Abstract
Prolonged nutrient limitation has been extensively studied due to its positive effects on life span. However, less is understood of how brief periods of starvation can have lasting consequences. In this study, we use genetics, biochemistry, pharmacology and behavioral analysis to show that after a limited period of starvation, the synthesis of egl-2-encoded ether-a-go-go (EAG) K+ channels and its C-terminal modifications by unc-43-encoded CaMKII have a perduring effect on C. elegans male sexual behavior. EGL-2 and UNC-43 interactions, induced after food deprivation, maintain reduced excitability in muscles involved in sex. In young adult males, spastic contractions occur in cholinergic-activated sex muscles that lack functional unc-103-encoded ERG-like K+ channels. Promoting EGL-2 and UNC-43 interactions in unc-103 mutant adult males by starving them for a few hours reduce spastic muscle contractions over multiple days. Although transient starvation during early adulthood has a hormetic effect of suppressing mutation-induced muscle contractions, the treatment reduces the ability of young wild-type males to compete with well-fed cohorts in siring progeny.
Keywords: Mating behavior, C. elegans, CaMKII, ether-a-go-go K+ channels, starvation, cholinergic signaling
Organisms commonly encounter varying levels of nutrient availability in their natural environment. Until the development of modern farming techniques, humans also did not have a stable food source and had to withstand periods of famine. Organisms developed mechanisms enabling them to respond to nutrient deprivation via down-regulating circuits not involved in food foraging behaviors while up-regulating those behaviors directly involved in food acquisition. For example, in C. elegans, this involves down-regulating male mating behavior, while in Drosophila, food acquisition behaviors are increased (Wu et al., 2005, Gruninger et al., 2006). In addition, restricting caloric intake has positive long-term effects and will extend lifespan in organisms from yeast to primates (Jiang et al., 2000, Lin et al., 2000, Colman et al., 2009). The nutrient-limited environment induces changes that increase the probability of survival.
Once the stress of food deprivation is lifted, an organism reverses the suppression of behaviors that would not be beneficial in a starved state. However, transient starvation, especially during development, has lasting consequences, from stunted growth to various brain abnormalities (Hulshoff Pol et al., 2000, Victora et al., 2008). Inadequate nutrient availability positively correlates with the development of schizophrenia, indicating that transient starvation results not only in morphological defects but has a lasting impact on behavior (Susser et al., 1996, Wahlbeck et al., 2001, Victora et al., 2008). Determining the mechanisms via which the lasting consequences of transient starvation are realized provides avenues for alleviation of these effects later in life.
The mechanisms activated by a period of starvation that persist once the organism is returned to food remain largely unknown. In an attempt to address this deficiency, we use the tractable model organism C. elegans. As in vertebrates and insects, food deprivation during adulthood extends lifespan regardless of when it is applied, indicating the organism is capable of responding to nutrient deprivation throughout its life (Mair et al., 2003, Dhahbi et al., 2004, Smith et al., 2008). Food deprivation during larval stages is also able to activate pathways that regulate adult lifespan (Lee and Ashrafi, 2008). In addition to extending life span, limited nutrient availability affects behavior, such as inhibiting hermaphrodite egg laying, increasing movement, and reducing male mating ability. This suggests that the excitability of some behavioral circuits is down regulated while others are up regulated in response to starvation (Croll, 1975, Horvitz et al., 1982, Trent et al., 1983, Sawin et al., 2000, Gruninger et al., 2008). Laboratory cultures of C. elegans are grown in nutrient-rich environments but C. elegans is often found starved in its natural environment (Barriere and Felix, 2005, Mohri et al., 2005). A period of transient starvation has been shown to impact behaviors, such as an increased ability to distinguish between odors and decreased male mate searching (Colbert and Bargmann, 1997, Lipton et al., 2004). The observed behavioral effects combined with the ease of molecular manipulation make C. elegans an ideal organism for studying the mechanisms used by transient starvation to influence behavioral response programs.
We used C. elegans male mating behavior to uncover the mechanisms responsible for the perduring effects of transient starvation. Successful mating requires the male to complete a series of stereotyped steps, starting with response to the hermaphrodite, then proceeding to vulva location, insertion of their copulatory spicules, and finally sperm transfer. Spicule insertion includes a series of sub-steps that result in vulva penetration. Protractor muscles attached to the base of the spicules undergo rapid, rhythmic contractions that result in the spicules prodding at the vulva until it is breached. Once the vulva is breached, the protractor muscles tonically contract, forcing the spicules into the vulva and allowing sperm transfer (Ward and Carrel, 1979, Sulston et al., 1980, Loer and Kenyon, 1993, Liu and Sternberg, 1995, Garcia et al., 2001). The ability to successfully impregnate a mate is reduced when males are food deprived, and spicule muscle response is reduced as well (Gruninger et al., 2008). Previous work established that starvation is able to attenuate spicule muscle spasms induced by the loss of the ether-a-go-go-related gene K+ channel unc-103 (Gruninger et al., 2006). In this study we report that increased expression of the ether-a-go-go K+ channel egl-2 in the male sex muscles, in response to a period of transient starvation, produces lasting consequences in the excitability of the male mating circuit. In addition, the activity of EGL-2 is promoted by the calcium/calmodulin-dependent protein kinase II (CaMKII) unc-43.
1. Experimental Procedures
1.1 Strains and culture methods
C. elegans strains used to obtain males for this study contained the allele him-5(e1490) on LGV (Hodgkin et al., 1979). The him-5(e1490) mutation results in a higher incidence of spontaneous males and allows one to obtain sufficient numbers of males in the hermaphroditic species. Additional alleles used were: unc-103(n1213) (Park and Horvitz, 1986), pha-1 (e2123) (Schnabel and Schnabel, 1990), unc-64 (e246) (Brenner, 1974) on LG III; unc-43(sy574) on LG IV (LeBoeuf et al., 2007); and fog-2(q71) (Schedl and Kimble, 1988), egl-2(rg4) (LeBoeuf et al., 2007), egl-2(n693) (Reiner et al., 1995), and egl-2(n693n904) on LG V (Weinshenker et al., 1999). The fog-2(q71) strain did not contain the him-5 mutation.
Animals were grown at 20°C on 5 cm NGM agar plates containing E. coli OP50 as the food source (Brenner, 1974). For behavioral assays, virgin males were isolated from non-crowded plates either at the late L4 stage (when cells in the male tail spike have completely migrated anteriorly) or after they newly crawled out of their L4 cuticle. They were kept solitary or in groups of 20 on 1–2 cm diameter lawns of bacteria. For experiments that required monitoring of behavior over multiple days, the males were transferred daily to new OP50-seeded NGM plates.
To starve males transiently, they were serially transferred to 3.5 cm E. coli-less NGM agar plates using a mouth pipette and water as a vehicle. To inhibit potential bacterial growth, the plates contained streptomycin at a final concentration of 30 µg/ml. An 8 M glycerol ring was applied to the edge of the agar to discourage males from crawling and desiccating on the sides of the plates. After the starvation period, males were transferred using a worm pick to NGM plates containing E. coli.
To assay the effects of translational inhibitors on male growth and behavior, cycloheximide (Sigma-Aldrich, St. Louis, MO) freshly dissolved in water at a concentration of 20 mg/ml was added to the surface of NGM plates with or without E. coli. The drug was allowed to soak into the plate overnight. The final concentration of cycloheximide in the plates was 250 µg/ml. This concentration was either lethal to the larva or stalls larval growth. We determined if the concentration of cycloheximide was effective in inhibiting protein translation in adult males by measuring how well the antibiotic interfered with the expression of a heat shock-induced transgene. syIs38 transgenic males contain a constitutively active allele of the Gαq G-protein controlled by the hsp-16 heat shock promoter (Bastiani et al., 2003). The behavioral effects of this transgene are rapidly potent, and all males in the presence or absence of food will artificially protrude their spicules in less than an hour after a 30 minute heat pulse at 30° C (n=10 animals for both conditions). When cycloheximide was present, males did not display the effects of the induced transgene well after two hours post induction (n=10 animals for both conditions). However, ~20 hr later, all the antibiotic-treated animals displayed the effects of the transgene; at 250 µg/ml, cycloheximide either breaks down, or small amounts translation can occur over time. To ensure that the effects of cycloheximide were due to its inhibition of protein translation, we also used puromycin (Sigma-Aldrich) freshly dissolved in water at a concentration of 40 mg/ml and added it to plates with or without E. coli to obtain a final concentration of 128 µg/ml. Puromycin is a compound that causes premature termination of the elongating polypeptide during protein translation. Like cycloheximide, puromycin prevented larval growth. Males responded similarly to puromycin as they did to cycloheximide. 8% of wild-type males protracted their spicules when they were fed for 18 hrs in the presence of puromycin (n=36). 72% of unc-103(0) fed 18 hrs males protracted their spicules on puromycin, indicating protein synthesis was necessary to compensate for the loss of unc-103 (n=72, p value = 0.0001 compared to unc-103(0) fed. 3% of unc-103(0) males starved for 18 hrs on puromycin protracted their spicules, which was similar to the 5% of unc-103(0) males starved for 18 hrs without puromycin (n=35 and 169, respectively). 32% of unc-103(0) males that had been starved for 18 hrs on puromycin and then fed for 7 hrs on puromycin protract their spicules (n=35), which was significantly higher than the 0% of unc-103(0) males that protracted their spicules after having been starved for 18 hrs and then fed for 7 hrs (n=20, p value = 0.004, Fisher’s Exact Test).
1.2 Statistical tests performed
GraphPad InStat v.3.06 and GraphPad Prism 5 software were used to perform statistics on all data. Fisher’s Exact Test was used when comparing two or more experiments where the outcome was a categorical variable (such as non-protracted spicules vs. protracted spicules (Table 1), expression of the marker gene vs. no expression (Figure 2A), sensitivity to the ACh agonist vs. resistance to the ACh agonist (Table 3) and siring progeny vs. siring no progeny (Figure 4)). Unpaired t test was used to compare the means of experimental replicates from two groups (Figure 3).
Table 1.
Effects of starvation and translation on the unc-103(0) phenotype.
| Genotype | Treatment | % spicule protract ed (n) |
p value Fisher’s exact test |
|---|---|---|---|
| wild type | fed 18 hrs | 10 (30) | |
| unc-103(0) | fed | 30 (439) | 0.02 vs wild type fed |
| unc-103(0) | starved | 5 (169) | 0.2 vs wild type fed |
| unc-103(0) | starved 18 hrs; fed 7 hrs | 0 (20) | 0.0016 vs unc-103(0) fed |
| unc-103(0) | starved 1 hr; fed 18 hrs | 42 (106) | 0.08 vs unc-103(0) fed |
| unc-103(0) | starved 2 hrs; fed 18 hrs | 19 (89) | 0.008 vs unc-103(0) fed |
| unc-103(0) | starved 3 hrs; fed 18hrs | 10 (59) | 0.0005 vs unc-103(0) fed |
| wild type | fed 18 hrs + 250 µg/ml cycloheximide | 3 (30) | 0.0005 vs unc-103(0) fed |
| unc-103(0) | fed 18 hrs + 250 µg/ml cycloheximide | 63 (129) | 0.0001 vs unc-103(0) fed |
| unc-103(0) | starved 18 hrs + 250 µg/ml cycloheximide | 12 (81) | 0.0006 vs unc-103(0) fed |
| unc-103(0) | starved 18 hrs + 250 µg/ml cycloheximide; fed 7hrs + 250 µg/ml cycloheximide | 34 (29) | 0.68 vs unc-103(0) fed |
| unc-103(0) | starved 18 hrs+ 250 µg/ml cycloheximide; fed7 hrs | 21 (42) | 0.28 vs unc-103(0) fed |
| unc-103(0) | starved 18 hrs; fed 7 hrs + 250 µg/ml cycloheximide plates | 0 (25) | <0.0001 vs unc-103(0) fed |
| egl-2(0) | fed | 10 (49) | 1 vs wild type |
| egl-2(0) | starved | 3 (110) | 0.1 vs egl-2(0) fed |
| egl-2(0) | starved 18 hrs; fed 7 hrs | 2 (46) | 0.2 vs egl-2(0) fed |
| egl-2(0) | fed 18 hrs + 250 µg/ml cycloheximide | 11 (44) | 1 vs egl-2(0) fed |
| egl-2(0) | starved 18 hrs + 250 µg/ml cycloheximide | 6 (90) | 0.3 vs egl-2(0) fed + cycloheximide |
| egl-2(0) | starved 18 hrs + 250 µg/ml cycloheximide; fed 7 hrs + 250 µg/ml cycloheximide | 15 (39) | 0.04 vs egl-2(0) starved 18 hrs; fed 7 hrs |
| egl-2(0) | starved 18 hrs + 250 µg/ml cycloheximide; fed 7 hrs | 13 (48) | 0.1 vs egl-2(0) starved 18 hrs; fed 7 hrs |
| egl-2(0) | starved 18 hrs; fed 7 hrs + 250 µg/ml cycloheximide plates | 6 (54) | 0.6 vs egl-2(0) starved 18 hrs; fed 7 hrs |
| unc-103(0); egl-2(0) | fed | 55 (272) | <0.0001 vs unc-103(0) |
| unc-103(0); egl-2(0) | starved | 37 (65) | 0.01 vs unc-103(0); egl-2(0) fed |
| unc-103(0); egl-2(0) | starved 18 hrs; fed 7 hrs | 33 (36) | 0.02 vs unc-103(0); egl-2(0) fed |
| unc-103(0); egl-2(0) | fed 18 hrs + 250 µg/ml cycloheximide | 84 (63) | <0.0001 vs unc-103(0); egl-2(0) fed |
| unc-103(0); egl-2(0) | starved 18 hrs + 250 µg/ml cycloheximide | 78 (116) | <0.0001 vs unc-103(0); egl-2(0) starved |
| unc-103(0); egl-2(0) | starved 18 hrs + 250 µg/ml cycloheximide; fed 7hrs + 250 µg/ml cycloheximide | 74 (38) | 0.04 vs unc-103(0); egl-2(0) fed |
| unc-103(0); egl-2(0) | starved 18 hrs + 250 µg/ml cycloheximide; fed 7 hrs | 86 (44) | <0.0001 vs unc-103(0); egl-2(0) fed |
| unc-103(0); egl-2(0) | starved 18 hrs; fed 7 hrs + 250 µg/ml cycloheximide plates | 45 (29) | 0.3 vs unc-103(0); egl-2(0) fed |
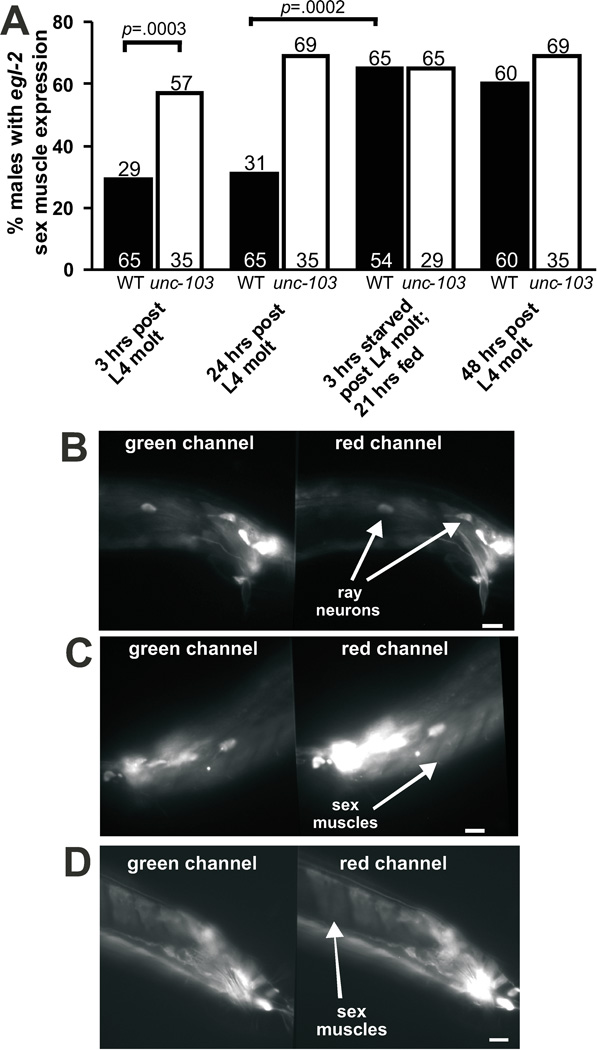
Figure 2.
egl-2 promoter expression in sex muscles increases after transient starvation. (A) Percentage of males with visible DsRed1-E5 fluorescent protein expression in the male sex muscles. The black and white bars represent the percentage of wild-type (WT) and unc-103(0) males that express DsRed1-E5 in their sex muscles, respectively. The values within and above the bars correspond to the number of males assayed and the percentage values, respectively. Fisher’s exact test was used to calculate statistical significance. (B)–(D) Fluorescence images of wild-type adult male tails. The left and right panels show fluorescence in the green and red channels, respectively. Scale bars depict 10 micrometers. (B) 24 hr adult male tail. egl-2 promoter expression of DsRed1-E5 is seen mainly in the male ray neurons. (C) 48 hr adult male tail. egl-2 promoter expression of the DsRed1-E5 can now be seen in male sex muscles in addition to the ray neurons. (D) 24 hr adult male tail where the male was starved for three hours after L4 molt. egl-2 promoter expression of DsRed1-E5 can be seen in male sex muscles.
Table 3.
Mutating potential CaMKII sites on EAG K+ channel EGL-2 reduces response to transient starvation.
| % spicule protracted (n)a | ||
|---|---|---|
| Genotype | Fed | Starved |
| Wild type | 94 (50) | 75 (71)c |
| egl-2(0) | 100 (38) | 91 (55) |
| egl-2(0); rgEx398[Phsp-16∷egl-2(gf)]b | 75 (52) | 42 (36)c |
| egl-2(0); rgEx390[Phsp-16∷egl-2(gf,-CaMKII sites)]b | 100 (43) | 79 (38)c,d |
Protraction after 5 min in 1 mM ARE. All males were heat-shocked for 30 min @ 33°C on food and allowed to recover for 1 hrs on food prior to drug testing.
Three independently obtained transmitting lines were scored for each construct. One representative line is shown.
p value < 0.05 compared to Fed of the same genotype, Fisher’s Exact Test.
p value = 0.0002 to rgEx398[Phsp-16∷egl-2(gf)] starved, Fisher’s Exact Test.
Figure 4.
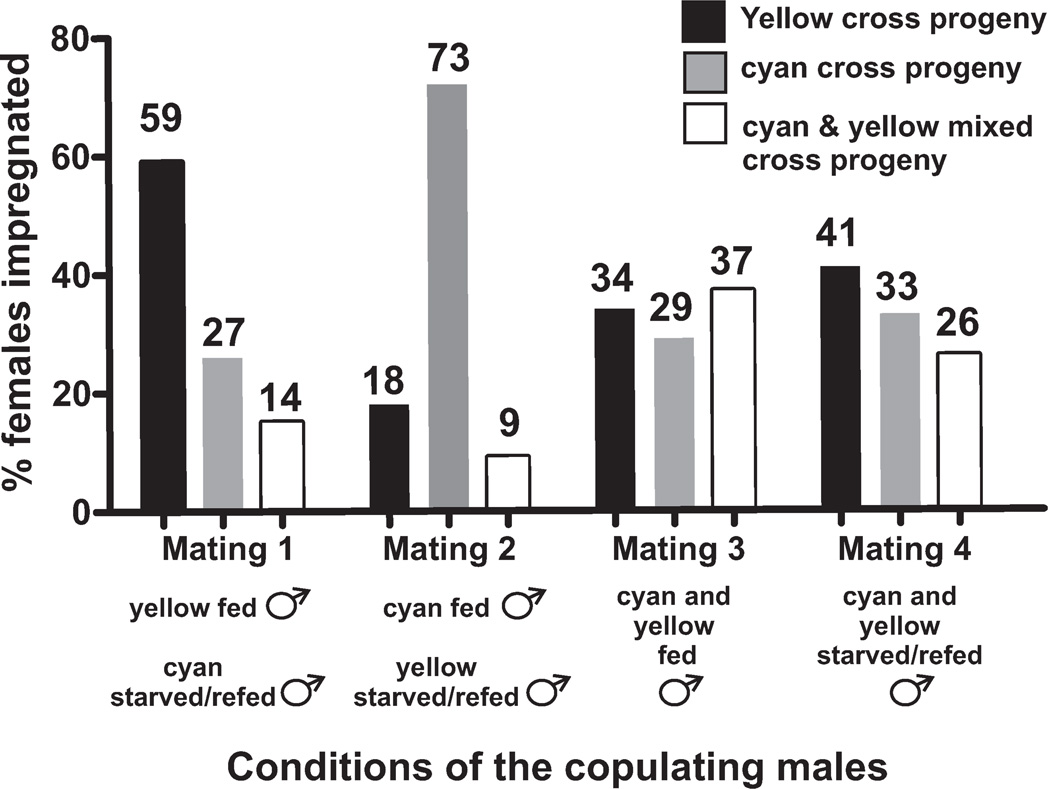
Competition mating assay. The y axis indicates the percentage of fog-2 females impregnated by a well-fed male, a starved/re-fed male or both. The x axis indicates whether the males tested were one-day-old males fed for 23 hrs or one-day-old males that were starved for 20 hrs and then re-fed for 3 hrs. Black bars represent females that produced cross progeny only containing a yellow fluorescent protein (YFP) transgenic marker, grey bars represent females that produced cross progeny only containing a cyan fluorescent protein (CFP) transgenic marker, and white bars represent females that produced mixed cross progeny that contain either markers. Numbers at the top of the bars are the measured percentages. Mating competitions 1, 2, 3 and 4 involved 34, 33, 62, and 66 females, respectively.
Figure 3.
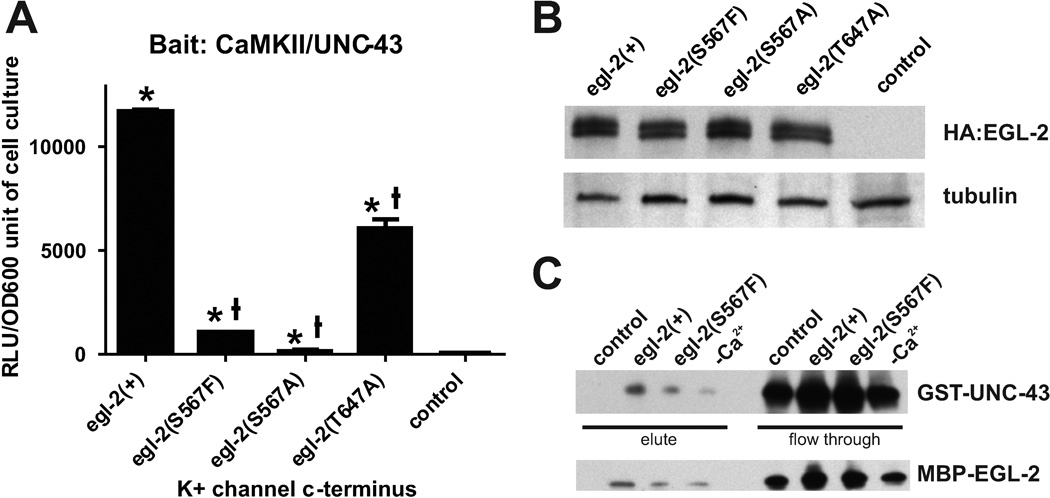
Direct interaction between CaMKII/UNC-43 and the EAG K+ channel EGL-2. (A) Protein interaction in a yeast two-hybrid assay. The x axis lists the EGL-2 constructs with amino acid substitutions at the c-terminus and the y axis depicts the amount of real light units (RLU) given off per unit of cell culture. The control column depicts the amount of RLU released from wild-type yeast cells that do not contain either EGL-2 or UNC-43 sequences. An * above the column indicates a p value < 0.005 compared to the control, while a † above the column indicates a p value < 0.005 compared to egl-2(+). p values were determined by the unpaired t test. (B) Western blot assaying for the presence of HA-tagged EGL-2 in yeast cells. α-tubulin was used as a loading control. (C) Western blots assaying for the presence of UNC-43-GST bound to amylose resin-associated EGL-2 –MBP (top panel) and for the presence of EGL-2-MBP bound to glutathione-associated UNC-43-GST (bottom panel).
1.3 Mutation and drug-induced spicule protraction
To score spontaneous spicule protraction, L4 males in groups of 20 or less animals were kept on NGM agar plates containing or lacking food. 18 to 24 hrs later, using a dissecting stereomicroscope, we counted the number of males with one or both spicules extending from their cloaca. We disposed of the spicule-protracted males and transferred the non-spicule protracted animals to fresh plates for rescoring 18 to 24 hrs later.
To assay agonist-induced spicule protraction, we dissolved the acetylcholine agonist arecoline (Indofine Chemical Company, Hillsborough, NJ) in water to make a stock solution of 1 M. We then serially diluted the stock solution in water as needed. We added 0.5 ml of the drug to a three well round-bottom Pyrex titer dish. Five to ten males were then transferred to the drug bath. The males were observed for five minutes at 20°C with a stereomicroscope; they were considered responsive to the drug if their spicules remained protracted for ≥ 10 seconds. Drug baths were changed after 30 males were observed.
1.4 Identification of the egl-2(n693n904) lesion
Weinshenker et al (1999) identified egl-2(n693n904) but did not report the molecular lesion. We sequenced the egl-2 coding region from egl-2(n693n904) genomic DNA and found that the n904 mutation changes GCATCTGAC to GCATTTGAC.
1.5 Yeast two-hybrid assay
The plasmids used in the yeast two-hybrid assay were constructed as follows. To generate a yeast expression vector containing the catalytic and regulatory domains of unc-43 tagged with a c-Myc epitope tag, unc-43 was amplified from pBL33 (LeBoeuf et al., 2007) using primers func43iecor1 and unc43iecor1r. The PCR product was cut with EcoRI and ligated to EcoRI-digested pGBKT7 (Clontech Laboratories, Inc.) to create plasmid pBL81. The UNC-43 kinase domain was inactivated by a D135N mutation via single site mutagenesis on pBL81 using primers FUnc43inact and Unc43inactr to prevent the kinase from killing the yeast host (Rosenberg et al., 2005), thus creating the plasmid pBL85. Single site mutagenesis with primers Unc43irev and ForPGBKT7 was used to remove the unc-43 autoinhibitory domain from pBL85 to create pBL88. To generate a yeast expression vector containing the egl-2 c-terminus tagged with a hemagglutinin (HA) epitope tag, egl-2 was amplified from pTG44 (LeBoeuf et al., 2007) using primers fegl2cterm and egl2cterm2r, cut with SacI and EcoRI and ligated to SacI/EcoRI-digested pGADT7 (Clontech Laboratories, Inc.) to create plasmid pBL93. To introduce the S567F point mutation, single site mutagenesis of pBL93, using primers Fegl2n904 and Egl2n904r, was used to create the plasmid pBL112. To create the S567A point mutation, primers Fegl2S567A and Egl2n904r were used to perform a single site mutagenesis of pBL93, creating plasmid pBL188. The T647A mutation was generated using primers Fegl2T647A and egl2T647GR to perform a single site mutagenesis of pBL93, creating plasmid pBL189.
The yeast two-hybrid assay was performed as described in Matchmaker GAL4 Two-Hybrid System 3 & Libraries User Manual (PT3247-1, Clontech Laboratories, Inc., Mountain View, CA). Briefly, pBL88 was co-transfected with either pBL93, pBL112, pBL188, or pBL189 into Y187 yeast cells and plated on -Leu/-Trp minimal media plates to select for the presence of the plasmids. Detection of protein interaction was performed using the Galacton-Star reaction kit to test for the presence of β-galactosidase activity as described in the Yeast Protocols Handbook (PT3024-1, Clontech Laboratories, Inc.). Chemiluminesence produced by the β-galactosidase cleavage of the Galacton-Star reagent was read by the TopCount Microplate Scintillation Counter (Packard). Three or more replicates were done for each interaction. Unpaired t tests were performed using GraphPad Prism software.
To detect the presence of the HA-tagged EGL-2 proteins, yeast containing the proteins were grown on -Leu/-Trp minimal media plates at 30°C for three days. The yeast cells were then collected with 2 mL of phosphate buffered saline. The yeast cells were spun down and resuspended in 500 µl 1× laemmli buffer, after which they were boiled for 5 min to release the proteins. The boiled yeast cells were then loaded and separated on an SDS-PAGE gel and transferred to a PVDF membrane. HA-tagged EGL-2 was detected using an anti-HA antibody (Roche). α-tubulin (Novus Biologicals, Littleton, CO) was used as a loading control.
1.6 Protein interaction
The plasmids used to generate tagged proteins were constructed as follows. egl-2 was PCR amplified from pBL93 using primers fegl2cterm and egl2hind3r, cut with EcoRI and HindIII, and ligated into pMalC2 (New England Biolabs, Ipswich, MA) cut with the same enzymes to create the plasmid pBL99. The S567F point mutation was introduced via single site mutagenesis on pBL99 using primers Fegl2n904 and Egl2n904r to create plasmid pBL114. pGEX-3T was cut with SmaI and Gateway Vector Conversion Reading Frame Cassette C.1 (RfC.1) (Invitrogen, Carlsbad, CA) was ligated to the plasmid, creating pBL117. pBL117 and pBL54 (LeBoeuf et al., 2007) were recombined using LR clonase to generate plasmid pBL120, a plasmid that contains full-length unc-43 cDNA attached to GST. To removed the unc-43 self-association domain, single site mutagenesis was performed on pBL120 using primers Fpbl333utr and Rpbl33stop (LeBoeuf et al., 2007), to create plasmid pBL123.
After plasmid generation, GenScript (Piscataway, NJ) generated proteins from plasmids pBL99, pBL114, and pBL123. 1 mg of UNC-43-GST was used for each reaction. 1.46 mg of EGL-2(+)-MBP and 1.38 mg of EGL-2(S567F)-MBP were used. UNC-43 is inactive in the absence of Ca2+, calmodulin, and ATP. 2 mM CaCl2, 1.2 µM Calmodulin, and 200 µM ATP were used in 1× CaMKII Reaction Buffer (New England Biolabs) to create a final volume of 100 µl. To allow time for UNC-43 and EGL-2 to interact, the reaction mixture was incubated at 20°C overnight. To purify UNC-43-GST or EGL-2-MBP, 50 µl of the reaction mixture was combined with 50 µl of glutathione resin (Sigma-Aldrich) or 50 µl of amylose resin (New England Biolabs) and incubated at 4°C for 1 hr with rotation. The resin was collected by centrifugation at 500 rpm for 2 min at 4°C. The resin was washed 3× with 150 µl column buffer (20 mM Tris-HCl, pH 7.4, 200 mM NaCl, 1 mM EDTA) via incubation at 4°C with rotation for 5 min and centrifuged. The flow-through was collected and concentrated to a final volume of 50 µl using Vivaspin 500 columns (Sartorius Stedim Biotech, Aubagne, France). Next, 50 µl of 100 mM glutathione (Sigma-Aldrich) or 50 µl of 10 mM maltose (Sigma-Aldrich) was added to the resin and the mixture was incubated at 4°C for 1 hr with rotation. The resin was collected and the supernatant removed. 5 µl of 10× Laemmli buffer was added to the collected protein samples and boiled for 1 min. The samples were separated on a SDS-PAGE gel and transferred to PVDF membrane using a mini Trans-Blot electrophoretic transfer cell (Bio-Rad). Anti-GST (Abgent, San Diego, CA) and anti-MBP (New England Biolabs) antibodies were used to detect UNC-43-GST and EGL-2-MBP.
1.7 Construction of DsRed1-E5 fluorescent protein expressed from the egl-2 promoter
The DsRed1-E5 is a DsRed variant that shifts its excitation/emission spectra from 483/500nm (green) to 558/583 nm (red) over time (Terskikh et al., 2000). A plasmid encoding DsRed1-E5 was purchased from Clontech under the product name pTimer. The DsRed1-E5 was removed from pTimer using XbaI. The gene was then cloned into the NheI site of the plasmid pTG24, a pBR322 based vector that contains multi-cloning sites, a synthetic intron and the unc-54 3’ UTR (LeBoeuf et al., 2007). The resulting construct was then cut with XbaI, blunt-ended and then ligated with the Gateway RfC.1 cassette (Invitrogen) to generate the Gateway destination vector pLR186. Using LR clonase (Invitrogen), this plasmid was then recombined with pLR11, which contains the 8.5 kb immediately upstream of the egl-2 coding region (LeBoeuf et al., 2007), to generate the plasmid pLR193. Injection mixtures of 50 ng/µl pLR193, 50ng/µl of the pha-1 rescuing plasmid pBX1 and 100 ng/µl of pUC18 was then injected into pha-1; him-5 hermaphrodites. pBX1 contains the wild-type pha-1 gene and is used as a marker to select for worms expressing the transgene (Granato et al., 1994).
1.8 Construction of egl-2 cDNA constructs expressed from the hsp-16 promoter
Separate plasmids containing the egl-2 cDNA and hsp-16 promoter were previously constructed (LeBoeuf et al., 2007). The egl-2 cDNA splice isoform in this paper differs from the previously reported isoform in two ways (Weinshenker et al., 1999): 3 amino acids, IKV, are added between TSSNFNQV and MNLGGDML, and 8 amino acids, LRPFYLIS, are removed from between FNIAFKNS and SRENPGGG. The numbers used to designate amino acids in this paper correspond to a protein that includes the above modifications. The egl-2(n693gf) point mutation was introduced via single site mutagenesis using primers fegl2n698gf and Regl2698gf. The plasmid containing the egl-2(gf) cDNA with several canonical CaMKII sites removed (designated egl-2(gf,-CaMKII sites) was constructed by inducing the various mutations using sequential single site mutagenesis with the following primers: Fegl2n904 and Egl2n904r for the S567G change, Fegl2T647G and egl2T647GR for the T647G change, Fegl2S811G and egl2S811GR for the S811G change, and fEgl2end and egl2rsvsr to remove the last 60 amino acids. The plasmids containing the egl-2(gf) and egl-2(gf,-CaMKII sites) cDNA also include the Gateway RfC.1 cassette, allowing them to be recombined with the hsp-16 promoter containing plasmid pBL60, using LR clonase to create plasmids pBL174 and pBL175, respectively (LeBoeuf et al., 2007). pBL174 and pBL175 were inject into egl-2(rg4) hermaphrodites at a concentration of 25 ng/µl, along with 50 ng/µl of pBL66 and 100 ng/µl of pUC18. pBL66 contains the Pgtl-1∷CFP construct which drives CFP expression in the intestine and serves as a marker for worms carrying a transgene (LeBoeuf et al., 2007). The functionality of pBL174 and pBL175 was determined via heat shocking hermaphrodites in a 33°C water bath for 30 min. Heat-shock expressed egl-2 transcript levels were monitored via quantitative real time PCR, see below. Hermaphrodites were observed every 30 min following heat shock for paralysis (n=29 for pBL174 and n=30 for pBL175). After 30 min, 60 min, and 90 min, no hermaphrodites carrying either pBL174 or pBL175 displayed paralysis. However, 120 min post-heat shock, 100% of pBL174 hermaphrodites and 40% of pBL175 hermaphrodites displayed complete paralysis. At 150 min post heat-shock, 87% of pBL175 hermaphrodites were paralyzed, and at 180 min 100% of pBL175 hermaphrodites were paralyzed. Thus, both constructs produced functional proteins. Males, regardless of their genotype, were heat-shocked in the following manner: L4 males were separated from hermaphrodites and kept on plates with E. coli (Fed) or without E. coli (Starved) for ~18 hours. The next day, the adult males were transferred to plates with food and were heat shocked at 33°C for 30 min, after which they were kept on food at 20°C for 1 hrs. The males were then exposed to 1 mM ARE for 5 min, and the number that maintain spicule protraction for at least 10 sec was recorded. Three independently obtained transmitting lines were analyzed for each transgenic construct. The results reported in Table 3 are from transmitting line 1 for both pBL174 and pBL175. The results for two additional transmitting lines for each construct are as follows: pBL174 TL2 fed 57% Prc (n=30), starved 33% Prc (n=30), p value = 0.1; pBL174 TL3 fed 41% Prc (n=32), starved 8% Prc (n=36), p value = 0.003; pBL175 TL2 fed 98% Prc (n=42), starved 90% Prc (n=41), p value = 0.2; pBL175 TL3 fed 87% Prc (n=30), starved 84% Prc (n=32), p value = 1. p values determined using Fisher’s Exact Test.
1.9 Real-Time Quantitative PCR Analysis of heat shock egl-2
L4 males containing pBL174 and pBL175 (500 and 398 males, respectively) were separated from hermaphrodites. The next day they were heat shocked for 30 min at 33°C. They were collected 1 hrs after heat shock. 250 µl of Tri Reagent (Sigma) and 0.5 mm zirconium oxide beads (Next Advance, Cambridge, MA) were added to the worms. They were broken open to release RNA using the Bullet Blender (Next Advance) mechanical agitator. The worms were stored at −80°C until RNA extraction.
After total RNA was extracted using the Tri Reagent solution, RNA samples were treated with Turbo DNA free kit (Ambion, Austin, TX) to eliminate genomic DNA. The first strand of cDNA was synthesized using SuperScript First-Strand kit (Invitrogen). Real time PCR reaction was performed on the ABI 7500 fast real time PCR machine (Applied Biosystems, Foster City, CA). cDNA was diluted to 1:1000 and 1:2000 for each transgenic line. pBL174 cDNA was diluted from 1:100, 1:500, 1:1000 and 1:5000 and served as a standard curve template, with arbitrary gene copy number assigned as 50, 10, 5 and 1 respectively. 5 µl of template was added to each well. Final concentration for the primer was 0.6 µM. 7.5 µl of power SYBR Green (Invitrogen) was added to each well. Actin-1 was amplified using primers qPCRact-1F and qPCRacy-1R as an endogenous control to normalize the cDNA of the samples. The normalized expression level is the number of copies of egl-2 normalized to the number of copies of actin for each transgene. The relative quantity was calculated using the CT method. There was 0.9 copy of pBL174 transcript for every 1 copy of pBL175 transcript (n=6 for each transgene, p value = 0.03, unpaired t test).
1.10 Determining EGL-2 protein levels in C. elegans transgenic lines
Worms for each strain tested were grown on 12 3.5 cm NGM plates seeded with E. coli OP50. When plates were packed with worms but prior to starvation, they were heat shocked for 30 min at 33°C and allowed to recover for 1 1/2 hrs. This was repeated 2×. After the final recovery period, worms were collected using water. The worms were allowed to settle, then as much water was possible was removed. 200 µl of laemelli buffer and 0.5 mm zirconium oxide beads (Next Advance) were added to each worm sample. Worms were lysed using the Bullet Blender (Next Advance) for 3 min at speed 8. Protein samples were then boiled for 5 min and spun down for 10 min. EGL-2 protein was detected using an antibody to rat EAG, KCNH1 (Novus Biologicals, Littleton, CO). However, protein levels were too faint to document for publication.
1.11 Mating competition
Males were transgenically marked with either yellow fluorescent protein or cyan fluorescent protein expressed from the tph-1 promoter. The tph-1 promoter expresses in neurons that secrete serotonin as a neurotransmitter (Sze et al., 2000). These markers were made for other purposes but were used in the experiments out of convenience. The tph-1 promoter was PCR amplified with the following primers: attb1tph1 and attb2tph1. The primers contain Gateway ATTB sites that allowed the PCR product to be recombined using BP clonase (Invitrogen) into pDONR221 to generate pTG9. pTG9 was then subsequently recombined into the CFP-containing destination vector pGW77C and the YFP destination vector pGW322YFP using LR clonase (Invitrogen) to make pTG10 and pTG11, respectively (Gruninger et al., 2006, Reiner et al., 2006). To obtain heritable transgenic lines, injection mixtures containing pTG10 (100 ng/µl pTG10, 50 ng/µl pBX1, 50 ng/µl pUC18) and pTG11 (100 ng/µl pTG10, 50 ng/µl pBX1, 50 ng/µl pUC18) were separately injected into the germ line of adult pha-1(e2123); him-5(e1490) hermaphrodites.
To conduct the competition assay, mid to late L4 fog-2(q71) females were separated from males (fog-2(q71) XX animals are considered females because they do not produce self-sperm). Twenty hours later, single females were added to 5 mm diameter lawns of bacteria. One 18–20 hr fed transgenic virgin male and one 18–20 hr starved-three hr re-fed male were added simultaneously to the lawn. The plates were incubated at 20° C for one hour, and then were inspected with a high-powered Stemi SV11 stereomicroscope (Zeiss, Thornwood, NY). If the female had sperm in the spermatheca, then both males were removed, otherwise the animals were allowed to mate for another hour. Majority of the females were mated within 1 hour; by 2 or 3 hours, all females were pregnant. The next day, we determined if the serotonergic neurons of L1 cross progeny fluoresced yellow or cyan.
To ensure that the yellow and cyan transgenic markers used to identify males did not affect the outcome of the competition assay, control experiments were performed. In one experiment, one 23 hr fed YFP-expressing male was placed with one 23 hr fed CFP-expressing male. 62 females were tested. 34% of females were impregnated by YFP-expressing males, 29% of females were impregnated by CFP-expressing males, and 37% of females were impregnated by both. In another experiment, one 20 hr starved/3 hr re-fed YFP-expressing male was placed with one 20 hr starved/3 hr re-fed CFP-expressing male. 62 females were tested. 41% of males expressing YFP sired progeny, while 33% of males expressing CFP sired progeny. 26% of females were impregnated by both YFP- and CFP-expressing males. These control experiments indicated that the transgenic marker used to identify males and their progeny does not influence the outcome of the competition assay.
1.12 Primer designations
func43iecor1: 5’- GCGCGAATTCATGATGAACGCAAGCACCAAGTTTAGT
unc43iecor1r: 5’- GCGCGAATTCTCACTCGTTACCTTTTTCCGAATCGTTG
FUnc43inact: 5’- CGGTATTGTTCACAGAAACTTGAAGCCAG
Unc43inactr: 5’- TTAGAGTGGCAATAAGCAATCGATTCGAG
Unc43irev: 5’- AATCCATGGGACTTTCAAGGCCTGATC
ForPGBKT7: 5’- GAATTCCCGGGGATCCGTCGAC
fegl2cterm: 5’- CGCGGAATTCCAACAAATGACATCCAGTACTGTGAGATATCA
egl2cterm2r: 5’- CGCGCGATCGTCATATCCGTGTTCTTGTCGGAGGAC
Fegl2n904: 5’- GTTGTTTTCGTCTTGCAGGTGACGGGTGT
Egl2n904R: 5’- TGTGCTCATTGAAAACCTTTCGATTCAAGTGAAC
egl2hind3r: 5’- CGCGAAGCTTTCATATCCGTGTTCTTGTCGGAGGAC
fegl2n698gf: 5’- CTCCGCACTTTTGTATGTTGCAATTTTTGGAC
Regl2698gf: 5’- ATGATCATCATGCAGACTCCGAATATCTTCTC
fEgl2end: 5’- CGTCCTCCGGCAAGAACACGGATATGA
egl2rsvsr: 5’- TGCGACAGGCGCCGCGTCAAGT
Fegl2T647G: 5’- CGAATGTTCGGGCTCTCGGGTACTCTGAT
egl2T647GR: 5’- CCGCAGACTGTCCAGTCGATCCGTTC
Fegl2S811G: 5’- GGATAAGAAAGATCGTGAATGGGGTTCACTTTCCAATATC
egl2S811GR: 5’- ATTCCCCAACTTGTACGGGATAGTGCGTC
Fegl2S567A: 5’- GTTGTTTTCGTCTTGCAGCTGACGGGTGT
Fegl2T647A: 5’- CGAATGTTCGGGCTCTCGCGTACTCTGAT
attb1tph1: 5’-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGGTTTTCACACAGCTTTGGGTATTC
attb2tph1: 5’- GGGACCACTTTGTACAAGAAAGCTGGGTCTGCGGATGCCATCTGAAACAACG
2qPCRegl-2f: 5’- GAGTAAACCGAAGAGACCTCC
2qPCRegl-2R: 5’- TCCATTCCCCAACTTGTACG
qPCRact-1F: 5’- TCCGTAAGGACTTGTACGCC
qPCRacy-1R: 5’- ATCCAGACGGAGTACTTGCG
2. Results
2.1 Transient starvation stably reduces unc-103(0)-induced seizures
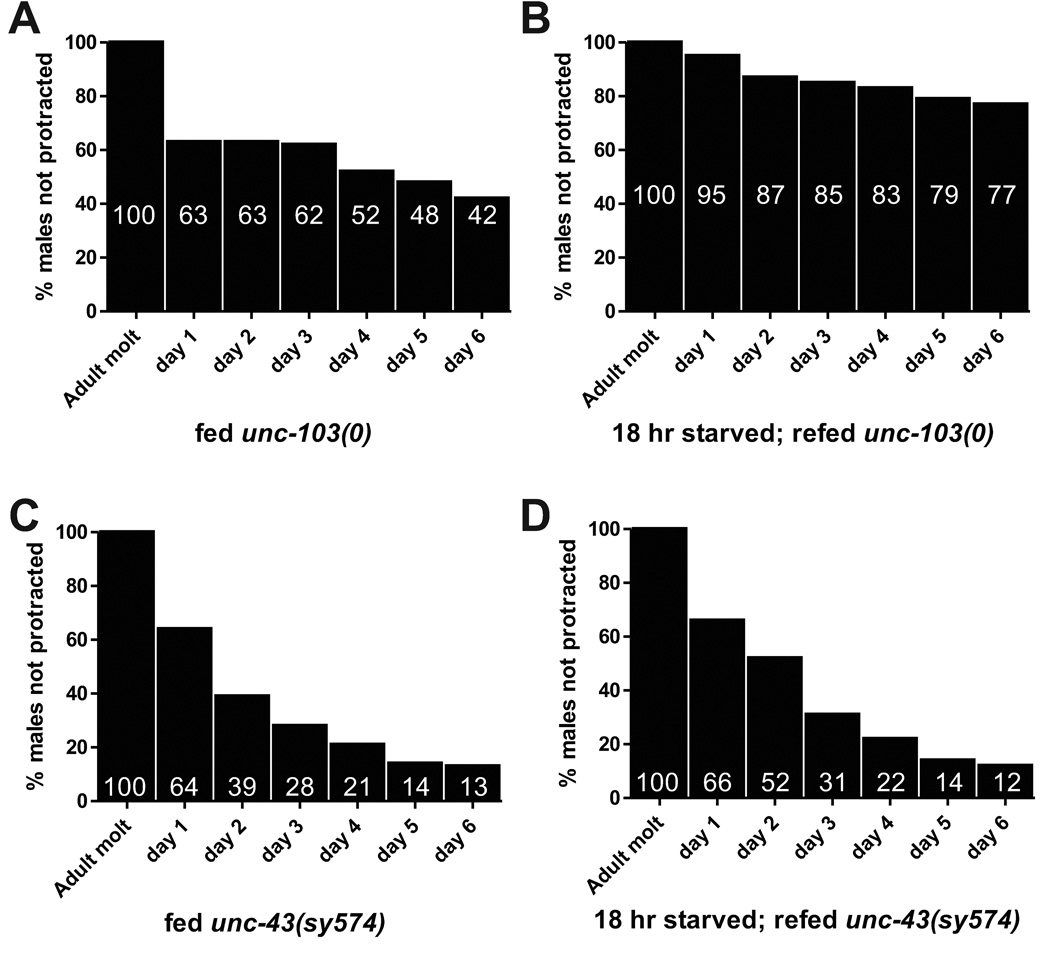
C. elegans males lacking the unc-103-encoded ether-a-go-go related gene K+ channel display sex muscle spasms, which can be measured by the number of one-day-old virgin males that spontaneously protract their copulatory spicules (Garcia and Sternberg, 2003). 30% of unc-103(0) males display this protraction constitutive (Prc) phenotype, which can be reduced via starvation (p value <0.0001, Fisher’s Exact Test) (Table 1) (Gruninger et al., 2006). Here, we asked if this suppression is maintained after the males are returned to food. We initially hypothesized that once the stress of starvation was alleviated, starvation-induced pathways would be turned off, and the mutant males would once again display the Prc phenotype. To test this hypothesis, we starved unc-103(0) males for 18 hrs and then re-fed them for 7 hrs. Contrary to our expectations, we found that transient starvation had lasting effects on the males, as none of them display the Prc phenotype (0%, n=20) (Table 1). In addition, 18 to 20 hours of starvation was not necessary to maintain suppression of the unc-103(0) Prc phenotype after re-feeding. We starved males immediately after they matured into adults for a period of 1 hr, 2 hrs and 3 hrs, and then returned them to food for 18 hrs before scoring the percentage of males that spontaneously protracted their spicules. Starving the newly adult males for 1 hr before re-feeding them had no effect on the instance of the Prc phenotype (42%, n=106); however, we found that only 2 hrs of starvation were required to reduce the unc-103(0)-induced Prc phenotype (19%, n=89) (Table 1). This suppression is maintained when the males are starved for 3 hr and then returned to food, as only 10% of males so treated display the mutant phenotype (n=59), a percentage that is similar to the 5% of males that protract their spicules if starved overnight and not re-fed (n=169, p value = 0.2 vs unc-103(0) starved 3 hrs; fed 18 hrs) (Table 1). Thus, we conclude that transient starvation has lasting effects on cell excitability.
Next, we asked how long the effects of transient starvation persisted. We first determined when the majority of fed unc-103(0) males protract their spicules. A population of L4 males was isolated from hermaphrodites and allowed to mature to adulthood overnight, and then the percentages of Prc males were determined every day over a period of six days. The majority of males protract their spicules within 24 hrs, indicating the presence of compensatory mechanisms that maintain the appropriate levels of sex muscle excitability as the male ages (Figure 1A). To determine how long the effects of transient starvation lasted, we starved unc-103(0) males for 18 hr as they mature into adults and then returned them to food, recording the instance of the Prc phenotype every day for 6 days. We found that the starvation-induced suppression of the unc-103(0)-induced Prc phenotype is maintained over time, as 77% of unc-103(0) males did not protract their spicules on day 6 after being starved, compared to 42% of their non-starved counterparts (p value < 0.0001, Fisher’s Exact Test) (Figure 1B). Thus for unc-103(0) males, food deprivation during the first few hours of adulthood induced long-lasting changes in the cell excitability of the mating circuit, such that when animals are returned to conditions of food abundance, UNC-103 is not essential to regulate muscle physiology.
Figure 1.
Changes in the frequency of mutation-induced spicule protraction as the males age. (A) Fed unc-103(0) males. Starting population was 141 males. (B) 18–20 hr starved/re-fed unc-103(0) males. Starting population was 103 males. (C) Fed unc-43(sy574) males. Starting population was 148 males. (D) 18–20 hr starved/re-fed unc-43(sy574) males. Starting population was 114 males.
2.2 Protein translation is required for the lasting effects of transient starvation
To address what changes occurred after starvation, we asked if protein synthesis during starvation was important in keeping spastic muscle contractions suppressed in the absence and subsequent presence of food. We grew unc-103(0) males in the presence of food until their final molt. After the males crawled out of their L4 cuticle, they were starved for 18 hrs on plates containing the protein translation inhibitor cycloheximide (250 µg/ml final concentration). Suppressed non-Prc males were then re-fed on E. coli lawns lacking or containing the translational inhibitor. Cycloheximide affects protein synthesis by stabilizing mRNAs’ half-life but interferes with their translation by blocking the ribosomal translocation step (Ernest, 1982, Dani et al., 1984, Laird-Offringa et al., 1990, Beelman and Parker, 1994). The dose of cycloheximide used in our experiments was lethal to larval animals (L4 and younger) within hours, suggesting that the dosage of drug is sufficient to reduce synthesis of essential proteins. In adults, 18 to 72 hr of drug exposure did not obviously reduce their health or gross behavior but the dose we used did affect the animals, since the drug could delay (for hours) the expression of an induced transgene (Experimental Procedures 1.1).
24 hr of growth on food containing cycloheximide did not affect spontaneous spicule protraction of fed wild-type males but the antibiotic doubled the penetrance of the unc-103(0) spicule protraction phenotype (from 30% to 63%) under the same conditions (Table 1). This suggests that under normal conditions, the ~70% of mutant males that did not display seized sex muscles underwent new protein synthesis, which allowed the male mating circuit to compensate for deficiency in unc-103. In the absence of food, we observed that the Prc phenotype was still suppressed in the starved unc-103(0) males, regardless if cycloheximide was present (12% Prc in cycloheximide n=81) (Table 1). This indicated that new or continued protein synthesis was not needed to attenuate cell excitability under food deprivation conditions. However, when the drug-treated starved non-Prc males were put back onto food with or without cycloheximide, the Prc phenotype returned. 34% of males that were not Prc during starvation conditions displayed the phenotype in the presence of food with cycloheximide (n=29), and 21% of males became Prc in the presence of food without cycloheximide (n=42) (Table 1). In contrast, when males were starved in the absence of cycloheximide but then transferred onto food plates containing the drug, the Prc phenotype remained suppressed (0% Prc, n=25) (Table 1). These results were confirmed using the translation inhibitor puromycin (Experimental Procedures 1.1). In summary, starvation suppresses the Prc phenotype in unc-103(0) males even if protein synthesis is inhibited, but protein synthesis during starvation is required for the lasting effects of transient starvation once the males are returned to food.
2.3 The lasting effects of transient starvation are dependent upon translation and the EAG K+ channel EGL-2
Our work with the protein synthesis inhibitor cycloheximide indicates additional proteins need to be made during or immediately after a period of transient starvation for its effects to last once the males are re-fed. To determine which proteins are required for this process, we first analyzed the contributions of the ether-a-go-go (EAG) K+ channel egl-2. egl-2 shows high homology to both human EAG1 and EAG2. Though human EAG1 was originally cloned from differentiating myocytes, detection of EAG1 in adults has been limited to the central nervous system while EAG2 is expressed in brain, skeletal muscle, heart, and several other tissues (Occhiodoro et al., 1998, Pardo et al., 1999, Ju and Wray, 2002). The functional role of EAG K+ channels has been most heavily studied in Drosophila, where the channel was originally cloned (Warmke et al., 1991, Ganetzky et al., 1999). Mutations in Drosophila EAG induce a leg-shaking phenotype when flies are exposed to ether (Kaplan and Trout, 1969). In recordings at the fly larva muscular junction, it was shown that EAG mutations induce hyper excitability in neurons (Ganetzky and Wu, 1983). Various proteins have been identified in Drosophila that modify the function of EAG and have behavioral consequences (Wilson et al., 1998, Wang et al., 2002, Marble et al., 2005). The C. elegans EAG K+ channel, EGL-2, is expressed in a subset of neurons and the sex muscles of both males and hermaphrodites. Mutations in this channel affect egg-laying and chemotaxis in hermaphrodites and sex muscle excitability in males (Weinshenker et al., 1999, Faumont et al., 2005, LeBoeuf et al., 2007).
Removing the EGL-2 K+ channel via a deletion does not result in increased spicule protraction (LeBoeuf et al., 2007). We looked at the effects of inhibiting protein synthesis in egl-2(0) mutants. Unlike in unc-103(0) males, cycloheximide did not increase the instance of Prc in egl-2(0) males on food (10% fed vs 11% fed + cycloheximide, p value = 1) (Table 1). Similar to unc-103(0) males, starving egl-2(0) males in the presence of cycloheximide reduced the instance of spicule protraction, though not significantly (11% starved vs 6% starved + cycloheximide) (Table 1). However, starving the males in cycloheximide and then re-feeding them with cycloheximide showed a significant increase in the number of males that protract their spicules versus starved and re-fed in the absence of the translation inhibitor (15% vs 2%, p value = 0.04) (Table 1). Thus, mechanisms exist that are able to compensate for the loss of egl-2 and these mechanisms rely, at least in part, on de novo protein translation.
Since egl-2(0) did not induce the Prc phenotype by itself, we used a double mutant lacking two EAG K+ channel family members, unc-103(0);egl-2(0), to explore the relationship between protein synthesis and egl-2 in regulating muscle excitability. We previously reported that unc-103(0);egl-2(0) males are Prc on food (LeBoeuf et al., 2007). Here, we show that the double mutant has a higher instance of Prc than either single mutant (Table 1). This indicates that egl-2 up-regulation partially compensates for the loss of unc-103. Thus, the egl-2 K+ channel can play a role in suppressing sex muscle excitability when males are well-fed. However, EGL-2 and UNC-103 are likely not the only proteins involved in suppressing spicule protraction when males are well-fed, as the incidence of spicule protraction seen in unc-103(0);egl-2(0) males increases when protein synthesis is inhibited (from 55% to 84%, p value <0.0001) (Table 1).
We next looked at the effects of inhibiting protein synthesis while unc-103(0);egl-2(0) males are starved. We previously reported that double mutant males protracted their spicules even in the absence of food (37%, n=65) (Table 1) (LeBoeuf et al., 2007). Inhibiting protein synthesis by growing unc-103(0);egl-2(0) males on plates containing cycloheximide and without food increases the instance of spicule protraction to 78% (n=116) (Table 1). If no other protein other than egl-2 was synthesized in response to starvation, then the percentage of unc-103(0);egl-2(0) males that protracted their spicules would be the same on starved plates, both with and without cycloheximide. However, this was not the case, indicating that when EGL-2 is absent, the synthesis of additional proteins can partially compensate for the loss of the K+ channel.
The high incidence of spicule protraction seen in unc-103(0);egl-2(0) double mutants when the males are starved on plates with cycloheximide is also indicative of the role of egl-2 under starvation conditions. Starvation still inhibited spontaneous sex muscle excitability in unc-103(0) males when cycloheximide was added to the plates (12%, n=81) (p value < 0.0001 vs unc-103(0);egl-2(0) starved with cycloheximide) (Table 1). Since removing egl-2 increased the incidence of the Prc phenotype from 12% to 78%, this indicated that in the absence of protein synthesis, the activity of the egl-2 EAG K+ channel was increased to reduce sex muscle excitability. This finding supports previous work that indicates that egl-2 is required in the sex muscles for males to respond to a period of starvation (LeBoeuf et al., 2007). The presence of EGL-2 alone, possibly due to some modification of the channel that increases its activity, is able to compensate for the loss of UNC-103, even when protein synthesis is inhibited under starvation conditions.
Next we looked at the effects of re-feeding starved unc-103(0);egl-2(0) males. Since starving double mutant males on plates with cycloheximide resulted in a high incidence of the Prc phenotype, we did not expect the Prc phenotype to change greatly once the males were re-fed with or without cycloheximide. This was indeed the case, as unc-103(0);egl-2(0) males that were starved for 18 hrs in the presence of cycloheximide and then fed for 7 hrs either with or without cycloheximide were 74% Prc (n=38) and 86% Prc (n=44), respectively (Table 1). Thus, egl-2 and protein synthesis are required for the normal response to starvation, both while the worms are being food deprived and after they are returned to a food source.
Finally, we looked at the effects of starving unc-103(0);egl-2(0) males on plates without cycloheximide and then re-feeding them on plates containing cycloheximide. We found that 45% of males protracted their spicules (n=29) under these conditions, which was similar to the 37% of double mutant males that were starved and not re-fed (n=65, p value = 0.4) (Table 1). Additionally, the percentage of unc-103(0);egl-2(0) males that protracted their spicules when re-fed (33%, n=36) was significantly higher than the percentage of unc-103(0) males that displayed the Prc phenotype when re-fed (0%, n=20, p value = 0.005) (Table 1). Thus, starving unc-103(0);egl-2(0) males on plates without cycloheximide allows for the synthesis of proteins that can partially compensate for the loss of egl-2 function, and the compensation persists once the males are returned to food.
2.4 EAG/EGL-2 K+ channel expression is increased following a period of starvation
Although inhibiting protein synthesis of unc-103(0);egl-2(0) males indicates that some EGL-2 is required prior to starvation, we asked if EGL-2 expression was also increased in response to food deprivation after re-feeding. We previously reported that the egl-2 promoter drives expression in the male tail in sex muscles and neurons (LeBoeuf et al., 2007). We constructed a reporter containing the egl-2 promoter driving DsRed1-E5, which shifts its emission spectra from green to red over time (Terskikh et al., 2000). With DsRed1-E5, we discovered that while neuronal expression exists in early adulthood, sex-muscle expression was faint until >24 hrs post L4 molt (Figure 2). We quantified the number of males with sex muscle expression at different time points, and found that at 3 and 24 hrs post L4 molt, only 29% and 31% of males had DsRed1-E5 expression in their sex muscles, respectively (Figure 2A–B). The incidence of expression increased to 60% 48 hrs post L4 molt (Figure 2A,C). To test if starvation had any effect on egl-2 expression, we starved males containing DsRed1-E5 driven from the egl-2 promoter for 3 hrs post L4 molt, re-fed them for 21 hrs, then determined the number of males that expressed DsRed1-E5 in their sex muscles. Compared to fed cohorts, we found that a 3 hr period of starvation increased the instance of sex-muscle expression from 31% to 65% (n=65 and n=54, respectively, p value = 0.0002, Fisher’s Exact Test) (Figure 2A,D). This indicates that EGL-2 expression is up-regulated after a period of transient starvation.
One of the functional consequences of increased EAG K+ channel/egl-2 sex-muscle expression could be to lower muscle excitability. As we previously reported, removing the unc-103 ERG-like K+ channel increased the incidence of spastic muscles contractions from 10% to 30% (Table 1) (Garcia and Sternberg, 2003). Our genetic data suggests that egl-2 can compensate for loss of unc-103 function, as a double mutant removing both K+ channels increased the incidence of the Prc phenotype from 30% to 55% (p value < 0.0001) (Table 1). We reasoned that the lower incidence of the Prc phenotype seen in unc-103(0) vs. unc-103(0);egl-2(0) mutant males could be due to the up-regulation of egl-2 when unc-103(0) is removed. To test this idea, we used the egl-2 promoter to drive DsRed1-E5 expression in unc-103(0) males and observed the number of males that expressed the construct in their sex muscles. We found that after 3 hrs of adulthood, 60% of unc-103(0) males expressed DsRed1-E5 in their sex muscles, compared to 29% of wild-type males (p value = 0.0003, Fisher’s Exact Test) (Figure 2A). egl-2 expression in unc-103(0) males did not change as they age nor after a period of starvation (Figure 2A). This indicates that egl-2 is expressed earlier in unc-103(0) male sex muscles and this expression could compensate, at least partially, for the loss of ERG-like K+ channel function.
2.5 Transient starvation does not inhibit spontaneous muscle contractions in CaMKII mutant males
In addition to the EAG/EGL-2 K+ channel down-regulating the male mating circuit in response to starvation, we previously reported that starvation suppresses the unc-103(0)-induced Prc phenotype through CaMKII activity (LeBoeuf et al., 2007). In C. elegans, CaMKII is encoded by the gene unc-43. unc-43 is broadly expressed in all neurons and muscles, and nonsense mutations induce spontaneous seizures affecting locomotion, egg-laying, defecation and male mating behavior (Reiner et al., 1999). A weak loss-of-function missense mutation (sy574), which changes a glycine to a glutamic acid in the substrate binding site of CaMKII, has subtler effects on the animals. The unc-43(sy574) mutation does not grossly affect locomotion, defecation or egg-laying behavior in hermaphrodites. However, ~50–60% of 18–24 hr well-fed males will display protracted spicules. Although the behavior of unc-43(sy574) males is similar to that of unc-103(0) males in the presence of food, transient starvation does not suppress unc-43(sy574)-induced spicule protraction (LeBoeuf et al., 2007).
When we assayed the behavior of aged unc-43(sy574) males, we found that they differ greatly from aged unc-103(0) males. A majority of unc-103(0) males displayed muscle spasms within 18 to 24 hr of adulthood; those that did not display the Prc phenotype maintained control of their spicule muscles for at least five additional days (Figure 1A). In contrast, 36% of unc-43(sy574) males displayed protruding spicules after 18–24 hr of adulthood, and additional males continued to displayed the Prc phenotype at a constant rate during the six-day experiment (n=148 males) (Figure 1C). When unc-103(0) males were starved for 18 hrs and then re-fed, the suppression of seized muscles continued for multiple days (n=103) (Figure 1B). In contrast, when unc-43(sy574) males were food deprived for 18 hr, the starvation period did not suppressed the Prc phenotype (34% males were Prc, n=114 males), and re-fed males displayed protraction at a similar rate to their fed cohorts (Figure 1D). Thus unlike UNC-103 K+ channels, CaMKII is required for the male to extend the effects of transient starvation.
2.6 CaMKII/UNC-43 directly interacts with the EAG/EGL-2 K+ channel
We showed using genetic evidence that both CaMKII/UNC-43 and EAG/EGL-2 K+ channels are required for the male to respond to transient starvation. Prior work in Drosophila established a physical link between CaMKII and EAG, where EAG phosphorylation by the kinase up-regulates channel activity (Wang et al., 2002, Sun et al., 2004). However, the C-terminal amino acid region of Drosophila EAG that binds CaMKII does not show much homology with the C-terminal region of C. elegans EGL-2. To investigate if there is a direct binding between the C. elegans EGL-2 and UNC-43 proteins, we performed a Yeast Two-Hybrid assay using the kinase domain of UNC-43 and the c-terminal domain of EGL-2. We used UNC-43 residues 1–270 and introduced an Asp 135 Asn mutation to inactivate the kinase so it would not kill the yeast host (Rosenberg et al., 2005). The EGL-2 c-terminus consists of residues 488–957, and contains several potential CaMKII phosphorylation sites (sequence RXXS/T) (Pearson et al., 1985, Weinshenker et al., 1999). Bait plasmids containing the UNC-43 kinase domain were co-transfected into yeast cells with prey plasmids containing the EGL-2 c-terminus. After selecting for colonies carrying both plasmids, we tested for protein interaction by measuring the chemiluminescence produced by β-galactosidase activity using the Galacton Star (Clontech) substrate. We found that there was a strong positive interaction between UNC-43 and EGL-2 (Figure 3A). Thus, UNC-43 could be binding EGL-2 in vivo to affect channel activity.
We next asked if this interaction was dependent on a potential CaMKII phosphorylation site located on the EGL-2 c-terminus. The egl-2(n693gf) allele is an A473V change in the sixth transmembrane domain (pore-forming region) that results in a channel with a higher open probability at lower membrane potential (Weinshenker et al., 1999). The n904 allele was generated via mutagenesis of egl-2(n693gf) to screen for suppression of the n693gf –induced egg-laying defective phenotype (Park and Horvitz, 1986). egl-2(n693n904) animals are grossly wild type; hermaphrodites do not retain eggs, and males do not display the Prc phenotype (0%, n=30). egl-2(n693gf) does not have an effect on the unc-103(0) Prc phenotype (LeBoeuf et al., 2007); however surprisingly, the intragenic reversion allele egl-2(n693n904) can suppress the unc-103(0)-induced spicule protraction. 14% of unc-103(0);egl-2(n693n904) males protract their spicules in the absence of mating cues (n=194), compared to 30% of unc-103(0) males (n=439, p value < 0.0001). We sequenced the genomic DNA from the strain and found that the n904 mutation affects a potential CaMKII site, which changes the serine at amino acid 567 to the bulky hydrophobic residue phenylalanine.
Since the egl-2(n693n904) mutation suppresses unc-103(0)-induced spicule protraction, we placed the S567F mutation in the egl-2 c-terminus and performed a Yeast Two-Hybrid assay. We found that the strong positive interaction between UNC-43 and EGL-2 is reduced when S567 is mutated (Figure 3A). Since changing a serine to phenylalanine can have a profound impact on the overall structure of the c-terminus, we mutated S567 to alanine, which is not as likely to drastically change the protein structure. The egl-2 c-terminus carrying the S567A mutation interacts with UNC-43 less than egl-2(S567F) (p value = 0.0001, unpaired t test) (Figure 3A). To show that the decreased interaction between the two proteins was caused by mutating S567 specifically and not due to protein miss-folding defects, we mutated another potential CaMKII binding site, T647, to alanine. The T647A mutation reduced interaction significantly, but not to the extent of either S567A or S567F (Figure 3A). The reduction in egl-2(T647A) binding to UNC-43 could be due to two reasons: (1) the T647 site plays a role in CaMKII binding to EGL-2 or (2) the mutation alters the ability of the protein to fold correctly and thereby reduces protein stability. Both arguments could be made for the S567 mutations as well. However, since mutating S567 has a greater impact on protein interaction, we favor the explanation that to abolish most of the interaction between the two proteins, the specific CaMKII binding site involved needs to be mutated. Mutating an amino acid in the EGL-2 c-terminus can affect protein folding and reduce the signal in our Yeast Two-Hybrid assay. However, we do not think it accounts for all the reduction in signal seen when mutating S567. To ensure that the loss of signal was not due to reduced protein expression, we measured protein levels in the yeast host via western blotting. We found that EGL-2 is expressed in the yeast cells at similar levels, regardless if an amino acid substitution was made or not (Figure 3B). Thus, S567 seems a likely target of UNC-43 in C. elegans.
To confirm the Yeast Two-Hybrid interaction, we looked at in vitro binding between EAG K+ channel/EGL-2 and CaMKII/UNC-43. The EGL-2 c-terminus attached to the maltose binding protein (MBP) was placed in a solution containing calcium, calmodulin, and ATP along with the kinase and regulatory domains of UNC-43 linked to glutathione S-transferase (GST). Using amylose resin, we selected for EGL-2 and any UNC-43 that might be bound to it. We then used antibodies against GST to detect the presence of UNC-43-GST that was associated with EGL-2. We used a control that contained UNC-43 and wild-type EGL-2 but no calcium, calmodulin, or ATP, so as not to activate the kinase. We found that UNC-43 does bind EGL-2; this association was reduced when the EGL-2(S567F) mutation is present (Figure 3C). We found similar results when we used glutathione resin to select for UNC-43 and probed for EGL-2-MBP using an antibody against MBP (Figure 3C). As an additional control, we used a reaction that contained both wild-type proteins plus ATP and calmodulin but not Ca2+, to ask if UNC-43 needs to be fully activated to get robust interaction. However, we found that even in the absence of Ca2+, some interaction between UNC-43 and EGL-2 occurred. This indicates that the interaction between the two proteins is very strong in vitro and helps explain why mutating S567 to F reduces but does not abolish protein interaction. In conclusion, C. elegans UNC-43 and EGL-2 interact, and this interaction is partly dependent upon EGL-2 S567.
2.7 Mutating a potential CaMKII binding site in EAG/EGL-2 interferes with the K+ channel’s response to starvation
Having established EGL-2 S567 as a CaMKII binding site in vitro, we wanted to determine the in vivo significance of this interaction. Thus, we measured the response of egl-2 mutants to the acetylcholine (ACh) agonist arecoline (ARE). We previously established that ACh is responsible for male sex muscle contractions during mating and determined that CaMKII/UNC-43 inhibits its effects through the EAG/EGL-2 K+ channel (Garcia et al., 2001). Specifically, we found that males protract their spicules in response to ARE, and this response is reduced in unc-43(gf) males. However, this effect is partially restored when the egl-2 K+ channel is deleted (LeBoeuf et al., 2007). Here, we looked at the response of wild type and egl-2 mutant males to 1 mM ARE under fed, starved, and starved + 1 hr fed conditions. Males were placed in 1 mM ARE for 5 min, and those that protracted their spicules were recorded. 100% of fed wild-type males protracted their spicules in 1 mM ARE (n=30) (Table 2). Consistent with previously published reports, the number of wild-type males that responded to ARE dropped to 71% when they were starved (n=55, p value = 0.0008, Fisher’s Exact Test) (Table 2) (Gruninger et al., 2008). When the starved males were fed for 1 hr prior to ARE exposure, the instance of protraction increased to 88% (n=33, p value = 0.07 to wild-type starved, p value = 0.1 to wild-type fed) (Table 2). Thus, wild-type males are able to partially recover from a transient period of starvation. Exposing egl-2(0) males to ARE under the same conditions produced similar results, except that more males responded to the drug when starved, and no males retained any resistance after 1 hr of re-feeding (Table 2). Interestingly, exposing egl-2(n693gf) males to 1 mM ARE resulted in a more profound response. Fed egl-2(n693gf) males responded similarly to wild-type males in 1 mM ARE (90% vs 100%, n=30) (Table 2). In contrast, fewer egl-2(n693gf) males responded to 1 mM ARE during starvation (13%, n=30, p value < 0.0001 to wild-type starved, Fisher’s Exact Test) and only 30% of males protracted their spicules after they were re-fed (n=33, p value < 0.0001 to wild-type re-fed 1 hr, Fisher’s Exact Test) (Table 2). This suggests that in wild type, egl-2 activity is up-regulated, and in egl-2(n693gf) males, the A473V mutation amplifies this effect. We used the egl-2(n693gf) allele for further study since it allowed for easier measurement of the changes that starvation has on the male.
Table 2.
EAG K+ channel egl-2 function affects ARE sensitivity in starved and re-fed males.
| % spicule protracted (n) | |||
|---|---|---|---|
| Genotype | After 5 min exposure to 1 mM ARE | ||
| Fed | Starved | Starved + fed 1hr |
|
| Wild type | 100 (30) | 71 (55) | 88 (33) |
| egl-2(0) | 100 (39) | 82 (72) | 100 (35) |
| egl-2(n693gf) | 90 (30) | 13 (30) | 30 (33) |
| egl-2(n693n904) | 97 (30) | 53 (30) | 85 (33) |
We next tested the effect of the S567F change in the EGL-2 C-terminus by measuring the response of egl-2(n693n904) males to 1 mM ARE. Fed egl-2(n693n904) males responded to the drug similar to wild type and egl-2(n693gf) males (97%, n=30) (Table 2). However, starved egl-2(n693n904) males did not show as dramatic resistance to ARE as egl-2(n693gf) (53%, n=30, p values = 0.2 to wild type starved and 0.0022 to egl-2(n693gf) starved, Fisher’s Exact Test) (Table 2). In addition, 85% of egl-2(n693n904) males that have been starved overnight and then fed for 1 hr responded to 1 mM ARE (n=33) with values similar to wild type and different from egl-2(n693gf) (88% and 30%, respectively, p values = 1 and < 0.0001, respectively, Fisher’s Exact Test) (Table 2). Therefore, mutating the S567 site on EGL-2 reduces the K+ channel’s ability to respond to or extend the effects of starvation.
2.8 Modification of EAG K+ channel activity occurs after a period of starvation
We hypothesize that if starvation stimulates CaMKII and its activity persists when the males encounter abundant nutrient conditions, then CaMKII might continue to modify EGL-2 function even after transient food deprivation. We tested this by expressing the egl-2(n693gf) cDNA from the hsp-16 heat-shock promoter in fed or starved/re-fed egl-2(0) males, and using the 1 mM ARE- spicule protraction assay, asked if the experience of nutrient restriction affected the function of newly made EGL-2 (Jones et al., 1989, Stringham et al., 1992). 90 minutes after heat shock, 94% (n=50) and 75% (n=71, p value < 0.05) of fed and starved-refed nontransgenic wild-type males protracted their spicules in ARE; in nontransgenic egl-2(0) males, 100% (n=38) and 91%(n=55) of fed and starved-refed males protracted their spicules (Table 3). These controls indicate that the combined effects of starvation and artificial heat shock reduced the males’ spicule muscles sensitivity to ARE via EGL-2 (p value = 0.02, Fisher’s Exact Test) (Table 3). Next we looked at the effect of heat shock on Phsp-16∷egl-2(gf) expressing egl-2(0) worms; 75% of fed males protracted their spicules (n=52, p value < 0.05 compared to fed wild type, Fisher’s Exact Test) (Table 3), indicating that egl-2(gf) over-expressed from the heat-shock promoter conferred ARE resistance. Importantly, starving the males prior to heat shock increased the ARE resistance, as only 42% of starved/refed males expressing egl-2(gf) protract their spicules in response to ARE (n=36, p value < 0.05 compared to fed males) (Table 3). Thus, a mechanism is activated during starvation that remains active once the males are re-fed and are capable of increasing the effect of EGL-2 on sex muscle excitability.
To explore the possibility that CaMKII might mediate these effects on EGL-2, we mutated several potential CaMKII phosphorylation sites in the EGL-2 c-terminus. The following sites were mutated in the egl-2(gf) cDNA: S567G, T647G, S811G; and 60 amino acids from position 888 to position 947 were also removed, deleting potential CaMKII sites S888, T902, T922, and T947 (Weinshenker et al., 1999, LeBoeuf et al., 2007). This construct was expressed via the hsp-16 promoter and is referred to as egl-2(gf,-CaMKII sites). In contrast to males expressing Phsp-16:egl-2(gf), fed males expressing Phsp-16:egl-2(gf,-CaMKII sites) did not display ARE resistance, as 100% of these males protracted their spicules when exposed to the ACh agonist (n=43, p value = 0.0002) (Table 3). Similarly, starving the males prior to heat shock did not have a large effect on egl-2(gf,-CaMKII sites)-expressing males, since 79% of previously starved males protracted their spicules (n=38), compared to 42% of males expressing egl-2(gf) (p value = 0.0017) (Table 3). Thus, abolishing potential CaMKII binding sites reduces EGL-2’s ability to respond to a period of starvation.
We wanted to determine if the differences in behavior induced by the two constructs was due to different levels of expression. We performed qPCR on heat-shocked worms and determined that mRNA transcripts from the Phsp-16:egl-2(gf,-CaMKII) and Phsp-16:egl-2(gf) constructs were expressed at similar levels. Also both constructs could induce paralysis, indicating that the expressed proteins are functional (Experimental Procedures section 1.8). We attempted to determine protein levels in C. elegans using an antibody to rat KCNH1. The antibody cross-reacted with C. elegans protein, but both EGL-2 proteins were expressed at very low levels and we were unable to determine the amount of protein expressed via the different constructs. Because of this, we can not rule out that the differences in behavior we see between these two constructs were due to unequal protein levels.
2.9 Transiently starved/re-fed males are less competitive at mating compared to well-fed cohorts
Since the pharmacological and genetic data indicate that transient starvation can have lasting effects on spicule muscle excitability, we asked how transient starvation might alter male mating behavior. We conducted a competition assay between 23 hr well-fed and 20 hr transiently starved/3 hr re-fed males and asked which type of animal can first impregnate a moving mate during a short mating period. To distinguish between fed and starved/re-fed males, we marked the animals with either transgenically expressed yellow or cyan fluorescent protein. Males were starved for 20 hr and then re-fed for three hours before they competed for a mate. We then paired one fed and one starved/re-fed male with a mobile fog-2(lf) female. fog-2(lf) females contain a mutation that disrupts the generation of self-sperm; thus unlike wild-type hermaphrodites, these worms are infertile until they become impregnated (Schedl and Kimble, 1988). After we added the pair of starved/re-fed and well-fed males, every hour (for three hours) we checked if the fog-2(lf) female contained male sperm in her spermatheca. If the female did, we removed both males to stop subsequent copulations. The next day, we checked if the cross progeny inherited the fluorescent marker from either the well-fed, starved/re-fed or from both types of males. We found that fed males significantly out-competed the starved/re-fed males (p value = 0.02, Fisher’s Exact test). From 67 impregnated females, 66% of them were impregnated by well-fed males, 22% were impregnated by starved/re-fed males and 12% were impregnated by both during the experimental window (Figure 4). This result indicates that although transient starvation might have beneficial effects on reducing mutation-induced spastic muscle contractions, for a wild-type male, being well-fed imbues a competitive mating advantage over males that experienced inconsistent feeding conditions.
3. Discussion
While predetermined programs drive an organism’s behavior, the behavioral output can be modified by past experiences. For this modification to occur, organisms must respond to environmental stimuli appropriately, and then store that experience. One environmental experience that is common in nature is periods of limited nutrient availability. Organisms that are most capable of coping with food shortages have better survivability. Once food abundance is restored, the coping mechanisms are relaxed. However, a period of limited nutrient availability can have lasting consequences, especially if it occurs during development. Lasting physical reminders of transient nutrient deprivation include stunted growth and brain abnormalities, which can persist throughout an individual’s life (Hulshoff Pol et al., 2000, Victora et al., 2008). In addition, behavioral consequences such as schizophrenia could be a result of nutrient deprivation during development, as studies have correlated the occurrence of schizophrenia with limited nutrient availability (Susser et al., 1996, Wahlbeck et al., 2001). It has been suggested that schizophrenia, due to its high prevalence in all human populations, represents an adaptive response to starvation, as individuals exposed to one period of harsh environmental conditions are more likely to encounter another. The hallmarks of schizophrenia that reduce an individual’s ability to cope in society, such as impulsive behavior and the inability to ignore extraneous stimuli, might be advantageous in a nutrient poor and stressful environment, heightening awareness and increasing responsiveness (Reser, 2007). Therefore, an organism capable of incorporating the consequences of nutrient deprivation could face both positive and negative adaptive advantages, depending on the environment it later encounters.
To study the lasting consequences of transitory nutrient deprivation and the mechanisms coordinating these responses, we analyzed the effects of short term starvation on C. elegans male mating behavior. We previously identified that starving males suppresses abnormal muscle contractions induced by deletion of the ERG-like K+ channel unc-103 (Gruninger et al., 2006). In this study, we report that this suppression persists after the males are returned to food, indicating that long-lasting changes occur in response to transient starvation.
3.1 CaMKII modifies EAG/EGL-2 in response to starvation
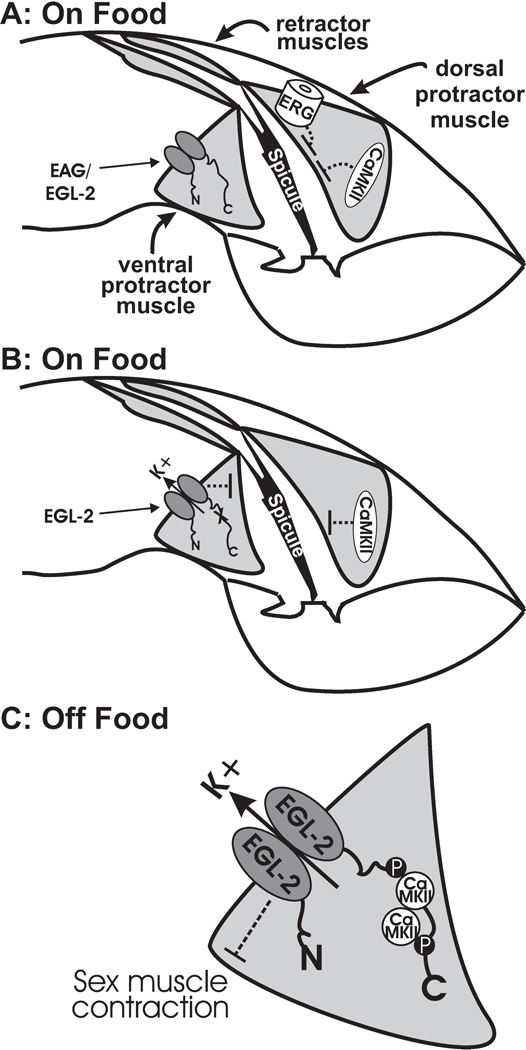
We propose that interactions between CaMKII/UNC-43 and the EAG K+ channel EGL-2 facilitate how males both respond to the period of starvation and are continually altered by it once they are returned to food. Both the EAG/EGL-2 K+ channel and CaMKII are involved in down-regulating the male mating circuit in response to food deprivation, and both function in the male sex muscles (LeBoeuf et al., 2007). In addition, both proteins are involved in the permanent changes that occur as a consequence of exposure to limited nutrient availability. However, unlike UNC-43, EGL-2 does not play a significant role in regulating male sex muscle excitability under well-fed conditions. We propose that under well-fed conditions, EGL-2 activity in the sex muscles is minimal (Figure 5A). This is supported by the low level of egl-2 expression seen in the sex muscles of young adult males and by the inability of an egl-2(gf) mutation to suppress the unc-103(0)-induced spicule protraction (LeBoeuf et al., 2007). However, since mutating serine 567 to phenylalanine in a potential CaMKII phosphorylation site causes EGL-2(gf) K+ channels to reduce the unc-103(0)-induced Prc phenotype, we suggest that the EGL-2(S567F) mutation increases the activity of the channel on food (Figure 5B). Interestingly, while mutating S567 seems to promote EGL-2 function in fed males, the mutation interferes with the K+ channel’s ability to respond when food is scarce. Starved egl-2(n693n904) males do not display similar resistance to ARE as compared to starved egl-2(n693gf) males. Additionally, the effect of starvation does not last after the egl-2(n693n904) males are transferred back to food. We propose this is due to the requirement for CaMKII to modify S567, so that it might then further modify other sites on the EGL-2 c-terminus to promote maximal function to the K+ channel (Figure 5C). In addition to kinase and regulatory domains, CaMKII also contains a self-association domain that allows it to form complexes containing up to 12 members in two stacked rings, with the self-association domains on the inside and the kinase domains on the outside (Kolodziej et al., 2000, Morris and Torok, 2001, Colbran, 2004). CaMKII, normally activated by Ca2+/calmodulin, maintains its function in the absence of Ca2+-bound calmodulin by self-phosphorylation. The initial CaMKII that modifies S567 might remain at this site, as has been shown with Drosophila CaMKII/EAG and vertebrate CaMKII/NMDAR (N-methyl D-aspartate receptor) interactions (Strack et al., 2000, Bayer et al., 2001, Wang et al., 2002, Sun et al., 2004, Pradeep et al., 2009). The targeting of CaMKII might also be important to phosphorylate neighboring substrates at the plasma membrane (Tsui et al., 2005). After food deprivation induces the influx of calcium via the insulin-like receptor/PLC-γ pathway (Gruninger et al., 2008), more CaMKII subunits within the same complex could be activated, thereby phosphorylating additional sites on the EGL-2 c-terminus and increasing channel activity in the absence of food. Due to the self-activating properties of CaMKII, it can then potentially modify newly made EGL-2 protein, even after the animal is re-fed. This hypothesis is supported by the experiments we performed where no EGL-2 was present during the starvation period, yet once EGL-2 was expressed during food presentation, cell excitability was significantly decreased. This effect was not as pronounced when potential CaMKII phosphorylation sites were removed from the EGL-2 c-terminus. Therefore, CaMKII and EGL-2 constitutes a molecular memory pathway by which a transient period of starvation is able to have lasting effects on cell excitability and behavior.
Figure 5.
Transient starvation activates a pathway that includes EAG K+ channels/EGL-2 and CaMKII/UNC-43. (A–B) Cartoons of the right half of the male tail. Under well-fed conditions, the EAG K+ channel EGL-2 is minimally active in the sex muscles, while the ERG-like K+ channel UNC-103 and CaMKII/UNC-43 suppress sex muscle contraction in the absence of mating cues. All three proteins are expressed in both dorsal and ventral protractor muscles. (B) Mutating the S567 site on the EGL-2 c-terminus results in a channel that is more active on food and able to inhibit muscle spasms caused by the loss of UNC-103. (C) Cartoon of a protractor muscle. CaMKII binds and phosphorylates EGL-2 at more than one site to activate the channel under starvation conditions. This activity persists after the males are returned to food.
3.2 The hormetic effects of developing under starvation conditions are balanced by a cost to mating competitiveness
Many studies report how caloric restriction promotes vigor and reproductive capacity of organisms ranging from bacteria to humans (Jenkins et al., 1988, Fontana et al., 2004, Burger et al., 2007, Selesniemi et al., 2008, Smith et al., 2008). However, other reports describe the difficulty in maintaining restricted diet regimes, and the detrimental physiological and behavioral trade-offs associated with these treatments (Vitousek et al., 2004, Polivy et al., 2008). Similar to many studies, transient starvation has a positive effect on C. elegans male muscle excitability, as a two hour period of food deprivation is sufficient to suppress ERG-like K+ channel unc-103(0)-induced muscle spasms for up to six days. However, this raises the question of why nature did not design the male neural circuitry to be able to compensate for the loss of unc-103, irrespective of the food conditions they develop from? During mating contests between young well-fed and starved/re-fed males, well-fed males are more successful. Thus the young well-fed male mating circuit out performs the starved/re-fed circuit. This observation suggests that reducing the excitability of the mating circuit comes at the cost of efficiency. C. elegans hermaphrodites are not behaviorally receptive to mating (Garcia et al., 2007), and when food is abundant, a male would want to impregnate as many reluctant hermaphrodites as possible before his cohorts. Through evolution, sexual competition between males might have reduced the excitation threshold of neurons and muscles, allowing for faster motor responses to impregnate hermaphrodites. However, that leaves the males less able to compensate for inappropriately excitable circuits. Thus, transient starvation induces lasting changes that result in both positive and negative consequences.
Acknowledgments
We thank Yishi Liu, Paola Correa, and Liusuo Zhang for reviewing the manuscript, and LaShundra Rodgers and Daisy Gualberto for technical help. Strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources (NCRR). LRG and BL were funded by the Howard Hughes Medical Institute. XG was supported by NIH grant GM-70531.
Glossary
Abbreviations
- ACh
acetylcholine
- ARE
arecoline
- CaMKII
calcium/calmodulin-dependent protein kinase II
- EGL
egg-laying defective
- UNC
uncoordinated
- YFP
yellow fluorescent protein
- CFP
cyan fluorescent protein
- EAG
ether-a-go-go
- ERG
ether-a-go-go related gene
- gf
gain-of-function
- Prc
protraction constitutive
- GST
glutathione S-transferase
- MBP
maltose binding protein
- WT
wild-type
- hsp
heat shock protein
- fog
feminization of the germ line
- NMDAR
N-methyl D-aspartate receptor
- HA
hemagglutinin
Glossary
- EAG K+ channel
ether-a-go-go K+ channel
- ERG-like K+ channel
ether-a-go-go related gene K+ channel
- EGL-2
C. elegans EAG K+ channel
- UNC-103
C. elegans ERG-like K+ channel
- CaMKII
calcium/calmodulin-dependent protein kinase II
- UNC-43
C. elegans CaMKII
- arecoline
acetylcholine agonist
- cycloheximide
protein translation inhibitor
- L4
C. elegans larval stage 4
- hsp-16
C. elegans heat shock protein
- fog-2
C. elegans gene for feminization of the germ line
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barriere A, Felix MA. Natural variation and population genetics of Caenorhabditis elegans. WormBook. 2005:1–19. doi: 10.1895/wormbook.1.43.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani CA, Gharib S, Simon MI, Sternberg PW. Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCbeta-independent and seroton-independent signaling pathway and likely functions both in the nervous system and in muscle. Genetics. 2003;165:1805–1822. doi: 10.1093/genetics/165.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Beelman CA, Parker R. Differential effects of translational inhibition in cis and in trans on the decay of the unstable yeast MFA2 mRNA. J Biol Chem. 1994;269:9687–9692. [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger JM, Hwangbo DS, Corby-Harris V, Promislow DE. The functional costs and benefits of dietary restriction in Drosophila. Aging Cell. 2007;6:63–71. doi: 10.1111/j.1474-9726.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learn Mem. 1997;4:179–191. doi: 10.1101/lm.4.2.179. [DOI] [PubMed] [Google Scholar]
- Colbran RJ. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll NA. Components and patterns in the behavior of the nematode Caenorhabditis elegans. J Zoology. 1975;176:159–176. [Google Scholar]
- Dani C, Blanchard JM, Piechaczyk M, El Sabouty S, Marty L, Jeanteur P. Extreme instability of myc mRNA in normal and transformed human cells. Proc Natl Acad Sci U S A. 1984;81:7046–7050. doi: 10.1073/pnas.81.22.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci U S A. 2004;101:5524–5529. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest MJ. Regulation of tyrosine aminotransferase messenger ribonucleic acid in rat liver. Effect of cycloheximide on messenger ribonucleic acid turnover. Biochemistry. 1982;21:6761–6767. doi: 10.1021/bi00269a022. [DOI] [PubMed] [Google Scholar]
- Faumont S, Miller AC, Lockery SR. Chemosensory behavior of semi-restrained Caenorhabditis elegans. J Neurobiol. 2005;65:171–178. doi: 10.1002/neu.20196. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B, Robertson GA, Wilson GF, Trudeau MC, Titus SA. The eag family of K+ channels in Drosophila and mammals. Ann N Y Acad Sci. 1999;868:356–369. doi: 10.1111/j.1749-6632.1999.tb11297.x. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1:17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- Garcia LR, LeBoeuf B, Koo P. Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics. 2007;175:1761–1771. doi: 10.1534/genetics.106.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LR, Mehta P, Sternberg PW. Regulation of distinct muscle behaviors controls the C. elegans male's copulatory spicules during mating. Cell. 2001;107:777–788. doi: 10.1016/s0092-8674(01)00600-6. [DOI] [PubMed] [Google Scholar]
- Garcia LR, Sternberg PW. Caenorhabditis elegans UNC-103 ERG-like potassium channel regulates contractile behaviors of sex muscles in males before and during mating. J Neurosci. 2003;23:2696–2705. doi: 10.1523/JNEUROSCI.23-07-02696.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, Schnabel H, Schnabel R. pha-1, a selectable marker for gene transfer in C. elegans. Nucleic Acids Res. 1994;22:1762–1763. doi: 10.1093/nar/22.9.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruninger TR, Gualberto DG, Garcia LR. Sensory perception of food and insulin-like signals influence seizure susceptibility. PLoS Genet. 2008;4:e1000117. doi: 10.1371/journal.pgen.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruninger TR, Gualberto DG, LeBoeuf B, Garcia LR. Integration of male mating and feeding behaviors in Caenorhabditis elegans. J Neurosci. 2006;26:169–179. doi: 10.1523/JNEUROSCI.3364-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin JA, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz HR, Chalfie M, Trent C, Sulston JE, Evans PD. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science. 1982;216:1012–1014. [Google Scholar]
- Hulshoff Pol HE, Hoek HW, Susser E, Brown AS, Dingemans A, Schnack HG, van Haren NE, Pereira Ramos LM, Gispen-de Wied CC, Kahn RS. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 2000;157:1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- Jenkins DE, Schultz JE, Matin A. Starvation-induced cross protection against heat or H2O2 challenge in Escherichia coli. J Bacteriol. 1988;170:3910–3914. doi: 10.1128/jb.170.9.3910-3914.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Jones D, Dixon DK, Graham RW, Candido EP. Differential regulation of closely related members of the hsp16 gene family in Caenorhabditis elegans. DNA. 1989;8:481–490. doi: 10.1089/dna.1.1989.8.481. [DOI] [PubMed] [Google Scholar]
- Ju M, Wray D. Molecular identification and characterisation of the human eag2 potassium channel. FEBS Lett. 2002;524:204–210. doi: 10.1016/s0014-5793(02)03055-7. [DOI] [PubMed] [Google Scholar]
- Kaplan WD, Trout WE., 3rd The behavior of four neurological mutants of Drosophila. Genetics. 1969;61:399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej SJ, Hudmon A, Waxham MN, Stoops JK. Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIalpha and truncated CaM kinase IIalpha reveal a unique organization for its structural core and functional domains. J Biol Chem. 2000;275:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- Laird-Offringa IA, de Wit CL, Elfferich P, van der Eb AJ. Poly(A) tail shortening is the translation-dependent step in c-myc mRNA degradation. Mol Cell Biol. 1990;10:6132–6140. doi: 10.1128/mcb.10.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf B, Gruninger TR, Garcia LR. Food deprivation attenuates seizures through CaMKII and EAG K+ Channels. PLoS Genetics. 2007;3:e156. doi: 10.1371/journal.pgen.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Ashrafi K. A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet. 2008;4:e1000213. doi: 10.1371/journal.pgen.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-J, Defossez P-A, Guarente L. Requirement of NAD and SIR2 for Life-Span Extension by Calorie Restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. Mate searching in Caenorhabditis elegans: a genetic model for sex drive in a simple invertebrate. J Neurosci. 2004;24:7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Loer CM, Kenyon CJ. Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans. J Neurosci. 1993;13:5407–5417. doi: 10.1523/JNEUROSCI.13-12-05407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Marble DD, Hegle AP, Snyder ED, 2nd, Dimitratos S, Bryant PJ, Wilson GF. Camguk/CASK enhances Ether-a-go-go potassium current by a phosphorylation-dependent mechanism. J Neurosci. 2005;25:4898–4907. doi: 10.1523/JNEUROSCI.4566-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri A, Kodama E, Kimura KD, Koike M, Mizuno T, Mori I. Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics. 2005;169:1437–1450. doi: 10.1534/genetics.104.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris EP, Torok K. Oligomeric structure of alpha-calmodulin-dependent protein kinase II. J Mol Biol. 2001;308:1–8. doi: 10.1006/jmbi.2001.4584. [DOI] [PubMed] [Google Scholar]
- Occhiodoro T, Bernheim L, Liu JH, Bijlenga P, Sinnreich M, Bader CR, Fischer-Lougheed J. Cloning of a human ether-a-go-go potassium channel expressed in myoblasts at the onset of fusion. FEBS Lett. 1998;434:177–182. doi: 10.1016/s0014-5793(98)00973-9. [DOI] [PubMed] [Google Scholar]
- Pardo LA, del Camino D, Sanchez A, Alves F, Bruggemann A, Beckh S, Stuhmer W. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EC, Horvitz HR. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics. 1986;113:821–852. doi: 10.1093/genetics/113.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RB, Woodgett JR, Cohen P, Kemp BE. Substrate specificity of a multifunctional calmodulin-dependent protein kinase. J Biol Chem. 1985;260:14471–14476. [PubMed] [Google Scholar]
- Polivy J, Herman P, Coelho JS. Caloric restriction in the presence of attractive food cues: External cues, eating, and weight. Physiol Behav. 2008;94:729–733. doi: 10.1016/j.physbeh.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Pradeep KK, Cheriyan J, Suma Priya SD, Rajeevkumar R, Mayadevi M, Praseeda M, Omkumar RV. Regulation of Ca2+/calmodulin-dependent protein kinase II catalysis by N-methyl-D-aspartate receptor subunit 2B. Biochem J. 2009;419:123–132. doi: 10.1042/BJ20081707. 124 p following 132. [DOI] [PubMed] [Google Scholar]
- Reiner DJ, Newton EM, Tian H, Thomas JH. Diverse behavioural defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature. 1999;402:199–203. doi: 10.1038/46072. [DOI] [PubMed] [Google Scholar]
- Reiner DJ, Weinshenker D, Thomas JH. Analysis of dominant mutations affecting muscle excitation in Caenorhabditis elegans. Genetics. 1995;141:961–976. doi: 10.1093/genetics/141.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Weinshenker D, Tian H, Thomas JH, Nishiwaki K, Miwa J, Gruninger T, LeBoeuf B, Garcia LR. Behavioral genetics of Caenorhabditis elegans unc-103-encoded erg-like K(+) channel. J Neurogenet. 2006;20:41–66. doi: 10.1080/01677060600788826. [DOI] [PubMed] [Google Scholar]
- Reser JE. Schizophrenia and phenotypic plasticity: schizophrenia may represent a predictive, adaptive response to severe environmental adversity that allows both bioenergetic thrift and a defensive behavioral strategy. Med Hypotheses. 2007;69:383–394. doi: 10.1016/j.mehy.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel H, Schnabel R. An Organ-Specific Differentiation Gene, pha-1, from Caenorhabditis elegans. Science. 1990;250:686–688. doi: 10.1126/science.250.4981.686. [DOI] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–629. doi: 10.1111/j.1474-9726.2008.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ED, Kaeberlein TL, Lydum BT, Sager J, Welton KL, Kennedy BK, Kaeberlein M. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev Biol. 2008;8:49. doi: 10.1186/1471-213X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- Stringham EG, Dixon DK, Jones D, Candido EP. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Albertson DG, Thomas JN. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Developmental Biology. 1980;78:542–576. doi: 10.1016/0012-1606(80)90352-8. [DOI] [PubMed] [Google Scholar]
- Sun XX, Hodge JJL, Zhou Y, Nguyen M, Griffith LC. The eag potassium channel binds and locally activates calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:10206–10214. doi: 10.1074/jbc.M310728200. [DOI] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- Terskikh A, Fradkov A, Ermakova G, Zaraisky A, Tan P, Kajava AV, Zhao X, Lukyanov S, Matz M, Kim S, Weissman I, Siebert P. "Fluorescent timer": protein that changes color with time. Science. 2000;290:1585–1588. doi: 10.1126/science.290.5496.1585. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui J, Inagaki M, Schulman H. Calcium/calmodulin-dependent protein kinase II (CaMKII) localization acts in concert with substrate targeting to create spatial restriction for phosphorylation. J Biol Chem. 2005;280:9210–9216. doi: 10.1074/jbc.M407653200. [DOI] [PubMed] [Google Scholar]
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek KM, Manke FP, Gray JA, Vitousek MN. Caloric restriction for longevity: II. The systematic neglect of behavioral and psychological outcomes in animal research. Eur Eat Disord Rev. 2004;12:338–360. [Google Scholar]
- Wahlbeck K, Forsen T, Osmond C, Barker DJ, Eriksson JG. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch Gen Psychiatry. 2001;58:48–52. doi: 10.1001/archpsyc.58.1.48. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wilson GF, Griffith LC. Calcium/calmodulin-dependent protein kinase II phosphorylates and regulates the Drosophila eag potassium channel. J Biol Chem. 2002;277:24022–24029. doi: 10.1074/jbc.M201949200. [DOI] [PubMed] [Google Scholar]
- Ward S, Carrel JS. Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol. 1979;73:304–321. doi: 10.1016/0012-1606(79)90069-1. [DOI] [PubMed] [Google Scholar]
- Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991;252:1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Wei A, Salkoff L, Thomas JH. Block of an ether-a-go-go-like K(+) channel by imipramine rescues egl-2 excitation defects in Caenorhabditis elegans. J Neurosci. 1999;19:9831–9840. doi: 10.1523/JNEUROSCI.19-22-09831.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GF, Wang Z, Chouinard SW, Griffith LC, Ganetzky B. Interaction of the K channel beta subunit, Hyperkinetic, with eag family members. J Biol Chem. 1998;273:6389–6394. doi: 10.1074/jbc.273.11.6389. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci U S A. 2005;102:13289–13294. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]