Abstract
Transforming Growth Factor β (TGF-β) is produced excessively by many solid tumors and can drive malignant progression through multiple effects on the tumor cell and microenvironment. TGF-β signaling pathway inhibitors have shown efficacy in pre-clinical models of metastatic cancer. Here we investigated the effect of systemic LY2109761, a type I /II receptor (TβRI/TβRII) kinase inhibitor, in both a tumor allograft model and in the mouse skin model of de novo chemically-induced carcinogenesis in vivo. Systemic LY2109761 administration disrupted tumor vascular architecture and reduced myofibroblast differentiation of E4 skin carcinoma cells in a tumor allograft. In the 7,12 dimethyl-benzanthracene plus phorbol-myristate-acetate -induced skin chemical carcinogenesis model, acute dosing of established naïve primary carcinomas with LY2109761 (100mg/Kg) every eight hours for ten days (100mg/kg) diminished P-Smad2 levels and marginally decreased the expression of inflammatory and invasive markers. Sustained exposure to LY2109761 (100mg/kg/day) throughout the tumor outgrowth phase had no effect on carcinoma latency or incidence. However, molecular analysis of resultant carcinomas by microarray gene expression, Western blot and immunohistochemistry suggests that long term LY2109761 exposure leads to the outgrowth of carcinomas with elevated P-Smad2 levels that do not respond to drug. This is the first description of acquired resistance to a small molecule inhibitor of the TGF-βRI/II kinase. Resultant carcinomas were more aggressive and inflammatory in nature, with delocalized E-Cadherin and elevated expression of Il23a, laminin V and MMPs. Therefore, TGF-β inhibitors might be clinically useful for applications requiring acute administration, but chronic patient exposure to such drugs should be undertaken with caution.
Keywords: TGF-β, Small molecule kinase inhibitor, Mouse skin, Chemical carcinogenesis, Drug Resistance
Introduction
TGF-β is a potent growth inhibitor of normal epithelial cells, but is produced in excessive quantities by many advanced tumor types (1). This excess TGF-β is thought to drive malignant progression through multiple effects on the tumor cell and on the tumor micro-environment (1–3). In accordance with this hypothesis, local or systemically elevated levels of TGF-β1 are associated with poor prognosis, and the TGF-β signaling pathway has become the focus for the design of specific inhibitors for cancer therapy (4, 5).
TGF-β1 signals via a heteromeric complex of type II and type I TGF-β receptors that activates the canonical Smad pathway by TβRI-mediated phosphorylation of Smad2 and Smad3. Nuclear shuttling of the resultant hexameric complex, composed of receptor-associated phospho-Smads bound to Smad4, culminates in TGF-β-driven transcriptional responses (6). The TGF-β receptor complex can also signal via non-Smad pathways to affect cell survival and EMT (7). During tumorigenesis, the TGF-β signaling pathway can be genetically inactivated within the cancer cell, particularly in microsatellite unstable gastrointestinal tract cancers, making the tumor cell resistant to TGF-β growth inhibitory effects (8). Epigenetic inactivation of the gene encoding the TGF-β type 2 receptor (TGFBR2) has been observed in lung and prostate cancer (9, 10). Alternatively, in TGFBR2-positive tumors, activation of a panoply of oncogenic signaling pathways within the cancer cell can blunt the negative growth response to TGF-β and redirect signaling output towards stimulation of tumor cell migration, invasion, and in some cases, increased proliferation and tumor growth. Both in vivo and in vitro, this multistage passage towards invasion and metastasis is often accompanied by transition from an epithelial towards a fibroblastoid tumor cell phenotype (11). In the chemically-induced mouse skin model of tumorigenesis, resultant fibroblastoid spindle cell tumors are the most aggressive cutaneous lesions, although distant metastases are frequently squamous in character.
The goal of TGF-β inhibition has been to target its tumor promoting properties, both cell autonomous and micro-environmental, whilst avoiding inhibition of its tumor suppression arm (5). There have been several preclinical reports on the use of both large and small molecule inhibitors of the TGF-β signaling pathway for various oncology applications. These inhibitors have been particularly efficacious in metastatic carcinoma models (12–15), utilizing multiple mechanisms to elicit effects. Clinical trials have commenced which, like their preclinical counterparts, show promise for treatment of metastatic melanoma, renal cell carcinoma and glioblastoma (16, 17).
In mice, negative consequences of long term exposure to a large molecule inhibitor of TGF-β, Fc:TβRII, were few (12), yet this drug was efficacious in reducing metastasis of injected melanoma cells (13). More importantly, this soluble receptor antagonist inhibited spontaneous metastasis of primary mammary tumors that arise in MMTV-Neu transgenics (12). Taken together with preliminary reports on clinical trials with an anti-TGF-β antibody (17), these data suggest that anti-TGF-β drugs are well tolerated in mice and humans.
The current study explores the therapeutic potential of LY2109761, a small molecule inhibitor of type I and type II TGF-β receptor kinase that targets activation of P-Smad2 and P-Smad3, and inhibits the related kinase receptors, Acvr1b (activin activated) and Acvrlc (nodal activated). We demonstrate in a mouse skin model of chemically-induced carcinogenesis that, despite the favorable outcomes of LY2109761 in inhibiting EMT in vitro and its short term effects on carcinomas in vivo, sustained pharmacological inhibition of TGF-β signaling may have a pro-tumor effect with expansion of a more aggressive inflammatory drug-resistant carcinoma phenotype expressing markers of invasion.
Materials and Methods
Cell culture
Murine cell lines used in this study were isolated from chemically-induced cutaneous carcinoma, and have been very well characterized with respect to genetic mutations and gene expression profiles. These were provided by Allan Balmain at UCSF. E4 and H11 cells were both single cell clones from SN161, derived from a lymph node metastasis of an F1.129×NIH mouse (18). D3 cells were isolated from a primary SCC from a F1 M.musculus NIH × M.spretus animal (19). Where indicated, cells were treated with 5 ng/mL rhTGF-β1 (R&D Systems) and/or the TGF-β RI/II kinase inhibitor LY2109761 (20). Media containing TGF-β LY2109761 and/or DMSO vehicle were refreshed every other day.
Animals
All animal work was done in accordance with a UCSF Institutional Animal Care and Use Committee protocol. Nude mouse tumor allografts were generated by subcutaneous injection of E4 cells (1×107 cells/ml) (2 sites/mouse). Tumors were harvested 10 days later.
Pharmacokinetic analysis
Plasma samples were harvested from blood collected at 0.5, 2, 4 and 8 hours after oral administration of LY2109761 (100 mg/kg of body weight). Plasma LY2109761 levels were determined by HPLC at the UCSF Drug Study Unit, Analytical Division.
Chemical carcinogenesis
Mice (8 week-old female inbred NIH/OlaHsd) underwent standard DMBA/PMA tumor induction as previously described (11, 21). Tumors were initiated with dimethylbenzanthracene (DMBA), 25ug/200ul acetone. The tumor promoter was phorbol myristate acetate (PMA), 2ug/200ul acetone. The experiment was terminated once carcinoma reached 1.5cm, became ulcerated, or if the mouse showed signs of morbidity. Carcinomas were diagnosed clinically, which was confirmed by histological analysis. LY2109761 (7.5 mg/ml) or vehicle alone was administered by oral gavage in a polytroned suspension in CMC/SLS/PVP/anti-foam. Two dosing regimens were used: the Sustained Dosing Regimen was a daily single 100mg/kg dose of LY2109761 every day, 7 days per week, from week 6 post-DMBA throughout tumor outgrowth, as specified. The Short Term Dosing Regimen was administered to animals that had already established DMBA/PMA-induced carcinoma i.e. 8–9 month old female mice ~ roughly 25 weeks after DMBA treatment. This regimen was 100mg/kg LY2109761 given every eight hours for 10 days.
Immunohistochemistry (IHC)
IHC was performed on 5µm 4% PFA fixed, paraffin-embedded tissue sections. Antibodies included anti: α-smooth muscle actin (Sigma), CD45 (Caltag Laboratories), E-cadherin (BD Biosciences), F4/80 (Caltag Laboratories), P-Smad2 (Cell Signaling), P-Smad2/3 (Santa Cruz) and MMP13 (Santa Cruz), Vimentin (Cell Signaling). CD31 staining was performed using a Biocare Medical® kit). TUNEL staining was performed using the DeadEnd Colorimetric TUNEL system (Promega). Quantification employed 10 fields of view per sample. NIH ImageJ was use to determine pixel density (P-Smad staining), tumor stroma area (Vimentin staining) and TUNEL positive cell counts.
RNA isolation, labeling and microarray hybridization
Total RNA was extracted from snap-frozen carcinomas by Trizol (Invitrogen) using Ambion’s DNA-free kit, and RNAs were purified using RNeasy (Qiagen). Quantified RNAs were amplified using the Illumina TotalPrep RNA amplification kit (Ambion). The effects of the Sustained Drug Dosing (LY2109761, n=3; Vehicle, n=3) and Short Term Drug Dosing (LY2109761, n=4; Vehicle, n=5) regimens on gene expression were analyzed using the Illumina Mouse-WG-6 v2 platform. Slides were scanned on an Illumina Beadstation, data were extracted using Illumina BeadStudio software and normalized by quantile normalization. Of 46,644 probes on the microarray, 32% (14,818) had a present P value of ≤ 0.05 in at least 80% of samples as assigned by BeadStudio, and these probes were used for further analysis. Gene expression in both treatment conditions was compared to that of matched vehicle-treated control mice, using the Signficance Analysis of Microarrays algorithm (22), with a False Discovery Rate cut-off of 10%.
Western blotting
Protein extraction and western blotting was performed as described previously (21). Antibodies included anti: P-Smad2 (Eli Lilly or Cell Signaling), Total Smad2/3 (BD Biosciences), E-cadherin (BD Biosciences), RBPjk (Santa Cruz), Lgr6 (Santa Cruz), GAPDH (Cell Signaling) or β-actin (Sigma-Aldrich).
Results
LY2109761 inhibits and reverses TGF-β1-induced EMT in vitro
Murine carcinoma cell lines isolated from different stages of chemically-induced mouse skin tumorigenesis were evaluated for their response to the LY2109761 inhibitor. Squamous E4 cells, isolated from a lymph node metastasis of a squamous cell carcinoma, convert to a fibroblastoid spindle phenotype on addition of TGF-β in vitro, or if grown as a subcutaneous tumor in vivo. This EMT is driven cell-autonomously by TGF-β signaling (23). 2µM LY2109761 was sufficient to prevent Smad2 phosphorylation of both squamous and spindle E4 cells (Figure S1A–B). Prolonged LY2109761 exposure elevated E-cadherin protein levels higher than in the absence of exogenous TGF-β (Figure S1A), and prevented and reversed (Figure S1B–C) TGF-β1-induced EMT.
The D3 and H11 carcinoma lines are both innately spindle, independently of exogenous TGF-β (18, 19) and both have higher basal TGF-β signaling levels compared to E4 cells (24) (Figure S1D–E). Acute or chronic treatment with LY2109761 reduced innate P-Smad2 levels in both cell lines (Figure S1D and E), supporting the view that, in these cell lines, TβRI, Acvrlb and/or Acvrlc actively signal to P-Smad2 via a positive autocrine loop (24). Additionally, the D3 cells demonstrated a LY2109761 dose-dependent increase in E-cadherin protein expression (Figure S1D), illustrating the partial contribution of Smad2 signaling to the maintenance of mesenchymal properties and the ability of LY2109761 to steer the D3 cells towards a more epithelial phenotype.
Pharmacokinetics and pharmacodynamics of LY2109761 in vivo
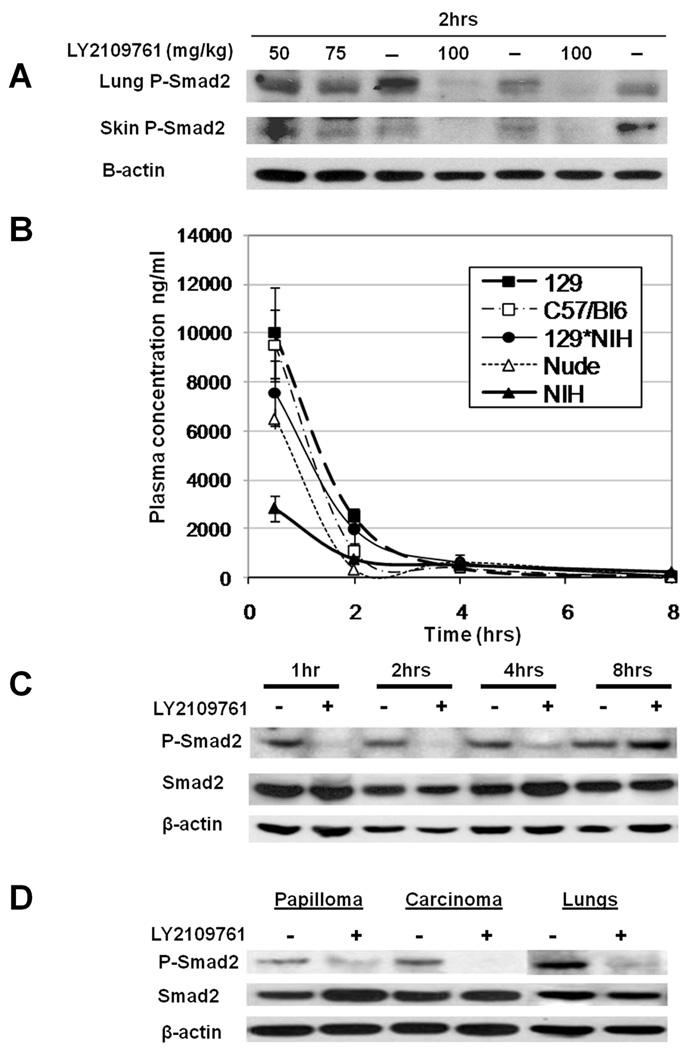
To determine LY2109761 effects on TGF-β signaling in vivo, drug was administered to mice at three doses (50, 75 or 100mg/kg body weight) and tissue P-Smad2 levels were assessed two hours post-oral gavage. In both lung and skin, P-Smad2 levels were suppressed dose-dependently with partial inhibition at 50 and 75 mg/kg and almost complete inhibition at 100mg/kg (Figure 1A). LY2109761 pharmacokinetics were determined by HPLC analysis of murine plasma after a single oral dose (100mg/kg). By four hours LY2109761 was rapidly cleared from the circulation of all mouse strains tested (Figure 1B). LY2109761 pharmacodynamics were assessed by examining pulmonary P-Smad2 levels. Western blot analysis demonstrated an acute down-regulation of P-Smad2 one hour post-oral gavage which was maintained for at least four hours post-LY2109761 dosing and returned to base levels by eight hours (Figure 1C). Therefore, LY2109761 inhibits TGF-β signaling for a few hours after the drug was cleared from the circulation. Administration of a single 100mg/kg LY2109761 dose to tumor-bearing mice resulted in decreased P-Smad2 levels within both carcinomas and papillomas (Fig. 1D), demonstrating that drug effectively penetrated both benign and malignant tumors.
Figure 1. Pharmacokinetics and pharmacodynamics of LY2109761 after oral dosing in mice.
A) Mice were administered a single oral bolus of LY2109761 at the indicated doses. P-Smad2 western blot analysis was performed on protein lysates from lung and skin two hours after dosing. B) Plasma concentrations of LY2109761 in different mouse strains were measured by HPLC at various time points following a single oral dose of the drug (100mg/kg). Mouse strains analyzed: 129, 129SvS2/Hsd; C57/BL6; C57BL/6NTac; NIH, NIH/OlaHsd; 129/NIH, F1 between 129SvS2/Hsd and NIH/OlaHsd; Nude, NCR.nu/nuTac. C) Inhibition of P-Smad2 levels by LY2109761 was determined by western blot analysis of lung tissue from mice after a single oral dose of 100mg/kg LY2109761. D) Tumor-bearing mice that had been subjected to tri-daily oral dosing with LY2109761 at 100mg/ml for 10 days (Short Term), were administered a single oral bolus of 100mg/kg LY2109761 and protein lysates from papilloma, carcinoma and lung were isolated 2 hours later. Western blot analysis was performed to detect P-Smad2, total Smad2 and β-actin levels.
LY2109761 treatment of E4 tumor allografts in vivo reduces carcinoma myofibroblasts and disrupts vascular integrity
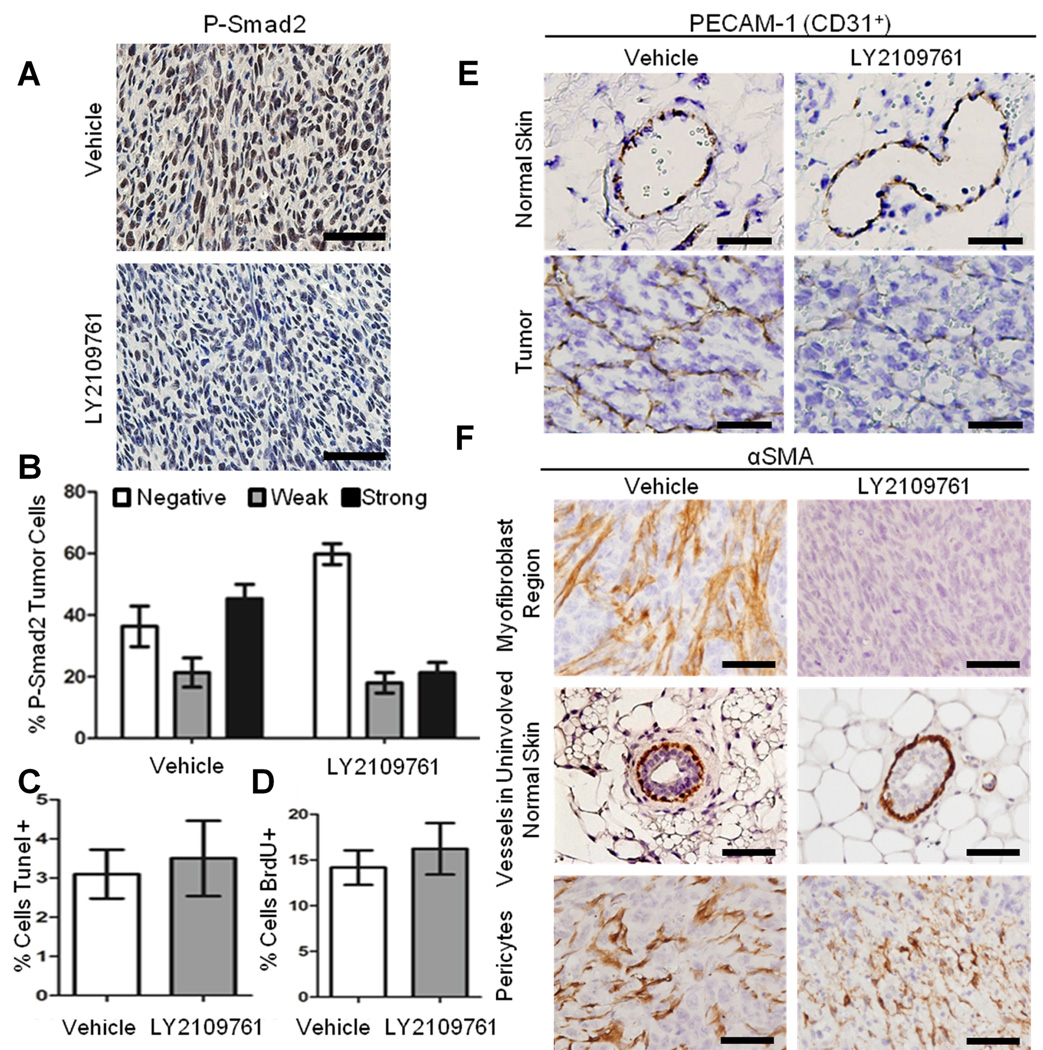
Subcutaneously injected E4 cells grow as aggressive highly vascularized spindle tumors. LY2109761 administered in drinking water (2mg/mL) ad libitum for 10 days significantly reduced nuclear P-Smad2 expression (Figure 2A, B), but had no effect on tumor outgrowth (data not shown), apoptosis or proliferation (Figure 2C–D). However, LY2109761 treatment did alter tumor histology. Whereas control tumors demonstrated a fine reticular network of CD31+ vessels invested with pericytes and features of active angiogenesis such as non-invested CD31+ cells, (Figure 2E), LY2109761-treated tumors displayed disrupted vascular architecture. Vessels present in drug-treated tumors were dilated, with weak or no CD31+ staining, and pooling of red blood cells suggestive of indolent blood flow and hemorrhage (Figure 2E).
Figure 2. LY2109761 reduces P-Smad2 expression, myofibroblast phenotype and vascular integrity without effects on proliferation or apoptosis in E4 carcinoma allografts in vivo.
Fourteen nude mice were inoculated bilaterally and sub-cutaneously with E4 carcinoma cells. For 10 days from the time of inoculation, LY2109761 2 mg/mL was provided ad libitum in drinking water to the experimental group (n=7) whereas the control group were fed normal water. A) Tumor sections from vehicle- and drug-treat were stained for P-Smad2. LY2109761 treatment led to a decrease in nuclear P-Smad2 expression. B) ImageJ was used to determine the average pixel intensity of the nuclear P-Smad2 staining. Nuclei were scored as strong, weak or negative for nuclear P-Smad2. Five sections each of five independent allografts were quantified from both the LY2109761 and vehicle treated mice. The decrease in P-Smad2 nuclear staining was significant (P<0.001 by 2-way ANOVA). C) Tumor sections were stained by TUNEL, and apoptosis was estimated as the average of TUNEL-positive cells per representative field (5 allografts/treatment). D) Mice were injected with BrdU two hours before harvest of the tumor. LY2109761 and vehicle treated mice were examined for BrdU incorruption by anti-BrdU staining (5 allografts/treatment). Proliferation was estimated as the average of the BrdU-positive cells per representative field. There was no significant difference between the drug and vehicle treatments in either the percentage of apoptotic or proliferating cells. E–F) Tumor sections from vehicle- and drug-treated mice, as indicated, were stained for E) CD31 /PECAM-1 or F) αSMA. Vehicle-treated tumors possessed a fine reticular capillary network of pericyte-invested, PECAM-1+ vessels, whereas the tumor vasculature of LY2109761-treated mice was poorly invested with α-SMA+ cells. Note that in E, αSMA staining of vascular smooth muscle cells was more intense than that of the myofibroblasts, and that vessels within uninvolved skin were unaffected by LY2109761 (E upper panels). Vessels within drug-treated tumor stained weakly for PECAM-1, were dilated, filled with pooling red blood cells, with hemorrhaging into tumor tissue. Scale bar = 50µM
It has been reported that increased carcinoma levels of α-smooth muscle actin (αSMA) in tumors may be predictive of an aggressive phenotype, and the most invasive tumor types take on a myofibroblast phenotype (24, 25). E4 spindle tumor cells from vehicle-treated mice showed high levels of αSMA staining, and treatment with LY2109761 decreased this expression (Figure 2F). However, unlike genetic inhibition of TGF-β signaling in this model (23), there was not a complete reversion from spindle to squamous morphology.
Sustained suppression of TGF-β signaling by LY2109761 increases chemically induced papilloma incidence
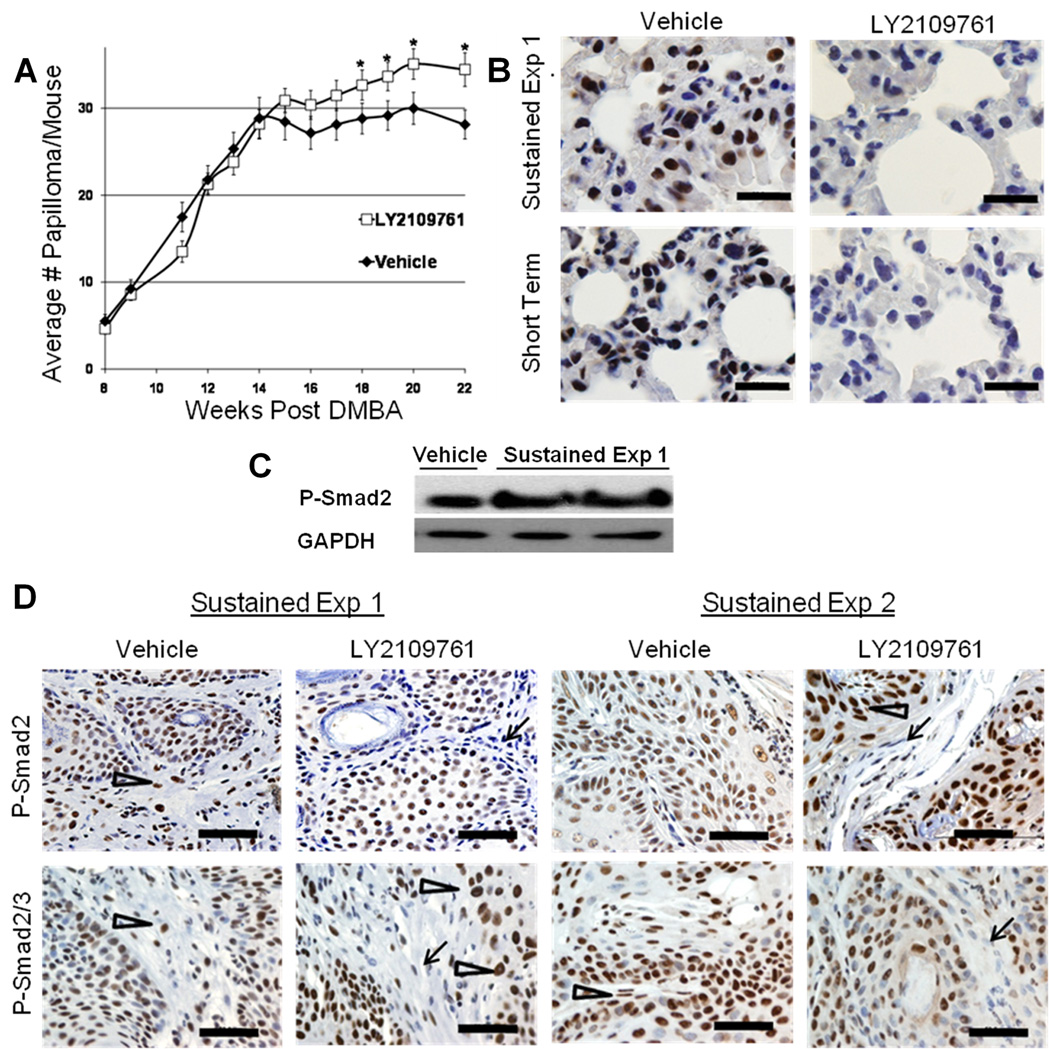
To determine the long term effects of LY2109761 treatment on DMBA-PMA-induced tumorigenesis, we dosed mice once daily with LY2109761 during the papilloma and carcinoma outgrowth phases of skin carcinogenesis. Three experiments were undertaken (Figures 3A and S2). A pilot experiment (75mg/kg dosing) showed a significant drug-induced increase in papilloma numbers (Figure S2A), but a larger replication study failed to show a difference (Figure S2B). In a third experiment, the drug dose was elevated to 100mg/kg, which significantly increased papilloma numbers in drug-treated compared to vehicle-treated mice (Figure 3A). These findings are concordant with our previous studies showing that elevation of TGF-β1 expression via transgenesis reduced benign papilloma number, but genetic reduction of TGF-β1 levels led to increased papilloma numbers (11, 21).
Figure 3. Sustained LY2109761 treatment increases papilloma incidence and results in resistance to P-Smad2 drug response in malignant but not normal cells.
A) Fifty two age-matched adult NIH female mice were subjected to a standard chemical carcinogenesis protocol with once daily oral treatment with LY2109761 at 100mg/kg or vehicle alone (Sustained Drug Treatment). Papilloma numbers were counted weekly. B) Representative western blot analysis for P-Smad2 (Cell Signaling) in chemically-induced primary carcinomas from the Sustained Drug Treatment regimen, harvested 2 hours after the last drug administration. GAPDH was used as loading control. C) IHC of P-Smad2 (Cell Signaling) and P-Smad2/3 (Santa Cruz) in carcinomas from two independent experiments of the Sustained Drug Treatment regimen. Sustained Exp 1 (LY2109761 100mg/kg/daily from week 6 to week 33 post DMBA) and Sustained Exp 2 (LY2109761 100mg/kg/daily from week 6 to week 17). All mice were dosed with LY2109761 or vehicle two hours prior to collection of tumors. Note the positive P-Smad2 staining of both stromal fibroblasts and carcinoma cells in vehicle-treated carcinomas (arrowheads) compared to down-regulation of P-Smad2 in stromal fibroblasts (arrows) but not in carcinoma cells (arrowheads) in the LY2109761-treated carcinoma. Quantification of data is shown in Supplementary Figure S4. D) IHC evaluation of the effect of both the Sustained and the Short Term drug regimens on lung P-Smad2 expression, two hours posted last drug dosing. Scale bar = 50µM
The LY2109761 increase in papilloma number was not due to a decrease in apoptosis (data not shown). Histological examination of papillomas revealed that, compared to vehicle-treated mice, Sustained LY2109761 Treatment increased the number of inflammatory (CD45+) cells within the papilloma stroma (Figure S3), suggesting that inflammation might contribute to increased papilloma incidence (26). By morphology, most of the infiltrate appeared to be neutrophilic, with a minority of macrophages. Interestingly, there was no significant change in carcinoma number, malignant conversion frequency or carcinoma latency in the LY2109761-treated mice compared to vehicle (Table S1).
Failure to down-regulate P-Smad2 levels in tumors after sustained LY2109761-treatment
P-Smad2 western blot analysis of carcinoma cell lysates (Figure 3B) and P-Smad2 and P-Smad2/3 IHC analysis of tumors (Fig. 3C) was undertaken on carcinoma that developed following Sustained LY2109761 treatment and that were collected two hours following the last drug dose. Surprisingly, this analysis revealed that P-Smad2 was not significantly down-regulated by LY2109761 (Figures 3C and S4A) and in several carcinomas P-Smad2 was in fact up regulated in drug-treated compared to vehicle-treated mice (Figures 3B, C and S4C). In contrast, non-malignant fibroblast cells of the carcinoma stroma showed decreased P-Smad2 staining in response to LY2109761 treatment, even after sustained drug exposure (Figures 3C and S4B).
We hypothesized that drug-refractile P-Smad2 signaling in the tumor parenchyma was a malignant adaptation to sustained TGF-β signaling inhibition, by outgrowth of a drug resistant carcinoma population or by activation of P-Smad2 by alternative intersecting pathways (27). To address this issue we investigated the effect of a shorter but more frequent drug-dosing regimen (Short Term) on established naïve DMBA/PMA-induced primary carcinoma. Mice bearing full blown DMBA/PMA-induced carcinoma 25 weeks post-initiation, were orally dosed with LY2109761 (100mg/kg) every eight hours for 10 days (n=7 drug-treated and n=7 vehicle treated). This treatment had no overt effect on carcinoma morphology, but IHC demonstrated variable degrees of P-Smad2 down-regulation (Fig. S4A). We also evaluated lung tissue from these carcinoma-bearing mice to determine the response of non-malignant tissue to LY2109761 treatment. Indeed, western analysis (not shown) and IHC of lung tissue from mice on both the Short Term or Sustained Treatment regimens showed reduced nuclear P-Smad2 levels in response to LY2109761 (Figure 3D). Our findings of high drug-insensitive nuclear P-Smad2 levels after Sustained LY2109761 Treatment was validated by IHC analysis of P-Smad2 levels in carcinoma from an independent study in which mice received LY2109761 on a Sustained Dosing regimen from week 6 until week 17 post-DMBA (Sustained Exp 2) (Figure 3C and S4C). Together, these data suggest that acquired drug resistance was limited to malignant tissue and occurred predominantly after Sustained Drug Dosing.
We investigated whether the high P-Smad2 levels seen after sustained treatment might be due to a rebound effect on TGF-β signaling leading to hyper-activation of P-Smad2 between drug doses. Mice were treated once daily for ≥ 7 days with the LY2109761 inhibitor (100mg/kg) and lung tissue was collected at various times after the last drug dose. Western blot analysis showed that P-Smad2 levels were suppressed at two hours, returning to near baseline by 16 hour post treatment, but even after 24 hrs, when the next drug dose would normally be administered, there was no increase in P-Smad2 levels above those observed in the control arm (Figure S5). Increased P-Smad2 levels in carcinomas following Sustained LY2109761 Treatment were therefore unlikely due to a rebound effect.
To investigate the stage of onset of acquired LY2109761-resistance, benign papillomas harvested from the same carcinoma-bearing mice described above were assayed for nuclear P-Smad2 levels. There was a significant decrease in nuclear P-Smad2 levels in papillomas from the Short Term Dosing Regimen compared to matched vehicle-treated controls, but no significant P-Smad2 response after Sustained LY2109761treatment compared to matched vehicle-treated papillomas (Figure S6). Therefore, drug resistance was observed even at the papilloma stage, albeit at experiment termination following many weeks of LY2109761 treatment. Papillomas at earlier stages were not collected.
Sustained suppression of TGF-β signaling induces a pro-invasive gene signature
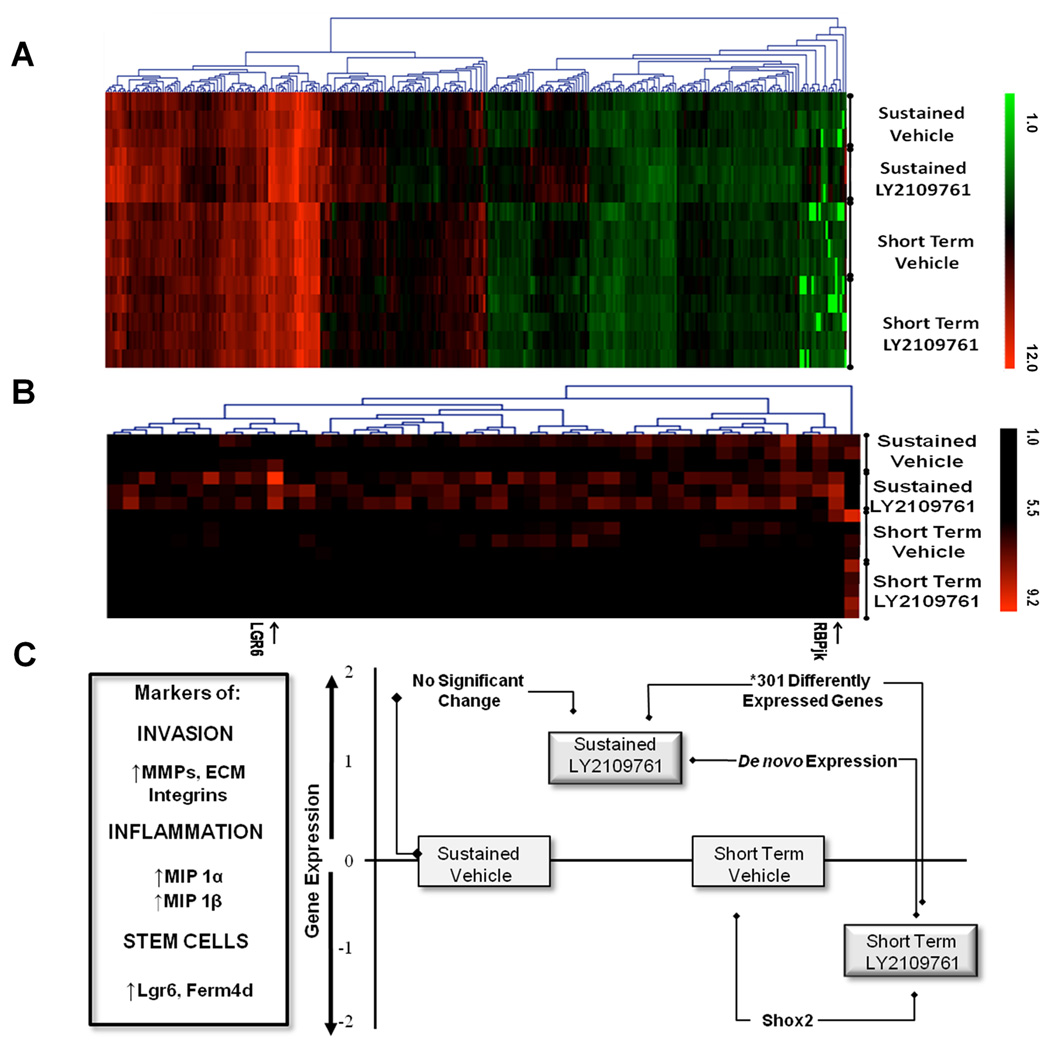
Since sustained LY2109761 administration appeared to paradoxically increase carcinoma P-Smad2 levels, we hypothesized that the resultant carcinomas may be more aggressive, despite the fact that there was no drug induced change in spindle to squamous character (Table S2). To probe the molecular make up of the carcinomas further, microarray gene expression analysis was performed on carcinomas from both drug dosing regimens and their matched vehicle controls. After statistical correction for genome-wide testing, there were no significant changes (≥1.5 fold) in gene expression between the two vehicle-treated arms of the two experiments, nor between matched drug- and vehicle-treated carcinoma on the Sustained Treatment Regimen (False Discovery Rate [FDR] ≤10%). The Short Term dosing regimen resulted in the significant reduction in expression of only one gene; short stature homebox 2 (Shox2; q ≤0.1%; Figure 4), a modulator of embryonic EMT (28). We speculated that, using the stringent statistical analysis employed, inherent heterogeneity between different carcinomas masked any drug-induced changes in gene expression.
Figure 4. Divergent molecular outcomes in carcinomas after LY2109761 treatment with the two drug dosing regimens.
A) Heat Map of 15 carcinoma expression profiles showing ~ 300 differentially expressed genes between the two drug dosing regimens (≥1.5 fold; Q value <10%). Note the most divergent expression profile of the Sustained LY2109761 treatment group compared to the other three groups. B) Heat Map of genes expressed below detection levels (< log2 5.0) in the Short Term dosing regimen, but up regulated to > log2 5.6 after Sustained LY2109761 treatment. Note that Lgr6 and RBPjk are within this latter group of genes expressed at relatively low levels (Table S4). C) A schematized cartoon representing microarray analysis of DMBA/PMA-induced carcinoma after Sustained and Short Term LY2109761 dosing regimens. Y axis represents extent of expression of genes associated with invasion, inflammation and stem cell markers. Blocks represent average expression levels of this class of gene. Note that the Sustained Drug Treatment regimen enhanced expression of this gene set, whereas the Short Term regimen decreased their expression.
In contrast, direct comparison between drug-treated carcinomas from both drug dosing regimens revealed more than 800 significant differences in gene expression (FDR ≤10%) between the two drug regimens (FDR ≤10%), of which approximately 300 were ≥1.5 fold (Figure 4, Table S3). These data indicate that sub-threshold changes in gene expression had occurred in response to drug treatment under both regimens, but that directionality of these changes was appositional such that they were only statistically detectable by direct comparison between the two drug arms (Figure 4 and Tables S3 and S4).
Gene Ontology analysis indicated enrichment for expression of genes encoding key extracellular matrix proteins and receptors, cytoskeletal proteins and chemokines in the drug-treated carcinomas of the Sustained versus Short Term Dosing regimens. All three components of Laminin 332 (Lama3, Lamb3 and Lamc2) were elevated approximately 2 fold by Sustained drug treatment. These same genes were down-regulated ~ 30% by the Short Term dosing regimen (Table S3). Moreover, the Itgb1 gene which encodes a component of a cognate Laminin 322 receptor exhibited increased expression following Sustained LY2109761 treatment compared to the Short Term dosing regimen (Table S3). Both Laminin 332 and Itgb1 have been shown to be centrally involved in SCC tumor invasion (29).
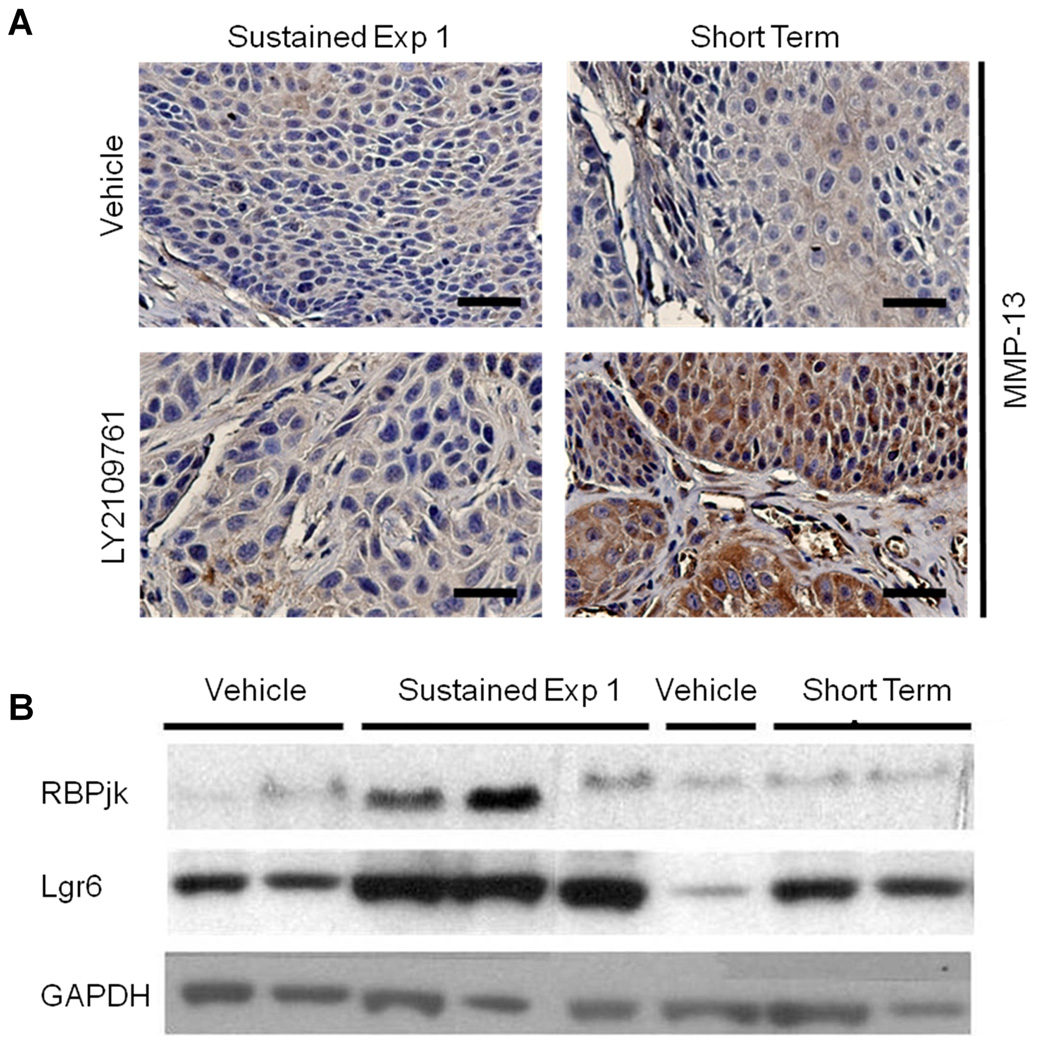
Increases in Matrix Metalloproteinase (MMP) expression and/or activity have also been linked to tumor cell invasion, and sustained LY2109761 treatment led to a 2.5 to 4 fold increase in Mmp3, Mmp10, Mmp9 and Mmp13 compared to the Short Term Dosing regimen (Table S3). In the case of MMP13, this observation was validated by IHC (Fig. 5A). Many other genes involved in extracellular matrix remodeling showed similar increases in expression after sustained drug treatment but slight decreases after the Short Term Dosing regimen, including Latent TGF-β binding protein 2 (Ltbp2), Bmp1, and Pcolce2 (Table S3). Additionally, markers of EMT, including Msx1 and Twisted2, were elevated by sustained drug-treatment but down-regulated by the Short Term dosing regimen. Intriguingly, Lgr6, reportedly a multi-potential keratinocyte stem cell marker (30), and Fermd4A, also reported to mark keratinocyte stem cells (31), were both found to be elevated in carcinoma after Sustained Drug Treatment (Figure 4C, Table S4). RbpjK, a component of the Notch signaling pathway that is important in stem cell fate, angiogenesis and EMT (32, 33), was also elevated in carcinomas after Sustained Drug Treatment. These findings from microarray analysis were validated by western blot estimation of protein levels for Lgr6 and RbpjK (Figure 5B).
Figure 5. Validation of microarray data by western blot analysis and immunohistochemistry.
A) Validation of microarray data by IHC detection of elevated MMP-13 after Sustained but not Short Term Drug dosing. Scale bar = 50µM. B) Validation of microarray data by western blot of carcinoma lysates using Lgr6 and RBPjk antibodies.
Lastly, inflammatory gene networks showed divergent trends in gene expression between the two drug regimens. Il23a which is known to promote inflammatory tumor progression in the DMBA/PMA model (26), was increased in carcinomas after sustained drug exposure. Several genes involved in leukocyte cell adhesion and cytoskeletal reorganization (Jam2, Icam, Nfat5 and Msn) demonstrated the same reciprocal gene expression pattern between the two drug dosing regimens. The potent macrophage chemo-attractants Ccl3 (MIP1-α) and Ccl4 (MIP1-β) were also elevated after Sustained Drug Treatment but suppressed following Short Term dosing. Similar observations were made for other inflammatory markers, including the tumor cell marker CD274 (increased 3.6 fold in the Sustained Drug Treatment group) which is involved in suppression of antitumor CD8+ T cell. In conclusion, Sustained drug treatment induced a gene expression signature indicative of a more aggressive and invasive carcinoma type with elevated inflammatory markers compared to the Short Term Dosing regimen, or to vehicle treated carcinoma.
Sustained LY2109761 exposure results in expansion of tumor stroma and delocalized E-cadherin within carcinoma cells in vivo
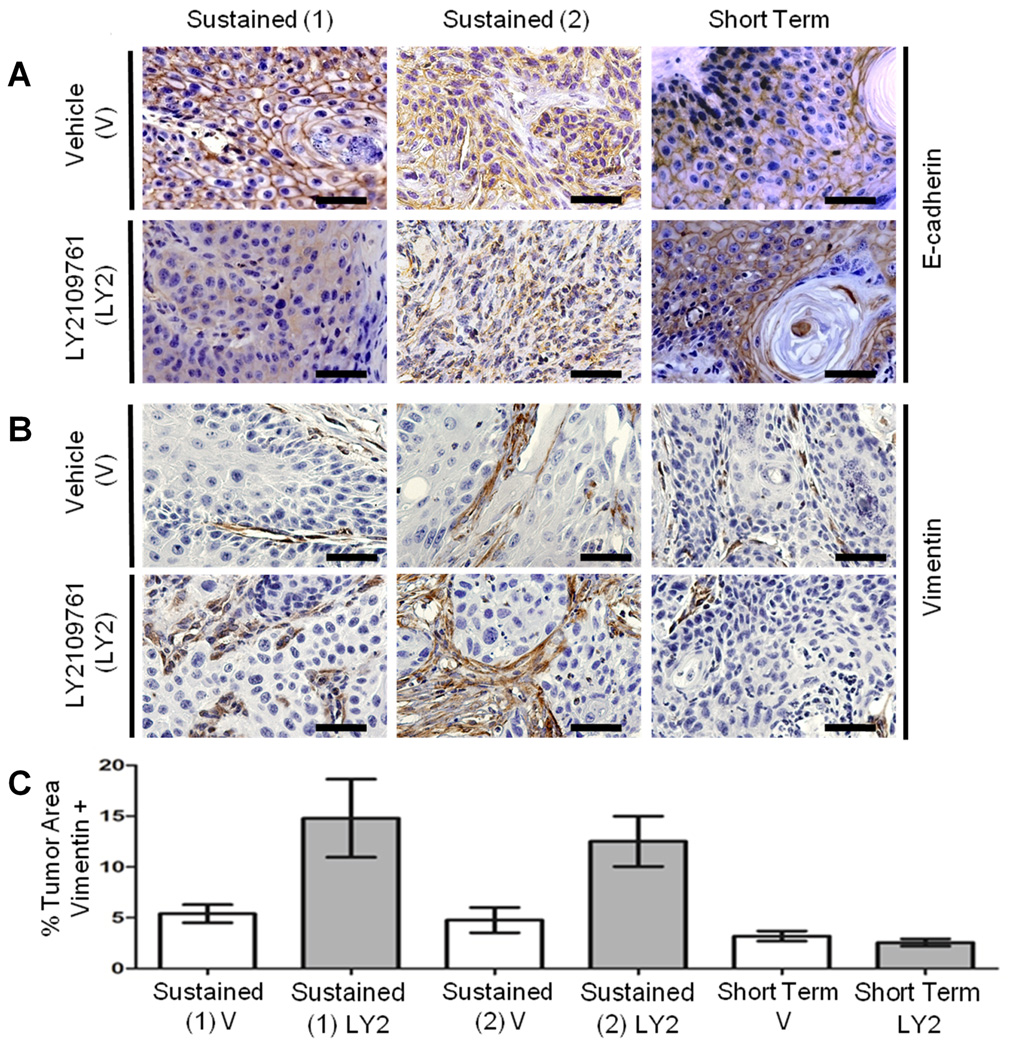
Although histological examination indicated no difference in spindle carcinoma incidence after sustained drug treatment (Table S2), we further investigated the possibility of paradoxical induction of EMT by LY2109761 using IHC for E-cadherin (epithelial) and vimentin (mesenchymal). There was consistent delocalization of E-cadherin in carcinoma cells after Sustained LY2109761-treatment compared to the characteristic cell-cell junctional localization of E-cadherin seen in carcinomas from either vehicle-treated mice or after the LY2109761 Short Term Dosing regimen (Figure 6A, Table S5). There was no carcinoma cell vimentin expression in any of the treatments. However, vimentin staining revealed a significant expansion of the tumor stroma compartment after Sustained LY2109761 Treatment compared to vehicle-treated carcinoma or to drug-dosed carcinoma on the Short Term Dosing regimen (Figure 6B, C). This data, showing delocalization of E-cadherin in carcinoma and an expanded vimentin-positive tumor stromal compartment was replicated in an independent DMBA/PMA study in which mice underwent 11 weeks of sustained drug dosing during the tumor outgrowth phase (Figure 6, Table S5).
Figure 6. Sustained LY2109761 leads to delocalized E-cadherin in carcinoma cells and expansion of vimentin+ stromal compartment.
Carcinomas from two independent LY2109761 Sustained Drug Treatment regimens and from the Short Term dosing regimen were stained for (A) E-cadherin and (B) vimentin. (C) Both Sustained treatment experiments showed a significant increase (P < 0.05) in the stroma: carcinoma ratio compared to vehicle or the Short Term dosing regimen, as determined by vimentin staining. Photomicrographs show representative staining in each arm of the two drug regimens. E-cadherin quantification is presented in Table S5. Scale bar = 50µM
Discussion
Here we reveal that sustained suppression of TGF-β signaling in vivo results in the outgrowth of carcinomas that are apparently resistant to the small molecule TβR1/TβRII inhibitor, LY2109761. We demonstrate marked differences in the outcome of LY2109761 treatment of carcinoma cells in vitro, tumor allografts in vivo and primary carcinomas in situ. Furthermore, the data illustrate that, in the same in vivo model of carcinogen-induced primary tumor outgrowth and progression, the outcome of LY2109761 treatment can be either anti- or pro-tumorigenic, dependent on the precise drug-dosing regimen.
Short Term, high dose LY2109716 treatment was unable to completely reverse TGF-β induced carcinoma EMT, in either the E4 allograft or primary chemically-induced carcinoma models. This data was in contrast with our previous finding using genetic inhibition of autocrine TGF-β signaling in E4 cells in vitro and in vivo (23). Nevertheless, Short Term LY2109716 treatment did reduce the levels of α-SMA and Shox2 in the allograft and DMBA/PMA models respectively, which suggests a trend towards a less myofibroblast phenotype in mice treated continuously with drug for 10 days. Failure to definitively replicate inhibition of EMT after blockade of TGF-β signaling inhibition (23) could be due to additional effects of LY2109761 on the tumor stroma that potentiates tumorigenesis (34, 35) and/or inadequate drug delivery to the tumor parenchyma, especially in the light of vascular disruption in response to LY2109761 in the tumor allograft model.
In concordance with predictions from earlier studies using genetically-manipulated TGF-β1 or TβRII (11, 21, 36), we show that long term daily LY2109761 treatment during tumor outgrowth increased papilloma number, supporting a suppressive effect of TGF-β in early tumorigenesis. Analysis of the papillomas suggests that these tumor-suppressive effects may be at least in part due to the immune suppressive action of TGF-β signaling. Inflammation has been suggested to act either protectively via immune surveillance or in a pro-tumorigenic manner, dependent on the nature of the inflammatory cell infiltrate (37). In the mouse skin model of chemical carcinogenesis, inflammation has clearly been shown to be pro-tumorigenic (26, 38). In agreement with these observations, we demonstrated that Sustained LY2109761 treatment resulted in a significant increase in CD45+ neutrophilic infiltrate within the papilloma stroma.
Importantly, we demonstrated that Sustained Treatment with LY2109761 during the tumor outgrowth phase resulted in resistance to drug induced P-Smad2 down-regulation, specifically in malignant tumor cells but not in tumor stromal fibroblasts or normal tissue. The development of acquired drug resistance to both conventional chemotherapeutics and targeted therapies is a common undesirable outcome in malignant disease. The mechanisms of acquired drug resistance are varied and, for targeted small molecule therapies, might be somewhat complex (39, 40). However, understanding these mechanisms of drug resistance will allow modification of therapeutic strategies that lead to more efficacious treatments. Mechanisms of acquired drug resistance include amplification of the target gene, as seen in acquired resistance to Met tyrosine kinase inhibitors (41), as well as activation of alternative signaling pathways (39, 42) and perturbations in the intermolecular cross talk between interacting ligands, tyrosine kinases or their kinase-inactive partners (39, 40, 43).
There are several possible explanations for our unexpected findings of outgrowth of tumor cells with high levels of LY2109761-resistant P-Smad2. The simplest would be inadequate drug delivery, possibly as a consequence of vascular disruption. However, this is unlikely, bearing in mind that LY2109761 can down modulate P-Smad2 in the tumor stroma, even after sustained drug treatment. Moreover, the Sustained Dosing Regimen resulted in a molecular carcinoma phenotype distinct from that of either vehicle-treated or Short Term Drug-treated mice. Mutation of the ATP/drug binding site of TβRI, Acvr1b or Acvr1c may confer drug resistance (see for example (44)), as would mutational hyperactivation of the kinase receptor (45). Genetic alterations that influence P-Smad2 levels downstream or parallel to TβRI (27) may also provide mechanisms to bypass LY2107961 effects. TGF-β signaling is known to be finely regulated by both negative and positive feedback mechanisms, and in tumor cells, signaling may be regulated by trans receptor interactions that might be perturbed in the presence of LY2109761. This drug is known to inhibit type I receptors that signal for activins, GDF3, nodal and myostatin. It is therefore conceivable that perturbation of one or more of these signaling pathways within the tumor cell or tumor microenvironment might lead to expansion of a more aggressive tumor type. In the current study, it was noted that the LY2109761 target, TβRI, was up-regulated more than 1.5 fold in the Sustained LY2109761-treated carcinomas. This might be due to disruption of a negative feedback loop on TβRI levels and/or indirectly caused by expansion of a cellular compartment, characterized by high TβRI expression (46, 47).
Importantly, not only was there resistance to LY2109761-mediated attenuation of carcinoma P-Smad2 levels, but these carcinomas took on a more aggressive molecular profile, with up-regulation of markers of EMT and inflammation, illustrated by delocalization of cell surface E-cadherin and expansion of a vimentin-positive tumor stroma, as well as elevated expression of pro-inflammatory markers. In contrast, carcinomas treated on the Short Term LY2109761 Dosing regimen showed the inverse trend, with down-regulation of markers of EMT, such as Msx1 and Shox2. It would appear that many of the pro-tumorigenic effects of LY2109761 are likely driven by the action of this drug on the tumor stroma, leading to immune cell infiltration, stromal cell expansion, and subsequent feedback via growth factors such as HGF, that drive the outgrowth of LY2109761-resistant carcinomas, as has been seen in genetic models of TGF-β signaling inhibition (34, 35). There is an increasing appreciation of the importance of the interaction between the carcinoma cell and its substratum in driving tumor progression, particularly with respect to matrix density and stiffness (48, 49). The expansion of the tumor stroma, the prevalence of ECM components, junctional adhesion molecules and integrins in genes up regulated after sustained exposure to LY2109761, would all support the concept of a stromal-driven enhancement in aggressive molecular phenotype.
In conclusion, the current study demonstrates that, although TGF-β inhibitors might be clinically useful for short term patient exposure (50), long term treatment with TGF-β inhibitors should be administered with caution. Dosing regimen is clearly critical, and recommended dosage might vary considerably based on tumor type and TGF-β signaling status. Further investigation of mechanism of acquired drug resistance might provide more efficacious routes to therapy.
Supplementary Material
Acknowledgements
We would like to thank Honrado Lopez, Bryan Young, the UCSF HDFCCC Preclinical Therapeutics and UCSF Biostatistical Cores for assistance and advice.
Funding: Sponsored research agreement between UCSF and Eli Lilly and Company: NIH grants R01-CA116019 and P01- AR050440, and a gift from the Bouque Estate to RJA. ECC was funded by an institutional research service award from the National Cancer Institute (T32 CA108462).
References
- 1.Barcellos-Hoff MH, Akhurst RJ. Transforming growth factor-beta in breast cancer: too much, too late. Breast Cancer Res. 2009;11:202. doi: 10.1186/bcr2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 4.Saunier EF, Akhurst RJ. TGFbeta inhibition for cancer therapy. Current Cancer Drug Targets. 2006;6:519–532. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 5.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 7.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 8.Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HT, Chen XF, Wang MH, Wang JC, Qi QY, Zhang RM, et al. Defective expression of transforming growth factor beta receptor type II is associated with CpG methylated promoter in primary non-small cell lung cancer. Clin Cancer Res. 2004;10:2359–2367. doi: 10.1158/1078-0432.ccr-0959-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Shiina H, Greene KL, Li LC, Tanaka Y, Kishi H, et al. CpG methylation at promoter site −140 inactivates TGFbeta2 receptor gene in prostate cancer. Cancer. 2005;104:44–52. doi: 10.1002/cncr.21135. [DOI] [PubMed] [Google Scholar]
- 11.Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 12.Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–1615. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008;68:3835–3843. doi: 10.1158/0008-5472.CAN-08-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, et al. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Cancer Res. 2006;12:4315–4330. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- 16.Schlingensiepen R, Goldbrunner M, Szyrach MN, Stauder G, Jachimczak P, Bogdahn U, et al. Intracerebral and intrathecal infusion of the TGF-beta2-specific antisense phosphorothioate oligonucleotide AP 12009 in rabbits and primates: Toxicology and Safety. Oligonucleotides. 2005;15:94–104. doi: 10.1089/oli.2005.15.94. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC, Shapiro GI, Tan AR, Lawrence DP, Olencki TE, Dezube BJ, et al. Phase I/II study of GC1008: A human anti-transfroming growth factor-beta (TGFB) monoclonal antibody in patients with advanced malignant melanoma (MM) or renal cell carcinoma (RCC). ASCO Annual Meeting; 2008. Journal of Clinical Oncology. 2008:9028. doi: 10.1371/journal.pone.0090353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bremner R, Balmain A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell. 1990;61:407–417. doi: 10.1016/0092-8674(90)90523-h. [DOI] [PubMed] [Google Scholar]
- 19.Burns PA, Kemp CJ, Gannon JV, Lane DP, Bremner R, Balmain A. Loss of heterozygosity and mutational alterations of the p53 gene in skin tumours of interspecific hybrid mice. Oncogene. 1991;6:2363–2369. [PubMed] [Google Scholar]
- 20.Sawyer JS, Anderson BD, Beight DW, Campbell RM, Jones ML, Herron DK, et al. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-beta type I receptor kinase domain. J Med Chem. 2003;46:3953–3956. doi: 10.1021/jm0205705. [DOI] [PubMed] [Google Scholar]
- 21.Mao JH, Saunier EF, de Koning JP, McKinnon MM, Higgins MN, Nicklas K, et al. Genetic variants of Tgfb1 act as context-dependent modifiers of mouse skin tumor susceptibility. Proc Natl Acad Sci U S A. 2006;103:8125–8130. doi: 10.1073/pnas.0602581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portella G, Cumming SA, Liddell J, Cui W, Ireland H, Akhurst RJ, et al. Transforming growth factor beta is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ. 1998;9:393–404. [PubMed] [Google Scholar]
- 24.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002;4:487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 25.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nature Cell Biology. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 26.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, Ruperez M, Egido J, Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111:2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Gu S, Alappat S, Song Y, Yan M, Zhang X, et al. Shox2-deficient mice exhibit a rare type of incomplete clefting of the secondary palate. Development. 2005;132:4397–4406. doi: 10.1242/dev.02013. [DOI] [PubMed] [Google Scholar]
- 29.Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 30.Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 31.Jensen KB, Watt FM. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc Natl Acad Sci U S A. 2006;103:11958–11963. doi: 10.1073/pnas.0601886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan X, Khaki L, Zhu TS, Soules ME, Talsma CE, Gul N, et al. Notch Pathway Blockade Depletes CD133-Positive Glioblastoma Cells and Inhibits Growth of Tumor Neurospheres and Xenografts. Stem Cells. 2009 doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zavadil J, Cermak L, Soto-Nieves N, Bottinger EP. Integration of TGF-beta/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. Embo J. 2004;23:1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhowmick NA, Chytil A, Plieth D, Govska AE, Dumont N, Shappell S, et al. TGFbeta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelial. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 35.Bierie B, Chung CH, Parker JS, Stover DG, Cheng N, Chytil A, et al. Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119:1571–1582. doi: 10.1172/JCI37480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest. 2005;115:1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 38.Quigley DA, To MD, Perez-Losada J, Pelorosso FG, Mao JH, Nagase H, et al. Genetic architecture of mouse skin inflammation and tumour susceptibility. Nature. 2009;458:505–508. doi: 10.1038/nature07683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431–435. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 41.Cepero V, Sierra JR, Corso S, Ghiso E, Casorzo L, Perera T, et al. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res. 2010;70:7580–7590. doi: 10.1158/0008-5472.CAN-10-0436. [DOI] [PubMed] [Google Scholar]
- 42.Corso S, Ghiso E, Cepero V, Sierra JR, Migliore C, Bertotti A, et al. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol Cancer. 2010;9:121. doi: 10.1186/1476-4598-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 44.Weisberg E, Choi HG, Ray A, Barrett R, Zhang J, Sim T, et al. Discovery of a small-molecule type II inhibitor of wild-type and gatekeeper mutants of BCR-ABL, PDGFRalpha, Kit, and Src kinases: novel type II inhibitor of gatekeeper mutants. Blood. 2010;115:4206–4216. doi: 10.1182/blood-2009-11-251751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wieser R, Wrana JL, Massague J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 49.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace A, Kapoor V, Sun J, Mrass P, Weninger W, Heitjan DF, et al. Transforming growth factor-beta receptor blockade augments the effectiveness of adoptive T-cell therapy of established solid cancers. Clin Cancer Res. 2008;14:3966–3974. doi: 10.1158/1078-0432.CCR-08-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.