Abstract
Suckling “F/A2” mice, which overexpress arginase-I in their enterocytes, develop a syndrome (hypoargininemia, reduced hair and muscle growth, impaired B-cell maturation) that resembles IGF1 deficiency. The syndrome may result from an impaired function of the GH-IGF1 axis, activation of the stress-kinase GCN2, and/or blocking of the mTORC1-signaling pathway. Arginine deficiency inhibited GH secretion and decreased liver Igf1 mRNA and plasma IGF1 concentration, but did not change muscle IGF1 concentration. GH supplementation induced Igf1 mRNA synthesis, but did not restore growth, ruling out direct involvement of the GH-IGF1 axis. In C2C12 muscle cells, arginine withdrawal activated GCN2 signaling, without impacting mTORC1 signaling. In F/A2 mice, the reduction of plasma and tissue arginine concentrations to ∼25% of wild-type values activated GCN2 signaling, but mTORC1-mediated signaling remained unaffected. Gcn2-deficient F/A2 mice suffered from hypoglycemia and died shortly after birth. Because common targets of all stress kinases (eIF2α phosphorylation, Chop mRNA expression) were not increased in these mice, the effects of arginine deficiency were solely mediated by GCN2.
Keywords: Amino Acid, ER Stress, Insulin-like Growth Factor (IGF), mTOR Complex (mTORC), Translation Regulation, Arginine, General Control Non-derepressible-2 Kinase, Growth Retardation, Integrated Stress Response, Somatotropic Axis
Introduction
Arginine is a substrate for the synthesis of proteins, creatine, agmatine, ornithine, and nitric oxide (1). In addition, it functions as a secretagogue for hormones, such as growth hormone (GH) and insulin (2–4). Under normal conditions, endogenous arginine synthesis in adult mammals suffices to sustain daily requirements (5), but a dietary source of arginine may become necessary when demand increases under anabolic or catabolic conditions (5). For this reason, arginine is considered a conditionally essential amino acid.
Arginine deficiency represents a significant metabolic problem in premature infants (6), but its cause remains unknown. In rapidly growing suckling rodents, endogenous arginine biosynthesis is crucial to compensate for the insufficient supply of arginine via the milk (7). The intestine rather than the kidney is primarily responsible for endogenous arginine synthesis in suckling piglets (8, 9). In suckling rodents, the enterocytes of the small intestine also express all enzymes necessary to synthesize arginine (10–13), while arginase is not expressed, thus allowing efficient de novo arginine biosynthesis. To examine the function of intestinal arginine synthesis, a transgenic mouse model that overexpresses arginase-I in the enterocytes of the small intestine was produced (14–17). During the suckling period, these “F/A2”4 mice suffer from a selective deficiency of circulating arginine (which declines to ∼25% of controls), reduced growth of hair and skeletal muscle, and inhibition of B-cell maturation (14). The phenotype can be rescued with arginine supplementation (14), but could not be ascribed to a deficiency of arginine metabolites, such as creatine or polyamines, or to arginine-dependent ADP-ribosylation of proteins (14–17). Furthermore, the features of the “arginine-deficiency syndrome” are not seen in mice that are deficient in all three nitric-oxide synthases (18). This leaves two functions of arginine as likely candidates to account for the highly characteristic phenotype of arginine deficiency in suckling mice, viz. its role as a building block in protein synthesis and as a secretagogue of hormones.
Amino acids, including arginine, control translation by two well-established mechanisms. Whenever the intracellular concentration of free arginine decreases below ∼20 μm, the charging process of its corresponding tRNA slows down and the concentration of uncharged tRNA increases (19). Uncharged tRNAs activate the ubiquitously expressed “general control non-derepressible2” (GCN2) stress kinase, which then phosphorylates the α-subunit of the eukaryotic initiation factor 2 (eIF2α) and thereby initiates the “integrated stress response” (20–22). This response blocks cap-dependent protein synthesis, facilitates the translation of specific mRNAs, such as Atf4 and Cat-1, from internal ribosome entry sites, and increases expression of specific transcription factors such as ATF2, ATF4, and CHOP (23), which, in turn, induce the transcription of genes necessary for amino acid synthesis and transport. The other mechanism senses, instead, increases in amino acid concentration, probably via the Rag GTPase complex (24), and results in the activation of the mTORC1 kinase. mTORC1, in turn, regulates cap-dependent translation through the phosphorylation of 4EBP1 and S6K1 (25, 26). The essential amino acid leucine is the prototypic regulator of both branches of amino acid-dependent translational regulation (22, 27). However, for a non-essential amino acid, arginine has an unexpectedly strong effect on mTORC1-dependent signaling (28) and quantitatively rivals leucine in vitro (29, 30). The F/A2 phenotype could, therefore, be mediated by activation of GCN2 signaling and/or inhibition of mTORC1-mediated signaling.
The more remarkable phenotypic features of F/A2 mice, namely impaired muscle and hair growth and impaired B-cell maturation, are all also affected by insulin-like growth factor-1 (IGF1) signaling (31–35). Like amino acids, growth factor signals, including those arising from IGF1, stimulate protein synthesis via the mTORC1 pathway (36). The production of IGF1, in turn, is largely dependent on growth-hormone (GH) signaling (37). Arginine, finally, is also a potent growth-hormone secretagogue (38). Arginine deficiency could, therefore, also cause insufficient signaling via the mTORC1 pathway due to an inactive somatotropic axis.
In the present study, we used a combination of in vivo and in vitro approaches to test the hypotheses that arginine deficiency activates GCN2 signaling or blocks mTORC1 signaling, or both. Our findings demonstrate that arginine deficiency in the neonatal and suckling period activates the GCN2 stress-kinase pathway, without affecting the mTORC1 pathway.
EXPERIMENTAL PROCEDURES
Transgenic Mouse Lines and Animal Husbandry
Transgenic mice that overexpress arginase-I in their small-intestinal enterocytes (F/A2 line (14)) were bred hemizygously. Gcn2−/− mice (39) originated from the colony of D. Ron (New York, NY). Control animals were littermates of the experimental animals. Animal studies were reviewed and approved by the committee for animal care and use of Maastricht University.
GH Administration
Litters were limited to 5 animals on neonatal day 1 (ND1) and weighed daily. Starting on ND3, 3 mg/kg body weight of human recombinant growth hormone (hrGH; Humatrope, Lilly, Indianapolis, IN) was administered subcutaneously in 25 μl of vehicle twice daily (40). Control animals were injected with vehicle alone.
Blood and Tissue Collection
Animals were sacrificed by decapitation. Blood was collected into heparin-containing tubes and centrifuged at 2,000 × g for 5 min at 4 °C. IGF-1 protein concentrations were measured in plasma, whereas amino acids were measured in deproteinized plasma. 80 μl of plasma was added to 6.4 mg of lyophilized sulfosalicylic acid, vortexed, and stored at −20 °C. For amino acid measurements, the tissues were homogenized with silica mini-beads in 0.33 m sulfosalicylic acid. Tissues were collected at ND0, ND10, ND17, and ND21. Amino acid concentrations were determined by HPLC (41). Tissues for protein and mRNA analysis were isolated, snap frozen in liquid nitrogen and stored at −80 °C. For histological analysis, tissues were fixed in 4% buffered formalin and embedded in paraffin.
IGF-1 Assay
Mouse IGF-1 concentration in plasma and muscle was measured by the m/r IGF-1 E25 enzyme immunoassay, as detailed by the manufacturer (Mediagnost, Reutlingen, Germany). This assay eliminates IGFBP interference with the IGF-1 measurement.
RNA Extraction and Quantitative PCR
Total RNA was extracted with Trizol (Sigma). 1 μg of total RNA was reverse transcribed with the iScript cDNA synthesis kit (Bio-Rad). Primer sequences (supplemental Table S1) were optimized for an annealing temperature of 60 °C, except for the Igf1 primers that were annealed at 70 °C. cDNA samples for mRNA determination were diluted 60-fold before use and those for 18 S rRNA 200-fold. Primary fluorescent data were exported and analyzed with the Lin-Reg Analysis program (42). If reverse transcriptase was omitted, no product formed. mRNA concentrations were expressed relative to 18 S rRNA content. Between session variations in replicate experiments were corrected using factor correction (43).
Western Blots, Immunostaining, and Immunofluorescence
For Western blot assays, 100 μg of protein were loaded per lane. Antibody (supplemental Table S2) binding was visualized using the Super Signal West Femto Maximum Sensitivity Substrate (Pierce). For detection of the phosphorylated and unphosphorylated forms of eIF2α, the Odyssey Infrared Imaging system (Li-cor Bioscience, UK) was used.
For immunostaining, 5-μm sections of tissues were prepared. After deparaffinization, sections were preincubated with Teng-T (10 mm Tris (pH 7.6), 5 mm EDTA, 150 mm NaCl, 0.25% gelatin, 0.05% Tween-20)/10% normal goat serum (NGS) for 30 min, followed by an overnight incubation with the primary antibody diluted in Teng-T/10% NGS. Slides were then washed with PBS and incubated with Teng-T/10% NGS for 15 min, followed by 90 min incubation with the secondary antibody (supplemental Table S2) in Teng-T/10% NGS. After washing, the slides were incubated with alkaline phosphatase substrate (NBT/BCIP tablets; Roche) at 37 °C. Digital images of the sections were analyzed with the Leica Quantimet 500 Image Analysis System, v3.2 (44).
Statistical Analysis
The significant difference for one variable between two distinct groups was analyzed using an unpaired Student's t test. For more complex analyses of significance for variables with different controls, each gene was analyzed using Gaussian linear regression, including 18 S as housekeeping gene. When appropriate, additional explanatory variables such as tissue type, strain, and time were included in the model. Finally, interactions among the included variables were considered. The inference criterion used for comparing the models is their ability to predict the observed data, i.e. models are compared directly through their minimized minus log-likelihood. When the numbers of parameters in models differ, they are penalized by adding the number of estimated parameters, a form of the Akaike information criterion (45). If the most likely model contains the treatment effect, the fold change representing the ratio between the variable of the treated group and the same variable for the control is computed, as well as its 95% confidence interval.
RESULTS
The GH/IGF1 Axis Is Functional but Repressed in F/A2 Mice
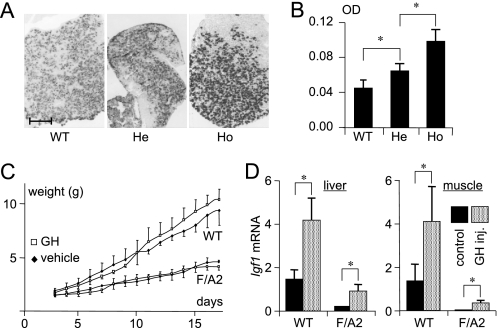
The phenotype of F/A2 mice is compatible with a deficiency of GH and/or IGF1. We, therefore, investigated the GH content in the pituitary gland by quantitative immunohistochemistry (Fig. 1A). The somatotropes of 3-week-old hemi- and homozygous F/A2 mice contained 1.4- and 2.2-fold more GH, respectively, than those of their wild-type counterparts (Fig. 1B), indicating an inverse correlation with the severity of the F/A2 phenotype (14, 16).
FIGURE 1.
Role of growth hormone in the phenotype of F/A2 mice. Panels A and B: Detection of GH in pituitary somatotropes of ND21 mice. A, sections of pituitary glands of ND21 WT, hemizygous F/A2 mice (He), and homozygous F/A2 mice (Ho) were stained for the presence of GH. Bar: 5 μm. B, optical density (OD) in GH-positive cells (n = 3 mice per genotype) increases from wild-type via hemizygous to homozygous F/A2 mice. Panels C and D, effect of GH treatment on weight gain in suckling F/A2 mice. C, change in body weight of F/A2 mice and their wild-type littermates during treatment with GH (twice daily 3 mg rhGH/kg body weight subcutaneously) between ND3 and ND21 (n = 6 mice per genotype). Note that F/A2 mice show a ∼3-fold lower growth rate compared with wild-type mice. D, quantification of Igf1 mRNA in liver (left) and muscle (right) of vehicle and GH-injected ND17 mice. n = 3 mice per genotype. *, p < 0.01.
We then used the rat pituitary cell line GH3, which produces GH (46, 47), to assess the direct effects of arginine on GH production and secretion. GH content in the GH3 cells was assessed by quantitative immunohistochemistry (supplemental Fig. S1, A and B) and ELISA (supplemental Fig. S1C). Both assays showed that, after 24 h of culture, the cellular GH content was inversely correlated with extracellular arginine concentration and decreased from ∼50 to ∼25 pg of GH per cell at 0 and 200 μm arginine in the medium, respectively (supplemental Fig. S1C; significant at ≥50 μm arginine). This decrease in cellular GH content was accounted for by a 1.5-fold increase in concentration of GH in the medium (significant at ≥25 μm arginine). Because the total GH content of the cells and the medium combined did not vary with arginine concentration in the medium, these findings reveal a pronounced concentration-dependent effect of arginine on GH secretion. The dependence of GH secretion on the ambient concentration of arginine after 72 h of culture was similar to that observed after 24 h (not shown). These data show that, below a concentration of 50 μm ambient arginine, GH secretion is reduced, with a half-maximal effect at ∼20 μm arginine.
As early as 3 days after birth (ND3), F/A2 mice can be distinguished from their wild-type littermates by a lower body weight (14). To assess whether the typically hypomorphic tissues of F/A2 mice (muscle, hair, and B-cells (15)) respond to GH supplementation, mice were subcutaneously injected twice daily with 3 mg/kg recombinant human (rh)GH from ND3 onwards. This dose of rhGH mediates growth in liver IGF-1-deficient mice (40). Fig. 1C shows that the growth rate of homozygous F/A2 mice was ∼50% of that of wild-type mice and that treatment with rhGH had no effect on the growth rate of either wild-type or F/A2 mice.
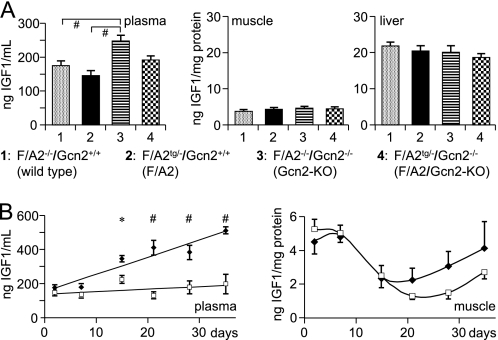
GH action is mediated by GH receptors (GHR) and results in the synthesis and secretion of IGF1 in target tissues. Ghr mRNA concentrations in the livers of ND1, ND10, and ND21 mice increased with age, but were not different in wild-type and F/A2 mice (supplemental Fig. S2). To assess the functionality of these GHRs and their downstream signaling pathway, Igf1 mRNA concentrations were quantified in liver and muscle of GH-treated and control mice (Fig. 1D). In both tissues, Igf1 mRNA concentrations in F/A2 mice were only 10–15% of those in wild-type mice. Although the rhGH injections increased Igf1 mRNA concentration in liver and muscle of both F/A2 and wild-type mice considerably (∼8- and ∼2.5-fold, respectively; p < 0.01), Igf1 mRNA levels in the liver and muscle of treated F/A2 mice increased to only ∼65 and ∼25%, respectively, of that in untreated wild-type mice. We then measured plasma and tissue IGF1 concentrations to assess whether F/A2 mice suffer from a low production of IGF1 (Fig. 2). In wild-type and F/A2 ND1 mice, no differences in plasma, muscle, or liver IGF1 protein were detected (Fig. 2A). However, plasma IGF1 levels increased ∼7-fold faster in wild-type than F/A2 mice during the first 5 postnatal weeks (Fig. 2B, left panel; p < 0.001)). Plasma IGF1 levels reflect hepatic IGF1 production, but liver IGF1 does not affect growth until 6 weeks postnatally (48, 49). We, therefore, measured IGF1 levels in an affected tissue (calf muscle) of the same mice (Fig. 2B, right panel). Muscular IGF1 concentration decreased gradually between 1 and 3 weeks after birth, without difference between F/A2 and wild-type mice. After weaning, the IGF1 concentration in muscle increased again, with a ∼1 week delay in F/A2 relative to wild-type mice. Although the age-dependent changes in muscle IGF1 content were significant (p < 0.01), they did not differ between F/A2 and wild-type mice. Because IGF1 in skeletal muscle affects growth only after the 3rd postnatal week (32), that is, coincident with the resumption of growth in F/A2 mice (14), we further concluded that, although the GH-IGF-1 axis did not function properly in F/A2 mice, its disturbed function could not account for the marked hypotrophy seen in these animals.
FIGURE 2.
Plasma and tissue IGF1 protein concentrations in F/A2 mice. Panel A, differences in plasma, muscle and liver IGF1 protein concentration in ND1 F/A2−/−/Gcn2+/+ (wild-type), F/A2tg/−/Gcn2+/+ (F/A2 transgenic), F/A2−/−/Gcn2−/− (Gcn2-KO), and F/A2tg/−/Gcn2−/− (F/A2/Gcn2-KO double mutant) mice (n = 6 per genotype). The plasma IGF1 concentration in F/A2tg/−/Gcn2−/− tended to be lower than in F/A2−/−/Gcn2−/− mice (p = 0.055). Panel B, changes in plasma and muscle IGF1 protein concentration during the first 5 postnatal weeks in wild-type (black diamonds) and F/A2 mice (white squares); (n = 3 per genotype except at ND1). *, p < 0.05; #, p < 0.01. The age-dependent changes in muscle IGF1 content were highly significant (p < 0.01).
Arginine Is Essential for Muscle Development
F/A2 skeletal muscles have an immature appearance (14). We, therefore, investigated if arginine directly affects muscle development in differentiating C2C12 myoblasts that were cultured in the presence of different concentrations of arginine. Supplemental Fig. S3A shows representative pictures after 4 days of myogenic differentiation in the presence (200 μm) or absence of arginine. Both the myogenic index (the fraction of nuclei residing in cells with three or more nuclei and considered an early differentiation parameter; supplemental Fig. S3B) and the cellular concentration of the late differentiation marker creatine kinase-M (supplemental Fig. S3C) increased with increasing arginine concentration to plateau at 25–50 μm arginine. Myogenic differentiation is, therefore, clearly dependent on arginine availability, with a half-maximal effect at 10–20 μm arginine in the medium.
Arginine Deficiency Induces the Integrated Stress Response in Vitro
Amino acid deficiency can activate the GCN2 stress kinase pathway (50). An early step is the phosphorylation of translation factor eIF2α (51). As anticipated, arginine deprivation of C2C12 cells at 80% confluence induced phosphorylation of eIF2α on serine 51 within 30 min. The protein remained phosphorylated for at least 2 h (supplemental Fig. S4A). Furthermore, arginine depletion induced the accumulation of the transcription factors ATF4 and CHOP, which are well-established downstream mRNA targets of the GCN2 stress-kinase pathway (23, 52), in a concentration-dependent way in both C2C12 and GH3 cells (supplemental Fig. S4B). In both cell types, Chop mRNA concentration increased more gradually with declining arginine concentration in the medium than Atf4 mRNA, indicating that Chop mRNA accumulation was more sensitive to arginine removal than that of Atf4 mRNA. The adaptive response of Atf4 and Chop mRNA expression in primary cultures of mouse B-lymphocytes, another hypomorphic tissue in F/A2 mice, to arginine deprivation was similar to that of C2C12 and GH3 cells (not shown). Supplemental Fig. S4C, finally, shows that ATF4 protein accumulates at arginine concentrations below 25 μm (see Ref. 52). These findings reveal that the integrated stress response becomes induced at arginine concentrations in the medium below 50 μm, with an EC50 of ∼12.5 μm.
Arginine Deficiency Does Not Affect Signaling through the mTORC1 Pathway
Amino acid deficiency can regulate protein synthesis by up-regulating signaling via the GCN2 pathway and by down-regulating signaling via the mTORC1 pathway. We, therefore, compared the effects of 0, 12.5, and 200 μm arginine in the culture medium on the degree of phosphorylation of the translation factors eIF2α as target of GCN2 kinase and 4EBP1, and S6K1 as targets of mTORC1 kinase in the same cell extracts (supplemental Fig. S5, column “Ctrl”). As expected, arginine deficiency caused a significant >2-fold increase in eIF2α phosphorylation, but did not significantly affect either S6K1- or 4EBP1 phosphorylation (p = 0.13 and 1.0, respectively). Furthermore, arginine deficiency increased the mRNA concentrations of Atf4, ATF4 target Chop, and CHOP target Gadd34, which dephosphorylates eIF4α-P. In addition, the expression of the ATF4 target Cat-1 was induced. Collectively, these data show that the ambient arginine concentration affects the activity or expression of all investigated parameters except the mTORC1 targets S6K1 and 4EBP1.
The eIF2αP-specific phosphatase inhibitor salubrinal was used to assess to what extent arginine deficiency induced maximal eIF2α phosphorylation and whether the degree of eIF2α phosphorylation affected the stress-kinase esponse only quantitatively or also qualitatively (supplemental Fig. S5, column “Sal”). At the concentration used (50 μm), salubrinal increased eIF2α phosphorylation ∼3-fold over basal levels (p < 0.001) and abolished the dependence of eIF2α phosphorylation and its downstream targets on arginine concentration. Furthermore, salubrinal decreased overall S6K1 phosphorylation levels ∼3-fold (p < 0.001) and increased the mRNAs of Atf4 (∼3-fold; p < 0.001), Chop (∼20-fold; p < 0.001), Gadd34 (∼6-fold; p < 0.001), and Cat-1 (∼3-fold; p < 0.001), that is, the ∼3-fold increase in eIF2α phosphorylation had quantitatively a disproportional effect on its downstream targets. These data reveal that the effects of a graded phosphorylation of eIF2α due to arginine deficiency differ both qualitatively and quantitatively from the effects of a maximal phosphorylation due to salubrinal treatment.
Constitutive Gcn2 knockdown in C2C12 muscle cells by integration of a viral vector carrying Gcn2-specific shRNA reduced Gcn2 mRNA expression to ∼25% of control values (not shown). It strongly inhibited myotube formation, suggesting that GCN2 activation is involved in myocyte differentiation. This level of knockdown did, however, not affect the arginine-dependent stress-kinase response (supplemental Fig. S5, column “Gcn2-kd”). Similarly, treatment with the muscle growth factor IGF1 did not mediate, at the concentration used (0.13 nm), a change in effects on the arginine-dependent phosphorylation of eIF2α, S6K1, or 4EBP1 (p < 0.05, p = 0.18, and p = 0.66, respectively) or their downstream targets.
Collectively, these data show that arginine deficiency induced a graded and comparatively mild increase in eIF2α phosphorylation and ATF4-dependent gene expression, but did not affect phosphorylation of S6K1 or 4EBP1. However, when eIF2α became hyperphosphorylated after treatment with the phosphatase inhibitor salubrinal, this specificity was lost. Gcn2 knockdown blocked myotube differentiation, without totally eliminating the dependence of eIF2α phosphorylation on ambient arginine concentrations. The muscle growth factor IGF1, finally, increased the effect of arginine deficiency.
The Stress Kinase Pathway Is Activated in F/A2 Mice
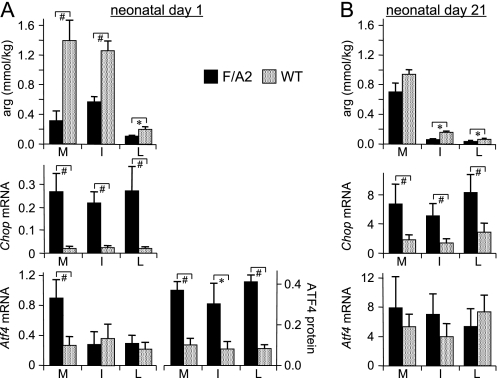
On neonatal day 1, the tissue concentration of arginine in skeletal muscle was only 20% (p < 0.01) and that in intestine and liver ∼50% (p < 0.01 and <0.05, respectively) in F/A2 compared with wild-type mice (Fig. 3A, top subpanel). The low concentration of arginine in liver can be ascribed to the high concentration of the urea cycle enzyme arginase-1 in the liver. The low tissue arginine concentration induced Chop mRNA expression >8-fold in all tissues investigated (p < 0.001; Fig. 3A, middle subpanel), but Atf4 mRNA expression in skeletal muscle only (∼3-fold; p < 0.05; Fig. 3A, bottom subpanel). These findings in vivo reveal that the tissue arginine concentration had decreased most in muscle and that Chop mRNA concentration was a more sensitive parameter to monitor the stress-kinase response to amino acid deprivation than Atf4 mRNA.
FIGURE 3.
The stress-kinase pathway is activated in neonatal F/A2 mice. Panel A, concentration of arginine, Chop mRNA, and Atf4 mRNA and ATF4 protein in skeletal muscle (M), small intestine (I), and liver (L) of ND1 F/A2 and wild-type mice. mRNA concentrations were corrected for 18 S rRNA content, while equal protein loading was checked by pre-staining Western blots with Ponceau-S. n = 8–12 mice per genotype for arginine and mRNA concentration, and n = 3 for ATF4 protein. Panel B, concentration of arginine, and Chop and Atf4 mRNA concentration in muscle, small intestine, and liver of ND21 F/A2 and wild-type mice. n = 8–12 mice per genotype. *, p < 0.05; #, p < 0.01.
Because phosphorylation of eIF2α facilitates translation of Atf4 mRNA (53), we also determined ATF4 protein concentration in ND1 mice (Fig. 3A, bottom subpanel). Western blot analysis of muscles of F/A2 mice showed the expected 38-kDa band (bold arrow in supplemental Fig. S6) and a much weaker band at ∼50kDa (small arrow), whereas these bands were absent in muscle of wild-type mice. The ∼50kDa band may correspond to an ubiquitinated form of ATF4 (54). ATF4 protein was 3–4-fold higher in muscle, small intestine, and liver of F/A2 than of wild-type animals (Fig. 3A, bottom subpanel; p < 0.01, <0.02, and <0.01, respectively). The data in Fig. 3A demonstrate that Chop mRNA and ATF4 protein are equally sensitive parameters to assess the response to arginine deficiency in muscle, intestine, and liver.
On neonatal day 21, the concentration of arginine in wild-type muscle was similar to that on ND1, but in the intestine and liver, it had decreased to ∼15 and ∼30% of that on ND1 (p < 0.01). This pronounced decline can be ascribed to the cessation of intestinal arginine biosynthesis just prior to weaning (10) and the continued maturation of the function of the urea cycle in the liver (55). The arginine concentration in F/A2 muscle had increased 2-fold relative to that on ND1 (p < 0.05) and was no longer different from that in controls (Fig. 3B, top subpanel). However, the concentration of arginine in F/A2 intestine and liver remained depressed (p < 0.05). Tissue Chop and Atf4 mRNA concentration had increased ∼20-fold and ∼10-fold, respectively, relative to ND1 (Fig. 3B, middle and bottom subpanels). Chop mRNA concentrations were still significantly elevated in F/A2 tissues (p < 0.01; Fig. 3B, middle subpanel, but Atf4 mRNA concentrations in F/A2 mice were no longer different from those in control mice (Fig. 3B, bottom subpanel). Together, these in vivo data show that the stress-kinase response pathway is strongly activated in neonatal F/A2 mice. This activation persists throughout the suckling period, although its severity appears to decline, in agreement with the disappearance of the phenotype after weaning.
GCN2 Mediates the Activation of the Stress Response in Arginine-deficient Neonatal Mice
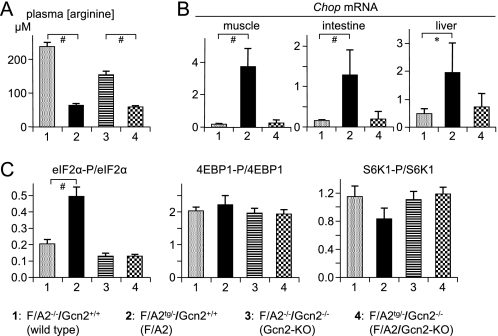
In addition to GCN2, three other stress kinases can phosphorylate eIF2α. To demonstrate that GCN2 is the only stress kinase that becomes activated in neonatal F/A2 mice, we crossed F/A2tg/− mice with Gcn2−/− mice. Table 1A lists the genotypes of the offspring and shows that the respective genotypes were born at the expected Mendelian frequency. This Table also shows that all F/A2 mice that were deficient for Gcn2 (F/A2tg/?/Gcn2−/−) died within 48 h after birth (p < 0.001). The plasma arginine concentration in F/A2 neonates was only 25–40% of that in control neonates, irrespective of whether Gcn2 was or was not expressed (p < 0.01; Fig. 4A). Gcn2-deficient F/A2 neonates did drink, but their plasma glucose concentration on ND1 was significantly lower than that in wild-type controls (p < 0.01), with Gcn2-deficient and F/A2 neonates taking intermediate positions (Table 1B).
TABLE 1.
Distribution of genotypes and plasma glucose concentrations in the offspring of crosses of hemizygous F/A2 (F/A2tg/−) mice and heterozygous Gcn2 (Gcn2+/−) mice
Panel A: six different genotypes could be identified by PCR (F/A2tg/− and F/A2tg/tg mice could not be distinguished; F/A2−/− mice denote wild-type animals). A total of 60 mice (dead and alive) were analyzed. Four newborn mice disappeared from the nests in the first two days and could not be analyzed. Panel B: blood was collected from mice at ND1.
| Genotype (A) | Expected | Day 0 |
Day 2 |
|
|---|---|---|---|---|
| Alive | Alive | Dead | ||
| F/A2−/−/Gcn2+/+ | 4 | 0 | ||
| F/A2−/−/Gcn2+/− | 8 | 10 | 10 | |
| F/A2−/−/Gcn2−/− | 4 | 6 | 5 | 1 |
| F/A2tg/?/Gcn2+/+ | 11 | 10 | 10 | |
| F/A2tg/?/Gcn2+/− | 22 | 23 | 23 | |
| F/A2tg/?/Gcn2−/− | 11 | 7 | 0 | 7 |
| F/A2?/?/Gcn2?/? | 4 | 4 | ||
| Total | 60 | 48 | 12 | |
| Genotype (B) | Plasma glucose |
|
|---|---|---|
| mm | N | |
| F/A2−/−/Gcn2+/+ | 3.3 ± 0.2 | 23 |
| F/A2−/−/Gcn2−/− | 2.8 ± 0.3 | 7 |
| F/A2tg/?/Gcn2+/+ | 3.2 ± 0.2 | 25 |
| F/A2tg/?/Gcn2−/− | 2.7 ± 0.2 | 15 |
FIGURE 4.
GCN2 is responsible for the induction of the integrated stress response in neonatal F/A2 mice. Panel A, arginine concentration in trunk blood of ND1 mice. Panel B, Chop mRNA expression in muscle, small intestine, and liver of the same mice as shown in panel A. Panel C, Ser51-eIF2α, Ser65–4EBP1, and Thr389-S6K1 phosphorylation in skeletal muscle of ND1 mice. n = 8–12 mice per genotype. *, p < 0.05; #, p < 0.01.
To establish whether Gcn2 deficiency prevented the activation of the stress response, we determined Chop mRNA concentrations in muscle, small intestine and liver of neonatal wild-type mice (F/A2−/−/Gcn2+/+), F/A2 mice (F/A2tg/?/ Gcn2+/+) and F/A2 mice that were also Gcn2-deficient (F/A2tg/?/Gcn2−/−) (Fig. 4B). As expected, Chop mRNA concentrations were low in muscle, liver and gut of wild-type (F/A2−/−/Gcn2+/+) mice and elevated in the same organs of F/A2 (F/A2tg/?/Gcn2+/+) mice (p < 0.01, <0.01, and <0.05, respectively). However, the elevation of Chop mRNA concentration was not found in muscle, liver, and gut of the F/A2 neonates that were, in addition, deficient for Gcn2 (F/A2tg/?/ Gcn2−/−). Similarly, the degree of phosphorylation of eIF2α in muscle was increased in F/A2 mice (p < 0.01), but not in F/A2 mice that were also deficient in Gcn2 (Fig. 4C, left subpanel, and supplemental Fig. S6). These findings demonstrate that GCN2 activation is necessary for postnatal survival in F/A2 mice and that the phosphorylation of eIF2α and the induction of Chop expression in these mice are solely dependent on GCN2 activation. Gcn2-deficient neonates further had a significantly higher plasma IGF1 level than wild-type or F/A2 mice (Fig. 2A, left subpanel). F/A2 neonates had a lower plasma IGF1 level than wild-type or Gcn2-deficient neonates, but the difference was significant for only the latter.
mTORC1-mediated Translational Control Is Not Affected in Arginine-deficient Neonatal Mice
The degree of phosphorylation of S6K1 and 4EBP1 in neonatal skeletal muscle was not significantly different in any of the four genotypes (Fig. 4C and supplemental Fig. S6; p = 0.36 and 0.71, respectively). These findings indicate that the reduction in circulating arginine concentration in F/A2 mice does not affect signaling through the mTORC1 signaling pathway. Ckm, Myhc1, and Myhc2B mRNA concentrations were not affected either by arginine or Gcn2 deficiency in neonates (supplemental Fig. S7).
DISCUSSION
Suckling F/A2 mice, which express arginase I from the Fabp1-promoter exclusively in the enterocytes of the small intestine (14), suffer from a severe reduction in plasma arginine levels (∼70 μm versus ∼230 μm), reduced hair and muscle growth, and a specific block in B-cell development (14–17). In this study, we show that the deficiency of arginine causes an impaired function of the endocrine GH/IGF1 axis of these mice, but that the compromised function of the somatotropic axis does not account for the observed growth deficiency. Instead, activation of the GCN2-dependent stress response was found to be responsible for the hampered growth and even to be necessary for postnatal survival. Arginine deficiency does not suppress growth factor (mTORC1)-dependent signaling.
The Somatotropic Axis Is Not Responsible for the Runting Phenotype of F/A2 Mice
The gradual accumulation of GH in the pituitary gland from wild-type mice via hemizygous to homozygous F/A2 mice suggests that decreasing circulating concentrations of arginine progressively suppresses GH synthesis or secretion. Using GH3 cells, we could demonstrate that arginine deficiency blocks GH secretion rather than synthesis. Our findings, therefore, complement the recent finding that chronic arginine supplementation stimulates GH secretion (56) and imply that a broad dose-response relation exists between circulating arginine and GH secretion. The severely reduced plasma IGF1 concentration in suckling and weanling F/A2 mice also underscores the presence of a GH deficiency in F/A2 suckling mice, as IGF1 production is dependent upon GH stimulation (38, 57) and >75% of plasma IGF1 is produced by the liver (48, 49). In vivo, the effects of arginine deficiency on growth in suckling mice become apparent when the circulating arginine concentration falls below ∼80 μm (14), which compares well with our present finding in vitro that arginine concentrations below 50 μm inhibit GH secretion (neonatal plasma arginine concentrations are ∼1.6-fold higher than those after weaning (14, 16)). However, neonatal F/A2 mice are not growth-retarded, even though their plasma arginine concentration is low, whereas Igf1-deficient mice are (58, 59). Furthermore, GH supplementation could not restore growth in suckling F/A2 mice. In addition, IGF1 concentration in skeletal muscle, one of the most severely affected tissues, was similar in F/A2 and wild-type mice. These findings demonstrate that the activity of the somatotropic axis is impaired in arginine-deficient suckling mice, but not directly responsible for the runted phenotype observed in these mice.
Arginine Deficiency Selectively Induces the Integrated Stress Response in Vitro
Because skeletal muscle is a severely affected tissue in F/A2 mice (14), we used the C2C12 muscle cell line to investigate the mechanism underlying the observed effects of arginine deficiency. Whereas arginine withdrawal increased, as expected, eIF2α phosphorylation and the expression of its established downstream targets Atf4, Chop, Gadd34, and Cat-1 (39, 60, 61), it did not significantly affect the degree of phosphorylation of S6K1 or 4EBP1, demonstrating that, in vitro, a deficiency of arginine activates the stress kinase GCN2, but does not block mTORC1 signaling. The pronounced effect of arginine deficiency on GCN2 activation contrasts with an earlier report that the depletion of all essential amino acids for 6 h does not activate the GCN2 pathway in C2C12 myotubes (62). It is well possible that, as the authors themselves suggest (62), the complete removal of all essential amino acids strongly induces intracellular proteolysis in myotubes, whereas the selective depletion of arginine does not, or to a much lesser extent, as shown by the absence of an effect of arginine withdrawal on the expression of the ubiquitin ligases Atrogin1 and Murf-1.5 These discrepant findings underscore, nevertheless, that results obtained after complete removal of amino acids (e.g. Ref. 62), sometimes followed by adding back a single amino acid (30), often differ from results obtained after selectively removing a single amino acid from a complete mixture (29, 63) and present study). We opted to selectively remove arginine, because that intervention best mimicked the difference in plasma amino acid concentration between wild-type and F/A2 mice (14, 16).
The restriction of the effects of arginine deficiency to activation of the GCN2 stress-kinase pathway appears to reflect a limited increase in eIF2α phosphorylation, because maximizing the degree of phosphorylation of eIF2α with salubrinal caused a severe suppression of S6K1 phosphorylation and a disproportionate up-regulation of Atf4, Chop, Gadd34, and Cat-1 expression. These data suggest that crosstalk of the stress-kinase and the mTORC1 pathways only occurs when eIF2α phosphorylation reaches an unphysiologically high level. Gcn2 knockdown in C2C12 myoblasts almost completely prevented myotube formation, but did not affect the degree of phosphorylation of eIF2α, S6K1, or 4EBP1. Treatment with the growth factor IGF1 also did not affect eIF2α, S6K1, or 4EBP1 phosphorylation in response to arginine withdrawal. Our findings in C2C12 cells, therefore, support the hypothesis that arginine deficiency selectively affects the GCN2 stress-kinase pathway.
Arginine Deficiency Selectively Induces the Integrated Stress Response in Vivo
Based on the degree of phosphorylation of eIF2α and the expression of Chop mRNA, the GCN2 signaling pathway was activated in all F/A2 tissues tested on postnatal day 1. Atf4 mRNA was only up-regulated in skeletal muscle of 1-day-old F/A2 mice, but ATF4 protein concentration was elevated in all tissues. These findings underline the severity of the muscle phenotype in F/A2 mice and the primarily translational regulation of ATF4 expression (52, 53). The concentration gradient of arginine between plasma and muscle was similar in control and F/A2 mice (4–5-fold), but the absolute concentrations were 3–4-fold lower in F/A2 than in wild-type mice, in agreement with an arginine supply rather than transport problem. F/A2 homozygotes resume growth around weaning (14), in all likelihood due to the increasing cellular arginine, IGF1, and IGF1R1 (64) concentrations in target tissues. In combination, these data, therefore, indicate that the GCN2 signaling pathway in muscle in vivo becomes activated when intracellular arginine concentrations in muscle decrease below ∼400 μmol/kg. GCN2 activation in the neonatal intestine appears to have a similar sensitivity to arginine, but despite much lower tissue arginine concentrations, Chop mRNA and ATF4 protein concentrations in liver are similar to those in muscle and intestine.
Neonatal F/A2 mice did not show a reduced degree of S6K1 or 4EBP1 phosphorylation, which demonstrates that the effects of arginine deficiency are restricted to GCN2 activation both in vitro and in vivo. In this respect, arginine deficiency differs from leucine deficiency, which, at least in adult mice, causes both a GCN2-dependent phosphorrylation of eIF2α and a hypophosphorylation of S6K1 and 4EBP1 (65).
GCN2 Is Necessary to Cope with and Survive Neonatal Arginine Deficiency
The absence of an increase in eIF2α phosphorylation and Chop mRNA expression in neonates of crosses of F/A2 and Gcn2-deficient mice confirmed our in vitro data that GCN2 was necessary to mediate the response to arginine deficiency. These data also highlighted the vital role of GCN2 activation for postnatal survival of arginine-deficient pups. When a leucine-deficient diet was fed to pregnant Gcn2+/− dams, neonatal survival of their Gcn2-deficient offspring was only ∼40% (22), but F/A2 neonates show a complete loss of vitality when the concentration of arginine falls to ∼30% of normal. We have not yet identified the dysfunction that kills Gcn2-deficient F/A2 pups, but, in all likelihood, the decline in plasma glucose concentration, possibly in conjunction with an increase in plasma IGF1 concentration, is a contributing factor to their untimely death. A similar correlation between stress-kinase signaling and lethal hypoglycemia was also noted in pups carrying the phosphorylation-resistant Ser51Ala mutation of eIF2α (66), but these latter pups died earlier (<18 h) and with lower blood glucose concentrations (≤1 mm) than Gcn2-deficient F/A2 neonates (<48 h and <3 mm, respectively). Apparently, GCN2-independent phosphorylation of eIF2α is also necessary to survive neonatally.
Arginine Is a Signaling Molecule in Its Own Right
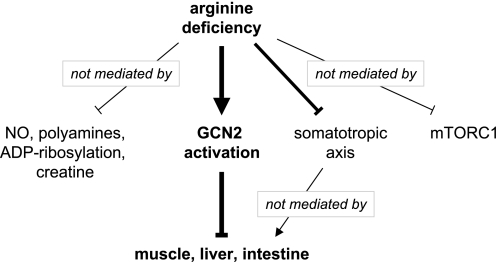
The discussion of arginine-dependent signaling in tissues often focuses on arginine metabolites, such as NO, agmatine, polyamines, creatine, or on arginine-mediated modifications such as ADP-ribosylation. This and earlier studies of the F/A2 mouse (14–17) demonstrate that the arginine-deficient phenotype is not mediated by a metabolite of arginine (Fig. 5). Instead, it is the low circulating concentration of arginine itself, which suppresses the somatotropic axis and activates the GCN2-signaling pathway without affecting mTORC1-mediated signaling. We would, therefore, like to propose that the striking phenotype of suckling F/A2 mice is a paradigm for selective GCN2 activation (Fig. 5).
FIGURE 5.
Proposed mechanism underlying the arginine-deficient phenotype in mice. Arginine is the precursor for NO, polyamines, and creatine synthesis, and regulates ADP-ribosylation, but these molecules do not mediate the developmental impairment of F/A2 mice. The somatotropic (GH/IGF1) axis does not function properly in arginine-deficient mice, but although GH supplementation increases Igf1 mRNA concentration to wild-type values, it cannot restore growth. Arginine deficiency, finally, does not block mTORC1 phosphorylation-dependent signaling, but does activate the stress kinase GCN2 in neonatal and suckling F/A2 mice. The sole activation of the GCN2-mediated integrated stress response is apparently necessary and sufficient to block growth in a highly distinctive way.
Supplementary Material
Acknowledgments
We thank Dr. W. Gerver (Pediatrics, azM, Maastricht) for providing us with recombinant human growth hormone, Dr. T. B. M. Hakvoort (Tytgat Institute for Liver and Gastrointestinal Diseases) for determining plasma arginine concentrations and Dr. H. A. M. Geerts (Dept. Human Genetics, AMC) and Ms. W. T. Labruyere (Tytgat Institute) for providing and growing the GCN2-antisense lentivirus, respectively.
This study was supported by the graduate school NUTRIM.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. S1–S7.
S. E. Kohler, unpublished observations.
- F/A2
- mice overexpressing arginase-I under control of the Fabpi promoter
- ATF4
- activating transcription factor 4 (Atf4)
- Atrogin1
- muscle atrophy F-box E3 ubiquitin-protein ligase or F-box-only protein 32 (Fbxo32)
- CAT-1
- cationic amino-acid transporter-1 or Solute carrier 7A1 (Slc7A1)
- CHOP
- C/EBP-homologous protein or growth arrest and DNA damage-inducible protein 135 or DNA-damage inducible transcript 3 (Ddit3)
- 4EBP1
- eukaryotic translation initiation factor (eIF)4E-binding protein-1 (Eif4ebp1)
- GADD34
- growth arrest and DNA damage-inducible protein 34 or protein phosphatase-1 regulatory subunit 15A (Ppp1r15a)
- GCN2
- general control non-derepressible 2 kinase or eukaryotic translation initiation factor 2α kinase 4 (Eif2ak4)
- IGF1
- insulin-like growth factor 1 (Igf1)
- CKM
- muscle-specific creatine kinase (Ckm)
- mTORC1
- mammalian target of rapamycin complex-1
- MURF1
- muscle-specific RING finger-1 or E3 ubiquitin-protein ligase (Trim63)
- MyHC1
- myosin heavy chain-I or heavy polypeptide 7 (Myh7)
- MyHC2B
- myosin heavy chain-IIb or heavy polypeptide 4 (Myh4)
- ND
- neonatal day
- S6K1
- p70 ribosomal protein S6 kinase-1 or polypeptide 5 (Rps6ka5).
REFERENCES
- 1. Wu G., Morris S. M., Jr. (1998) Biochem. J. 336, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alba-Roth J., Müller O. A., Schopohl J., von Werder K. (1988) J. Clin. Endocrinol. Metab. 67, 1186–1189 [DOI] [PubMed] [Google Scholar]
- 3. Rosati B., Marchetti P., Crociani O., Lecchi M., Lupi R., Arcangeli A., Olivotto M., Wanke E. (2000) Faseb. J. 14, 2601–2610 [DOI] [PubMed] [Google Scholar]
- 4. Van Haeften T. W., Van Faassen I., Van der Veen E. A. (1988) Diabetes Res. 9, 187–191 [PubMed] [Google Scholar]
- 5. Visek W. J. (1986) J. Nutr. 116, 36–46 [DOI] [PubMed] [Google Scholar]
- 6. Wu G., Jaeger L. A., Bazer F. W., Rhoads J. M. (2004) J. Nutr. Biochem. 15, 442–451 [DOI] [PubMed] [Google Scholar]
- 7. Davis T. A., Fiorotto M. L., Reeds P. J. (1993) J. Nutr. 123, 947–956 [DOI] [PubMed] [Google Scholar]
- 8. Wu G. (1997) Am. J. Physiol. 272, G1382–G1390 [DOI] [PubMed] [Google Scholar]
- 9. Bertolo R. F., Brunton J. A., Pencharz P. B., Ball R. O. (2003) Am. J. Physiol. Endocrinol. Metab. 284, E915–922 [DOI] [PubMed] [Google Scholar]
- 10. De Jonge. W. J., Dingemanse M. A., de Boer P. A., Lamers W. H., Moorman A. F. (1998) Pediatr. Res. 43, 442–451 [DOI] [PubMed] [Google Scholar]
- 11. Hurwitz R., Kretchmer N. (1986) Am. J. Physiol. 251, G103–G110 [DOI] [PubMed] [Google Scholar]
- 12. Riby J. E., Hurwitz R. E., Kretchmer N. (1990) Pediatr. Res. 28, 261–265 [DOI] [PubMed] [Google Scholar]
- 13. Windmueller H. G. (1982) Adv. Enzymol. Relat. Areas Mol. Biol. 53, 201–237 [DOI] [PubMed] [Google Scholar]
- 14. de Jonge W. J., Hallemeesch M. M., Kwikkers K. L., Ruijter J. M., de Gier-de Vries C., van Roon M. A., Meijer A. J., Marescau B., de Deyn P. P., Deutz N. E., Lamers W. H. (2002) Am. J. Clin Nutr. 76, 128–140 [DOI] [PubMed] [Google Scholar]
- 15. de Jonge W. J., Kwikkers K. L., te Velde A. A., van Deventer S. J., Nolte M. A., Mebius R. E., Ruijter J. M., Lamers M. C., Lamers W. H. (2002) J. Clin. Invest. 110, 1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Jonge W. J., Marescau B., D'Hooge R., De Deyn P. P., Hallemeesch M. M., Deutz N. E., Ruijter J. M., Lamers W. H. (2001) J. Nutr. 131, 2732–2740 [DOI] [PubMed] [Google Scholar]
- 17. Kwikkers K. L., Ruijter J. M., Labruyère W. T., McMahon K. K., Lamers W. H. (2005) Br. J. Nutr. 93, 183–189 [DOI] [PubMed] [Google Scholar]
- 18. Tsutsui M., Shimokawa H., Morishita T., Nakashima Y., Yanagihara N. (2006) J. Pharmacol. Sci. 102, 147–154 [DOI] [PubMed] [Google Scholar]
- 19. Vellekamp G., Sihag R. K., Deutscher M. P. (1985) J. Biol. Chem. 260, 9843–9847 [PubMed] [Google Scholar]
- 20. Ron D. (2002) J. Clin. Invest. 110, 1383–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wek R. C., Jiang H. Y., Anthony T. G. (2006) Biochem. Soc Trans 34, 7–11 [DOI] [PubMed] [Google Scholar]
- 22. Zhang P., McGrath B. C., Reinert J., Olsen D. S., Lei L., Gill S., Wek S. A., Vattem K. M., Wek R. C., Kimball S. R., Jefferson L. S., Cavener D. R. (2002) Mol. Cell Biol. 22, 6681–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Averous J., Bruhat A., Jousse C., Carraro V., Thiel G., Fafournoux P. (2004) J. Biol. Chem. 279, 5288–5297 [DOI] [PubMed] [Google Scholar]
- 24. Shaw R. J. (2008) Trends Biochem. Sci. 33, 565–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Avruch J., Long X., Ortiz-Vega S., Rapley J., Papageorgiou A., Dai N. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E592–E602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma X. M., Blenis J. (2009) Nat. Rev. Mol. Cell Biol. 10, 307–318 [DOI] [PubMed] [Google Scholar]
- 27. Drummond M. J., Rasmussen B. B. (2008) Curr. Opin. Clin. Nutr. Metab. Care 11, 222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yao K., Yin Y. L., Chu W., Liu Z., Deng D., Li T., Huang R., Zhang J., Tan B., Wang W., Wu G. (2008) J. Nutr. 138, 867–872 [DOI] [PubMed] [Google Scholar]
- 29. Hara K., Yonezawa K., Weng Q. P., Kozlowski M. T., Belham C., Avruch J. (1998) J. Biol. Chem. 273, 14484–14494 [DOI] [PubMed] [Google Scholar]
- 30. Nakajo T., Yamatsuji T., Ban H., Shigemitsu K., Haisa M., Motoki T., Noma K., Nobuhisa T., Matsuoka J., Gunduz M., Yonezawa K., Tanaka N., Naomoto Y. (2005) Biochem. Biophys. Res. Commun. 326, 174–180 [DOI] [PubMed] [Google Scholar]
- 31. Foulstone E. J., Huser C., Crown A. L., Holly J. M., Stewart C. E. (2004) Exp. Cell Res. 294, 223–235 [DOI] [PubMed] [Google Scholar]
- 32. Kim H., Barton E., Muja N., Yakar S., Pennisi P., Leroith D. (2005) Endocrinology 146, 1772–1779 [DOI] [PubMed] [Google Scholar]
- 33. Landreth K. S., Narayanan R., Dorshkind K. (1992) Blood 80, 1207–1212 [PubMed] [Google Scholar]
- 34. Sumita K., Hattori N., Inagaki C. (2005) J Pharmacol. Sci. 97, 408–416 [DOI] [PubMed] [Google Scholar]
- 35. Weger N., Schlake T. (2005) J. Invest. Dermatol. 125, 873–882 [DOI] [PubMed] [Google Scholar]
- 36. Soulard A., Hall M. N. (2007) Cell 129, 434. [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez S., Gaunt T. R., Day I. N. (2007) Hum. Genet. 122, 1–21 [DOI] [PubMed] [Google Scholar]
- 38. Laron Z. (1995) Drugs 50, 595–601 [DOI] [PubMed] [Google Scholar]
- 39. Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. (2000) Mol. Cell 6, 1099–1108 [DOI] [PubMed] [Google Scholar]
- 40. Liu J. L., Yakar S., LeRoith D. (2000) Endocrinology 141, 4436–4441 [DOI] [PubMed] [Google Scholar]
- 41. van Eijk H. M., Rooyakkers D. R., Deutz N. E. (1993) J. Chromatogr. 620, 143–148 [DOI] [PubMed] [Google Scholar]
- 42. Ruijter J. M., Ramakers C., Hoogaars W. M., Karlen Y., Bakker O., van den Hoff M. J., Moorman A. F. (2009) Nucleic Acids Res. 37, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ruijter J. M., Thygesen H. H., Schoneveld O. J., Das A. T., Berkhout B., Lamers W. H. (2006) Retrovirology 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Straaten H. W., He Y., van Duist M. M., Labruyère W. T., Vermeulen J. L., van Dijk P. J., Ruijter J. M., Lamers W. H., Hakvoort T. B. (2006) Biochem. Cell Biol. 84, 215–231 [DOI] [PubMed] [Google Scholar]
- 45. Akaike H. (1973) Second International Symposium on Information Theory, Akademia Kiado [Google Scholar]
- 46. Adrião M., Chrisman C. J., Bielavsky M., Olinto S. C., Shiraishi E. M., Nunes M. T. (2004) Neuroendocrinology 79, 26–33 [DOI] [PubMed] [Google Scholar]
- 47. Liu Y. L., Zhong Y. Q., Chi S. M., Zhu Y. L. (2005) Sheng. Li Xue Bao. 57, 254–258 [PubMed] [Google Scholar]
- 48. Sjögren K., Liu J. L., Blad K., Skrtic S., Vidal O., Wallenius V., LeRoith D., Törnell J., Isaksson O. G., Jansson J. O., Ohlsson C. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7088–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yakar S., Liu J. L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gebauer F., Hentze M. W. (2004) Nat. Rev. Mol. Cell Biol. 5, 827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D. F., Bell J. C., Hettmann T., Leiden J. M., Ron D. (2003) Mol. Cell 11, 619–633 [DOI] [PubMed] [Google Scholar]
- 52. Ameri K., Harris A. L. (2008) Int. J. Biochem. Cell Biol. 40, 14–21 [DOI] [PubMed] [Google Scholar]
- 53. Holcik M., Sonenberg N. (2005) Nat. Rev. Mol. Cell Biol. 6, 318–327 [DOI] [PubMed] [Google Scholar]
- 54. Lassot I., Ségéral E., Berlioz-Torrent C., Durand H., Groussin L., Hai T., Benarous R., Margottin-Goguet F. (2001) Mol. Cell Biol. 21, 2192–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Illnerová H. (1965) Biol. Neonat. 9, 197–202 [DOI] [PubMed] [Google Scholar]
- 56. de Castro Barbosa T., de Carvalho J. E., Poyares L. L., Bordin S., Machado U. F., Nunes M. T. (2009) Endocrinology 150, 2080–2086 [DOI] [PubMed] [Google Scholar]
- 57. Le Roith D., Bondy C., Yakar S., Liu J. L., Butler A. (2001) Endocr. Rev. 22, 53–74 [DOI] [PubMed] [Google Scholar]
- 58. Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. (1993) Cell 75, 59–72 [PubMed] [Google Scholar]
- 59. Powell-Braxton L., Hollingshead P., Warburton C., Dowd M., Pitts-Meek S., Dalton D., Gillett N., Stewart T. A. (1993) Genes Dev. 7, 2609–2617 [DOI] [PubMed] [Google Scholar]
- 60. Fernandez J., Yaman I., Sarnow P., Snider M. D., Hatzoglou M. (2002) J. Biol. Chem. 277, 19198–19205 [DOI] [PubMed] [Google Scholar]
- 61. Kilberg M. S., Shan J., Su N. (2009) Trends Endocrinol. Metab. 20, 436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Deval C., Talvas J., Chaveroux C., Maurin A. C., Mordier S., Cherasse Y., Parry L., Carraro V., Jousse C., Bruhat A., Fafournoux P. (2008) Biochimie 90, 1716–1721 [DOI] [PubMed] [Google Scholar]
- 63. Deldicque L., Sanchez Canedo C., Horman S., De Potter I., Bertrand L., Hue L., Francaux M. (2008) Amino Acids 35, 147–155 [DOI] [PubMed] [Google Scholar]
- 64. Sakuma K., Watanabe K., Sano M., Uramoto I., Totsuka T. (2000) Acta Neuropathol. 99, 169–176 [DOI] [PubMed] [Google Scholar]
- 65. Anthony T. G., McDaniel B. J., Byerley R. L., McGrath B. C., Cavener D. R., McNurlan M. A., Wek R. C. (2004) J. Biol. Chem. 279, 36553–36561 [DOI] [PubMed] [Google Scholar]
- 66. Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. (2001) Mol. Cell 7, 1165–1176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.