Abstract
The mercury(II) complexes formed in neutral aqueous solution with glutathione (GSH, here denoted AH3 in its tri-protonated form) were studied using Hg LIII-edge extended X-ray absorption fine structure (EXAFS) and 199Hg NMR spectroscopy, complemented with electrospray ionization mass spectrometric (ESI-MS) analyses. The [Hg(AH)2]2− complex, with the Hg-S bond distances 2.325 ± 0.01 Å in linear S-Hg-S coordination and the 199Hg NMR chemical shift −984 ppm, dominates except at high excess of glutathione. In a series of solutions with CHg(II) ~17 mM and GSH/Hg(II) mole ratios rising from 2.4 to 11.8, the gradually increasing mean Hg-S bond distance corresponds to an increasing amount of the [Hg(AH)3]4− complex. ESI-MS peaks appear at −m/z values of 1208 and 1230 corresponding to the [Na4Hg(AH)2(A)]− and [Na5Hg(AH)(A)2]− species, respectively. In another series of solutions at pH = 7.0 with CHg(II) ~50 mM and GSH/Hg(II) ratios from 2.0 to 10.0, the Hg LIII-edge EXAFS and 199Hg NMR spectra show that at high excess of glutathione (~0.35 mol·dm−3) about ~ 70% of the total mercury(II) concentration is present as the [Hg(AH)3]4− complex, with the average Hg-S bond distance 2.42 ± 0.02 Å in trigonal HgS3 coordination. The proportions of HgSn species, n = 2, 3 and 4, quantified by fitting linear combinations of model EXAFS oscillations to the experimental EXAFS data in our present and previous studies, were used to obtain stability constants for the [Hg(AH)3]4− complex, and also for the [Hg(A)4]10− complex that is present at high pH. For Hg(II) in low concentration at physiological conditions (pH = 7.4, CGSH = 2.2 mM) the relative amounts in the HgS2 species [Hg(AH)2]2−, [Hg(AH)(A)]3− and the HgS3 complex [Hg(AH)3]4− was calculated to be 95 : 2 : 3. Our results are not consistent with the formation of dimeric Hg(II)-GSH complexes proposed in a recent EXAFS study.
Keywords: Mercury(II), glutathione, structure, solution, Hg LIII-edge EXAFS, ESI-MS, 199Hg NMR
Introduction
Human exposure to the toxic heavy metal mercury remains a major concern because of its previous and still existing use in a number of commercial and medical products. Elemental mercury (Hg0) has been widely utilized for dental amalgams (1), and organic mercury compounds are found in fish as methyl mercury (CH3Hg+) (2), and in vaccine preservatives in the form of ethyl mercury (CH3CH2Hg+). The elemental and organic forms of mercury are in part metabolized to inorganic Hg(II) species in the body (3). Mercury toxicity is known to target the central nervous system and the kidneys, although its role in neurodegenerative disorders such as multiple sclerosis, Alzheimer's and Parkinson's diseases, and autism is a controversial subject (4).
The strong affinity of mercury(II) to the cysteinyl thiol groups in proteins and peptides can be detrimental to their normal function. One of the intracellular mechanisms of protection involves the abundant tripeptide glutathione (GSH, γ-L-glutamyl-L-cysteinyl-glycine, Scheme 1), which is able to bind to heavy metals and transport them out of the cell (5). The complex formation between mercury(II) and glutathione has been well characterized by polarographic and potentiometric titrations (6–8), 1H, 13C and 199Hg NMR (9–11), and ESI-MS (12–15). Two separate 13C NMR studies showed strong binding to the cysteinyl thiolate group of GSH forming the [Hg(AH)2]2− complex for aqueous solutions of GSH/Hg(II) = 2 (AH3 denotes the tri-protonated form of glutathione) (16, 17).
Scheme 1.
The major form of glutathione at pH = 7.0 (AH2−).
In their 13C NMR and polarimetric investigations, Cheesman et al. showed that for solutions with CGSH = 0.2 M and molar ratios of GSH/Hg(II) ≥ 3 at pH ~ 7, a three-coordinated [Hg(AH)3]4− complex forms with an estimated lifetime of 1.4 × 10−4 s (18, 19). They discussed the high lability of Hg(II) in biological systems, exchanging between sulfhydryl groups of enzymes and other molecules, and proposed that the rapid exchange of thiol ligands between free and Hg(thiol)2 forms proceeds via a mechanism involving Hg(thiol)3 complexes. They concluded that considering the ubiquity of glutathione in cellular systems those reactions play a major role in the mobility of Hg(II) in biological systems (18).
In a recent Hg LIII-edge EXAFS study we showed that the [Hg(GS)(GSH)]ClO4 compound, which precipitates from acidic mercury(II)–glutathione solutions (pH ~ 2.0), has linear S-Hg-S coordination geometry with a mean Hg–S distance of 2.33 ± 0.01 Å, and a Raman band at 331 cm−1 for the symmetric stretching v(S–Hg–S). Additionally, the [HgA2]4− complex was characterized in alkaline solutions at pH = 10.5 (CHg(II) ~18 mM, CGSH = 40 mM) with the Hg-S distance 2.32 ± 0.01 Å and the 199Hg chemical shift −961 ppm. For the higher complexes [HgA3]7− and [HgA4]10−, which formed at large excess of GSH, CGSH ≥ 160 mM, the mean Hg-S bond distances 2.42 ± 0.02 Å and 2.52 ± 0.02 Å, respectively, were obtained (20).
To gain a better understanding of the speciation and structures of the Hg(II)-GSH complexes as a continuation of our studies of mercury(II) complexes to bio-relevant ligands (20–22), and to provide a basis for detailed studies of the reaction mechanisms for the exchange of thiol-containing ligands in biological systems, we have characterized the [Hg(AH)2]2− and [Hg(AH)3]4− complexes formed at near physiological pH (~7.0) in aqueous solution, combining Hg LIII-edge XAS and 199Hg NMR spectroscopic techniques with ESI-MS. To evaluate the effect of mercury(II) and glutathione concentrations on the Hg(II) speciation, two series of solutions were prepared with different mercury(II) concentration (CHg(II) ~17 mM and 50 mM) with molar ratios of GSH/Hg(II) ranging from ~2 to ~12. The higher mercury(II) concentration (50 mM) made 199Hg NMR measurements possible, as complement to the EXAFS spectra. The relative amounts of HgS2 and HgS3 species in the form of [Hg(AH)2]2− and [Hg(AH)3]4− complexes in neutral aqueous solution were obtained, which allowed the free GSH concentration at pH 7.0, [AH2−], to be derived as: [AH2−] = CGSH − 2[Hg(AH)2]2− − 3[Hg(AH)3]4−.
Experimental
Sample preparation
Glutathione, sodium hydroxide and Hg(ClO4)2·~3.26H2O were purchased from Sigma-Aldrich and used without further purification. An acidic stock solution with CHg(II) = 0.17 M was prepared in ~ 0.5 M HClO4, where the total Hg(II) concentration was measured by the inductively coupled plasma (ICP) technique. The Hg(II)-GSH solutions were prepared in degassed distilled water under inert Ar atmosphere, and their pH was measured with a Corning Semi-Micro electrode calibrated with standard buffers. Our attempts to crystallize complexes of Hg(II)-GSH failed due to sample decomposition.
The series of mercury(II)-GSH solutions A1–F1 containing CHg(II) ~17 mM was synthesized by dissolving glutathione (0.2–1 mmol) in ~3.5 mL O2-free water (pH 2.7–2.8), followed by adding 0.5 mL of the stock Hg(II) solution (0.085 mmol Hg2+). The pH decreased to < 2.0 and a white precipitate formed that dissolved with further stirring. Sodium hydroxide solution (2.0 M) was added dropwise to adjust the solution pH to 7.0. Six solutions with GSH/Hg(II) mole ratios of 2.4 (A1), 3.5 (B1), 4.7 (C1), 5.9 (D1), 9.4 (E1) and 11.8 (F1) were prepared with a final volume of 5.0 mL and pH = 7.0 (Table 1). A similar procedure was used to prepare a series of solutions with total CHg(II) ~50 mM (1.5 mL stock solution) containing 10 % v/v D2O, and with GSH/Hg(II) mole ratios of 2.0 (A2), 3.0 (B2), 4.0 (C2), 5.0 (D2), 8.0 (E2), and 10.0 (F2). The 199Hg NMR spectra of solutions A1 and B1 were measured after addition of 10 % D2O, reducing their concentration to CHg(II) ~15 mM.
Table 1.
Composition of the Hg(II)-GSH Solutions Studied at pH = 7.0.
| Solution | CGSH/CHg(II) | CHg(II) (mM) | CGSH (mM) |
|---|---|---|---|
| A1 | 2.4 | 17a | 40 |
| B1 | 3.5 | 17a | 60 |
| C1 | 4.7 | 17 | 80 |
| D1 | 5.9 | 17 | 100 |
| E1 | 9.4 | 17 | 160 |
| F1 | 11.8 | 17 | 200 |
|
| |||
| A2 | 2.0 | 50 | 100 |
| B2 | 3.0 | 50 | 150 |
| C2 | 4.0 | 50 | 200 |
| D2 | 5.0 | 50 | 250 |
| E2 | 8.0 | 50 | 400 |
| F2 | 10.0 | 50 | 500 |
For 199Hg NMR measurements, 10% (v/v) D2O was added to these solutions, reducing CHg(II) to ~15 mM.
Mass spectrometry
Mass spectra were collected in negative ion mode by direct infusion of solution F1 into the electrospray ionization (ESI) source of a Bruker Esquire 3000 mass spectrometer using a continuous injection flow rate of 0.06 mL/min. The drying gas temperature was 300 °C, with a 4 L/min flow rate. The capillary voltage was set at ~3.1 kV, the skimmer voltage was −47.5 V. The mass spectrum of the most concentrated solution F2 could not be measured for technical reasons (contamination of the sample path in the instrument).
Nuclear Magnetic Resonance Spectroscopy
199Hg NMR spectra were collected at a resonance frequency of 53.76 MHz using a Bruker AMX 300 spectrometer equipped with a 10 mm broadband probe (BB10) for the Hg(II)-GSH solutions A1 and B1 (diluted to CHg(II) ~15 mM after adding 10 % D2O), and A2 – F2 (containing 10 % D2O and CHg(II) ~50 mM). The 199Hg chemical shift was externally calibrated relative to a saturated HgCl2 standard solution in D2O, resonating at −1550 ppm relative to the Hg(CH3)2 resonance at 0 ppm (20–23). NMR data were acquired using a 90° pulse, a sweep width of 59.2 kHz, and 32 K data points. A 1 s delay was used between scans, and 47000–186000 scans were collected. Spectra were processed using exponential line broadening: 10 % of v1/2 (25 – 250 Hz).
X-ray Absorption Spectroscopy Data Collection
Hg LIII-edge X-ray absorption spectra, averaging 3 to 4 scans for each sample, were collected on beamline 7-3 at the Stanford Synchrotron Radiation Lightsource (SSRL) under dedicated conditions (3 GeV, 85–100 mA). The ion chambers I0 (monitoring the incident beam) and I1 (after the sample) were filled with N2 gas for transmission measurements at ambient temperature, where each Hg(II)-GSH solution was held in a 10 mm (A1 – F1) or 5 mm (A2 – F2) Teflon spacer with 5 μm polypropylene windows. Higher order harmonics were rejected by a rhodium coated mirror on the fully tuned beam. To calibrate the energy of the X-rays, the first inflection point of the HgCl2 standard, measured simultaneously between I1 and I2 (filled with Ar gas), was set to 12284.0 eV.
X-ray Absorption Spectroscopy Data Analysis
The scans for each sample were visually compared using the EXAFSPAK suite of programs (24), prior to averaging. The EXAFS oscillation was extracted using the WinXAS 3.1 program (25), and converted to k-space, where k = [(8π2me/h2)(E−E0)]1/2, using the threshold energy E0 = 12285.0 eV. Effective amplitude, mean free path and phase shift functions were obtained by means of ab initio calculations with the FEFF 8.1 code (26, 27), applied on the atomic coordinates of the linearly coordinated Hg(cysteamine)2 complex (28), and used to simulate the theoretical EXAFS oscillations χ(k). The amplitude reduction factor, S02, was fixed at 1.0, the value obtained for the EXAFS data analysis of the solid [Hg(GS)(GSH)]ClO4 compound (20). Further details on EXAFS data analysis can be found elsewhere (21). The estimated errors for the coordination numbers and bond distances obtained from least squares curve-fitting of the EXAFS model functions are within ± 20 % and ± 0.02 Å, respectively.
Principal component analysis (PCA) implemented in the EXAFSPAK program, was applied to the k3-weighted EXAFS spectra of solutions C1 – F1 and B2 – F2 over the range of 3.9–11.9 Å−1, and indicated the presence of two major components in all these solutions (Figure S-1 and Table S-1). To quantify the relative proportion of the [Hg(AH)2]2− and [Hg(AH)3]4− complexes, linear combinations of the EXAFS model oscillations representing the [Hg(AH)2]2− and [Hg(AH)3]4− species, respectively, were fitted to the experimental EXAFS spectra for solutions C1 – F1 and B2 – F2, using DATFIT in the EXAFSPAK suite of programs (Figures S-2 and S-3) (24). The EXAFS oscillation derived from the model fitting for solutions A1 and A2 represented the [Hg(AH)2]2− species (Tables 2 and 3). The EXAFS oscillation for the [Hg(AH)3]4− complex was simulated according to the procedure described previously (20).
Table 2.
Structural Parameters Derived from EXAFS Least-Squares Curve Fitting for the Hg(II)-GSH Solutions A1 – E1 at pH = 7.0 (CHg(II) ~17 mM; see Figure 2).a
| Solution (GSH/Hg(II)) | Hg-S | Additional contributions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | R (Å) | σ2 (Å2) | Nb | R (Å) | σ2 (Å2) | ΔE0 (eV) | Rc | ||
| A1 (2.4) | 2.1 | 2.325 | 0.0041 | S-Hg-S | 2.1 | 4.66 | 0.0104 | 10.6 | 18.8 |
| B1 (3.5) | 2.0 | 2.331 | 0.0034 | S-Hg-S | 2.0 | 4.65 | 0.0071 | 10.7 | 19.0 |
| C1 (4.7) | 2.1 | 2.335 | 0.0049 | S-Hg-S | 2.1 | 4.65 | 0.0118 | 10.3 | 14.2 |
| D1 (5.9) | 2.1 | 2.340 | 0.0053 | S-Hg-S | 2.1 | 4.65 | 0.0125 | 9.8 | 15.0 |
| E1 (9.4) | 2.2 | 2.354 | 0.0063 | S-Hg-S | 2.2 | 4.65 | 0.0195 | 9.6 | 17.4 |
| F1 (11.8) | 2.5 | 2.368 | 0.0084 | 9.1 | 18.2 | ||||
Fitting k-range = 3.9 – 12.0 Å−1; S02 = 1.0; estimated error limits: N ± 20 %, R ± 0.02 Å, σ2±2 0.001 Å2;
Correlated to the coordination number N of Hg-S path.
Table 3.
Structural Parameters Derived from EXAFS Least-Squares Curve Fitting for the Hg(II)-GSH Solutions A2 – F2 at pH = 7.0 (CHg(II) ~50 mM; see Figure 3).a
| Solution (GSH/Hg(II)) | Hg-S | Additional contributions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | R (Å) | σ (Å2) | Nb | R (Å) | σ (Å2) | ΔE0 (eV) | Rc | ||
| A2 (2.0) | 2.1 | 2.324 | 0.0035 | S-Hg-S | 2.1 | 4.66 | 0.0078 | 10.7 | 12.3 |
| B2 (3.0) | 2.2 | 2.336 | 0.0051 | S-Hg-S | 2.2 | 4.64 | 0.0122 | 9.4 | 13.1 |
| C2 (4.0) | 2.2 | 2.352 | 0.0063 | S-Hg-S | 2.2 | 4.63 | 0.0120 | 8.9 | 16.9 |
| D2 (5.0) | 2.4 | 2.359 | 0.0075 | 8.4 | 21.1 | ||||
| E2 (8.0) | 2.6 | 2.388 | 0.0086 | 8.9 | 19.2 | ||||
| F2 (10.0) | 2.5 | 2.393 | 0.0081 | 8.9 | 20.1 | ||||
Fitting k-range = 3.7 – 12.0 Å−1; S02 = 1.0; estimated error limits: N ± 20 %, R ± 0.02 Å, σ2 ± 0.001 Å2;
Correlated to the coordination number N of Hg-S path.
Residual.
Results
199Hg NMR spectroscopy
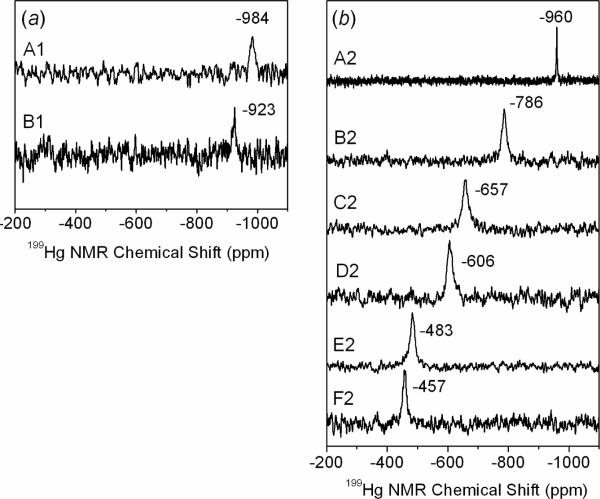
The 199Hg NMR chemical shifts for HgS2, HgS3 and HgS4 coordination environments span over a wide range, generally within −800 to −1200 ppm for HgS2, −79 to −159 ppm for HgS3, and −275 to −374 ppm for HgS4, depending on the type of ligand, coordination number, and nature of the solvent (29–32). The 199Hg resonance for solutions A1–B1 and A2–F2 (Figure 1) can therefore serve as a guideline to the Hg(II) coordination. By increasing the mercury(II) concentration to ~50 mM (solutions A2 – F2), broad 199Hg NMR signals could be obtained for the entire series of solutions. The increasing deshielding of the 199Hg chemical shift with higher glutathione concentration in this series corresponds to an increasing number of thiolate groups coordinated to the Hg(II) ions.
Figure 1.
199Hg NMR spectra for the Hg(II)-GSH solutions at pH = 7.0: (a) A1 and B1 (CHg(II) ~ 15 mM, GSH/Hg(II) mole ratios = 2.4 and 3,5, respectively), (b) A2 – F2 (CHg(II) ~50 mM, GSH/Hg(II) mole ratios = 2.0–10.0).
Hg LIII-edge X-ray absorption spectroscopy
In crystal structures the Hg-S bond distances for mercury(II) complexes with thiolate ligands typically occur within discrete ranges for HgS2, HgS3 and HgS4 coordination geometries, 2.30–2.38 Å (Rave = 2.34 ± 0.02 Å), 2.40–2.51 Å (Rave = 2.44 ± 0.04 Å), and 2.49–2.58 Å (Rave = 2.54 ± 0.02 Å), respectively (22). The mean Hg-S bond length obtained from the EXAFS curve fitting for the Hg(II)-GSH solutions thus provides another indication of the mercury(II) coordination geometry (20–22, 33).
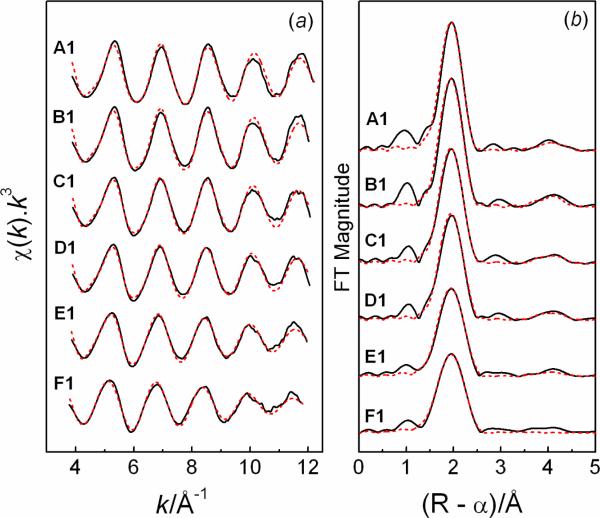
The k3-weighted EXAFS spectra for the Hg(II)-GSH solutions A1 – F1 are shown in Figure 2, with the structural parameters obtained from curve fitting listed in Table 2. The corresponding Fourier transforms (FTs) show a single peak at ~2.0 Å (without phase shift correction), attributed to the Hg-S backscattering in the first coordination shell. The peak gradually becomes smaller and broader as the GSH concentration increases.
Figure 2.
(a) Hg LIII-edge EXAFS spectra for the Hg(II)-GSH solutions with GSH/Hg(II) mole ratios 2.4 (A1), 3.5 (B1), 4.7 (C1), 5.9 (D1), 9.4 (E1), and 11.8 (F1), CHg(II) ~17 mM, pH = 7.0 ( experimental data; --- model). Structural parameters are listed in Table 2. (b) Corresponding Fourier transforms.
experimental data; --- model). Structural parameters are listed in Table 2. (b) Corresponding Fourier transforms.
The k3-weighted EXAFS spectrum of solution A1 (CHg(II) ~17 mM, GSH/Hg(II) = 2.4) fitted well to a model with two Hg-S bonds at 2.325 ± 0.01 Å and two S-Hg-S multiple scattering paths (nleg = 4) at 4.66 ± 0.02 Å (approximately twice the Hg-S distance), characteristic of a linear coordination geometry in the [Hg(AH)2]2− complex. Solutions B1–D1 with GSH/Hg(II) mole ratios of 3.5 – 5.9 also showed very similar EXAFS oscillations, with the mean Hg-S bond distances refined to 2.33 – 2.34 Å (Figure 2). For solutions E1 and F1 with large excess of glutathione (CGSH ≥ 160 mM) the increase in the mean Hg-S distances, 2.35 ± 0.02 Å and 2.37 ± 0.02 Å, respectively, indicated formation of a significant amount of the [Hg(AH)3]4− complex (see above).
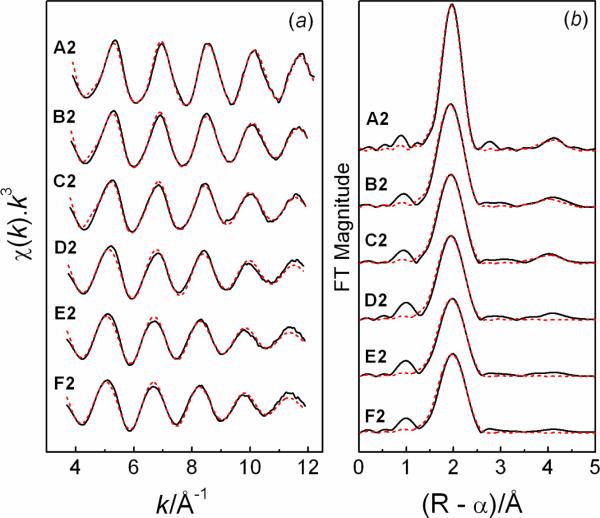
The Hg LIII-edge EXAFS spectra for the series of solutions A2 – F2 with CHg(II) ~50 mM and GSH/Hg(II) mole ratios 2.0 – 10.0 at pH = 7.0 are shown in Figure 3, with the structural parameters of the Hg(II)-GSH complexes listed in Table 3.
Figure 3.
(a) Hg LIII-edge EXAFS spectra for Hg(II)-GSH solutions with CHg(II) ~50 mM and GSH/Hg(II) mole ratios 2.0 (A2), 3.0 (B2), 4.0 (C2), 5.0 (D2), 8.0 (E2), 10.0 (F2), at pH = 7.0 (— experimental data; --- model). Structural parameters are listed in Table 3. (b) Corresponding Fourier transforms.
The EXAFS oscillations for solutions A2 and B2 (CHg(II) ~50 mM; CGSH ~ 100 – 150 mM) were modeled over the k-range 3.7 – 12.0 Å−1 by introducing two Hg-S bonds at a distance of 2.32 – 2.34 Å and a S-Hg-S multiple scattering contribution (nleg = 4) at 4.64 – 4.66 Å as expected for a linear entity, in a similar way as for solutions A1 and B1 (CHg(II) ~17 mM, CGSH ~ 40 – 60 mM). For solutions E2 and F2 with the highest excess of glutathione (CGSH = 0.4 – 0.5 M), the average Hg-S bond length was refined to 2.39 ± 0.02 Å, intermediate to the average values for crystalline HgS2 (Rav = 2.34 Å) and HgS3 (Rav = 2.44 Å) compounds; see above. At intermediate GSH concentration (CGSH = 0.2 – 0.25 M) in solutions C2 – D2 the mean Hg-S distance, 2.35–2.36 Å, is comparable to the mean Hg-S distances of 2.35–2.37 Å obtained for solutions E1 and F1 (CHg(II) ~17 mM; CGSH ~ 0.16 – 0.2 M) with a similar excess of free glutathione. In the less concentrated solutions C1 and D1 (CHg(II) ~17 mM; CGSH ~ 80 – 100 mM), the lower excess of free GSH results in lower average Hg-S coordination numbers corresponding to the slightly shorter refined average Hg-S distances, 2.33 – 2.34 Å (Table 2).
Electrospray Ion Mass Spectrometry
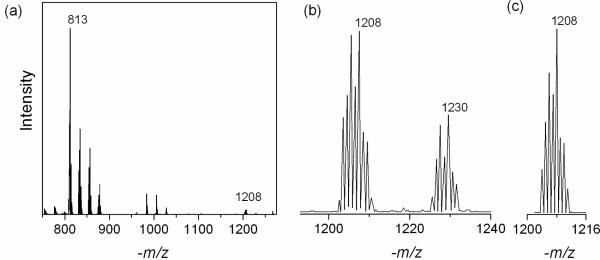
The presence of the tris-glutathionyl [Hg(AH)3]4− complex in the neutral solutions was also verified by measuring the ESI mass spectrum, even though the Na+ ion pairs with mercury(II) glutathionyl complexes with partially deprotonated −NH3+ groups in the gas phase do not represent the equilibrium state in solution. The ESI-MS for solution F1, with an excess of free glutathione of about 0.16 M, displayed strong peaks for ionic species with mass/charge ratios −m/z of 812.7, 834.7, 856.7 and 878.7, assigned as the bis-glutathionyl mercury(II) complexes [Hg(AH)(AH2)]−, [NaHg(AH)2]−, [Na2Hg(AH)(A)]−, and [Na3Hg(A)2]−, respectively (Figure 4, Table S-2). Bis-glutathionyl mercury(II) complexes have previously been characterized in acidic solutions by ESI-MS (12–15). In the present work, the tris-glutathionyl mercury(II) complexes [Na4Hg(AH)2(A)]− and [Na5Hg(AH)(A)2]− could be identified by peaks with −m/z values of 1208 and 1230, respectively (Figure 4). Even though their intensities are much lower than for the signals of the bis-glutathionyl mercury(II) complexes, the calculated isotopic distribution closely matches the experimental one.
Figure 4.
(a) ESI-MS measured in the negative ion mode for solution F1 with GSH/Hg(II) = 11.8 at pH = 7.0; (b) expanded region showing peaks labeled −m/z 1208 and 1230 assigned as the most abundant 202Hg isotopomers of the [Na4Hg(AH)2(A)]− and [Na5Hg(AH)(A)2]− species. (c) Calculated isotopic distribution for [Na4Hg(AH)2(A)]−.
Discussion
The previously reported formation constants for the [Hg(AH)2]2− and [Hg(AH)3]4− species, log β = 40.95 and 44.18, respectively, show that a tris-thiolate Hg(II) complex with weaker bonding to the (AH)2− groups than in [Hg(AH)2]2−, can form at physiological pH in excess of glutathione (8, 18, 19). In the present work, the structure and composition of the Hg(II)-glutathione complexes formed in solution at physiological pH have been studied by combining Hg LIII-edge EXAFS and 199Hg NMR spectroscopy, complemented by ESI-MS.
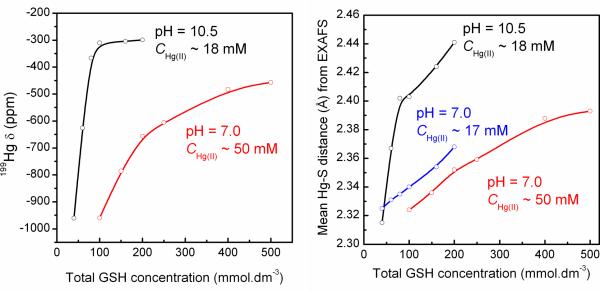
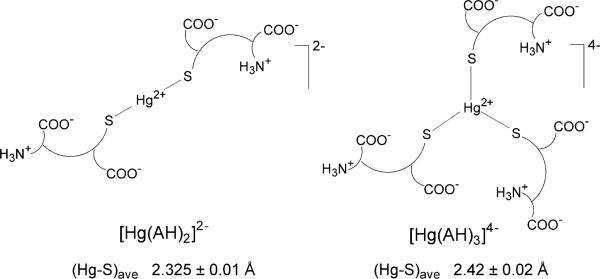
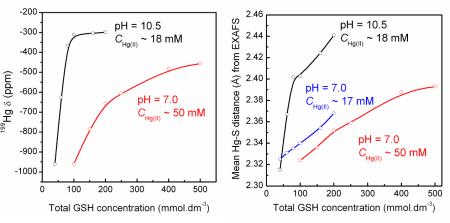
We recently reported the 199Hg chemical shift −961 ppm for the [Hg(A)2]4− complex in a solution containing CHg(II) ~ 18 mM and CGSH ~ 40 mM at pH 10.5 (20). In the current study, solution A1 (pH 7.0) with rather similar composition shows a 199Hg NMR resonance at −984 ppm, which is within the range typical for Hg(II)-dithiolates (about −800 to −1200 ppm) (32), and can be attributed to the [Hg(AH)2]2− complex. This resonance is close to the value reported previously (−993 ppm) for a solution with mole ratio GSH/Hg(II) = 2 and CHg(II) = 0.25 M at pH ~ 7 (11). By increasing the Hg(II) concentration to ~ 50 mM in solution A2 (GSH / Hg(II) = 2.0), the 199Hg NMR signal shifts to −960 ppm. We therefore conclude that the [Hg(AH)2]2− complex completely dominates in solutions A1 and A2. From the curve-fitting analyses of their EXAFS spectra, a Hg-S bond distance of 2.325 ± 0.01 Å with a relatively small disorder parameter, σ2 = 0.0035 ± 0.001 Å2, emerged for the [Hg(AH)2]2− species, which can be compared with the Hg-S distance 2.315 ± 0.01 Å obtained for the [Hg(A)2]4− complex with deprotonated amine groups at pH = 10.5 (20).
In a recent EXAFS study (data limit kmax = 11 Å−1) of the complexes formed in a dilute mercury(II)–glutathione frozen glass at pH 7.5, formation of a (GS)2-Hg⋯Hg-(GS)2 dimer in aqueous solution was proposed for the first time, with two Hg–S bond distances of 2.334(4) Å and a Hg⋯Hg interaction at 2.884(6) Å. It was suggested that “this Hg species could be implicated in the mammalian toxicology of Hg2+” (34). However, in the current study, EXAFS spectra were obtained for solutions A1 and A2 at room temperature in a wide k-range (up to 14.5 Å−1) and no such Hg⋯Hg scattering contribution could be observed (Figure S-4). In a survey of the Cambridge Structural Database (CSD, version 5.31, Nov. 2009) (35), we found dimeric complexes (such as COHWEB and DAXRAV) with two bridging thiolate groups, Hg2(μ-SR)2, leading to HgII⋯HgII distances of ~ 3.6 Å (36, 37). Single oxygen bridges between two mercury(II) atoms have been found in several crystal structures, leading to HgII⋯HgII distances of 3.5–3.6 Å (38). Mercury(I) forms dimeric Hg22+ species with direct HgI-HgI bonds of ~ 2.5 Å (39). Therefore, the proposed dimeric structure with such a short HgII⋯HgII distance as 2.9 Å seems unlikely when considering possible bridging groups.
The 199Hg NMR resonance that appears at −923 ppm for solution B1 (CHg(II) ~ 15 mM, CGSH ~ 53 mM) is about ~ +60 ppm deshielded relative to that of A1 (GSH / Hg(II) = 2.4), but still within the chemical shift range for Hg(SR)2 complexes. For the more concentrated solution B2 (CHg(II) ~ 50 mM, CGSH ~ 150 mM), the 199Hg NMR chemical shift deshielded to −786 ppm was considerably broader as compared with A2 (−960 ppm). Since Hg-S bonds are quite labile in solution, the 199Hg chemical shift is often representative of two or more species in fast exchange (20–22). Three-coordinated aliphatic Hg(II)-thiolate complexes are typically highly deshielded, with 199Hg NMR signals between −79 to −179 ppm (29, 31, 32, 40, 41). The chemical shift difference of +137 ppm for solution B2 versus B1 implicates a significant difference in the speciation, consistent with the higher free GSH concentration in B2 that favors formation of some amount of the [Hg(AH)3]4− complex. Curve-fitting of EXAFS oscillations for solutions B1 and B2, however, resulted in mean Hg-S bond lengths of 2.33 – 2.34 Å, similar to that obtained for A1 and A2 (2.325 ± 0.01 Å) implicating that 199Hg NMR is a more sensitive probe of small changes in the Hg(II) speciation.
For the series of solutions C2 – F2 (CHg(II) ~ 50 mM, pH = 7.0), the deshielding of the 199Hg NMR chemical shifts gradually increased relative to B2 (−786 ppm; CGSH ~ 0.1 M) as the glutathione concentration increased from 0.2 to 0.5 M: C2 (−657 ppm), D2 (−606 ppm), E2 (−483 ppm) and F2 (−457 ppm). In addition, the mean Hg-S bond distance obtained from the model fitting of the EXAFS spectra increased from 2.34 Å (B2) to 2.39 ± 0.02 Å (F2), accompanied with a corresponding substantial increase in the disorder parameter (σ2) from 0.0051 Å2 to 0.0081 ± 0.0010 Å2 (Table 3), indicating a wider distribution of the Hg-S distances. Also, for the solutions C1 – F1 with GSH / Hg(II) ratios increasing from 4.7 to 11.8 for CHg(II) ~17 mM, the EXAFS data analyses show a gradual increase in the mean Hg-S bond length from 2.33 to 2.37 ± 0.02 Å, while the disorder parameter (σ2) for the Hg-S path increases from 0.0049 (C1) to 0.0084 (F1) ± 0.0010 Å2.
For both series of solutions, the increase in the Hg-S bond distance, and also in the σ2 value that represents a large variation around the mean Hg-S distance, especially for solutions F1 and F2, can be attributed to the formation of a significant amount of the [Hg(AH)3]4− complex at increasing ligand concentration. Since the Hg-S distances differ significantly in the [Hg(AH)2]2− and [Hg(AH)3]4− complexes, their corresponding EXAFS oscillations interfere and reduce the amplitude of the EXAFS spectra for solutions F1 and F2 in comparison with A1 and A2, and also their Fourier transforms (Figure S-5). Furthermore, a 199Hg resonance could not be detected for the dilute solutions C1 – F1 due to chemical exchange broadening in the mixture of HgS2 and HgS3 complexes. The presence of tris-thiolate complexes was further confirmed by the ESI-MS of solution F1 (Figure 4).
Speciation of Hg(II)-GSH complexes using PCA of EXAFS spectra
Principal component analysis applied on the nine experimental EXAFS spectra obtained for solutions C1–F1 and B2–F2 showed the presence of two major Hg(II) species (Figure S-1 and Table S1). To quantify the relative proportion of [Hg(AH)2]2− and [Hg(AH)3]4− complexes formed in the Hg(II)-GSH solutions B1 – F1 at pH = 7.0, the experimental EXAFS spectra were fitted with a linear combination of model oscillations representing these two species. Solution A1 was assumed to contain 100 % of the [Hg(AH)2]2− complex, and the fitted curve from the least squares model fitting was used in the linear combinations. The model oscillation for the [Hg(AH)3]4− complex that gave the best fit to the experimental EXAFS spectra was obtained for 3 Hg-S distances at 2.42 Å, σ2 = 0.0060 Å2, S02 = 1.0 and ΔE0 = 9.0. To summarize, the Hg-S distances 2.325 Å (σ2 = 0.0040 Å) for [Hg(AH)2]2− , and 2.42 Å (σ2 = 0.0060 Å) for [Hg(AH)3]4−, resulted in the best linear combination fits. The results are provided in Table 4 (see Figure S-2).
Table 4.
Percentage of [Hg(AH)2]2− and [Hg(AH)3]4− Complexes Obtained by Linear Combination Fitting of EXAFS data for the Hg(II)-GSH Solutions A1 - F1 and A2–F2.a
| Solution (GSH/Hg2+) | CGSH (mM) | δHg(II) (ppm) | % HgS2 | % HgS3 |
|---|---|---|---|---|
| A1 (2.4) | 40 | −984 | 100 | 0 |
| B1 (3.5) | 60 | −923 | 95 | 5 |
| C1 (4.7) | 80 | 87 | 13 | |
| D1 (5.9) | 100 | 79 | 21 | |
| E1 (9.4) | 160 | 65 | 35 | |
| F1 (11.8) | 200 | 52 | 48 | |
| A2 (2.0) | 100 | −960 | 100 | 0 |
| B2 (3.0) | 150 | −786 | 78 | 22 |
| C2 (4.0) | 200 | −657 | 62 | 38 |
| D2 (5.0) | 250 | −606 | 54 | 46 |
| E2 (8.0) | 400 | −483 | 35 | 65 |
| F2 (10.0) | 500 | −457 | 30 | 70 |
The percentages shown are for the best fits with Hg-S bond distances 2.325 Å for [Hg(AH)2]2− and 2.42 Å for [Hg(AH)3]4− complexes. The estimated error is ±15 %.
The [Hg(AH)2]2− complex predominates (87–100 %, Table 4) for solutions A1–C1 where the free GSH concentration is less than ~0.04 M. Even though the least-squares refinement procedure resulted in quite similar mean Hg-S distances of 2.33 ± 0.02 Å for solutions A1 – C1 (Table 2), the linear combination fitting indicates that a minor amount of the [Hg(AH)3]4− complex is present in B1 and C1 (~5–13 %). For solutions D1 and F1, with free GSH concentrations of ~0.06 and 0.16 M, the amount of the [Hg(AH)3]4− complex increases to about 21 and 48%, respectively.
The relative amounts of [Hg(AH)2]2− and [Hg(AH)3]4− complexes formed in the Hg(II)-GSH solutions B2 – F2 at pH = 7.0 were quantified by a similar method (Figure S-3, Table 4), assuming that solution A2 contains 100 % of the [Hg(AH)2]2− complex. The results from the linear combination fitting show somewhat higher amounts (~20–30 %) of the [Hg(AH)3]4− complex in the solutions B2 – F2 (free GSH concentration ~0.04 – 0.35 M, CHg(II) ~50 mM) than in the solutions B1 – F1 (free GSH concentration ~ 0.02 – 0.16 M, CHg(II) ~17 mM) for similar ratios of GSH/Hg(II). Solution B2 with free GSH concentration ~0.04 M has ~78 % [Hg(AH)2]2− and ~22 % [Hg(AH)34− complexes, consistent with the deshielded 199Hg chemical shift −786 ppm, while the more dilute solution B1 with similar ratio GSH/Hg(II) = 3.5 (free GSH concentration ~0.02 M), only has ~5 % of the [Hg(AH)3]4− complex and the 199Hg chemical shift −923 ppm (Figure 1). In the concentrated solutions C2 and D2 with GSH/Hg(II) = 4.0 and 5.0 (free GSH concentration ~0.08 M and ~0.13 M, respectively), approximately 40–45 % of the total Hg(II) amount is present as the three-coordinated complex. Also in solution F1 (CHg(II) ~17 mM, GSH/Hg(II) = 11.8) with lower Hg(II) concentration and similar free GSH concentration (~0.16 M) as D2, almost half is present as the [Hg(AH)3]4− complex. With large excess of free GSH (> 0.35 M) as for solution F2 (CGSH = 500 mM, CHg(II) ~50 mM), the [Hg(AH)3]4− complex predominates (~70 % of the Hg(II) species). Figure 5 visualizes the percentage of the [Hg(AH)3]4− complex formed in Hg(II)-glutathione solutions A1 – F1, and A2 – F2 at pH = 7.0, as obtained from a linear combination fitting of HgS2 and HgS3 models to their EXAFS spectra (Table 4). For both series approximately equal concentrations of HgS2 and HgS3 species are reached for an excess amount of free GSH of ~0.17 M at pH 7.0.
Figure 5.
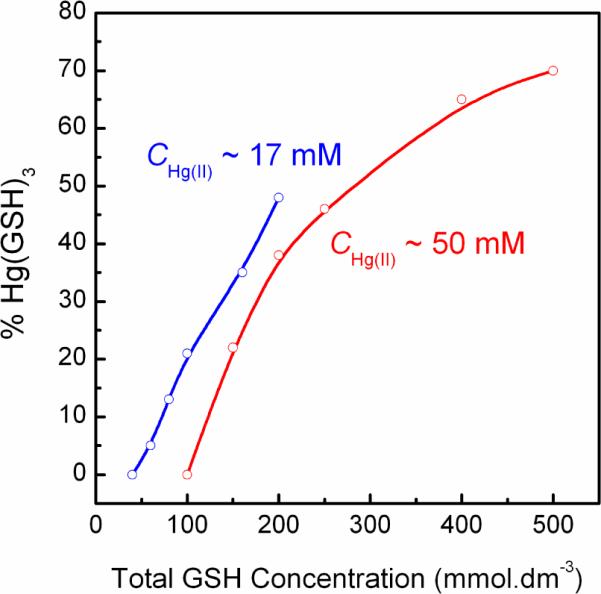
Percentage of the tris-glutathionyl Hg(II) complex [Hg(AH)3]4− in solutions A1–F1 (CHg(II) ~ 17 mM) and A2–F2 (CHg(II) ~ 50 mM) at pH 7.0, as obtained from fitting linear combinations of HgS2 and HgS3 EXAFS oscillations to their spectra (Table 4).
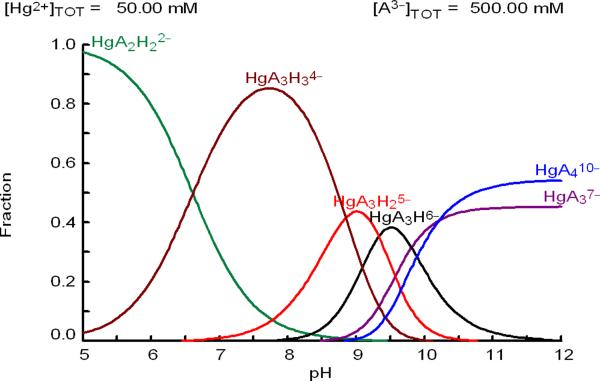
The relative proportions of HgS2 and HgS3 species obtained by the fitting of the EXAFS data (Table 4) are in qualitative agreement with distribution diagrams of the major Hg(II)-glutathione species vs. pH, calculated by means of the available stability constants from the literature (see Appendix I and Refs. (8, 19). Such calculated diagrams confirm that the [Hg(AH)2]2− and [Hg(AH)3]4− complexes are the major Hg(II)-GSH species at neutral pH, with the glutathione amine group still being protonated. However, the literature values gave slightly different ratios between the major HgS2 and HgS3 species (at pH = 7.0 [Hg(AH)2]2− and [Hg(AH)3]4− complexes) than those obtained experimentally for the solutions in the current study. Assuming that the reported stability constant well characterizes the stable [Hg(AH)2]2− complex, we therefore adjusted the stability constant of the [Hg(AH)3]4− complex to obtain calculated [Hg(AH)2]2− : [Hg(AH)3]4− ratios close to those in Table 4 (see Appendix I; Figure S-6a). The formation constant for the [Hg(AH)3]4− complex, defined as Hg2+ + 3A3− + 3H+ ↔ HgA3H34−, with the reported value log β7 = 72.75 (19), then attained the value log β7 72.22 for both series of solutions in Table 4. Since the deprotonation of the amine group of the HgS3 species is independent of the Hg-S coordination, the formation constants for all HgS3 species were shifted with the same amount, −0.53 logarithmic units (see Appendix I). Fraction diagrams in Figures 6, S-7a and S-7b, calculated using the adjusted stability constants, demonstrate the distribution of Hg(II)-glutathione complexes vs. pH for solutions B1, B2, F1 and F2. The calculated distribution of complexes for solution F2 shows that the amino groups of the [Hg(AH)3]4− complex has started to deprotonate at pH = 7.0 with about 3% of the mercury(II) amount present in the [Hg(AH)2(A)]5− complex (Figure 6). The stability constant for the formation of the HgA410− complex could be estimated to log β10 = 44.8 for Hg2+ + 4A3− ↔ HgA410− (which then also includes possible contributions from deprotonated HgS4 species) from the previously determined distributions of HgS2, HgS3 and HgS4 species at pH = 10.5 (20); see below and Appendix 1, Figure S-6b.
Figure 6.
Fraction diagram showing the distribution of major Hg(II) complexes vs. pH for an aqueous solution containing CHg(II) = 0.050 M and CGSH = 0.5 M, as in solution F2, calculated according to the adjusted formation constants (see Appendix I, Supporting Information).
Effect of pH on Hg(II)-GSH speciation
In our previous EXAFS study of Hg(II)-GSH complex formation in alkaline aqueous solution (pH = 10.5, CHg(II) ~18 mM), the deprotonated [Hg(A)2]4−, [Hg(A)3]7− and [Hg(A)4]10− complexes were characterized. By increasing the CGSH from 40 mM to 200 mM in the alkaline solutions, the average Hg-S distance obtained from EXAFS spectra elongated from 2.315 to 2.44 Å (20), while for the neutral solutions A1 – F1 with similar chemical composition the variation of the mean Hg-S distances was smaller, from 2.325 to 2.37 Å. Furthermore, for the alkaline solutions containing CHg(II) ~18 mM and excess glutathione (CGSH = 160 – 200 mM), a deshielded 199Hg resonance at ~ −300 ppm could be detected, which is due to the predominating [Hg(A)3]7− and the minor (20–30%) [Hg(A)4]10− species (see Figure 7) (20). The formation of HgS3 and HgS4 complexes at pH = 10.5 (GSH/Hg(II) > 11), is promoted by the deprotonation of the cysteinyl thiol group to thiolate in alkaline solution, which occurs between pH 9 to 10 according to sulfur K-edge XANES measurements (42). At pH = 7.0 the thiol group in free GSH is only deprotonated to a minor extent. Thus, even at high glutathione concentration as in the currently studied solutions, the thiolate concentration is not sufficient (Figure S-7c) to promote a detectable amount of a four coordinated HgS4 species, which presumably would be the [Hg(AH)4]6− complex in neutral solutions.
Figure 7.
(left) Variation of the 199Hg NMR chemical shift (ppm) with the total glutathione concentration for two series of Hg(II)-GSH solutions at neutral (CHg(II) = 50 mM) and alkaline pH (CHg(II) = 18 mM); (right) Variation of the mean Hg-S bond distance obtained from EXAFS spectra of these solutions (see Tables 2 and 3) (20).
Conclusions
The Hg LIII-edge EXAFS and 199Hg NMR results for two series of Hg(II)-GSH solutions with CHg(II) = 17 and 50 mM at pH 7.0 show that [Hg(AH)2]2− and [Hg(AH)3]4− complexes (Scheme 2) form at physiological pH in aqueous solutions. The [Hg(AH)2]2− complex with linear S-Hg-S coordination and the Hg-S bond distances 2.325 ± 0.01 Å and the 199Hg NMR resonance at −984 ppm, dominates except at high excess of glutathione. In solutions with free glutathione concentration higher than ~0.17 M at pH = 7.0 the [Hg(AH)3]4− complex with trigonal HgS3 coordination and an average Hg-S bond distance of 2.42 ± 0.02 Å, dominates. Tris-glutathionyl Hg(II) complexes were also detected by ESI-MS. For a corresponding series of alkaline solutions (pH = 10.5), for which our previous results showed that also HgS4 species formed (20), we have estimated a formation constant of log β = 44.8 (Hg2+ + 4A3− ↔ HgA410−) for the [Hg(A)4]10− complex with deprotonated amino groups. However, at pH 7.0 the four-coordinated [Hg(AH)4]6− complex does not form because of the highly protonated thiol groups in the free GSH ligands (42). For the series of solutions containing CHg(II) ~50 mM and GSH/Hg(II) = 3.0–10.0 at pH 7.0, a somewhat higher proportion of the [Hg(AH)3]4− complex is formed as compared with solutions containing CHg(II) ~17 mM despite similar ligand to metal mole ratios, because of the lower concentration of free GSH.
Scheme 2.
Proposed structures for the major Hg(II)- glutathione complexes at pH = 7.0.
Following Cheesman and coworkers, the equilibrium properties of the mercury(II) complexes in vivo are predicted, i.e. for the total glutathione concentration 2.2 mM and the pH = 7.4, as in human erythrocytes (18). Calculations performed for Hg(II) concentrations from 0 to 1 mM with our adjusted stability constants show that at low Hg(II) concentrations the [Hg(AH)2]2− complex dominates at pH = 7.4, and that the highest proportion of the [Hg(AH)3]4− complex, about 3%, occurs in media with CHg(II) < ~ 0.1 mM (Figure S-8a), which is less than that previously proposed by Cheesman et al (18) (~11%). The percentage is slightly reduced at higher Hg(II)-concentration because of the lower free GSH concentration. About 2% of the total Hg(II) amount is present as the deprotonated HgS2 complex [Hg(AH)(A)]3− at pH 7.4 (Figure S-8).
Synopsis.
Combined 199Hg NMR, Hg LIII-edge X-ray absorption and mass spectroscopic studies reveal the amount of two and three-coordinated Hg(II)-glutathione complexes, with the mean Hg-S bond distances 2.325 and 2.42 ± 0.02 Å, respectively, that form in aqueous solution at pH 7.0.
Supplementary Material
Acknowledgement
We thank Mrs. Qiao Wu for her skillful assistance with the ESI-MS measurements. We are grateful to Professor Jürgen Gailer and Shawn Manley for ICP analyses. X-ray absorption measurements were carried out at the Stanford Synchrotron Radiation Laboratory, SSRL, (proposal No. 2848), a US national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program. We are grateful to the National Science and Engineering Research Council (NSERC) of Canada, the Canadian Foundation for Innovation (CFI) and the Province of Alberta (Department of Innovation and Science) for their financial support. F.J. is a recipient of a NSERC University Faculty Award (UFA).
Footnotes
Supporting Information Available: PCA of Hg(II)-GSH solution EXAFS C1–F1 and B2-F2; linear combination fitting for Hg(II)-GSH solutions B1–F1 and B2-F2; table for assignment of mass ions in the ESI-MS of solution F1 (GSH/Hg(II) = 11.8); fraction diagrams for solutions B1, B2 and F1, showing distribution of Hg(II)-GSH complexes vs. pH using adjusted stability constant for the HgS3 complexes, together with the calculated distribution of Hg(II) species at low concentration under physiological conditions (pH 7.4, [GSH]tot = 2.2 mM). This material is available free of charge via the Internet at http://pubs.acs.org.”
References
- (1).George GN, Singh SP, Hoover J, Pickering IJ. The chemical forms of mercury in aged and fresh dental amalgam surfaces. Chem Res Toxicol. 2009;22:1761–1764. doi: 10.1021/tx900309c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Korbas M, Blechinger SR, Krone PH, Pickering IJ, George GN. Localizing organomercury uptake and accumulation in zebrafish larvae at the tissue and cellular level. Proc Natl Acad Sci U S A. 2008;105:12108–12112. doi: 10.1073/pnas.0803147105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).George GN, Singh SP, Myers GJ, Watson GE, Pickering IJ. The chemical forms of mercury in human hair: a study using X-ray absorption spectroscopy. J Biol Inorg Chem. 2010;15:709–715. doi: 10.1007/s00775-010-0638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110(Suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- (6).Kapoor RC, Doughty G, Gorin G. The Reaction and Assay of Glutathione with Hg2+ and Alkali. Biochim Biophys Acta. 1965;100:376–383. doi: 10.1016/0304-4165(65)90006-1. [DOI] [PubMed] [Google Scholar]
- (7).Oram PD, Fang X, Fernando Q, Letkeman P, Letkeman D. The formation of constants of mercury(II)--glutathione complexes. Chem Res Toxicol. 1996;9:709–712. doi: 10.1021/tx9501896. [DOI] [PubMed] [Google Scholar]
- (8).Stricks W, Kolthoff IM. Reactions between Mercuric Mercury and Cysteine and Glutathione. Apparent Dissociation Constants, Heats and Entropies of Formation of Various Forms of Mercuric Mercapto-Cysteine and -Glutathione. J. Am. Chem. Soc. 1953;75:5673–5681. [Google Scholar]
- (9).Neville GA, Drakenberg T. Acta Chem. Scand. B. 1974;28:473–477. doi: 10.3891/acta.chem.scand.28b-0473. [DOI] [PubMed] [Google Scholar]
- (10).Rabenstein DL, Isab AA. Biochim. Biophys. Acta. 1982;721:374–384. doi: 10.1016/0167-4889(82)90092-1. [DOI] [PubMed] [Google Scholar]
- (11).Sudmeier JL, Birge RR, Perkins TG. J. Magn. Reson. 1978;30:491–496. [Google Scholar]
- (12).Burford N, Eelman MD, Groom K. J. Inorg. Biochem. 2005;99:1992–1997. doi: 10.1016/j.jinorgbio.2005.06.019. [DOI] [PubMed] [Google Scholar]
- (13).Rubino FM, Verduci C, Giampiccolo R, Pulvirenti S, Brambilla G, Colombi A. J. Am. Soc. Mass Spectrom. 2004;15:288–300. doi: 10.1016/j.jasms.2003.10.013. [DOI] [PubMed] [Google Scholar]
- (14).Krupp EM, Milne BF, Mestrot A, Meharg AA, Feldmann J. Anal. Bioanal. Chem. 2008;390:1753–1764. doi: 10.1007/s00216-008-1927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Rubino FM, Pitton M, Brambilla G, Colombi A. J. Mass Spectrom. 2006;41:1578–1593. doi: 10.1002/jms.1143. [DOI] [PubMed] [Google Scholar]
- (16).Fuhr BJ, Rabenstein DL. J. Am. Chem. Soc. 1973;95:6944–6950. doi: 10.1021/ja00802a013. [DOI] [PubMed] [Google Scholar]
- (17).Katōno Y, Inoue Y, Chûjô R. Polymer J. 1977;9:471–478. [Google Scholar]
- (18).Cheesman BV, Arnold AP, Rabenstein DL. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. 25. Hg(thiol)3 complexes and HG(II)-thiol ligand exchange kinetics. J. Am. Chem. Soc. 1988;110:6359–6364. [Google Scholar]
- (19).Shoukry MM, Cheesman BV, Rabenstein DL. Polarimetric and nuclear magnetic resonance studies of the complexation of mercury by thiols. Can. J. Chem. 1988;66:3184–3189. [Google Scholar]
- (20).Mah V, Jalilehvand F. Mercury(II) complex formation with glutathione in alkaline aqueous solution. J. Biol. Inorg. Chem. 2008;13:541–553. doi: 10.1007/s00775-008-0342-2. [DOI] [PubMed] [Google Scholar]
- (21).Jalilehvand F, Leung BO, Izadifard M, Damian E. Mercury(II) Cysteine Complexes in Alkaline Aqueous Solution. Inorg. Chem. 2006;45:66–73. doi: 10.1021/ic0508932. [DOI] [PubMed] [Google Scholar]
- (22).Leung BO, Jalilehvand F, Mah V. Mercury(II) penicillamine complex formation in alkaline aqueous solution. Dalton Trans. 2007:4666–4674. doi: 10.1039/b711436b. [DOI] [PubMed] [Google Scholar]
- (23).Klose G, Volke F, Peinel G, Knobloch G. 199Hg NMR of Aqueous Solutions of Inorganic Mercury salts. Chemical shifts of HgCln2-n with n = 0–4. Magn. Reson. Chem. 1993;31:548–551. [Google Scholar]
- (24).George GN, Geroge SJ, Pickering IJ. EXAFSPAK. Stanford Synchrotron Radiation Lightsource (SSRL); Menlo Park, CA: 2001. [Google Scholar]
- (25).Ressler T. WinXAS: a Program for X-ray Absorption Spectroscopy Data Analysis under MS-Windows. J. Synchrotron Rad. 1998;5:118–122. doi: 10.1107/S0909049597019298. [DOI] [PubMed] [Google Scholar]
- (26).Ankudinov AL, Rehr JJ. Relativistic calculations of spin-dependent x-ray-absorption spectra. Phys. Rev. B. 1997;56:R1712. [Google Scholar]
- (27).Zabinsky SI, Rehr JJ, Ankudinov A, Albers RC, Eller MJ. Multiple-scattering calculations of x-ray-absorption spectra. Phys. Rev. B. 1995;52:2995. doi: 10.1103/physrevb.52.2995. [DOI] [PubMed] [Google Scholar]
- (28).Kim C-H, Parkin S, Bharara M, Atwood D. Linear coordination of Hg(II) by cysteamine. Polyhedron. 2002;21:225–228. [Google Scholar]
- (29).Bowmaker GA, Harris RK, Oh S-W. Solid-state NMR spectroscopy of mercury compounds. Coord. Chem. Rev. 1997;167:49–94. [Google Scholar]
- (30).Natan MJ, Millikan CF, Wright JG, O'Halloran TV. Solid-state mercury-199 nuclear magnetic resonance as a probe of coordination number and geometry in Hg(II) complexes. J. Am. Chem. Soc. 1990;112:3255–3257. [Google Scholar]
- (31).Utschig LM, Bryson JW, O'Halloran TV. Mercury-199 NMR of the metal receptor site in MerR and its protein-DNA complex. Science. 1995;268:380–385. doi: 10.1126/science.7716541. [DOI] [PubMed] [Google Scholar]
- (32).Wright JG, Natan MJ, MacDonnell FM, Ralston DM, O'Halloran TV. Prog. Inorg. Chem. 1990;38:323–412. [Google Scholar]
- (33).Manceau A, Nagy KL. Relationships between Hg(II)-S bond distance and Hg(II) coordination in thiolates. Dalton Trans. 2008:1421–1425. doi: 10.1039/b718372k. [DOI] [PubMed] [Google Scholar]
- (34).Percy AJ, Korbas M, George GN, Gailer J. J. Chromatogr. A. 2007;1156:331–339. doi: 10.1016/j.chroma.2006.12.061. [DOI] [PubMed] [Google Scholar]
- (35).Allen FH. The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Cryst. B. 2002;B58:380–388. doi: 10.1107/s0108768102003890. [DOI] [PubMed] [Google Scholar]
- (36).Bowmaker GA, Dance IG, Dobson BC, Rogers DA. Syntheses and Vibrational-Spectra of Some Tris(Alkanethiolato)Mercurate(Ii) Complexes, and Crystal-Structure of the Hexakis(Methanethiolato)Dimercurate(Ii) Dianion. Australian Journal of Chemistry. 1984;37:1607–1618. [Google Scholar]
- (37).Henkel G, Betz P, Krebs B. [Hg3(Sch2ch2s)4]2- and ([Hg2(Sch2ch2s)3]2-)-Normal - Examples of Trinuclear and Quasi-Isolated Binuclear Polymeric Mercury Thiolate Anions. Journal of the Chemical Society-Chemical Communications. 1985:1498–1499. [Google Scholar]
- (38).Sandström M. An X-ray Diffraction and Raman Study of Chloride, Bromide and Iodide Complexes of Mercury(II) in Dimethyl Sulfoxide Solution and of Mercury(II) Chloride in Methanol Solution. Acta Chem. Scand. A. 1978;32:627–641. [Google Scholar]
- (39).Rosdahl J, Persson I, Kloo L, Ståhl K. On the solvation of the mercury(I) ion. A structural, vibration spectroscopic and quantum chemical study. Inorg. Chim. Acta. 2004;357:2624–2634. [Google Scholar]
- (40).Dieckmann GR, McRorie DK, Tierney DL, Utschig LM, Singer CP, O'Halloran TV, Penner-Hahn JE, DeGrado WF, Pecoraro VL. De Novo Design of Mercury-Binding Two- and Three-Helical Bundles. J. Am. Chem. Soc. 1997;119:6195–6196. [Google Scholar]
- (41).Matzapetakis M, Farrer BT, Weng T-C, Hemmingsen L, Penner-Hahn JE, Pecoraro VL. Comparison of the Binding of Cadmium(II), Mercury(II), and Arsenic(III) to the de Novo Designed Peptides TRI L12C and TRI L16C. J. Am. Chem. Soc. 2002;124:8042–8054. doi: 10.1021/ja017520u. [DOI] [PubMed] [Google Scholar]
- (42).Risberg ED, Jalilehvand F, Leung BO, Pettersson LGM, Sandstrom M. Theoretical and experimental sulfur K-edge X-ray absorption spectroscopic study of cysteine, cystine, homocysteine, penicillamine, methionine and methionine sulfoxide. Dalton Trans. 2009:3542–3558. doi: 10.1039/b819257j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.