Abstract
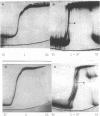
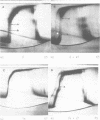
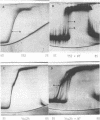
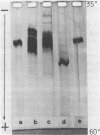
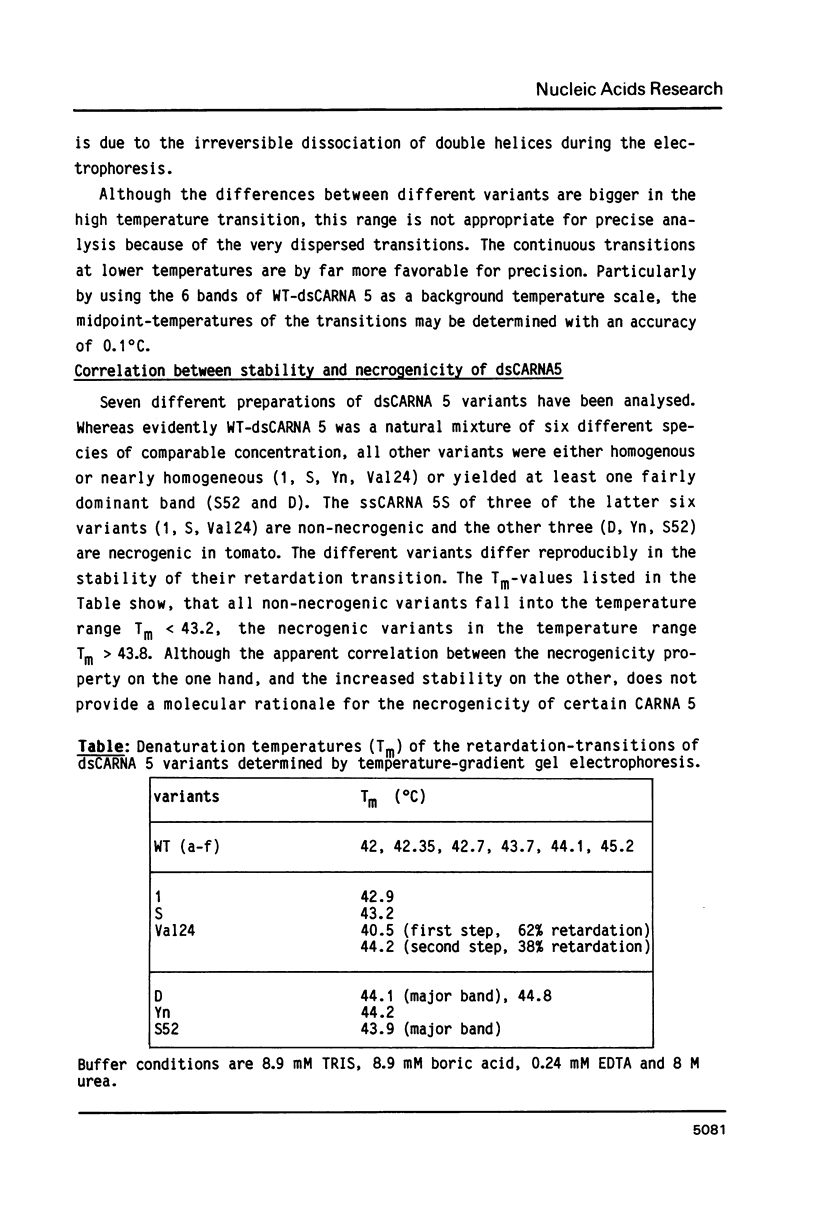
Cucumber mosaic virus (CMV) and peanut stunt virus (PSV) each contain a fifth major RNA in the size range of 334 to 393 nucleotides. This fifth RNA is a satellite capable of modulating the expression of viral disease symptoms. It is present in infected tissue in single-stranded and double-stranded form. Nucleotide sequence variants of the double-stranded CMV-associated RNA 5 (dsCARNA 5) and PSV-associated RNA 5 (dsPARNA 5) were analysed by temperature-gradient gel electrophoresis. Gels were 5% polyacrylamide, containing 8 M urea in 8.9 mM Tris-borate buffer, with temperature differences of 25-40 degrees C establishing gradients either perpendicular or parallel to the direction of the electric field. For dsCARNA 5 two characteristic transitions were detected with increasing temperature: at temperatures between 40 degrees C and 46 degrees C a drastic retardation in electrophoretic mobility induced by partial dissociation of the duplex structure from the ends and at temperatures above 52 degrees C an abrupt increase in mobility due to complete strand dissociation. dsPARNA 5 exhibited both transitions at up to 10 degrees C higher temperatures and an additional retardation between the transitions mentioned. Seven different variants of dsCARNA 5, 4 necrogenic and 3 non-necrogenic, were analysed. Some showed only one single band, others gave rise to up to six well separated bands corresponding to six molecular species. From all experimental results a correlation between the temperature of the retardation transition and the necrogenicity of CARNA 5 was derived. The diagnostic application of the temperature-gradient gel analysis in agriculture, particularly for the use of non-necrogenic variants as biological control agents to impede CMV-infections, is discussed.
Full text
PDF





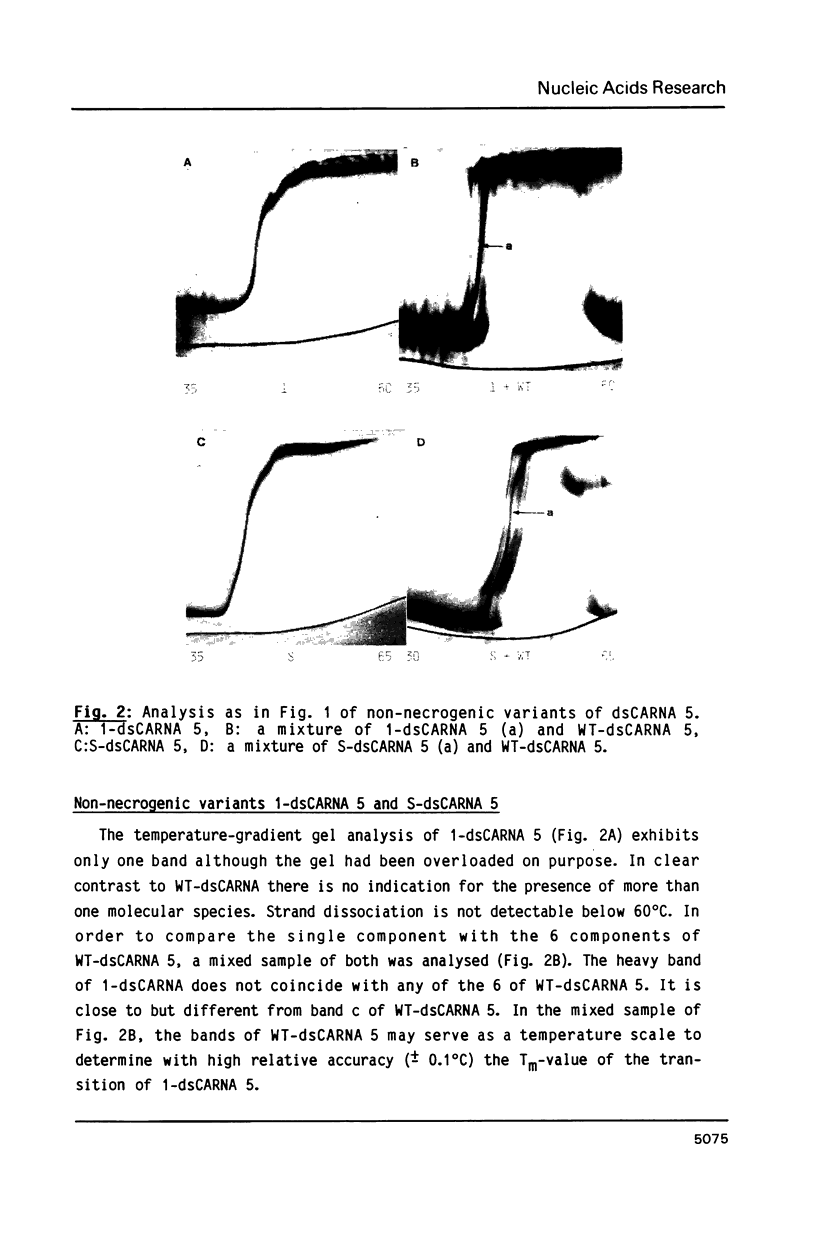
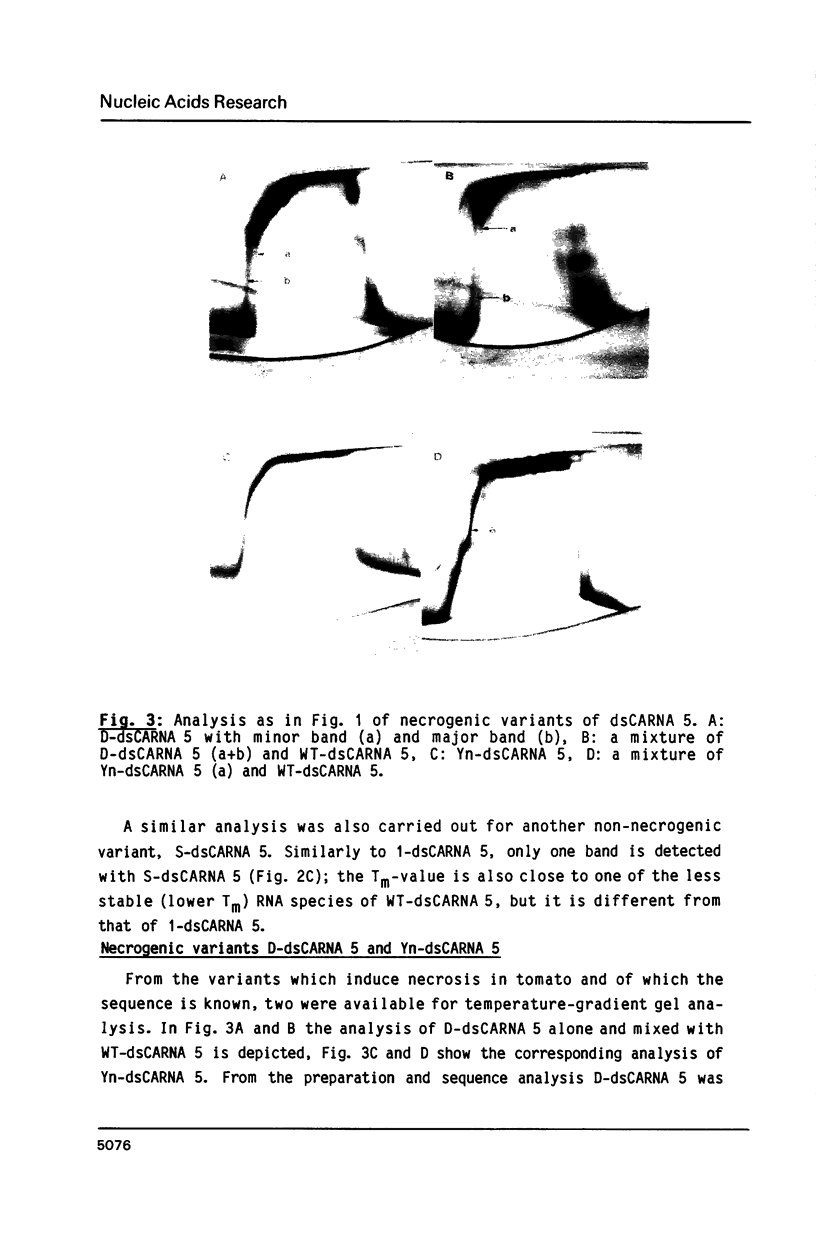
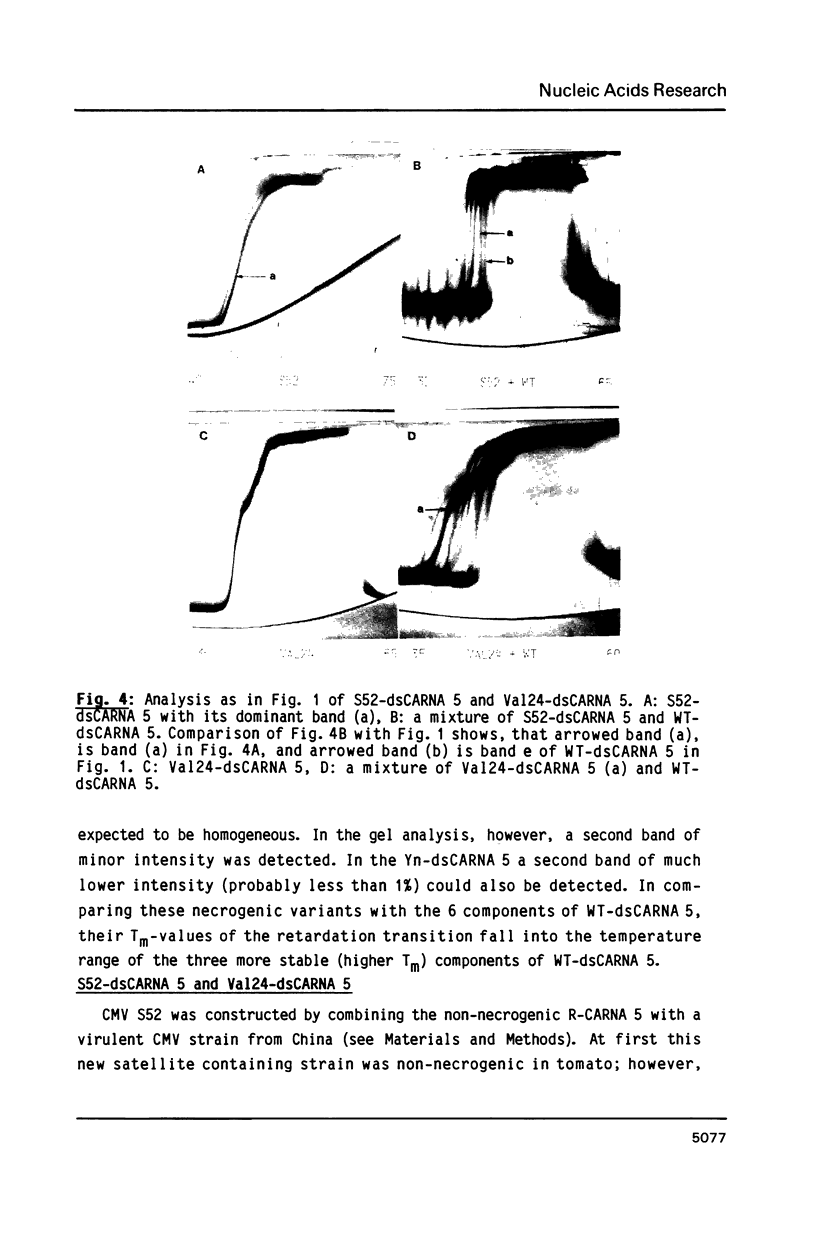

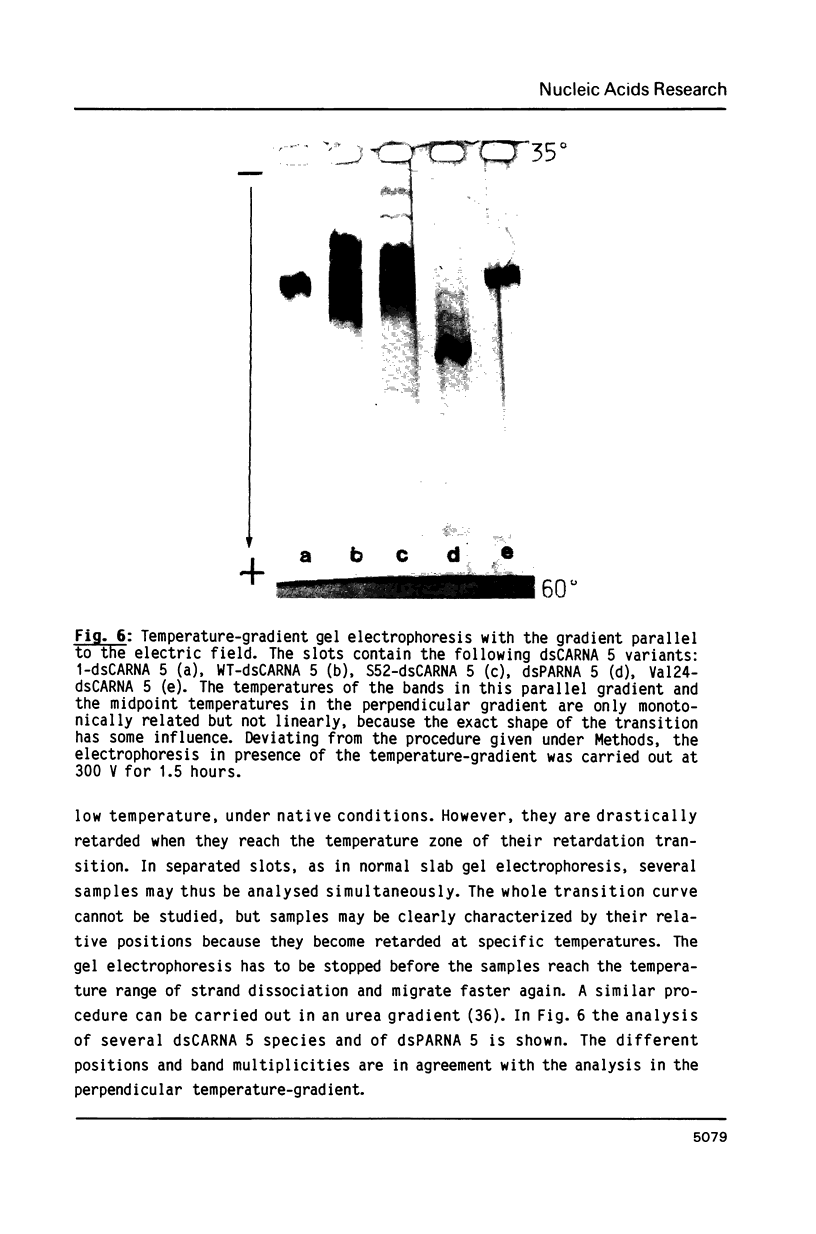



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collmer C. W., Hadidi A., Kaper J. M. Nucleotide sequence of the satellite of peanut stunt virus reveals structural homologies with viroids and certain nuclear and mitochondrial introns. Proc Natl Acad Sci U S A. 1985 May;82(10):3110–3114. doi: 10.1073/pnas.82.10.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz J. R., Kaper J. M. Cucumber mosaic virus-associated RNA 5. III. Little nucleotide sequence homology between CARNA 5 and helper RNA. Virology. 1977 Jul 1;80(1):204–213. doi: 10.1016/0042-6822(77)90393-2. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz J. R., Kaper J. M. Cucumber mosaic virus-associated RNA 5. VI. Characterization and denaturation-renaturation behavior of the double-stranded form. Biochim Biophys Acta. 1979 Sep 27;564(2):275–288. doi: 10.1016/0005-2787(79)90225-9. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Desselberger U. Cocirculation of different rotavirus strains in a local outbreak of infantile gastroenteritis: monitoring by rapid and sensitive nucleic acid analysis. J Med Virol. 1983;11(1):39–52. doi: 10.1002/jmv.1890110106. [DOI] [PubMed] [Google Scholar]
- Francki R. I. Plant virus satellites. Annu Rev Microbiol. 1985;39:151–174. doi: 10.1146/annurev.mi.39.100185.001055. [DOI] [PubMed] [Google Scholar]
- Gordon K. H., Symons R. H. Satellite RNA of cucumber mosaic virus forms a secondary structure with partial 3'-terminal homology to genomal RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):947–960. doi: 10.1093/nar/11.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. M. Rapid synthesis of double-stranded cucumber mosaic virus-associated RNA 5: mechanism controlling viral pathogenesis? Biochem Biophys Res Commun. 1982 Apr 14;105(3):1014–1022. doi: 10.1016/0006-291x(82)91071-3. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E. Cucumber mosaic virus-associated RNA 5. V. Extensive nucleotide sequence homology among CARNA 5 preparations of different CMV strains. Virology. 1978 Mar;85(1):323–327. doi: 10.1016/0042-6822(78)90438-5. [DOI] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E. Viral satellites: parasitic nucleic acids capable of modulating disease expression. Endeavour. 1984;8(4):194–200. doi: 10.1016/0160-9327(84)90084-x. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Fischer S. G., Hurley I., Silverstein K., Lumelsky N. Sequence-determined DNA separations. Annu Rev Biophys Bioeng. 1984;13:399–423. doi: 10.1146/annurev.bb.13.060184.002151. [DOI] [PubMed] [Google Scholar]
- Lot H., Kaper J. M. Physical and chemical differentiation of three strains of cucumber mosaic virus and peanut stunt virus. Virology. 1976 Oct 1;74(1):209–222. doi: 10.1016/0042-6822(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Lot H., Marchoux G., Marrou J., Kaper J. M., West C. K., van Vloten-Doting L., Hull R. Evidence for three functional RNA species in several strains of cucumber mosaic virus. J Gen Virol. 1974 Jan;22(1):81–93. doi: 10.1099/0022-1317-22-1-81. [DOI] [PubMed] [Google Scholar]
- Rosenbaum V., Riesner D. Temperature-gradient gel electrophoresis. Thermodynamic analysis of nucleic acids and proteins in purified form and in cellular extracts. Biophys Chem. 1987 May 9;26(2-3):235–246. doi: 10.1016/0301-4622(87)80026-1. [DOI] [PubMed] [Google Scholar]
- Steger G., Müller H., Riesner D. Helix-coil transitions in double-stranded viral RNA. Fine resolution melting and ionic strength dependence. Biochim Biophys Acta. 1980 Feb 29;606(2):274–284. doi: 10.1016/0005-2787(80)90037-4. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R., Hodson B. Denaturation of proteins and nucleic acids by thermal-gradient electrophoresis. Biochem J. 1981 Jul 1;197(1):105–109. doi: 10.1042/bj1970105. [DOI] [PMC free article] [PubMed] [Google Scholar]