Abstract
The three mammalian bombesin (Bn) receptors (gastrin-releasing peptide [GRP] receptor, neuromedin B [NMB] receptor, BRS-3) are one of the classes of G protein-coupled receptors that are most frequently over-express/ectopically expressed by common, important malignancies. Because of the clinical success of somatostatin receptor-mediated imaging and cytotoxicity with neuroendocrine tumors, there is now increasing interest in pursuing a similar approach with Bn receptors. In the last few years then have been more than 200 studies in this area. In the present paper, the in vitro and in vivo results, as well as results of human studies from many of these studies are reviewed and the current state of Bn receptor-mediated imaging or cytotoxicity is discussed. Both Bn receptor-mediated imaging studies as well as Bn receptor-mediated tumoral cytotoxic studies using radioactive and non-radioactive Bn-based ligands are covered.
Keywords: bombesin, gastrin-releasing peptide, neuromedin B, BRS-3, receptor-mediated imaging, tumor cytotoxicity, DOTA, DTPA, NOTA
I. Bombesin (Bn) receptor family-General (Table 1,2)
Table 1.
Abbreviations.
| (NαHis)Ac | = | Nα-histidinyl acetyl |
| Aba | = | γ-aminobutyric acid |
| Ac | = | acetyl |
| Aca | = | aminohexanoic acid |
| ACMpip | = | 4-aminocarboxymethylpiperidine |
| Acp | = | 1-aminoethy-l,4-carboxymethylpiperazine |
| ADS | = | amino-3-oxapentyl-succinamic acid |
| Ado | = | 12-aminododecanoic acid |
| Ahx | = | 6-aminohexanoic acid |
| AM2BA | = | p-aminomethylbenzoic acid |
| AMBA | = | Aminobenzoyl |
| Aoc | = | aminooctanoic acid |
| AOS | = | amino-3,6-dioxaoctyl-succinamic acid |
| 11-Aun | = | 11-aminoundecanoic acid |
| Ava | = | 5-aminopentanoic acid |
| βAla | = | Beta-Alanine |
| Bomproamide | = | [DPhe6,Leu-NHEt13,des-Met14]Bn(6-14) |
| Bn | = | Bombesin |
| BRS-3 | = | Bombesin receptor subtype 3 |
| Bzdig | = | p-aminobenzyldiglycolic acid |
| BZH3 | = | [DTyr6,βAla11,Thi13,Nle14]Bn(6-14) |
| CB-TE2A | = | 1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diacetic acid |
| Cha | = | cyclohexylalanine |
| CNS | = | Central nervous system |
| CT | = | Computed tomography |
| DADT | = | diaminedithiol |
| DPhe | = | D-phenylalanine |
| Demobesin 1 | = | [(N4-bzlg)0,DPhe6,LeuNHEt13,desMet14]Bn(6–14)] |
| Demobesin 3 | = | [N40,Pro1,Tyr4]Bn |
| Demobesin 4 | = | [N40,Pro1,Tyr4,Nle14]Bn |
| Demobesin 5 | = | [(N4Bzdig)0]Bn(7–14) |
| Demobesin 6 | = | [(N4Bzdig)0,Nle14]Bn(7–14) |
| Des-Met | = | Methionine removed |
| Desmosin 1 | = | [N40,DPhe6,LeuNHEt13,desMet14]Bn(6-14) |
| Desmosin 4 | = | [N40,Pro1,Tyr4,Nle14]Bn(6-14) |
| DMTA | = | 2-(N,N″-bis(tert-butoxycarbonyl)diethylenetriamine)acetic acid |
| DO3A | = | 1,4,7-tris(carboxymethyl)10-(aminoethyl)-1,4,7,10-tetraazacyclododecaneOH |
| DOTA | = | 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraaceticacid |
| Dpr | = | 1,2-diaminopropionic acid |
| DPR | = | 2,3-diaminopropionic acid |
| DTPA | = | 2-[bis[2-[bis(carboxymethyl)amino]ethyl]amino]acetic |
| EDDA | = | diorthohydroxyphenyl acetic acid |
| FA01010 | = | (4R,5S)-4-amino-5-methylheptanoic acid |
| FB | = | fluorobenzoato |
| GABA | = | γ-aminobutyric acid |
| GEG | = | Glycine-Glutammate-Glycine |
| GI | = | Gastrointestinal |
| GRP | = | Gastrin-releasing peptide |
| GRPR | = | Gastrin-releasing peptide receptor |
| GSS | = | Glycine-Serine-Serine |
| GSG | = | Glycine-Serine-Glycine |
| GGG | = | Glycine-Glycine - Glycine |
| HSA | = | Human serum albumin |
| HYNIC | = | 6-hydrazinonicotinic acid |
| Lys(Acm) | = | Amadori-Product |
| Lys(sha) | = | Lysine-coupled shikimic acid |
| Mac | = | mercaptoacetic acid |
| MAG3 | = | mercaptoacetyltriglycine |
| MeGly | = | Methylglicine |
| Me2Gly | = | Dimethylglicine |
| mIP | = | meta-phenylalanine |
| MP2248 | = | DPTA-[Pro1,Tyr4]Bn(1-14) |
| MP2346 | = | DOTA-[Pro1,Tyr4]Bn(1-14) |
| MP2653 | = | [ACMpip5,Tha6,βAla11,Tha13,Nle14]Bn(5–14) |
| MRI | = | Magnetic resonance imaging |
| MTT | = | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| N2S2 | = | Cys(Acm)-Gly-Cys(Acm) |
| N3S | = | dimethylglycyl-L-seryl-L-cysteinglycinamide |
| N4 | = | tetramine |
| Nle | = | Norleucine |
| NMB | = | Neuromedin B |
| NMBR | = | Neuromedin B receptor |
| NOTA | = | 1,4,7-triazacyclononanetriacetic acid |
| NO2A | = | 1,4,7-triazacyclononane-1,4-diacetate |
| NS3 | = | 2′,2″,2‴-nitrotriethanethiol |
| NTG | = | triazole-couple glucose |
| PADA | = | [pyridin-2-yl-methyl-amino]-diacetic acid |
| PBS | = | Phosphate buffered saline |
| PEG | = | ethylene glycol [2-aminoethylcarboxymethylether] |
| PEG2 | = | (2-aminoethyl)-carboxymethyl ether |
| PEG3 | = | 11-amino-3,6,9-trioxaundecanoic acid |
| PEG4 | = | 15-amino-4,7,10,13-tetraoxapentadecanoic acid |
| PET | = | Positron emission tomography |
| PNP6 | = | N,N-bis[2-(bis(3-ethoxypropyl)phosphino)ethyl]ethoxyethylamine |
| Pra | = | Propargylglycine |
| PZ1 | = | pyrazolyl |
| RGD | = | RGDyK (Arginine-Glycine-Aspartic Acid-Lysine) |
| RM1 | = | H-DPhe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2 |
| RP527 | = | N3S-5-Ava-Bn(7-14) |
| SPECT | = | Single photon emission computed tomography |
| SRS | = | Somatostatin receptor scintigraphy |
| Sta | = | Statine: (3S,4S)-4-amino-3-hydroxy-6-methylheptanoic acid |
| TACN | = | 2-[4,7-bis(2-pyridylmethyl)-1,4,7-triazacyclononan-1-yl]acetic acid |
| Tat (49-57) | = | Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg (HIV-peptide) |
| Tha | = | β-(2-thienyl)alanine |
| Thi | = | 3-(2-thienyl)alanine |
| TPPS | = | trisodium triphenylphosphine-3,3′,3″-trisulfonate |
| Tricine | = | N-(2-Hydroxy-1,1-bis(hydroxymethyl)ethyl)glycine |
| Z-070 | = | DOTA-PEG40[DTyr6,βAla11,Thi13,Nle14]Bn(6–14) |
Table 2.
Structure of Bn-related peptides used in various imaging studies(a)
| Peptide | Structure (Position relative to Bn) | Ag/Ant(b) | RM # | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N° | Name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| 1 | Bombesin (Bn) | Pyr | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [119,164–166] |
| 2 | GRP (13-27) | Tyr | Pro | Arg | Leu | Gly | Asn | His | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [48] |
| 3 | Litorin [pGlu6, Phe13]Bn(6-14) | pGlu | Gln | Trp | Ala | Val | Gly | His | Phe | Met-NH2 | Ag | [71] | |||||
| 4 | Demobesin 3 [N40,Pro1,Tyr4]Bn | N4 -Pro | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [70] |
| 5 | Demobesin 4 [N40,Pro1,Tyr4,Nle14]Bn | N4 -Pro | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Nle-NH2 | Ag | [70] |
| 6 | [Lys3]Bn | Pyr | Gln | Lys | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [52,54,85,86,98,99,106,129,167] |
| 7 | [Tyr4]Bn | Pyr | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [48,76,102,119] |
| 8 | [εLys3,Tyr4]Bn | Pyr | Gln | εLys | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [76] |
| 9 | [Gln1,Tyr4]Bn | Gln | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [168] |
| 10 | [Gly1]Bn | Gly | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [169] |
| 11 | [Pro1,Tyr4]Bn (MP2346) | Pro | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [2,48,74,76,168] |
| 12 | [Pro1,Tyr4,Nle14]Bn | Pro | Gln | Arg | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Nle-NH2 | Ag | [45,48] |
| 13 | [Cys0,Aca1]Bn(2-14) | Cys-Aca | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [122,124,126,127,170] |
| 14 | Bn(2-14) | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [55,106,107,171] | |
| 15 | [Lys14]Bn(2-14) | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Lys-NH2 | Ag | [172] | |
| 16 | [Lys3,Tyr4]Bn(2-14) | Gln | Lys | Tyr | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [80] | |
| 17 | [mIP]Bn(2-14) | mIP | Gln | Arg | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [119] |
| 18 | Bn(4-14) | Leu | Gly | Asn | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [106] | |||
| 19 | [Ser4,5,6]Bn(4-14) | Ser | Ser | Ser | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [57] | |||
| 20 | [ACMpip5,Tha6,βAla11, Tha13,Nle14]Bn(5–14) (MP2653) | ACMpip | Tha | Gln | Trp | Ala | Val | βAla | His | Tha | Nle-NH2 | Ag | [2,87] | ||||
| 21 | [Tyr5,DPhe6,ψPhe14]] Bn(5-14) | Tyr | DPhe | Gln | Trp | Ala | Val | Gly | His | Leuψ (c) | Phe-NH2 | Ant | [48] | ||||
| 22 | [DPhe6,Leu13ψPhe14] Bn(6-14) | DPhe | Gln | Trp | Ala | Val | Gly | His | Leuψ | Phe-NH2 | Ant | [48] | |||||
| 23 | [DPhe6,Leu-NHEt13,des-Met14] Bn(6-14)(d) | DPhe | Gln | Trp | Ala | Val | Gly | His | Leu-NHEt (e) | Ant | [47] | ||||||
| 24 | [DTyr6,βAla11,Thi13,Nle14] Bn(6-14) | DTyr | Gln | Trp | Ala | Val | βAla | His | Thi | Nle-NH2 | Ag | [50,79,104,130,173] | |||||
| 25 | Demobesin 1 [N40–1,bzdig0,DPhe6,Leu-NHEt13,des-Met14]Bn(6–13) | N4-bzdig | DPhe | Gln | Trp | Ala | Val | Gly | His | Leu-NHEt | Ant | [45,174] | |||||
| 26 | RM1 [N40DPhe6,Sta13,Leu14] Bn(6-14) | N4-DPhe | Gln | Trp | Ala | Val | Gly | His | Sta | Leu-NH2 | Ant | [46,49] | |||||
| 27 | [Lys6]Bn(6-14) | Lys | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [168] | |||||
| 28 | Bn(7-14) | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH2 | Ag | [2,18,46,56–62,65,66,72,73,83,84,84,86,88,91,99–101,105,108–113,116,117,121,128,168,175–183] | ||||||
| 29 | Demobesin 5 [(N4Bzdig)0]Bn(7–14) | N4-bzdig | Gln | Trp | Ala | Val | Gly | His | Leu | Met-NH11 | Ag | [70] | |||||
| 30 | Demobesin 6 [(N4Bzdig)0,Nle14] Bn(7–14) | N4-bzdig | Gln | Trp | Ala | Val | Gly | His | Leu | Nle-NH2 | Ag | [70] | |||||
| 31 | [Cha13,Nle14]Bn(7-14) | Gln | Trp | Ala | Val | Gly | His | Cha | Nle-NH2 | Ant | [66,67,67,69,93,94,176,184] | ||||||
| 32 | [Cha13]Bn(7-14) | Gln | Trp | Ala | Val | Gly | His | Cha | Met-NH2 | Ant | [66,176] | ||||||
| 33 | [Nle14]Bn(7-14) | Gln | Trp | Ala | Val | Gly | His | Leu | Nle-NH2 | Ag | [66,176] | ||||||
| 34 | [NMeGly11,Sta13,Leu14] Bn(7-14) | Gln | Trp | Ala | Val | NMeGly | His | Sta | Leu-NH2 | Ant | [51,102] | ||||||
| 35 | [FA0101013,Leu14] Bn(7-14) | Gln | Trp | Ala | Val | Gly | His | FA01010 | Leu-NH2 | ND(f) | [102] | ||||||
| 36 | [βAla11, Phe13,Nle14]Bn(7-14) | Gln | Trp | Ala | Val | βAla | His | Phe | Nle-NH2 | Ag | [77] | ||||||
| 37 | [His(3Me)11,Sta13,Leu14]Bn(7-14) | Gln | Trp | Ala | Val | His(3Me) | His | Sta | Leu-NH2 | Ant | [51] | ||||||
| 38 | [des-Met14] Bn(7-14)NH2 | Gln | Trp | Ala | Val | Gly | His | Leu-NH2 | Ant | [118,120] | |||||||
| 39 | [DTyr6,des-Met14] Bn(6-13)NHEt | DTyr | Gln | Trp | Ala | Val | Gly | His | Leu-NHEt | Ant | [48] | ||||||
| 40 | [Tyr5,DPhe6] Bn(5-13)NHEt | Tyr | DPhe | Gln | Trp | Ala | Val | Gly | His | Leu-NHEt | Ant | [50] | |||||
| 41 | RC-3095 [D-Tpi6,Leu13, ψLeu14]Bn(6-14)(g) | D-Tpi | Gln | Trp | Ala | Val | Gly | His | Leuψ (c) | Leu-NH2 | Ant | [142,155] | |||||
All the abbreviations are listed in Table 1;
Aminoacid variations compared to Bn sequence are bold;
Ag: agonist; Ant: antagonist;
ψindicates a reduced peptide bond (-CH2NH- instead of –CONH-);
des-Met indicates the deletion of the Bn 14th aminoacid, Methionine;
NHEt, Et=ethyl;
ND: no data.
Tpi: 2,3,4,9-tetrahydro-1H-pyridol[3,4-b]indol-3-carboxylic acid
The mammalian Bn receptor family is receiving increased attention as a means of localizing tumors or other disease processes by receptor-mediated imaging or for receptor-mediated cytotoxicity of tumors [1–5]. This family got its unusual name, because the original members of this peptide family were isolated from various frog skins and were named after the frog they were isolated from, with the original amidated tetradecapeptide isolated from the European frog, Bombina bombina in 1970 [6–8] (Table 2). Subsequently, a large number of related peptides were isolated which were divided into three groups: the Bn-related peptides with a COOH terminal, Gly-His-Leu-Met-NH2, the ranatesin-litorin group with a COOH terminus of Gly-His-Phe-Met-NH2 and the phyllolitorin group with a COOH terminus ending in Gly-Ser-Phe/Leu-Met-NH2 (Table 2) [6–8]. Subsequently two mammalian equivalent peptides were isolated, gastrin-releasing peptide (GRP), a 27 amino acid peptide which shares the same seven COOH terminal amino acids with Bn (Table 2)[9] and the decapeptide, neuromedin B (NMB) (Tables 1,2) which shares 6 of the 7 COOH terminal amino acids with litorin (Table 2)[10]. Each of these peptides is widely distributed in both the central nervous system (CNS) and peripheral tissues, especially in the gastrointestinal (GI) tract [8]. Numerous studies demonstrate these two peptides are involved in a wide range of physiological and pathophysiological processes which include: in the CNS (circadian rhythm, TSH release, behavior control, thermoregulation, satiety), in the immune system [effects on macrophages, lymphocytes, leukocytes, dendritic cells], endocrine effects [release of numerous hormones/neurotransmitters], GI tract [motility, secretion, growth], as well as urogenital tract and respiratory system [8,11–13]. They have important pathophysiological effect on growth and differentiation of a number of important human tumors [colon, prostate, lung, head/neck squamous cell, CNS, pancreatic and some gynecologic cancers] and in some cases function as autocrine growth factors [5,11,14,15]. In mammals, the Bn receptor family consists of three hepata-helical, G-protein-coupled receptors, which include the 384 amino acid gastrin-releasing peptide receptor (GRPR), which has 55% amino acid identities to the 390 amino acid neuromedin B receptor (NMBR), and a 399 amino acid orphan receptor, bombesin receptor subtype 3 (BRS-3) [8]. The BRS-3 receptor is included in the mammalian Bn receptor family because it has 47–52% homology to the GRPR and NMBR even though its natural ligand is still unknown [5,8,15]. The BRS-3 has a more limited distribution than the GRPR and NMBR, but is found in both the CNS and peripheral tissues, especially the GI tract [8]. Each of these receptors is coupled to phospholipase C signaling cascades as well as activates a number of tyrosine kinase cascades [5,8,13,15].
II. Why there is special interest in Bombesin (Bn) receptor family-mediated imaging/cytotoxicity
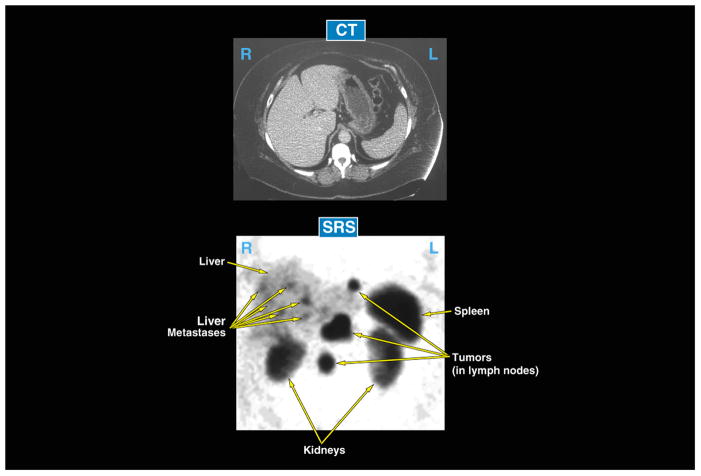
The presence of bombesin receptors (Bn) receptors on tumor tissues is receiving increased attention, both for its possible utilization to image tumors as well as to target cytotoxic agents either using radiolabeled Bn analogues or other cytotoxic agents formed by coupling various Bn receptor ligands by with various linkers to various cytotoxic agents[1–5,16–18](Fig. 1, Table 1). While this receptor-mediated targeting approach is being used with many regulatory peptides [1–5,17,18], there is particularly interest with this receptor family for a number of reasons. First, the Bn receptor family of receptors, particularly GRPR, has been shown to be one of most over-expressed or ectopically expressed family of G protein-coupled receptors by small lung cancer cells [GRPR - 85–100%, NMBR-55%, BRS-3–25%]; nonsmall cell lung cancer [GRPR – 74–78%, NMBR-67%, BRS-3–8%]; pancreatic cancer [GRPR - 75%, NMBR-100%]; prostate cancer [GRPR – 60–100%, 0%-NMBR, BRS-3]; head/neck squamous cell cancers [GRPR - 100%]; glioblastomas [GRPR - 85%]; neuroblastomas [GRPR-72% NMBR - 46%, 0% BRS-3]; breast cancer [GRPR – 40–70%, NMBR-0%, BRS-3]; intestinal carcinoids [NMPR - 46%, 0%-GRPR, BRS-3]; and bronchial carcinoids [35%-BRS-3, 4%-NMBR, GRPR -0%][11,16,19,21]. Many of these malignancies have a poor prognosis with advanced disease, current treatments are suboptimal, and therefore there is heightened interest in developing newer, novel treatments, of which the utilization of the over-expression/ectopic expression of this family of receptors could be one useful approach. Second, this approach has proven merit. In the case of somatostatin receptors, receptor-mediated imaging and cytotoxicity has been shown to be safe, clinically useful and is now being widely used in clinical practice [22,23]. In the case of neuroendocrine tumors (carcinoids, pancreatic endocrine tumors), in most studies the majority (>80%) over-express or ectopically express one or more of the five classes of G protein-coupled somatostatin receptors (sst1–5), usually the sst2 subtype [17,22–25], in an analogous fashion to the tumors listed above, over-expressing one of the Bn receptor family. The use of 111In-penetreotide for somatostatin receptor scintigraphy (SRS) is now a standard clinical method to image these tumors [26–28]. Studies have shown SRS is more sensitive than conventional methods used for neuroendocrine tumor localization (computed tomographic scanning, MRI scanning ultrasound) of the primary tumor and metastatic disease [26–29]. Figure 2 shows an example of its sensitivity and usefulness in a typical patient with a neuroendocrine tumor. In this patient the CT scan was negative however, the SRS showed tumor presence in the liver and lymph nodes. This figure illustrates the selectivity and sensitivity of using somatostatin receptor over-expression to target these tumors [26–29]. A similar strategy is now being used to target tumoricidal doses of radiolabeled somatostatin analogs (90Y, 177Lu, 111In-labeled) to treat patients with advanced malignant neuroendocrine tumors [22,23]. Such a strategy could also be used to target nonradioactive cytotoxic agents (i.e. chemotherapeutic agents, toxins, immunological agents, etc) to tumor cells [30–34]. Unfortunately, many of the common lethal tumors do not over-express somatostatin receptors, as occurs in the neuroendocrine tumors. Therefore if receptor-mediated imaging or cytotoxicity is going to be used for these tumors, some other family of receptors needs to be considered. As discussed above, the Bn family of receptors could fulfill this requirement for a number of these tumors [1–5,16–18]. Third, Bn-related peptides also function as potent growth factors, sometimes in an autocrine fashion, for many common malignant tumors including those of lung, pancreas, head/neck, CNS (glioblastomas), kidney, prostate, breast, colon/rectum, ovary and stomach [11,21,35,36]. This raises the possibility that receptor antagonists of Bn receptors may have cytotoxic effects for a number of theses tumors, as well as raises the possibility that targeting Bn receptors on these tumor cells may have additional cytotoxic effects by interrupting this autocrine stimulatory effect. Four, although there are no effective nonpeptide antagonists or agonists for GRPR or NMBR, which are primarily over-expressed by tumors, the pharmacology of these receptors has been well studied, especially in nonhuman cells. Both selective agonists and at least eleven chemical classes of antagonists, with varying degrees of selectivity, have been described [8,14,37]. Therefore, pharmacological, both agonists and antagonists exist that can be used for Bn receptor targeting strategies.
Fig. 1.
Chemical structure of common linkers used in studies to couple Bombesin (Bn) analogs to various radioisotopes for Bn-receptor-mediated imaging or cytotoxicity. For listing of abbreviations see Table 1.
Fig. 2.
Example of usefulness of receptor-mediated imaging for targeting and imaging tumors. The upper panel shows a computed tomographic scan (CT). The lower panel shows the abdominal nuclear medicine images (SPECT image) (from a patient with metastatic neuroendocrine tumor taken 24 hours after injection of 6 mCi of [111In-DTPA,DPhe1]octreotide, to image over-expression of somatostatin receptors on the tumor. In this patient the CT scan was negative, whereas the somatostatin receptor scan was positive for tumor in a number of lymph nodes and the liver. This illustrates the higher sensitivity of somatostatin receptor imaging than conventional imaging (CT, MRI), the precise targeting to the tumor and the clinical usefulness of such an approach.
III. Bn receptor-mediated imaging/cytotoxicity. Review of studies and current status
III. A. General (Fig. 1, Tables 1–12)
Table 12.
Studies with Bn analogs conjugated to non-radioactive cytotoxic agents.
| In vitro | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Drug | Linker | Bn Analog | Cell used | Binding affinity IC50 (nM) | Stability | Amnt of Rcptr Int | Cytotoxicity | Ref. N |
| 1 | Camptothecin | N-(N-Me-aminoethyl)-glycine carbamate | [DTyr6, β Ala11, Phe13, Nle14)Bn(6–14)] | Balb 3T3, NCI-H1299, MOLT-4, HT-29, PC-3, NCI-H69, SKNSH | Ki: GRPR=0.012±0.001; NMBR= 0.035±0.003; BRS-3=0.031±0.008. | 20 min half life in mouse plasma | 33±2% (1h) | Reduced cell growth depends on the cell expression of GRPR, NMBR or BRS-3. Tumoricidal IC50 from 90 to 1923 nM. In NCI-H1299, CFPAC-1 or PC-3 xenografted nude mice reduced tumor growth 55–84%. | [30,34] |
| 2 | Camptothecin | [N-(N-methyl-amino-ethyl)-glycine carbamate | [DSer5, DTyr6, β Ala11, Phe13, NLe14]Bn(5–14) | HUVECs, PC-3, MCF-7, H29, SKNSH | IC50 for cytotoxicity: PC-3=429.85 nM, MCF-7: 1.70 μM, NCI-H69= 2.71 μM, H29= 2269 nM, SKNSH= 1610 nM | Bn conjugated in PC-3 inhibits adhesion to collagen type I, αVβ3 and αVβ5, dose 10–20 μM. In HUVECs inhibits capillary-like tube formation and in vivo angiogenesis, dose 10–20 μM and 40 μM, respectively. | [136] | ||
| 3 | Paclitaxel | PEG | Bn(7–13) | H1299 | 24h incubation=14±1.1 nM, 96h=6±0.9 nM | T1/2 in PBS: 154 min, t1/2 in human plasma 113 min | Conjugated tumoricidal IC50 is 2.5-fold lower than with Paclitaxel alone | [144] | |
| 4 | Glu and PEG | Bn(6–14) | MO59J, JNPRSLT1, Hutu-80, FADU, SKNAS | Addition of PEG as linker produces an increase in the solubility but a decrease in the cytotoxicity. The best cytotoxicity results were obtained with Paclitaxel-Glu-(Bn(6–14)2): 64–93% | [145] | ||||
| 5 | 2-Pyrrolino-DOX | Glutaric acid | [DTpi6, Leu13, ψiLeu14]Bn(6–14) (Antagonist) and 15 other [Leu13, ψiLeu14]Bn(6–14) analogs | CFPAC-1, DMS-53, PC-3 and MKN-45 cells | 2-pyrrolino-DOX-14-O-glt-[13ψ14, CH2-NH, Leu14]Bn(6–14) Ki: 1.6 in Bn/GRP Swiss 3T3 cells | Tumoricidal IC50 of 2-pyrrolino-DOX-14-O-glt-[13ψ14, CH2-NH, Leu14]Bn(6–14) ranges from 0.4 to 6.8 nM in cell tested, and 2-Pyrrolino-DOX from 0.22–3.6 nM | [141] | ||
| 6 | Hemiasterlin | ALALAEGEGEG | [DPhe6, βAla11, Phe13, Nle14)Bn(6–14) | NCI-H1299 | 15±2 | From 0.1–1 μM inhibited proliferation in a dose-related manner. | [33] | ||
| ALALANG | 25±3 | ||||||||
| Dolastatin | LALAEGEGEG | 150±18 | No cytotoxic activity in NCI-H1299 cells. | ||||||
| G | 20±2 | ||||||||
| LALAG | 15±1 | ||||||||
| 7 | KLAKLAKKLAKLAKGG (KLA) | Bn(2–14) | Raji, NB4, CEM, K562, Molt4 and Jurkat | Tumoricidal IC50 in μM range in all tumor cell line, from solid tumors or leukemia. In vivo K562 xenografted BALB/C nude mice treatment with each Bn conjugated produce a reduction in tumor volume. | [151] | ||||
| GRFKRFRKKFKKLFKKLS (B27) | |||||||||
| GGLRSLGRKILRAWKKYG (B28) | |||||||||
| 8 | DAB389 | GRP | AR42J, HuTu 80 | GRP conjugated peptide inhibited protein synthesis in cell lines expressing GRPR or NMBR. | [150] | ||||
| 9 | OKT3 (anti-CD3 antibody) | SPDP | [Cys5, DPhe6, Leu-NHEt13, des-Met14] Bn(5-14) (Antagonist) | NCI-H345, DMS273 | Specific binding of Bn conjugated to NCI-H345 and DMS273 | Specific and dose dependent inhibition of SCLC growth by Bn conjugate, increasing apoptosis by cleavage of caspase-3, -9 and PARP. In vivo DMS276 xenografted mice treated with the Bn conjugated showed a reduction of tumor size. | [152] | ||
| 10 | FcγT | SATA/Sulfo-SMCC | [Lys3]Bn | NCI-H69, NCI-H345, SHP-77, DMS273 | NCI-H69 binds 5036 immnuconjugates/cell, NCI-H345 binds 6116, SHP-77 binds 2399 and DMS273 to 9473 5–50 μg/ml FcγT-[Lys3]Bn =50–85% positive cells | The amount of compound internalized remain inside the cells for 4 h | Co-culture of tumor cells line with activated monocytes and immunoconjugate produces >80% of cell tumor lysis, and 75% with neutrophils and SHP-77 cells. | [154] | |
| 11 | FcγT or FcγTII | SATA/SMCC | [DTrp6, Leu13-ψ(CH2NH)Phe14]Bn(6–14) (Antagonist) | NCI-H69, NCI-H345, SHP-77, DMS273 | Both immnunoconjugates binds in a dose related manner to the SCLC cells, 50–85% positive cells | Bn agonist immunoconjugated has no effect on clonogenic growth of SCCL cells. Both (agonist and antagonist) immunoconjugated produced the lysis of SCLC when incubated with monocytes previously activated | [153] | ||
| 12 | Lys3]Bn | ||||||||
| 13 | [DPhe6, desMet14]Bn(6–14) | MiaPaCa-2, SW620, HT29, PTC | In vitro MTT results: in MiaPaCa-2 cell best cytotoxicity with analog 17 at 0.1 nM, SW620 analog 18 at 0.1 nM, HT29 analog 16 at 1 μM. PTC analog 19 at 0.01 nM. In vivo PTC cell tumor xenofraft mice analog 18 produced a inhibition of tumor growth of 44.3% | [163] | |||||
| 14 | [DPhe6, Aib11, desMet14]Bn(6–14) | ||||||||
| 15 | [DPhe6, Aib9, desMet14]Bn(6–14) | ||||||||
| 16 | [DPhe6, Aib9, Ile13, desMet14]Bn(6–14) | ||||||||
| 17 | [DPhe6, Aib11, Ile13, desMet14]Bn(6–14) | ||||||||
| 18 | [DPhe6, Aib9, Aib11, Ile13, desMet14]Bn(6–14) | ||||||||
| 19 | Butanoyl[DPhe6, Aib11, desMet14]Bn(6–14) (All antagonists) | ||||||||
| 20 | Lys-Lys between peptides | SS analog-Substance P antagonist-VIP receptor binding inhibitor-Bn antagonist | MOLT-4, MCF-7, MiaPaCa-2, KB, PTC | EC50 (μM) for MTT
assay: MOLT-4=0.29, MCF-7=0.34,
MiaPaCa-2=0.21, KB=2.1, PTC>10. In vivo experiments with PTC tumor bearing mice showed a 73.7% tumor regression. |
[156] | ||||
| Colo-205, MiaPaCa-2, ECV304 | Analog 20 decreases cAMP, EGF stimulated growth and pMAPKs, also reduces p53 and Bcl-2 but increases caspase 3. It also inhibits capillary-like tube formation and secretion of VEGF in endothelial cells. | [157] | |||||||
| 21 | Mono-carbohexyl-tetrasulfonated aluminium phthalocyanine | 8-Aoc-Bn(7–14) | PC-3 | 8-Aoc-Bn(7–14)=3.73×10−10 M AlPcS4-8-Aoc-Bn(7–14)=2.94×10−8 M | AlPcS4-8-Aoc-Bn(7–14) showed higher phototoxicity than AlPcS4 alone and 2–3 fold increase photodynamic efficacy over AlPcS4 at lower doses. | [155] | |||
| 22 | Maleimide-PEG | Bn(7–14) | CHO-d1EGFP | Bn analog combined with EHCO/siRNA nanoparticles produces a high efficient cell-specific siRNA system (cell uptake=73.9%; gene silencing effiency=91.9%) | [188] | ||||
| 23 | GRP-MH20 (GRP bound to the N′ side of MH20) | HeLa, Colo 205, Swiss 3T3 ans NIH 3T3 | It showed a significant enhancement (8–15- fold) of adenovirus mediated gene transfer in the 3 cell lines. This increase is proportional to the GRPR in cell. | [158] | |||||
| 24 | MH20-GRP (GRP bound to the C′ side of MH20) | It had not activity on adenovirus infection and gene transfer. | |||||||
All peptides not indicated as antagonist are agonist at human Bn receptors.
Abbreviations: SATA= N-succinimidyl S-acetylthioacetate; Sulfo-SMCC= sulfosuccinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate; Ail= α-aminoisobutyric acid; EHCO= 1(-aminoethyl)imino-bis[N-(oleicyl-cysteinyl-histinyl-1-aminoethyl)propio-n-amide for peptide 19: Somatostain analog= D-Phe-Cys-Tyr-D-Trp-Orn-CyLeu-Pen-Thr-NH2; Substance P antagonist= D-Arg-Pro-Lys-Pro-DPhe-Gln-D-Trp-Phe-D-Trp-Leu-CyLeu-NH2 VIP receptor binding inhibitor=Leu-Met-Tyr-Pro-Thr-Tyr-Leu-Lys-OH; Bn anatagonist=[DPhe6,Aib11,desMet14]Bn(6–14); MH-20=Lys-Met-Tyr-Pro-Arg-Gly-Asn-Hys-Trp-Ala-Val-Gly-His-Leu-Met; SPDP= N-succinimidyl 3-[2-pyridyldithio] propionate. OKT3= anti-CD3 monoclonal antibody; DOX= doxorubicin. Cell lines: Rat pancreatic cancer= AR42J, mouse embryonic fibroblasts= Balb-3T3 and Swiss 3T3 and Chinese hamster ovary cell line= CHO.
Human breast cancer= MCF-7, colon cancer= Colo-205, HT29 and SW620, epidermoid carcinoma= KB, gastric cancer= MKN-45, gliobalstoma= MO59J, hypopharyngeal carcinoma=FADU, intestine carcinoma= Hutu, leukemia= CEM, Jurkat, KS62, MOLT-4 and NB4, lymphoma= Raj, neuroblastoma= SKNAS and SKNSH, pancreatic cancer= CFPAC-1 and MiaPaCa-2, papillary thyroid cancer= PTC, prostate cancer=PC-3, small cancer lung cell= DMS273, DMS-53, JNPRSLT1, NCI-H345, NCI-H69 and SHP-77, umbilical vein endothelial cell= ECV304 and HUVECS, non-small cell lung cancer cell line= NCI-H1299, human cervical adenocarcinoma cell line= HeLa
In this paper the important results of studies of Bn receptor-mediated imaging or cytotoxicity are summarized in the accompanying tables and briefly reviewed in the text. Both studies dealing with radiolabeled Bn analogs for either imaging or cytotoxic studies (Table 3–11) and studies investigating nonradioactive Bn receptor-mediated cytotoxicity are reviewed (Table 12). In the tables, results of in vitro and in vivo studies are considered in separate tables, because of the different questions frequently addressed. Furthermore, human studies are included in a separate section (Table 11). The radiolabeled Bn peptide studies (Tables 3–11) are divided by the type of isotope used. A wide range of different linkers to couple the isotopes to the Bn-related peptides were used in different studies and their structures are shown in Fig. 1. Their abbreviations as well as those of various spacers used in the different studies are summarized in Table 1.
Table 3.
In vitro studies with 99mTc bombesin analogs.
| In vitro | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Binding Affinity | |||||||||
| 99mTc N | Linker | Peptide | Cell used | IC50 | Memb bound | Stability | Amnt of Rcptr Int | Comment | Ref. N |
| 1 | DPR-βAla | Bn(7-14) | T47-D/MDA-MB-231 | 2.0/1.1 | Stable 24 h in PBS±HSA | 45 min:
80/88% 120 min: 50/69% |
PZ1 Derivatives showed higher binding affinities in vitro | [65] | |
| 2 | DPR-GGG | ||||||||
| 3 | DPR-GSG | 8.1/6.1 | |||||||
| 4 | DPR-PEG5 | ||||||||
| 5 | DPR-PEG8 | ||||||||
| 6 | DPR-Ser-Gly-Ser | 0.8/0.3 | |||||||
| 7 | DPR-Ser-Ser-Ser | 5.9/2.2 | |||||||
| 8 | PZ1-βAla | 0.7/0.3 | 45 min:
69/83% 120 min: 34/69% |
||||||
| 9 | PZ1-Gly-Gly-Gly | 6.1/2.0 | |||||||
| 10 | PZ1-Gly-Ser-Gly | ||||||||
| 11 | PZ1-PEG5 | 2.0/0.7 | |||||||
| 12 | PZ1-PEG8 | 3.2/0.3 | |||||||
| 13 | PZ1-Ser-Gly-Ser | ||||||||
| 14 | PZ1-Ser-Ser-Ser | 1.4/0.5 | |||||||
| 15 | N4-Gly-4-aminobenzoyl | [HDPhe6,Sta13,Leu 14]Bn(6-14) “RM1” [Antagonist] | GRPR binding by autoradiography on cancer sections of prostate. | 3.7±1.3 | 40% (2h) | Low internalization (10%, 2 h). Antagonist | [49] | ||
| 16 | NS3-Gly-Gly-Cys | Bn(2-14) | PC-3 | 3.0±0.7 | 17% (2h) | After 2 h stability in plasma 60%, in PC-3 30%. Kidney homogenates, after 5 min 40% intact, after 15 min 20%. Liver homogenates, after 5 min, almost totally degradated. | 78% (2 h) | Introduction of the spacer Orn-Orn-Orn compared to non spacer produced a higher stability and internalization into the cells. | [171] |
| 17 | NS3-Gly-Gly-Cys-Orn-Orn-Orn | 2.2±0.1 | 4%(2h) | After 2 h stability in plasma 60%, in PC-3 50%. Kidney homogenates, after 5 min 45% intact, after 15 min 30%. Liver homogenates, after 5 min, 60% intact. | 88% (2 h) | ||||
| 18 | Gly-Gly-Cys-Aca | BN(2-14) | PC-3 | 1.1±0..1 | In human plasma 2h: 80%. Kidney and liver homogenates: total degradation after 15 min. | 75% (30 min) | The length of the Bn sequence did not alter binding, internalization or efflux rate. | [56] | |
| 19 | BN(7-14) | 1.9±0.1 | In human plasma 2h: 35%. Kidney and liver homogenates: total degradation after 15 min. | 65% (30 min) | |||||
| 20 | (NαHis)Ac-Pra(Glu)-βAla-βAla | [Cha13,NLe14]Bn(7-14) | PC-3 | IC50:4.2±0.1, KD: 0.3±0.1 | [69] | ||||
| 21 | EDDA/HYNIC | [Lys3]Bn(1-14) | PC-3/MCF7/MDA-MB231 | 17.6±1.9(4h)/19.0±0.9(2h)/5.5±0.2(1h)/ | The hybrid Tat-Bn analog’s cell binding and internalization is higher than with the Bn analog. | [54] | |||
| 22 | N2S2 | [Tat(49-57)-Gly-Gly-Cys-Gly-[Lys3]]Bn(1-14) | PC-3/MCF7/MDA-MB231 | 65% in human serum (24h); 41% after cys challenge in molar ratio 500:1 (cys:pept) | 28.1±3.9(4h)/18.3±2.1(2h)/19.4±1.3(1h) | ||||
| 23 | Tetraamine-benzylaminodiglycolic acid | Demobes0in 1 (Antagonist) | PC-3/GRPR-HEK293/human prostate cancer | 2.1±0.5/2.4±0.5/2.6±0.2 | 25% (2h) | No internalization (Antagonist) | [45] | ||
| 24 | Demobesin 4 | 0.8±0.1/2.1±0.3/2.0±0.5 | 10% (2h) | 0.2±0.1 (30min)(Agonist) | |||||
| 25 | ≡N(PNP6)-Cys-βAla- | Bn(7-14) | PC-3 | After 4h cys challenge in molar ratio 100:1 (cys:pept): 93.6% | 15.5% (2h) | [63] | |||
| 26 | HYNIC-βAla | After 4h cys challenge in molar ratio 100:1 (cys:pept): 98.3% | 18.5% (2h) | ||||||
| 27 | (NαHis)Ac-β3hGlu-β3Glu-β3Glu | [Cha13,NLe14]Bn(7-14) | PC-3 | IC50:634±221.7 kD:nd |
t1/2 human plasma: 16h; in PC-3: 30–40min | 1% (1h) | A positive charge may favor internalization of the tricarbonyl-labeled analogues, while more than one negative charge would have an adverse influence. | [67] | |
| 28 | (NαHis)Ac-β3hGlu-β3Glu-βAla | IC50:16.3±8.3 kD:0.4±0.1 |
15% (1h) | ||||||
| 29 | (NαHis)Ac-β3hGlu-βAla-βAla | IC50:13.3±3.0 kD: 0.08±0.01 |
30% (1h) | ||||||
| 30 | (NαHis)Ac-β3hLys-βAla-βAla | IC50:23.6±12.0 kD:0.14±0.06 |
t1/2 human plasma: 16h; in PC-3: 80min | 40% (1h) | |||||
| 31 | (NαHis)Ac-β3hSer-βAla-βAla | IC50:6.8±3.2 kD:0.05±0.03 |
t1/2 human plasma: 16h; in PC-3: 30–40min | 30% (1h) | |||||
| 32 | (NαHis)Ac-βAla-βAla | IC50:5.1±1.7 kD: 0.19±0.12 |
30% (1h) | ||||||
| 33 | DTMA-βAla- | Bn(7-14) | PC-3 | 0.28±0.02 | 23.8±0.003% (2h) | βAla analog had the highest IC50 and internalization rates. | [62] | ||
| 34 | DTMA-Gly-Gly-Gly | 2.56±1.3 | 2.37±0.01% (2h) | ||||||
| 35 | DTMA-Gly-Ser-Gly | 0.68±0.3 | 6.59±0.04% (2h) | ||||||
| 36 | DTMA-Ser-Ser-Ser | 0.74±0.2 | 11.46±0.03% (2h) | ||||||
| 37 | (NαHis)Ac-βAla-βAla | [Cha13,NLe14]Bn(7-14) | PC-3 | IC50:5.1±1.7 kD:0.18±0.12 |
t1/2 in PC-3: 30±6 min | 29–37% of the total radioactivity/mg | [68] | ||
| 38 | (NαHis)Ac-Lys(sha)-βAla-βAla | IC50:6.5±1.7 kD:0.02±0.01 |
t1/2 in PC-3: 35±11 min | ||||||
| 39 | (NαHis)Ac-Lys(Amd)-βAla-βAla | IC50:3.7±1.7 kD:0.18±0.03 |
t1/2 in PC-3: 37±8 min | ||||||
| 40 | (NαHis)Ac-Ala(NTG)-βAla-βAla | IC50:3.2±1.2 kD:0.29±0.16 |
t1/2 in PC-3: 38±5 min | ||||||
| 41 | Litorin | 67.5±5.0% in human plasma after 24h. 83.2±2.7% after cys challenge ratio molarity 1:1000 | [71] | ||||||
| 42 | (NαHis)Ac | Bn(7-14) | PC-3 | IC50:1.9±0.7 kD:0.19±0.09 |
t1/2 human plasma:0.5±0.2h, in PC-3: <0.1h | Internalization increased in the first 30 min and remained constant for 2 h. Cell related activity: 71–92% was internalized after 2 h. | The difference in the linker did not have a significant effect on stability or receptor affinity. However, substitutions in 13 and 14 positions increased stability. | [66] | |
| 43 | [Cha13]Bn(7-14) | IC50:5.1±1.4 kD:0.08±0.04 |
t1/2 human plasma:16±3h, in PC-3:0.3±0.1h | ||||||
| 44 | [Cha13,NLe14]Bn(7-14) | IC50:14.2±3.0 kD:0.39±0.23 |
t1/2 human plasma:6±1.5h, in PC-3:0.25±0.1h | ||||||
| 45 | [NLe14]Bn(7-14) | IC50:15.7±6.0 kD:0.51±0.28 |
t1/2 human plasma:16±3h, in PC-3:0.25±0.1h | ||||||
| 46 | (NαHis)Ac-βAla-βAla | [Cha13,NLe14]Bn(7-14) | IC50:5.1±1.7 kD:0.18±0.12 |
t1/2 human plasma:10±1.45h, in PC-3:0.5±0.1h | |||||
| 47 | (NαHis)Ac-NH-CH2-CH2-O-CH2-CH2-O- | [Cha13,NLe14]Bn(7-14) | IC50:8.9±0.5 kD:0.25±0.06 |
t1/2 human plasma:8±3h, in PC-3:0.35±0.1h | |||||
| 48 | NS3-4-(isocyanomethyl)benz oic acid-βAla | Bn(7-14) | PC-3 | 0.20±0.04 | 10–20% (2h) | [61] | |||
| 49 | NS3-4-(isocyanomethyl)benz oic acid-Gly-Gly-Gly | 1.9±0.4 | |||||||
| 50 | NS3-4-(isocyanomethyl)benz oic acid-Ser-Ser-Ser | 1.2±0.1 | |||||||
| 51 | NS3-4-isocyanobutanoic acid-βAla | 0.35±0.03 | 65% (2h) | ||||||
| 52 | NS3-4-isocyanobutanoic acid-Gly-Gly-Gly | 1.4±0.1 | 10–20% (2h) | ||||||
| 53 | NS3-4-isocyanobutanoic acid-Ser-Ser-Ser | 0.65±0.32 | |||||||
| 54 | DPR-Asn-Asn-Asn | Bn(7-14) | PC-3 | 1.3±0.2 | 20% (2h) | >75% after 4h in human serum | The different spacer linker used did not produce changes in the binding or stability characteristics between them. | [60] | |
| 55 | DPR-Asn-Asn-Asn-βAla | 2.2±0.1 | |||||||
| 56 | DPR-Asn-Asn-Asn-5Ava | 3.6±2.2 | |||||||
| 57 | DPR-Arg-Arg-Arg | 0.6±0.1 | |||||||
| 58 | DPR-Arg-Arg-Arg-βAla | 0.2±0.02 | |||||||
| 59 | DPR-Arg-Arg-Arg-5Ava | 0.4±0.1 | |||||||
| 61 | HYNIC/Tricine/TPPS–βAla | Bn(7-14) | PC-3/HT-29 | 38±1 in PC-3 | In saline solution with Cys after 12h incubation, 95% stable. | 20±3% (1h) | [64] | ||
| 62 | EDDA-HYNIC | [Lys3]Bn | PC-3 | >90% after 24h in human serum. >91% after cys challenge ratio molarity 1:500 | 11.5% (4h) | [167] | |||
| 63 | Gly-Gly-Cys-Aca | Bn(2-14) | PC-3 | 1.13 | [55] | ||||
| 64 | MeGly-Gly-Cys-Aca | 0.76 | |||||||
| 65 | Me2Gly-Gly-Cys-Aca | 0.76 | |||||||
| 66 | Mac-Gly-Cys-Aca | 1.42 | |||||||
| 67 | PZ1-βAla | Bn(7-14) | PC-3 | 0.7±0.04 | 25% (2h) | 90% (90min) | [59] | ||
| 68 | PZ1-Gly-Gly-Gly | 0.2±0.02 | 53% (2h) | 56% (90min) | |||||
| 69 | PZ1-Ser-Ser-Ser | 1.9±0.1 | 30% (2h) | 48% (90min) | |||||
| 70 | Pm-DADT | [DTPA1,Lys3,Tyr4]Bn | PC-3 | Kiα (nM): 4.1±1.4 | >90% after 6h in human serum. | [53] | |||
| 71 | Demobesin 3 | PC-3/autoradiography in human tumor biopsy samples. | 0.06±0.04/0.47 | In mouse plasma: 50% (2h) | 75% (2h) | [70] | |||
| 72 | Demobesin 4 | 0.15±0.04/1.94 | |||||||
| 73 | Demobesin 5 | 0.08±0.05/0.65 | |||||||
| 74 | Demobesin 6 | 0.60±0.05/1.3 | |||||||
| 75 | Demobesin 1 (Antagonist) | PC-3/AR42J/autoradiography in human prostate cancer samples/mouse pancreas | PC-3=0.35±0.32AR42J=0.45±0.18, human tumor: 3.2±0.7, mouse pancreas: 7.1±1.1. | PC-3=37% (6h), AR42J=19% (6h) | Demobesin 1 had IC50 11-14 fold lower than Z-070 in PC-3 | [50] see also 111In tables | |||
| 76 | 99mTc(H2O)(CO)3-Dpr-Ser-Ser-Ser | Bn(7-14) | PC-3 | 0.86±0.22 | 16% (40min) | 55% (90min) | [57] | ||
All peptides not indicated as antagonist are agonist at human Bn receptors.
Abbreviations: T47-D, MDA-MB-231, MCF7=human breast cancer cell line, PC-3=human prostate cancer cell line, HEK293=transformed human embryonic kidney cell line, HT29=colon rectal adenocarcinoma cell line.
Table 11.
Studies in human using radiolabeled bombesin analogs.
| Isotope | Linker | Peptide | No | Patients studied | Imaging technique | Results | Ref. N |
|---|---|---|---|---|---|---|---|
| 99mTc | Cys0-Aca1 | “[Leu13]Bn” [Cys0-Aca1, Bn(2–14)] |
1 | 3 normal; 1 prostate cancer, 1 SCLC | SPECT and planar scintigraphy | No side effects, visualization of both tumors for 3h, radioactivity accumulation in liver, kidneys and thyroid gland. Tumor uptake higher than with 99mTc sestamibi alone. | [123] |
| 2 | Biopsies from 5 suspicious for breast cancer | Biopsy driven by Imaging Probe combined with X-ray | 48/48 biopsies high, 19/21 intermediate and 2/8 low radioactivity uptake positive for cancer. | [124] | |||
| 3 | 14 patients positive for prostatic lesions | SPECT and planar scintigraphy | 100% cancer and lymph nodes visualized. Results confirmed by pathologic evaluation while 111In-Octreotide only detected 2/3 cases. | [125] | |||
| 4 | 5 suspicious for breast cancer | Planar scintigraphy | No sides effects. 100% cancer
and lymph nodes visualized. Radioactivity accumulated in liver, kidneys and thyroid gland. Tumor/breast uptake ratio higher than with 99mTc sestamibi alone. |
[122] | |||
| 5 | 10 suspected and 1 proven with prostate cancer | SPECT and planar scintigraphy | 100% cancer and lymph nodes visualized. Results confirmed by pathologic evaluation. Detection of the lymph nodes affected better than with MRI. | [126] | |||
| 6 | 13 (6 suspected+7 known to have rectal cancer) | SPECT and planar scintigraphy | Cancer detected in 11/13 and 2 false
positives. 5/5 positive lymph nodes detected. Results confirmed by
pathologic evaluation. After 60 min all radiopeptide is in intestine. |
[127] | |||
| 99mTc | EDDA/HYNIC | [Lys3]Bn | 7 | 11 (3 with proven breast cancer and 8 with possible cancer) | SPECT and planar scintigraphy | No side effects. Predominant renal clearance. Patients with breast cancer showed asymmetrical uptake by breast tissue, with higher accumulation in patients with breast cancer. | [129] |
| 99mTc | N3S | “RP527”
5-Ava-Bn(7–14) |
8 | 4 patients with bone metastasis with
androgen-resistant prostate
cancer + 6 suspected breast carcinoma |
SPECT and planar scintigraphy | No side effects. Hepatic and renal clearance non blood accumulation. Radiopeptide uptake in 1/4 prostate cancer bone metastases and 4/6 breast cancer metastases and affected lymph nodes. | [58] |
| 9 | 6 healthy subjects | Study of dosimetry by time/course (planar scintigraphy, blood and urine samples) | No side effects. Hepatobiliary and renal clereance, non blood accumulation. Low uptake by brain, myocardium, lungs, brest and testes. Possible radiotracer for the supradiaphragmatic region and favorable dosimetry for SPECT. | [128] | |||
| 10 | 14 patients (9 suspected breast carcinoma+5 tamoxifen-resistant bone metastasized breast carcinoma) | Planar scintigraphy | 8/9 suspected patients tumor and lymph nodes were detected. 0/5 resistant patients detected. | [187] | |||
| 68Ga | DOTA-PEG2 | “BZH3”
[DTyr6,β Ala11,
Thi13, Nle14]
Bn(6–14) |
11 | 17 GIST patients | PET scans comparing 68Ga-BZH3 to 18F-FDG | FDG discovered 25/30 lessions, BZH3 8/30. Tumor uptake is lower with BZH3 than with FDG. In 1 case the lesion was seen with BZH3 and not with FDG. | [130] |
| 12 | 9 low grade glioma patients | PET scans comparing 68Ga-BZH3 to 18F-FDG | 6/6 patients with increase with BZH3 and FDG uptake had malignant transformation. 2/2 with decreased BZH3 and no FDG uptake had malignant transformation. | [131] |
III. B. Pharmacology of Bn analogs used for receptor-mediated imaging/cytotoxicity studies
Bn is a 14 amino acid COOH terminal amidated peptide; GRP has 27 amino acids and NMB 10 amino acids (Table 2)[8]. The COOH terminus with amidation is needed for high affinity and biological activity, whereas the NH2 terminal is not need for high affinity receptor interaction, and COOH modified analogues can function as potent antagonists [8,37–39]. Therefore the NH2 terminal of various Bn COOH terminal peptides can be attached to coupling agents or radiolabeled with full retention of biological activity. Bn and GRP have 8- and 1000-fold selectivity for the hGRPR over the hNMBR, respectively, whereas NMB has 1000-fold selectivity for the hNMBR over the hGRPR [14]. GRP, NMB, as well as all other natural occurring Bn-related peptides interact with hBRS-3 only with very low affinity (>1 uM)[8,40–42]. The novel, synthetic Bn analog, [D-Tyr6, β-Ala11, Phe13, Nle14]Bn(6–14)(Table 2) has the unique property of having high affinity for all three human receptor subtypes, functions as a potent agonist at each of these receptors and is rapidly internalized by each receptor [30,34,40–42].
Because the GRPR is the principal Bn receptor subtype over-expressed by most cancers, almost all Bn receptor-mediated imaging studies and cytotoxicity studies have concentrated on developing ligands that interact with this receptor with high affinity (Tables 2–12). Because previous studies demonstrate that the COOH terminal Bn hepapetide is largely inactive, most studies used at least Bn octapeptide (BN (7–14) or longer Bn peptides for their studies (Tables 2–12). Bn (7–14) analogs were the most frequently used Bn related peptide for these studies[8,43], followed by longer Bn peptides (Tables 2–12). Most studies used Bn receptor agonists for both Bn receptor-mediated imaging and cytotoxicity studies, however, a number of more recent studies used radiolabeled antagonists for imaging studies (Tables 2–12). Recent studies have reported that radiolabeled antagonists with various G protein-coupled receptors, even though not internalized, may give better images than radiolabeled agonists [44,45]. Similarly, with Bn analogs, a number of radiolabeled Bn antagonists were reported in various studies to give excellent imaging of various tumors in vivo [2,45–51].
III. C. Review of 99MTc-labeled Bn analog in vitro (Table 3) and in vivo (Table 4) studies of Bn receptor-mediated imaging/cytotoxicity studies
Table 4.
In vivo studies with 99mTc bombesin analogs.
| In vivo | ||||||||
|---|---|---|---|---|---|---|---|---|
| 99mTc N | Linker | Peptide | Stability | Animal | Biodistribution | Imaging | Comment | Ref. N |
| 1 | DPR-βAla | Bn(7-14) | Normal CF-1 and SCID bearing T47D or MDA-MB-231 tumors | High level of pancreas uptake12.2–15.0±0.7/2.7% ID/g in normal CF-1 mice, but low tumor uptake. | DPR showed superior target tissue accumulation and pharmacokinetic properties in vivo. | [65] | ||
| 2 | DPR-GGG | |||||||
| 3 | DPR-GSG | SPECT, favorable tumor/background ratio, clear visualization of tumor tissue. Kidneys (1.0–1.8) and GI (2.3–4.8) predominant source of radioactivity. | ||||||
| 4 | DPR-PEG5 | |||||||
| 5 | DPR-PEG8 | |||||||
| 6 | DPR-Ser-Gly-Ser | |||||||
| 7 | DPR-Ser-Ser-Ser | High level of pancreas uptake12.2–15.0±0.7/2.7% ID/g in normal CF-1 mice. High tumor upake. Selected for imaging study due to good tumor uptake and retention, and rapid elimination from non-target tissue by the renal-urinary system. | Favorable tumor/background ratio, clear visualization of tumor tissue. Kidneys (0.8–0.9) and GI (1.3–2.7) predominant source of radioactivity. | |||||
| 8 | PZ1-βAla | Normal CF-1 | 8.5±0.2.2% ID/g pancreas uptake, but low tumor uptake. | |||||
| 9 | PZ1-Gly-Gly-Gly | Low pancreas uptake | ||||||
| 10 | PZ1-Gly-Ser-Gly | |||||||
| 11 | PZ1-PEG5 | |||||||
| 12 | PZ1-PEG8 | |||||||
| 13 | PZ1-Ser-Gly-Ser | |||||||
| 14 | PZ1-Ser-Ser-Ser | |||||||
| 15 | N4-Gly-4-aminobenzoyl | [HDPhe6,Sta13,Leu14]Bn(6-14)“RM1” [Antagonist] | Human PC-3 xenograft-bearing nude mice | Tumor uptake at 4 h:29.9±4.0 %IA/g. Rapid and very high uptake in the pancreas and other GRPR expressing organs was also found but it washes out from these abdominal organs quickly, which results in good tumor/non-tumor tissue ratios at early time points. | SPECT/CT after 12 h injection of radiopeptide. Clear delineation of the tumor, low abdominal uptake, kidneys faintly visible. | [49] | ||
| 16 | NS3-Gly-Gly-Cys | Bn(2-14) | Normal Swiss and PC-3 tumor-bearing SCID mice | In normal mice: fast blood clearance, no uptake or retention in the stomach, low accumulation in liver and GI, excretion by kidneys and high accumulation in pancreas. In PC-3 tumor bearing SCID mice: “NS3-Gly-Gly-Cys” showed lower accumulation in pancreas and tumor than “NS3-Gly-Gly-Cys-Orn-Orn-Orn” which shows higher tumor/non-tumor ratios. | Introduction of the spacer Orn-Orn-Orn compared to non spacer produced a better uptake in target specific pancreatic and tumor tissue, and also higher quality SPECT images. | [171] | ||
| 17 | NS3-Gly-Gly-Cys-Orn-Orn-Orn | Dynamic γ camera: The PC-3 tumor was visible as early as 10 min after injection and remained observable up to 120 min p.i. Prominent uptake was also observed in the kidneys. Clearance of the radioactivity through the urinary bladder was evident. | ||||||
| 18 | Gly-Gly-Cys-Aca | BN(2-14) | Normal Swiss and PC-3 tumor-bearing SCID mice | In normal mice: fast blood clearance, no uptake or retention in the stomach, low accumulation in liver but high uptake by intestine for 4 h, and high accumulation in pancreas. In PC-3 tumor bearing SCID mice: good tumor uptake by both analogs. | Dynamic planar view: tumors clearly viewed from min 15 in both cases. | Bn (7-14) radiolabeled analog had a slower washout from pancreas, but a slightly higher liver excretion rate. | [56] | |
| 19 | BN(7-14) | |||||||
| 20 | (NαHis)Ac-Pra(Glu)-βAla-βAla | [Cha13,NLe14]Bn(7-14) | Nude mice bearing PC-3 tumors | Introduction of carbohydrate moiety Pra(Glu) produces an increase in the tumor uptake and retention. | [69] | |||
| 21 | EDDA/HYNIC | [Lys3]Bn(1-14) | Balb C normal mouse and athymic mice bearing PC-3 tumors. | 99mTc-Tat-BN clearance is predominantly renal. Pancreas shows higher uptake than non-excretory organs such as muscle. The tumor/muscle ratio for 99mTc-BN was 7 and for 99mTc-Tat-BN was 8.5 | γ camera: Clear tumor uptake and a dissection process to eliminate internal viscera, highlighted the 99mTc-BN and 99mTc-Tat-BN uptake in tumor PC-3 cells.. | Although 99mTc-Tat-BN has better tumor/muscle ratio, it also has a high uptake by kidneys and non-target organs that should be reduced for a lower background. | [54] | |
| 22 | N2S2 | [Tat(49-57)-Gly-Gly-Cys-Gly-[Lys3]]Bn(1-14) | ||||||
| 23 | Tetraamine-benzylaminodiglycolic acid | Demobesin 1 (Antagonist) | PC-3 tumor-bearing SCID mice | Both Bn analogs targeted well the pancreas and PC-3 tumor. [99mTc]Demobesin1 showed higher PC-3 tumor accumulation at all times. Pancreas uptake/accumulation of [99mTc]Demobesin1 declined faster. Blood and background clearance was fast for both agents, excreted predominantly via kidneys. [99mTc]Demobesin1 showed a higher of hepatobiliary excretion with higher liver and bowel values. | The radiolabeled Bn antagonist (Demobesin1) may be is a preferable tool for radioimagining due to its higher tumor accumulation and uptake. | [45] | ||
| 24 | Demobesin 4 | |||||||
| 25 | ≡N(PNP6)-Cys-βAla- | Bn(7-14) | Normal Swiss and PC-3 tumor-bearing SCID mice | Both analogs had rapid blood clearance. High uptake of 99mTcN(PNP6) by liver, pancreas and intestine expected considering the lipophilic character of the conjugate. 99mTc-HYNIC excreted primarily by the renal-urinary system and 99mTcN(PNP6) via the hepatobiliary system. The highest tumor uptake using the HYNIC conjugate: 3.0±0.5%ID/g vs 1.2±0.3%ID/g for nitrido conjugate. The best ratios tumor/non tumor achieved with 99mTc-HYNIC. | Scintigraphy: Tumor uptake was higher with 99mTc-HYNIC than 99mTcN(PNP6). Higher uptake of 99mTcN(PNP6) by the hepatobiliary excretory system. | The best radiotracer was 99mTc-HYNIC due its high radiochemical yield, fast radiolabeling procedure without need of purification step, and more consistent tumor uptake. | [63] | |
| 26 | HYNIC-βAla | |||||||
| 27 | (NαHis)Ac-β3hGlu-β3Glu-β3Glu | [Cha13,NLe14]Bn(7-14) | PC-3 tumor-bearing SCID mice | No tested | A positive charge in the linker resulted in higher uptake in kidney and liver. A hydroxyl group and especially a single negative charge in form of a β3homoglutamic acid considerably ameliorated the biodistribution profile, with higher tumor uptake, and significantly improved tumor-to-background ratios. However, additional negative charges led to a loss of affinity and internalization, and unfavorable biodistribution. | |||
| 28 | (NαHis)Ac-β3hGlu-β3Glu-βAla | SPECT/CT: high uptake in the kidneys, pancreas, and bowel. | ||||||
| 29 | (NαHis)Ac-β3hGlu-βAla-βAla | Highest tumor uptake with a longer retention. Fast clearance from normal tissues. Higher pancreas and tumor uptake | SPECT/CT: Clearer visualizatin of tumors xenograft, lower renal and hepatic uptakes, abdominal uptake corresponds to pancreas and intestinal tract. | |||||
| 30 | (NαHis)Ac-β3hLys-βAla-βAla | Not tested | Not tested | |||||
| 31 | (NαHis)Ac-β3hSer-βAla-βAla | Not tested | ||||||
| 32 | (NαHis)Ac-βAla-βAla | SPECT/CT: high uptake in the abdominal cavity due to the high hepatic, pancreatic, and intestinal uptakes. | ||||||
| 33 | DTMA-βAla- | Bn(7-14) | Normal CF-1 and PC-3 tumor-bearing SCID mice | Normal CF-1 mice: rapid clearance from blood in the 4 analogs, except in the case of Ser-Ser-Ser. βAla and Gly-Gly-Gly analogs excreted by hepatobiliary system, Ser-Ser-Ser and Gly-Ser-Gly analogs by the kidneys. βAla has the highest uptake by the pancreas. In PC-3 bearing tumor mice: βAla was the only one tested, high tumor uptake and very rapid clearance from the whole body. | Although βAla analog has the higher tumor uptake rate, it had low accumulation in tumor tissue due to rapid accumulation in the hepatobiliary system. | [62] | ||
| 34 | DTMA-Gly-Gly-Gly | |||||||
| 35 | DTMA-Gly-Ser-Gly | |||||||
| 36 | DTMA-Ser-Ser-Ser | |||||||
| 37 | (NαHis)Ac-βAla-βAla | [Cha13,NLe14]Bn(7-14) | CF-1 nu/nu PC-3 tumor bearing mice | All new analogs exhibited higher tumor/background ratios compared to the nonglycated peptide. The best results were obtained with the triazole coupled glucose with a 4-fold increased uptake and retention in tumor tissue and a significantly reduced accumulation in the liver. Apart from higher tumor-to-liver ratios, both tumor-to-kidney and tumor-to-blood ratios could be significantly improved. | SPECT/CT: reduction of abdominal background, tumor xenografts could clearly be visualized. | The introduction of a carbohydrated linker improved the biodistribution of Bn analogues labeled with the 99mTc-tricarbonyl core. | [68] | |
| 38 | (NαHis)Ac-Lys(sha)-βAla-βAla | |||||||
| 39 | (NαHis)Ac-Lys(Amd)-βAla-βAla | |||||||
| 40 | (NαHis)Ac-Ala(NTG)-βAla-βAla | |||||||
| 41 | Litorin | Normal Wistar rat | High and specific pancreas uptake. Excretion by kidneys. | [71] | ||||
| 42 | (NαHis)Ac | Bn(7-14) | CF-1 nu/nu PC-3 tumor bearing mice | All analogues had low blood and stomach accumulation. Higher kidney uptake than liver and high colon and pancreas uptake. Tumor uptake was lower than pancreas in all cases. | The analogues including a spacer (46 and 47) had an improved biodistribution, and higher tumor-to-blood ratios. Tumor-to-kidney and tumor-to-liver ratios also increased when the -βAla βAla-spacer was used. | [66] | ||
| 43 | [Cha13]Bn(7-14) | |||||||
| 44 | [Cha13,NLe14]Bn(7-14) | |||||||
| 45 | [NLe14]Bn(7-14) | |||||||
| 46 | (NαHis)Ac-βAla-βAla | [Cha13,NLe14]Bn(7-14) | ||||||
| 47 | (NαHis)Ac-NH-CH2-CH2-O-CH2-CH2-O-CH2CO | [Cha13,NLe14]Bn(7-14) | ||||||
| 48 | NS3-4-(isocyanomethyl)benzoic acid-βAla | Bn(7-14) | CF-1 mice | Minimal uptake by stomach, rapid accumulation in the liver and excretion to the intestines, and low accumulation in pancreas after 1h. No good candidate. | [61] | |||
| 51 | NS3-4-isocyanobutanoic acid-βAla | |||||||
| 54 | DPR-Asn-Asn-Asn | Bn(7-14) | Normal CF-1 and PC-3 tumor-bearing SCID mice | In Normal CF-1: Highest pancreas uptake after 1h by Bn analog 56 and 55. At 4h and 24h pancreas uptake was higher with 55 analog. In PC-3 tumor-bearing SCID mice: the analog tested was number 55 and showed a good tumor uptake. | Analogs including Asn had no liver accumulation but kidney clearance, the contrary was observed in Arg derivatives. Among them the more promising is number 55. | [60] | ||
| 55 | DPR-Asn-Asn-Asn-βAla | SPECT/CT and MRI: Clearly visualized the tumors, but GI uptake was higher than with a previous described analog (DPR-Ser-Ser-Ser). | ||||||
| 56 | DPR-Asn-Asn-Asn-5Ava | |||||||
| 57 | DPR-Arg-Arg-Arg | |||||||
| 58 | DPR-Arg-Arg-Arg-βAla | |||||||
| 59 | DPR-Arg-Arg-Arg-5Ava | |||||||
| 60 | Cys-Aca-Gln-Arg-Leu-Gly-Asn | [Lys14]Bn(2-14) | Normal rats | SPECT: amygdala is clearly visualized. | [172] | |||
| 61 | HYNIC/Tricine/TPPS –βAla | Bn(7-14) | This analog was completely metabolized in urine, kidney, and liver samples at 1 h p.i. The majority of the radioactivity was found in the urine sample at 1 h p.i. | BALB/c normal and BALB/c nude mice bearing HT-29 tumors | The analog had a rapid renal clearance. Tumor uptake was the highest at 30 min p.i., with a steady decrease over the 4 h. study period. It had good T/B ratios for blood, liver and muscle at 1 h p.i | γ camera: Tumor is clearly visualized at 1 h p.i. with excellent tumor/background contrast. At 1h p.i., the highest uptake areas were tumor, kidneys, and bladder. By 4h p.i., the radioactivity in the chest region almost completely disappears while the tumor is still clearly seen. | [64] | |
| 62 | EDDA-HYNIC | [Lys3]Bn | Athymic mice bearing PC-3 tumors. | 2 h p.i. the analog exhibited a rapid renal clearance. The highest non-specific uptake was found in kidneys. A significant uptake of radioactivity was observed in pancreas. Tumor also exhibited specific uptake of radioactivity. | γ camera: clear tumor uptake and a dissection process to eliminate internal viscera, highlighted the Bn analog uptake in tumor. | [167] | ||
| 63 | Gly-Gly-Cys-Aca | Bn(2-14) | Normal Swiss mice | The 4 Bn analogs showed renal clearance. Pancreas uptake was high and specific. Intestinal uptake can be attributed mainly to the GRP-R expressed in this tissue. | [55] | |||
| 64 | MeGly-Gly-Cys-Aca | |||||||
| 65 | Me2Gly-Gly-Cys-Aca | |||||||
| 66 | Mac-Gly-Cys-Aca | |||||||
| 67 | PZ1-βAla | Bn(7-14) | PC-3 tumor-bearing SCID mice | Tumor uptake and retention were lower when compared to other 99mTc-Bn conjugates of this type. The 3 analogs tested showed comparable accumulation of radiotracers in PC-3 xenografted tumors. The uptake of radioactivity in a normal pancreas was not different from other studies with 99mTc-Bn conjugates. | [59] | |||
| 68 | PZ1-Gly-Gly-Gly | |||||||
| 69 | PZ1-Ser-Ser-Ser | |||||||
| 70 | Pm-DADT | [DTPA1,Lys3,Tyr4]Bn | CD-1 normal mice and PC-3 tumor-bearing SCID mice | In normal mice: fast clearance with low radioactivity excreted through the hepatobiliary system. Small amount of radioactivity was found in stomach, but high uptake in the pancreas. In C3 tumor-bearing SCID mice: specific and clear uptake by the tumor. | γ camera: clear and specific tumor uptake after 12h. | DTPA or its combination with Pm-DADT is important for the analog to be excreted by kidneys produces a low background imaging in the abdominal region. | [53] | |
| 71 | Demobesin 3 | In urine samples from animal after being inyected with the analogs showed the presence of 3 metabolites and no intact analog. | PC-3-tumor-bearing CD-1 nu/nu mice;human ileal carcinoids | Demobesin 5 and 6 were rapidly cleared via the liver and Demobesin 3 and 4 via kidneys, showing low background activity. [99mTc]Demobesin 5 and 6 show a high percentage of intestinal uptake. All four radiopeptides had high and slowly declining pancreas uptake. Uptake of radiopeptides in the PC-3 human prostate cancer xenograft was high, especially for [99mTc]Demobesin 3 and 4 (9–11%ID/g at 1 h pi), remaining high (7–9%ID/g) at 4 h pi. | γ camera:clear tumour uptake, low background and low kidney uptake. | [70] | ||
| 72 | Demobesin 4 | |||||||
| 73 | Demobesin 5 | |||||||
| 74 | Demobesin 6 | |||||||
| 75 | Demobesin 1 (Antagonist) | Nude mice bearing PC-3 and AR42J tumors. | In both cases rapid blood clearance, Demobesin1 is excreted by renal and hepatobiliar systems. In AR4-2J and PC-3 tumor-bearing mice, [99mTc]Demobesin 1 and [111In]Z-070 displayed similar uptake in the rat tumor. However, in the human PC-3 xenografts, [99mTc]Demobesin 1 showed a 2- to 3-fold higher uptake than [111In]Z-070. | Tumor uptakes depends on the origin of the tumor, this should be taking into account in the selection of experimental tools. | [50] see also 111In tables | |||
| 76 | 99mTc(H2O)(CO)3-Dpr-Ser-Ser-Ser | Bn(7-14) | Normal CF-1 and PC-3 tumor-bearing SCID mice | In normal mice: in both cases, fast clearance from blood, with no uptake or retention in the stomach, very high uptake by normal pancreas, excretion by renal system. In PC-3 tumor bearing mice: high uptake and accumulation in the tumor. Being the tumor uptake by 99mTc(H2O)(CO)3-analog higher than than the other analog and than the previously described in 99mTc-N3S-analog. | [57] | |||
| 77 | 99m TC(CH2CH3)(CO)3-Dpr-Ser-Ser-Ser | |||||||
99mTc is the most used radioisotope worldwide for diagnosis in nuclear medicine, as it is used in 85% of diagnostic imaging. This is due to its availability (99Mo/99mTc generator system), well-established labeling chemistry, good labeling efficiency, half life (6.01 h) and 140 keV gamma energy. Among its applications are included: bone scanning (99mTc-MDP), myocardial perfusion imaging (99mTc-retrofosmin and 99mTc-sestambi), functional brain imaging (99mTc-HMPAO and 99mTc-EC), immunoscintigraphy (99mTc-scintium), red cells blood labeling to localize gastrointestinal bleeding, imaging of heart damage (99mTc-pyrophosphate) and liver-spleen scanning (99mTc-sulfur colloids).
Bn (1–14)
The first study using a 99mTc radiolabeled bombesin analog is from 1998 [52]. The authors tested the agonist [Lys3]Bn (Table 2) coupled to the isotope through 2 different linkers (Pm-DADT or Hx-DADT, DADT= diaminedithiol, Table 1, Fig. 1). Binding studies in rat brain membranes with 99mTc-Pm-DADT-[Lys3]Bn or 99mTc-Hx-DADT-[Lys3]Bn showed Ki values not different (3.5±0.7 nM and 5.2±1.5 nM, respectively) from natural bombesin (4.3±1.0 nM). In vivo biodistribution experiments in normal animals showed that the 99mTc-Hx-DADT linked [Lys3]Bn analog had 4-fold greater accumulation in the intestine due to its more lipophilic character than 99mTc-Pm-DADT-[Lys3]Bn, making the latter more suitable for imaging in the abdominal area.
In another study from the same group [53], 99mTc-Pm-DADT-[Lys3]Bn showed high accumulation in the intestine, which the authors attempted to decreased by introducing a DTPA moiety (Fig. 1, Table 2) in position 1 of [Lys3, Tyr4]Bn [Analog #70, Table 3] (Table 2). Binding experiments [Analog #70, Table 3] with PC-3 membranes showed Ki values for the new Bn agonist of 4.1±1.4 nM, slightly higher than Bombesin (1.7±0.6 nM). In vivo biodistribution experiment with normal and PC-3 cell xenografts bearing rats demonstrated the introduction of DTPA in this Bn analog produced decreased radioactivity accumulation in the abdominal region, increased renal clearance, as well as, high and specific uptake by pancreas and PC-3 tumor cells, which could be clearly observed by scintigraphy [Analog #70, Table 4].
Another study using the Bn agonist [Lys3]Bn (Table 2) coupled to 99mTc by the linker EDDA/HYNIC (Fig. 1, Table 1) using an instant freeze-dried kit formulation [Analog #62, Table 3], showed high stability either in human serum or a cysteine solution. In vivo biodistribution and imaging studies [Analog #62, Table 4] with 99mTc-EDDA/HYNYC- [Lys3]Bn in normal and PC-3 tumor bearing rats demonstrated rapid clearance from blood with renal excretion of the Bn analog, and significant uptake by both the pancreas and the tumor cells, which could be observed by scintigraphy and highlighted after the removal of the internal viscera. This study proved the possibility of creating a 99mTc Bn analog using this instant freeze-dried kit.
In a study from the same group [54], 99mTc-EDDA/HYNYC- [Lys3]Bn (Table 2) was compared with 99mTc- N2S2-Tat (49–57) -[Lys3]Bn (N2S2=Cys (Acm)-Gly-Cys (Acm); Tat (49–57)=Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg, Table 1) [Analogs #21–22, Table 3 and 4]. This hybrid Bn analog was obtained by coupling the Bn agonist to the Tat (49–57) HIV peptide through the spacer Glu-Gly-Cys-Gly and the linker N2S2 bound to 99mTc. With this approach the authors tried to increase the internalization of the Bn analog using the HIV peptide Tat, because it has been used to deliver a large variety of cargoes into cells. In fact, this hybrid Bn analog showed higher internalization values than 99mTc-EDDA/HYNYC- [Lys3]Bn in 3 different cell lines: PC-3, MCF7 and MDA-MB231 [Analogs #21–22, Table 3], although it presented lower stability in human serum and/or cysteine solution. When comparing these two radiolabeled peptides in biodistribution and imaging studies [Analogs #21–22, Table 4], the hybrid Bn analog also showed rapid clearance from blood with renal excretion, significant uptake by the pancreas and tumor, but a higher uptake in non-targeted organs and kidneys, thus producing a higher background.
Bn (2–14)
Bn agonist Bn (2–14) (Table 2) has also been radiolabeled with 99mTc and studied for its possible use in nuclear medicine. One study from Gourni et al. [55] studied this Bn agonist linked through 4 different amino acid sequences to the 99mTc isotope (Gly-Gly-Cys-Aca, MeGly-Gly-Cys-Aca, Me2Gly-Gly-Cys-Aca or Mac-Gly-Gly-Aca; Aca=aminohexanoic acid, Table 1) [Analogs #63–69, Tables 3 and 4]. Binding studies with these Bn (2–14) analogs in PC-3 cells showed no difference in the IC50 values. In vivo biodistribution studies performed in normal animals showed that the 4 radiolabeled Bn (2–14) analogs had rapid blood clearance, were elimination mainly through the renal/urinary pathway, and had high and specific pancreatic uptake [Analogs #63–69, Table 4].
In later paper from Gourni et al. [56], the previously tested 99mTc- Gly-Gly-Cys-Aca-Bn (2–14) was compared to 99mTc- Gly-Gly-Cys-Aca-Bn (7–14), in order to determine if the shorter sequence in the latter compound produced any improve in the properties of the radiopeptide [Analogs #18–19, Tables 3 and 4]. It was found that the IC50’s, internalization and efflux values were not different between them, and the Bn (2–14) analog was more stable after 2 h in human plasma [Analogs #18–19, Table 3]. Biodistribution studies with both 99mTc-Bn analogs demonstrated they had fast blood clearance, no uptake or retention in the stomach, low accumulation in the liver, but high uptake in the intestine, high accumulation in the pancreas and good tumor uptakes, but also both produced clear images of the tumors in dynamic planar studies. The only difference found between them was a slower washout from the pancreas and slightly higher liver excretion rate by 99mTc-Gly-Gly-Cys-Aca-Bn(7–14) [Analogs #18–19, Table 4].
The Bn analog Gly-Gly-Cys-Aca-Bn (2–14) also has been studied using as a linker, N3S (2‴,2″,2‴-nitrotriethanethiol, Table 1) and the effect of the introduction of 3 basic amino acids (Orn-Orn-Orn) in the sequence of the spacer [Analogs #16–17, Tables 3,4] studied. It was found that the analog N3S-Orn-Orn-Orn-Gly-Gly-Cys-Aca-Bn (2–14) had higher stability and internalization rate in PC-3 cells than the N3S-Gly-Gly-Cys-Aca-Bn (2–14) [Analogs #16–17, Table 3]. In vivo biodistribution/imaging experiments [Analogs #16–17, Table 4]. showed that the radiopeptide 99mTc-N3S-Orn-Orn-Orn-Gly-Gly-Cys-Aca-Bn(2–14), compared to 99mTc-N3S-Gly-Gly-Cys-Aca-Bn(2–14), produced a better uptake in pancreas and tumor tissues and had a higher tumor/non tumor ratio, as well as produced quality SPECT images with clear PC-3 cell tumor visualization and low background as early as 10 min p.i.
Bn (7–14)
Among the 99mTc radiolabeled Bn analogs studied, Bn (7–14) (Table 2) has been the most widely used, being studied with different linkers in 41% of the publications [Tables 3 and 4].
Smith et al.[57] tested the radiolabeled Bn analog Dpr-Ser-Ser-Ser-Bn (7–14) (Dpr=1,2-diaminopropionic acid, Table 1) coupled to 99mTc by the moiety (H2O)(CO)3 or (CH2CH3)(CO)3. They observed that (H2O)(CO)3-Dpr-Ser-Ser-Ser-Bn (7–14) had an IC50 value in the nanomolar range (0.86±0.22), was stable in aqueous solution for more than 24 h and had a 55% internalization rate after a 90 min incubation [Analogs #76, Table 3]. In vivo experiments in normal or PC-3 cell tumor bearing mice [Analog #76–77, Table 4] comparing these two radiolabeled Bn agonists showed fast blood clearance, high renal excretion, high pancreatic and tumor uptake with both, but the 99mTc-(H2O)(CO)3 Bn analog’s values were higher than those of either 99mTc-(CH2CH3)(CO)3-Bn analog or the previously reported Bn analog, 99mTc-N3S-5-Ava-Bn(7–14) [58].
In another study from the same group [59], Alves et al. studied 3 different Bn (7–14) analogs, coupled to 99mTc with the same linker PZ1 (pyrazolyl, Table 1), but using 3 different spacers (Gly-Gly-Gly, Ser-Ser-Ser or β-Ala=β-Alanine, Table 1) [Analogs #67–69, Table 3 and 4]. Among them, the one with the highest affinity was the PZ1-Gly-Gly-Gly-Bn (7–14) agonist (IC50: 0.2±0.02 nM), which was 10-fold higher than Bn (7–14). 99mTc-PZ1-Ala-Ala-Ala-Bn(7–14) showed the highest internalization value with 90% of the cell-associated activity remaining internalized even at 90 min [Analogs #67–69, Table 3]. When these Bn (7–14) agonists were tested in in vivo biodistribution/imaging studies [Analogs #67–69, Table 4] in SCID mice bearing xenografted human PC-3 cell tumors, all of them showed rapid blood clearance, minimal gastric accumulation, renal excretion and high accumulation in pancreas, but the tumor uptake observed was lower than seen with 99mTc-(H2O)(CO)3-Dpr-Ser-Ser-Ser-Bn (7–14) [57].
One year later, the same group [60] studied the possibility of improving the characteristics of the Bn (7–14) analog by coupling it to the linker DPR (Table 1) using different spacers (Asn-Asn-Asn, Asn-Asn-Asn-βAla, Asn-Asn-Asn-5Ava, Arg-Arg-Arg, Arg-Arg-Arg-βAla or Arg-Arg-Arg-5Ava; where βAla: β-Alanine and 5Ava: 5-aminovaleric acid, Table 1) [Analogs #54–59, Tables 3, 4]. The different spacers did not produce any significant change either in binding affinity [IC50 ranging from 0.2±0.02 for DPR-Arg-Arg-Arg-βAla-Bn(7–14) to 3.6±2.2 nM for DPR- Asn-Asn-Asn-5Ava-Bn(7–14)], or stability (>75% after 4h in human serum). When all analogs were tested for 1 h p.i. in vivo biodistribution experiments in normal CF-1 mice [Analogs #54–59, Table 4], the one with the amino acid Arg in the spacer showed hepatobiliary clearance, while those with Asp had renal excretion of the radiolabeled peptide. All of them showed high pancreas uptake with the highest values with 99mTc-DPR-Asn-Asn-Asn-βAla-Bn(7–14) and 99mTc-DPR-Asn-Asn-Asn-βAla-Bn (7–14) [Analogs #55–56, Table 4]. The latter two Bn analogs were tested for longer periods of time (4h and 24h), and demonstrated that the radiolabeled agonist, 99mTc-DPR-Asn-Asn-Asn- βAla-Bn (7–14) showed the best pancreatic uptake, so it was chosen for in vivo biodistribution experiments with animal bearing PC-3 cell tumor xenografts and for imaging studies [Analog #55, Table 4]. This Bn analog had good tumor uptake values and the tumors were clearly visualized, however the GI uptake was higher than with the previously studied Bn analog 99mTc-DPR-Ser-Ser-Ser-Bn(7–14) [57].
In another study with 99mTc and a Bn (7–14) analog [61], the analog was bound to the isotope 99mTc by a different linker, NS3 (2′,2″,2‴-nitrotriethanethiol, Table 1, Fig. 1) and 3 different spacers (βAla, Gly-Gly-Gly and Ser-Ser-Ser) combined with 4-(isocyanomethy)benzoic acid or 4-isocyonobutanoic acid [Analogs #48–53, Table 3, 4]. When the 6 Bn analogs were tested in binding studies in PC-3 cells [Analogs #48–53, Table 3], no significant difference in the IC50 values were found [values ranging from 0.2±0.04 for Analog #48 to 1.9±0.4 nM for Analog #49] and the highest internalization value was observed with Analog #51. The two Bn analogs showing the best values for cellular binding and internalization [Analogs #48, Table 4): 99mTc- NS3-4-(isocyanomethy)benzoic acid- βAla-Bn (7–14) and analog #51, Table 4): 99mTc-NS3-4-(isocyanomethy)benzoic acid- βAla-Bn (7–14), were used in in vivo biodistribution studies in normal animals. These analogs showed rapid accumulation in the liver, excretion to the intestine, and low pancreatic uptakes, making poor candidates to be used in the nuclear medicine.
Another linker, DMTA (2-(N,N″-bis(tert-butoxycarbonyl)diethylenetriamine)acetic acid, (Table 1, Fig. 1), has been used to bind 99mTc. In this study [62], DMTA was linked to Bn (7–14) through 4 different spacers: βAla, Gly-Gly-Gly, Gly-Ser-Gly or Ser-Ser-Ser [Analog #33–36, Tables 3, 4]. Binding studies were performed with each Bn analog [Analog #33–36, Table 3], and in all cases a nM IC50 were obtained, ranging from 0.28±0.2 with DMTA-βAla-Bn (7–14) to 2.56±1.3 nM with DMTA-Gly-Gly-Gly -Bn (7–14). The highest internalization value, though, was observed with 99mTc-DMTA-βAla-Bn(7–14) (23.8±0.03% after 2h incubation). All of the Bn analogs were tested for in vivo biodistribution studies in normal animals [Analog #33–36, Table 4]. 99mTc-DMTA-Ser-Ser-Ser-Bn(7–14) had prolonged retention in the circulation, the more hydrophilic radiolabeled agonist (serine-containing spacer) were predominantly excreted by the kidneys, while the more hydrophobic conjugates were excreted by the hepatobiliary system. The highest pancreas uptake was observed with the agonist 99mTc-DMTA-βAla-Bn(7–14). This radiolabeled Bn agonist was selected for biodistribution studies in animals bearing PC-3 tumors. This radioconjugate showed high affinity and internalization values in PC-3 cells. In another study from the same group [63], two different 99mTc radiolabeled βAla-Bn (7–14) analogs were studied and compared, one coupled to the linker HYNIC (Table 1, Fig. 1) and the other to N(PN6)-Cys, [Analogs #25–26, Tables 3,4]. Comparing the results obtained with each Bn analog, the value of all the parameters studied (stability, amount of receptor internalization in PC-3 cells, pancreas and tumor uptake in normal and PC-3 tumor bearing animals, higher uptake in scintigraphy image studies) were more favorable with 99mTc-HYNIC-βAla-Bn(7–14) than 99mTc≡N(PN6)-Cys- βAla-Bn (7–14).
HYNIC (Fig. 1) has been also used in another study as a linker between for 99mTc and a Bn (7–14) analog. 99mTc-HYNIC/Tricine/TPPS-Bn (7–14) [Analog #61, Tables 3, 4] [64] had a nM IC50 value in PC-3 cells, although it was16-fold higher than that of Bn (7–14) analog; it showed good stability and internalization values. In vivo biodistribution studies/imaging with this Bn analog radio-conjugate demonstrated that xenografted HT-29 tumors in BALB/c nude mice were clearly visualized at 1 h p.i. with excellent tumor/background ratio, although at this time the highest uptake areas were in the kidneys and bladder due to the renal excretion. After 4 h p.i. the background radioactivity in the chest region disappeared due to the high renal excretion rate, but the tumors were still clearly seen.
In a recent study [65], 14 Bn (7–14) analogs were studied [Analogs #1–14, Tables 3,4], comparing the linker DPR or PZ1 and 7 different spacers (βAla, Gly-Gly-Gly, Gly-Ser-Gly, PEG5, PEG8, Ser-Gly-Ser, Ser-Ser-Ser; PEG=ethylene glycol [2-aminoethylcarboxymethylether] (Table 1). In vitro binding experiments with T47-D and MDA-MB-231 cells [Analogs #1–14, Table 3] showed that, although all of the had IC50 values in the nM range, the PZ1-Bn (7–14) analogs had higher binding affinities. In all cases these analogs were stable more than 24h in PBS with or without HSA (human serum albumin) and the amount of receptor internalized was similar. In vivo biodistribution experiments in normal and tumor bearing animals were performed with all the analogs. All the PZ1-Bn (7–14) radioconjugates showed low tumor uptake, and among the DPR linked radiopeptides all of them showed high pancreatic uptake, but only one showed high tumor uptake and accumulation, which was 99mTc-DPR-Ser-Ser-SerBn(7–14). This radiolabeled Bn analog when used in imaging studies and produced favorable tumor/background ratios and clear visualization of the tumor tissue.
In several studies the possibility of using the linker (NαHis)Ac (Nα-carboximethylhistidine, Table 1, Fig. 1) to the Bn (7–14) analog through several spacers has been examined. In one study from 2006 [66] Bn (7–14) and 3 other Bn analogs ([Cha13]Bn (7–14), [Nle14]Bn (7–14) and [Cha13, Nle14]Bn (7–14)) were tested using none or 2 different spacers (βAla-βAla or NH-CH2-CH2-O-CH2-CH2O-CH2CO) [Analogs #42–47, Tables 3, 4]. Bindings studies showed that all the Bn analogs showed IC50 values in the nM range, with (NαHis)Ac-Bn (7–14) having the highest affinity (IC50: 0.19±0.09 nM) and (NαHis)Ac- [Nle14]Bn (7–14) the lowest (IC50: 15.7±6.0 nM), but stability studies in human plasma or using PC-3 cells showed the contrary, with the more stable molecule being (NαHis)Ac- [Nle14]Bn (7–14) [Analogs #42–47, Table 3]. Internalization/efflux experiments showed a similar pattern for all the Bn (7–14) analogs. When all of the radiopeptides were tested in in vivo biodistribution studies, the 99mTc-(NαHis)Ac- NH-CH2-CH2-O-CH2-CH2O-CH2CO- [Cha13, Nle14]Bn (7–14) and 99mTc-(NαHis)Ac-βAla-βAla- [Cha13, Nle14]Bn (7–14) showed improved biodistribution and much higher tumor/blood ratios, with the latter one also showing increased tumor/kidney and tumor/liver values [Analogs #42–47, Table 4].
A later study from the group of Garcia-Garayoa et al. [67] tried to improve the biodistribution of the 99mTc-(NαHis)Ac-βAla-βAla- [Cha13, Nle14]Bn (7–14) analog (Fig. 1, Table 2) by introducing a positive or negative charged amino acid in the spacer sequence (β3hGlu-β3Glu-β3Glu, β3hGlu-β3Glu-βAla, β3hGlu-βAla-βAla, β3hLys-βAla-βAla, β3hSer-βAla-βAla and βAla-βAla) [Analogs #27–32 Tables 3, 4]. They found that the binding affinity of (NαHis)Ac-β3hGlu-β3Glu-β3Glu- [Cha13, Nle14]Bn (7–14) to PC-3 cells was reduced (IC50: 634±221.7 nM) having an affinity 317-fold lower than Bn (7–14) and 124-fold lower than (NαHis)Ac-βAla-βAla- [Cha13, Nle14]Bn (7–14), while the latter’s affinity, was not different from that of (NαHis)-βAla-βAla- [Cha13, Nle14]Bn (7–14) (IC50: 5.1±1.7 nM) or (NαHis)Ac-β3hSer-βAla-βAla- [Cha13, Nle14]Bn (7–14) (IC50: 6.8±3.2 nM). Introduction of the negative or positive charged amino acid in the spacer structure did not produce any significant modification in the stability of the molecules, but did alter the internalization values [Analogs #27–32 Table 3]. In fact, 99mTc-(NαHis)Ac-β3hGlu-β3Glu-β3Glu -[Cha13, Nle14]Bn (7–14) was not internalizated (1% after 1 h incubation), 99mTc-(NαHis)Ac-β3hGlu-β3Glu-βAla- [Cha13, Nle14]Bn (7–14) had half of the internalization value (15%) observed with 99mTc-(NαHis)Ac-β3hGlu-βAla-βAla- [Cha13, Nle14]Bn (7–14) (30%) and 99mTc-(NαHis)Ac-β3hLys-βAla-βAla-[Cha13, Nle14]Bn (7–14) had a higher rate (40%). In vivo biodistribution/SPECT imaging studies performed in normal and PC-3 cell bearing tumor SCID mice showed that 99mTc-(NαHis)Ac-β3hGlu-β3Glu-βAla- [Cha13, Nle14]Bn(7–14) demonstrated the best properties compared to the other radiopeptides. In fact, this radiolabeled bombesin agonist had the highest tumor uptake and retention, and fast clearance from non-targeted tissues, with clearer visualization of the tumors and lower renal and hepatic uptakes [Analogs #27–32 Table 4].
Another strategy to improve the characteristics of the Bn analog 99mTc-(NαHis)Ac-βAla-βAla- [Cha13, Nle14]Bn (7–14) (Fig. 1, Table 2) was performed by Schweinsberg et al. [68] by the insertion of a polar carbohydrate (Lys (sha), Lys (Amd) or Ala(NTG), Table 1) between the linker ((NαHis)Ac) and the spacer (βAla-βAla) [Analogs #37–40, Tables 3, 4]. Binding affinities and internalization studies in PC-3 cells showed no differences among the 4 Bn agonists, but differences were found in vivo biodistribution and imaging studies. In fact, all of the glycated analogues showed higher tumor/background ratio compared with the non-glycated. The best results were obtained with the 99mTc-(NαHis)Ac-Ala(NTG)-βAla-βAla- [Cha13, Nle14]Bn(7–14) which had a 4-fold increase in uptake and retention in the tumor and significant less accumulation in the tumor. All 3 new glycated Bn analogs produced a clear image of the tumor with a low abdominal background [Analogs #37–40, Table 4].
Maes et al. [69] also tried to improved the characteristics of the radiolabeled Bn analog 99mTc-(NαHis)Ac-βAla-βAla-[Cha13, Nle14]Bn(7–14) (Fig. 1, Table 2) by introducing the carbohydrate moiety Pra(Glu) (Table 1). This modification did not change the IC50 value obtained with 99mTc-(NαHis)Ac-βAla-βAla- [Cha13, Nle14]Bn (7–14), but produced an increase in the tumor uptake, tumor retention and excretion via kidneys [Analog #20, Tables 3, 4].
Bn Antagonist
In different studies 99mTc radiolabeled Bn antagonists have been used to study the possibility they could be used as a radiotracer in prostate and breast tumor cancer over-expressing bombesin receptors due to their proven high affinities.
In a paper from Maina et al. the Bn antagonist Demobesin 1 (Table 1) was coupled to 99mTc and its binding capacity and biodistribution properties in a mouse (AR42J) and a human (PC-3) tumor cell line were measured and compared to the radiopeptide, 111In-Z-070 (Table 1). Binding studies showed that Demobesin 1 had IC50 11–14-fold lower than Z-070 in PC-3. In vivo biodistribution studies showed that although both Bn analogs showed similar tumor uptake in AR42J bearing mice, 99mTc-Demobesin 1 had 2–3-fold higher tumor uptake in PC-3 xenografted mice, revealing the importance of the selection of the experimental tools testing the radiolabeled peptide [Analog #75, Tables 3, 4 and #7, Tables 3, 4].
Table 7.
In vitro studies with 125I, 185/187Re, 18F, 64Cu, 68Ga and 90Y bombesin analogs.
| Peptide # | Iso. | Linker | Peptide | In vitro | Comment | Ref. N | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Binding affinity | Membrane bound | Degrad. | Receptor Intern. | |||||||
| Cell used | IC50/Kd (nM) | Amnt.(%) | Amnt.(%) | |||||||
| 1 | 125I | no | [Tyr4]Bn | SKOV3.ip1-AdCMV-GPRR | no | 80.3±5.9 | no | no | Exp. Performed over-expressing AdCMVGRPR construct in each cell line | [119] |
| 2 | 131I | mIP | Bn | no | no | no | no | |||
| 3 | 125I | no | [Tyr4]Bn | SKOV3.ip1-AdCMV-GPRR | no | ~ 75 | no | no | [118] | |
| 4 | 125I | no | Balb/B1 GRPR, (BnR11) | no | 25.6±1.6 | no | 37.3±10.9 (4h) | |||
| 5 | 125I | no | Hela | no | ~70 | no | no | |||
| 6 | 125I | no | A427 | no | ~ 88 | no | no | |||
| 7 | 125I | mIP | [des Met14]Bn [Antagonist] | SKOV3.ip1-AdCMV-GPRR | no | ~ 68 | no | no | ||
| 8 | 125I | mIP | Balb/B1 GRPR, (BnR11) | no | 38.3±11.6 | no | 32±9 (4h) | |||
| 9 | 125I | mIP | Hela | no | ~ 60 | no | no | |||
| 10 | 125I | mIP | A427 | no | ~ 65 | no | no | |||
| 11 | 185/187Re | Aca-Gly-Gly-Cys(Bn1.1) | Bn(2–14) | PC-3 | 1.13 | no | no | no | High affinity for Bn1.1 cmpd | [55] |
| 12 | 185/187Re | Aca-MeGly-Gly-Cys (Bn1.2) | 0.76 | no | no | no | ||||
| 13 | 185/187Re | Aca-Me2Gly-Gly-Cys (Bn1.3) | nd | no | no | no | ||||
| 14 | 185/187Re | Aca-Mac-Gly-Cys (Bn1.4) | 1.42 | no | no | no | ||||
| 18 | 188Re | Tris | [des Met14] Bn(7–14) [Antagonist] | BnR-11 | no | 13.9±0.1 | no | no | [120] | |
| 19 | 188Re | Tris-C6 | no | 12.8±0.2 | no | no | ||||
| 20 | 188Re | Tris | PC-3 | no | 9.9±0.3 | no | no | |||
| 21 | 188Re | Tris-C6 | no | nd | no | no | ||||
| 22 | 18F | 3-Cyano-4-[18F] fluorobenzoyl-Ava | [NMeGly11, Sta13, Leu14] Bn(7–14) [Antagonist] | PC-3 | 2.71 | no | ~ 100 (2h) | no | High affinity and plasma stability | [102] |
| 23 | 18F | [FA(01010)13, Leu14] Bn(7–14) | PC-3 | 9.2 | no | ~ 100 (2h) | no | |||
| 24 | 18F | 2-(4-(di-tert-butylfluorosilyl)phenyl) acetyl-Arg-Ava- | [NMeGly11, Sta13, Leu14] Bn(7–14) (4b)[Antagonist] | PC-3 | 22.9 | no | no | no | 4b has higher affinity, lower stability both in vitro and ex vivo experiments than 3b cmpd | [51] |
| 25 | 18F | [His(3Me)11, Sta13, Leu14] Bn(7–14) (3b) [Antagonist] | PC-3 | 267.7 | no | no | no | |||
| 26 | 18F | FB | Bn | PC-3 | no | no | no | 7 (30 min) | FB-dual GRPR/integrin avb3 cmpd, has slighty lower uptake than FB-Bn compd. | [100] |
| 27 | 18F | Bn (7–14)-RGD | PC-3 | no | no | no | 6 (30 min) | |||
| 28 | 18F | FB-PEG3-Glu | Bn (7–14)-RGD | PC-3 | 73.3±1.6 | no | no | 6.65±0.3 (2h) | Rapid internalization. Similar affinity. | [101] |
| 29 | 18F | PEG3-Glu | PC-3 | 85.4± 1.9 | no | no | ||||
| 30 | 18F | FB | [Lys3]Bn | PC-3 | 5.3±0.6 | no | no | 61 (2h) | Aca-linker reduces affinity and uptake of Bn(7–14) | [99] |
| 31 | 18F | FB-Aca | Bn(7–14) | 48.7± 0.1 | no | no | 50 (2h) | |||
| 32 | 18F | no | [Lys3]Bn | 3.3± 0.4 | no | no | no | |||
| 33 | 18F | Aca | Bn(7–14) | 20.8± 0.3 | no | no | no | |||
| 34 | 64Cu | DOTA | MP2346 | PC-3 | no | no | no | 34 (1h) | Lower internalization and retention compared to the same cmpd Labeled with 86Y. | [87] |
| 35 | 64Cu | DOTA | [Lys3]Bn | PC-3 | 2.2±0.5 | no | no | 65±10 (30min) | DOTA-[Lys3]Bn has affinity and internalization rate similar to Bn | [85] |
| 37 | 64Cu | DOTA-8-Aoc | Bn(7–14) | PC-3 | 1.4±0.1 | no | no | no | Both cmpds have a steady increase in the amount of activity in the cell over time | [90] |
| 38 | 64Cu | CB-TE2A-8-Aoc | 0.5±0.01 | ~600cpm | no | ~1000cpm | ||||
| 39 | 64Cu | TACN-βAla-βAla | [Cha13, Nle14] Bn(7–14) | No | no | no | in vitro/in vivo | no | Stable in presence of a large excess of a competing ligand or copper seeking superoxide dismutase and in rat plasma. | [93] |
| 40 | 64Cu | bispidine derivatives | [Cha13, Nle14] Bn(7–14) | no | no | no | in vitro/in vivo | no | Cmpd Show high stability both in vitro and in vivo. | [94] |
| 41 | 64Cu | [Cha13, Nle14] Bn(7–14) | no | no | no | no | no | |||
| 42 | 64Cu | DOTA | Bn (7–14)-RGD | PC-3 | 85.8± 2.1 | no | no | 3.7±0.02 | NOTA cmpd has faster in vitro kinetic than DOTA cmpd | [95] |
| 43 | 64Cu | NOTA | 92.7± 3.5 | no | no | 2.9±0.5 | ||||
| 44 | 64Cu | cage amine ligand (CuL1) | [Lys3] Bn(1–14) | no | no | no | no | no | High serum stability. | [98] |
| 45 | 64Cu | DOTA-GGG | Bn(7–14) | PC-3 | 50±16.2 | no | no | 670 fmol/mg (20h) | The presence of glutamic acids in the linker reduces binding affinity and internalization | [89] |
| 46 | 64Cu | DOTA-GSG | 81.8± 34.3 | no | no | 830 fmol/mg (20h) | ||||
| 47 | 64Cu | DOTA-GSS | 31.7±6 | no | no | 1550 fmol/mg (20h) | ||||
| 48 | 64Cu | DOTA-GEG | 2160± 718 | no | no | 160 fmol/mg (20h) | ||||
| 49 | 64Cu | DOTA-GEE | 19300±4050 | no | no | no sign. | ||||
| 50 | 64Cu | DOTA-Aba | Bn(7–14) | T-47D | 78.5± 15.1 | no | no | 1300±198 fmol/mg (4h) | Low binding affinity values. The longer linker Ado allows binding but interferes with internalization | [88] |
| 51 | 64Cu | DOTA-Ava | 41.5± 7.8 | no | no | n.d. | ||||
| 52 | 64Cu | DOTA-Ahx | 15.8± 5.4 | no | no | 1312±100 fmol/mg (4h) | ||||
| 53 | 64Cu | DOTA-Aoc | 6.7±1.1 | no | no | 1419±109 fmol/mg (4h) | ||||
| 54 | 64Cu | DOTA-Ado | 27±5.4 | no | no | 5.6 fmol/mg (2h) | ||||
| 55 | 64Cu | NOTA-8-Aoc | Bn(7–14) | PC-3 | 3.1±0.5 | no | no | no | NOTA chelator improves binding affinity. | [91] |
| 57 | 64Cu | NO2A-8-Aoc | Bn(7–14) | T-47D | 7.6±2.2 | no | no | 90 (45min) | Promising pharmacokinetic values. | [92] |
| 59 | 64Cu | DOTA-Aoc | Bn(7–14) | PC-3 | Kd 6.1±2.5 | 3.3 to 4.6 | no | 18.2±0.4 (2h) | High affinity and internalization rates. | [83] |
| 60 | 64Cu | DOTA-PEG | Bn(7–14) | PC-3 | 3.9±0.6 mM | 9.3 to 12.4 | no | ~ 45 (2h) | Differences in internalization due to different affinity. | [84] |
| 61 | 64Cu | DOTA-Aoc | 90.5±22 | 2.4 to 3.1 | no | ~ 12 (2h) | ||||
| 62 | 64Cu | DOTA | [Lys3 ]Bn | PC-3 | 2.2±0.5 | no | no | ~ 84 (2h) | DOTA- Aca compd has reduced pharmacokinetic properties. | [86] |
| 63 | 64Cu | DOTA-Aca | Bn(7–14) | 18.4±0.2 | no | no | ~ 75 (2h) | |||
| 66 | 68Ga | NOTA | Bn (7–14)-RGD | PC-3 | 55.9±4.2 | no | no | 1.7±0.3 (15 min) | High affinity and internalization rates. | [105] |
| 68 | 68Ga | DOTA-PEG2 | [DTyr6,βAla11, Thi13, Nle14] Bn(6–14) (BZH3) | AR42J | Kd 0.5 | no | no | 88 (2h) | Good affinity. Rate of internalization shows an agonistic nature of the compd. | [104] |
| 69 | 68Ga | DOTA-PEG4 | Bn(7–14) | human cancer tissue | 6.6±0.1 | no | no | 43.7±1.8 (6h) | Affinity analysis for receptor subtypes [NMBR 12.5±0.5; GRPR 10±0.0;BB3R >1000] | [103] |
| 70 | 90Y | DOTA-GABA | [DTyr6,βAla11, Thi13, Nle14] Bn(6–14) (BZH2) | human cancer tissue | 4.9±1 NMBr; 1.4±0.1 GRPr; 10.7±4.2 BB3 | no | no | no | 90Y-BHZ2 had significantly higher affinity for all receptor subtypes, due to the extra negative charge at the NH2 terminus. | [79] |
| 71 | 90Y | DOTA-GABA | [DTyr6,βAla11, Thi13, Nle14] Bn(6–14) (BZH2) | ARJ42 | no | no | no | Rapidly internalyzed | ||
| 72 | 86Y | DOTA | MP2346 | PC-3 | no | no | no | 13 (1h) | Long incubation period shows higher levels of internalization | [87] |
| 74 | 90Y | DOTA | Bn(2–14) | PC-3 | 1.99±0.1 | no | 79.1 (24h) | 23.3±0.03 (4h) | [107] | |
All peptides not indicated as antagonist are agonist at human Bn receptors.
Cell lines: SKOV3.ip1, Ovarian carcinoma; Hela, Human cervical cancer; A427, Human lung carcinoma; BnR11, Mouse embryonic fibroblast cell line stably transfected with GRP receptor; PC-3, Human prostate cancer cells; T47D, Human breast cancer cells; AR42J, Rat pancreatic acinar cell tumor
Another study from the same group [70] compared 4 different Demobesin analogs (Demobesin 3–6, Table 1) coupled to 99mTc. All of these are agonists and each had similar IC50 values (in the nM range), stability and internalization rates [Analogs #71–75, Table 3]. When comparing the results from biodistribution studies (Table 4), radiolabeled Demobesin 5 and 6 were rapidly cleared via liver and had a high percentage of intestinal uptake, while 99mTc-Demobesin 3 and 4 were renally excreted and which produced lower background activity. Uptake by PC-3 tumors in xenografted animals was higher with 99mTc-Demobesin 3 and it was selected for imaging studies which showed clear tumor uptake and low kidney retention.
Cescato et al. [45] compared Demobesin 1 (Bn antagonist) and Demobesin 4 (Bn agonist) (Tables 1,2) coupled to 99mTc through the linker tetraamine-benzyllaminidiglycolic acid. In vitro experiments showed that both of them had similar IC50 values, however, Demobesin 1 showed a higher percent of membrane binding, but this Bn antagonist was not internalized [Analogs #23–24, Table 3]. Biodistribution studies (Table 4) in normal and PC-3 bearing SCID mice showed good pancreatic uptake and fast clearance in blood and non-target tissues. 99mTc-Demobesin 1 (antagonist) had a higher tumor accumulation and faster pancreatic washout, but also had higher liver and intestinal uptake values than 99mTc-Demobesin 4 [Analogs #23–24, Table 4]. It was concluded that the radiolabeled antagonist is the preferable candidate as radiotracer.
RM1 (Tables 1,2), another Bn antagonist, has also been tested as a possible 99mTc radiotracer [49] coupled to the isotope by the linker N4-Gly-aminobenzoyl. IC50 values of 3.7±1.3 nM were measured, not different from that with Bn (7–14), and high membrane bound values were obtained, but it was not internalized [Analog #15, Table 3]. When 99mTc-RM1 was injected into PC-3 bearing nude mice, high tumor and pancreatic uptake was observed with fast washout in the non-expressing GRPR tissues producing high tumor/non tumor ratios. SPECT/CT studies in animal bearing PC-3 tumors resulted in images with a clear delineation of the tumor with low abdominal and kidney uptake 12 h p.i. [Analog #15, Table 4].
Litorin (Table 2)
Litorin (Table 2) belongs to the ranatensin peptide family and it has high amino acid sequence similar to bombesin. This bombesin related peptide has been also coupled to 99mTc and its possibility as a radiopeptide investigated. Durkan et al.[71] coupled litorin directly, with no linker or spacer, to 99mTc. The resulting agonist radiopeptide had high stability after incubation with high concentration of Cys solution. Injecting normal Wistar rats with this radiolabeled Bn-related peptide produced a high and specific uptake in the pancreas, and excretion of 99mTc-Litorin by kidneys [Analog #41, Tables 3, 4].
III. D. Review of 111In-labeled Bn analog in vitro (Table 5) and in vivo (Table 6) studies of Bn receptor-mediated imaging/cytotoxicity studies
Table 5.
In vitro studies with 111In bombesin analogs.
| In vitro | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Binding affinity | Membrane bound | Stability | Amount of Receptor Internalization | ||||||
|
111In N |
Linker | Peptide | Cell used | IC 50/Kd (nM) | Comment | Ref. N | |||
| 1 | DO3A-CH2CO-G-4-aminobenzoyl | Bn(7–14) “AMBA” | GRPR binding by autoradiography on cancer sections of prostate. | 0.8±0.1 | 4.3±0.3% (4h) | 29±2.3% (4h) | [46] | ||
| 2 | [HDPhe6, Sta13, Leu14]Bn(6–14) “RM1” [Antagonist] | 35±13 | 21.8±0.93% (4h) | 4.7±0.1% (4h) | |||||
| 3 | DOTA | [Pro1, Tyr4]Bn | CA20948 and AR42J | IC50: 3–9 for the 4 Bn analogs. Binding and internalization depends on temperature. DPTA-[Pro1, Tyr4]Bn has highest binding and internalization. | [76] | ||||
| 4 | [ε-Lys3, Tyr4]Bn | ||||||||
| 5 | DPTA | [Pro1, Tyr4]Bn | Stable after heating for 25 min 100 °C | ||||||
| 6 | [ε-Lys3, Tyr4]Bn | ||||||||
| 7 | DOTA0–1PEG40 | [DTyr6, β Ala11, Thi13, Nle14]Bn(6–14) “Z-070” | PC-3 and AR42J | PC-3=3.87±0.97, AR42J=0.17±0.04 | PC-3<7.5%, AR42J<7.5% (both 6 h) | PC-3=37% AR42J=19% (both 6h) |
Demobesin1= affinity 11–14 fold>Z-070 (PC3) | [50] | |
| 12 | DOTA | Bn(7–14) | PC-3 | 110.6±32.3 | [72] | ||||
| 13 | DOTA-βAla | 2.1±0.3 | |||||||
| 14 | DOTA-5-Ava | 1.7±0.4 | |||||||
| 15 | DOTA-8-Aoc | 0.6±0.1 | 26% (1 h) | t1/2: 17.3 h in human serum | 72% (1 h) | ||||
| 16 | DOTA-11-Aun | 64±11.2 | |||||||
| 17 | DOTA-GABA | [DTyr6,βAla11, Thi13, Nle14]Bn(6–14): “BZH2” | Autoradiography on sections of cancers with BnRs | GRPR: 1.4±0.1, NMBR: 4.93±1.03, BRS-3: 10.7±4.2 | AR42J<7%. | t1/2: 2.3 h in human serum | 35% (6 h). | [79] | |
| 18 | DPTA-GABA | [DTyr6,βAla11, Thi13, Nle14]Bn(6–14): “BZH1” | GRPR: 3.47±0.32, NMBR: 10.5±3.03, BRS-3: 41.7±22.2 | AR42J<7% | t1/2: 2.0 h in human serum | 40% (6 h). | |||
| 19 | DOTA-8-Aoc | Bn(7–14) | PC-3 | 0.5±0.1 | [73] | ||||
| 20 | DOTA-5-ADS | 3.2±0.3 | |||||||
| 21 | DOTA-8-AOS | 6.2±0.3 | |||||||
| 22 | DOTA-AMBA | 1.1±0.1 | |||||||
| 23 | DOTA-Gly-AMBA | 1.9±0.1 | Lowest internalization | ||||||
| 24 | DOTA-Gly-AM2BA | 0.7±0.1 | |||||||
| 26 | DOTA-aminohexanoyl | [DPhe6, Leu-NHEt13, des-Met14]Bn(6–14): “Bomproamide” [Antagonist] | PC-3 | 1.4±0.1 | >internalizated | 14% (45 min) | [47] | ||
| 30 | DPTA | [Lys3, Tyr4]Bn(2–14) | PC-3 | Kd:
22.9±6.8 Bmax: 880 fmol per 106 PC-3 cells |
After 1 h incubation: 17% radiactivity in cell surface | 87.0±5.7% (72 h normal saline),66.0 ±5.35% and 59.4±3.40 % (8 h in human or rat plasma, respectively) | 83% (1 h) | [80] | |
| 33 | DPTA | [Pro1, Tyr4]Bn(1–14) | Autoradiography in 12 different prostate tumor xenografts in male nuce mice | High-density of GRPR in androgen-dependent tumors, low GRPR in androgen- responsive and -independent tumors. Castration results in GRPR downregulation in the 3 androgen-dependent tumors. | [78] | ||||
| 34 | DTPA | [Pro1, Tyr4]Bn | 7315b rat pituitary tumor cells | 8 nM | With AR42J and CA20948 cells, binding with agonist>antagonist. Agonist was internalizated, not antagonist. | [48] | |||
| 35 | [Tyr5, DPhe6]Bn(5–13)NHEt [Antagonist] | 11 nM | |||||||
| 36 | DPTA | [Pro1, Tyr4]Bn | PC-295 human prostate tumor xenografts and rat colon sections. | Rat: 2.4±0.6, human: 1.4±0.6 | human serum: 67% (4 h) | [77] | |||
| 37 | DDpr(DPTA) | [βAla11, Phe13, Nle14]Bn(7–14) | Rat:0.2±0.1, human: 0.5±0.1 | ||||||
| 38 | DPTA-ACMpip-Tha | [βAla11, Phe13, Nle14]Bn(7–14) | Rat: 0.1±0.01, human: 0.4±0.1 | human serum: 74% (4 h) | |||||
| 39 | DPTA-ACMpip-Tha | [βAla11, Tha13, Nle14]BN(7–14) | Rat: 0.3±0.1, human: 0.4±0.01 | ||||||
| 40 | DPTA-Acp | [βAla11, Phe13, Nle14]Bn(7–14) | Rat: 0.4±0.1, human: 2.5±0.3 | ||||||
| 41 | DPTA-DTha | [βAla11, Tha13, Nle14]BN(7–14) | Rat: 1.2±0.1, human: 15.4±2.8 | ||||||
All peptides not indicated as antagonist are agonist at human Bn receptors.
Cell line: CA20948 (rat pancreatic tumor cell line), AR42J (rat acinar pancreatic tumor cell line), PC-3 (human prostate cancer), 7315b (rat pituitary tumor cell line), PC-295 (human prostate cancer).
Table 6.
In vivo studies with 111In bombesin analogs.
| In vivo | ||||||||
|---|---|---|---|---|---|---|---|---|
|
111In N |
Linker | Peptide | Stability | Animal | Biodistribution | Imaging | Comment | Ref. N |
| 1 | DO3A-CH2CO-G-4-aminobenzoyl | Bn(7–14) “AMBA” | Nude mice+PC-3 | RM1 has a higher tumor uptake than AMBA (13.4±0.8% vs 3.7±0.8% IA/g at 4 h after inyection) as well as to all tumor-to-normal tissues ratio. | [46] | |||
| 2 | “RM1” [HDPhe6, Sta13, Leu14]Bn(6–14) [Antagonist] | SPECT showed a high uptake by the tumor lasting more than 72 h | ||||||
| 3 | DOTA | [Pro1, Tyr4]Bn | Nude rats+AR42J | Although [111In-DOTA-Pro1, Tyr4]Bn showed the highest uptake, of radioactivity in GRP receptor-positive tissues as well as higher target-to-blood ratios, 111In-DTPA [Pro1, Tyr4]Bn was easier to handle and is more practical to use. | [76] | |||
| 4 | [ε-Lys3, Tyr4]Bn | |||||||
| 5 | DPTA | [Pro1, Tyr4]Bn | 111In-DPTA-[Pro1, Tyr4]Bn can visualized tumor in rat by scintigraphy. | |||||
| 6 | [ε-Lys3, Tyr4]Bn | |||||||
| 7 | DOTA0–1-PEG40 | [DTyr6, β Ala11, Thi13, Nle14]Bn(6–14): Z-070 | Normal Lewis rats and Nude mice+AR42J and PC-3 xenografts | In AR4-2J and PC-3 tumor-bearing mice, [99mTc]Demobesin 1 and [111In]Z-070 have a similar tumor uptake. PC-3 xenografts, [99mTc]Demobesin 1 showed a 2–3x> uptake than [111In]Z-070. | [50] | |||
| 8 | DOTA | PESIN | 23.3±1.4% (5 min), 1.8±0.6% (15 min) | Swiss nu/nu mice+PC-3 | PC-3 uptake at 1 h was comparable for Demobesin1, AMBA, PESIN and MP2346 (3.0±0.4, 2.7±0.5, 2.3±0.5 and 2.1±0.9%ID/g, respectively). AMBA, MP2346 and PESIN revealed favourable increases in tumor to blood ratios over time. | All analogues visualised PC-3 tumours by SPECT/CT and autoradiography. | Comparing this Bn analogs with 99mTc-Desmosin 1: Stability: 64.1±1.6% (5 min) and 41.5±0.5 (15 min). Changes in tumor to kidney and pancreas ratios for Demobesin1 from 1 to 24 h after injection were significantly better than for the other analogues. | [2] |
| 9 | AMBA | 36.1±2.7% (5 min), 9.8±0.5% (15 min) | ||||||
| 10 | MP2346 | 21.2±0.8% (5 min), 3.4±1.3% (15 min) | High tumor uptake: 2.1±0.9%ID/g (1h), but high uptake by the kidneys (7.9±1.9%ID/g) | |||||
| 11 | MP2653 | 9.8±0.5% (5 min), 2.8±0.4% (15 min) | Very low tumor uptake: 0.9±0.25ID/g (1h) | |||||
| 12 | DOTA | Bn(7–14) | CF-1 healthy mice and PC-3 in ICR SCID mice for DOTA-8-Aoc | Radioactivity was cleared efficiently from blood by renal/urinary pathway. Pancreatic uptake increased as a function of hydrocarbon spacer length. 111In-DOTA-8-Aoc-BBN(7–14) conjugate conducted on PC-3 xenografts in SCID mice showed a specific uptake of radioactivity in tumor, with 3.63±1.11 %ID/g observed 1 h after injection. High tumor-to-blood and tumor-to-muscle ratios (6:1 and 45:1, respectively) were achieved at 1 h after injection. Radioactivity in the tumor was 43,19, and 9% of the radioactivity retained 24, 48, and 72 h after injection vs 1h. | [72] | |||
| 13 | DOTA-βAla | |||||||
| 14 | DOTA-5-Ava | |||||||
| 15 | DOTA-8-Aoc | |||||||
| 16 | DOTA-11-Aun | |||||||
| 17 | DOTA-GABA | [DTyr6,βAla11, Thi13, Nle14]Bn(6–14): BZH2 | Lewis rats+ARJ42 | Biodistribution studies of 111In-BZH1 and 111In-BZH2 (177Lu-BZH2) in AR4–2J tumor-bearing rats showed specific and high uptake in GRPR–positive organs and in the AR4–2J tumor. A fast clearance from blood and all of the non-target organs except the kidneys was found. | [79] | |||
| 18 | DPTA-GABA | [DTyr6,βAla11, Thi13, Nle14]Bn(6–14): BZH1 | ||||||
| 19 | DOTA-8-Aoc | Bn(7–14) | Normal CF-1 mice and SCID mice+PC-3 xenografts | In CF-1 mice, the BB2R uptake in the pancreas of radioconjugates containing aromatic linking groups was found to be significantly higher at 1 h postinjection than with radioconjugates with ether linker moieties. By 24 h postinjection, the radioconjugates containing aromatic groups exhibited the highest percentage tumor retention. | Fused Micro-SPECT/CT imaging studies at 24 h showed accumulation of radioactivity in the tumor with all radioconjugates. In both biodistribution and Micro-SPECT/CT imaging studies, the radioconjugates containing aromatic linking groups typically exhibited higher G.I. retention than hydrocarbon or ether linking moieties. | [73] | ||
| 20 | DOTA-5-ADS | |||||||
| 21 | DOTA-8-AOS | |||||||
| 22 | DOTA-AMBA | |||||||
| 23 | DOTA-Gly-AMBA | |||||||
| 24 | DOTA-Gly-AM2BA | |||||||
| 25 | DOTA-Sar5 | [DTyr5,6,βAla11, Thi13, Nle14]Bn(6–14) | Normal Wistar rat and autoradiography of kidney slides | Injection of polyglutamic acid or gelofusine but not that of Lys reduce kidney accumulation of the radiopeptide. | [173] | |||
| 26 | DOTA-aminohexanoyl | [DPhe6, Leu-NHEt13, des-Met14]Bn(6–14): Bomproamide [Antagonist] | Normal CF-1 mice and SCID mice with PC-3 xenografts | Rapid (0.25 h p.i.) and high (12.2±3.2%ID/g) pancreatic uptake was observed in healthy CF-1 mice. Rapid (0.25 h p.i.) and high uptake (6.9± 1.1%ID/g) was observed in PC-3 prostate cancer xenografts in SCID mice. | PC-3 xenografts were well observed in SCID mice by SPECT/CT. | [47] | ||
| 27 | DPTA | [Pro1, Tyr4]Bn: MP2248 | Nude NMRI mice, with or without surgical castration and resupplementation of testosterone with PC-82, PC-295 and PC-310 xenografts | Expression of human GRPR in androgen-dependent PC xenografts is reduced by androgen ablation and is reversed by restoring the hormonal status of the animals. | [185] | |||
| 28 | No linker | [ACMpip5, Tha6,βAla11, Tha13, Nle14]Bn(5–14): MP2653 | ||||||
| 29 | DPTA | [Pro1, Tyr4]Bn | Lewis rats with CA20948 and AR42J | CA20948 tumor, both in cell culture and as solid tumor in rats, is a very useful model for peptide receptor scintigraphy and radionuclide therapy studies. | This radiopeptde was compared with CCK, substance P and octreotide radiopeptide. | [75] | ||
| 30 | DPTA | [Lys3, Tyr4]Bn(2–14) | SCID mice with PC-3 xenografts | Accumulated in tumor, adrenal, pancreas, small intestine, and large intestine. Fast blood clearance and fast excretion from the kidneys were observed. The levels of radioactivity within the tumor peaked at 8 hours and then declined rapidly. | Micro-SPECT/CT showed uptake in the tumors at 8 and 24 h. High accumulation in the kidney, pancreas, and GI at 4, 8, 24, and 48 h. The trend of uptake seen in the imaging data was similar to the results of the biodistribution study. | [80] | ||
| 31 | DPTA | [Pro1, Tyr4]Bn: MP2248 | Lewis rats+CC531 (Colon CAR.) or CA20948 xenografts | Highest uptake by pancreas and kidneys. Rapid clearance of radioactivity from blood. Urinary excretion amounted to 70% ID after 24 hr, with a total body retention of 10% ID. Specific uptake was found in the CA20948 pancreas tumour and CC531 colon carcinoma in tumour bearing rats. | The CA20948 tumour, inoculated in the hindleg, was also visualized scintigraphically. | [74] | ||
| 32 | DPTA | [Pro1, Tyr4]Bn(1–14) | Lewis rats and C57B16 or NMRI nude mice | Kidney uptake is higher in mouse than in rat males. | Autoradiography in kidney slices showed, in both mice and rats, high uptake in cortex, less in outer medulla and none in inner medulla. | [186] | ||
| 34 | DTPA | [Pro1, Tyr4]Bn | Bufallo rats+7315b prolactinoma xenografts | The agonist [111In-DTPA-Pro1, Tyr4]Bn showed much higher specific uptake in Bn receptor-positive tissues and tumor than the antagonist [111In-DTPA-Tyr5, DPhe6]Bn(5–13)NHEt, with concordant target to background ratios. | Scintigraphy of isolated 7315b tumors from rat showed tumor uptake | [48] | ||
| 35 | [Tyr5, DPhe6]Bn(5–13)NHEt [Antagonist] | |||||||
| 36 | DPTA | [Pro1, Tyr4]Bn | Rats bearing CA20948 tumors and Nude mice bearing PC-3 tumors. | High receptor-mediated uptake in receptor-positive organs and tumors. | Using this Bn analog tumours could be visualised using planar gamma camera and microSPECT/CT imaging after 1 min during 4 h. | [77] | ||
| 38 | DPTA-ACMpip-Tha | [βAla11, Phe13, Nle14]Bn(7–14) | ||||||
111Indium is a very useful tool in nuclear medicine for the imaging of tumors due to its half life (67 h), gamma energy of 247 keV and the type of disintegration producing an electron making it suitable for SPECT studies. In fact it is the isotope used for the radiolabeling of the first somatostatin analog available in the market for scintigraphic localization of primary and metastatic neuroendocrine tumors bearing somatostatin receptors, Octreoscan [111In-pentreotide]. Another routinely used application of this radioisotope is for evaluating patients suspected of having abscesses with autologous human leukocytes labeled in vitro with (111In)-oxine.
In the literature there are 17 experimental studies using this isotope in combination with different Bn analogs and linkers in order to obtain a radiopeptide suitable for its use in nuclear medicine for imaging, primarily concentrating on the detection of prostate or breast cancer by SPECT imaging (Tables 5, 6). Among all the Bn analogs tested two agonist were present in 71% of the studies: Bn (7–14) or [Pro1, Tyr4]Bn (Table 2, Fig. 1).
In an early study [72] with Bn (7–14) the characteristics of 5 111In-DOTA-radiolabeled peptides [Analogs #12–16, Tables 5,6], which were conjugated by different spacers, were analyzed. In each of the 5 peptides 111In was bound to the linker DOTA (Fig. 1), but the spacer between DOTA and the Bn analog ranged between no spacer to one of 5 to 8 carbon atoms. Three of them showed a nanomolar IC50 [β-alanine [βAla], 5-aminopentanoic acid [5-Ava] and 8-aminooctanoic acid [8-Aoc] spacers, Table 1], which was similar to Bn (7–14) (IC50: 1–3 nM), one had a 32.5-fold decreased affinity (65 nM) (11-aminoundecanoic acid [11-Aun], Table 1) and the DOTA-peptide without a spacer showed a 55-fold decrease (IC50: 111 nM) compared to Bn (7–14). Cell internalization was measurement with just one of the analogs [111In-DOTA-8-Aoc-Bn(7–14)] [Analog #15, Table 5] and was high (72% after 2h). Biodistribution was studied with all 5 Bn derivatives in normal animals [Analogs #12–16, Tables 6] [72], and the 8-Aoc derivative demonstrated the highest pancreatic uptake. In mice bearing PC-3 xenografts biodistribution was determined just for the 111In-DOTA-8-Aoc-Bn(7–14) [Analog #15, Table 6] and high tumor uptake and tumor/non-tumor ratio were found [72].
In another study from the same group [73], the same radiolabeled Bn derivative (111In-DOTA-8-Aoc) was compared with another set of Bn derivatives [Analogs #19–24, Table 5, 6], each containing DOTA as the linker, but varying in the spacer structure (5-amino-3, 6-dioxaoctyl-succinamic acid [5-Aos], 8-amino-3-oxapentyl-succinamic acid [8-Ads], aminobenzoyl [AMBA], glycine-aminobenzoyl [Gly-AMBA] (Table 1) and glycine-p-aminomethylbenzoic acid [Gly-AM2BA] (Table 1). In most cases the IC50 obtained was in the nM range and was similar to Bn (7–14), however with 5-Aos as the spacer, a 3-fold decrease in affinity (6.2 nM) was seen compared to Bn (7–14). The 8-Aoc and Gly-AM2BA derivatives demonstrated a 4 and 2.9-fold increase in affinity compared to Bn (7–14) (0.51 and 0.7 nM respectively) [Analogs #19 and 24, Table 5]. The Gly-AM2BA analog showed the lowest rate of internalization. When in vivo biodistribution and imaging studies of these analogs were performed [Analogs #19–24, Table 6], in normal animals the highest pancreas uptake was observed with Gly-AMBA and AMBA, followed by the 8-Aoc derivative, which had the highest tumor uptake rate. When SPECT studies were performed with 8-Aoc, AMBA, Gly-AMBA and Gly-AMB2A radiolabeled analogs [Analogs #19, 23 and 24, Table 6], each produced a clear image of the tumor. The analogs with an aromatic group demonstrated higher GI retention (AMBA, Gly-AMBA and Gly-AM2BA).
In another set of studies [2,46] different Bn (7–14) radioconjugate derivatives [Analogs #1,2 and #8–11, Table 5, 6] were compared with each other and also with the 111In labeled Bn antagonist [H-DPhe6, Sta13, Leu14]Bn (7–14) [RM1, Table 1,2] and a 99mTc labeled Bn antagonist [(N4-bzlg)0, DPhe6, LeuNHEt13, desMet14]Bn (6–14), Fig. 1 [99mTc-Demobesin1, Table 1,2]. When studies of binding were performed, affinity was high (IC50: 0.8 nM) for the Bn agonist (AMBA, #1, Table 5), near that seen with Bn(7–14), and was 40-fold greater than with the antagonist RM1 (IC50: 35 nM). The same difference was observed for the internalization rates, but when the in vivo biodistribution and imaging studies were performed [Analogs #1,2 and #8–11, Table 6], the radiolabeled antagonists (111In-RM1 and 99mTc-Demobesin1) [Analog #2, Table 6] had greater tumor uptake, tumor/non tumor ratio and tumor retention.
The first study using a [Pro1, Tyr4]Bn analog radiolabeled with 111In by the linker DTPA (Table 2, Fig. 1) was published in 1999 [48][Analog #34, Table 5], in which this radiolabeled Bn analog was compared with a Bn antagonist [[Tyr5, DPhe6]Bn(5–13)ethyl amide, (Table 1, Analog #35, Table 5)], also radiolabeled with 111In using a DTPA linker, and both were tested in the 7315b rat pituitary tumor cell line, AR42J cells and CA20948 cells. The results demonstrated that the Bn agonist ([Pro1, Tyr4]Bn) had a slightly higher affinity for the GRP receptor (8 vs 11 nM) and the agonist was internalizated, whereas the radiolabeled antagonist [Tyr5, DPhe6]Bn(5–13)ethyl amide, was not. This was also demonstrated by biodistribution studies [Analog #34, Table 6] that the Bn agonist showed a much higher specific uptake by GRP receptor tissues and by tumors, which could be detected by ex-vivo scintigraphy.
The same group published another biodistribution study [74] [Analog #31, Table 6] with this radiolabeled Bn agonist (111In-DPTA-[Pro1, Tyr4]Bn), using as xenograft tumors the colon cancer cell line CC531 and the pancreatic cancer cell line CA20948. The results showed that in normal animals the highest uptake was observed in the pancreas, followed by kidneys and bladder (demonstrating a renal clearance of the radiopeptide). When the radioconjugate was injected into animals bearing the CC531 cancer cell line or the CA20948 cancer cell line, it was taken up by the tumor in a specific way and it was visualized scintigraphically. When this Bn radiopeptide was compared with other 111In hormone radiopeptides (111In-DPTA-Octreotide, 111In-DOTA-CCK, 111In-DTPA-[Arg1]Substance P) [75] [Analog #29, Table 5] in the pancreatic cancer cell line CA20948, the Bn derivative had the highest internalization rate, and the second highest tumor uptake ratio in Lewis rat bearing CA20948 tumors, after 111In-DPTA-Octreotide [Analog #29, Table 6].
In another study four [76] 111In radiolabeled [Tyr4]Bn analogs [Analogs #3–6, Table 5] were tested ([Pro1, Tyr4]Bn or [εLys1, Tyr4]Bn) (Table 2) which were linked to the isotope with DOTA or DPTA. All the Bn analogs had an IC50 of 3–9 nM near that of the [4Tyr]Bn (Table 2) value (1 nM). When the analogs were labeled with the isotope through the linker, the 4 resultant radiopeptides agonists were all internalized with the DPTA-[Pro1, Tyr4]Bn having the highest value. The biodistribution study [Analogs #3–6, Table 6] showed that DOTA-[Pro1, Tyr4]Bn had the highest tumor uptake and tumor/blood ratio, followed by the DPTA-[Pro1, Tyr4]Bn radiopeptide. However, the authors concluded that, as 111In labeled DTPA-[Pro1, Tyr4]Bn is easier to handle, this should be the radiopeptide chosen for future studies.
Attempting to improve some characteristics of this radiopeptide (111In- DTPA- [Pro1, Tyr4]Bn), it was compared with 2 others Bn agonist analogs ([βAla11, Phe13, Nle14]Bn(7–14) and [βAla11, Tha13, Nle14]Bn(7–14); Nle: Norleucine, Tha: β-(2-thienyl)alanine; Tables 1,2) [Analogs #36–41, Table 5] [77] which were combined with different DPTA-spacers (1,2-diaminopropionic acid (DDpr), 4-aminocarboxymethylpiperidine-Tha (ACMpip-Tha), 1-aminoethy-l,4-carboxymethylpiperazine (Acp) and DTha, Table 1). Among them, the best affinity, internalization and stability values were obtained with DPTA-ACMpip-Tha-[βAla11, Phe13, Nle14]Bn(7–14) in both prostate PC-295 or pancreatic CA20948 cancer cell lines, with a 25-fold (IC50: 0.1 nM) and 5.6-fold increase (IC50: 0.4 nM) in the GRP receptor affinity with respect that of Bn(7–14). In vivo biodistribution studies in rats bearing PC-3 cell xenografts [Analogs #36 and #38, Table 6] comparing 111In-DPTA-ACMpip-Tha-[βAla11, Phe13, Nle14]Bn(7–14) with 111In-DPTA-[Pro1, Tyr4]Bn, demonstrated that the former analog had higher pancreatic and tumor uptake, tumor/non-tumor ratio and lower kidney retention. Furthermore with 111In-DPTA-ACMpip-Tha-[βAla11, Phe13, Nle14]Bn(7–14), tumors were clearly visualized by SPECT/CT, suggested this new radiolabeled Bn analog as a new candidate for use in nuclear medicine.
111In-DPTA-[Pro1, Tyr4]Bn has been also use in one study [Analog #33, Table 5][78]. The results showed that androgen ablation in animals bearing PC-3 tumor cells produced a decreased in the expression of GRP receptor in the tumor, and it also reduced the radiopeptide uptake by the tumor. This suggests that hormonal therapy may affect GRP receptor expression in prostate cancer tissue making GRP receptor-based imaging and therapy especially suitable for non-hormonally treated prostate cancer patients.
Another Bn analog that has been tested as a 111In-radiolabeled agonist peptide is [DTyr6, βAla11, Thi11, Nle14]Bn(6–14) Table 2; Thi: 3-(2-thienyl)alanine, Table 1). In one study [50] [Analog #7, Table 5] this Bn analog was linked to the isotope by the linker DOTA0–1, PEG0 (Z-070) and it was compared with another Bn analog that was labeled with 99mTc (99mTc-Demobesin1, Table 1,2). The results demonstrated that Demobesin1 had 11–14 fold higher affinity for the GPRP receptor in PC-3 cells, but had a similar affinity in AR42J cells compared to Z-070. The same result was observed in the case of mice bearing PC-3 or AR42J xenografts [Analog #7, Table 6]. In another study with [DTyr6, βAla11, Thi11, Nle14]Bn(6–14) [79][Analogs 17–18, Table 5] the effect of two different linkers (DOTA-γ-aminobutyric acid (GABA) or DPTA-GABA, Table 1) was examined. The DOTA linked Bn analog had a lower affinity (1.4±0.1 nM vs. 3.5±0.32 nM), but both of them showed similar internalization rates and their half-life were similar. In vivo biodistribution studies [Analogs #17–18, Table 6] showed a high uptake by GRP receptor expressing tissues and tumor with also high values in the ratio tumor/non-tumor.
Another 111In labeled Bn antagonist studied was Bomproamide [47] ([DPhe6, Leu-NHEt13, des-Met14]Bn(6–14), Table 2), a GRP receptor antagonist, coupled to the isotope by the linker DOTA-aminohexanoyl. This radiolabeled Bn antagonist analog [Analog #26, Table 5] showed a nM IC50 value (1.36±0.09 nM) similar to that of Bn(7–14), but had a low internalization level. In vivo experiments with mice bearing PC-3 xenografts demonstrated that the Bn antagonist radiopeptide [Analog #26, Table 6] showed high and rapid uptake by pancreas and tumor, which could be clearly visualized by SPECT/CT.
111In-DPTA-[Lys3, DTyr4]Bn(2–14) [Analog #32, Table 5, Table 2] is another radiopeptide Bn agonist tested [80] for its possible use in imaging and treating tumors expressing GRP receptors. The experiments with this compound showed an IC50 of 1.05 nM in PC-3 cells, similar to that of Bn(7–14), with internalization values near 60% and a long half-life (59.4% after 8 h in rat plasma). When the radiopeptide was injected into mice bearing PC-3 cell xenografts [Analog #32, Table 6], a high uptake by tumor, adrenal gland, pancreas and intestine was observed.
III. E. Review of 64Cu -labeled Bn analog in vitro (Table 7) and in vivo (Table 8) studies of Bn receptor-mediated imaging/cytotoxicity studies
Table 8.
In vivo studies with 125I, 185/187Re, 18F, 64Cu, 68Ga and 90Y bombesin analogs.
| Peptide # | Isotope | Linker | Peptide | In vivo | Ref. N | |||
|---|---|---|---|---|---|---|---|---|
| Animal | Biodistribution | Imaging | Comment | |||||
| 1 | 125I | mIP | Bn | SKOV3.ip1-AdCMV-GRPR tumor bearing Athymic nu/nu mice BALB/c | Similar biodistribution between 125I and 131I. | no | 131I cmpd has good tumor uptake but a short effective half life in tumor, too. Therapeutic response with repeated administration. | [119] |
| 2 | 131I | Bn | no | |||||
| 6 | 125I | no | [Tyr4]Bn | SKOV3.ip1 tumor bearing athymic nude mice | In normal mice clearance for both cmpds was rapid. mIp cmpd undergoes a slower deiodination than the other cmpd In tumor bearing mice mIP showed a faster and better tumor uptake than the other cmpd | no | SKOV3.ip1 injected in mice oveexpressed AdCMVGRPR construct. Difference in tumor uptake between two cmpds is likely due to the different rate of deiodination, | [118] |
| 7 | 125I | no | Balb/c mice | no | ||||
| 8 | 125I | mIP | [des-Met14]Bn [Antagonist] | no | ||||
| 9 | 125I | mIP | SKOV3.ip1 tumor bearing athymic nude mice | no | ||||
| 15 | 188Re | N3S-5-Ava | Bn(7–14) | CF-1 mice | Similar distribution for all compd. Differences observed at 4h PI. Clearance via renal and liver pathways. | no | 188,186Re coniugates are useful targeting GRPR +tissues. NCA186,188Re have higher specificity than CA 186Re | [121] |
| 16 | 186Re | N3S-5-Ava carried | no | |||||
| 17 | 186Re | N3S-5-Ava non-carried | no | |||||
| 24 | 18F | 2-(4-(di-tert-butylfluorosilyl)phenyl)acetyl-Arg-Ava- | [NMeGly11, Sta13, Leu14] Bn(7–14) (4b) [Antagonist] | PC-3 tumor bearing nude mice | Low uptake and clearance. High accumulation in liver, gallbladder, intestine | no | Cmpd synthesized by silicon-based one-step labeling protocols a new labeling method for synthesis of new 18F-labeled Bn derivatives. | [102] |
| 26 | 18F | FB | Bn | PC-3 tumor bearing nude mice | High tumor specificity of dual FB-Bn/RGD cmpd | microPET for distribution and metabolic stability | dual tracer has more than additive effect in vivo for tumor uptake. | [100] |
| 28 | 18F | FB-PEG3-Glu | Bn (7–14)-RGD | PC-3 tumor bearing nude mice | Hight tumor specificity. Low liver uptake. Excretion by kidney |
microPET for distribution and metabolic stability | PEG3 spacer improves renal clearance respect to non-PEGylated cmpd (see [100]) | [101] |
| 30 | 18F | FB | [Lys3]Bn | PC-3 tumor bearing nude mice | Tumor-to-nontarget ratios of Lys3-Bn cmpd were higher that those of Aca-Bn(7–14) cmpd Renal clearance, with higher hepatobiliary accumulation for Aca-Bn(7–14). | microPET 10 min after injection. PET and CT imaging of orthopic PC-3 tumor model 17min after injection. | FB-Lys3Bn has higher efficacy in targeting the tumor. Useful for orthopic prostate Ca. imaging and GRPR+ tumors. | [99] |
| 31 | 18F | FB-Aca | Bn(7–14) | |||||
| 34 | 64Cu | DOTA | MP2346 | PC-3 tumor athymic nude mice | Rapid clearance, within 24h. Uptake and specificity lower than 86Y-labeled cmpd | PET/CT imaging 1 and 24 h after injection. | Low tumor/non-tumor ratios. High background and liver accumulation. | [87] |
| 35 | 64Cu | DOTA | [Lys3]Bn | PC-3 tumor athymic nude mice | Rapid and predominant
renal clearance. Significant uptake in tumor and pancreas. |
microPET and
autoradiographic imaging. Comparable uptake indices between microPET ROI and quantitative autoradiography analyses. |
PET imaging of prostate Ca. with 64Cu-labeled Bn analogs is useful to determine dosimentry and tumor response to the same ligand labeled with therapeutic amounts of 67Cu for internal radiotherapy. | [85] |
| 36 | 64Cu | CWR22 tumor athymic nude mice | ||||||
| 37 | 64Cu | DOTA-8-Aoc | Bn(7–14) | PC-3 tumor bearing SCID mice | Clearance through renal system. | microPet, fused
microPET/CT and axial images. Significantly reduced activity of CB-TEA-8-Aoc cmpd in kidney and gastrointestinal tract |
Better clearance of CB-TEA-8-Aoc than DOTA-8-Aoc cmpd Even the last shows an higher retention. | [90] |
| 38 | 64Cu | CB-TE2A-8-Aoc | ||||||
| 39 | 64Cu | TACN-β Ala-β Ala | [Cha13, NLe14] Bn(7–14) | Wistar rats | high uptake and rapid renal clearance 5′ and 60′ single ID | PET images 60′ after single ID | Further exp. are necessary to evaluate specificity of the binding and tumor uptake. TACN as linker showed interesting results for developing new Cu-radiopharmaceuticals. | [93] |
| 40 | 64Cu | bispidine derivatives | [Cha13, NLe14] Bn(7–14) | Wistar rats | Significant retention in kidney; high stability | no | Bifunctional bispidine cmpds are new versatile Bn-derivatives of copper radiopharmaceuticals useful for both diagnosis and therapy. | [94] |
| 41 | 64Cu | PC-3 tumors bearing mice | no | PET imaging. | ||||
| 42 | 64Cu | DOTA | Bn (7–14)-RGD | PC-3 tumors bearing mice and Balb normal mice | Analysis in Balb normal mice. High pancreatic uptake. | Small animal PET in PC-3 tumor bearing mice. Rapid clearance by blood. High kidney uptake and tumor/non-tumor ratio. | NOTA-RGD-Bn has an higher tumor uptake and tumor/non-tumor ratio and lower liver uptake than DOTA-RGD-Bn cmpd | [95] |
| 43 | 64Cu | NOTA | ||||||
| 45 | 64Cu | DOTA-GGG | Bn(7–14) | PC-3 tumors bearing mice | Good blood clearance except for GSG cmpd which has constant but low values. GSG cmpd has a low tumor uptake | Small animal PET/CT images 1 and 24h after I.V. | Agreement bethween
biodistribution and imaging studies. Additional serines in the linker cause lower liver uptake. |
[89] |
| 46 | 64Cu | DOTA-GSG | ||||||
| 47 | 64Cu | DOTA-GSS | ||||||
| 50 | 64Cu | DOTA-Aba | Bn(7–14) | T-47D tumor bearing SCID mice | Rapid bood clearance. High pancreas and tumor uptake. Very similar tumor/non tumor ratios between cmpds | no | A reduced carbon linker length reduced both liver uptake and tumor uptake. | [88] |
| 52 | 64Cu | DOTA-Ahx | MicroPET imaging 1h after I.V. | |||||
| 53 | 64Cu | DOTA-Aoc | no | |||||
| 54 | 64Cu | DOTA-Ado | ||||||
| 55 | 64Cu | NOTA-8-Aoc | Bn(7–14) | CF-1 mice | Rapid blood clearance and excretion by kidney. | no | Promising cmpd for both molecular imaging and therapy. | [91] |
| 56 | 64Cu | PC-3 tumors bearing SCID mice | Good tumor uptake. High specificity and affinity | MicroPET 24h after P.I. Strong site direct PET targeting agent for prostate Ca. | ||||
| 57 | 64Cu | NO2A-8-Aoc | Bn(7–14) | CF-1 mice | High pancreas uptake after 1h I.V. | no | NO2A-compd. useful for breast therapeutic analyses. Very fine structure. | [92] |
| 58 | 64Cu | T-47D tumor bearing SCID mice | High pancreas uptake with a low intestine uptake. | MicroMR and microPET/CT imaging
analyses. Pancreas most visible. |
||||
| 59 | 64Cu | DOTA-Aoc | Bn(7–14) | PC-3 tumors bearing mice | Excellent liver and pancreas uptake. Good tumor uptake. Rapid blood clearance. | MicroPET imaging. Clear tumor visualization | Necessary to reduce liver and normal tissue uptake for imaging and therapeutic purposes. | [83] |
| 60 | 64Cu | DOTA-PEG | Bn(7–14) | Normal athymic nude mice | Only significant difference in uptake and retention is in the bone, kidneys and blood. High pancreas uptake. | no | Higher PEG-cmpd uptake than expected. | [84] |
| 61 | 64Cu | DOTA-Aoc | no | |||||
| 62 | 64Cu | DOTA | [Lys3]Bn | PC-3 tumor athymic nude mice | Obtained by PET images. Liver uptake higher that other organs. Aca-linker reduces pancreas affinity | micoPET images | DOTA-[Lys3]Bn has high affinity and moderate metabolic stability. Specific tumor localization with good tumor/non tumor ratios. It is superior to DOTA-Aca-Bn(7–14) cmpd | [86] |
| 63 | 64Cu | DOTA-Aca | Bn(7–14) | |||||
| 64 | 64Cu | DOTA | [Lys3]Bn | 22Rv1 tumor athymic nude mice | ||||
| 65 | 64Cu | DOTA-Aca | Bn(7–14) | |||||
| 66 | 68Ga | NOTA-Aca | Bn (7–14)-RGD | PC-3 tumor bearing mice | High tumor and pancreas uptake. | MicroPET images. Low uptake in PC-3 tumors. Excretion by kidneys. | Dual receptor binding cmpd Demonstrated to be useful in tumors where only one of the receptors is over-expressed. | [105] |
| 67 | 68Ga | MDA-MB-435 tumor bearing mice | MicroPET images. Low expressing GRPR system. Good tumor uptake. | |||||
| 68 | 68Ga | DOTA-PEG2 | [D-Tyr6,β-Ala11, Thi13, Nle14] Bn(6–14) (BZH3) | AR42J tumor bearing mice | Dose dependent uptake of high expressing GRPR tissues. Fast blood clearance. | PET images 1h after injection. Clear definition of tumor tissue and low uptake of non target tissues. | With well defined and low background images, BZH3 has prerequisites as a helpful cmpd in GRPR+ tumors. | [104] |
| 69 | 68Ga | DOTA-PEG4 | Bn(7–14) | PC-3 tumor bearing mice | Rapid blood clearance. High uptake in tumor and pancreas. High retention in kidneys. | PET and Scintigraphy imaging show high
uptake in tumor, pancreas and kidneys. Scintigraphy |
PEG4 spacer form suitable cmpd for clinical studies. | [103] |
| 72 | 86Y | DOTA | MP2346 | PC-3 tumor bearing athymic mice | High level of tumor/non tumor ratios. Specific uptake. No optimal kidneys uptake. More favorable distribution than 64Cu cmpd | PET/CT images. Low background uptake. Results consistent with biodistribution studies. | To reduce renal uptake and improve clearance 86Y-MP2346 has to be altered to have a neutral or negative charge. | [87] |
| 73 | 86Y | DOTA | AR42J tumor bearing rats | PET/CT images. Low bakcground uptake. Higher quality than 64Cu cmpd | ||||
| 74 | 90Y | DOTA | Bn(214) | normal Swiss mice | Efficent clearance from blood Excretion by kidneys. Good pancreatic uptake. | no | Preference toward 177Lu cmpd rather than 90Y cmpd | [107] |
All peptides not indicated as antagonist are agonist at human Bn receptors.
Cell lines: SKOV3.ip1, Ovarian carcinoma; T47D, Human breast cancer cell; CWR22, Human prostate cancer cells; 22RV1, Human prostate carcinoma; PC-3, Human prostate cancer cells; AR42J, Rat pancreatic acinar cell tumor
Studies attempting to identify useful Bn analogs for imaging studies using Positron emission tomographic scanning (PET) of bombesin receptors started lately compared to the development of Bn radiolabeled analogs for SPECT imaging. 64Cu is a positive charged radioisotope, which is used both for imaging (PET scanning) and therapy [81,82]. It is produced using a medical cyclotron, and has a half-life of 12.7 h, with an emission at 0.651MeV and decay at 0.578 MeV β− [81]. The first study that attempted to identify a Bn analog labeled with 64Cu was in 2003[83], subsequently 13 additional studies (Tables 7 and 8) reported different 64Cu-radiolabeled Bn analogues and their pharmacokinetics properties and imaging efficacy. Finding in these studies are summarized in Tables 7 and 8, for in vitro and in vivo results, respectively.
Roger et al., studied the in vitro and in vivo characteristics of the 64Cu-DOTA-Aoc-Bn(7–14) (Aoc=aminooctanoic acid) analog [Analog #59. Table 7, 8] (Tables 1, 2,, Fig. 1.) [83]. This analog showed in the prostate cancer cell line, PC-3, high affinity for the hGRP receptor (Kd 6.1±2.5 nM) and a rapid internalization rate [Analog #59, Table 7]. In vivo biodistribution studies and microPET imaging, performed in athymic mice bearing human prostate cancer xenografts, PC-3, showed a rapid tumor uptake as well as in other tissues such as liver, pancreas and intestine, resulting in a low tumor-non-tumor ratio [Analog #59, Table 8] [83]. Therefore, the authors performed an in vivo determination of blood flow, finding that it is 2.6-fold lower to the PC-3 tumor than to the pancreas. These results could explain a limited diffusion of the 64Cu-DOTA-Aoc-Bn(7–14) analog and, consequently, a low uptake and binding of the radioligand by the tumor [83]. Finally, the authors suggest modifying the charge of the peptide and the peptide linker group, in order to reduce the amount of the 64Cu-radiolabeled peptide taken up by normal tissues [83].
To improve the specificity of the 64Cu- DOTA-Aoc-Bn(7–14) analog the same group modified it by substituting PEG (ethylene glycol [2-aminoethylcarboxymethylether]) instead of Aoc (Table 1, Fig. 1.). The hypothesis was that this modification could improve the pharmacokinetics of the conjugate, in particular, for its delivery to the tumor site [84]. The authors evaluated in vivo and in vivo properties of 64Cu-DOTA-PEG–Bn(7–14) vs 64Cu-DOTA-Aoc-Bn(7–14) analogs [Analogs #60–61, Tables 7, 8], using the PC-3 cell line and normal athymic nude mice. Competitive binding assay results for [Tyr4]-Bn (used as control), DOTA-Aoc-Bn(7–14) and DOTA-PEG-Bn(7–14) showed a strong reduction of binding affinity toward hGRP receptor of the new conjugates (IC50 18.8±2.3 nM, 90.5±22 nM, 3.9±0.6 μM, respectively) [Analogs #60–61, Table 7]. Furthermore, 64Cu-DOTA-PEG–Bn(7–14) also showed a reduction in the internalization rate compared to 64Cu-DOTA-Aoc–Bn(7–14) analog, mostly due to the lack of affinity of PEG-conjugate [84]. Finally, in vivo studies found 64Cu-DOTA-PEG-Bn(7–14) had a faster blood clearance than expected and a specific, but reduced pancreatic uptake compared to 64Cu-DOTA-Aoc-Bn(7–14) (1.3-fold less) [Analogs #60–61, Table 8] [84].
Chen et al. studied the in vitro and in vivo characteristics of the 64Cu-DOTA-[Lys3]Bn agonist conjugate [Analogs #35–36, Tables 7, #35 Table 8] (Tables 1,2, Fig. 1.) [85]. Using the human androgen independent (AI) prostate cancer cell line PC-3, they found a high binding affinity to hGRP receptor (IC50 2.2±0.5 nM) and internalization rate [Analogs #35–36, Table 7] [85]. In vivo studies were performed in human prostate cancer carcinoma xenografts induced by injection of either the AI-PC-3 cell line or the androgen dependent (AD) CWR22 cell line. The radiopeptide uptake was specific and it displayed a predominant renal clearance. Interestingly, the uptake was higher in the AI-PC-3 xenografts than in AD-CWR22 xenografts [Analog #35, Table 8] [85]. MicroPET and autoradiography imaging for both models showed a very high tumor-to- background ratio, although tumor and pancreas accumulation was lower compared to normal biodistribution studies [Analog #35, Table 8] [85].
To investigate the possibility whether a truncated coupled Bn analog, Bn(7–14) was more suitable for PET imaging purposes than full-length Bn, Yang et al. performed a compared in vivo and in vitro evaluation of 64Cu-DOTA-Aoc-Bn(7–14) and 64Cu-DOTA-[Lys3]Bn agonist conjugates [Analogs #62–63, Table 7; #62–65, Table 8] (Tables 1,2, Fig. 1.) [86]. In PC-3 cells the DOTA-[Lys3]Bn compound displayed a higher binding affinity than the DOTA-Aca-Bn(7–14) compound (IC50 2.2±0.5 vs. 18.4±0.2 nM) [Analogs #62–63, Table 7]. Moreover, the internalization rate and retention was much higher for the full-length compound [Analogs #62–63, Table 7] [86]. In vivo studies showed more stability in mouse blood, urine, tumor, liver and kidney samples from PC-3 tumor bearing mice for the full length Bn compound and a higher liver and intestinal uptake for the Bn(7–14) analog [Analogs #62–65, Table 8] [86]. MicroPET images showed a low background radioactivity for 64Cu-DOTA-[Lys3]Bn but it still displayed a significant accumulation in intestine and rapid renal clearance [Analogs #62–65, Table 8] [86].
In a study aimed to identify a new Bn radioligand labeled with 111In, the agonist DOTA-[Pro1, Tyr4]Bn(1–14) (MP23436) was synthesized and characterized (Tables 1,2. Fig. 1.) [76]. Next, Biddlecombe et al. evaluated both 64Cu and 86Y-radiolabeld MP2346 conjugates [Analogs #34 and 71, Table 7; #34, 71,72, Table 8] [87]. An in vitro study in the PC-3 cell line showed a good internalization rate for both compounds, with an initial slower rate for 86Y-MP23436 that becomes 3-fold higher at 20 h with the 64Cu-radioligand [Analogs #34 and 69, Table 7] [87]. The in vivo biodistribution in PC-3 tumor bearing mice was associated with a higher uptake for the 86Y-conjugate and consistent levels of 64Cu compound in liver, mostly caused by transchelation of the copper [Analogs #34 and 69, Tables 8]. Finally, PET images showed a better tumor-normal tissue ratio for 86Y-MP23436. These results were attributed to the physical and chemical properties of 64Cu and 86Y metals [Analogs #34 and 69, Table 8] [87].
Parry et al. evaluated a series of Bn analogs coupled to 4 to 12-carbon linkers in the human breast cancer cell line, T-47D [Analogs #50–54, Tables 7, 8] [88]. In vitro binding affinity in these cells showed a high affinity of the DOTA-Aoc-Bn(7–14) compound (Table 1,2. Fig. 1.) (IC50 6.7 nM) compared to others and a very low affinity for the Aba-linker containing compound (4-carbons) (IC50 78.5 nM) [Analogs #50–54, Table 7] [88]. The internalization rate displayed a very low value for DOTA-Ado-Bn(7–14) compound (12-carbons), in spite of its binding affinity. The authors concluded that the presence of 12 carbon spacer in Ado-compound could improve the GRP receptor affinity, but lead to a tridimensional conformation that inhibited its internalization [Analogs #50–54, Table 7] [88]. In vivo experiments in T-47D tumor bearing mice showed that Aoc, the 8-carbon linker compound had the highest tumor uptake, but also had high liver uptake. Moreover, PET images showed that 6-and 8- carbon containing linkers had a good tumor uptake suggesting that further modifications are necessary to optimize the use of Bn radiolabeled analogs for breast cancer imaging [Analogs #50–54, Table 8] [88].
The same group studied the effect of the presence of various amino acid spacers between DOTA and truncated Bn(7–14) compound when labeled with 64Cu [89]. 64Cu-DOTA-X-Bn(7–14) (Tables 1,2. Fig. 1.) containing in the X-position three amino acid combinations of non polar glycine (G), polar serine (S) or negatively charged glutamic acid (E) [Analogs #45–49, Table 7; #45–47, Table 8] [89]. The presence of a negative charged E strongly reduced GRP receptor binding affinity and the internalization rate of the compound in PC-3 cell lines, so the author focused their in vivo studies using GGG, GSG and GSS- containing conjugates [Analogs #45–49, Table 7] [89]. MicroPET images from PC-3 tumor bearing mice, displayed a high tumor, liver and kidney uptake for the GGG-containing radioligand, while the two GSG- and GSS- radioligand showed a better tumor-normal tissues ratio. In particular, 64Cu-DOTA-GSS-Bn(7–14) had the longer retention compared to the other two conjugates [Analogs #45–47, Table 8] [89]. The presence of a serine amino acid linker seemed to decrease the lipophilicity and liver uptake; on the other hand, there was an increase of abdominal accumulation, compromising the tumor-non-tumor ratio [89].
To reduce transchelaton of 64Cu, Garrison et al. attempted to use a different chelator, CB-TE2A (1,4,8,11-tetraazabicyclo[6.6.2]hexadecane-4,11-diacetic acid) (Table 1, Fig. 1) [90]. They compared in vitro and in vivo properties of -8-Aoc-Bn(7–14) either chelated with CB-TE2A or DOTA [Analogs # 37–38, Tables 7, 8]. In PC-3 cells, 64Cu-CB-TE2A-8-Aoc-Bn(7–14) showed higher binding affinity than 64Cu-DOTA-8-Aoc-Bn(7–14) (IC50 0.5 vs. 1.4 nM, respectively) [Analogs # 37–38, Table 7] [90]. Furthermore, in internalization experiments performed in the same cell line, the 64Cu-CB-TE2A radioconjugate showed a faster internalization rate than 64Cu-DOTA radioconjugate [Analogs # 37–38, Table 7] [90]. In vivo experiments conducted in SCID mice bearing PC-3 xenografts, showed a rapid uptake for both compounds, but a significant rapidly clearance for the 64Cu-CB-TE2A-compound. Moreover, microPET images displayed a tumor-non-tumor ratio higher for 64Cu-CB-TE2A-8-Aoc-Bn(7–14) [Analogs #37–38, Table 8] [90].
Another group conjugated the 8-Aoc-Bn(7–14) compound with 64Cu-NOTA (1,4,7-triazacyclononanetriacetic acid) [Analog #55, Table 7; analogs #55–56, Table 8] (Table 1, Fig. 1.) [91]. In vitro binding affinity of 64Cu-NOTA -8-Aoc-Bn(7–14) in PC-3 cell line displayed an IC50 of 3.1 nM, very close to the Bn and Bn(7–14) affinities to hGRP receptors [Analog #55, Table 7] [91]. In vivo studies in CF-1 normal mice demonstrated very fast blood clearance and a significance renal excretion [Analogs #55–56, Table 8]. Moreover, 64Cu-NOTA-8-Aoc-Bn(7–14) (Fig. 1, Table 2) displayed a specific uptake in GRP receptor-expressing tissues such as mouse pancreas. MicroPET images and in vivo biodistribution studies in SCID mice bearing PC-3 tumors also showed high and specific uptake of 64Cu-NOTA -8-Aoc-Bn(7–14) in tumors, with a reduced liver accumulation, which points out that the use of NOTA as chelator strongly reduces the possibility of any dissociation phenomenon for 64Cu [Analogs #55–56, Table 8] [91]. The same group, has recently reported another study in a breast cell cancer model, using the 64Cu-NO2A-8-Aoc-Bn(7–14) conjugate [Analog #57, Table 7; Analogs #57–58, Table 8], where the chelator is NO2A (1,4,7-triazacyclononane-1,4-diacetate) (Table 1, Fig. 1) and compared it with a 64Cu-DOTA-8-Aoc-Bn(7–14) compound[92]. In vitro binding affinity studies were performed in the human breast cancer cell line T-47D and showed a 3-fold decrease in affinity compared to Bn(7–14) (i.e. 7.6 nM), but a very fast internalization rate [92] [Analog #57, Table 7]. In vivo evaluation of 64Cu-NO2A-8-Aoc-Bn(7–14) in normal CF-1 mice showed a fast and specific uptake in tissues expressing GRP receptors [Analogs #57–58, Table 8]. Moreover, microPET/CT and microMRI images of SCID mice bearing T-47D tumors showed high tumor-non tumor ratios for most of the tissues, with a reduced abdominal and liver accumulation, probably due to the hydrophobicity of the NOA2 chelators [Analogs #57–58, Table 8] [92].
Gasser et al. recently reported in a study a new ligand derivative TACN ((2-[4,7-bis(2-pyridylmethyl)-1,4,7-triazacyclononan-1-yl]acetic acid) (Table 1,, Fig. 1) coupled to the Bn agonist analog, βAla-βAla – [Cha13, Nle14]Bn(7–14) (Tables 1, 2) [Analog #39, Tables 7, 8] [93]. In vitro stability studies showed that 64Cu-TACN- βAla-βAla–[Cha13, Nle14]Bn(7–14) had high stability in presence of an excess of either the competing ligand (cyclam) or the copper-seeking superoxide dismutase (SOD). Similar results were obtained by in vivo stability studies in rat plasma [Analog #39, Table 7] [93]. Furthermore, the authors performed in vivo biodistribution studies in Wistar rats and showed a high uptake in pancreas with a predominant renal excretion [Analog #39, Table 8] [93].
A similar study was also performed using the same Bn agonist analog [Cha13, Nle14]Bn(7–14) (Tables 1,2) but using as a chelator, a bispidine derivative (3,7-diazabicyclo[3.3.1]nonane) [Analogs #40–41, Tables 7, 8] [94]. In vivo studies in Wistar rats showed a very fast blood clearance and renal excretion, with low liver accumulation. Furthermore, NMRI nu/nu mice bearing PC-3 humane prostate tumors, displayed a tumor accumulation [Analogs #40–41, Tables 8] [94].
Liu et al. analyzed the synergistic effect of the dual-receptor targeting of a 64Cu-X-RGD-Bn radioligand targeting GRP receptors and αvβ3 integrin, using as chelators either DOTA or NOTA [Analogs #42–42, Tables 7, 8] (Table 1,2. Fig. 2) [95]. In PC-3 cells both 64Cu-DOTA-RGD-Bn and 64Cu-–NOTA-RGD-Bn conjugates displayed a comparable affinity to both the integrin αvβ3 and the hGRP receptor. They targeted αvβ3 integrin, because it has been demonstrated this integrin is involved in tumor angiogenesis in several tumors [95–97]. Although the IC50 values for hGRP receptor were relatively high (85.8 nM for DOTA compound and 92.7 nM for NOTA compound) [Analogs #42–42, Table 7], in vivo PET images in PC-3 bearing tumor mice showed a specific tumor uptake and lower liver accumulation for NOTA-compound (Fig. 1) [Analogs #42–42, Table 8]. In addition, biodistribution studies in normal Balb/c mice, confirmed high uptake in GRP receptors-expressing tissues, such as murine pancreas and a fast excretion, mostly by kidneys [Analogs #42–42, Table 8] [95].
Finally, Ma et al., recently, reported a study in which the Bn agonist [Lys3]Bn (Tables 1,2) and the somatostatin derivative [Tyr3]octreotate were compared. Each showed high stability in human serum for a long time period [Analog #44, Table 7] [98].
III. F. Review of 18F -labeled Bn analog in vitro (Table 7) and in vivo (Table 8) studies of Bn receptor-mediated imaging/cytotoxicity studies
18F is a suitable isotope used in positron emission tomography (PET) with a short half-life (110 minutes), used for labeling small molecules such as biologically active peptides and produced in small biomedical cyclotrons [82,99]. The radiolabeling process is complex but, since 18F has a small prosthetic group, coupling to the peptide or the chelator, strongly reduces the chance that alterations in the coupled peptides change biological properties [82]. Findings from studies reporting in vitro and in vivo use of 18F-Bn-radiolabeled conjugates are summarized in Tables 7 and 8.
Zhang et al. analyzed the properties of two Bn-derivatives, [Lys3]Bn and Aca-Bn(7–14), both coupled with 18F-SFB (N-succinimidyl-4–18F-fluorobenzoate) [Analogs #30–33, Table 7; analogs #30–31, Table 8] (Table 2, Table 1, Fig. 1)[99]. In vitro studies, performed in the human prostate cell line PC-3, showed that 18F-[Lys3]Bn had a higher affinity for GRP receptors than 18F-Aca-Bn(7–14) (IC50 3.3 vs 20.8 nM, respectively) [Analogs #30–33, Table 7]. Moreover, when coupling with FB (fluorobenzoate), the resulting conjugates, 18F-FB-[Lys3]Bn and 18F-FB-Aca-Bn(7–14) (Table 2, Table 1, Fig. 1) had IC50 values of 5.6 nM and 48.7 nM, respectively [Analogs #30–33, Table 7] [99]. Internalization experiments in PC-3 cells demonstrated these radioligands showed a rapid uptake rate, although this was followed by a high efflux rate, probably due to the strong lipophilic properties of the radioconjugates [Analogs #30–33, Table 7]. In vivo studies were performed only with 18F-FB-[Lys3]Bn and 18F-FB-Aca-Bn(7–14) conjugates. Biodistribution and microPET images studies in PC-3 tumor bearing athymic mice displayed a rapid blood clearance, an excretion through kidneys for both radiolabeled compounds and a significant liver and gallbladder accumulation for 18F-FB-Aca-Bn(7–14) compound [Analogs #30–31, Table 8]. This latter finding strongly reduces the suitability of its use for detecting orthopic prostate cancer, located very close to the urinary bladder [99]. The tumor uptake and the tumor-non tumor ratios were higher for 18F-FB-[Lys3]Bn than for 18F-FB-Aca-Bn(7–14) [99].
The same group reported another study aimed to increase the tumor-non-tumor tissues ratios synthesizing a dual-receptor targeting radioligand by targeting the GRP receptor and αvβ3 integrin, since αvβ3 is involved in the angiogenesis of most solid tumors [96,97,100]. The resulting ligand, 18F-FB-Bn-RGD (FB=fluorobenzoate; RGD=arginine-glycine-aspartate) (Table 2, Table 1, Fig. 1) was studied in comparison with the monomeric forms, 18F-FB-Bn and 18F-FB-RGD [Analogs #26–27, Tables 7, 8]. In vitro internalization rate in the human prostate cancer cell line PC-3, showed comparable behavior between 18F-FB-Bn and 18F-FB-Bn-RGD conjugates [Analogs #26–27, Table 7] [100].
Moreover, in vivo experiments in PC-3 tumor bearing mice, displayed a higher tumor uptake compared to the monomeric forms [Analogs #26–27, Table 8] [100]. To improve the in vivo kinetics, the same group synthesized another radioconjugate adding PEG3 (11-amino-3,6,9-trioxaundecanoic acid) (Table 1) as a spacer [Analogs #28–29, Tables 7, 8] [101]. In PC-3 cell line, the authors found that FB-PEG3-Glu-RGD-Bn (Glu=glutamate) (Table 2, Table 1, Fig. 1) had comparable binding affinity to GRP receptor with Aca-Bn(7–14) and PEG3-Glu-RGD-Bn (Tables 1,2) [Analogs #28–29, Table 7] [101]. Moreover, internalization studies in PC-3 cells showed that 18F-FB-PEG3-Glu-RGD-Bn had a fast internalization rate, mostly due to the binding to GRP receptors rather then integrin binding [Analogs #28–29, Table 7]. MicroPET images in PC-3 tumor bearing mice showed that 18F-FB-PEG3-Glu-RGD-Bn showed renal excretion, with a fast accumulation in kidneys. It also had a low accumulation in liver and high tumor-non-tumor tissues ratios [Analogs #28–29, Table 8] [101].
Höhne et al. synthesized 18F-Bn-derivatives radioligand using a silicon one-step method [Analogs #24–25, Tables 7, 8] [51]. The in vitro behavior of the GRP receptor antagonists 18F-2-(4-(di-tert-butylfluorosilyl)phenyl) acetyl-Arg-Ava- [NMeGly11, Sta13, Leu14] Bn(7–14) and the 18F-2-(4-(di-tert-butylfluorosilyl)phenyl) acetyl-Arg-Ava- [His(3Me)11, Sta13, Leu14]Bn(7–14) (Tables 1,2) was studied in PC-3 cells and revealed a very different GRP receptor affinity, (IC50 22.9 and 267.7 nM, respectively) [Analogs #24–25, Table 7] [51]. In vitro stability studies in PBS (phosphate buffered saline), mouse or human plasma showed no degradation products within 2 h for 18F-2-(4-(di-tert-butylfluorosilyl)phenyl) acetyl-Arg-Ava-[NMeGly11, Sta13, Leu14] Bn(7–14). Moreover, this result was supported by ex vivo stability studies in mouse blood at 10 and 30 minutes post-injection [Analogs #24–25, Table 7] [51]. Finally, in vivo biodistribution studies with the 18F-2-(4-(di-tert-butylfluorosilyl)phenyl) acetyl-Arg-Ava-[NMeGly11, Sta13, Leu14] Bn(7–14) antagonist, performed in PC-3 tumor bearing mice, displayed a low tumor uptake, however pancreatic uptake was high [Analogs #24–25, Table 8]. This study confirmed the use of a silicon-based one-step method to synthesize 18F-labeled Bn derivatives. Furthermore, the authors suggested an increase of lipophilic characteristics be considered to improve tumor uptake and, consequently, suitability for prostate tumor imaging, for these Bn radiolabeled compounds [51].
Becaud et al., from the same group, recently reported a study on the synthesis of two new 18F-radiolabeled Bn-derivatives: the antagonist ligand, 3-Cyano-4-[18F] fluorobenzoyl-Ava-[NMeGly11, Sta13, Leu14]Bn(7–14) and the agonist ligand, 3-Cyano-4-[18F] fluorobenzoyl-Ava- [FA(01010)13, Leu14] Bn(7–14) [Analogs #22–23, Table 7] (Tables 1,2) [102]. In vitro binding assay in PC-3 cells, showed a good affinity of these two radioligands for the GRP receptor compared to Bn and Bn(7–14) with IC50 of 2.7 nM for 3-Cyano-4-[18F] fluorobenzoyl-Ava-[NMeGly11, Sta13, Leu14]Bn(7–14) and 9.2 nM for 3-Cyano-4-[18F] fluorobenzoyl-Ava- [FA(01010)13, Leu14] Bn(7–14) [Analogs #22–23, Table 7] [102]. Mouse plasma stability studies indicated an excellent stability for both radioligands, especially for 3-Cyano-4-[18F] fluorobenzoyl-Ava-[NMeGly11, Sta13, Leu14]Bn(7–14). The results of this study pointed out that the one-step 18F-fluorination of Bn peptides is practical under mild conditions and produces a good yield of radiochemical compounds [102].
III. G. Review of 68Ga -labeled Bn analog in vitro (Table 7) and in vivo (Table 8) studies of Bn receptor-mediated imaging/cytotoxicity studies
The positron emitter 68Ga is a high β+ energy emitter (Eβ +max=1.9 MeV) with a short half-life (t1/2= 68 minutes) [82,103]. It has been used to label Bn analogs for PET imaging purposes because of its ease of production from 68Ge/68Ga generators. There are only three studies assessing the in vitro and in vivo properties of various 68Ga-Bn analogs conjugates and their results are summarized in Tables 7 and 8 [Analogs #66–68] [103–105].
In 2004, Shuhmacher et al. analyzed the Bn radiolabeled agonists 68/67Ga-DOTA-PEG2-[D-Tyr6,β-Ala11, Thi13, Nle14] Bn(6–14) (DOTA=1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraaceticacid; PEG2 = (2-aminoethyl)-carboxymethyl ether; 68/67Ga-BZH3) [Analog # 68, Table 7, 8] (Tables 1,2, Fig. 1) in vitro and in vivo behavior and compared the results obtained with the same peptide radiolabeled with the radiolanthanide 177Lu [104]. In vitro studies were performed in the pancreatic tumor rat cell line AR42J, using the 67Ga-BZH3 conjugate. They reported an hGRP receptor binding affinity of 0.46 nM for the new Bn analog [Analog #68, Table 7], which is three times higher affinity than that for the 125I-[Tyr4]Bn radiocompound, used as standard control. The internalization rate, in AR42J cells, was rapid and high (88% after 2 hours), confirming the agonistic nature of 67Ga-BZH3 radiopeptide [104]. Moreover, 67Ga-BZH3, as well as the 177Lu-BZH3 peptide, showed a high retention rate (t1/2=13.5 hours compared to 125I-[Tyr4]Bn for 3 hours), probably due to the linker used for coupling. Biodistribution studies, in AR42J tumor bearing mice, displayed a dose-dependent uptake for 67Ga-BZH3 in hGRP receptor positive tissues (tumor and pancreas), with a fast blood clearance. However, the intestinal uptake was still high [Analog #68, Table 8]. PET images were obtained using the 68Ga-BZH3 radioconjugate and indicated a very sensitive localization of hGRP receptor positive tumors in the mediastinal area [Analog #68, Table 8]. On the other hand, this 67/68Ga-radiolabeled compound could show some limitations in detection of metastatic prostate carcinoma, because of its high background signal in the abdomen [104].
The same group reported another study in which they designed and developed the new Bn analogue agonist, DOTA-PEG4-Bn(7–14) (PEG4=15-amino-4,7,10,13 tetraoxapentadecanoic acid; DOTA-PESIN) [Analog #69, Table 7, 8] (Tables 1,2, Fig. 1) and coupled it with either 68Ga or 177Lu[103]. In an in vitro analysis in the human prostate cancer PC-3 cell line, they found a high hGRP receptor binding affinity for 68Ga-DOTA-PESIN conjugate (IC50 6.6±3.0 nM). Moreover, as well as the 177Lu-radio-compound, a specific analysis demonstrated selectivity for the hGRP receptor subtype (10.0 nM), and the hNMB receptor (12 nM) over the hBRS-3 receptor (>1000 nM) [103]. The internalization and retention rates, after 2 hours, were much higher for 177Lu-DOTA-PESIN than 68Ga-DOTA-PESIN. On the other hand, a longer time period (4 hours) showed a higher 68Ga-DOTA-PESIN uptake [Analog #69, Table 7] [103]. In vivo biodistribution and scintigraphy experiments using PC-3 cell xenografts showed a fast tumor uptake and high level of tumor-liver ratio [Analog #69, Table 8]. Moreover, they found a fast renal excretion and a lower background for PET imaging [103].
Finally, Liu et al., recently, reported a study with the dual radiocompound 68Ga-NOTA-RGD-Bn [Analog # 69, Table 7, 8; NOTA=1,4,7-triazacyclononanetriacetic acid; RGD=arginine-glycine-aspartate] (Tables 1,2, Fig. 1), directed against both the αvβ3 integrin and hGRP receptors. The use of αvβ3 integrin is justified by several studies that demonstrated its involvement in the angiogenesis of most solid tumors [96,97,100]. The in vitro affinity for hGRP receptor was evaluated in the human prostate cancer cell line, PC-3. Compared to the native Bn and the compound with no linker (RGD-Bn), 68Ga-NOTA-RGD-Bn showed comparable affinity with IC50 values of 67.9 nM for RGD-Bn, 55.9 nM for NOTA-RGD-Bn and 78.9 nM for Bn [Analog #66, Table 7] [105]. Cell uptake in PC-3 cells was higher for 68Ga-NOTA-RGD-Bn than for those of 64Cu and 18F labeled Bn reported previously [95,100], but lower than 68Ga-NOTA-Bn compound. MicroPET images, biodistribution studies and immunofluorescence analysis in PC-3 tumor bearing mice showed slightly higher tumor uptake of 68Ga-NOTA-RGD-Bn than 68Ga-NOTA-Bn [Analog #66–67, Table 8]. The difference between in vitro and in vivo results was probably due to several factors including the presence of integrin receptors, which is much higher in the PC-3 tumor than in in vitro cells and the possibility that RGD was able to recognize the murine integrin β3, which is strongly expressed on tumor vasculature (as demonstrated by the immunofluorescence study). Moreover, the author hypothesized an in vivo synergistic interaction of the two motifs of the heterodimer compound that improved its binding affinity [105].
III. H. Review of 177Lu-labeled Bn analog in vitro (Table 9) and in vivo (Table 10) studies of Bn receptor-mediated imaging/cytotoxicity studies
Table 9.
In vitro studies with 177Lu bombesin analogs.
| 177Lu-Peptide# | Linker | Peptide | In vitro | Comment | Ref. N | ||||
|---|---|---|---|---|---|---|---|---|---|
| Binding affinity | Membrane bound | Degrad. | Receptor Intern. | ||||||
| Cell used | IC50/Kd (nM) | Amnt.(%) | Amnt.(%) | ||||||
| 1 | DOTA | di=[Lys3]
Bn(1–14)(09) |
PC-3 | no | no | no | 35.9±1.5 | 08 and 09 cmpd. are divalent. Increased targeting propertes. Potential probes for MRI | [106] |
| 2 | DOTA | mono=[Lys3]
Bn(1–14)(07) |
18.3± 1.1 | ||||||
| 3 | DOTA-Ahx | mono=Bn(4–14)(06) | 26.5± 0.8 | ||||||
| 4 | DOTA-Ahx | di=Bn(4–14)(08) | 41.9± 2.1 | ||||||
| 7 | DOTA | Bn(2–14) | PC-3 | 1.3±0.1 | 5.2±0.01 | 85.6% after 24h in h. serum | 30.7±0.07 (4h) | Adding 177Lu increases affinity compared to 90Y-labeling | [107] |
| 8 | Bn8 (DO3A-CH2CO-8-Aminooctanoyl) | Bn(714) | PC-3 | 3.1±1 | 34.6 | no | 72.9±0.08 (40min) | 177Lu-Amba displays high affinity, retention rather than other pan-Bn or Bn8 | [110] |
| 9 | DO3A-CH2CO-G-(4-aminobenzoyl) [AMBA] | Bn(7–14) | PC-3 | 2.5±0.5/Kd 1.02 | 34.1 | t1/2=38.8h (h) 3.1h (m) | 76.8±1.8 (40min) | ||
| 10 | DOTA-8-Aoc | Bn(7–14) | PC-3 | 0.3± 0.1 | 10 (40min) | no | 85 (40min) | Superior pharmacokinetic properties to 99Tc-N3S-5-Ava-Bn (7–14) | [108] |
| 11 | DOTA-PEG4 | Bn(7–14) | h. cancer tissues | 6.1±3.0 | no | no | 39.1±1.1 (6h) | Low affinity respect to unlabeled cmpd. | [103] |
| 12 | DO3A-CH2CO-G-4-aminobenzoyl [AMBA] | Bn(7–14) | PC-3 | 5 | no | t1/2=38.8±1.3h (human); 8.1±3.8h (rat); 3.1±0.1h (mouse) | no | In vitro slow degradation | [111] |
| 13 | DO3A-CH2CO-G-4-aminobenzoyl [AMBA] | Bn(7–14) | LNCaP | Kd 0.6±0.2 | 19.6±3.3 (2h) | no | 47.9±5.3 | Good affinity even in low expressing GRPR tumors (LNCaP and DU145) | [112] |
| 14 | Bn(7–14) | DU145 | Kd 0.5±0.1 | 16.4±3.4 (2h) | 63.9±6.9 | ||||
| 15 | Bn(7–14) | PC-3 | no | 15.3±2.3 (2h) | 74.7±15.3 | ||||
| 16 | DOTA-GABA | [DTyr6, β
Ala11, Thi13, Nle14]
Bn(6–14): “BZH2” [Antagonist] |
Autoradiography on sections of cancers with BnRs | no | no | no | no | [79] | |
| 17 | DO3A-CH2CO-G-4-aminobenzoyl [AMBA] | Bn(7–14) | Autoradiography on sections of cancers with BnRs | no | no | no | no | 177Lu-AMBA identifies hGRPR and hNMBR, but not BRS-3. | [116] |
| 18 | DO3A-CH2CO-G-4-aminobenzoyl [AMBA] | Bn(7–14) | CHO expr. hNMBR; | Kd 0.025 | no | no | no | No BRS-3 specific binding observed. Competition exp. On hunan cancer tissue revealed high GRPR affinity over NMBR and BRS-3. | [117] |
| 19 | HEK293 expr. hGRPR | Kd 0.035 | no | no | no | ||||
| 20 | BALB 3T3 exp. BRS-3 | Kd>1000 | no | no | no | ||||
| 21 (149Pm) | DO3A-amide | Bn(7–14) | PC-3 | no | no | ~95 (more 5 days) | no | β-alanine increasaes affinity | [113] |
| 22 (149Pm) | DO3A-amide-β-Ala | Bn(7–14) | no | no | ~95 (more 5 days) | no | |||
| 23 (153 Sm) | DO3A-amide-β-Ala | Bn(7–14) | 59.8± 23.1 | no | ~95 (more 5 days) | no | |||
| 24 (153 Sm) | DO3A-amide | Bn(7–14) | 1.8±0.2 | no | ~95 (more 5 days) | no | |||
All peptides not indicated as antagonist are agonist at human Bn receptors.
Cell lines: SKOV3.ip1, Human ovarian carcinoma; AR42J, Rat pancreatic acinar cell tumor; LNCaP, Human prostate cancer cells; DU145, Human metastatic prostate cancer cells; PC-3, Human prostate cancer cells; HEK293, Human Embryonic kidney cells; CHO, Chinese hamster ovary; BALB 3T3, Mouse embryonic fibroblast cell line
Table 10.
In vivo studies with 177Lu bombesin analogs.
| 177Lu-Peptide # | Linker | Peptide | In vivo | Ref. N | |||
|---|---|---|---|---|---|---|---|
| Animal | Biodistribution | Imaging | Comment | ||||
| 5 | DO3A-amide-β-Ala | Bn(7–14) | CF-1 mice | Only with the β-Ala cmpd.
Rapid tissue clearance |
no | β-Ala increases lipophilicity with faster clearance | [113] |
| 6 | DOTA-8-Aoc | Bn(7–14) | PC-3-tumor-bearing SCID mice | no | scintigraphy 48hr post IV. | GRPR radiolabeled cmpd.+chemo suppressed prostate Ca. | [109] |
| 7 | DOTA | Bn(2–14) | mice | Cleared from the blood within 24hr; rapidly excreted in urine; low kidney retention | no | 177Lu compd. had slower kinetics. | [107] |
| 8 | Bn8 (DO3A-CH2CO-8-Aminooctanoyl) | Bn(7–14) | PC-3-tumor-bearing SCID mice | 40–50% excreted by urine after 24hr. High cellular retention. | no | 177Lu-Amba better biodistribution for radiotherapeutic purpose than pan-Bn or Bn8 | [110] |
| 9 | DO3A-CH2CO-G-(4-aminobenzoyl) [AMBA] | ||||||
| 10 | DOTA-8-Aoc | Bn(7–14) | CF-1 and PC-3-tumor-bearing SCID mice | Cleared from blood within 1 h, mainly renal. High pancreatic accumulation in CF-1 and specific tumor targeting in PC-3 xenografts. | no | Potential peptide for therapeutic radiopharmaceuticals for GRPR+ cancers. | [108] |
| 11 | DOTA-PEG4 | Bn(7–14) | PC-3 tumor bearing athymic nude mice | Blood clearance at 1 h post IV; faster from pancreas than tumor. Excreted by kidney and high pancreas and tumor uptake. | PET and scintigraphic images; accumulation mostly in pancreas, kindey and tumor. | 177Lu AMBA is more effective for clinical studies than 99Tc-labelled Bn analogues. | [103] |
| 12 | DO3A-CH2CO-G-4-aminobenzoyl [AMBA] | Bn(7–14) | PC-3 tumor bearing nude mice | Renal excretion. High uptake in pancreas and tumors. | no | Rapidly metabolized in vivo but strong efficacy in targeting GRPR+ tumors. | [111] |
| 13 | DO3A-CH2CO-G-4-aminobenzoyl [AMBA] | Bn(7–14) | LNCaP tumor bearing nude mice | Renal clearance (50% after 24hr). Main targets= pancreas and tumor for PC-3 tumor bearing mice. For the others types of xenograft the principal target is only the pancreas | γ-imaging for tumor uptake and retention; Autoradiography for for viable tumor cells. | Limits cell proliferation and re-establish the normal vascular phenotype in LNCaP and DU145 xenografts. Therapeutic potential in low-GRPR expressing prostate Ca. | [112] |
| 14 | DU145 tumor bearing nude mice | ||||||
| 15 | PC-3 tumor bearing nude mice | ||||||
| 16 | DOTA-GABA | [DTyr6,β
Ala11, Thi13, Nle14]
Bn(6–14): “BZH2” [Antagonist] |
ARJ42 tumor bearing Lewis rats | 177Lu-BHZ2 showed specific and high uptake in GRPR–positive organs and in the AR42J tumor. A fast clearance from blood and all of the non-target organs except the kidneys was found. | no | [79] | |
| 18 | DO3A-CH2CO-G-4-aminobenzoyl [AMBA] | Bn(7–14) | Autoradiography on human neoplastic and non-neoplastic tissues | no | no | Specific binding of various types of tumor tissues and chroinc pancreatitis pancreas. | [117] |
| 22 (149Pm) | DO3A-amide-β-Ala | Bn(7–14) | CF-1 mice | Only with the
β-Ala cmpd. Rapid tissue clearance |
no | Same retention compared to other tho
lanthanides. (see in this table peptide
#6.). Potential therapeutic cmpd. |
[113] |
| 23 (153 Sm) | no | See peptide # 6 and 17. | |||||
All peptides not indicated as antagonist are agonist at human Bn receptors.
Cell lines: SKOV3.ip1, Human ovarian carcinoma; AR42J, Rat pancreatic acinar cell tumor; LNCaP, Human prostate cancer cells; DU145, Human metastatic prostate cancer cells; PC-3, Human prostate cancer cells.
Radiolanthanides are a family of trivalent radiometals (*M3+), β- and γ-emitters, primarily used for both imaging and therapy. They are easily available and possess high stability in aqueous solutions creating stable conjugates [81].
In order to coupled to peptides and inhibit their in vivo transchelation activity, radiolanthanides require multidentate chelators, usually macrocyclic, poliamino-carboxilate ligands such as DTPA (2-[bis[2-[bis(carboxymethyl)amino]ethyl]amino]acetic) or DOTA (1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraaceticacid) (Tables 1,2. Fig. 1)[103,106–109]. 177Lu coupling has been also reported with the use of the asymmetrically substitute chelator DO3A (1,4,7-tris(carboxymethyl)10-(aminoethyl)-1,4,7,10-tetraazacyclododecaneOH) (Tables 1,2, Fig. 1). Coupling to DO3A is reported to have some favorable features compared to DTPA and DOTA chelators, in terms of greater stability and inertia to metal dissociation [3,110–113]. 177Lu is the lanthanide that is frequently used as a “no carrier added” isotope, and is widely used for imaging studies, although it is also used as a therapeutic agent [82,114]. It has excellent stability (t1/2=6.7 days) and emits both medium energy β-emissions (133 and 412 keV) and γ-emissions (113 and 208 keV) [114].
To try to develop targeting and therapeutic 177Lu labeled Bn analogues, there have been 12 studies attempting to optimize the balance between tumor uptake, tumor retention and chemical properties (Tables 10 and 9). The in vitro results of these studies are summarized in Table 9 and in vivo results in Table 10.
Since the conjugate DOTA-8-Aoc-Bn(7–14) (Aoc=aminooctanoic acid) (Tables 1, 2), in previous studies, showed very high affinity for GRP receptors and stability, Smith et al., labeled it with 177Lu. [72,108]. 177Lu -DOTA-8-Aoc Bn(7–14) [Analog #10, Tables 9, 10] has high affinity and specificity, displaying an IC50 value of 0.3 ± 0.1nM, in PC-3 cells, which possess hGRPR receptors and which is similar to the affinity of Bn and Bn(7–14) for hGRP receptors [108]. Moreover, it showed 73% cell retention after 2 hours post-internalization and very low efflux levels [108]. In vivo studies in CF-1 and PC-3 bearing tumors mice models demonstrated good tumor uptake in GRP receptor expressing tissues and in tumors, as well as an efficient clearance by renal system [Analog #10, Tables 9, 10] [108]. Subsequently, the same group evaluated the efficacy of a combined GRP receptor targeted radiotherapy (TRT)/chemotherapy approach, using this new conjugate, in androgen independent prostate cancer [109]. Using as a model, the GRP receptor containing prostate cancer cells, PC-3 in xenografts, they found that a combined therapy with the two microtubule inhibitors, docetaxel and estamustine with177Lu -DOTA-8-Aoc-Bn(7–14) [Analog #6, Table 10] increased by 30% the mean survival compared to targeted radiotherapy or chemotherapy used as single agents [109].
Based on well-known Bn analogs, which have high affinity for all human Bn, receptors [40,115], Zhang et al., developed radiolabeled 111In-, 90Y- and 177Lu- pan-Bn agonist conjugates having high affinity for all the three Bn receptors [79]. Specifically, they studied, in vivo, the properties of 177Lu-DOTA-BZH2 (BZH2=[DTyr6,βAla11, Thi13, Nle14])Bn(6–14))[Analog # 16, Tables 9,10; Table 2] and found that it had specific, high uptake in rat pancreatic GRP receptor bearing AR-42J tumors in rats, both in GRP receptor positive tissues and in xenograft tumors. Moreover, it displayed a rapid blood clearance with a <0.015% ID/g remaining amount at 4 hours [79]. Unfortunately, preclinical studies showed this compound had a fast washout. To overcome that disadvantage, the same group designed a new conjugate, DOTA-PEG4-Bn(7–14) (PEG4=15-amino-4,7,10,13-tetraoxapentadecanoic acid; DOTA-PESIN) [Analog #11, Table 9; Tables 1,2, Fig. 1] and labeled it either with 177Lu or 68Ga [103]. In PC-3 cells, using the universal agonist ligand 125I- ([DTyr6,βAla11, Thi13, Nle14]Bn(6–14) which has high affinity for human Bn receptors [103] as preferring ligand in competitive experiments, both 177Lu-and 68Ga- radiolabeled Bn showed high affinity for human GRP receptors (IC50 6.1±3.0 nM and 6.6±3.0 nM, respectively) and demonstrated some selectivity for the hGRP receptor subtype (8.3±1.7 nM and 10.0 nM, respectively), over than hNMB receptor (15±4, nM and 12±4.0 nM, respectively) and had no affinity for the hBRS-3 receptor (>1000 nM) [103]. The internalization and retention rates were much higher compared to other pan-Bn analogues [103]. These latter results were confirmed by in vivo biodistribution and scintigraphy experiments in PC-3 xenografts, in which they revealed a superior tumor to liver ratio than seen with other radiolabeled peptides and a clear contrast from the background [Analog # 11, Table 10] [103].
A recent study reported divalent DOTA-Bn conjugates could improve targeted imaging compared to monovalent analogues [106]. Specifically, in this study the authors evaluated the amount of receptor internalization in PC-3 cells, of four different 177Lu-labeled compounds: monovalent DOTA-Ahx-Bn(4–14) (06), DOTA-[Lys3]Bn(1–14) (07) and divalents DOTA-Ahx-Bn(4–14) (08) and DOTA-[Lys3]Bn(1–14) (09) [Analogs #1–4, Table 9]. Interestingly, after 4 hours, the 08 and 09 divalent conjugates showed a better internalization rate (41.9± 2.1% and 35.9±1.5%, respectively) than the monovalent compounds. At the same time, 08 and 09 compounds showed high cell retention [106].
Recently, Koumarianou et al. analyzed and compared the in vitro and in vivo behavior of 90Y- and 177Lu-DOTA-Bn(2–14) agonist analogues [Analog #7, Tables 9,10] (Table 2) [107]. The 177Lu-labeled compound showed high affinity with an IC50 of 1.34± 0.1 nM and good in vitro stability (85.6% in serum after 24 hours) [Analog #8, Table 9]. In vivo studies in normal mice demonstrated a rapid blood clearance, primarily by renal excretion [Analog #7, Table 10]. However, they found a high uptake in large intestine, probably due to the expression of GRP receptors in this organ. The authors concluded that the 177Lu- labeled compound was preferable compared to the 90Y labeled compound [107].
The use of an asymmetric chelator such as DO3A was first evaluated by Hu et al. in a comparative analysis between DO3A-amide-Bn(7–14) and DO3A-amide-βAla-Bn(7–14) (three carbon spacer) conjugates [Analogs #21–24, Table 9] (Tables 1,2, Fig. 1) [113]. The authors labeled these compounds with three different lanthanides, 149Promethium [149Pm], 153Samarium [153Sm] and 177Lu. In vitro analysis in PC-3 cells showed that the β-alanine spacer-containing compound had a higher binding affinity (IC50 1.8±0.2 nM) compared with the no spacer compound (IC50 59.8±23.1 nM) [Analogs # 23, 24, Table 9]. On that basis, in vivo analyses in normal mice were performed only with the DO3A-amide βAla-Bn [7–14] compound [Analogs # 5 and 22, 23, Table 10]. The 149Pm -labeled compound showed very similar behavior compared to the same compound labeled with 153Sm and 177Lu. The authors considered that the use of the three different radiolanthanides for the same conjugate could be interchangeable, depending on the nuclear properties required for a particular disease target [113].
Lantry’s group synthesized and characterized a new conjugate radiolabeled Bn agonist analog, 177Lu-DO3A-glycyl-4-aminobenzoyl-Bn(7–14) (177Lu-AMBA) and compared both its in vitro and in vivo characteristics to 177-Lu-DOTA-8-Aoc-Bn(7–14) (177Lu-Bn8) [Analogs # 8, 9, Tables 9,10] (Tables 1,2, Fig. 1)[110]. Binding affinity assessed in PC-3 cells showed that 177Lu-AMBA had a similar affinity, compared to the 177Lu-Bn8 compound (IC50 2.50±0.5 vs 3.10±0.99, respectively), as well as, a similar degree of internalization and retention [110]. In vivo studies in athymic mice showed a similar biodistribution and mechanism of excretion, however 177Lu-AMBA displayed higher levels of accumulation and retention after 1 h and 24 h, respectively [Analogs # 8, 9, Table 10] [110]. In PC-3 tumor-bearing mice treatment with 177 Lu-AMBA resulted in a significant increase in survival and a reduction of PC-3 growth rate in treated mice vs non-treated, in a single dose treatment [Analogs #8. 9, Table 9]. Moreover, the survival rate and the tumor growth rate of tumor-bearing mice increased with a second dose treatment after 14 days [Analogs #8, 9, Table 9] [110].
Since the prostate cancer cell line PC-3 displays a high expression of GRP receptors (2.5×105/cells), Maddalena et al. evaluated 177Lu-AMBA tumor binding and imaging potential in low GRP receptors expressing models, such as the prostate cancer cell lines, LNCaP and DU145 cells (an early androgen-sensitive prostate cancer and an androgen insensitive metastatic cell line, respectively) [Analogs #13–15, Table 9] [112]. LNCaP expressed 5.9×103 GRP receptors per cell, while DU145 expresses 1.2×104 GRP receptors per cell. 177Lu-AMBA showed a very high affinity for all the cell lines tested (Kd of LNCaP, 0.65 nM, of DU145, 0.53 nM, of PC-3 1.01 nM) [Analogs # 13–15, Table 9]. Moreover, both using autoradiography and γ-images, in LNCaP and DU145 xenografts models, 177Lu-AMBA showed a clear identification of tumors [Analogs #13–15, Table 10]. Finally, radiotherapy studies using either LNCaP- or DU145- tumor-bearing mice demonstrated a strong effect in decreasing tumor proliferation rates compared to PC-3 xenografts models [110,112]. Interestingly, in LNCaP model, 177Lu-AMBA was able to normalize tumor microvasculature phenotype, reducing tumoral blood pooling [Analog #13, Table 10] [112].
To further investigate the in vitro binding properties of 177Lu-AMBA [Analog #17, Table 9], a series of human neoplastic and non-neoplastic tissues were evaluate by autoradiography, for their bombesin-related receptor expression [116]. 177 Lu-AMBA demonstrated a number of GRP and NMB receptors- expressing tumors, including various prostate, mammary and renal cell carcinomas, as well as gastrointestinal stromal tumors. On the other hand, 177Lu-AMBA was not able to identify BRS-3 receptor expressing tumors and Bn receptors on pancreatic islets [116]. Interestingly, this compound demonstrated no binding to normal human pancreas, unless chronic pancreatitis was present [116]. Thomas et al., performed a similar analysis using GRP- or NMB- or BRS-3- receptors over-expressing cell lines and human normal and tumor tissues, obtaining very similar results [Analogs#18–20, Table 9 and analog #18, Table 10] [117].
To further explore the in vitro and in vivo behavior of 177Lu-AMBA, its stability was studied in mouse, rat and human [Analog #12, Table 9] by analyzing the generation of a series of metabolites derived from this compound [111]. A rapid cleavage of the peptide was seen in mouse, rat and human plasma as well as mouse kidney homogenates. A rapid in vivo clearance of the entire conjugate and radioactivity was also found in mouse and rat blood [Analog #12, Table 10]. No unmetabolized drug was excreted in mouse and rat urine [111]. Furthermore, in vitro binding affinity in PC-3 cells and in vivo biodistribution and clearance in PC-3 xenografts of all metabolites derived from 177Lu-AMBA were evaluated [Analog #13, Table 10]. The results showed the 177Lu-AMBA metabolites all had a low affinity for hGRP receptors and a very fast renal excretion (within an hour), demonstrating that the tumor uptake observed in this study and in previous ones, was only due to 177Lu-AMBA and not to any radiolabeled metabolites [Analog #12, Table 10] [111].
III. I. Review of 125I, 86,90Y, 186,187,188Re -labeled Bn analog in vitro (Table 7) and in vivo (Table 8) studies of Bn receptor-mediated imaging/cytotoxicity studies
III. I. 1. 125I studies
These are a few studies in which Bn-analogs were radiolabeled with 125I, 86/90Y or 186/188Re. The in vitro and in vivo results of these studies are summarized in Tables 7 and 8, respectively.
Two studies investigated the possibility of radiolabeled Bn-analogs involved the use of radio-iodinated conjugates [118,119]. Rogers et al., analyzed the Bn analog antagonist 125I-mIP-[des-Met14]Bn(7–14) (mIP=meta-iodophenyl and desMet= methionine removed) (Table 1,2) compared with the agonist 125I-[Tyr4]Bn [Analogs #3–10, Table 7; analogs #6–9, Table 7] [118]. Using as model the BALB/B1 mouse fibroblast cell line, BNR-11 that has a stably transfected murine GRP receptor, the authors found that the in vitro internalization rate was high for both compounds (≈40% after 5 minutes) [Analogs #3 and 8, Table 7]. However, they found a high efflux rate for both, but it was much higher with 125I-[Tyr4]Bn [118]. Next, the authors evaluated the in vitro binding affinity of 125I-mIP-[des-Met14]Bn(7–14) in the human carcinoma cell lines A427 (lung), SKOV3.ip1 (ovary), and HeLa cells (cervical epithelium) over-expressing a recombinant adenoviral vector containing the murine GRP receptor gene (AdCMVGRPr), using BNR-11 cells as control. For both radioconjugates in all the transfected cell lines the binding was greater than in BNR-11 cells [Analogs #3–10, Table 7]. In vivo studies were, initially, performed in normal BALB/c mice and showed a fast uptake and clearance from normal tissues. Moreover, 125I-mIP-[des-Met14]Bn(7–14) displayed lower levels of deiodination [Analogs #7 and 8, Table 8]. Biodistribution studies in SKOV3.ip1 tumor bearing mice showed that 125I-mIP-[des-Met14]Bn(7–14) and 125I-[Tyr4]Bn had high uptake with a greater tumor localization for the first radioligand [Analogs #6 and 9, Table 8] [118].
The same group reported another study in the human ovarian cancer cell line SKOV3.ip1 over-expressing the AdCMVGRPr vector, comparing the two Bn agonist radioligands 125I-mIP-Bn and 125I-[Tyr4]Bn [Analogs #1 and 2, Tables 7, 8] (Tables 1,2) [119]. The live-cell binding assay showed high binding in these cells for 125I-[Tyr4]Bn with 80% of radioligand bound after 2 days [Analog #1, Table 7] [119]. Biodistribution studies in SKOV3.ip1 tumor bearing nude mice indicated a greater tumor localization of 125I-mIP-Bn than 125I-[Tyr4]Bn, although the tumor uptake was comparable [Analogs #1 and 2, Table 8] [119].
III. I. 2. 86,90Y studies
86Y is a pure β-emitter, with half-life of t1/2=64 h and Eβmax=2.27 MeV, used for imaging purposes in PET scanning as a surrogate for 90Y, which is also used as a therapeutic nuclide. Unfortunately, since it has a large range in tissues and can cause hematological toxicity, its use in radiotherapeutical procedures is not still clear [87,107].
As described in the 177Lu –radioligands paragraph, Zhang et al. performed a comparative study of the Bn agonist, BZH2 (BZH2=DOTA-GABA-[DTyr6,βAla11, Thi13, Nle14]Bn(6–14)) (DOTA= 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraaceticacid; GABA=γ-aminobutyric acid) (Tables 1,2, Fig. 2) compound, labeling it either with 90Y, 111In or 177Lu [Analogs # 70, 71, Table 7 and # 16, Tables 9,10 and # 17, Tables 5, 6]. In vitro binding receptor autoradiography on human tumors, each expressing a single bombesin receptor subtype, showed higher affinity and selectivity of 90Y-BZH2 for the GRP receptor, NMB receptor and BB3 (IC50 1.4 nM, 4.9 nM and 10.7 nM, respectively), probably due to the extra negative charge at the NH2 terminus of 90Y-BZH2[79]. Internalization studies performed in the pancreatic tumor rat cell line AR42J, showed that radiolabeled BHZ2 Bn-analog had a comparable internalization rate when radiolabeled either with 90Y, 111In or 177Lu [Analogs # 70–71, Table 7 and # 16, Table 9 and # 17, Table 7] [79].
Similarly, in the comparative study between 177Lu-and 90Y-DOTA-Bn(2–14) agonist analogues [Analog #7, Tables 9,10; analog #73, Tables 7, 8] (Tables 1,2. Fig. 2) Koumarianou et al. found in vitro, similar affinity to hGRP receptor (IC50 1.3 and 1.99 nM, respectively) and serum stability (85.6% and 79.1%, in serum after 24 hours. respectively) [107]. However, the 90Y-radioligand had a faster efflux rate than the 177Lu-Bn analog [Analog #7, Table 10–1; analog #74, Table 7]. Moreover, in vivo studies in normal mice demonstrated specific binding to the GRP receptor, a fast blood clearance and renal excretion [Analog #7, Table 9; analog #74, Table 8]. Nevertheless, 177Lu-DOTA-Bn(2–14) had more specific uptake in in vivo blocking experiments with the native Bn, than 90Y-DOTA-Bn(2–14) [107].
Biddlecombe et al. compared both 64Cu and 86Y-DOTA-[Pro1, Tyr4]Bn(1–14) (DOTA-[Pro1, Tyr4]Bn(1–14) =MP23436) (Table 1,2 Fig. 2) [Analogs #34 and 72, Table 7; #34, 72 and 73, Table 8] [87]. The in vitro study in the PC-3 cell line displayed 3-fold higher internalization rate for 86Y-MP23436 at 20 h than 64Cu-radioligand [Analogs #34 and 72, Table 7] [87]. An in vivo biodistribution in the PC-3 tumor bearing mice showed a higher uptake for the 86Y- than 64Cu-MP23436 [Analogs #34, 72 and 73, Table 8]. Biodistribution results were confirmed by PET images where 86Y-MP23436 had a better tumor-normal tissue ratio [Analogs #34, 72 and 73, Table 8] [87].
III. I. 3. 186,187,188Re studies
Rhenium is a transition metal that shares several chemical properties with technetium (99mTc). Rhenium radioisotopes, 186Re and 188Re, are β-emitters with half-lives of t1/2=90.6 and 16.9 hours, respectively [114,120]. 188W/188Re generators produce both radioisotopes. The drawback of 186Re is its low specific activity due to the production method, while for 188Re, its short half-life is a drawback for some studies [121].
Safavy et al. coupled the trihydroxamate bifunctional chelating agent trisuccin to the Bn-analog antagonist [des Met14] Bn(7–14) (Tables 1,2) and radiolabeled with 188Re [Analogs #18–21, Table 7] [120]. In a cell binding assay in the BNR-11 cell line, which is a 3T3 mouse fibroblast cell line stably transfected with the murine GRP receptor, the 188Re-Tris-[des Met14] Bn(7–14) and 188Re-Tris-C6-[des Met14]Bn(7–14) (C6 = 6 carbons, as linker) radioligands showed 14% and 13% binding, respectively, compared with 21% for the control, 125I-[Tyr4]Bn [Analogs #18–19, Table 7] [120]. Comparable results were obtained using the PC-3 cell line for 188Re-Tris-[Des Met14] Bn(7–14) which bound a 10% compared with 20% for 125I-[Tyr4]Bn [Analog #20, Table 7]. The reduced binding could be a consequence of the labeling process [120].
Moustapha et al. performed an in vivo study in CF-1 normal mice, with the radiolabeled compounds 188Re-N3S -5-Ava-Bn(7–14) non-carried (NCA), 186Re-N3S-5-Ava-Bn(7–14) carried (CA) and 186Re-N3S-5-Ava- Bn(7–14) non-carried (NCA) (N3S= dimethylglycyl-L-seryl-L-cysteinglycinamide; Ava=5-aminopentanoic acid; [Analogs #15–17, Table 8] (Tables 1,2, Fig. 2) [121]. Biodistribution studies of all three radioligands displayed an efficient blood clearance after 4 hours with high affinity and specificity in pancreas [Analogs #15–17, Table 8] rising the possibility to use NCA 188/186Re-Bn-derivatives for targeting GRP receptor expressing tumors [121].
Finally, Gourni et al. evaluated the in vitro features of a series of Bn compounds labeled either with 99mTc or 185/187Re, which have similar chemical properties [55]. They synthesized four labeled Bn agonists, where the pyroglutamic acid of Bn was replaced by different chemical groups that were able to bind radiometals: Aca-Gly-Gly-Cys-Bn(2–14) (Bn1.1), Aca-MeGly-Gly-Cys-Bn(2–14) (Bn1.2), Aca-Me2Gly-Gly-Cys-Bn(2–14) (Bn1.3) and Aca-Mac-Gly-Cys-Bn(2–14) (Bn1.4) (MeGly=methylglicine; Me2Gly=dimethylglycine; Mac=mercaptoacetic acid; [Analogs #11–14, Table 7] (Tables 1,2. Fig. 2) [55]. In vitro binding to the hGRP receptor, in PC-3 cells, showed high affinity for the three radioligands (IC50 1.13 nM for Bn1.1; 0.76 nM for Bn1.2 and 1.42 nM for Bn1.4) [55]. Assuming that 99mTc and 185/187Re are comparable radioisotopes and consequently, give similar GRP receptor affinity to the radioligands, the authors performed in vivo experiments in mice with the all four ligands labeled with 99mTc, concluding that the Bn1.1 derivative seems to be the more promising compound (see the 99mTc paragraph)[55].
IV. Review of human radiolabeled studies of Bn receptor-mediated imaging/cytotoxicity studies (Table 11)
IV. A. Human studies using radiolabeled bombesin analog (Table 11)
12 studies has been published (Table 11) where different radiolabeled bombesin analogues were tested in human healthy volunteers or patients suspected or confirmed to have breast, prostate, gastrointestinal or lung cancer, the radiolabeled Bn analogs were examined for diagnostic purposes, alone or comparing with another radiolabeled compound used in nuclear medicine. In 83% of studies 99mTc was used and in 17% 68Ga was the isotope selected to be coupled to the Bn analog. The most frequent Bn analog used was [Cys0-Aca1]Bn(2–14) also called [Leu13]Bn (Table 2) (50% of cases), which is a Bn receptor agonist.
IV. B. Human 99mTc bombesin analog studies
In 2002 Scopinaro et al. [122], tested for the first time 99mTc-[Leu13]Bn [Study #4, Table 11] in 5 patients suspected to have breast cancer, and after 2 days the same patients were injected with 99mTc-Sestamibi, a routinely used radiotracer for the detection of breast cancer, in order to compare the results with that obtained with radiolabeled [Leu13]Bn. They observed by planar scintigraphy that 99mTc-Sestamibi detected 4 of the lesions, while 99mTc-[Leu13]Bn showed 5 lesions (100%) and all the lymph node affected. No side effects were observed. Apart from the tumor, the radiolabeled bombesin agonist was also taken up by the thyroid gland, liver and kidneys. In both cases, with sestamibi and [Leu13]Bn, there was no uptake by a fibroadenoma lesion.
The same group [123] studied 3 healthy subjects and 2 patients, 1 with prostate cancer and 1 with small cell lung cancer (SCLC), injected with 99mTc-[13Leu]Bn, and images were obtained by SPECT and planar scintigraphy [Study #1, Table 11]. The patient with SCLC was also studied with 99mTc-Sestamibi. Side-effects after the injection of the radiolabeled Bn analog were not observed. 99mTc-[Leu13]Bn imagined both prostate cancer and SCLC. Prostate cancer was visualized as soon as 1 and 2 min after injection and then was progressively masked by radioactivity accumulating in the bladder. The patient with SCLC after injection with 99mTc-[Leu13]Bn four uptake zones were detected by SPECT and 3 by planar scan, while after 99mTc-Sestamibi 3 and 2 hot zones were detected, respectively. 99mTc-[Leu13]Bn imagined SCLC from minute 1 to 3 hours. 99mTc-[Leu13]Bn was also taken up by liver and kidneys, faintly by the thyroid gland, and appeared into duodenum and jejunum at 3 h p.i.
In 2003 99mTc-[13Leu]Bn was used for biopsy site localization driven by the use of an imaging probe combined with X-ray in 5 patients, suspicious for breast cancer [124][Study #2, Table 11]. Patients were injected with 99mTc-[Leu13]Bn and the biopsy samples obtained were measured for radioactivity. 48 samples were obtained, 19 of them with high 99mTc-[Leu13]Bn, 21 with intermediate and 8 with low uptake. Histochemical studies performed in these samples showed that cancer was found in all the samples with high 99mTc-[13Leu]Bn uptake and 19/21 with intermediate and 2/8 with low uptake.
In 2004, De Vincentis et al. [125] studied 14 patients with a prostatic lesion by performing trans-rectal ultrasonography-guided biopsy, CT, MRI and as well as 99mTc-[Leu13]Bn scintigraphy [Study #3, Table 11]. The radiolabeled bombesin analog detected all the cancer cases (12/14 cases, confirmed by histopathology), and also the lymph nodes involved (4 cases, confirmed by histopathology study after operation), while CT and MRI only were positive in 3/4 cases. Studies in 5 patients with 111In-Octreoscan detected only 2/3 cases of cancer and no lymph node involvement.
Scopinaro et al. [126][Study #5, Table 11] used the same approach as the previous study to assess whether or not 99mTc-[Leu13]Bn scan was able to detect not only prostate cancer but also invasion of pelvic lymph nodes, in 10 patients suspected to have prostate cancer. They observed that 99mTc-[Leu13]Bn visualized 100% of the cancers (8/10 patients) and lymph node invasion (3/10). No positive uptake was seen in the 2 cases of adenoma, with results confirmed by pathology evaluation. With MRI no lymph node invasion was found.
In a later study Scopinaro et al. [127] [Study #6, Table 11] used 99mTc-[Leu13]Bn to test whether or not this radiolabeled Bn analog can detect colon cancer, as it is known this type of cancer, as well as breast and prostate cancer, can over-express bombesin receptors. For that, 13 patients, 7 of them known to have colon cancer, were subjected to SPECT and planar scintigraphy with 99mTc-[Leu13]Bn. Images were taken before 1 h p.i., before discharge of radioactivity from the liver to the duodenum. Cancer was detected in 11/11 patients; it showed 2 false positives (1 Crohn’s disease and 1 with polyp showing mild dysplasia). 99mTc-[Leu13]Bn detected invasion of lymph nodes in 5 patients (100% of the cases).
Another Bn analog (N3S-5Ava-Bn(7–14) also know as RP527, Table 1) coupled to 99mTc has been used 3 in human studies. In the first study, from 2000 (Van de Miele et al. [58][Study #8, Table 11]), 10 patients with prostate (4 patients with bone metastasis with androgen resistant prostate cancer) or suspected of breast cancer (6 patients) were subjected to planar scintigraphy after injection of 99mTc-RP527. None of the patients had an adverse reaction. After injection of the radiolabeled Bn analog renal and hepatic clearance and pancreatic uptake, but not blood accumulation was observed. Positive imaging of the bone metastasis in a patient with androgen resistant prostate cancer with 99mTc-RP527 was obtained (1/4 patients) and in this case just half of the lesions were visualized. However, in 4/5 patients 99mTc-RP527 showed positive uptake by breast cancer and all lymph nodes involved. In the breast cancer patient with bone metastasis there was no clear 99mTc-RP527 uptake by the primary tumor, lymph nodes involved or the metastasis.
The same group published a second study [128] [Study #9, Table 11] with the radiolabeled Bn analog 99mTc-RP527, but in this case just healthy subjects were included in order to study the biodistribution and dosimetry of the radiotracer by planar scintigraphy images from 30 min to 24h p.i., and to assess blood and urine samples. They found low accumulation of 99mTc-RP527 in brain, lung, myocardium, breast and testis, with hepatobiliary and renal clearance and extensive bowel uptake. The authors concluded that the biodistribution characteristics of 99mTc-RP527 made it suitable for the tumor detection in the suprabdominal region, but imaging of the abdominal region more problematic, due to the intestinal accumulation.
In 2008 Van de Wiele et al. [4] published another human study [Study #10, Table 11] using 99mTc-RP527. 9 patients with suspected breast cancer and 5 with tamoxifen-resistant bone-metastasized breast carcinoma underwent 99mTc-RP527 scintigraphy. The results showed that in 8/9 patients the radiolabeled Bn analog visualized the tumor, lymph node involved and part of the bone metastasis when present (1 patient). However, 99mTc-RP527 did not visualize any of the bone metastasis in the 5 tamoxifen-resistant bone-metastasized breast carcinoma patients. In no case was any adverse reaction to 99mTc-RP527 seen.
Another 99mTc-Bn analog has been studied [129] [Study #7] in humans, [Lys3]Bn (Table 2) coupled to the isotope by the linker EDDA/HYNIC (Table 1, Fig, 1). It was injected in 11 patients (3 with proven and 8 suspected to have breast cancer) and SPECT and planar scintigraphy images were taken from 20 min to 24 h p.i.. After injection none of the patients suffered from side-effects. 99mTc-EDDA/HYNIC-[Lys3]Bn had a rapid blood clearance and was mainly renal excretion. The images obtained showed that patients with cancer presented asymmetrical uptake by the breast tissue and higher accumulation in the breasts with malignant tumors.
IV. C. Human 68Ga bombesin analog studies
The Bn analog BZH3 ([DTyr6, βAla11, Thi13, Nle14]Bn(6–14), Tables 1,2) coupled to the 68Ga isotope through the linker DOTA-PEG2 has been tested in 2 studies with cancer patients [130,131]. One study involved 17 patients with gastrointestinal stromal tumors (GIST) [Study #11, Table 11] and the other [Study #12, Table 11] involved 9 patients with low grade gliomas. In both cases patients underwent PET scans with 68Ga-BZH3 and the radiotracer 18F-FDG and results were compared. In the study with the GIST patients [130] 68Ga-BZH3 localized 8/30 lesion (positive tumor uptake in 7/17 patients) while 18F-FDG was positive in 25/30 lesion (14/17 patients). In one case the radiolabeled Bn analog was able to detect one tumor in the stomach not detected by 18F-FDG. In the study performed in low grade gliomas [131], in all cases (9/9) the combination of both radiotracer, 68Ga-BZH3 and 18F-FDG, was able to detect the tumors.
V. Nonradioactive Bn cytotoxic analogs (Table 12)
Many cancer patients are treated with cytotoxic chemotherapeutic drugs. For example, each year over 170,000 patients in the United States are diagnosed with lung cancer but unfortunately 160,000 lung cancer patients die from this disease annually [132]. Small cell lung cancer (SCLC), which kills approximately 25,000 patients, is treated with chemotherapy and/or radiation therapy, but relapse frequently occurs and the median survival time is less than 1 year. Non-SCLC (NSCLC), which kills approximately 135,000 patients, is treated with combination chemotherapy but the 5-year survival rate is only approximately 15%. Cancer cells take up chemotherapeutic drugs; however, they are internalized by rapidly growing normal cells such as white blood cells causing toxic side effects. Chemotherapy effectiveness in cancer patients is limited by toxicity to normal cells and multidrug resistance [4]. It is possible to target drugs to cancer cells using cell surface peptide receptors. Bombesin (Bn)-like peptides and receptors are present in many cancer cells including lung cancer and Bn stimulates their growth [11,19,21,133,134]. Thus attempts have been made to develop cytotoxic Bn-conjugates, which will kill cancer cells, but not normal cells.
Camptothecin is a topoisomerase I inhibitor binding directly to the topoisomerase I-DNA complex, resulting in DNA damage and apoptosis. Two camptothecin analogs (topotecan and irinotecan) are used for ovarian, cervical, SCLC and colon cancer treatment. Apart from its anticancer properties, camptothecin has low aqueous solubility. BA0, (DTyr6, β-Ala11, Phe13, Nle14)Bn-(6–14) (Table 2), is a universal agonist, which binds with high affinity to BRS-3 as well as NMB and GRP receptors [30,34,40,135]. In 2007, in order to improve camptothecan’s solubility Shun et al. [136] coupled camptothecin to the Bn agonist analog, [DSer5, DTyr6, βAla11, Phe13, NLe14]Bn(5–14) (BA3) [Analog #2, Table 12] using various built in nucleophile-assisted releasing (BINAR) linkers (L1-L3)): analogs of N-(N-methyl-amino-ethyl)-glycine carbamate. The carbamate linkage in CPT-L2-BA3 is metabolized by P450 enzymes, which are enriched in cancer cells, resulting in L2-BA3 and CPT [137]. CPT diffuses into the nucleus where it can inhibit cancer cell replication [30]. In particular, CPT inhibits topoisomerase I, which unwinds DNA prior to replication. In vitro cytotoxicity studies [136] in different cell lines were performed and results showed that the conjugated Bn analog had a tumoricidal IC50 in the μM range with values 10-fold higher than camptothecin alone [Analog #2, Table 12]. The CPT-Bn analog in PC-3 cells inhibited adhesion to collagen type I, αVβ3 and αVβ5, at 10–20 μM, also in HUVECs inhibited capillary-like tube formation and in vivo angiogenesis, at 10–20 μM and 40 μM, respectively [Analog #2, Table 12].
The synthetic analog of Bn, (DSer5, DTyr6, β-Ala11, Phe13, Nle14)Bn-(5–14) (BA3) coupled at the N-terminal to camptothecin (CPT), was studied in more detail for its ability to interact with Bn receptors in other studies [30,34,138]. Results in study #1 (Table 12) [30] demonstrate CPT-L2-BA3 can inhibit specific binding of 125I-BA0 to Balb/c 3T3 cells transfected with GRP receptors, NMB receptors or BRS-3 (IC50 = 0.012, 0.035 and 0.031 nM, respectively) [Analog #1, Table 12]. Because BA0 had IC50 values of 0.32, 0.74 and 0.25 nM for GRP receptors, NMB receptors or BRS-3 respectively, CPT-L2-BA3 bound with approximately 1-order of magnitude greater affinity than did BA0 [Analog #1, Table 12]. This may result because CPT interacts with additional hydrophobic amino acids present in Bn receptors in addition to the essential Bn receptor amino acids, which interact with Bn agonists. For GRP receptors Gln122, Phe185, Ala198, Pro199, Arg288 and Ala308 are important for residues for binding GRP with selectivity and with high affinity [8,139,140].
CPT-L2-BA3 was internalized at 37°C but not 4°C [Analog #1, Table 12]. [30]. Using Balb-3T3 cells transfected stably with the GRP receptor, NMB receptor or BRS-3 33% of the 125I-CPT-L2-BA3 added to the cells was internalized after 10 min at 37°C [30] [Analog #1, Table 12]. As a control, CPT-L2-(DSer5, 125I-DTyr6, β-Ala11, DPhe13, Nle14)Bn-(5–14) was not internalized. CPT-L2-(DSer5, DTyr6, β-Ala11, Phe13, Nle14)Bn-(5–14) functioned as a weak Bn receptor agonist which bound with over 2-orders of magnitude lower affinity to GRP receptors than did CPT-L2-BA3 [Analog #1, Table 12]. [30].
CPT-L2-BA3 is a potent Bn receptor agonist [30,34] [Analog #1, Table 12]. CPT-L2-BA3 increased phosphatidylinositol turnover and the ED50 for GRP receptors, NMB receptors and BRS-3 was 1, 1 and 11 nM respectively [Analog #1, Table 12] [30]. CPT-L2-BA3 inhibited the growth of lung cancer cells in vitro using the MTT assay as well as the clonogenic assay (IC50 = 70 nM) [30]. CPT-L2-BA3 (0.8 mg/kg, subcutaneous injection) slowed the growth of NCI-H1299 xenografts in nude mice in vivo by 38%[30][Analog #1, Table 12]. In addition, CPT-L2-BA3 inhibited the growth of CFPAC-1 (pancreatic cancer) and PC-3 cells (prostate cancer) in vitro and in vivo [30] [Analog #1, Table 12). Nanomolar concentrations of CPT-L2-BA3 were present in the plasma of nude mice treated with CPT-L2-BA3 and the half-life of CPT-L2-BA3 was approximately 20 min. in the mouse plasma [30] [Analog #1, Table 12]. In a separate study the mechanism of action CPT-L2-BA3 was investigated by comparing its behavior to a chemically identical compound, but with very low affinity for Bn receptors and which failed to activate Bn receptors (DPhe13-CPT-2-BA3)[34]. This study demonstrated that the inactive analog was not internalized and in both the MTT assay and clonogenic assays on NCI-1299 cells, which possess Bn receptors, the active analog was more potent than the inactive analog [34]. Furthermore, in vivo studies of the growth of H1229 xenografts in nude mice demonstrated that the active compound CPT-L2-BA3 was more potent than the inactive compound, at inhibiting tumor growth [34]. These results provide strong evidence that the cytotoxicity of CPT-L2-BA3 is mediated by interaction with Bn receptors on the tumor. A goal is to develop a high affinity Bn conjugate that has a prolonged half-life in vivo. It remains to be determined if CPT-L2-BA3 will benefit patients with lung cancer.
A chemotherapeutic doxorubicin (DOX) analog was conjugated to GRP receptor antagonist RC-3095 (Table 3) using an ester linkage [141,142] [Analog #5, Table 12]. Specifically, the cytotoxic Bn conjugate, AN-215 was prepared by coupling the NH2 terminus of des-DTpi-RC-3095 through a glutaric spacer to the 14-OH group of 2-pyrrolino-DOX (AN201) giving the structure: 2-pyrrolino-DOX-14-O-gl- [Leu13ψ-Leu14]Bn (7–14) [141,142]. The resulting AN-215 bound with high affinity to Swiss 3T3 cells containing BB2 receptors (IC50 = 1.6 nM) whereas RC3095 alone had an IC50 value of 1.6 nM for the GRP receptor [14,141]. AN-215 inhibited the growth of CFPAC-1 pancreatic cancer, DMS-53 SCLC, PC-3 prostate cancer and MKN-45 gastric cancer cell lines with IC50 values of 0.3, 0.04, 0.7 and 0.2 nM respectively [141]. AN-215 also inhibited growth of H-69 small cell lung cancer cells, U-87-MG glioblastoma tumors, as well as NCI-N87 and HS-746 gastric cancers [Analog #5, Table 12] [141]. AN215 inhibited the growth of PC-3 tumors in nude mice whereas the DOX analogue (AN201) had little effect on tumor growth, but was toxic [142,143]. A key question is if AN-215, which is a Bn receptor antagonist, is internalized by cancer cells. Also, it remains to be determined if AN-215 is rapidly degraded by blood esterases.
The mitotic inhibitor paclitaxel (Taxol) is widely used in the treatment of breast, ovarian, lung and head and neck cancer, and also in advanced forms of Kaposi’s sarcoma, but it has limited aqueous solubility and it is not targeted to any particular tissue. In order to improve solubility and efficiency in the drug delivery, Safavy et al. [144] coupled the Bn analog Bn(7–13)-NH2[Analog #3, Table 12] (Table 2) directly to paclitaxel using PEG as linker, and studied the binding properties of the molecule and its cytotoxic activity. They found that the conjugated Bn analog was soluble in water at a concentration of 250 mg/mL, in GRP receptor bearing BNR-11 cells it inhibited 125I-[Tyr4]Bn-binding to the same extent as Bn(7–13)NH2, and it had t1/2 of 154 min and 113 in PBS (phosphate buffered saline) and human plasma, respectively. When cytotoxic activity of the conjugated Bn analog was studied in NCI-H1299 cells, an increase in the cytotoxicity was found, with tumoricidal IC50 values after 24 h incubation of 14±1.1 nM vs 35±1.8 nM with paclitaxel alone.
In 2006, the same group published a study [145] using the same Bn analog (7–13)-NH2 [Analog #4, Table 12], but as dipeptide, coupled to paclitaxel through PEG or Glu, and studied their cytotoxic activity in different cell lines. They found that the highest cytotoxic effect was obtained with paclitaxel-Glu-(Bn(6–14)2) achieving 64–93% of growth inhibition.
The Bn receptor antagonists RC-3095 (Table 2) or RC-3940, (Hca6, Leu13, ψTac14)Bn(6–14) inhibited the growth of non-small cell lung cancer (NSCLC) cell lines NCI-H460 and A549 in orthotopically xenografted mice [146]. This resulted in a reduction of K-ras, COX-2 and pAkt in the tumors. Similarly, RC-3940 inhibited the growth of PC-3 and DU-145 prostate cancer tumors in nude mice [147]. This treatment resulted in a reduction in VEGF, bFGF, EGFR and HER2. In NSCLC, Bn and NMB were found to increase transactivation of the EGFR, which is inhibited by the tyrosine kinase inhibitor gefitinib. The Bn receptor antagonists PD176252 and PD168368 potentiate the growth inhibitory effects of gefitinib on cancer cells [148,149]. These results suggest that many of the effects of Bn may be mediated by the EGFR. The marine toxins hemiasterlin (Hem) and dolastatin (Dol) were coupled to a universal Bn agonist using an amide linkage [33]. The resulting Hem-LA-BA1 inhibited specific binding to NCI-H1299 lung cancer with an IC50 value of 15 nM [Analog #6, Table 12]. Hem-LA-BA1 was an agonist, which elevated cytosolic Ca2+ after addition to lung cancer cells. Hem-LA-BA1, but not BA1 inhibited the proliferation of NCI-H1299 cells in vitro [33]. The results indicate that marine toxin Bn conjugates kill cancer cells enriched in GRP receptors in vitro [33]. It remains to be determined if Hem-LA-BA1 inhibits lung cancer growth in vivo.
A diphtheria toxin-GRP fusion protein was cytotoxic and inhibited protein synthesis in cancer cells [150] [Analog #8, Table 12]. The catalytic and transmembrane domains of diphtheria toxin (DAB) were fused to GRP using molecular biology techniques. DAB389GRP inhibited protein synthesis (IC50 = 0.02 nM) [150]. The cytotoxicity of DAB389GRP resulted from receptor-mediated endocytosis through acidic vesicles and was blocked by 10 uM chloroquine [150] [Analog #8, Table 12]. DAB389GRP inhibited the proliferation of SCLC cell line NCI-H345 which possess GRP receptors, with an IC50 of 1 nM, whereas the IC50 was 100 nM for NIH/3T3 cells, which lack GRP receptors [150]. It remains to be determined if DAB389GRP inhibits the growth of SCLC tumors in vivo and does not cause toxic side effects in the host.
Mitochondria-disrupting peptides such as KLAKLAKKLAKLAK (KLA), GRFKRFRKKRKKLFKKLS (B27) and GGLRSLGRKILRAWKKYG (B28) were coupled to the N-terminal of Bn (2–14) [151] [Analog #7, Table 12] (Table 2). The resulting KB, BB27 and BB28 were evaluated [151]. BB28 half maximally inhibited the death of MCF-7 and CEM cells at 4 and 6 μM, respectively. Treated CEM cells had increased numbers of apoptotic and necrotic cells, loss of mitochondrial membrane potential and release of cytochrome C [151] [Analog #7, Table 12]. The effects of BB28 on CEM cells were inhibited by z-VAD-Fmk, a pan-caspase inhibitor. Intratumoral or intraperitoneal injection of BB28 (10 mg/kg) significantly slowed the growth of K562 tumors in nude mice [151]. Unfortunately relatively high doses of BB28 were used and it remains to be determined if lower doses are effective [151].
Another approach is to couple Bn antagonists to agents, which activate polyclonal T lymphocytes [152][Analog #9, Table 12]. Monoclonal antibody to OKT3 (anti-CD3) was coupled to [Cys5, DPhe6, Bn(5–13)ethyl amide](EA)[151]. EA binds with high affinity (IC50 = 1.7 nM) to Balb/3T3 cells containing GRP receptors and similar to other Bn des Met14 ethyl amide analogs functions as a Bn receptor antagonist [14,37,38,152]. The resulting bispecific molecule caused apoptosis and necrosis of NCI-H345 and DMS273 cells [152]. The bispecific molecule killed SCLC cells in vivo by activating allogeneic T cells through the CD2/TCR complex utilizing Il-2 [152] [Analog #9, Table 12]. The results suggest that bispecific molecules may stimulate the immune response against SCLC tumors.
In 1995 Chen et al. published a paper [153,154] using Bn analog ([Lys3]Bn, (Table 2) [Analog #10, Table 12] coupled to an antibody anti-FcγRI as an immunotherapeutic approach to the treatment of SCLC. This bispecific immunoconjugate should bind by one side to the GRP receptor expressed in SCLC cells and by the other to the FcγRI expressed in cells such as activated monocytes or neutrophils and produce lysis of the cancer cells. In fact, the authors found [154] that binding to the SCLC cells was proportional to the immunoconjugate concentration and to the number of GRP receptors on the cell surface, and it also bound to monocytes and neutrophils. When the monocytes/neutrophils were previously activated and then coincubated with the SCLC and in the presence of the immunoconjugate, an increase in the lysis of the cancer cells was observed. In another study from the same group [155] they examined the effect of a different immunoconjugate composed of a Bn antagonist ([DTrp6, Leu13-ψ(CH2NH)Phe14]Bn(6–14), [Analog #11, Table 12] or agonist ([Lys3]Bn, (Table 2) [Analog #9 Table 12] coupled to an antibody, anti- FcγRI or- FcγRIII, and the results showed that both immunoconjugates bound to SCLC cells in a dose-related manner, and none of them produced an alteration in the clonogenic growth of the cells. When the SCLC cells were coincubated in the presence of either immunoconjugate with activated monocytes or natural killer cells, a clear increase in cytotoxicity of SCLC was observed.
Another approach to attempt to decrease growth of tumor cells expressing GRP receptors was the synthesis of a 40 residue precursor peptide by linking together 4 designed anticancer peptide analogs including a VIP binding receptor inhibitor, a somatostatin agonist, a substance P receptor antagonist and a Bn receptor antagonist ([DPhe6, Aib11, desMet14]Bn(6–14), [Analog #20, Table 12] through enzyme cleavable Lys-Lys linkers [156,157]. Treatment with this precursor peptide produced the release of each individual peptide analog by the action of enzymes such as PC1 or PC2, so each neuropeptide analog will bind its receptor and inhibit tumor growth. In fact, it was found that incubation of the precursor peptide with trypsin produced the release of all the individual peptides. Also treatment with this precursor peptide inhibited cell proliferation in all cancer cell lines tested. When Balb C nude mice xenografted with primary colon tumor cells were treated with the peptide precursor [Analog #20, Table 12], inhibition in tumor growth of 73.7% vs no treated animals was found. When the molecular pathway used to inhibit cellular proliferation in tumors by the precursor peptide was studied [157] in different cancer cell lines, the results showed that the precursor peptide down-regulated cAMP, EGF-dependent cell proliferation and the phosphorylation pERK1/2 in GI carcinomas. It also produced an activation of the apoptotic caspase-3 dependent pathway and induced the p53 tumor suppressor protein. In endothelial cells it inhibited the formation of capillary-like tubes and reduced VEGF levels.
Bn analog 8-Aoc-Bn(7–14) [Analog #21, Table 12] has been studied coupled to the photosensitizer Mono-carbohexyl-tetrasulfonated aluminium phthalocyanine in order to improve the site-delivery of the drug in prostate cancer [155]. Binding studies revealed that conjugated Bn analog [Analog #21, Table 12] had lower affinity than Bn analog alone in PC-3 cells (IC50: 29.4 nM vs 0.37 nM, respectively), but when photodynamic efficacy was tested in PC-3 cells in vitro, it was improved by a 2.5-fold compared to the Mono-carbohexyl- tetrasulfonated aluminium phthalocyanine alone.
Bn analogs have been also used to improve gene delivery into the cells by siRNA or adenovirus. In the first case [158], the Bn agonist analog Bn(7–14) [Analog #22, Table 12] (Table 2) was coupled to maleimide-PEG and combined with EHCO nanoparticles containing siRNA; the inclusion of the Bn conjugated to the nanoparticle produced a gene silencing efficiency of 91.9% and cell uptake of 73.9%, which was significantly higher than with EHCO nanoparticles without the Bn analog. In order to increase adenovirus-mediated gene delivery Hong et al. [158] conjugated a human GRP analog (13–27) [Analog #22, Table 12] to the N or C terminal of MH20 (an icosapeptide that mimics a portion of the α2 domain of human MHC class I molecules which are receptors for the entrance of the adenovirus in the cell). With this approach it was proposed that the conjugated GRP analog [Analog #22, Table 12] would bind the GRP receptor with the MH20 free to bind the adenovirus which will increase the number of adenovirus receptors in the cell and enhance virus entrance and gene delivery. In fact, while the GRP analog bound to the C side of the MH20 had no effect on adenovirus infection and gene transfer, the GRP-N′-MH20 showed a significant enhancement (8–15-fold) in adenovirus-mediated gene transfer in all cell lines tested when cells were pretreatment with the GRP conjugate [Analog #22, Table 12] at 25 μM. The increase in entry was proportional to the amount GRP receptor in the cell membrane.
Eleven different classes of Bn receptor antagonists have been developed [8,14,37–39] and members of some classes have been shown to be cytostatic for lung cancer cells. Peptide receptor antagonists for GRP receptors were developed by eliminating the C-terminal methionine or reducing the penultimate peptide bond before the C-terminal methionine [8,14,37–39,159]. BW2258U89 and RC-3095 (Table 2) are two such Bn antagonists which bind with high affinity (IC50 = 0.2 nM and 1.4 nM respectively) to GRP receptors [160]. BW2258U89, 1 uM, inhibited the growth of lung cancer cells. In nude mice bearing NCI-H1299 xenografts, tumor growth was slowed significantly if BW2258U89 was administered subcutaneously (0.8 mg/kg) [160]. The tumors rapidly regrew, however, if BW2258U89 administration was discontinued [160]. For NMB receptors, nonpeptide antagonists have been developed such as PD168368 and PD176252 (Table 2) [14,161]. PD168368 and PD176252 bind with high affinity (IC50 = 0.51 and 0.53 nM respectively) to Balb/3T3 cells transfected with NMB receptors [14]. PD168368 is slowly metabolized by proteolytic enzymes and hence can be administered orally. Gavage administration of PD168368 (0.8 mg/kg) inhibited the growth of tumors in nude mice [162]. PD168368 has limited solubility in water and permeates the brain after crossing the blood-brain barrier. In contrast, BW2258U89 is water-soluble and does not readily cross the blood-brain barrier.
In another study [163] using the Bn antagonists [DPhe6, Aib11, desMet14]Bn(6–14) and [DPhe6, desMet14]Bn(6–14) analogs [Analogs #13–19, Table 12] (Table 2), the anti-proliferative properties of these peptides were tested in different cancer cell lines [163]. In vitro MTT results showed that all Bn antagonists had cytotoxic effects in these cancer cells, specifically the highest values in cell proliferation inhibition were found with [DPhe6, Aib11, Ile13, desMet14]Bn(6–14) at 0.1 nM in MiaPaCa-2 cell line, [DPhe6, Aib9, Aib11, Ile13, desMet14]Bn(6–14) at 0.1 nM in SW620, [DPhe6, Aib9, Ile13, desMet14]Bn(6–14) at 1 μM in HT29 cells and Butanoyl[DPhe6, Aib11, desMet14]Bn(6–14) at 0.01 nM in PTC[Analogs #13–19, Table 12]. Butanoyl[DPhe6, Aib11, desMet14]Bn(6–14) [Analog #19, Table 12] was chosen to treat PTC cell tumor xenograft mice as the butanoyl moiety may protect the N′ terminal end of the molecule and improve its in vivo stability and biodistribution. After 29 days of treatment, tumor growth was inhibited by 44.3% in the treated animals.
VI. Conclusions
The discovery of the frequent over-expression and/or ectopic expression of various peptide/neurotransmitter receptors on many neoplasms have opened the potential for a new approach to both imaging these tumors and for targeted delivery of cytotoxic agents. The results using radiolabeled somatostatin analogs to target somatostatin receptors (sst1–5) which are over-expressed/ectopically expressed in various neuroendocrine tumors (primarily carcinoids, pancreatic endocrine tumors) [1,22,23] have clearly established the clinical usefulness of somatostatin receptor-mediated imaging and cytotoxicity for treatment of these malignancies. Unfortunately, many of the common neoplasms (lung, gastric, pancreatic, breast, prostate, CNS tumors, etc) for which limited treatments exist for advanced disease, do not over-express/ectopically express somatostatin receptors, however they frequently over-express other G protein-coupled receptors, particularly those of the Bn family of receptors (GRPR, NMBR, BRS-3) [11,19,20].
Unfortunately, at present except for the use of radiolabeled somatostatin analogs in patients with neuroendocrine tumors, the approach of using the tumor receptor over-expression/ectopic expression for receptor-mediated imaging or cytotoxicity with any of the other peptide receptors, including Bn receptors, has not yet been demonstrated to be clinically useful. The studies reviewed here have identified a number of radiolabeled Bn analogs that could be used for standard nuclear medicine imaging (99mTc-, 111In-, 185/7Rh-labeled analogs, 125I), for PET imaging (18F, 68Ga, 64Cu) or for radiation-mediated cytotoxicity (90Y, 111In, 64Cu, 177Lu, 125I). Both in vitro and in vivo studies in animals show a number of these radiolabeled Bn analogs have many properties that should allow them to be useful for studies in patients with these neoplasms. These results including identifying a number of radiolabeled analogs with high Bn receptor affinity, particularly for the GRP receptor, which is most frequently over-expressed in these tumors; that show stability in plasma or in vivo and that function as Bn receptor agonists and are rapidly internalized by the tumors. Furthermore, in in vivo studies, a number of these radiolabeled Bn analogs imaged tumors, were internalized and the radiolabeled ligand was retained in the tumor, properties that are thought necessary for effective receptor-mediated cytotoxicity. Although the clinically relevant studies in patients with neuroendocrine tumors using radiolabeled somatostatin analogs have all used radiolabeled agonists, recent studies show that radiolabeled antagonists of the somatostatin receptor give even better imaging results even though they are not internalized [44]. Whether they will also be more effective for radiation-induced cytotoxicity in patients with neuroendocrine tumors is not established at present. Similarly, a number of radiolabeled Bn analogs, which function as receptor antagonists, are also reported to give excellent imaging, although they are not internalized [2,45–51].
At present there are only 12 studies of radiolabeled Bn analogs (all agonists) in humans, which in most cases are performed on patients with known Bn receptor containing tumors and are either standard nuclear medicine imaging or PET imaging studies (Table 11). While some of these studies show promising results, they include only small numbers of patients and much more systematic studies involving larger numbers of patients will need to be performed to assess the potential value of this approach. Future studies will need to establish the sensitivity of the radioligands used; their specificity because false positives can have a marked effect on their general utility, and whether their use changes clinical management to justify the full development of Bn receptor-mediated imaging agents. Furthermore, the question of whether a radiolabeled agonist or Bn antagonist is best will need to be resolved. Additional detailed studies will need to be done to evaluate the possible use of radiolabeled Bn analogs for receptor-mediated cytotoxic in human malignancies.
A number of in vitro and in vivo studies are suggesting various approaches for Bn receptor-mediated cytotoxicity using nonradiolabeled analogues (Table 12). These include coupling of Bn analogs to chemotherapeutic agents (camptothecin, paclitaxel); to novel marine cytotoxic peptides (hemiasterlin, dolistatin); to various cytotoxins (diphtheria toxin); to mitochondria disruptive peptides; to immune activating agents; to agents enhancing sensitivity to the effects of photodynamic therapy; to agents that enhance interfering mRNA delivery or to agents that enhance adenovirus-mediated gene delivery (Table 12). At present there are very limited in vivo studies on this approach, and no studies examining their possible clinical efficacy in human diseases. Nevertheless, this approach may lead to agents that allow targeted delivery of cytotoxic agents with much less toxicity, that are either effective alone or in combination with existing treatments and therefore should be more extensively investigated. Because Bn receptors are so frequently over-expressed on various common malignancies, they are an excellent model to use to investigate this novel, therapeutic approach.
Acknowledgments
This work was partially supported by intramural research funds of NIDDK and NIH.
Footnotes
Conflict of Interest: None
References
- 1.Breeman WA, Kwekkeboom DJ, de Blois E, de Jong M, Visser TJ, Krenning EP. Radiolabelled regulatory peptides for imaging and therapy. Anticancer Agents Med Chem. 2007;7:345–357. doi: 10.2174/187152007780618171. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder RP, Muller C, Reneman S, Melis ML, Breeman WA, de Blois E, Bangma CH, Krenning EP, van Weerden WM, de Jong M. A standardised study to compare prostate cancer targeting efficacy of five radiolabelled bombesin analogues. Eur J Nucl Med Mol Imaging. 2010 doi: 10.1007/s00259-010-1388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tweedle MF. Peptide-targeted diagnostics and radiotherapeutics. Acc Chem Res. 2009;42:958–968. doi: 10.1021/ar800215p. [DOI] [PubMed] [Google Scholar]
- 4.Okarvi SM. Peptide-based radiopharmaceuticals and cytotoxic conjugates: potential tools against cancer. Cancer Treat Rev. 2008;34:13–26. doi: 10.1016/j.ctrv.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez N, Moody TW, Igarashi H, Ito T, Jensen RT. Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diabetes Obes. 2008;15:58–64. doi: 10.1097/MED.0b013e3282f3709b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erspamer V, Erpamer GF, Inselvini M. Some pharmacological actions of alytesin and bombesin. J Pharm Pharmacol. 1970;22:875–876. doi: 10.1111/j.2042-7158.1970.tb08465.x. [DOI] [PubMed] [Google Scholar]
- 7.Erspamer V. Discovery, isolation and characterization of bombesin-like peptides. Ann N Y Acad Sci. 1988;547:3–9. doi: 10.1111/j.1749-6632.1988.tb23870.x. [DOI] [PubMed] [Google Scholar]
- 8.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LVIII. Mammalian Bombesin Receptors: Nomenclature, distribution, pharmacology, signaling and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald TJ, Jornvall H, Nilsson G, Vagne M, Ghatei M, Bloom SR, Mutt V. Characterization of a gastrin-releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979;90:227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- 10.Minamino N, Kangawa K, Matsuo H. Neuromedin B: a novel bombesin-like peptide identified in porcine spinal cord. Biochem Biophys Res Commun. 1983;114:541–548. doi: 10.1016/0006-291x(83)90814-8. [DOI] [PubMed] [Google Scholar]
- 11.Jensen RT, Moody TW. Bombesin-related peptides and neurotensin: effects on cancer growth/proliferation and cellular signaling in cancer. In: Kastin AJ, editor. Handbook of Biologically active peptides. Vol. 1. Elsevier; Amsterdam: 2006. pp. 429–434. [Google Scholar]
- 12.Ohki-Hamazaki H. Neuromedin B. Prog Neurobiol. 2000;62:297–312. doi: 10.1016/s0301-0082(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 13.Jensen RT, Coy DH, Saeed ZA, Heinz-Erian P, Mantey S, Gardner JD. Interaction of bombesin and related peptides with receptors on pancreatic acini. Ann N Y Acad Sci. 1988;547:138–149. doi: 10.1111/j.1749-6632.1988.tb23882.x. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez N, Mantey SA, Pradhan TK, Sancho V, Moody TW, Coy DH, Jensen RT. Characterization of putative GRP- and NMB-receptor antagonist’s interaction with human receptors. Peptides. 2009;30:1473–1486. doi: 10.1016/j.peptides.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber HC. Regulation and signaling of human bombesin receptors and their biological effects. Curr Opin Endocrinol Diabetes Obes. 2009;16:66–71. doi: 10.1097/med.0b013e32831cf5aa. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Chen J, Mokotoff M, Ball ED. Targeting gastrin-releasing peptide receptors for cancer treatment. Anticancer Drugs. 2004;15:921–927. doi: 10.1097/00001813-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 17.de Visser M, Verwijnen SM, de Jong M. Update: improvement strategies for peptide receptor scintigraphy and radionuclide therapy. Cancer Biother Radiopharm. 2008;23:137–157. doi: 10.1089/cbr.2007.0435. [DOI] [PubMed] [Google Scholar]
- 18.Okarvi SM. Peptide-based radiopharmaceuticals: future tools for diagnostic imaging of cancers and other diseases. Med Res Rev. 2004;24:357–397. doi: 10.1002/med.20002. [DOI] [PubMed] [Google Scholar]
- 19.Reubi JC, Wenger S, Schumuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radoligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14) Clin Cancer Res. 2002;8:1139–1146. [PubMed] [Google Scholar]
- 20.Reubi JC, Macke HR, Krenning EP. Candidates for peptide receptor radiotherapy today and in the future. J Nucl Med. 2005;46(Suppl 1):67S–75S. [PubMed] [Google Scholar]
- 21.Moody TW, Chan D, Fahrenkrug J, Jensen RT. Neuropeptides as autocrine growth factors in cancer cells. Curr Pharm Des. 2003;9:495–509. doi: 10.2174/1381612033391621. [DOI] [PubMed] [Google Scholar]
- 22.Forrer F, Valkema R, Kwekkeboom DJ, de Jong M, Krenning EP. Neuroendocrine tumors. Peptide receptor radionuclide therapy. Best Pract Res Clin Endocrinol Metab. 2007;21:111–129. doi: 10.1016/j.beem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Van Essen M, Krenning EP, Kam BL, de Jong M, Valkema R, Kwekkeboom DJ. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol. 2009;5:382–393. doi: 10.1038/nrendo.2009.105. [DOI] [PubMed] [Google Scholar]
- 24.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WAP, Kooij PPM, Oei HY, van Hagen M, Postema PTE, de Jong M, Reubi JC, Visser TJ, Reijs AEM, Hofland LJ, Koper JW, Lamberts SWJ. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 25.Jensen RT. Peptide therapy. Recent advances in the use of somatostatin and other peptide receptor agonists and antagonists. In: Lewis JH, Dubois A, editors. Current Clinical Topics in Gastrointestinal Pharmacology. Blackwell Science, Inc; Malden, MA: 1997. pp. 144–223. [Google Scholar]
- 26.Gibril F, Jensen RT. Diagnostic uses of radiolabelled somatostatin-receptor analogues in gastroenteropancreatic endocrine tumors. Dig Liver Dis. 2004;36:S106–S120. doi: 10.1016/j.dld.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and therapy. J Nucl Med. 2000;41:1704–1713. [PubMed] [Google Scholar]
- 28.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors:; Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibril F, Reynolds JC, Doppman JL, Chen CC, Venzon DJ, Termanini B, Weber HC, Stewart CA, Jensen RT. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas: a prospective study. Ann Intern Med. 1996;125:26–34. doi: 10.7326/0003-4819-125-1-199607010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Moody TW, Mantey SA, Pradhan TK, Schumann M, Nakagawa T, Martinez A, Fuselier J, Coy DH, Jensen RT. Development of high affinity camptothecin-bombesin conjugates that have targeted cytotoxicity for bombesin receptor-containing tumor cells. J Biol Chem. 2004;279:23580–23589. doi: 10.1074/jbc.M401938200. [DOI] [PubMed] [Google Scholar]
- 31.Nagy A, Schally AV. Targeting cytotoxic conjugates of somatostatin, luteinizing hormone-releasing hormone and bombesin to cancers expressing their receptors: a “smarter” chemotherapy. Curr Pharm Des. 2005;11:1167–1180. doi: 10.2174/1381612053507594. [DOI] [PubMed] [Google Scholar]
- 32.Schally AV. New approaches to the therapy of various tumors based on Peptide analogues. Horm Metab Res. 2008;40:315–322. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- 33.Moody TW, Pradhan T, Mantey SA, Jensen RT, Dyba M, Moody D, Tarasova NI, Michejda CJ. Bombesin marine toxin conjugates inhibit the growth of lung cancer cells. Life Sci. 2008;82:855–861. doi: 10.1016/j.lfs.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moody TW, Sun LC, Mantey SA, Pradhan T, Mackey LV, Gonzales N, Fuselier JA, Coy DH, Jensen RT. In vitro and in vivo antitumor effects of cytotoxic camptothecin-bombesin conjugates are mediated by specific interaction with cellular bombesin receptors. J Pharmacol Exp Ther. 2006;318:1265–1272. doi: 10.1124/jpet.106.104141. [DOI] [PubMed] [Google Scholar]
- 35.Preston SR, Miller GV, Primrose JN. Bombesin-like peptides and cancer. Crit Rev Oncol Hematol. 1996;23:225–238. doi: 10.1016/1040-8428(96)00204-1. [DOI] [PubMed] [Google Scholar]
- 36.Yegen BC. Bombesin-like peptides: candidates as diagnostic and therapeutic tools. Curr Pharm Des. 2003;9:1013–1022. doi: 10.2174/1381612033455134. [DOI] [PubMed] [Google Scholar]
- 37.Jensen RT, Coy DH. Progress in the development of potent bombesin receptor antagonists. Trends Pharmacol Sci. 1991;12(1):13–19. doi: 10.1016/0165-6147(91)90483-9. [DOI] [PubMed] [Google Scholar]
- 38.Wang LH, Coy DH, Taylor JE, Jiang NY, Kim SH, Moreau JP, Huang SC, Mantey SA, Frucht H, Jensen RT. Desmethionine alkylamide bombesin analogues: a new class of bombesin receptor antagonists with a potent antisecretory activity in pancreatic acini and antimitotic activity in Swiss 3T3 cells. Biochemistry (Mosc) 1990;29(3):616–622. doi: 10.1021/bi00455a004. [DOI] [PubMed] [Google Scholar]
- 39.Wang LH, Coy DH, Taylor JE, Jiang NY, Moreau JP, Huang SC, Frucht H, Haffar BM, Jensen RT. Des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists. J Biol Chem. 1990;265(26):15695–15703. [PubMed] [Google Scholar]
- 40.Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan TK, Searles RP, Spindel ER, Battey JF, Coy DH, Jensen RT. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3: which demonstrates it has a unique pharmacology compared to other mammalian bombesin receptors. J Biol Chem. 1997;272(41):26062–26071. doi: 10.1074/jbc.272.41.26062. [DOI] [PubMed] [Google Scholar]
- 41.Ryan RR, Weber HC, Hou W, Sainz E, Mantey SA, Battey JF, Coy DH, Jensen RT. Ability of various bombesin receptor agonists and antagonists to alter intracellular signaling of the human orphan receptor BRS-3. J Biol Chem. 1998;273:13613–13624. doi: 10.1074/jbc.273.22.13613. [DOI] [PubMed] [Google Scholar]
- 42.Ryan RR, Weber HC, Mantey SA, Hou W, Hilburger ME, Pradhan TK, Coy DH, Jensen RT. Pharmacology and intracellular signaling mechanisms of the native human orphan receptor BRS-3 in lung cancer cells. J Pharmacol Exp Ther. 1998;287:366–380. [PubMed] [Google Scholar]
- 43.Lin JT, Coy DH, Mantey SA, Jensen RT. Comparison of the peptide structural requirements for high affinity interaction with bombesin receptors. Eur J Pharmacol. 1996;294:55–69. doi: 10.1016/0014-2999(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 44.Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X, Erchegyi J, Rivier J, Macke HR, Reubi JC. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci U S A. 2006;103:16436–16441. doi: 10.1073/pnas.0607761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cescato R, Maina T, Nock B, Nikolopoulou A, Charalambidis D, Piccand V, Reubi JC. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med. 2008;49:318–326. doi: 10.2967/jnumed.107.045054. [DOI] [PubMed] [Google Scholar]
- 46.Mansi R, Wang X, Forrer F, Kneifel S, Tamma ML, Waser B, Cescato R, Reubi JC, Maecke HR. Evaluation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-conjugated bombesin-based radioantagonist for the labeling with single-photon emission computed tomography, positron emission tomography, and therapeutic radionuclides. Clin Cancer Res. 2009;15:5240–5249. doi: 10.1158/1078-0432.CCR-08-3145. [DOI] [PubMed] [Google Scholar]
- 47.Abd-Elgaliel WR, Gallazzi F, Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Hoffman TJ, Lever SZ. Design, synthesis, and biological evaluation of an antagonist-bombesin analogue as targeting vector. Bioconjug Chem. 2008;19:2040–2048. doi: 10.1021/bc800290c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breeman WA, Hofland LJ, de Jong M, Bernard BF, Srinivasan A, Kwekkeboom DJ, Visser TJ, Krenning EP. Evaluation of radiolabelled bombesin analogues for receptor-targeted scintigraphy and radiotherapy. Int J Cancer. 1999;81:658–665. doi: 10.1002/(sici)1097-0215(19990517)81:4<658::aid-ijc24>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 49.Abiraj K, Mansi R, Tamma ML, Forrer F, Cescato R, Reubi JC, Akyel KG, Maecke HR. Tetraamine-Derived Bifunctional Chelators for Technetium-99m Labelling: Synthesis, Bioconjugation and Evaluation as Targeted SPECT Imaging Probes for GRP-Receptor-Positive Tumours. Chemistry. 2010;16:2115–2124. doi: 10.1002/chem.200902011. [DOI] [PubMed] [Google Scholar]
- 50.Maina T, Nock B, Mather S. Targeting prostate cancer with radiolabelled bombesins. Cancer Imaging. 2006;6:153–157. doi: 10.1102/1470-7330.2006.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hohne A, Mu L, Honer M, Schubiger PA, Ametamey SM, Graham K, Stellfeld T, Borkowski S, Berndorff D, Klar U, Voigtmann U, Cyr JE, Friebe M, Dinkelborg L, Srinivasan A. Synthesis, 18F-labeling, and in vitro and in vivo studies of bombesin peptides modified with silicon-based building blocks. Bioconjug Chem. 2008;19:1871–1879. doi: 10.1021/bc800157h. [DOI] [PubMed] [Google Scholar]
- 52.Baidoo KE, Lin KS, Zhan Y, Finley P, Scheffel U, Wagner HN., Jr Design, synthesis, and initial evaluation of high-affinity technetium bombesin analogues. Bioconjug Chem. 1998;9:218–225. doi: 10.1021/bc9701959. [DOI] [PubMed] [Google Scholar]
- 53.Lin KS, Luu A, Baidoo KE, Hashemzadeh-Gargari H, Chen MK, Brenneman K, Pili R, Pomper M, Carducci MA, Wagner HN., Jr A new high affinity technetium-99m-bombesin analogue with low abdominal accumulation. Bioconjug Chem. 2005;16:43–50. doi: 10.1021/bc049820h. [DOI] [PubMed] [Google Scholar]
- 54.Santos-Cuevas CL, Ferro-Flores G, Arteaga de Murphy C, Ramirez Fde M, Luna-Gutierrez MA, Pedraza-Lopez M, Garcia-Becerra R, Ordaz-Rosado D. Design, preparation, in vitro and in vivo evaluation of (99m)Tc-N2S2-Tat(49–57)-bombesin: a target-specific hybrid radiopharmaceutical. Int J Pharm. 2009;375:75–83. doi: 10.1016/j.ijpharm.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 55.Gourni E, Paravatou M, Bouziotis P, Zikos C, Fani M, Xanthopoulos S, Archimandritis SC, Livaniou E, Varvarigou AD. Evaluation of a series of new 99mTc-labeled bombesin-like peptides for early cancer detection. Anticancer Res. 2006;26:435–438. [PubMed] [Google Scholar]
- 56.Gourni E, Bouziotis P, Benaki D, Loudos G, Xanthopoulos S, Paravatou-Petsotas M, Mavri-Vavagianni M, Pelecanou M, Archimandritis SC, Varvarigou AD. Structural assessment and biological evaluation of two N3S bombesin derivatives. J Med Chem. 2009;52:4234–4246. doi: 10.1021/jm900360d. [DOI] [PubMed] [Google Scholar]
- 57.Smith CJ, Sieckman GL, Owen NK, Hayes DL, Mazuru DG, Kannan R, Volkert WA, Hoffman TJ. Radiochemical investigations of gastrin-releasing peptide receptor-specific [(99m)Tc(X)(CO)3-Dpr-Ser-Ser-Ser-Gln-Trp-Ala-Val-Gly-His-Leu-Met-(NH2)] in PC-3, tumor-bearing, rodent models: syntheses, radiolabeling, and in vitro/in vivo studies where Dpr = 2,3-diaminopropionic acid and X = H2O or P(CH20H)3. Cancer Res. 2003;63:4082–4088. [PubMed] [Google Scholar]
- 58.Van de Wiele C, Dumont F, Vanden Broecke R, Oosterlinck W, Cocquyt V, Serreyn R, Peers S, Thornback J, Slegers G, Dierckx RA. Technetium-99m RP527, a GRP analogue for visualisation of GRP receptor-expressing malignancies: a feasibility study. Eur J Nucl Med. 2000;27:1694–1699. doi: 10.1007/s002590000355. [DOI] [PubMed] [Google Scholar]
- 59.Alves S, Correia JD, Santos I, Veerendra B, Sieckman GL, Hoffman TJ, Rold TL, Figueroa SD, Retzloff L, McCrate J, Prasanphanich A, Smith CJ. Pyrazolyl conjugates of bombesin: a new tridentate ligand framework for the stabilization of fac-[M(CO)3]+ moiety. Nucl Med Biol. 2006;33:625–634. doi: 10.1016/j.nucmedbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Prasanphanich AF, Lane SR, Figueroa SD, Ma L, Rold TL, Sieckman GL, Hoffman TJ, McCrate JM, Smith CJ. The effects of linking substituents on the in vivo behavior of site-directed, peptide-based, diagnostic radiopharmaceuticals. In Vivo. 2007;21:1–16. [PubMed] [Google Scholar]
- 61.Kunstler JU, Veerendra B, Figueroa SD, Sieckman GL, Rold TL, Hoffman TJ, Smith CJ, Pietzsch HJ. Organometallic 99mTc(III) ‘4 + 1’ bombesin(7–14) conjugates: synthesis, radiolabeling, and in vitro/in vivo studies. Bioconjug Chem. 2007;18:1651–1661. doi: 10.1021/bc700197m. [DOI] [PubMed] [Google Scholar]
- 62.Lane SR, Veerendra B, Rold TL, Sieckman GL, Hoffman TJ, Jurisson SS, Smith CJ. 99mTc(CO)3-DTMA bombesin conjugates having high affinity for the GRP receptor. Nucl Med Biol. 2008;35:263–272. doi: 10.1016/j.nucmedbio.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faintuch BL, Teodoro R, Duatti A, Muramoto E, Faintuch S, Smith CJ. Radiolabeled bombesin analogs for prostate cancer diagnosis: preclinical studies. Nucl Med Biol. 2008;35:401–411. doi: 10.1016/j.nucmedbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Shi J, Jia B, Liu Z, Yang Z, Yu Z, Chen K, Chen X, Liu S, Wang F. 99mTc-labeled bombesin(7–14)NH2 with favorable properties for SPECT imaging of colon cancer. Bioconjug Chem. 2008;19:1170–1178. doi: 10.1021/bc700471z. [DOI] [PubMed] [Google Scholar]
- 65.Retzloff LB, Heinzke L, Figureoa SD, Sublett SV, Ma L, Sieckman GL, Rold TL, Santos I, Hoffman TJ, Smith CJ. Evaluation of [99mTc-(CO)3-X-Y-Bombesin(7–14)NH2] Conjugates for Targeting Gastrin-releasing Peptide Receptors Over-expressed on Breast Carcinoma. Anticancer Res. 2010;30:19–30. [PubMed] [Google Scholar]
- 66.Garcia Garayoa E, Ruegg D, Blauenstein P, Zwimpfer M, Khan IU, Maes V, Blanc A, Beck-Sickinger AG, Tourwe DA, Schubiger PA. Chemical and biological characterization of new Re(CO)3/[99mTc](CO)3 bombesin analogues. Nucl Med Biol. 2007;34:17–28. doi: 10.1016/j.nucmedbio.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 67.Garcia Garayoa E, Schweinsberg C, Maes V, Brans L, Blauenstein P, Tourwe DA, Schibli R, Schubiger PA. Influence of the molecular charge on the biodistribution of bombesin analogues labeled with the [99mTc(CO)3]-core. Bioconjug Chem. 2008;19:2409–2416. doi: 10.1021/bc800262m. [DOI] [PubMed] [Google Scholar]
- 68.Schweinsberg C, Maes V, Brans L, Blauenstein P, Tourwe DA, Schubiger PA, Schibli R, Garcia Garayoa E. Novel glycated [99mTc(CO)3]-labeled bombesin analogues for improved targeting of gastrin-releasing peptide receptor-positive tumors. Bioconjug Chem. 2008;19:2432–2439. doi: 10.1021/bc800319g. [DOI] [PubMed] [Google Scholar]
- 69.Maes V, Brans L, Schweinsberg C, Garcia-Garayoa E, Blauenstein P, Schubiger PA, Tourwe D. Carbohydrated [99mTc(CO)3](NalphaHis)Ac-bombesin(7–14) analogs. Adv Exp Med Biol. 2009;611:409–410. [PubMed] [Google Scholar]
- 70.Nock BA, Nikolopoulou A, Galanis A, Cordopatis P, Waser B, Reubi JC, Maina T. Potent bombesin-like peptides for GRP-receptor targeting of tumors with 99mTc: a preclinical study. J Med Chem. 2005;48:100–110. doi: 10.1021/jm049437y. [DOI] [PubMed] [Google Scholar]
- 71.Durkan K, Lambrecht FY, Unak P. Radiolabeling of bombesin-like peptide with 99mTc: 99mTc-litorin and biodistribution in rats. Bioconjug Chem. 2007;18:1516–1520. doi: 10.1021/bc060400x. [DOI] [PubMed] [Google Scholar]
- 72.Hoffman TJ, Gali H, Smith CJ, Sieckman GL, Hayes DL, Owen NK, Volkert WA. Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. J Nucl Med. 2003;44:823–831. [PubMed] [Google Scholar]
- 73.Garrison JC, Rold TL, Sieckman GL, Naz F, Sublett SV, Figueroa SD, Volkert WA, Hoffman TJ. Evaluation of the pharmacokinetic effects of various linking group using the 111In-DOTA-X-BBN(7–14)NH2 structural paradigm in a prostate cancer model. Bioconjug Chem. 2008;19:1803–1812. doi: 10.1021/bc8001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breeman WA, de Jong M, Bernard BF, Kwekkeboom DJ, Srinivasan A, van der Pluijm ME, Hofland LJ, Visser TJ, Krenning EP. Pre-clinical evaluation of [(111)In-DTPA-Pro(1), Tyr(4)]bombesin, a new radioligand for bombesin-receptor scintigraphy. Int J Cancer. 1999;83:657–663. doi: 10.1002/(sici)1097-0215(19991126)83:5<657::aid-ijc15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 75.Bernard BF, Krenning E, Breeman WA, Visser TJ, Bakker WH, Srinivasan A, de Jong M. Use of the rat pancreatic CA20948 cell line for the comparison of radiolabelled peptides for receptor-targeted scintigraphy and radionuclide therapy. Nucl Med Commun. 2000;21:1079–1085. doi: 10.1097/00006231-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 76.Breeman WA, de Jong M, Erion JL, Bugaj JE, Srinivasan A, Bernard BF, Kwekkeboom DJ, Visser TJ, Krenning EP. Preclinical comparison of (111)In-labeled DTPA- or DOTA-bombesin analogs for receptor-targeted scintigraphy and radionuclide therapy. J Nucl Med. 2002;43:1650–1656. [PubMed] [Google Scholar]
- 77.de Visser M, Bernard HF, Erion JL, Schmidt MA, Srinivasan A, Waser B, Reubi JC, Krenning EP, de Jong M. Novel (111)In-labelled bombesin analogues for molecular imaging of prostate tumours. Eur J Nucl Med Mol Imaging. 2007;34:1228–1238. doi: 10.1007/s00259-006-0356-3. [DOI] [PubMed] [Google Scholar]
- 78.de Visser M, van Weerden WM, de Ridder CM, Reneman S, Melis M, Krenning EP, de Jong M. Androgen-dependent expression of the gastrin-releasing peptide receptor in human prostate tumor xenografts. J Nucl Med. 2007;48:88–93. [PubMed] [Google Scholar]
- 79.Zhang H, Chen J, Waldherr C, Hinni K, Waser B, Reubi JC, Maecke HR. Synthesis and evaluation of bombesin derivatives on the basis of pan-bombesin peptides labeled with indium-111, lutetium-177, and yttrium-90 for targeting bombesin receptor-expressing tumors. Cancer Res. 2004;64:6707–6715. doi: 10.1158/0008-5472.CAN-03-3845. [DOI] [PubMed] [Google Scholar]
- 80.Ho CL, Chen LC, Lee WC, Chiu SP, Hsu WC, Wu YH, Yeh CH, Stabin MG, Jan ML, Lin WJ, Lee TW, Chang CH. Receptor-binding, biodistribution, dosimetry, and micro-SPECT/CT imaging of 111In-[DTPA(1), Lys(3), Tyr(4)]-bombesin analog in human prostate tumor-bearing mice. Cancer Biother Radiopharm. 2009;24:435–443. doi: 10.1089/cbr.2008.0616. [DOI] [PubMed] [Google Scholar]
- 81.Smith CJ, Volkert WA, Hoffman TJ. Radiolabeled peptide conjugates for targeting of the bombesin receptor superfamily subtypes. Nucl Med Biol. 2005;32:733–740. doi: 10.1016/j.nucmedbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Ananias HJ, de Jong I, Dierck RA, Van de Wiele C, Helfrich W, Elsinga PH. Nuclear imaging of prostate cancer with gastrin-releasing-peptide-receptor targeted radiopharmaceuticals. Curr Pharm Des. 2008;14:3033–3047. doi: 10.2174/138161208786404335. [DOI] [PubMed] [Google Scholar]
- 83.Rogers BE, Bigott HM, McCarthy DW, Della Manna D, Kim J, Sharp TL, Welch MJ. MicroPET imaging of a gastrin-releasing peptide receptor-positive tumor in a mouse model of human prostate cancer using a 64Cu-labeled bombesin analogue. Bioconjug Chem. 2003;14:756–763. doi: 10.1021/bc034018l. [DOI] [PubMed] [Google Scholar]
- 84.Rogers BE, Manna DD, Safavy A. In vitro and in vivo evaluation of a 64Cu-labeled polyethylene glycol-bombesin conjugate. Cancer Biother Radiopharm. 2004;19:25–34. doi: 10.1089/108497804773391649. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, Park R, Hou Y, Tohme M, Shahinian AH, Bading JR, Conti PS. microPET and autoradiographic imaging of GRP receptor expression with 64Cu-DOTA-[Lys3]bombesin in human prostate adenocarcinoma xenografts. J Nucl Med. 2004;45:1390–1397. [PubMed] [Google Scholar]
- 86.Yang YS, Zhang X, Xiong Z, Chen X. Comparative in vitro and in vivo evaluation of two 64Cu-labeled bombesin analogs in a mouse model of human prostate adenocarcinoma. Nucl Med Biol. 2006;33:371–380. doi: 10.1016/j.nucmedbio.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 87.Biddlecombe GB, Rogers BE, de Visser M, Parry JJ, de Jong M, Erion JL, Lewis JS. Molecular imaging of gastrin-releasing peptide receptor-positive tumors in mice using 64Cu- and 86Y-DOTA-(Pro1, Tyr4)-bombesin(1–14) Bioconjug Chem. 2007;18:724–730. doi: 10.1021/bc060281l. [DOI] [PubMed] [Google Scholar]
- 88.Parry JJ, Andrews R, Rogers BE. MicroPET Imaging of Breast Cancer Using Radiolabeled Bombesin Analogs Targeting the Gastrin-releasing Peptide Receptor. Breast Cancer Res Treat. 2007;18:110–117. doi: 10.1007/s10549-006-9287-8. [DOI] [PubMed] [Google Scholar]
- 89.Parry JJ, Kelly TS, Andrews R, Rogers BE. In vitro and in vivo evaluation of 64Cu-labeled DOTA-linker-bombesin(7–14) analogues containing different amino acid linker moieties. Bioconjug Chem. 2007;18:1110–1117. doi: 10.1021/bc0603788. [DOI] [PubMed] [Google Scholar]
- 90.Garrison JC, Rold TL, Sieckman GL, Figueroa SD, Volkert WA, Jurisson SS, Hoffman TJ. In vivo evaluation and small-animal PET/CT of a prostate cancer mouse model using 64Cu bombesin analogs: side-by-side comparison of the CB-TE2A and DOTA chelation systems. J Nucl Med. 2007;48:1327–1337. doi: 10.2967/jnumed.107.039487. [DOI] [PubMed] [Google Scholar]
- 91.Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ. [64Cu-NOTA-8-Aoc-BBN(7–14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci U S A. 2007;104:12462–12467. doi: 10.1073/pnas.0705347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prasanphanich AF, Retzloff L, Lane SR, Nanda PK, Sieckman GL, Rold TL, Ma L, Figueroa SD, Sublett SV, Hoffman TJ, Smith CJ. In vitro and in vivo analysis of [(64)Cu-NO2A-8-Aoc-BBN(7–14)NH(2)]: a site-directed radiopharmaceutical for positron-emission tomography imaging of T-47D human breast cancer tumors. Nucl Med Biol. 2009;36:171–181. doi: 10.1016/j.nucmedbio.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gasser G, Tjioe L, Graham B, Belousoff MJ, Juran S, Walther M, Kunstler JU, Bergmann R, Stephan H, Spiccia L. Synthesis, Copper(II) Complexation, (64)Cu-Labeling, and Bioconjugation of a New Bis(2-pyridylmethyl) Derivative of 1,4,7-Triazacyclononane. Bioconjug Chem. 2008;19:719–730. doi: 10.1021/bc700396e. [DOI] [PubMed] [Google Scholar]
- 94.Juran S, Walther M, Stephan H, Bergmann R, Steinbach J, Kraus W, Emmerling F, Comba P. Hexadentate bispidine derivatives as versatile bifunctional chelate agents for copper(II) radioisotopes. Bioconjug Chem. 2009;20:347–359. doi: 10.1021/bc800461e. [DOI] [PubMed] [Google Scholar]
- 95.Liu Z, Li ZB, Cao Q, Liu S, Wang F, Chen X. Small-animal PET of tumors with (64)Cu-labeled RGD-bombesin heterodimer. J Nucl Med. 2009;50:1168–1177. doi: 10.2967/jnumed.108.061739. [DOI] [PubMed] [Google Scholar]
- 96.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 97.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 98.Ma MT, Karas JA, White JM, Scanlon D, Donnelly PS. A new bifunctional chelator for copper radiopharmaceuticals: a cage amine ligand with a carboxylate functional group for conjugation to peptides. Chem Commun (Camb) 2009:3237–3239. doi: 10.1039/b903426a. [DOI] [PubMed] [Google Scholar]
- 99.Zhang X, Cai W, Cao F, Schreibmann E, Wu Y, Wu JC, Xing L, Chen X. 18F-Labeled Bombesin Analogs for Targeting GRP Receptor-Expressing Prostate Cancer. J Nucl Med. 2006;47:492–501. [PubMed] [Google Scholar]
- 100.Li ZB, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49:453–461. doi: 10.2967/jnumed.107.048009. [DOI] [PubMed] [Google Scholar]
- 101.Liu Z, Yan Y, Chin FT, Wang F, Chen X. Dual integrin and gastrin-releasing peptide receptor targeted tumor imaging using 18F-labeled PEGylated RGD-bombesin heterodimer 18F-FB-PEG3-Glu-RGD-BBN. J Med Chem. 2009;52:425–432. doi: 10.1021/jm801285t. [DOI] [PubMed] [Google Scholar]
- 102.Becaud J, Mu L, Karramkam M, Schubiger PA, Ametamey SM, Graham K, Stellfeld T, Lehmann L, Borkowski S, Berndorff D, Dinkelborg L, Srinivasan A, Smits R, Koksch B. Direct one-step 18F-labeling of peptides via nucleophilic aromatic substitution. Bioconjug Chem. 2009;20:2254–2261. doi: 10.1021/bc900240z. [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Schuhmacher J, Waser B, Wild D, Eisenhut M, Reubi JC, Maecke HR. DOTA-PESIN, a DOTA-conjugated bombesin derivative designed for the imaging and targeted radionuclide treatment of bombesin receptor-positive tumours. Eur J Nucl Med Mol Imaging. 2007;34:1198–1208. doi: 10.1007/s00259-006-0347-4. [DOI] [PubMed] [Google Scholar]
- 104.Schuhmacher J, Zhang H, Doll J, Macke HR, Matys R, Hauser H, Henze M, Haberkorn U, Eisenhut M. GRP receptor-targeted PET of a rat pancreas carcinoma xenograft in nude mice with a 68Ga-labeled bombesin(6–14) analog. J Nucl Med. 2005;46:691–699. [PubMed] [Google Scholar]
- 105.Liu Z, Niu G, Wang F, Chen X. (68)Ga-labeled NOTA-RGD-BBN peptide for dual integrin and GRPR-targeted tumor imaging. Eur J Nucl Med Mol Imaging. 2009;36:1483–1494. doi: 10.1007/s00259-009-1123-z. [DOI] [PubMed] [Google Scholar]
- 106.Abiraj K, Jaccard H, Kretzschmar M, Helm L, Maecke HR. Novel DOTA-based prochelator for divalent peptide vectorization: synthesis of dimeric bombesin analogues for multimodality tumor imaging and therapy. Chem Commun (Camb) 2008:3248–3250. doi: 10.1039/b805281f. [DOI] [PubMed] [Google Scholar]
- 107.Koumarianou E, Mikolajczak R, Pawlak D, Zikos X, Bouziotis P, Garnuszek P, Karczmarczyk U, Maurin M, Archimandritis SC. Comparative study on DOTA-derivatized bombesin analog labeled with 90Y and 177Lu: in vitro and in vivo evaluation. Nucl Med Biol. 2009;36:591–603. doi: 10.1016/j.nucmedbio.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 108.Smith CJ, Gali H, Sieckman GL, Hayes DL, Owen NK, Mazuru DG, Volkert WA, Hoffman TJ. Radiochemical investigations of 177Lu-DOTA-8-Aoc-BBN[7–14]NH2: an in vitro/in vivo assessment of the targeting ability of this new radiopharmaceutical for PC-3 human prostate cancer cells. Nucl Med Biol. 2003;30:101–109. doi: 10.1016/s0969-8051(02)00391-8. [DOI] [PubMed] [Google Scholar]
- 109.Johnson CV, Shelton T, Smith CJ, Ma L, Perry MC, Volkert WA, Hoffman TJ. Evaluation of combined (177)Lu-DOTA-8-AOC-BBN (7–14)NH(2) GRP receptor-targeted radiotherapy and chemotherapy in PC-3 human prostate tumor cell xenografted SCID mice. Cancer Biother Radiopharm. 2006;21:155–166. doi: 10.1089/cbr.2006.21.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lantry LE, Cappelletti E, Maddalena ME, Fox JS, Feng W, Chen J, Thomas R, Eaton SM, Bogdan NJ, Arunachalam T, Reubi JC, Raju N, Metcalfe EC, Lattuada L, Linder KE, Swenson RE, Tweedle MF, Nunn AD. 177Lu-AMBA: Synthesis and characterization of a selective 177Lu-labeled GRP-R agonist for systemic radiotherapy of prostate cancer. J Nucl Med. 2006;47:1144–1152. [PubMed] [Google Scholar]
- 111.Linder KE, Metcalfe E, Arunachalam T, Chen J, Eaton SM, Feng W, Fan H, Raju N, Cagnolini A, Lantry LE, Nunn AD, Swenson RE. In vitro and in vivo metabolism of Lu-AMBA, a GRP-receptor binding compound, and the synthesis and characterization of its metabolites. Bioconjug Chem. 2009;20:1171–1178. doi: 10.1021/bc9000189. [DOI] [PubMed] [Google Scholar]
- 112.Maddalena ME, Fox J, Chen J, Feng W, Cagnolini A, Linder KE, Tweedle MF, Nunn AD, Lantry LE. 177Lu-AMBA Biodistribution, Radiotherapeutic Efficacy, Imaging, and Autoradiography in Prostate Cancer Models with Low GRP-R Expression. J Nucl Med. 2009 doi: 10.2967/jnumed.109.064444. [DOI] [PubMed] [Google Scholar]
- 113.Hu F, Cutler CS, Hoffman T, Sieckman G, Volkert WA, Jurisson SS. Pm-149 DOTA bombesin analogs for potential radiotherapy. in vivo comparison with Sm-153 and Lu-177 labeled DO3A-amide-betaAla-BBN(7–14)NH(2) Nucl Med Biol. 2002;29:423–430. doi: 10.1016/s0969-8051(02)00290-1. [DOI] [PubMed] [Google Scholar]
- 114.Varvarigou A, Bouziotis P, Zikos C, Scopinaro F, De Vincentis G. Gastrin-releasing peptide (GRP) analogues for cancer imaging. Cancer Biother Radiopharm. 2004;19:219–229. doi: 10.1089/108497804323072002. [DOI] [PubMed] [Google Scholar]
- 115.Pradhan TK, Katsuno T, Taylor JE, Kim SH, Ryan RR, Mantey SA, Donohue PJ, Weber HC, Sainz E, Battey JF, Coy DH, Jensen RT. Identification of a unique ligand which has high affinity for all four bombesin receptor subtypes. Eur J Pharmacol. 1998;343:275–287. doi: 10.1016/s0014-2999(97)01527-6. [DOI] [PubMed] [Google Scholar]
- 116.Waser B, Eltschinger V, Linder K, Nunn A, Reubi JC. Selective in vitro targeting of GRP and NMB receptors in human tumours with the new bombesin tracer (177)Lu-AMBA. Eur J Nucl Med Mol Imaging. 2007;34:95–100. doi: 10.1007/s00259-006-0229-9. [DOI] [PubMed] [Google Scholar]
- 117.Thomas R, Chen J, Roudier MM, Vessella RL, Lantry LE, Nunn AD. In vitro binding evaluation of 177Lu-AMBA, a novel 177Lu-labeled GRP-R agonist for systemic radiotherapy in human tissues. Clin Exp Metastasis. 2009;26:105–119. doi: 10.1007/s10585-008-9220-0. [DOI] [PubMed] [Google Scholar]
- 118.Rogers BE, Rosenfeld ME, Khazaeli MB, Mikheeva G, Stackhouse MA, Liu T, Curiel DT, Buchsbaum DJ. Localization of iodine-125-mIP-Des-Met14-bombesin (7–13)NH2 in ovarian carcinoma induced to express the gastrin releasing peptide receptor by adenoviral vector-mediated gene transfer. J Nucl Med. 1997;38:1221–1229. [PubMed] [Google Scholar]
- 119.Rogers BE, Curiel DT, Mayo MS, Laffoon KK, Bright SJ, Buchsbaum DJ. Tumor localization of a radiolabeled bombesin analogue in mice bearing human ovarian tumors induced to express the gastrin-releasing peptide receptor by an adenoviral vector. Cancer. 1997;80:2419–2424. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2419::aid-cncr13>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 120.Safavy A, Khazaeli MB, Qin H, Buchsbaum DJ. Synthesis of bombesin analogues for radiolabeling with rhenium-188. Cancer. 1997;80:2354–2359. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2354::aid-cncr4>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 121.Moustapha ME, Ehrhardt GJ, Smith CJ, Szajek LP, Eckelman WC, Jurisson SS. Preparation of cyclotron-produced 186Re and comparison with reactor-produced 186Re and generator-produced 188Re for the labeling of bombesin. Nucl Med Biol. 2006;33:81–89. doi: 10.1016/j.nucmedbio.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 122.Scopinaro F, Varvarigou AD, Ussof W, De Vincentis G, Sourlingas TG, Evangelatos GP, Datsteris J, Archimandritis SC. Technetium labeled bombesin-like peptide: preliminary report on breast cancer uptake in patients. Cancer Biother Radiopharm. 2002;17:327–335. doi: 10.1089/10849780260179297. [DOI] [PubMed] [Google Scholar]
- 123.De Vincentis G, Scopinaro F, Varvarigou A, Ussof W, Schillaci O, Archimandritis S, Corleto V, Longo F, Delle Fave G. Phase I trial of technetium [Leu13] bombesin as cancer seeking agent: possible scintigraphic guide for surgery? Tumori. 2002;88:S28–S30. doi: 10.1177/030089160208800332. [DOI] [PubMed] [Google Scholar]
- 124.Soluri A, Scopinaro F, De Vincentis G, Varvarigou A, Scafe R, Massa R, Schillaci O, Spanu A, David V. 99MTC [13LEU] bombesin and a new gamma camera, the imaging probe, are able to guide mammotome breast biopsy. Anticancer Res. 2003;23:2139–2142. [PubMed] [Google Scholar]
- 125.De Vincentis G, Remediani S, Varvarigou AD, Di Santo G, Iori F, Laurenti C, Scopinaro F. Role of 99mTc-bombesin scan in diagnosis and staging of prostate cancer. Cancer Biother Radiopharm. 2004;19:81–84. doi: 10.1089/108497804773391711. [DOI] [PubMed] [Google Scholar]
- 126.Scopinaro F, De Vincentis G, Varvarigou AD, Laurenti C, Iori F, Remediani S, Chiarini S, Stella S. 99mTc-bombesin detects prostate cancer and invasion of pelvic lymph nodes. Eur J Nucl Med Mol Imaging. 2003;30:1378–1382. doi: 10.1007/s00259-003-1261-7. [DOI] [PubMed] [Google Scholar]
- 127.Scopinaro F, De Vincentis G, Corazziari E, Massa R, Osti M, Pallotta N, Covotta A, Remediani S, Paolo MD, Monteleone F, Varvarigou A. Detection of colon cancer with 99mTc-labeled bombesin derivative (99mTc-leu13-BN1) Cancer Biother Radiopharm. 2004;19:245–252. doi: 10.1089/108497804323072020. [DOI] [PubMed] [Google Scholar]
- 128.van de WC, Dumont F, Dierck RA, Peers SH, Thornback JR, Slegers G, Thierens H. Biodistribution and dosimetry of (99m)Tc-RP527, a gastrin-releasing peptide (GRP) agonist for the visualization of GRP receptor-expressing malignancies. J Nucl Med. 2001;42:1722–1727. [PubMed] [Google Scholar]
- 129.Santos-Cuevas CL, Ferro-Flores G, Arteaga de Murphy C, Pichardo-Romero PA. Targeted imaging of gastrin-releasing peptide receptors with 99mTc-EDDA/HYNIC-[Lys3]-bombesin: biokinetics and dosimetry in women. Nucl Med Commun. 2008;29:741–747. doi: 10.1097/MNM.0b013e3282ffb45c. [DOI] [PubMed] [Google Scholar]
- 130.Dimitrakopoulou-Strauss A, Hohenberger P, Haberkorn U, Macke HR, Eisenhut M, Strauss LG. 68Ga-labeled bombesin studies in patients with gastrointestinal stromal tumors: comparison with 18F-FDG. J Nucl Med. 2007;48:1245–1250. doi: 10.2967/jnumed.106.038091. [DOI] [PubMed] [Google Scholar]
- 131.Seiz M, Mitrakopoulou-Strauss A, Schubert GA, Weinmann C, Strauss LG, Eisenhut M, Tuettenberg J. Differentiation between malignant transformation and tumour recurrence by (68)Ga-bombesin and (18)F-FDG-PET, in patients with low grade gliomas. Hell J Nucl Med. 2008;11:149–152. [PubMed] [Google Scholar]
- 132.Sekido Y, Fong KM, Minna JD. Cancer of the lung. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer: Princiles and Practice of Oncology. 7. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 745–752. [Google Scholar]
- 133.Carney DN, Moody T, Cuttitta F. Bombesin: a potent mitogen for small cell lung cancer. Ann N Y Acad Sci. 1988;547:303–309. doi: 10.1111/j.1749-6632.1988.tb23898.x. [DOI] [PubMed] [Google Scholar]
- 134.Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer cells. Nature. 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 135.Pradhan TK, Katsuno T, Weber HC, Mantey SA, Coy DH, Jensen RT. Discovery of a universal ligand that interacts with high affinity with all four classes of bombesin (BN) receptors. Gastroenterology. 1997;112:A474. Ref Type: Abstract. [Google Scholar]
- 136.Sun L, Fuselier JA, Coy DH. Effects of camptothecin conjugated to a somatostatin analog vector on growth of tumor cell lines in culture and related tumors in rodents. Drug Deliv. 2004;11:231–238. doi: 10.1080/10717540490446125. [DOI] [PubMed] [Google Scholar]
- 137.Patterson LH, McKeown SR, Robson T, Gallagher R, Raleigh SM, Orr S. Antitumour prodrug development using cytochrome P450 (CYP) mediated activation. Anticancer Drug Des. 1999;14:473–486. [PubMed] [Google Scholar]
- 138.Fuselier JA, Sun L, Woltering SN, Murphy WA, Vasilevich N, Coy DH. An adjustable release rate linking strategy for cytotoxin-Peptide conjugates. Bioorg Med Chem Lett. 2003;13:799–803. doi: 10.1016/s0960-894x(03)00016-7. [DOI] [PubMed] [Google Scholar]
- 139.Akeson M, Sainz E, Mantey SA, Jensen RT, Battey JF. Identification of four amino acids in the gastrin-releasing peptide C receptor that are required for high affinity agonist binding. J Biol Chem. 1997;272:17405–17409. doi: 10.1074/jbc.272.28.17405. [DOI] [PubMed] [Google Scholar]
- 140.Nakagawa T, Hocart SJ, Schumann M, Tapia JA, Mantey SA, Coy DH, Tokita K, Katsuno T, Jensen RT. Identification of key amino acids in the gastrin-releasing peptide receptor (GRPR) responsible for high affinity binding of gastrin-releasing peptide (GRP) Biochem Pharmacol. 2005;69:579–593. doi: 10.1016/j.bcp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 141.Nagy A, Armatis P, Cai RZ, Szepeshazi K, Halmos G, Schally AV. Design, synthesis, and in vitro evaluation of cytotoxic analogs of bombesin-like peptides containing doxorubicin or its intensely potent derivative, 2-pyrrolinodoxorubicin. Proc Natl Acad Sci U S A. 1997;94:652–656. doi: 10.1073/pnas.94.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schally AV, Nagy A. Chemotherapy targeted to cancers through tumoral hormone receptors. Trends Endocrinol Metab. 2004;15:300–310. doi: 10.1016/j.tem.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 143.Plonowski A, Nagy A, Schally AV, Sun B, Groot K, Halmos G. In vivo inhibition of PC-3 human androgen-independent prostate cancer by a targeted cytotoxic bombesin analogue, AN-215. Int J Cancer. 2000;88:652–657. doi: 10.1002/1097-0215(20001115)88:4<652::aid-ijc21>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 144.Safavy A, Raisch KP, Khazaeli MB, Buchsbaum DJ, Bonner JA. Paclitaxel derivatives for targeted therapy of cancer: toward the development of smart taxanes. J Med Chem. 1999;42:4919–4924. doi: 10.1021/jm990355x. [DOI] [PubMed] [Google Scholar]
- 145.Safavy A, Raisch KP, Matusiak D, Bhatnagar S, Helson L. Single-drug multiligand conjugates: synthesis and preliminary cytotoxicity evaluation of a paclitaxel-dipeptide “scorpion” molecule. Bioconjug Chem. 2006;17:565–570. doi: 10.1021/bc050224c. [DOI] [PubMed] [Google Scholar]
- 146.Hohla F, Schally AV, Kanashiro CA, Buchholz S, Baker B, Kannadka C, Moder A, Aigner E, Datz C, Halmos G. Growth inhibition of non-small-cell lung carcinoma by BN/GRP antagonist is linked with suppression of K-Ras, COX-2, and pAkt. Proc Natl Acad Sci U S A. 2007;104:18671–18676. doi: 10.1073/pnas.0709455104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stangelberger A, Schally AV, Varga JL, Hammann BD, Groot K, Halmos G, Cai RZ, Zarandi M. Antagonists of growth hormone releasing hormone (GHRH) and of bombesin/gastrin releasing peptide (BN/GRP) suppress the expression of VEGF, bFGF, and receptors of the EGF/HER family in PC-3 and DU-145 human androgen-independent prostate cancers. Prostate. 2005;64:303–315. doi: 10.1002/pros.20262. [DOI] [PubMed] [Google Scholar]
- 148.Lui VW, Thomas SM, Wentzel AM, Siegfried JM, Li JY, Grandis JR. Mitogenic effects of gastrin-releasing peptide in head and neck squamous cancer cells are mediated by activation of the epidermal growth factor receptor. Oncogene. 2003;22:6183–6193. doi: 10.1038/sj.onc.1206720. [DOI] [PubMed] [Google Scholar]
- 149.Moody TW, Berna MJ, Mantey S, Sancho V, Ridnour L, Wink DA, Chan D, Giaccone G, Jensen RT. Neuromedin B receptors regulate EGF receptor tyrosine phosphorylation in lung cancer cells. Eur J Pharmacol. 2010 doi: 10.1016/j.ejphar.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.vanderSpek JC, Sutherland JA, Zeng H, Battey JF, Jensen RT, Murphy JR. Inhibition of protein synthesis in small cell lung cancer cells induced by the diphtheria toxin-related fusion protein DAB389 GRP. Cancer Res. 1997;57:290–294. [PubMed] [Google Scholar]
- 151.Cai H, Yang H, Xiang B, Li S, Liu S, Wan L, Zhang J, Li Y, Cheng J, Lu X. Selective Apoptotic Killing of Solid and Hematologic Tumor Cells by Bombesin-Targeted Delivery of Mitochondria-Disrupting Peptides. Mol Pharm. 2010 doi: 10.1021/mp900280s. [DOI] [PubMed] [Google Scholar]
- 152.Zhou J, Chen J, Zhong R, Mokotoff M, Shultz LD, Ball ED. Targeting gastrin-releasing peptide receptors on small cell lung cancer cells with a bispecific molecule that activates polyclonal T lymphocytes. Clin Cancer Res. 2006;12:2224–2231. doi: 10.1158/1078-0432.CCR-05-1524. [DOI] [PubMed] [Google Scholar]
- 153.Chen J, Zhou JH, Mokotoff M, Fanger MW, Ball ED. Lysis of small cell carcinoma of the lung (SCCL) cells by cytokine-activated monocytes and natural killer cells in the presence of bispecific immunoconjugates containing a gastrin-releasing peptide (GRP) analog or a GRP antagonist. J Hematother. 1995;4:369–376. doi: 10.1089/scd.1.1995.4.369. [DOI] [PubMed] [Google Scholar]
- 154.Chen J, Mokotoff M, Zhou JH, Fanger MW, Ball ED. An immunoconjugate of Lys3-bombesin and monoclonal antibody 22 can specifically induce FcgammaRI (CD64)-dependent monocyte- and neutrophil-mediated lysis of small cell carcinoma of the lung cells. Clin Cancer Res. 1995;1:425–434. [PubMed] [Google Scholar]
- 155.Dubuc C, Langlois R, Benard F, Cauchon N, Klarskov K, Tone P, van Lier JE. Targeting gastrin-releasing peptide receptors of prostate cancer cells for photodynamic therapy with a phthalocyanine-bombesin conjugate. Bioorg Med Chem Lett. 2008;18:2424–2427. doi: 10.1016/j.bmcl.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 156.Prasad S, Mathur A, Jaggi M, Mukherjee R. Delivering multiple anticancer peptides as a single prodrug using lysyl-lysine as a facile linker. J Pept Sci. 2007;13:458–467. doi: 10.1002/psc.867. [DOI] [PubMed] [Google Scholar]
- 157.Singh AT, Jaggi M, Prasad S, Dutt S, Singh G, Datta K, Rajendran P, Sanna VK, Mukherjee R, Burman AC. Modulation of key signal transduction molecules by a novel peptide combination effective for the treatment of gastrointestinal carcinomas. Invest New Drugs. 2008;26:505–516. doi: 10.1007/s10637-008-9119-2. [DOI] [PubMed] [Google Scholar]
- 158.Hong SS, Galaup A, Peytavi R, Chazal N, Boulanger P. Enhancement of adenovirus-mediated gene delivery by use of an oligopeptide with dual binding specificity. Hum Gene Ther. 1999;10:2577–2586. doi: 10.1089/10430349950016627. [DOI] [PubMed] [Google Scholar]
- 159.Coy DH, Jiang NY, Sasaki Y, Taylor J, Moreau JP, Wolfrey WT, Gardner JD, Jensen RT. Probing peptide backbone function in bombesin. A reduced peptide bond analogue with potent and specific receptor antagonist activity. J Biol Chem. 1988;263(11):5056–5060. [PubMed] [Google Scholar]
- 160.Moody TW, Venugopal R, Hu V, Gozes Y, McDermed J, Leban JJ. BW 1023U90: a new GRP receptor antagonist for small-cell lung cancer cells. Peptides. 1996;17:1337–1343. doi: 10.1016/s0196-9781(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 161.Tokita K, Hocart SJ, Katsuno T, Mantey SA, Coy DH, Jensen RT. Tyrosine 220 in the fifth transmembrane domain of the neuromedin B receptor is critical for the high selectivity of the peptoid antagonist PD168368. J Biol Chem. 2001;276:495–504. doi: 10.1074/jbc.M006059200. [DOI] [PubMed] [Google Scholar]
- 162.Moody TW, Jensen RT, Garcia L, Leyton J. Nonpeptide neuromedin B receptor antagonists inhibit the proliferation of C6 cells. Eur J Pharmacol. 2000;409:133–142. doi: 10.1016/s0014-2999(00)00828-1. [DOI] [PubMed] [Google Scholar]
- 163.Prasad S, Mathur A, Gupta N, Jaggi M, Singh AT, Rajendran P, Sanna VK, Datta K, Mukherjee R. Bombesin analogs containing alpha-amino-isobutyric acid with potent anticancer activity. J Pept Sci. 2006 doi: 10.1002/psc.799. [DOI] [PubMed] [Google Scholar]
- 164.Okarvi SM, al-Jammaz I. Synthesis, radiolabelling and biological characteristics of a bombesin peptide analog as a tumor imaging agent. Anticancer Res. 2003;23:2745–2750. [PubMed] [Google Scholar]
- 165.Pu Y, Wang WB, Tang GC, Zeng F, Achilefu S, Vitenson JH, Sawczuk I, Peters S, Lombardo JM, Alfano RR. Spectral polarization imaging of human prostate cancer tissue using a near-infrared receptor-targeted contrast agent. Technol Cancer Res Treat. 2005;4:429–436. doi: 10.1177/153303460500400410. [DOI] [PubMed] [Google Scholar]
- 166.Varvarigou AD, Scopinaro F, Leondiadis L, Corleto V, Schillaci O, De Vincentis G, Sourlingas TG, Sekeri-Pataryas KE, Evangelatos GP, Leonti A, Xanthopoulos S, Delle Fave G, Archimandritis SC. Synthesis, chemical, radiochemical and radiobiological evaluation of a new 99mTc-labelled bombesin-like peptide. Cancer Biother Radiopharm. 2002;17:317–326. doi: 10.1089/10849780260179288. [DOI] [PubMed] [Google Scholar]
- 167.Ferro-Flores G, Arteaga de Murphy C, Rodriguez-Cortes J, Pedraza-Lopez M, Ramirez-Iglesias MT. Preparation and evaluation of 99mTc-EDDA/HYNIC-[Lys 3]-bombesin for imaging gastrin-releasing peptide receptor-positive tumours. Nucl Med Commun. 2006;27:371–376. doi: 10.1097/01.mnm.0000202863.52046.7f. [DOI] [PubMed] [Google Scholar]
- 168.Achilefu S, Jimenez HN, Dorshow RB, Bugaj JE, Webb EG, Wilhelm RR, Rajagopalan R, Johler J, Erion JL. Synthesis, in vitro receptor binding, and in vivo evaluation of fluorescein and carbocyanine peptide-based optical contrast agents. J Med Chem. 2002;45:2003–2015. doi: 10.1021/jm010519l. [DOI] [PubMed] [Google Scholar]
- 169.Montet X, Weissleder R, Josephson L. Imaging pancreatic cancer with a peptide-nanoparticle conjugate targeted to normal pancreas. Bioconjug Chem. 2006;17:905–911. doi: 10.1021/bc060035+. [DOI] [PubMed] [Google Scholar]
- 170.Scopinaro F, Varvarigou A, Ussof W, De Vincentis G, Archimandritis S, Evangelatos G, Corleto V, Pulcini A, Capoccetti F, Remediani S, Massa R. Breast cancer takes up 99mTc bombesin. A preliminary report. Tumori. 2002;88:S25–S28. doi: 10.1177/030089160208800331. [DOI] [PubMed] [Google Scholar]
- 171.Fragogeorgi EA, Zikos C, Gourni E, Bouziotis P, Paravatou-Petsotas M, Loudos G, Mitsokapas N, Xanthopoulos S, Mavri-Vavayanni M, Livaniou E, Varvarigou AD, Archimandritis SC. Spacer Site Modifications for the Improvement of the in vitro and in vivo Binding Properties of (99m)Tc-N(3)S-X-Bombesin[2–14] Derivatives. Bioconjug Chem. 2009 doi: 10.1021/bc800475k. [DOI] [PubMed] [Google Scholar]
- 172.Scopinaro F, Massari R, Varvarigou AD, D’Alessandria C, Trotta C, Di Santo GP, Soluri A. High resolution small animal single photon emission computed tomography: uptake of [99mTc]bombesin and [123I]ioflupane by rat brain. Q J Nucl Med Mol Imaging. 2007;51:204–210. [PubMed] [Google Scholar]
- 173.Gotthardt M, van Eerd-Vismale J, Oyen WJ, de Jong M, Zhang H, Rolleman E, Maecke HR, Behe M, Boerman O. Indication for different mechanisms of kidney uptake of radiolabeled peptides. J Nucl Med. 2007;48:596–601. doi: 10.2967/jnumed.106.036020. [DOI] [PubMed] [Google Scholar]
- 174.Nock B, Nikolopoulou A, Chiotellis E, Loudos G, Maintas D, Reubi JC, Maina T. [99mTc]Demobesin 1, a novel potent bombesin analogue for GRP receptor-targeted tumour imaging. Eur J Nucl Med Mol Imaging. 2003;30:247–258. doi: 10.1007/s00259-002-1040-x. [DOI] [PubMed] [Google Scholar]
- 175.Ma L, Yu P, Veerendra B, Rold TL, Retzloff L, Prasanphanich A, Sieckman G, Hoffman TJ, Volkert WA, Smith CJ. In vitro and in vivo evaluation of Alexa Fluor 680-bombesin[7–14]NH2 peptide conjugate, a high-affinity fluorescent probe with high selectivity for the gastrin-releasing peptide receptor. Mol Imaging. 2007;6:171–180. [PubMed] [Google Scholar]
- 176.Garcia Garayoa E, Schweinsberg C, Maes V, Ruegg D, Blanc A, Blauenstein P, Tourwe DA, Beck-Sickinger AG, Schubiger PA. New [99mTc]bombesin analogues with improved biodistribution for targeting gastrin releasing-peptide receptor-positive tumors. Q J Nucl Med Mol Imaging. 2007;51:42–50. [PubMed] [Google Scholar]
- 177.Paterson BM, Karas JA, Scanlon DB, White JM, Donnelly PS. Versatile new bis(thiosemicarbazone) bifunctional chelators: synthesis, conjugation to bombesin(7–14)-NH(2), and copper-64 radiolabeling. Inorg Chem. 2010;49:1884–1893. doi: 10.1021/ic902204e. [DOI] [PubMed] [Google Scholar]
- 178.Alves S, Paulo A, Correia JD, Gano L, Smith CJ, Hoffman TJ, Santos I. Pyrazolyl derivatives as bifunctional chelators for labeling tumor-seeking peptides with the fac-[M(CO)3]+ moiety (M = 99mTc, Re): synthesis, characterization, and biological behavior. Bioconjug Chem. 2005;16:438–449. doi: 10.1021/bc0497968. [DOI] [PubMed] [Google Scholar]
- 179.Gali H, Hoffman TJ, Sieckman GL, Owen NK, Katti KV, Volkert WA. Synthesis, characterization, and labeling with 99mTc/188Re of peptide conjugates containing a dithia-bisphosphine chelating agent. Bioconjug Chem. 2001;12:354–363. doi: 10.1021/bc000077c. [DOI] [PubMed] [Google Scholar]
- 180.La Bella R, Garcia-Garayoa E, Bahler M, Blauenstein P, Schibli R, Conrath P, Tourwe D, Schubiger PA. A 99mTc(I)-postlabeled high affinity bombesin analogue as a potential tumor imaging agent. Bioconjug Chem. 2002;13:599–604. doi: 10.1021/bc015571a. [DOI] [PubMed] [Google Scholar]
- 181.La Bella R, Garcia-Garayoa E, Langer M, Blauenstein P, Beck-Sickinger AG, Schubiger PA. In vitro and in vivo evaluation of a 99mTc(I)-labeled bombesin analogue for imaging of gastrin releasing peptide receptor-positive tumors. Nucl Med Biol. 2002;29:553–560. doi: 10.1016/s0969-8051(02)00314-1. [DOI] [PubMed] [Google Scholar]
- 182.Smith CJ, Gali H, Sieckman GL, Higginbotham C, Volkert WA, Hoffman TJ. Radiochemical investigations of (99m)Tc-N(3)S-X-BBN[7–14]NH(2): an in vitro/in vivo structure-activity relationship study where X = 0-, 3-, 5-, 8-, and 11-carbon tethering moieties. Bioconjug Chem. 2003;14:93–102. doi: 10.1021/bc020034r. [DOI] [PubMed] [Google Scholar]
- 183.Bugaj JE, Achilefu S, Dorshow RB, Rajagopalan R. Novel fluorescent contrast agents for optical imaging of in vivo tumors based on a receptor-targeted dye-peptide conjugate platform. J Biomed Opt. 2001;6:122–133. doi: 10.1117/1.1352748. [DOI] [PubMed] [Google Scholar]
- 184.Brans L, Maes V, Garcia-Garayoa E, Schweinsberg C, Daepp S, Blauenstein P, Schubiger PA, Schibli R, Tourwe DA. Glycation methods for bombesin analogs containing the (NalphaHis)Ac chelator for 99mTc(CO)3 radiolabeling. Chem Biol Drug Des. 2008;72:496–506. doi: 10.1111/j.1747-0285.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 185.Schroeder RP, van Weerden WM, Bangma C, Krenning EP, de Jong M. Peptide receptor imaging of prostate cancer with radiolabelled bombesin analogues. Methods. 2009;48:200–204. doi: 10.1016/j.ymeth.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 186.Melis M, Krenning EP, Bernard BF, de Visser M, Rolleman E, de Jong M. Renal uptake and retention of radiolabeled somatostatin, bombesin, neurotensin, minigastrin and CCK analogues: species and gender differences. Nucl Med Biol. 2007;34:633–641. doi: 10.1016/j.nucmedbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 187.Van de Wiele C, Phonteyne P, Pauwels P, Goethals I, Van den Broecke R, Cocquyt V, Dierck RA. Gastrin-releasing peptide receptor imaging in human breast carcinoma versus immunohistochemistry. J Nucl Med. 2008;49:260–264. doi: 10.2967/jnumed.107.047167. [DOI] [PubMed] [Google Scholar]
- 188.Wang XL, Xu R, Lu ZR. A peptide-targeted delivery system with pH-sensitive amphiphilic cell membrane disruption for efficient receptor-mediated siRNA delivery. J Control Release. 2009;134:207–213. doi: 10.1016/j.jconrel.2008.11.010. [DOI] [PubMed] [Google Scholar]