Abstract
This article provides a classification of primary progressive aphasia (PPA) and its 3 main variants to improve the uniformity of case reporting and the reliability of research results. Criteria for the 3 variants of PPA—nonfluent/agrammatic, semantic, and logopenic—were developed by an international group of PPA investigators who convened on 3 occasions to operationalize earlier published clinical descriptions for PPA subtypes. Patients are first diagnosed with PPA and are then divided into clinical variants based on specific speech and language features characteristic of each subtype. Classification can then be further specified as “imaging-supported” if the expected pattern of atrophy is found and “with definite pathology” if pathologic or genetic data are available. The working recommendations are presented in lists of features, and suggested assessment tasks are also provided. These recommendations have been widely agreed upon by a large group of experts and should be used to ensure consistency of PPA classification in future studies. Future collaborations will collect prospective data to identify relationships between each of these syndromes and specific biomarkers for a more detailed understanding of clinicopathologic correlations.
A progressive disorder of language associated with atrophy of the frontal and temporal regions of the left hemisphere was first described in the 1890s by Pick1 and Serieux.2 In the modern literature, Mesulam3 described a series of cases with “slowly progressive aphasia,” subsequently renamed primary progressive aphasia (PPA).4 Warrington5 described a progressive disorder of semantic memory in 1975. This condition was also described by Snowden et al.6 as semantic dementia. In the early 1990s, Hodges and colleagues7 provided a comprehensive characterization of semantic dementia. Subsequently, Grossman et al.8 described a different form of progressive language disorder, termed progressive nonfluent aphasia. A consensus meeting attempted to develop criteria for these conditions in relation to frontotemporal lobar degeneration.9 For about 2 decades, cases of PPA were generally categorized as semantic dementia or progressive nonfluent aphasia, or in some studies as “fluent” vs “nonfluent.” However, there were a number of PPA cases that did not seem to fit a binary classification,10 and a third clinical variant was empirically described and termed logopenic progressive aphasia by Gorno-Tempini et al.11 Despite these advances, the typical features of these main PPA clinical presentations have not been clearly defined and agreed upon. Adopting a common clinical classification scheme is the first step for effective scientific exchange across centers studying the cognitive, neuroanatomic, and molecular basis of PPA. The collection of data on the presence or absence of specific clinical features, along with neuroimaging, autopsy, and genetic data, will allow determination of the best approach for predicting pathology. Widely accepted clinical criteria, likely supplemented by other biomarkers, will become essential in the coming years as a new generation of disease-modifying treatment options emerges.
The aim of this article is to provide a common framework for the classification of PPA and its main clinical variants, in order to facilitate uniformity in clinical diagnosis and multicenter studies.
DEVELOPMENT OF THE PROPOSED CLASSIFICATION OF PPA AND ITS VARIANTS
A group of experienced clinicians met 3 times between 2006 and 2009 with the aim of identifying a potential common classification system for PPA subtypes. The group reviewed video presentations of 12 PPA cases from different sites. A list of 17 salient speech and language features was provided and each clinician rated whether specific language features were present or not. Videos included a component of spontaneous speech and various portions of formal language evaluations. The analysis of the responses revealed a high level of agreement. There were 15 clinical features in which experts agreed over 80% of the time. Thirteen of these features were included in an operationalized classification scheme listing the main features of the 3 most commonly recognized clinical presentations of PPA. Group members examined the criteria within their own research programs and discussed them during a third meeting and via e-mail discussions.
Investigators agreed that the proposed classification is intended to be most applicable at the relatively early stages of the disease and identifies only the 3 most commonly reported clinical presentations of PPA. A minority of patients may present with single, isolated language symptoms (such as anomia or dyslexia) or some will show mixed features. Although these patients will remain as “PPA unclassifiable,” their clinical syndrome may become clearer as disease progresses.
Relationship between behaviorally defined PPA variants and biomarkers.
Considerable advances have been made in characterizing neuroimaging and biological features of PPA and it was collectively decided to incorporate these findings into the classification system.
Brain atrophy in PPA was initially thought to encompass widespread perisylvian regions within the left hemisphere.3 Later studies reported associations between different PPA clinical presentations and particular patterns of neuroanatomic damage: left posterior frontal and insular regions in nonfluent forms,8,11–13 anterior temporal region in semantic dementia,7,14 and left temporo-parietal regions in the logopenic variant.11 These data showed that site of maximal anatomic damage within the language network determines the different clinical presentations in PPA, similar to aphasia caused by stroke, and prompted the introduction of neuroimaging findings in the proposed classification.
PPA is a clinical syndrome with heterogeneous neuropathologic causes. Current data show that most patients with PPA have been found to have tau-positive, ubiquitin/TDP43-positive frontotemporal lobar degeneration (FTLD) pathology,13,15,16 or Alzheimer disease (AD) pathology.17,18 Clinicopathologic studies have most often linked nonfluent progressive aphasia to tau-positive pathology,13,19,20 semantic dementia to ubiquitin-positive, TDP43-positive pathology,15,20–23 and the logopenic form to AD pathology20 and to in vivo biomarkers suggestive of AD, such as PET-PIB positivity and decreased Aβ42 and increased tau in the CSF.20,24 However, clinical-pathologic correlations in PPA reflect group-wide probabilities and there is no direct correspondence between each clinical and anatomic subtype and pathology, suggesting only relative vulnerability of certain neural networks for different pathologies.25 In fact, each pathologic FTLD subtype and even AD pathology changes have been associated to each of the clinical presentations, although with different frequencies. Furthermore, PPA clinical-pathologic studies coming from different laboratories can be difficult to interpret in a unitary fashion because of underspecification and variability of the clinical criteria adopted across centers. Consistent clinical descriptions, supplemented by neuroimaging and other biomarkers, and larger patient groups are necessary to determine reliable frequency of associations between clinical and anatomic data and pathology.
The last decade has provided significant advances in the field of PPA genetics. PPA can be inherited in an autosomal dominant manner; the majority of these patients have mutations in the progranulin (GRN) gene.26–28 The PPA phenotype associated with a GRN mutation has not been studied in detail. Initial reports indicated prominent anomia without development of motor speech impairment and relatively early single-word comprehension impairment.26,27,29–31 Other genes related to FTLD, such as the microtubule-associated protein tau (MAPT) gene, may be associated with PPA as well.26,28 It is not yet clear how the classification reported here will apply to genetic cases but genetic information is included as definite etiologic evidence, equivalent to pathology.
Terminology.
The terms used to label the 3 variants were a matter of extensive discussion during the group meetings. The terminology proposed here represents a compromise between the existing literature and current understanding of the phenomenology. The terms were selected to parallel the behavioral variant FTD terminology since PPA has long been considered one of the possible clinical presentations of the frontotemporal spectrum disorders. It is advisable to include alternative terms in the key words of new articles, to facilitate the use of literature search engines. Furthermore, this classification scheme can be referred to even when applying older terminology.
WORKING RESEARCH CRITERIA
Diagnostic process and the PPA classification.
Establishing a classification or “clinical diagnosis” involves a 2-step process. Patients should first meet basic PPA criteria, based on Mesulam's32,33 initial and current guidelines (table 1). A PPA clinical diagnosis requires a prominent, isolated language deficit during the initial phase of the disease. There is an insidious onset and gradual progressive impairment of language production, object naming, syntax, or word comprehension that is apparent during conversation or through speech and language assessments. Activities of daily living are maintained except those related to language (e.g., using the telephone). Aphasia should be the most prominent deficit at symptom onset, for the initial phases of the disease and at time of examination.34 Other cognitive functions may be affected later on, but language remains the most impaired domain throughout the course of the illness.33,35
Table 1.
Inclusion and exclusion criteria for the diagnosis of PPA: Based on criteria by Mesulam32
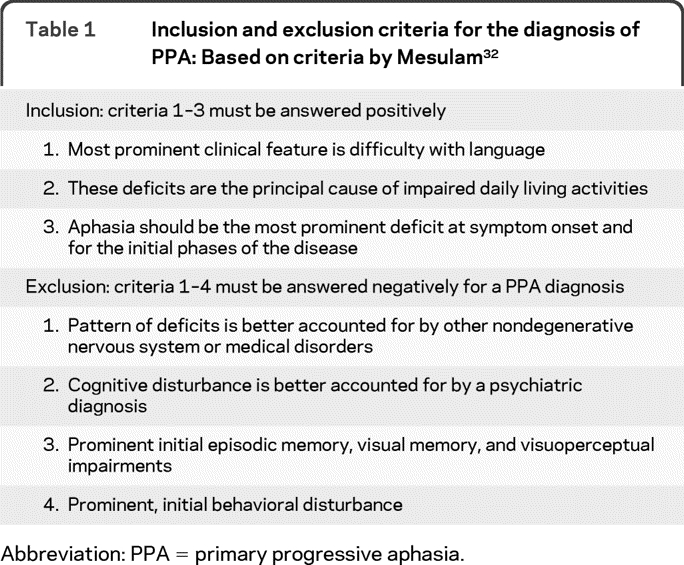
Abbreviation: PPA = primary progressive aphasia.
Exclusionary criteria include prominent episodic and nonverbal memory loss and visuospatial impairment during the initial stages of the illness. Specific causes of aphasia, such as stroke or tumor, are absent, as ascertained by neuroimaging. Behavioral disturbances can be early features in PPA (especially in the nonfluent and semantic variants), but they should not be the main complaint or the main cause of functional impairment. Similarly, a clear parkinsonian syndrome (rigidity, tremor) should not be present at time of diagnosis although mild limb apraxia and difficulty with fine finger movements can be noted. Cases with severe, isolated spastic dysphonia or repetitive language behaviors such as palilalia or echolalia should be excluded from the PPA syndrome because their deficit is nonlinguistic in nature.
Classification into PPA variants.
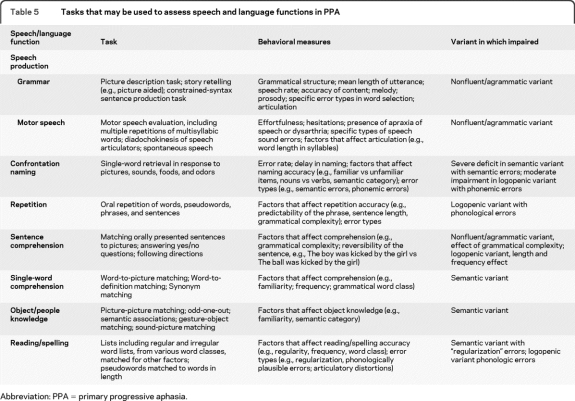
Once a PPA diagnosis is established, the relative presence or absence of salient speech and language features should be considered to classify PPA variants. The main language domains considered are speech production features (grammar, motor speech, sound errors, and word-finding pauses), repetition, single-word and syntax comprehension, confrontation naming, semantic knowledge, and reading/spelling. Clinical criteria for each variant are detailed in tables 2–4. Brief clinical evaluation of all these domains is necessary to correctly classify patients into PPA subtypes. A 20-minute bedside language examination could be sufficient, although detailed evaluation by a speech and language pathologist is likely to be more reliable. Suggested tasks for assessing speech and language functions are presented in table 5. Specific tests and cutoffs would be useful and have been proposed by Mesulam et al.34 They are not suggested here because different clinical and research sites are likely to apply different measures.
Table 2.
Diagnostic features for the nonfluent/agrammatic variant PPA
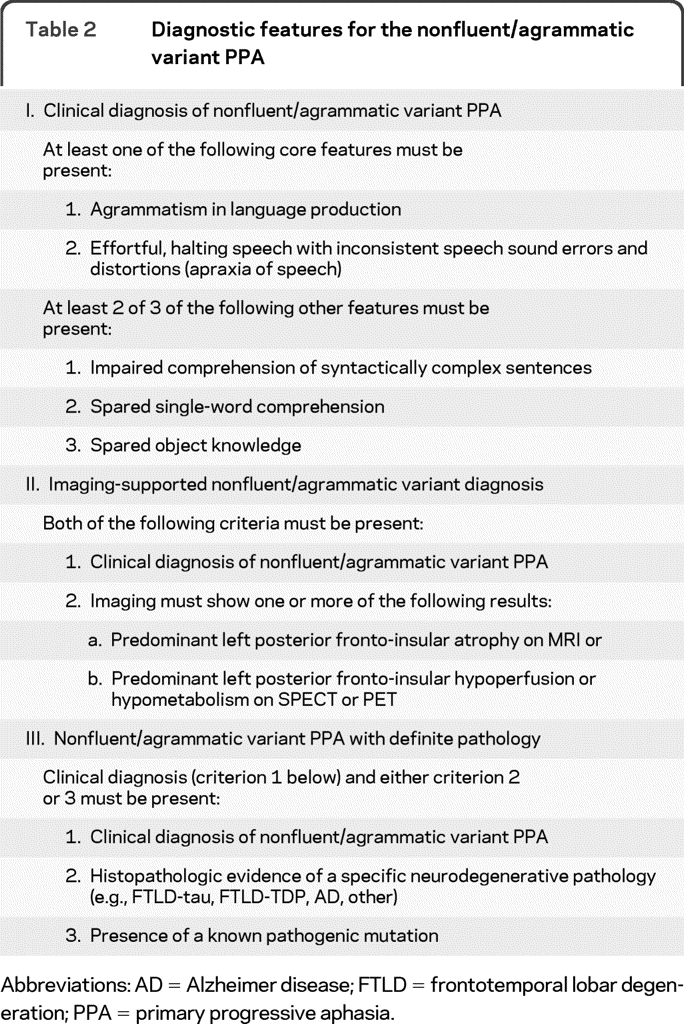
Abbreviations: AD = Alzheimer disease; FTLD = frontotemporal lobar degeneration; PPA = primary progressive aphasia.
Table 4.
Diagnostic criteria for logopenic variant PPA

Abbreviations: AD = Alzheimer disease; FTLD = frontotemporal lobar degeneration; PPA = primary progressive aphasia.
Table 5.
Tasks that may be used to assess speech and language functions in PPA
Abbreviation: PPA = primary progressive aphasia.
The classification of PPA into one of the variants may occur at one of 3 levels: clinical, imaging-supported, or definite pathologic diagnosis. Clinical diagnosis occurs when a case presents with speech and language features that are characteristic of a specific variant. At least one of the core features should be present for the nonfluent/agrammatic variant, while both must be present for semantic and logopenic variants. At least 2 (for the nonfluent variant) or 3 (for the semantic and logopenic variants) other features should be present in order to make a clinical diagnosis of a specific syndrome.
For an imaging-supported diagnosis, the next level of classification, a case should meet clinical criteria but should also show the distribution of neuroimaging changes (structural or functional imaging) previously associated with each variant. Since there is a direct correspondence between language symptoms and site of anatomic damage, a consistent pattern of imaging change supports the clinical classification.
The third level, a definite pathology diagnosis, refers to cases that present with typical clinical characteristics (with or without neuroimaging evidence) of each variant and pathologic or genetic mutations associated with definite or FTLD spectrum, AD, or other specific etiology. The presence of definite pathology does not imply that the clinical syndrome is better defined clinically, but only that it has been associated with a known biological feature.
In the future, biological markers, such as PET-PIB and CSF, might also be considered here.
Nonfluent/agrammatic variant PPA (also known as progressive nonfluent aphasia and as PPA-agrammatic).
The criteria for the nonfluent/agrammatic variant (for convenience hereafter called nonfluent) are summarized in table 2. Agrammatism in language production and effortful speech are the core criteria, and at least one should be present. Agrammatism typically consists of short, simple phrases and omissions of grammatical morphemes (e.g., function words, inflections). Effortful speech refers to slow, labored speech production. An articulation planning deficit, i.e., apraxia of speech, is often the most common disturbance, and can be the initial sign of the disease.11,13 Patients with the nonfluent variant typically make inconsistent speech sound errors, consisting of distortions, deletions, substitutions, insertions, or transpositions of speech sounds, of which they are often aware. Prosody is disrupted, and rate of speech is markedly reduced.11,36 Effortful speech and production errors can be the first symptoms of this variant, even before clear apraxia of speech or agrammatic errors occur. In these cases, a written language production test (such as a written description of a picture) or syntax comprehension tasks can often reveal early, mild grammatical errors.
At least 2 of the other 3 features of the nonfluent variant should be present. Deficits in syntax comprehension are evidenced by impairment of sentence comprehension, initially only for the most difficult syntactic constructions, such as negative passives and object relative clauses (e.g., “The car that the truck hit was green”).8,9,11,37,38 While sentence comprehension can also be impaired in logopenic variants, in the nonfluent presentation, the impairment is clearly influenced by the grammatical complexity of the sentence. Single-word comprehension and object knowledge are usually relatively spared in nonfluent patients and this feature is helpful in early differential diagnosis. It is now known that many patients with the nonfluent variant will eventually progress to a syndrome encompassing generalized motor problems compatible with a diagnosis of corticobasal syndrome or progressive supranuclear palsy.13,19,23 A clinical diagnosis of nonfluent variant PPA, therefore, should be limited to patients who do not present with a clear motor syndrome that impacts daily activities, such as generalized rigidity or tremor. However, as stated above, the presence of mild apraxia or slowing of fine finger movements does not exclude a PPA diagnosis.
Imaging abnormalities in the left posterior fronto-insular region, i.e., inferior frontal gyrus, insula, premotor, and supplementary motor areas, are necessary to make a diagnosis of imaging-supported nonfluent variant.8,11–13
Cases will be defined as nonfluent with definite pathology when patients present with the above reported clinical features, imaging-supported or not, and a known histopathologic diagnosis. Based on the literature, nonfluent patients with agrammatism or motor speech disturbance will most often show FTLD-tau or, less often, FTLD-TDP type of pathologic changes.
Semantic variant PPA (also known as semantic dementia, or as PPA-semantic).
The semantic variant is probably the most consistently defined PPA clinical syndrome. The criteria are summarized in table 3. In the current guidelines, anomia and single-word comprehension deficits are the core features, both essential for diagnosis. Although naming problems are present in other variants of PPA and in other neurodegenerative conditions causing aphasia, in the semantic variant the disturbance is severe, particularly when compared to relative sparing of other language domains. Single-word comprehension is severely impaired, especially for low-frequency items (e.g., “zebra” vs the more familiar/frequent “cat”). Inability to comprehend low-familiarity words can be the only symptom accompanying anomia at the earliest stages. Poor comprehension of single words is usually the earliest and most obvious manifestation of a widespread semantic memory deficit that causes impairments in object and person recognition, even when presented to other modalities of input such as visual (pictorial representations and real objects), tactile, olfactory, and gustatory.6,7,39–41 Semantic deficits in other modalities of input are therefore included among the other diagnostic features of the semantic variant. Semantic deficits are usually present for most categories (i.e., tools, animals, people), although rarer cases have been described with greater, or even selective, deficits for people and animals.42,43 Others describe worse performance with concrete object concepts than abstract concepts.5,44 These cases are usually associated with greater right temporal atrophy and early behavioral changes, such as loss of empathy and compulsions.45
Table 3.
Diagnostic criteria for the semantic variant PPA

Abbreviations: AD = Alzheimer disease; FTLD = frontotemporal lobar degeneration; PPA = primary progressive aphasia.
Surface dyslexia and dysgraphia are features of the semantic variant and refer to an impairment in reading and writing words with “irregular” or atypical relationship between spelling and pronunciation.46 Patients typically “regularize” such words, so that “sew” is read as /su/.
Sparing of repetition and motor speech, even when semantic deficits are prominent, are the other 2 features. Although language production is usually grammatically accurate, it can at times contain some “paragrammatic” errors, such as substituting less appropriate closed class words or inflections, for instance, “I know what they're doing but I can't think the words what they're doing.”47
Anatomically, the semantic variant has been associated with atrophy in the ventral and lateral portions of the anterior temporal lobes bilaterally, although damage is usually greater on the left.11,14,34,48,49
Cases will be defined as semantic variant PPA with definite pathology when patients present with the above mentioned clinical features, imaging-supported or not, and a known histopathologic diagnosis. Based on the literature, in the case of semantic variant PPA, FTLD-TDP type pathologic changes will be the most common finding.15,20–23
Logopenic variant PPA (also known as logopenic progressive aphasia or as logopenic PPA).
The logopenic variant is the most recently described variant of PPA.11,50 The criteria are summarized in table 4. Word retrieval (in spontaneous speech and confrontation naming) and sentence repetition deficits are the core features of the logopenic variant. Spontaneous speech is characterized by slow rate, with frequent pauses due to significant word-finding problems, but there is no frank agrammatism. Speech production deficits are therefore distinct from those of patients with the nonfluent variant, who also speak in a slow and halting manner, but with output that is dysprosodic, and marked by motor speech errors or agrammatism.8,50,51 The confrontation naming impairment is usually less severe in the logopenic than in the semantic variant, and errors are usually phonologic in nature.11 A useful differentiating feature between these 2 variants is also the relative sparing of single-word comprehension in logopenic patients. Consistent with the hypothesis that a phonologic short-term memory deficit is a key cognitive mechanism underlying most language deficits in the logopenic variant,50 sentence and phrase repetition is characteristically impaired, while reproduction of short, single words can be spared. This same mechanism can cause impairment in sentence comprehension, which is influenced more by length and probability of a sentence than by its grammatical complexity.
Other diagnostic features include phonologic paraphasias in spontaneous speech and naming. The sound substitutions that result in phonologic paraphasias in logopenic patients are usually well articulated, without distortions. Lack of frank agrammatic errors and preservation of articulation and prosody help distinguish the logopenic from the nonfluent variants.52
Imaging abnormalities in the left temporo-parietal junction area, i.e., posterior temporal, supramarginal, and angular gyri, are necessary to make a diagnosis of imaging-supported logopenic variant.11
Cases will be defined as having logopenic PPA with definite pathology when they present with the clinical features, imaging-supported or not, and a known histopathologic picture. Recent evidence shows that AD might be the most common underlying pathology.20,24
DISCUSSION
We have delineated a classification system that can be adopted in the diagnosis of PPA and its main variants for clinical and research purposes. Uniformity in diagnosis across centers is essential for ensuring reliable results in future studies that will seek to define the underlying biology of disease in specific PPA.
Currently, data indicate that there are only groupwise probabilities and no absolute correspondence between PPA clinical phenotypes and pathologic or genetic findings. This is not surprising since clinical presentation depends on site of anatomic damage and the selectivity of specific neurodegenerative diseases for certain neural networks is only relative. However, heterogeneity in the clinical criteria adopted for clinical diagnosis across centers can also contribute to produce inconsistent results between studies. The classification scheme proposed here attempts to correct this latter problem by proposing a classification scheme that has been accepted by most researchers in the field. Ultimately, improved clinicopathologic correlations will be obtained by incorporating in the diagnostic process biomarkers (e.g., molecular PET imaging or CSF markers) that will allow underlying tau, TDP-43, or AD pathology to be diagnosed in life. For this purpose, we encourage investigators to collect biofluid, neuropathologic and genetic data, along with a checklist of all clinical and neuroimaging features that were observed in the patient for future large-scale collaborative investigations.
There are a number of outstanding challenges in the field of PPA research. Determining the primary cognitive deficit in the nonfluent variant is a matter of much debate. Agrammatism and motor speech errors may be quite subtle initially and difficult to detect with clinical measures. Also, some patients may present with predominant apraxia of speech, which has led many to question whether the introduction of a separate syndrome of progressive apraxia of speech may be more appropriate for these cases,13 although such cases most often do appear to eventually develop an aphasia as the disease progresses. Developing reliable and objective measures that capture patients early in the disease process is very important. Toward this end, more longitudinal studies of PPA are required, particularly for the logopenic variant, which is the least consistently defined presentation. In addition, the features of genetic forms of PPA need to be studied in more detail, to identify whether the phenotypes fit into one of the 3 variants or present as a separate or mixed PPA phenotype.
Despite these outstanding challenges, the proposed classification is an attempt to provide a common approach to PPA patient classification across centers. Future studies aimed at identifying associations between subtypes and specific imaging, genetic, or neuropathologic findings will be more interpretable if these guidelines are utilized across centers.
- AD
- Alzheimer disease
- FTLD
- frontotemporal lobar degeneration
- PPA
- primary progressive aphasia
Editorial, page 942
DISCLOSURE
Dr. Gorno-Tempini receives research support from the NIH (NINDS, NIA), the John Douglas French Alzheimer's Foundation, the Alzheimer's Association, the Larry L. Hillblom Foundation, the Koret Family Foundation, and the McBean Family Foundation. Dr. Hillis serves as an Associate Editor for Brain and Aphasiology, Co-Editor-in-Chief for Behavioural Neurology, a Section Editor for Nature Reviews Neurology, and on the editorial boards of Brain and Language and Cognitive Neuropsychology; serves as a consultant for the Charles Dana Foundation; and receives research support from the NIH (NINDS, NIDCD). Dr. Weintraub serves on the editorial boards of Alzheimer's and Dementia, the Turkish Journal of Neurology, Dementia & Neuropsychologia, and the Journal of the Brazilian Academy of Neurology; and receives research support from the NIH (NIA, NIDCD). Dr. Kertesz serves on a scientific advisory board for Pfizer Inc.; serves on the editorial boards of Cognitive and Behavioral Neurology and Aphasiology; receives royalties from the publication of The Western Aphasia Battery (Grune and Stratton, 1982); and has received research support from Elan Corporation, Pfizer Inc., Lundbeck Inc., the Lawson Research Institute, the American Neurological Society, and the Whitaker professorship. Dr. Mendez serves on the editorial boards of Brain, Annals of Neurology, and Human Brain Mapping and on the Advisory Boards of the Cure Alzheimer Fund and the Association for Frontotemporal Dementia. Dr. Cappa has received speaker honoraria from Novartis; serves as Co-Editor-in-Chief for Behavioural Neurology, an Associate Editor for Neurological Sciences, Action Editor for Cortex, and on the editorial boards of Aphasiology, Neuropsychological Rehabilitation, and Future Neurology; receives publishing royalties for Cognitive Neurology: A Textbook (Oxford University Press, 2009) and Cognitive Neurology (Imperial College Press, 2009); and has received speaker honoraria from Janssen and Novartis. J.M. Ogar receives research support from the NIH. Dr. Rohrer has received research support from the Wellcome Trust and from a Brain Exit Scholarship. Dr. Black serves on a scientific advisory board for Pfizer Inc.; has received funding for travel or speaker honoraria from Pfizer Inc., Eisai Inc., and Myriad Genetics, Inc.; serves on the editorial board of Alzheimer's Research & Therapy; is an inventor on a patent re: INCAS (Integrated NeuroCognitive Assessment System): Cognitive Assessment Tool and Method; has served as a consultant for Pfizer Inc., Janssen, Novartis, Lundbeck Inc., Myriad Genetics, Inc., GlaxoSmithKline, Schering-Plough Corp., Elan Corporation, Wyeth, and Bristol-Myers Squibb; serves on speakers' bureaus for Janssen, Novartis, Lundbeck Inc., Pfizer Inc., Eisai Inc., and Myriad Genetics, Inc.; and receives research support from Roche, GlaxoSmithKline, Pfizer Inc., Novartis, Lundbeck Inc., Myriad Genetics, Inc., Boehringer Ingelheim, Sanofi-Aventis, Eisai Inc., the CIHR, the NIH, the Canada Foundation for Innovation, the Heart and Stroke Foundation of Ontario, Canadian Stroke Network, Sunnybrook Health Sciences Centre, NSERC, the Alzheimer Society of Canada, and the Alzheimer's Association US. Dr. Boeve receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge University Press, 2009); has served as a consultant for GE Healthcare; and receives research support from Myriad Genetics, Inc., Cephalon, Inc., the NIH, and the Alzheimer's Association. Dr. Manes reports no disclosures. Dr. Dronkers receives research support from the NIH and the US Department of Veterans Affairs. Dr. Vandenberghe serves on a scientific advisory board for AC Immune SA; serves on the editorial board of Frontiers in Neuroscience and Translational Neuroscience; receives research support from GE Healthcare, Pfizer Inc., Eli Lilly and Company, Medivation, Inc., Novartis, Research Foundation Flanders, Interuniversity Attraction Pole P6/29, KU Leuven, and Stichting Alzheimer Onderzoek. Dr. Rascovsky reports no disclosures. Dr. Patterson serves as Consulting Editor for Cortex and receives publishing royalties for Pyramids and Palmtrees Test (Thames Valley Publishing Company, 1992–present). Dr. Miller serves on a scientific advisory board for the Alzheimer's Disease Clinical Study, serves as an Editor for Neurocase, and as an Associate Editor of ADAD; receives royalties from the publication of Behavioral Neurology of Dementia (Cambridge, 2009), Handbook of Neurology (Elsevier, 2009), and The Human Frontal Lobes (Guilford, 2008); serves as a consultant for Lundbeck Inc., Allon Therapeutics, Inc., and Novartis; has served on speakers' bureaus for Novartis and Pfizer Inc.; and receives research support from Novartis, the NIH, and the State of California Alzheimer's Center. Dr. Knopman serves as Deputy Editor of Neurology®; has served on data safety monitoring boards for Sanofi-Aventis, GlaxoSmithKline, and Eli Lilly and Company; is an investigator in clinical trials sponsored by Elan Corporation, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH. Dr. Hodges serves on editorial boards of Aphasiology, Cognitive Neuropsychiatry, and Cognitive Neuropsychology; receives royalties from publication of Cognitive Assessment for Clinicians (Oxford University Press, 2007) and Frontotemporal Dementia Syndromes (Cambridge University Press, 2007); and receives fellowship support from the Australian Research Council Federation. Dr. Mesulam serves on the scientific advisory boards for the Cure Alzheimer Fund and the Association on Frontotemporal Dementia; serves on the editorial boards of Brain, Annals of Neurology, Human Brain Mapping, and Journal of Cognitive Neuroscience; receives royalties from the publication of Principles of Behavioral and Cognitive Neurology (Oxford University Press, 2000); and receives research support from the NIH (NIDCD, NIA). Dr. Grossman serves on a scientific advisory board for Allon Therapeutics, Inc.; serves as Editor for Cognitive and Behavioral Neurology; has served as a consultant for Pfizer Inc. and Forest Laboratories, Inc.; and receives research support from the NIH.
REFERENCES
- 1. Pick A. Uber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prager Medizinische Wochenschrift 1892;17:165–167 [Google Scholar]
- 2. Serieux P. Sur un cas de surdite verbale pure. Rev Med 1893;13:733–750 [Google Scholar]
- 3. Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol 1982;11:592–598 [DOI] [PubMed] [Google Scholar]
- 4. Mesulam MM, Weintraub S. Spectrum of primary progressive aphasia. Baillieres Clin Neurol 1992;1:583–609 [PubMed] [Google Scholar]
- 5. Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol 1975;27:635–657 [DOI] [PubMed] [Google Scholar]
- 6. Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behav Neurol 1989;2:167–182 [Google Scholar]
- 7. Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain 1992;115:1783–1806 [DOI] [PubMed] [Google Scholar]
- 8. Grossman M, Mickanin J, Onishi K, et al. Progressive non-fluent aphasia: language, cognitive and PET measures contrasted with probable Alzheimer's disease. J Cogn Neurosci 1996;8:135–154 [DOI] [PubMed] [Google Scholar]
- 9. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554 [DOI] [PubMed] [Google Scholar]
- 10. Grossman M, Ash S. Primary progressive aphasia: a review. Neurocase 2004;10:3–18 [DOI] [PubMed] [Google Scholar]
- 11. Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 2004;55:335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centered on the left anterior insula. Brain 2003;126:2406–2418 [DOI] [PubMed] [Google Scholar]
- 13. Josephs KA, Duffy JR, Strand EA, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 2006;129:1385–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol 2000;47:36–45 [PubMed] [Google Scholar]
- 15. Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol 2004;56:399–406 [DOI] [PubMed] [Google Scholar]
- 16. Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain 2005;128:1996–2005 [DOI] [PubMed] [Google Scholar]
- 17. Mesulam MM, Grossman M, Hillis A, Kertesz A, Weintraub S. The core and halo of primary progressive aphasia and semantic dementia. Ann Neurol 2003;54(suppl 5):S11–S14 [DOI] [PubMed] [Google Scholar]
- 18. Forman MS, Farmer J, Johnson JK, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol 2006;59:952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol 2006;59:156–165 [DOI] [PubMed] [Google Scholar]
- 20. Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008;63:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain 2005;128:1984–1995 [DOI] [PubMed] [Google Scholar]
- 22. Grossman M, Wood EM, Moore P, et al. TDP-43 pathologic lesions and clinical phenotype in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Arch Neurol 2007;64:1449–1454 [DOI] [PubMed] [Google Scholar]
- 23. Nestor PJ, Balan K, Cheow HK, et al. Nuclear imaging can predict pathologic diagnosis in progressive nonfluent aphasia. Neurology 2007;68:238–239 [DOI] [PubMed] [Google Scholar]
- 24. Rabinovici GD, Jagust WJ, Furst AJ, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol 2008;64:388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009;62:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Snowden JS, Pickering-Brown SM, Mackenzie IR, et al. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain 2006;129:3091–3102 [DOI] [PubMed] [Google Scholar]
- 27. Mesulam M, Johnson N, Krefft TA, et al. Progranulin mutations in primary progressive aphasia: the PPA1 and PPA3 families. Arch Neurol 2007;64:43–47 [DOI] [PubMed] [Google Scholar]
- 28. van Swieten J, Spillantini MG. Hereditary frontotemporal dementia caused by tau gene mutations. Brain Pathol 2007;17:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krefft TA, Graff-Radford NR, Dickson DW, Baker M, Castellani RJ. Familial primary progressive aphasia. Alzheimer Dis Assoc Disord 2003;17:106–112 [DOI] [PubMed] [Google Scholar]
- 30. Snowden JS, Pickering-Brown SM, Du Plessis D, et al. Progressive anomia revisited: focal degeneration associated with progranulin gene mutation. Neurocase 2007;13:366–377 [DOI] [PubMed] [Google Scholar]
- 31. Rohrer JD, Warren JD, Barnes J, et al. Mapping the progression of progranulin-associated frontotemporal lobar degeneration. Nat Clin Pract Neurol 2008;4:455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mesulam MM. Primary progressive aphasia. Ann Neurol 2001;49:425–432 [PubMed] [Google Scholar]
- 33. Mesulam MM. Primary progressive aphasia: a language-based dementia. N Engl J Med 2003;349:1535–1542 [DOI] [PubMed] [Google Scholar]
- 34. Mesulam M, Weineke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol 2009;66:1545–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Libon DJ, Xie SX, Wang X, et al. Neuropsychological decline in frontotemporal lobar degeneration: a longitudinal analysis. Neuropsychology 2009;23:337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord 2007;21:S23–S30 [DOI] [PubMed] [Google Scholar]
- 37. Hodges JR, Patterson K. Nonfluent progressive aphasia and semantic dementia: a comparative neuropsychological study. J Int Neuropsychol Soc 1996;2:511–524 [DOI] [PubMed] [Google Scholar]
- 38. Peelle JE, Troiani V, Gee J, et al. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics 2008;21:418–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia 2000;38:1207–1215 [DOI] [PubMed] [Google Scholar]
- 40. Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon RMA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 2007;45:1823–1831 [DOI] [PubMed] [Google Scholar]
- 41. Adlam AL, Patterson K, Rogers TT, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain 2006;129:3066–3080 [DOI] [PubMed] [Google Scholar]
- 42. Evans JJ, Heggs AJ, Antoun N, Hodges JR. Progressive prosopagnosia associated with selective right temporal lobe atrophy: a new syndrome? Brain 1995;118:1–13 [DOI] [PubMed] [Google Scholar]
- 43. Gainotti G. Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia 2007;45:1591–1607 [DOI] [PubMed] [Google Scholar]
- 44. Yi HA, Moore P, Grossman M. Reversal of the concreteness effect for verbs in patients with semantic dementia. Neuropsychology 2007;21:9–19 [DOI] [PubMed] [Google Scholar]
- 45. Seeley WW, Bauer AM, Miller BL, et al. The natural history of temporal variant frontotemporal dementia. Neurology 2005;64:1384–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wilson SM, Brambati SM, Henry RG, et al. The neural basis of surface dyslexia in semantic dementia. Brain 2009;132:71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meteyard L, Patterson K. The relation between content and structure in language production: an analysis of speech errors in semantic dementia. Brain Lang 2009;110:121–134 [DOI] [PubMed] [Google Scholar]
- 48. Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology 2001;57:216–225 [DOI] [PubMed] [Google Scholar]
- 49. Rosen HJ, Kramer JH, Gorno-Tempini ML, Schuff N, Weiner M, Miller BL. Patterns of cerebral atrophy in primary progressive aphasia. Am J Geriatr Psychiatry 2002;10:89–97 [PubMed] [Google Scholar]
- 50. Gorno-Tempini ML, Brambati SM, Ginex V, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology 2008;71:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ash S, Moore P, Vesely L, et al. Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics 2009;22:370–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilson S, Henry M, Besbri M, et al. Connected speech production in three variants of primary progressive aphasia. Brain Epub 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]