Abstract
Background
The relations between chronic kidney disease (CKD) and incident heart failure remain unclear.
Methods and Results
We related CKD to incident nonfatal heart failure and cardiovascular (CVD) death (as separate and combined endpoints) in 10,181 male participants (mean age, 67years). Kidney function was assessed by estimated glomerular filtration rate (eGFR) using Modification of Diet in Renal Disease equation in clinically relevant categories of <60 and ≥60 ml/min/1.73 m2 (referent); and <45, 45 to 60, 60 to 90 and ≥90 ml/min/1.73 m2 (referent). During follow up (mean 10.1years; range 0.03-12.2), 439 developed heart failure and 832 had CVD death/heart failure. In multivariable models, men with eGFR <60ml/min/1.73 m2 had a 2-fold risk of heart failure (95% confidence interval [CI], 1.62-2.56, p<0.0001) compared to referent category. The hazard ratio [HR] (with corresponding 95% CI) for development of heart failure according to eGFR categories of 60-90, 45-60, and <45ml/min/1.73m2 compared to referent category were 1.24 (0.98-1.56), 2.58 (1.91-3.49) and 1.52 (0.92-2.76) respectively. In the analyses restricted to subgroup of non-diabetics and normotensive individuals at baseline (n=7545), men with eGFR <60 ml/min/1.73 m2 had 2.2-fold risk of heart failure (95% CI 1.66-2.95), compared to men with eGFR≥60 ml/min/1.73 m2. Additionally, risk of heart failure or CVD death was >2.5-fold higher among individuals with eGFR 45-60 and <45 ml/min/1.73 m2, compared to referent category.
Conclusion
Moderate level of CKD, even in absence of diabetes and hypertension at baseline, is associated with a higher risk of developing heart failure and CVD death/heart failure in men.
Keywords: Heart Failure, Congestive, Epidemiology, Renal disease
Introduction
Compared to those who have end stage renal failure, individuals with mild-to-moderate kidney disease have a higher risk of cardiovascular disease (CVD).1 Epidemiologic definition of CVD 2 includes ‘heart failure’ though the mechanisms of development of heart failure are not well established.3 Heart failure is increasing in prevalence and incidence with an average middle-aged individual having 1 in 5 chances of developing heart failure in his/her lifetime.4
Cross-sectionally, individuals with mild-to-moderate kidney disease have higher prevalence of left ventricular hypertrophy (LVH) – a precursor for heart failure.5 In fact, data from US National Kidney Foundation estimated that 75% of individuals have LVH, at the time of dialysis initiation.6 In longitudinal studies, few investigators have separately evaluated the risk of heart failure according to different markers of renal function in selected individuals (i.e. older individuals).7-11 In addition, prior epidemiologic studies evaluating the association between CKD and CVD (including heart failure) have shown conflicting results with most reporting a significant increased risk of CVD in CKD patients 12,13 whereas some observed no association of CKD to incident CVD in the community.14,15 Moreover, data from NHANES shows a lack of association between proteinuria (a marker of early renal disease) and incident heart failure.16 Overall, it remains unanswered whether there is any increased risk of heart failure in those with mild-to-moderate renal dysfunction.1
Therefore, in the present investigation, we evaluated the relations of estimated glomerular filtration rate (eGFR) to the incidence of heart failure in the Physicians’ Health Study participants who were free of heart failure and myocardial infarction (MI) at baseline. As a secondary aim, we analyzed if the association of CKD to incident heart failure is influenced by prevalent diabetes and hypertension by excluding participants with diabetes and hypertension at baseline. Additionally, we also evaluated the relations of CKD to CVD death or incident heart failure as a combined endpoint.
Methods
Study Participants
The design and selection criteria for Physicians’ Health Study have been described previously.17 Briefly, 22,071 apparently healthy male physicians without history of cardiovascular disease, cancer, liver or kidney disease were enrolled in this study. Between 1995 and 2000, all current participants (n= 14,642) were asked to provide a blood sample by mail. For the present investigation, we selected individuals with available information on measured levels of serum creatinine (n=11,105). A simplified version of Modification of Diet in Renal Disease equation was used to calculate the eGFR.18 We excluded participants who reported heart failure at baseline or did not report any information about heart failure at the time of blood collection on their annual questionnaire (n=224), participants with history of MI (n=375) and those with missing covariates (n=325), therefore, for the present study our final sample included 10,181 participants. There were no significant differences between participants who provided blood sample from those who did not. Additionally, participants with missing covariates were similar in characteristics to those with all information available (data not shown). All participants signed informed consent and the protocol for study was approved by Institutional Review Board of Brigham and Women’s Hospital.
Measurement of Risk Factors
All information on demographics, medical history including history of diabetes mellitus, hypertension, heart failure and other life style variables were obtained annually. Therefore, information on body weight, presence or absence of diabetes mellitus, blood pressure, smoking habits, alcohol consumption, exercise routine, any use of anti-cholesterol medications and interim MI were obtained by questionnaires. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters square. Alcohol consumption was ascertained by calculating the average number of drinks consumed in categories of rarely or never, 1-3/month, ≥1/week and ≥1/day. Smoking history was reported in categories of never smokers, past history of smoking and current smokers.
Medical records from >95% of participants were obtained after each self-reported nonfatal MI and on receipt of their consent or consent from their next of kin (in case of death) after a fatal MI. Thereafter, an end point committee (by using World Health Organization criteria) 19 confirmed all MI diagnoses by inspection of medical records and other available information. Participants reported physical activity to a point of breaking into sweat and were categorized into <1 day/week (referent), 1-3 days/week, 3-4 days/week and 5-7days/week. In the present study, hypertension was present based on self-reported blood pressure of ≥140 systolic or ≥90 diastolic or history of hypertension or if on anti-hypertensive medications.
Blood Collection and Measurement of Serum Creatinine
Upon receipt of all EDTA blood specimens in the mail, samples were centrifuged, divided into aliquots, and frozen. Measurement of serum creatinine was performed in Oxford, England using Jaffe technique by a Synchronon LX20 autoanalyzer (Beckman Coulter, Fullerton, CA) as previously described.20 Coefficient of variation for blinded duplicate samples was 7.1% and intra-batch variations ranged from 1.4 to 2.3%.
Ascertainment of Heart Failure and CVD death
Information on heart failure was collected through self-reports from participants on the annual PHS questionnaire. We examined the validity of these self-reported heart failure events in a subset of alive, randomly selected 88 cases who had reported recent new episodes of heart failure. These selected individuals were mailed a questionnaire to obtain additional information on their symptoms, signs and laboratory investigations performed at the time of presentation with heart failure. After two mailings and further follow-ups by telephone all collected information was reviewed to verify a diagnosis of heart failure based on Framingham heart failure criteria.21 Of the 88 self-reported heart failure cases, 76 (86%) returned their completed questionnaires. Overall, we successfully confirmed heart failure in 68 (89%) individuals which suggested reasonably high validity for an epidemiologic study as described earlier.22 Additionally, a separate validation using information on invasive and other non-invasive imaging modalities to ascertain the diagnosis of heart failure for this sample of individuals showing 91% accuracy has also been published elsewhere.23
All deaths attributed to cardiovascular disease were confirmed by an endpoint committee using information from death certificates, next of kin and medical records and have been published in detail previously.24
Statistical Analyses
Baseline characteristics of the participants were assessed according to eGFR of <60 and ≥ 60ml/min/1.73 m2. We estimated the cumulative incidence of heart failure per 1000 person-years in each category of eGFR. We also constructed cumulative incidence curves using Kaplan Meier method according to eGFR categories. Assumptions for proportional hazard models were tested by fitting a product term eGFR × log (person-time of follow-up) and we found no evidence of violation (p =0.18). Then, after confirming that the assumption of proportionality of hazards was met, we used Cox regression models to relate eGFR levels to the incidence of heart failure. eGFR was analyzed in categories of <60 and ≥60 ml/min/1.73 m2 (referent); and <45, 45 to 60, 60 to 90 and ≥ 90 ml/min/1.73 m2 (referent). All multivariable models were adjusted for age (as part of eGFR calculation), BMI, systolic and diastolic blood pressure, diabetes mellitus, smoking history, alcohol intake and physical activity (exercise per week). We chose not to adjust for serum cholesterol or treatment of cholesterol because in the PHS dataset, serum cholesterol levels have not been associated with incident heart failure.22
We assessed for any effect modification by hypertension, diabetes, and BMI using interaction terms in multivariable models adjusting for all covariates (as above) to assess the risk of heart failure according to eGFR categories of <60 and ≥60ml/min/1.73 m2.
Subgroup Analyses
In a subgroup analyses, we excluded all participants with diabetes mellitus (n= 599) and hypertension at baseline (n=2037) and additionally adjusted for diabetes mellitus, hypertension and myocardial infarction as time-dependent covariates (in addition to other covariates as described above for primary analyses). These subgroup analyses were performed because individuals with hypertension and diabetes are highly predisposed to develop CKD and heart failure. We did not have enough events for separate analysis in subjects with diabetes or hypertension.
Additional Analyses
Because a fatal heart failure endpoint was not available in our dataset, we examined the combined risk of heart failure and CVD death (whichever came first), according to renal function categories. We estimated the cumulative incidence of CVD death or heart failure per 1000 person-years, constructed cumulative incidence curves and examined the relations of CKD to CVD death or heart failure using Cox regression models adjusting for all variables as in our primary analyses (see above) according to each category of eGFR.
All analyses were performed using SAS 9.2 (Cary, NC) software. A two-sided p value of <0.05 was considered statistically significant.
Results
Baseline characteristics of the participants according to eGFR levels of <60 and ≥60 ml/min/1.73 m2 are displayed in Table 1. As expected, individuals with eGFR <60 ml/min/1.73 m2 were older, had higher systolic and diastolic blood pressure, and had higher prevalence of cholesterol lowering medications use.
Table 1.
Baseline Characteristics of Participants According to eGFR Levels
| Characteristic | Glomerular Filtration Rate, ml/min/1.73 m2 |
|
|---|---|---|
| ≥ 60 | < 60 | |
| Number of participants (n) | 9012 | 1169 |
| eGFR, ml/min/1.73 m2 | 84.8 ± 16.5 | 49.9 ± 9.1 |
| Age, years | 66.5 ± 8.2 | 72.2 ± 9.1 |
| Body mass index, kg/m2 | 25.5 ± 3.1 | 25.6 ± 3.2 |
| Diabetes mellitus, % | 5.7 | 7.3 |
| Systolic blood pressure, mmHg | 126 ± 12 | 131 ± 14 |
| Diastolic blood pressure, mmHg | 78 ± 7 | 80 ± 7 |
| Total cholesterol mg/dl | 204 ± 34 | 205 ± 37 |
| Treatment for cholesterol, % | 18.9 | 22.2 |
| Exercise days per week, % | ||
| <1 day/week | 5.1 | 4.0 |
| 1-2 days/week | 31.3 | 30.0 |
| 3-4 days/week | 39.7 | 40.6 |
| 5-7 days/week | 23.9 | 25.4 |
| Alcohol drinks, % | ||
| ≥ 1/day | 18.3 | 16.8 |
| ≥ 1/week | 49.1 | 48.1 |
| 1-3/day | 12.8 | 13.9 |
| Rarely or never | 19.8 | 21.3 |
| Cigarette smoking, % | ||
| Past | 46.8 | 46.5 |
| Current | 3.2 | 2.6 |
Values are mean ± standard deviation or otherwise indicated
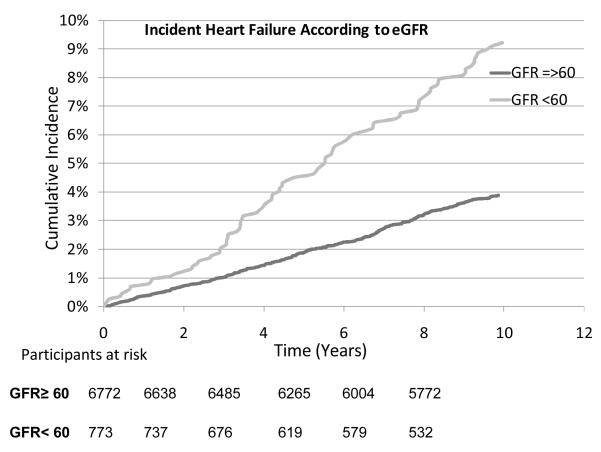
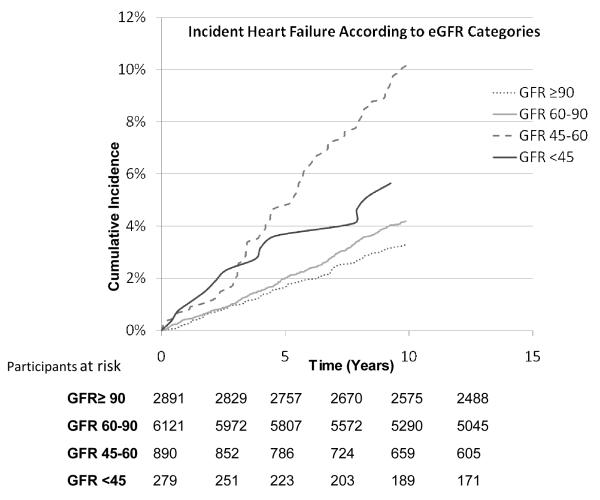
On follow up (mean 10.1 years, range 0.03-12.2), 439 participants developed heart failure and 1558 died. Men with eGFR <60 ml/min/1.73 m2 and especially those with eGFR between 45 and 60 ml/min/1.73 m2 had the highest crude cumulative incident rates per 1000 person-years of follow up compared to other categories (Table 2). Cumulative incident curves (truncated to ≤10 years) demonstrated a graded increase in incidence of heart failure according to different categories of eGFR (log rank P<0.0001 for both curves, Figure 1 and 2). Notably, those with eGFR between 45-60 ml/min/1.73 m2 showed graded and lowest survival rates compared to other categories (Figure 2). In multivariable models, individuals with eGFR <60 ml/min/1.73 m2 had >2-fold and those with eGFR between 45 and 60 ml/min/1.73 m2 had >2.5-fold higher risk of heart failure (HR 2.58, 95% CI 1.91-3.48; Table 3) when compared to referent categories. Further, when age was included as a variable (in addition to using age in calculation of eGFR) in multivariable models, the risk of heart failure was attenuated however, it remained statistically significant for individuals with eGFR between 45-60 ml/min/1.73 m2 (HR 1.45, 95% CI 1.07-1.97). The risk of heart failure was attenuated, and was not statistically significant in individuals with eGFR <45 ml/min/1.73 m2, perhaps due to fewer individuals in that category, compared to referent category (Table 3). Additionally, when we excluded men with eGFR<30 ml/min/1.73 m2(n=44; 3 heart failure events), the risk of heart failure was further attenuated among those with eGFR <45 ml/min/1.73 m2 (HR 1.49, 95% CI 0.80-2.78; p =0.21).
Table 2.
Cumulative Incident Rates for Heart Failure and CVD death or Heart Failure as Composite Endpoint According to eGFR Levels
| Glomerular Filtration Rate (ml/min/1.73 m2) |
Entire sample | Subgroup * | ||
|---|---|---|---|---|
| No. of HF/No. at Risk (%) |
Incident Rates/1000 person-years |
No. of HF/No. at Risk (%) |
Incident Rates/1000 person-years |
|
| Incident rates of Heart Failure | ||||
|
| ||||
| ≥ 60 | 345/9012 (3.8) | 3.7 | 222/6772 (3.3) | 3.2 |
| < 60 | 94/1169 (8.0) | 8.9 | 62/773 (8.0) | 8.8 |
|
| ||||
| ≥ 90 | 97/2891 (3.4) | 3.2 | 62/2187 (2.8) | 2.7 |
| ≥ 60 and <90 | 248/6121 (4.0) | 4.0 | 160/4585 (3.5) | 3.4 |
| ≥ 45 and <60 | 80/890 (9.0) | 9.8 | 51/588 (8.7) | 9.3 |
| < 45 | 14/279 (5.0) | 5.9 | 11/185 (6.0) | 7.0 |
|
| ||||
| Incident rates of CVD Death or Heart Failure | ||||
|
| ||||
| ≥ 60 | 641/9012 (7.1) | 9.2 | 410/6772 (6.1) | 4.5 |
| < 60 | 191/1169 (16.3) | 27.0 | 118/773 (15.3) | 11.2 |
|
| ||||
| ≥ 90 | 162/2891 (5.6) | 7.1 | 110/2187 (5.0) | 3.7 |
| ≥ 60 and <90 | 479/6121 (7.8) | 10.2 | 300/4585 (6.5) | 4.8 |
| ≥ 45 and <60 | 146/890 (16.4) | 26.5 | 86/588 (14.6) | 10.5 |
| < 45 | 45/279 (16.1) | 28.5 | 32/185 (17.3) | 13.5 |
Subgroup of participants without diabetes mellitus and hypertension at baseline (n=7545)
Figure 1.
Cumulative incidence of heart failure according to eGFR levels of < 60 and ≥ 60 ml/min/1.73 m2.
Figure 2.
Cumulative incidence of heart failure according to eGFR categories of <45, 45-60, 60-90 and ≥ 90 ml/min/1.73 m2
Table 3.
Cox Proportional Hazard Regression Models Examining the Risk of Heart Failure and CVD death or Heart Failure According to eGFR levels
| Glomerular Filtration Rate (ml/min/1.73 m2) |
Entire sample* | Subgroup† | ||
|---|---|---|---|---|
| Hazard Ratio (CIs) |
P value | Hazard Ratio (CIs) |
P value | |
| Incidence of Heart Failure | ||||
|
| ||||
| ≥ 60 | Referent | Referent | ||
| < 60 | 2.05 (1.62-2.59) | <0.0001 | 2.22 (1.66-2.95) | <0.0001 |
|
| ||||
| ≥ 90 | Referent | Referent | ||
| ≥ 60 and <90 | 1.24 (0.98-1.56) | 0.08 | 1.19 (0.88-1.59) | 0.26 |
| ≥ 45 and <60 | 2.58 (1.91-3.49) | <0.0001 | 2.66 (1.82-3.87) | <0.0001 |
| < 45 | 1.58 (0.92-2.76) | 0.11 | 1.96 (1.03-3.75) | 0.04 |
|
| ||||
| Incidence of CVD Death or Heart Failure | ||||
|
| ||||
| ≥ 60 | Referent | Referent | ||
| < 60 | 2.07 (1.76-2.44) | <0.0001 | 2.18 (1.76-2.69) | <0.0001 |
|
| ||||
| ≥ 90 | Referent | Referent | ||
| ≥ 60 and <90 | 1.42 (1.19-1.70) | 0.0001 | 1.26 (1.01-1.56) | 0.04 |
| ≥ 45 and <60 | 2.74 (2.19-3.44) | <0.0001 | 2.28 (1.71-3.04) | <0.0001 |
| < 45 | 3.05 (2.19-4.25) | <0.0001 | 2.65 (1.77-3.97) | <0.0001 |
CIs denotes confidence intervals
Adjusted for age (as part of calculated eGFR), body mass index, diabetes mellitus, systolic and diastolic blood pressure, smoking, alcohol intake and exercise days.
Subgroup analyses of participants with no hypertension or diabetes mellitus at baseline (n=7545) adjusting for all covariates as for the entire sample and with additional adjustment for hypertension, diabetes mellitus and myocardial infarction as time-dependent variables.
We could not detect any effect modification by hypertension, diabetes or BMI when heart failure risk was evaluated for individuals with eGFR <60 ml/min/1.73 m2 compared to the referent category (all p values >0.10).
Subgroup analyses
In the subgroup of individuals without diabetes and hypertension at baseline, 532 individuals developed diabetes, 2424 developed hypertension, 398 had interim MI and 284 participants had heart failure on follow up. Cumulative incident rates per 1000 person-years of follow up were similar to that of the entire sample (Table 2). In multivariable models with adjustment for all covariates including diabetes, hypertension and MI as time-dependent variables, the risk of heart failure remained robust with >2.0-fold among individuals with eGFR <60 ml/min/1.73 m2 and >2.5-fold for those with eGFR between 45-60 ml/min/1.73 m2, compared to respective referent categories (Table 2). The risk of heart failure in individuals with eGFR <45 ml/min/1.73 m2 was statistically significant and almost reached 2-fold, compared to referent category.
Additional analyses
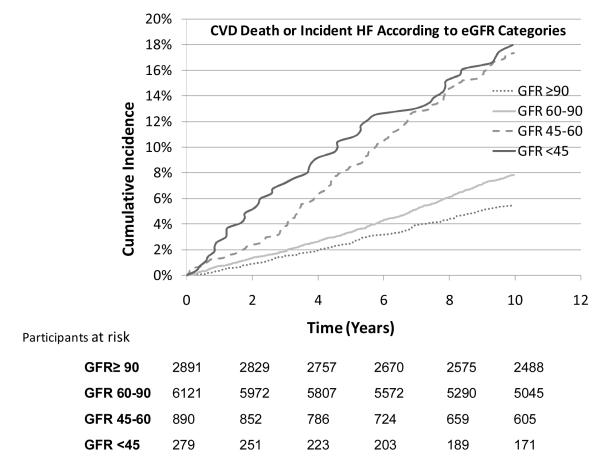
Since we did not have information on fatal heart failure events we also examined the relations of eGFR categories to the incidence of heart failure and CVD death as a combined end point. There were 832 combined events on follow up (mean 10 years). Cumulative incidence curves showed graded increasing risk (Figure 3) and incident rates increased with decreasing eGFR categories (Table 2). In multivariable models, individuals with eGFR between 45-60 ml/min/1.73 m2 had a 2.7-fold risk of an event (HR 2.74, 95% CI 2.19-3.44) whereas those with eGFR <45 ml/min/1.73 m2 had a 3-fold higher risk of incident heart failure or CVD death, (HR 3.05 95% CI 2.19-4.25), compared to the referent category (eGFR ≥90 ml/min/1.73 m2; Table 3). These associations remained robust and were slightly attenuated after excluding men with diabetes and hypertension at baseline (Table 3). Lastly, CVD death or new-onset heart failure was >2-fold higher among individuals with eGFR < 60 ml/min/1.73 m2, even in the subgroup of normotensive and non-diabetics, compared to referent category.
Figure 3.
Cumulative incidence of CVD death or incident heart failure (combined endpoint) according to eGFR categories of <45, 45-60, 60-90 and ≥ 90 ml/min/1.73 m2
Discussion
Principal findings
Our primary results were 3-fold. First, in individuals who were free of heart failure at baseline, eGFR between 45-60 ml/min/1.73 m2 was associated with higher risk of heart failure on follow up. Categorical models showed approximately 2-2.5 fold higher risk of heart failure in individuals within this category. Although in the multivariable models, age was a confounder of the association between CKD and heart failure risk. Second, the subgroup of non-diabetic and normotensive men, with eGFR between 45-60 ml/min/1.73 m2 had the greatest risk of developing new-onset heart failure. Third, among individuals with eGFR <45 ml/min/1.73 m2 and those with eGFR 45-60 ml/min/1.73 m2 the risk of CVD death or heart failure was >2.5-fold compared to the referent category and was attenuated minimally with exclusion of diabetics and normotensive individuals at baseline.
Comparison with prior literature
Prior results from two large epidemiologic studies did not show any increased risk of heart failure with higher serum creatinine, 14,15 but others have observed higher risk of heart failure with increasing serum creatinine levels 9-11 and with lower eGFR.8,12,13 Recently, newer but costlier markers such as cystatin C are also been studied to measure kidney function decline in assessment of heart failure risk 25 where researchers have observed differences by race and blood pressure such that blacks were at higher risk compared to white individuals 7 and hypertensives were more prone to develop heart failure compared to normotensives.26
Mechanisms
There are several possible mechanisms which can increase the risk of heart failure in individuals with CKD. First, as postulated earlier, individuals with CKD have higher prevalence of hypertension, diabetes mellitus, higher BMI, and other coronary risk factors which are also known to increase the risk for heart failure. Moreover, many researchers evaluating the risk of CVD in CKD patients have also postulated that most of the CVD risk is likely due to higher prevalence of traditional coronary risk factors in CKD patients.14,15,27 In the present study however, the risk of heart failure in participants with eGFR <60 ml/min/1.73 m2 was maintained after adjustment for traditional risk factors. Subgroup analyses also revealed that there was no difference when the risk for heart failure was evaluated among non-diabetics, normotensive individuals with adjustment for MI on follow up.
Second, damage to nephrons in renal dysfunction leads to high blood pressure through mechanisms such as plasma volume expansion, over-activity of sympathetic nervous system and renin-angiotensin-aldosterone axis,28 which sets a vicious circle of higher blood pressure thereby leading to left ventricular enlargement,29 a known precursor for heart failure.30 A regression of hypertrophy is associated with reduced cardiac failure outcomes.31 Of note, a tighter control of blood pressure is also known to decrease the progression of kidney disease in some but not all studies.32 However, in the present study, non-diabetics, normotensive individuals with moderate to severe kidney disease exhibited similar and higher risk of heart failure compared to the entire sample, independent of developing hypertension on follow up.
Third, moderate to advanced kidney disease is associated with higher serum phosphorus which is further linked to development of LVH in experimental studies 33,34 and higher incidence of CVD mortality in some 35,36 but not all 37 epidemiologic studies. However, recent data also suggests that a high but ‘normal’ level of serum phosphorus are associated with greater risk of developing CVD (including heart failure) in the community.38 Therefore, it is plausible that mild to moderate CKD may lead to elevated serum phosphorus which then can lead to an increased risk for the development of LVH and clinical heart failure.
Lastly, chronic kidney disease patients have decreased erythropoietin formation which leads to development of anemia. A graded decrease in hemoglobin concentration is significantly associated with increases in left ventricular mass.39 Conversely, even partial correction of anemia in dialysis patients has been shown to produce regression of LVH.40
Strengths and Limitations
The present study has the largest sample size including a large subgroup of non-diabetics and normotensive individuals as compared to previous studies. In the present analyses, we accounted for all traditional coronary risk factors as well as CHD, diabetes and hypertension on follow up which strengthens our study. Some limitations also merit discussion. First, measurement of renal function based on different formulas to measure eGFR has been questioned and some have found novel markers such as cystatin C to be better predictors of renal dysfunction 25 however, eGFR still remains the cheaper and precise method of measuring kidney function.41 CKD is defined by National Kidney Foundation as eGFR <60 ml/min/1.73 m2 for a period of at least 3 months. In the present study (and perhaps in most other large epidemiologic studies) a single measurement is often used. Moreover, any misclassification in the present study would likely underestimate the relations and would bias results towards the null. It is plausible that the risk of heart failure and CVD death may vary with time, especially in individuals with impaired kidney function. Second, in our dataset, we did not have information on fatal heart failure events therefore we performed additional analyses using CVD death or heart failure as a combined event. Individuals within eGFR categories of 45-60 ml/min/1.73 m2 and <45ml/min/1.73 m2 had a statistically significant higher risk of combined event (heart failure and CVD death). Of note, the hazard ratio for incident CVD death is even higher among men with eGFR <45ml/min/1.73 m2 compared to those with eGFR between 45-60 ml/min/1.73 m2. Therefore, it is plausible that individuals in the category of eGFR<45ml/min/1.73 m2 had more number of first fatal heart failure episodes. But, data from US renal data system indicates that only 13% of CVD deaths are associated with heart failure which also includes deaths among chronic heart failure patients.42 Lastly, participants in our study were predominantly white men who were physicians hence results may have limited generalizability for women and may differ with socioeconomic class.
Conclusion
In this sample of men free of heart failure at baseline, moderate CKD was associated with a higher incidence of heart failure and CVD death or heart failure on follow up. Additionally, subgroup of normotensive and non-diabetic individuals with moderate CKD also experienced similar and higher risk of heart failure as for the whole sample.
Clinical Perspective.
Individuals with chronic kidney disease (CKD) have higher prevalence of left ventricular hypertrophy and cardiovascular disease however, it remains unclear if development of CKD is associated with higher incidence of heart failure. We analyzed the relations of CKD to incident heart failure and to CVD death or heart failure (combined end point) in 10,181 Physicians’ health study male participants (mean age, 67years). Kidney function was assessed by estimating the glomerular filtration rate (eGFR) using Modification of Diet in Renal Disease equation in clinically relevant categories of <60 and ≥60 ml/min/1.73 m2 (referent); and <45, 45 to 60, 60 to 90 and ≥90 ml/min/1.73 m2 (referent). During follow up, 439 participants developed heart failure and 832 had a combined endpoint of CVD death or heart failure. In multivariable models, men with eGFR 45-60 and <45ml/min/1.73m2 had nearly 2-2.5-fold higher risk for heart failure compared to referent category. Further, these relations remained robust in the analyses restricted to subgroup of non-diabetics and normotensive individuals at baseline (n=7545). In addition, men with eGFR 45-60 and <45ml/min/1.73m2 had >2.5-fold risk of CVD death or heart failure compared to referent category. In summary, our results show that moderate level of CKD, even in the absence of diabetes and hypertension is associated with a higher risk of developing heart failure and CVD death/heart failure in men.
Acknowledgement
All authors have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Funding: The Physicians’ Health Study is supported by grants CA-34944, CA-40360, CA-097193, HL-26490, and HL-34595 from the NIH, Bethesda, MD.
Financial Disclosure: Dr Gaziano reported receiving investigator-initiated research funding from the NIH, the Veterans Administration, Veroscience, and Amgen; serving as a consultant or receiving honoraria from Bayer AG and serving as an expert witness for Merck. Dr Djoussé reported that he is currently the recipient of investigator-initiated grants from the NIH and that over the past 5 years he has received investigator-initiated grant from the Biomedical Research Institute, Brigham and Women’s Hospital.
References
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Garrison RJ, editors. The Framingham Study: an epidemiological investigation of cardiovascular disease. Section 34. Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements: Framingham Heart Study, 30-year follow up. National Heart, Lung and Blood Institute; Bethesda, MD: Feb, 1987. (NIH publication no. 87-2703) [Google Scholar]
- 3.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 5.Middleton RJ, Parfrey PS, Foley RN. Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol. 2001;12:1079–1084. doi: 10.1681/ASN.V1251079. [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 7.Bibbins-Domingo K, Chertow GM, Fried LF, Odden MC, Newman AB, Kritchevsky SB, Harris TB, Satterfield S, Cummings SR, Shlipak MG. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166:1396–1402. doi: 10.1001/archinte.166.13.1396. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 9.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 10.Chae CU, Albert CM, Glynn RJ, Guralnik JM, Curhan GC. Mild renal insufficiency and risk of congestive heart failure in men and women > or =70 years of age. Am J Cardiol. 2003;92:682–686. doi: 10.1016/s0002-9149(03)00822-1. [DOI] [PubMed] [Google Scholar]
- 11.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 12.Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63:1121–1129. doi: 10.1046/j.1523-1755.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- 13.Manjunath G, Tighiouart H, Ibrahim H, Macleod B, Salem DN, Griffith JL, Coresh J, Levey AS, Sarnak MJ. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 14.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 15.Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 16.Muntner P, He J, Hamm L, Loria C, Whelton PK. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 17.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization World Health Organization; Copenhagen, Denmark: Ischaemic heart disease registers: report of the Fifth Working Group, including a second revision of the operating protocol. 1971
- 20.Rexrode KM, Buring JE, Glynn RJ, Stampfer MJ, Youngman LD, Gaziano JM. Analgesic use and renal function in men. JAMA. 2001;286:315–321. doi: 10.1001/jama.286.3.315. [DOI] [PubMed] [Google Scholar]
- 21.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 22.Dhingra R, Sesso HD, Kenchaiah S, Gaziano JM. Differential effects of lipids on the risk of heart failure and coronary heart disease: the Physicians’ Health Study. Am Heart J. 2008;155:869–875. doi: 10.1016/j.ahj.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarnak MJ, Katz R, Stehman-Breen CO, Fried LF, Jenny NS, Psaty BM, Newman AB, Siscovick D, Shlipak MG. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 26.Djousse L, Kurth T, Gaziano JM. Cystatin C and risk of heart failure in the Physicians’ Health Study (PHS) Am Heart J. 2008;155:82–86. doi: 10.1016/j.ahj.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 28.Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–152. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- 29.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27:347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 30.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left Ventricular Dilatation and the Risk of Congestive Heart Failure in People without Myocardial Infarction. N Engl J Med. 1997;336:1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 31.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. J Am Soc Nephrol. 2000;11:912–916. doi: 10.1681/ASN.V115912. [DOI] [PubMed] [Google Scholar]
- 32.Sarnak MJ, Greene T, Wang X, Beck G, Kusek JW, Collins AJ, Levey AS. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 33.Amann K, Tornig J, Kugel B, Gross ML, Tyralla K, El Shakmak A, Szabo A, Ritz E. Hyperphosphatemia aggravates cardiac fibrosis and microvascular disease in experimental uremia. Kidney Int. 2003;63:1296–1301. doi: 10.1046/j.1523-1755.2003.00864.x. [DOI] [PubMed] [Google Scholar]
- 34.Neves KR, Graciolli FG, dos Reis LM, Pasqualucci CA, Moyses RM, Jorgetti V. Adverse effects of hyperphosphatemia on myocardial hypertrophy, renal function, and bone in rats with renal failure. Kidney Int. 2004;66:2237–2244. doi: 10.1111/j.1523-1755.2004.66013.x. [DOI] [PubMed] [Google Scholar]
- 35.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 36.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca × PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 37.Menon V, Greene T, Pereira AA, Wang X, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am J Kidney Dis. 2005;46:455–463. doi: 10.1053/j.ajkd.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB, Sr., Gaziano JM, Vasan RS. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 39.Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, Burgess E, Jindal K, Barrett B, Singer J, Djurdjev O. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125–134. doi: 10.1016/s0272-6386(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 40.Pascual J, Teruel JL, Moya JL, Liano F, Jimenez-Mena M, Ortuno J. Regression of left ventricular hypertrophy after partial correction of anemia with erythropoietin in patients on hemodialysis: a prospective study. Clin Nephrol. 1991;35:280–287. [PubMed] [Google Scholar]
- 41.Smilde TD, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–1580. doi: 10.1161/CIRCULATIONAHA.105.610642. [DOI] [PubMed] [Google Scholar]
- 42.US Renal Data System . USRDS 2008 Annual Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; Bethesda, MD: 2008. [Google Scholar]