Diamond MS. Alpha/beta IFN protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daffis S, et al. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J. Virol. 2008;82:8465–8475. doi: 10.1128/JVI.00918-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daffis S, Suthar MS, Szretter KJ, Gale M, Jr, Diamond MS. Induction of IFN-β and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog. 2009;5:e1000607. doi: 10.1371/journal.ppat.1000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J. Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl Acad. Sci. USA. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wacher C, et al. Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses. J. Virol. 2007;81:860–871. doi: 10.1128/JVI.01167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daffis S, Samuel MA, Keller BC, Gale M, Jr, Diamond MS. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and -independent mechanisms. PLoS Pathog. 2007;3:e106. doi: 10.1371/journal.ppat.0030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fensterl V, White CL, Yamashita M, Sen GC. Novel characteristics of the function and induction of murine p56 family proteins. J. Virol. 2008;82:11045–11053. doi: 10.1128/JVI.01593-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumpter R, Jr, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 2005;79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Burke CW, Ryman KD, Klimstra WB. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 2007;81:11246–11255. doi: 10.1128/JVI.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terenzi F, Hui DJ, Merrick WC, Sen GC. Distinct induction patterns and functions of two closely related interferon-inducible human genes, ISG54 and ISG56. J. Biol. Chem. 2006;281:34064–34071. doi: 10.1074/jbc.M605771200. [DOI] [PubMed] [Google Scholar]
- 20.Hui DJ, Terenzi F, Merrick WC, Sen GC. Mouse p56 blocks a distinct function of eukaryotic initiation factor 3 in translation initiation. J. Biol. Chem. 2005;280:3433–3440. doi: 10.1074/jbc.M406700200. [DOI] [PubMed] [Google Scholar]
- 21.Sarkar SN, Sen GC. Novel functions of proteins encoded by viral stress-inducible genes. Pharmacol. Ther. 2004;103:245–259. doi: 10.1016/j.pharmthera.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Latner DR, Thompson JM, Gershon PD, Storrs C, Condit RC. The positive transcription elongation factor activity of the vaccinia virus J3 protein is independent from its (nucleoside-2′-O-) methyltransferase and poly(A) polymerase stimulatory functions. Virology. 2002;301:64–80. doi: 10.1006/viro.2002.1538. [DOI] [PubMed] [Google Scholar]
- 23.Decroly E, et al. Coronavirus nonstructural protein 16 is a cap-0 binding enzyme possessing (nucleoside-2′O)-methyltransferase activity. J. Virol. 2008;82:8071–8084. doi: 10.1128/JVI.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui DJ, Bhasker CR, Merrick WC, Sen GC. Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2.GTP.Met-tRNAi. J. Biol. Chem. 2003;278:39477–39482. doi: 10.1074/jbc.M305038200. [DOI] [PubMed] [Google Scholar]
- 25.Coley SE, et al. Recombinant mouse hepatitis virus strain A59 from cloned, full-length cDNA replicates to high titers in vitro and is fully pathogenic in vivo. J. Virol. 2005;79:3097–3106. doi: 10.1128/JVI.79.5.3097-3106.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Figures 1-8 with legends, Supplementary Methods and additional references. (PDF 2375 kb)


