Abstract
Six family transcription factors play important roles in craniofacial development. Their transcriptional activity can be modified by co-factor proteins. Two Six genes and one co-factor gene (Eya1) are involved in the human Branchio-otic (BO) and Branchio-otic-renal (BOR) syndromes. However, mutations in Six and Eya genes only account for about half of these patients. To discover potential new causative genes, we searched the Xenopus genome for orthologues of Drosophila co-factor proteins that interact with the fly Six-related factor, SO. We identified 33 Xenopus genes with high sequence identity to 20 of the 25 fly SO-interacting proteins. We provide the developmental expression patterns of the Xenopus orthologues for 11 of the fly genes, and demonstrate that all are expressed in developing craniofacial tissues with at least partial overlap with Six1/Six2. We speculate that these genes may function as Six-interacting partners with important roles in vertebrate craniofacial development and perhaps congenital syndromes.
Keywords: Branchio-otic syndrome, Branchio-otic-renal syndrome, placodes, craniofacial development, Eya, Groucho, Sobp, Gadd45, Six1, Six2, sine oculis, neural crest
Introduction
Placodes are patches of thickened non-neural ectoderm that form lateral to the neural crest in the head of the vertebrate embryo. They give rise to sensory organs (olfactory epithelium, lens, auditory-vestibular organs and lateral line), and contribute to the anterior pituitary and cranial sensory ganglia (Webb and Noden, 1993; Schlosser and Northcutt, 2000; Baker and Bonner-Fraser, 2001; Schlosser, 2006; Moody, 2007). Placodes arise from a common precursor domain of non-neural ectoderm that surrounds the cranial portion of the neural plate, called the pre-placodal ectoderm (PPE) (Knouff, 1935; Streit, 2004, 2007; Schlosser, 2006). Among the earliest genes expressed throughout the PPE are Six1, Six2 and Six4 (Esteve et al., 1999; Kobayashi et al., 2000; Pandur and Moody, 2000; Ghanbari et al., 2001; Schlosser and Ahrens, 2004; Sato et al., 2010), suggesting that they have important roles in placodal development. The expression of Six1 in the PPE dominantly promotes a placodal fate, whereas knock-down of Six1 or repression of its targets results in craniofacial defects in frog, chick and mouse (Brugmann et al., 2004; Laclef et al., 2003; Christophorou et al., 2009); Six2- or Six4-null mice do not have reported craniofacial defects (Ozaki et al., 2001; Fogelgren et al., 2009; Self et al., 2009), but mis-expression of Six2 leads to frontonasal dysplasia (Fogelgren et al., 2008). Two human syndromes, the branchio-otic (BO) and branchio-oto-renal (BOR) syndromes, which are characterized by craniofacial defects and hearing loss, can be caused by mutations in SIX1, SIX5 or EYA1 (Abdelhak et al., 1997; Kumar et al., 1997; Rodriguez-Soriano, 2003; Ruf et al., 2004; Hoskins et al., 2007). Six1- and Eya1-null mutant mice likewise exhibit severe defects in the development of some placodal structures (Oliver et al., 1995; Vincent et al., 1997; Johnson et al., 1999; Xu et al., 1999; 2002; Laclef et al., 2003; Zheng et al., 2003; Ozaki et al., 2004). However, mutations in these genes account for less than half of the cases of BO or BOR, indicating that defects in other genes contribute to these syndromes.
The founding member of the Six gene family is Drosophila sine oculis (so; Cheyette et al., 1994; Serikaku and O’Tousa, 1994). In vertebrates, there are 6 different members of the Six gene family (Six1-6) that are expressed in a variety of tissues during development (reviewed in Kawakami et al., 2000; Brugmann and Moody, 2005). Each of the different Six proteins contains two highly conserved regions: a Six-type homeodomain (Six-HD) that binds to DNA and an N-terminal Six domain (SD) that can bind with other proteins (co-factors) that are unable to interact directly with DNA (Pignoni et al., 1997; Kawakami et al., 2000; Kobayashi et al., 2001). These co-factors increase the DNA binding specificity of the Six proteins and modulate Six function as either co-activators or co-repressors of transcription (Zhu et al., 2002; Tessmar et al., 2002). For example, during PPE development when either Eya1 or Eya2 is co-expressed with Six1, other placode genes are up-regulated, and when Groucho is co-expressed, neural crest and epidermal genes are down-regulated (Brugmann et al., 2004; Christophorou et al., 2009). Eya1 and Six1 also cooperatively regulate the onset of neurogenesis in the placodes that give rise to the cranial ganglia (Schlosser et al., 2008).
Searches for additional Six1 partners in vertebrates have met with very limited success (Rual et al., 2005), whereas yeast 2-hybrid screens of the smaller, more manageable Drosophila proteome have resulted in the identification of 25 factors that interact with the fly SO protein (Pignoni et al., 1997; Giot et al., 2003; Kenyon et al., 2005). Seven factors, including Eya and Groucho, are nuclear/transcriptional regulators, 14 are cytoplasmic and 4 are of unknown function (Table 1). However, there is very little information available regarding the developmental expression of most of these genes or their protein functions in conjunction with Six proteins; the exceptions are Eya, Groucho and SOBP (Pignoni et al., 1997; Heanue et al., 1999; Ohto et al., 1999; Kobayashi et al., 2001; Giot et al., 2003; Kenyon et al., 2005). To identify new, potential Six-interacting proteins that might have roles in vertebrate craniofacial development, we used the sequences of the Drosophila SO-interacting proteins to search for putative Xenopus orthologues. Herein, we identify 33 Xenopus genes related by sequence to 20 of the Drosophila SO-interacting proteins, and demonstrate that a large number of these are expressed during Xenopus craniofacial development. Thereby, these genes are potential candidates for regulating normal craniofacial development and being involved in craniofacial birth defects.
Table 1.
Drosophila SO-interacting proteins and their putative Xenopus orthologues
| SO-interacting proteins1 | Protein Activity2 | Interaction Confidence Score1 | Putative Xenopus Orthologues3 (Xl = X. laevis; Xt = X. tropicalis) |
|---|---|---|---|
| Eyes absent | Transcription factor | 0.624 | Eya1 (gi:13591786, Xl) Eya2 (gi:134024326, Xt) Eya3 (gi:58702065, Xl) Eya4 (Xt genomic sequence ENSXETG00000000110 in Ensembl database) |
| CG15459 | Unknown, only expressed in testis and adult in fly | 0.430 | Identified only in Drosophila |
| CG6688 | Ubiquitin-dependent protein catabolism, PDZ domain | 0.421 | Identified only in Drosophila |
| CG9083 | Unknown | 0.415 | Novel Ensembl predication (Xt genomic sequence ENSXETT00000000661 in Ensembl database) |
| Groucho | Transcription factor | 0.415 | Grg4/Tle4 (gi:50925318, Xl) ESG1/Tle3 (gi:50414464, Xl) ESG2 (gi:2408194, Xl) Grg5/AES (gi:38174466, Xl) |
| CG17265 | RNA interference | 0.413 | Coiled coil domain protein ccd85c (gi:169642663, Xt; gi:66912014, Xl)4 |
| CG5466 | Cell growth, protein binding | 0.406 | Identified only in Drosophila |
| C15 | Transcription factor | 0.404 | Hox11 (gi:10185813, Xl) Hox11L2 (gi:10185811, Xl) |
| CG1218 | Zn finger protein, unknown | 0.403 | Similar to C4orf27 EST (gi:8579074 Xl) |
| Pif1B | PFTAIRE-interacting factor 1B, protein binding, unknown | 0.402 | Identified only in Drosophila |
| SOBP | SO Binding Protein, transcription factor | 0.394 | SOBP (gi:159155685, Xt) Zinc finger protein 198 (gi:49903401, Xl) Zinc finger protein LOC414497 (gi:80479434, Xl) |
| CG4807 (Abrupt) | Transcription factor, BTB domain for protein dimerization | 0.393 | Identified only in Drosophila |
| CG1135 | Forkhead associated domain, putative nuclear signaling domain | 0.389 | LOC100049093 (gi:126631279, Xl) |
| CG7878 | Deadbox RNA helicase | 0.386 | Ddx43 (gi:112419338, Xt; gi:118404345, Xt)5 |
| CG5665 | Lipoprotein lipase | 0.373 | Lipase member H(Xt genomic sequence ENSXETG00000005849 ENSEMBL database) Phospholipase A1 member A (Xt genomic sequence ENSXETG00000018070 ENSEMBL database) |
| CG33090 | Hydroxymethylglutaryl-CoA reductase class I/II, bile acid glucosidase | 0.356 | GBA2 (gi: 169642398 Xt) |
| RhoGEF2 | Rho guanine nucleotide exchange factor | 0.331 | Guanine nucleotide exchange factor (gi:148223172, Xl) |
| Chc | Clathrin heavy chain, vesicle mediated transport | 0.330 | MCG80936 (gi:49115531, Xl) |
| CKIIβ | Casein kinase II regulatory subunit, role in Wnt signaling | 0.327 | Casein kinase II β subunit (gi:50414548, Xl) |
| CG10576 | Methionyl aminopeptidase activity; proteolysis | 0.325 | Proliferation-associated 2G4 (gi:54311186, Xl) |
| VhaAC39 | Proton transport, ATPase | 0.322 | ATPase Proton transporting lysosomal 38kD VO subunit d1 (gi:27769219, Xl) |
| RhoGAP93B | Rho GTPase activator protein | 0.312 | RhoGAP93B (Xt genomic sequence ENSXETG00000007422 Ensembl database) |
| CG5033 | Ribonucleoprotein binding, transcription factor | 0.294 | Hypothetical MGC68939 (gi:33416659, Xl) Block of proliferation (gi:2877251, Xl) |
| Gadd45 | Cell cycle regulator | 0.254 | gadd45α (gi:27694852, Xl) gadd45γ (gi:148223518, Xl) gadd45β (Xt genomic sequence ENSXETT00000008029 ENSEMBL database) |
| RpS27 | Cytosolic small ribosomal subunit | 0.202 | RpS27 (gi:297124, Xl) |
DroID, The Drosophila Interactions Database, Version 5.0, http://www.droidb.org/Index.jsp
Flybase, FB2010_04, http://flybase.org
If the X. laevis gene was identified in the BLAST search, the X. tropicalis gene is not listed (except for CG17265, see note 4).
Both X. laevis and X. tropicalis genes are listed for CG17265 (ccd85c) because the X. tropicalis gene has a higher amino acid sequence identity to CG17265 over its entire coding region.
These two NCBI GenBank entries for Ddx43 are identical in sequence, and thus are counted as only one gene in the text.
Results and Discussion
Drosophila SO and Xenopus Six1 and Six2 are highly similar in the Six Domain
It is well documented that SO/Six proteins bind to Eya and Groucho proteins (Pignoni et al., 1997; Heanue et al., 1999; Ohto et al., 1999; Kobayashi et al., 2001; Zhu et al., 2002; Lopez-Rios et al., 2003), and we previously showed in Xenopus that co-expression of either Eya1 or Groucho causes Six1 to have significantly different effects on PPE/placode development (Brugmann et al., 2004). Drosophila SO and Xenopus Six1/Six2 proteins are highly conserved through their Six-HD domains (Pandur and Moody, 2000), which is consistent with the observations that SO, Six1 and Six2 share DNA binding site specificity (Kawakami et al., 1996; Spitz et al., 1998; Silver et al., 2003; F. Pignoni unpublished). Figure 1 illustrates that SO, Six1 and Six2 also are highly conserved throughout their protein-protein interaction domains (the SD). In fact, only 4 amino acid substitutions are non-conserved; all other changes in sequence involve conserved or semi-conserved amino acids. This suggests that these three proteins are likely to share co-factor binding specificity.
Figure 1.
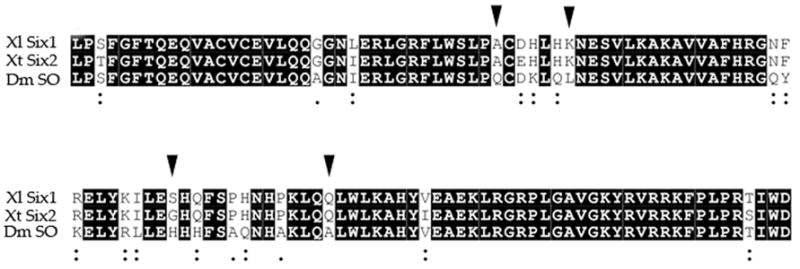
Fly SO, Xenopus Six1 and Xenopus Six2 proteins have a high level of amino acid identity (82.5%; black boxes) throughout their Six domain (SD, aa 1-120), the protein-protein interaction domain. Only 4 amino acid substitutions are non-conserved (arrowheads). All other changes in sequence involve conserved (:) or semi conserved (.) amino acids. The alignment was performed using Clustal W version 2.0 (Larkin et al., 2007). Dm, Drosophila melanogaster; Xl, Xenopus laevis; Xt, Xenopus tropicalis.
Identification of putative Xenopus orthologues of Drosophila SO-interacting proteins
In Drosophila, 25 factors that can interact with SO have been identified by yeast 2-hybrid screens (Pignoni et al., 1997; Giot et al., 2003; Kenyon et al., 2005). We performed database searches for putative Xenopus orthologues and identified between one and four genes with amino acid sequence homology to 20 of the fly proteins (Table 1). Two members of the Eya gene family (Eya1, David et al., 2001; Eya3, Kriebel et al., 2007), four Groucho-related genes (Grg4, Grg5, ESG1, ESG2; Choudhury et al., 1997; Molenaar et al., 2000), two C15-related genes (Hox11, Hox11L2; Patterson and Krieg, 1999) and one Gadd45-related gene (Gadd45γ; de la Calle-Mustienes et al., 2002) are previously reported in Xenopus. In addition to these, we identified two additional Eya genes (Eya2, Eya4), three genes with homology to SOBP, two genes each with homology to CG5033 and CG5665, two additional Gadd45 genes (Gadd45α, Gadd45β) and one gene each with homology to CG9083, CG17265, CG1218, CG1135, CG7878, CG33090, RhoGEF2, Chc, CKIIβ, CG10576, VhaAC39, RhoGAP93B, and RpS27 (Table 1).
Xenopus candidate Six-interacting genes are expressed in developing craniofacial tissues
Xenopus Six1 is initially expressed diffusely in the dorsal embryonic ectoderm, and then is highly expressed throughout the PPE (Pandur and Moody, 2000); weak expression has been noted in the early neural plate as well (Schlosser and Ahrens, 2004). As individual placodes form, Six1 is expressed in each except the lens, whereas Xenopus Six2 expression is initially concentrated in the olfactory and otic placodes (Pandur and Moody, 2000; Ghanbari et al., 2001). At tail bud to larval stages both are additionally expressed in the cranial ganglia, somites, hypaxial muscle precursors and nephric mesoderm, and Six1 expression is reported in the migrating lateral line primordium. Ghanbari et al. (2001) report Six1 and Six2 expression in the branchial arch mesoderm, whereas Pandur and Moody (2000) did not observe this for Six1.
To determine whether the putative Xenopus orthologues of Drosophila SO-interacting proteins are expressed in tissue domains that overlap with Six1/Six2 gene expression, we performed in situ hybridization (ISH) assays for candidates of 11 of the Drosophila genes. We did not study the expression patterns of the putative Xenopus orthologues of the remaining Drosophila genes because they are: 1) very broadly expressed in nearly all tissues in other organisms (RhoGEF2, Chc, RpS27); 2) reported in the Xenopus Unigene EST expression profile to not overlap with Six1 expression (CG33090, Unigene Str.38506; VhaAC39, Unigene Xl.77521); 3) only available as genomic sequence in the X. tropicalis database (Eya4, CG9083, CG5665, RhoGAP93B, Gadd45β); or 4) only available as an EST with homology to only a very short region of the Drosophila protein (CG1218), which reduced our confidence that this is a true orthologue. As summarized in Table 2, we found that the candidate Xenopus Six-interacting genes representing 11 of the Drosophila SO-interacting proteins are expressed in craniofacial tissues, most in patterns that overlap extensively with Six1/Six2 gene expression. In the following sections, we present the expression pattern of each of the 11 sets of putative Xenopus orthologues, and summarize what is know about their expression patterns and function in fly and in other vertebrates.
Table 2.
Developmental expression patterns of Xenopus putative Six-interacting proteins
| Drosophila gene | Xenopus candidates | Maternal O: st V/VI C: st 2–5 |
Blastula st 8–9 |
Gastrula E: st 10–11 L: st 12–13 |
Neural Plate E; st 14–15 L: st 16–18 |
Neural Tube Closure st 19–23 |
Tail bud st 24–32 |
Larva st 34–39 |
|---|---|---|---|---|---|---|---|---|
| Eya | Eya1* | O: ND C: ND |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE L: NP, PPE |
NT, AH, Olf, Vp, dlp | All placodes except lens, Oto, somites, hypaxial ms, tail bud | Same as tail bud; plus lateral line, nephric meso |
| Eya2 | O: ND C: ND |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE L: NP, PPE |
NT, AH, Olf, Vp, dlp, somites | All placodes except lens, Oto, lateral line, somites, hypaxial ms | Same as tail bud; plus nephric meso | |
| Eya3* | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE L: NP, PPE, NC |
NT, AH, Olf, Vp, dlp, NC, somites | Brain, retina, all placodes including lens, Oto, BA, somites, tail bud | Same as tail bud; plus hypaxial ms | |
| Groucho | Grg4* (Tle4) | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE L: NP, PPE, NC, CG, D. epi |
NT, AH, Olf, Vp, dlp, lens, NC, D. epi | Brain, retina, all placodes, Oto, BA, somites, nephric meso Loss of CG staining |
Same as tail bud; plus cardiac meso, ventral gut Loss of somitic, nephric staining |
| ESG1* (Es-gro) (Tle3) | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: Ant NP, PPE L: Ant NP, PPE, NC, D. epi |
Ant NT, AH, Olf, Vp, dlp, lens, NC, somites, D. epi | Brain, retina, all placodes, Oto, BA, somites, tail bud | Same as tail bud; plus lateral line, cardiac meso, ventral gut Loss of lens and somite staining |
|
| Grg5* (Aes) | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: Ant NP, PPE, CG L: Ant NP, PPE, NC, CG, D. epi |
Ant NT, AH, Olf, Vp, dlp, lens, NC, CG, epi | Brain, retina, all placodes, Oto, BA, CG, somites, nephric meso, tail bud | Same as tail bud; plus cardiac meso, ventral gut Loss of lens and CG staining |
|
| CG17265 | Ccd85c | O: ND C: ND |
ND | E: ND L: Dorsal ecto |
E: Ant NP, PPE L: Ant NP, PPE, NC |
Ant NT | Retina, Mb, Hb, pineal, lens, BA | Same as tail bud; plus Fb |
| C15 | Hox11* | O: ND C: ND |
ND | E: ND L: ND |
E: ND L: ND |
Dorsal-lateral NT, paraxial meso | Same as NT stage; plus hindbrain patch, Vg, VII/VIIIg, central BA, thyroid | Same as tail bud; plus IX/Xg Loss of Vg |
| Hox11L2* | O: ND C: ND |
ND | E: ND L: ND |
E: ND L: ND |
Dorsal-lateral spinal cord, Vg | Same as NT stage; plus hindbrain patch, VII/VIIIg, IX/Xg | Same as tail bud | |
| SOBP | Zfp198 | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE L: NP, PPE, NC, D. epi |
NT, AH, Olf, Vp, dlp, lens, NC | Ant NT, all placodes, Oto, BA, tail bud | Same as tail bud; plus somites Loss of lens staining |
| LOC414497 | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE L: NP, PPE, NC, D. epi |
NT, AH, Olf, Vp, dlp, lens, NC | Ant NT, all placodes, Oto, BA, tail bud, nephric meso | Same as tail bud; plus somites Loss of lens staining |
|
| Xt Sobp | O: ND C: ND |
ND | E: ND L: Dorsal ecto |
E: NP, PPE, D. epi L: NP, PPE, NC, D. epi |
NT, Olf, dlp | Mb, Hb, Olf, Oto, Vg, VIIg | Same as tail bud; plus Fb, IX/Xg | |
| CG1135 | LOC100049093 | O: ND C: Animal |
ND | E: ND L: Dorsal ecto |
E: NP, PPE, D. epi L: NP, PPE, NC, D. epi |
NT, AH, Olf, Vp, dlp, lens, NC | Brain, retina, lens, Olf, Vg, VIIg, IX/Xg Oto, BA | Same as tail bud; plus somites, nephric meso |
| CG7878 | Ddx43 | O: ND C: ND |
ND | E: ND L: Dorsal ecto |
E: NP, PPE, D.epi L: NP, PPE, NC, D. epi |
NT, dlp, lens, NC | Brain, retina, spinal cord, Olf, Oto, BA | Same as tail bud; plus somites, nephric meso, cardiac, hypaxial ms. |
| CKIIβ | CKIIβ* | O: Yes C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE L: NP, PPE, NC |
NT, AH, Olf, Vp, dlp, lens, NC | Brain, retina, spinal cord, Olf, Oto, Vg, lens, VIIg, BA | Same as tail bud; plus IX/Xg, somites, nephric meso, hypaxial ms. |
| CG10576 | 2G4 | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE L: NP, PPE, NC, D. epi |
NT, AH, Olf, Vp, dlp, lens, NC, D. epi | Brain, retina, all placodes, Oto, BA, nephric meso, D. epi | Same as tail bud |
| CG5033 | MGC68939 | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE, D. epi L: Ant NP, PPE, NC, D. epi |
Ant NT, AH, Olf, Vp, dlp, lens, NC | Brain, retina, all placodes, Oto, BA, somites, nephric meso, tail bud | Same as tail bud; plus hypaxial ms, ventral gut |
| Bop-1 | O: Animal C: Animal |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE, D. epi L: Ant NP, PPE, NC, D. epi |
Ant NT, AH, Olf, Vp, dlp, lens, NC, somites | Brain, retina, all placodes, Oto, BA, somites, nephric meso, tail bud | Same as tail bud; plus hypaxial ms, ventral gut | |
| Gadd45 | Gadd45α | O: ND C: ND |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: NP, PPE, CG, D. epi L: NP, PPE, NC, CG, D. epi |
CG, pineal, peri-cloacal meso, paraxial meso | Head and Ant trunk, paraxial meso patch in tail Loss of CG staining |
Same as tail bud; plus ventral gut |
| Gadd45γ* | O: ND C: ND |
Animal cap ecto | E: Ectoderm L: Dorsal ecto |
E: primary neurons, PPE L: primary neurons, PPE |
NT, pineal, AH, Olf, Vp, dlp, lens, CG, somites, D. epi | Same as NT stage; plus epibranchial placodes | Same as tail bud; plus somites Loss of lens and Olf staining |
AH, adenohypophyseal placode; Ant, anterior; BA, branchial arches; BZ, border zone; C, cleavage; CG, cement gland; D. epi, dorsal epidermis; dlp, dorso-lateral placode; E, early; ecto, ectoderm; Fb, forebrain; Hb, hindbrain; lat, lateral; IX/Xg, hypoglossal/vagal ganglion; L, late; Mb, midbrain; meso, mesoderm; ms, muscle; NC, neural crest; ND, not detected; NP, neural plate; NT, neural tube; O, oocyte; Olf, olfactory placode; Oto, otocyst; PPE, pre-placodal ectoderm; Vg, trigeminal ganglion; VII/VIIIg, facial/acouticovestibular ganglion; Vp, trigeminal placode.
Data in part from Choudhury et al., 1997; David et al., 2001; de la Calle-Mustienes et al., 2002; Dominguez et al., 2004; Kriebel et al., 2007; Molenaar et al., 2000; Patterson and Krieg, 1999; Schlosser and Ahrens, 2004; Schlosser, 2006; Wilhelm et al. 1995, and in part from our observations.
Eya genes
We identified three members of the Xenopus Eya gene family (Eya1, Eya2, Eya3); the Xenopus tropicalis genomic database annotates an Eya4 gene (Table 1), but no cDNA clones are currently available. The expression of Eya1 was previously reported (David et al., 2001; Schlosser and Ahrens, 2004; Schlosser, 2006), and is summarized in Table 2. Our ISH analyses confirm the reported patterns and additionally show weak expression throughout the blastula animal cap ectoderm (Fig. 2A), early gastrula ectoderm (Fig. 2B), dorsal ectoderm of the late gastrula and nephric mesoderm of the larva (Table 2). The expression of Xenopus Eya3 was previously reported (Kriebel et al., 2007). Unlike Eya1, it is detected maternally in the animal region of oocytes (Table 2) and animal blastomeres of cleavage stage embryos (Fig. 2C). It should be noted that the lack of vegetal pole staining for these genes (and all subsequent genes described) could result from poor probe penetration into the yolky endodermal cells rather than lack of expression. However, maternal animal hemisphere expression is consistent with the later zygotic expression in the ectodermal precursors in the animal half of blastula stage embryos and throughout the embryonic ectoderm in the early gastrula. Like Eya1, we observed that Eya3 is expressed in the blastula animal cap ectoderm (Fig. 2D) and gastrula ectoderm, most prominently on the dorsal side (Fig. 2E). In addition, Eya3 is strongly expressed in the neural plate and PPE (Fig. 2F), anterior neural tube, neural crest and the branchial arches derived from them, and the lens (Table 2; Kriebel et al., 2007). Xenopus Eya2 expression has not previously been reported. Maternal Eya2 mRNA is not detected. Like the other Eya genes, Eya2 is weakly expressed in the blastula animal cap, early gastrula ectoderm, and enhanced in the dorsal ectoderm of the late gastrula (Table 2). By neural plate stages, it is detected diffusely throughout the neural plate and in the PPE (Fig. 2G). As the neural tube closes, it is detected in the neural tube, multiple placodes and somites (Figs. 2H, I). During early tail bud stages, it is additionally expressed in the otocyst and nephric mesoderm (Fig. 2J), and by late tail bud/larval stages it is additionally expressed in the cranial ganglia, epibranchial placodes and hypaxial muscle precursors (Fig. 2K–O).
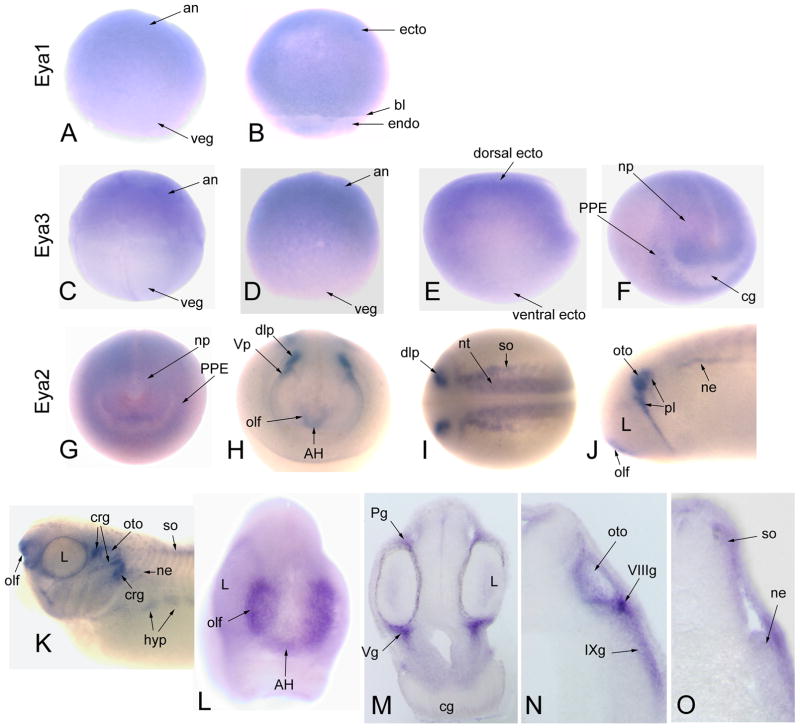
Figure 2.
Expression of Eya genes. Eya1 is diffusely expressed throughout the ectoderm (ecto) of (A) the animal (an) cap of the blastula and (B) the gastrula [Side views]. veg, vegetal pole; bl, blastopore lip; endo, endoderm. Eya3 is (C) detected in the animal blastomeres of the 32-cell embryo, (D) the animal cap of the blastula, and (E) enhanced in the dorsal ectoderm of the late gastrula [Side views]. (F) At neural plate stages, Eya3 is expressed throughout the neural plate (np) and pre-placodal ectoderm (PPE), but not the cement gland (cg) [Antero-lateral view]. (GO) Eya2 expression. (G) Eya2 is diffusely expressed throughout the neural plate and PPE [Anterior view]. Upon neural tube closure, Eya2 is (H) strongly expressed in the adeno-hypophyseal (AH), olfactory (olf), trigeminal (Vp) and dorso-lateral (dlp) placodes. (I) It also is detected in the caudal neural tube (nt) and somites (so) [H, Anterior view; I, Dorsal view]. At tail bud (J) and larval (K, L) stages, Eya2 is strongly expressed in the olfactory (olf), otic (oto), and various other placodes (pl) and their cranial ganglion derivatives (crg). Note that the lens placode (L) does not express Eya2. There is additional expression in the nephric mesoderm (ne) and hypaxial muscle precursors (hyp) [J, K, Side views; L, anterior view]. In transverse sections at the levels of the diencephalon (M), otocyst (N) and caudal hindbrain (O), Eya2 expression is notable in the cranial ganglia (profundal, Pg; maxillo-mandibular branch of the trigeminal, Vg; VIIIg, IXg), otocyst, somitic and nephric mesoderm.
These expression patterns are consistent with reports in other vertebrates that Eya genes are expressed in ectodermal placodes, somites, brain, nephric mesoderm and hypaxial muscle precursors, i.e., tissues that express Six genes (Xu et al., 1997; 2003; Sahly et al., 1999; Schonberger et al., 2005; Soker et al., 2008; Ishihara et al., 2008). The developmental functions of Eya are well-studied in Drosophila; it plays a key role in early eye formation, head morphogenesis, gametogenesis and muscle development (Bonini et al., 1993; 1997; 1998; Pignoni et al., 1997; Bai and Montell, 2002; Fabrizio et al, 2003; Lui et al., 2009). The involvement of vertebrate Eya proteins in craniofacial development also is well-established. In Xenopus and chick, Eya1 and Eya2 are involved in establishing the PPE domains (Brugmann et al., 2004; Christophorou et al., 2009). Mouse Eya1 is necessary for normal development of the ear, kidney, thymus, parathyroid, thyroid and somitic muscle (Xu et al., 1999; 2002; 2003; Johnson et al., 1999), and mutations in human Eya1 cause some cases of BO/BOR (Abdelhak et al., 1997). Eya4-deficient mice exhibit abnormal middle ear cavities, Eustachian tube dysmorphology and are a model for heritable otitis media (Depreux et al., 2008); mutations in human Eya4 result in sensorineural hearing loss (Wayne et al, 2001; Pfister et al., 2002). However, the roles for mammalian Eya2 and Eya3 in craniofacial development have not yet been established, although they are required in other tissues (Grifone et al., 2007; Soker et al., 2008); there also are no reported human defects involving Eya2 or Eya3 to date. Nonetheless, the expression of Eya2 and Eya3 in the Xenopus and chick PPE and placodes, and the demonstrated ability of each of the four vertebrate Eya proteins to interact with Six1, Six2 and/or Six4 (Heanue et al., 1999; Ohto et al., 1999; Ikeda et al., 2002; Li et al., 2003; Schonberger et al., 2005; Abe et al., 2009) predict that all four Eya genes are likely to act as Six gene co-factors, perhaps with redundant roles, during craniofacial development.
Groucho-related genes
Four Groucho-related (Grg) genes have been reported in Xenopus. ESG2 is not expressed in embryos (Choudhury et al., 1997), whereas the other three (Grg4/Tle4, Grg5/Aes, ESG1/Grg3/Tle3) have over-lapping, but not identical, embryonic expression patterns (Table 2; Fig. 3). All three mRNAs are detected by Northern blots to be maternally expressed (Choudhury et al., 1997; Molenaar et al., 2000); we confirmed this by ISH and show that each mRNA is localized to the animal hemisphere of oocytes and animal blastomeres of cleavage embryos (Fig. 3A, B; Table 2). All three Grg genes have enhanced expression in the animal cap ectoderm of the blastula (Fig. 3C; Table 2) and gastrula (Fig. 3D). At early neural plate stages, Grg4/Tle4 is expressed throughout the neural plate and PPE (Fig. 3E), whereas Grg5/AES and ESG1/Tle3 expression is more pronounced in the anterior neural plate (Fig. 3F, G); Grg5/AES also is expressed in the cement gland (Fig. 3G, O). At late neural plate and neural tube closure stages, all three Grg genes are additionally detected in the neural crest and dorsal epidermis (Fig. 3H, I). Grg4/Tle4 becomes weakly expressed in the cement gland (Fig. 3J) and ESG1/Tle3 is additionally expressed in somites (Fig. 3H, I). All three Grg genes are expressed throughout the head ectoderm, including the placodes (Fig. 3I). At tail bud and larval stages, all three Grg genes are expressed in the brain and retina (Fig. 3K, L, N–R), all placodes and cranial ganglia (Fig. 3K, L, N, Q, R), neural crest and branchial arch mesoderm (Fig. 3K, L, O–R), and somites. All three Grg genes are additionally expressed in the nephric mesoderm, heart and ventral gut (Fig. 3L, M, Q, R); ESG1/Tle3 is additionally detected in the initial outgrowth of the lateral line (Fig. 3L).
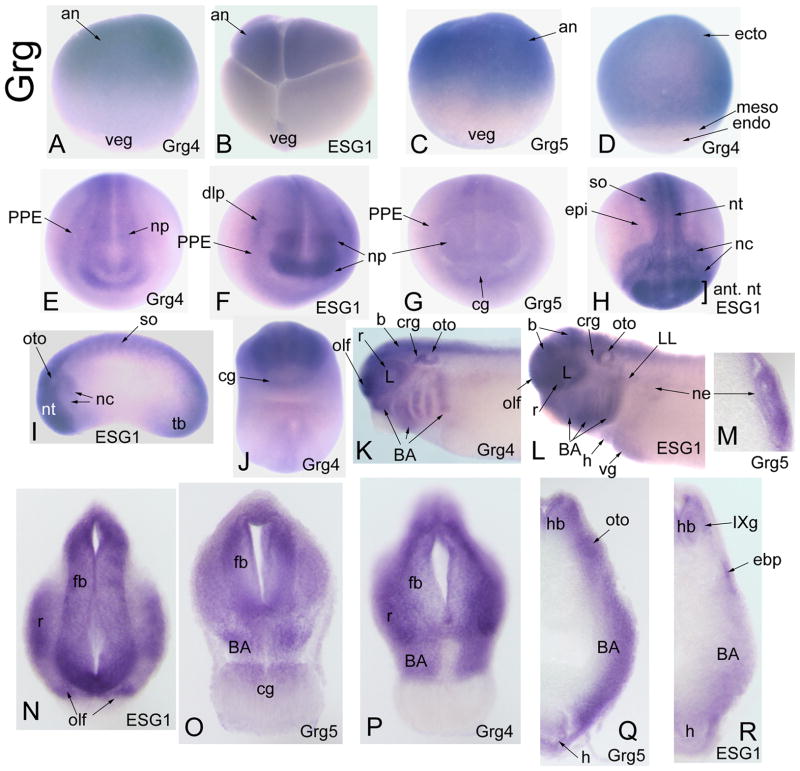
Figure 3.
Expression of Groucho-related genes (Grg). (A) Stage VI oocyte with Grg4 transcripts enhanced on animal (an) side [Side view]. (B) 8-cell embryo with maternal ESG1 transcripts enhanced in the animal blastomeres [Side view]. (C) In the blastula, animal cap ectoderm expresses Grg5 [Side view]. (D) In the gastrula, the entire ectoderm expresses Grg4, whereas the pre-involuted mesoderm (meso) and endoderm do not [Side view]. At neural plate stages, Grg4 (E), ESG1 (F) and Grg5 (G) are expressed in the neural plate and PPE; Grg5 is also expressed in the cement gland [Anterior views]. (H) ESG1 is expressed throughout the neural tube, but most strongly in the anterior part (ant. nt). It is also detected in the neural crest (nc), dorsal epidermis (epi) and somites [Dorsal view]. (I) At tail bud stages, ESG1 expression is strong in the entire neural tube, migrating neural crest, somites, otocyst and tail bud (tb) [Side view]. (J) At tail bud stages, Grg4 is weakly detected in the cement gland [Ventral view]. (K) At larval stages, Grg4 is expressed throughout the brain (b), retina (r), placode derivatives (crg, L, olf, oto) and the branchial arches (BA) [Side view]. (L) ESG1 is expressed in the same tissues, and also is detected in the heart (h), ventral gut (vg), nephric mesoderm and the initial outgrowth of the lateral line (LL) [Side view]. In transverse sections at the levels of the anterior trunk (M), forebrain (N, O, P) and hindbrain (Q, R), expression of the various Grg-related genes is noted in the nephric mesoderm, forebrain (fb), hindbrain (hb), retina, branchial arches, cement gland, various placodes (olf, oto, IXg, epibranchial {ebp}) and heart.
Groucho is a well-characterized transcriptional co-factor in fly that does not bind to DNA directly and requires protein-protein interactions to assert its repressive activity (reviewed by Courey and Jia, 2001). Yeast 2-hybrid analyses show that vertebrate Six proteins physically interact with Xenopus, zebrafish, mouse and human Grg proteins via the SD (Kobayashi et al., 2001; Zhu et al., 2002; Lopez-Rios et al., 2003). Xenopus Grg4/Tle4 is thought to be functionally homologous to Drosophila Groucho, whereas Grg5/AES is a naturally truncated form that functions as a dominant-negative (Roose et al., 1998; Molenaar et al., 2000). Xenopus Grg4/Tle4 inhibits β-catenin/Tcf-3 mediated axis duplication (Roose et al., 1998), and enhances the transcriptional repressive activity of FoxD3 required for mesoderm induction (Yaklichkin et al., 2007). Mouse and human Grg4/Tle4 are expressed in brain and muscle (Koop et al., 1996), mouse and human Grg5/AES in muscle, heart, brain and placenta (Miyasaka et al., 1993), and mouse ESG1/Grg3/Tle3 in the brain, cranial ganglia, olfactory and otic structures (Dehni et al, 1995; Leon and Lobe, 1997). To date there is no information regarding the functional role of vertebrate Grg co-factors in craniofacial development, but co-expression of Drosophila Groucho with Xenopus Six1 causes repression of cranial neural crest and epidermal genes (Brugmann et al., 2004). The expression of Xenopus Grg4/Tle4, Grg5/AES and ESG1/Tle3 overlaps extensively with Six1 and Six2 in PPE and placodes; they also are expressed in cranial neural crest and neural tube. Thus, these three Grg genes are likely to have roles in craniofacial development.
CG17265 (ccd85c)-related genes
We identified two putative Xenopus orthologues: Xenopus tropicalis (Xt) ccd85c and Xenopus laevis (Xl) ccd85c (Table 1). Since Xt-ccd85c showed the highest amino acid sequence identity to CG17265 over its entire coding region, we used the Xt-ccd85c probe to study expression patterns in X. laevis embryos; it should be noted that many ISH probes can be interchanged between the two Xenopus species. Subsequent studies with the Xl-ccd85c probe gave identical results (data not shown). Staining is not detected prior to stage 12 (Table 2). Throughout neural plate stages, the Xt-ccd85c probe diffusely stains the anterior neural plate and the PPE (Fig. 4A). At neural tube closure stages, diffuse expression is detected in the anterior neural tube, retina, otocyst, dorsal epidermis and branchial arches (Fig. 4B). At tail bud stages, the pineal, midbrain and hindbrain are strongly stained and the retina, lens, otocyst and branchial arches are weakly stained (Fig. 4C). By larval stages, forebrain staining intensifies (Fig. 4D, E); in some embryos expression extends into the spinal cord. Lens and retina expression is maintained, but branchial arch and otocyst expression is barely detected (Fig. 4E, F). Unlike other candidate co-factor genes, there is no detectable expression of Xt-ccd85c in nephric or somitic mesoderm (Fig. 4G).
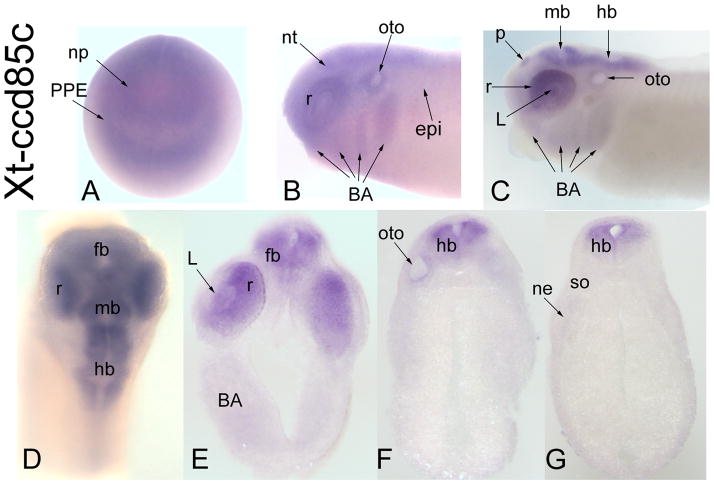
Figure 4.
Expression of a CG17265-related gene, Xt-ccd85c. (A) Diffuse expression of Xt-ccd85c throughout the anterior neural plate and PPE [Anterior view]. (B) At neural tube closure, there is diffuse expression throughout the neural tube, retina dorsal epidermis, otocyst and branchial arches [Side view]. (C) At late tail bud stages the pineal (p), retina, midbrain and hindbrain are stained. There also is weak staining in the lens, otocyst and branchial arches [Side view]. (D) Dorsal view at larval stage showing expression extending into the forebrain. Transverse sections at forebrain (E), hindbrain (F) and caudal hindbrain (G) demonstrate restricted neural expression.
Drosophila CG17265 encodes a nuclear protein that is thought to be involved in RNA interference (Dorner et al., 2006). There are no functional studies of this gene in Xenopus or any other vertebrate to date. However, a related protein, Ccd85b, was identified as a binding partner of human Six1 in the human protein-protein interaction network (Rual et al., 2005). Xenopus ccd85c expression only partially overlaps those of Six1 and Six2 (PPE, lens, otocyst), suggesting that it may have a restricted role in craniofacial development.
C15-related genes
C15 is assigned to Xenopus Unigene Xl.679 and shares 57.1% amino acid identity with Xenopus Hox11L2, the Xenopus homolog of the mammalian T-cell leukemia homeobox 3 (Tlx3) proteins (Table 1). The expression patterns of Hox11L2 and the related gene, Hox11 (mammalian Tlx1) were previously described in detail (Patterson and Krieg, 1999; summarized in Table 2). Their expression patterns are similar to those reported in mouse and chick embryos (Raju et al., 1993; Roberts et al., 1994, 1995; Logan et al., 1998; Uchiyama et al., 1999), although the prominent Hox11 expression in mouse spleen was not seen in Xenopus (Patterson and Krieg, 1999). Mis-expression of Hox11L2 or Hox11 is associated with some forms of human T-cell leukemia (Hatano et al., 1991; Lu et al., 1991; Bernard et al., 2001; Ferrando et al., 2002), and their knock-down in mice results in abnormal development of the spleen, colon and the ventral medullary respiratory center (Roberts et al., 1994; Dear et al., 1995; Hatano et al, 1997; Shirasawa et al., 2000); these studies do not report craniofacial defects. The expression of these genes overlaps only minimally with Six1/Six2; however, because they are expressed in cranial sensory ganglia and branchial arches, Hox11 and Hox11L2 may have late roles in craniofacial development.
Sobp-related genes
We identified two Sobp-related Xenopus laevis (Xl) genes (Zfp198, LOC414497) with homology in the regions designated as box2 and box3 in Drosophila SOBP (Kenyon et al, 2005), and one Xenopus tropicalis gene (Xt-Sobp) with homology in boxes 1–3. Maternal mRNAs for both Xl-Sobp-related genes are detected in the animal blastomeres of cleavage embryos (Fig. 5A; Table 2). They are expressed in the animal cap ectoderm of the blastula (Table 2), throughout the embryonic ectoderm at early gastrula stages (Fig. 5B) and begin to be dorsally concentrated in the ectoderm at late gastrula stages (Table 2). In contrast, maternal Xt-Sobp mRNA is not detected, nor is Xt-Sobp expression detected at blastula or early gastrula stages (Table 2). All three Sobp-related genes are expressed in the neural plate, PPE and dorsal epidermis at neural plate stages (Fig. 5C; Table 2). At neural tube closure, the two Xl-Sobp-related genes are detected throughout the neural tube, in all placodes and the neural crest (Fig. 5D; Table 2), whereas Xt-Sobp is only weakly expressed in the neural tube and in the olfactory and dorso-lateral placodes (Table 2). Epidermal staining of all three genes is lost at this stage. At tail bud and larval stages, the two Xl-Sobp-related genes are strongly expressed in brain and cervical spinal regions (Fig. 5E, F, I, J, K), whereas the Xt-Sobp gene is expressed only in lateral patches in the midbrain and hindbrain (Fig. 5G, L). All three genes are expressed in various placodes and placode derivatives, but the Xt-Sobp gene is more restricted (Fig. 5G, L). The two Xl-Sobp-related genes are additionally expressed in the branchial arches (Fig. 5E, F, I, J). At late larval stages, the two Xl-Sobp-related genes no longer stain the lens (Table 2), and begin to be expressed in the somites (Fig. 5K). At late larval stages, Xt-Sobp expression expands into the forebrain (Fig. 5H).
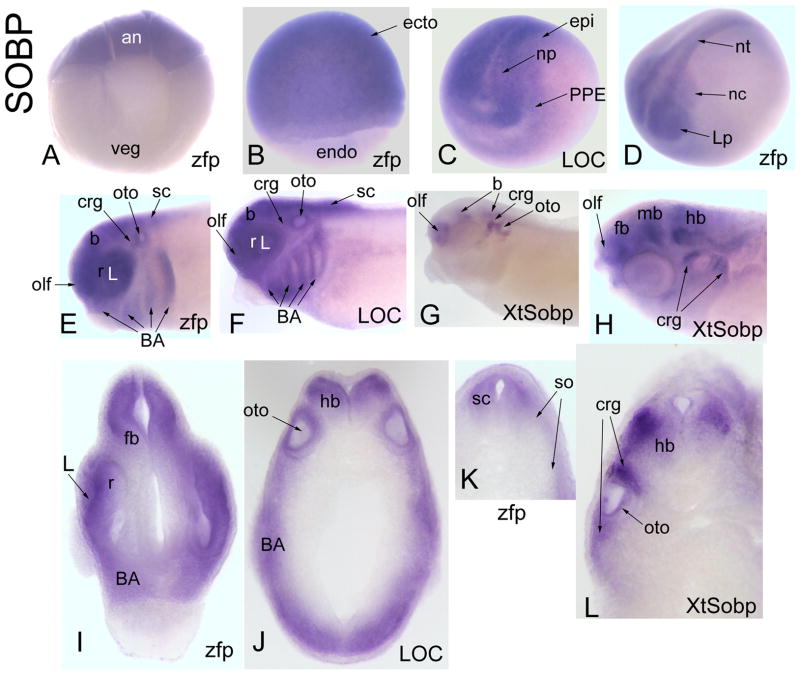
Figure 5.
Expression of SOBP-related genes. (A) zfp198 mRNA is concentrated in the animal blastomeres at the 8-cell stage [Side view]. (B) zfp198 is expressed throughout the ectoderm of the gastrula [Side view]. (C) At early neural plate, LOC414497 is expressed throughout the neural plate, PPE and dorsal epidermis [Antero-lateral view]. (D) At neural tube closure, neural crest and lens placode (Lp) expression of zfp198 becomes apparent [Antero-lateral view]. (E, F, G) At late tail bud, zfp198 (E) and LOC414497 (F) are expressed throughout the brain, retina, placode derivatives (olf, L, crg, oto) and branchial arches, whereas Xt-Sobp (G) is expressed in patches in the brain and in several placode derivatives (olf, crg, oto) [Side view]. (H) At larval stages, Xt-Sobp expression extends into the forebrain [Side view]. Transverse sections at forebrain (I), hindbrain (J) and spinal (K) levels demonstrate expression of zfg198 and LOC414497 in neural (fb, r, hb, sc), branchial arch, otocyst and somites. (L) Transverse section at hindbrain demonstrates Xt-Sobp more restricted expression in lateral hindbrain, otocyst and cranial ganglia.
Drosophila SOBP is a known binding partner of SO and is expressed in the differentiating eye field (Kenyon et al., 2005). In mouse, Sobp is expressed in the otocyst, retina, olfactory epithelium, trigeminal ganglion and hair follicles, and the Jackson circler mouse, which is deaf, carries a recessive mutation in Sobp (Chen et al., 2008). The expression of the Xenopus Sobp genes partially overlap with Six genes (PPE, placodes), and their additional expression in the cranial neural crest, branchial arches and anterior neural tube suggest they will have roles in craniofacial development.
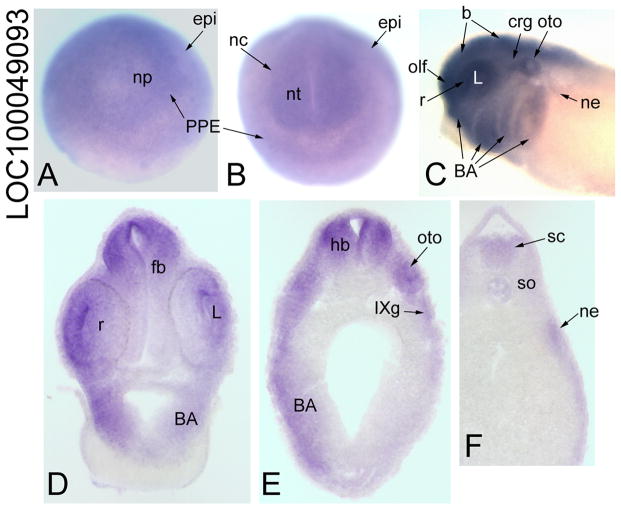
CG1135-related gene
Maternal LOC100049093 mRNA is weakly detected in animal blastomeres of cleavage embryos but is not detected by ISH at blastula or early gastrula stages (Table 2). By late gastrula and early neural plate stages, it is expressed throughout the dorsal epidermis, with enhancement in the neural plate and PPE (Table 2; Fig. 6A). At neural tube closure, it is expressed throughout the neural tube, and diffusely in the PPE, neural crest and epidermis (Fig. 6B). At tail bud and larval stages, it is strongly expressed throughout the brain and retina in all embryos (Fig. 6C), and weakly expressed in the spinal cord in a few embryos (Fig. 6F). In addition, it is strongly expressed in placodes, cranial ganglia and branchial arches (Fig. 6C, D, E), and weakly expressed in nephric mesoderm (Fig. 6C, F). By larval stages, it is additionally expressed throughout the head mesoderm and anterior somites (Table 2).
Figure 6.
Expression of a CG1135-related gene. (A) LOC100049093 is expressed diffusely through the neural plate, PPE and dorsal epidermis (epi) [Anterior view]. (B) At neural tube closure it is slightly enhanced in the neural tube, PPE and neural crest [Anterior view] (C) At larval stages, it is detected in the brain, retina, several placode derivatives (L, olf, oto, crg), branchial arches and nephric mesoderm [Side view]. Transverse sections at forebrain (D), hindbrain (E) and spinal (F) levels demonstrate extensive neural (fb, r, hb, sc), placodal (L, oto, IXg), and branchial arch, and weak expression in the nephric mesoderm.
In Drosophila, CG1135 contains a Forkhead-associated domain and was identified as a component of the Hh signal pathway in a large-scale RNA interference screen (Nybakken et al., 2005). Its molecular function is unknown, but it is annotated as having a role in centriole replication. The Xenopus orthologue was identified as a component of the origin of replication (Carpenter and Dunphy, 1998). The human orthologue, microspherule protein-1: 1) is part of a centrosomal complex that is essential for cell viability (Hirohashi et al., 2006); 2) modulates Daxx-dependent transcriptional repression (Lin and Shih, 2002); and 3) interacts with the fragile X mental retardation protein in polyribosomal mRNPS from neurons (Davidovic et al., 2006). LOC100049093 expression overlaps with that of Six1/Six2 (PPE, placodes, somites, nephric mesoderm) and also is expressed in the cranial neural crest, branchial arches and neural tube, suggesting a role in craniofacial development.
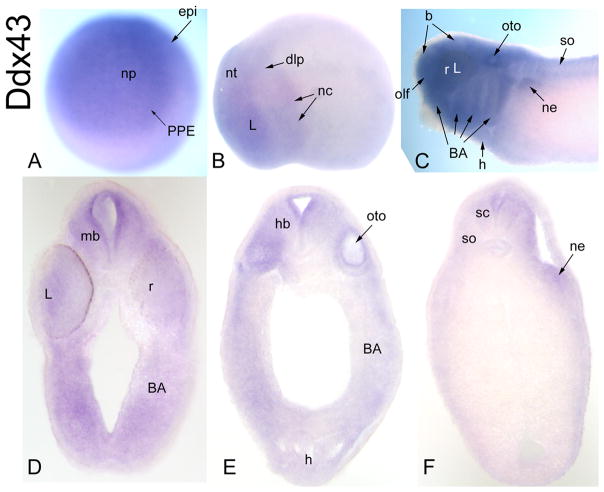
CG7878-related gene
There are two entries in GenBank for Xenopus tropicalis orthologues (Xt-Ddx43) of Drosophila CG7878 (Table 1), but their sequences are identical. Maternal mRNA for Xt-Ddx43 is not detected, nor is expression detected at blastula or early gastrula stages (Table 2). At neural plate stages, it is expressed diffusely through the neural plate, PPE and dorsal epidermis (Fig. 7A). At neural tube closure, it is weakly expressed throughout the neural tube, the lens and dorso-lateral placodes and migrating neural crest (Fig. 7B). At tail bud and larval stages, it is expressed throughout the brain and retina and eventually extends into the rostral spinal cord (Fig. 7C–F; Table 2). In addition, there is staining in the olfactory pit, otocyst and branchial arches (Fig. 7C, D, E; Table 2). By larval stages, there is additional diffuse staining in the head mesenchyme, somites, heart and nephric mesoderm (Fig. 7C–F).
Figure 7.
Expression of a CG7878-related gene. (A) Ddx43 is diffusely expressed throughout the neural plate, PPE and dorsal epidermis [Anterior view]. (B) At neural tube closure, it is very weakly expressed in the neural tube, a few placodes (dlp, L) and migrating neural crest [Side view]. (C) At larval stages it is expressed throughout the brain, retina, several placode derivatives (olf, oto, L), branchial arches, somites, heart and nephric mesoderm [Side view]. Transverse sections at midbrain (D), hindbrain (E) and spinal (F) levels demonstrate neural (mb, r, hb, sc), lens, branchial arch, heart, otocyst, somite and nephric mesoderm expression.
Drosophila CG7878 is predicted to function as an RNA helicase. The human orthologue, HAGE, is expressed at high levels in a variety of cancers and in normal adult testes (Martelange et al., 2000; Mathieu et al., 2010). Xt-Ddx43 expression overlaps with that of Six1/Six2 (PPE, placodes, somites, nephric mesoderm) and is expressed in the cranial neural crest, branchial arches and neural tube, suggesting a role in craniofacial development.
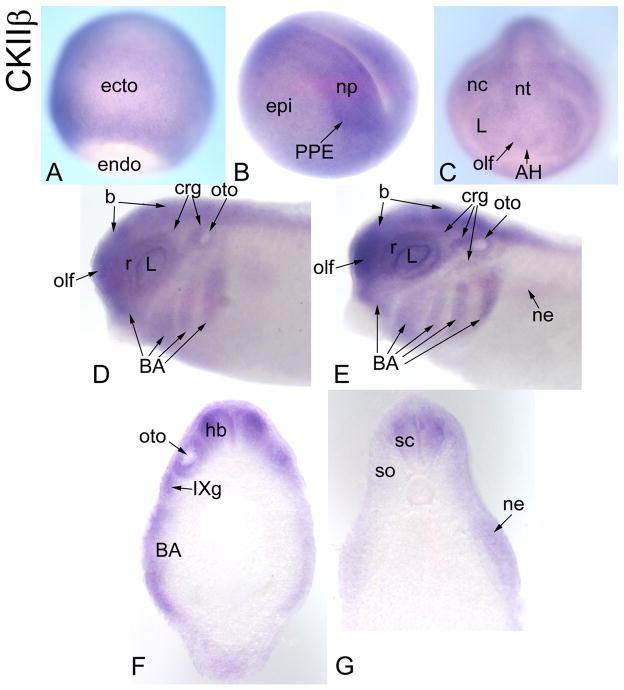
CKIIβ-related gene
We identified one Xenopus laevis gene related to Drosophila CKIIβ. Previous studies demonstrate that Xenopus CKIIβ is expressed maternally (Wilhelm et al., 1995) and in the animal hemispheres at cleavage and blastula stages (Dominguez et al., 2004) (Table 2). We detected its mRNA throughout the gastrula ectoderm (Fig. 8A), with a slight enhancement on the dorsal side. At neural plate stages it is expressed in the dorsal epidermis, neural plate and PPE (Fig. 8B). At neural tube closure, it is expressed throughout the neural tube, in all placodes and the neural crest (Fig. 8C). By tail bud stages, the branchial arches are additionally stained (Fig. 8D). By larval stages, there is additional expression in the somites and nephric mesoderm (Fig. 8E, F, G), and hypaxial muscle precursors (Table 2).
Figure 8.
Expression of a CKIIβ-related gene. (A) CKIIβ is expressed throughout the ectoderm of the gastrula [Dorsal view]. (B) At neural plate stages, epidermal staining continues, but expression is enhanced in the neural plate and PPE [Antero-lateral view]. (C) At neural tube closure, CKIIβ is expressed throughout the neural tube, in placodes (AH, olf, L) and neural crest [Anterior view]. (D) At tail bud stages, brain, retina, placodal structures (olf, L, crg, oto) and branchial arches are stained [Side view]. (E) At larval stages, the nephric mesoderm is additionally stained [Side view]. Transverse sections at hindbrain (F) and spinal (G) levels demonstrate neural (hb, sc), branchial arch, otocyst, cranial ganglion (IXg), somite and nephric mesoderm expression.
CKIIβ is the regulatory subunit of the pleiotropic and highly conserved protein kinase CK2 that is involved in a wide variety of cellular functions such as gene expression, protein synthesis, cell cycle and proliferation (reviewed in Pinna and Meggio, 1997; Guerra and Ossinger, 1999). In the fly, it is required for proper formation of both central and peripheral nervous systems. CKIIβ knock-out mice fail to survive beyond E6.5 (Buchou et al., 2003). In Xenopus embryos, CKIIβ: 1) inhibits the ability of Mos to arrest cells in mitosis (Lieberman et al., 2004); 2) is a positive regulator of the Wnt signaling pathway (Willert et al., 1997; Song et al., 2000; Dominguez et al., 2004); and 3) promotes dorsal axis development (Dominguez et al., 2004). Xenopus CKIIβ expression overlaps extensively with that of Six1/Six2 and it is expressed in the cranial neural crest, branchial arches and neural tube, suggesting a role in craniofacial development.
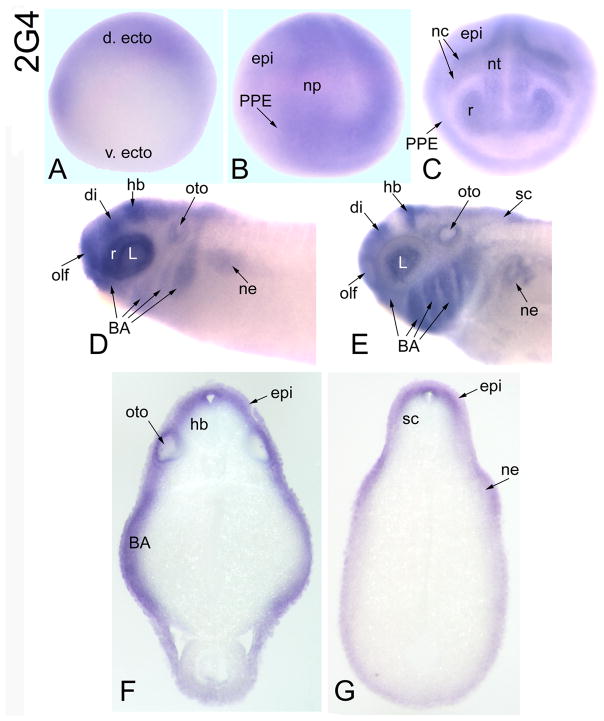
CG10576-related gene
Maternal 2G4 mRNA is barely detected in the animal regions of oocytes and cleavage blastomeres, and it is only weakly detected in the animal cap ectoderm of blastulas (Table 2). At early gastrula, it is expressed throughout the embryonic ectoderm and becomes enhanced in the dorsal ectoderm by late gastrula (Fig. 9A). At early neural plate stages, it is expressed throughout the neural plate, PPE and dorsal epidermis (Fig. 9B). At neural tube closure, it is detected throughout the neural tube, PPE, cranial neural crest and dorsal epidermis (Fig. 9C). At tail bud stages, it is strongly expressed in the retina, a band in the diencephalon at the mesencephalic border, a band in the hindbrain at the mesencephalic border, multiple placodes and branchial arches (Fig. 9D). At late tail bud and larval stages, staining in the otocyst and nephric mesoderm become apparent (Fig. 9D–G). At larval stages, staining in the neural tube becomes confined to dorsal domains and extends into the spinal cord; strong epidermal expression continues at these stages (Fig. 9F, G).
Figure 9.
Expression of a CG10576-related gene. (A) At late gastrula stages, the ectodermal expression of 2G4 is enhanced on the dorsal side (d. ecto) [v. ecto, ventral ectoderm; Side view]. (B) At neural plate stages, 2G4 is expressed diffusely throughout the neural plate, PPE and dorsal epidermis [Antero-lateral view]. (C) 2G4 is expressed throughout the neural tube, retina, PPE, cranial neural crest and epidermis [Anterior view]. (D) At tail bud stages, 2G4 is intensely expressed in the retina, diencephalon (di), hindbrain, placodes (olf, L, oto), branchial arches and weakly in the nephric mesoderm [Side view]. (E) At larval stages, the neural tube expression expands to the dorsal spinal cord, and nephric mesoderm staining is prominent [Side view]. Transverse sections at hindbrain (F) and spinal (G) levels demonstrate neural (hb, sc), branchial arch, otocyst, epidermal and nephric mesoderm expression.
In Drosophila, CG10576 acts as a modifier of muscle and heart fate specification (Bidet et al., 2003). Members of the mammalian proliferation-associated 2G4 family of RNA/DNA binding proteins are implicated in regulation of cell growth and differentiation (Kowalinski, et al., 2007; Sithanandam and Anderson, 2008; Zhang et al., 2008). The human orthologue, Ebp-1, is widely expressed (Xia et al., 2001), may play a role in ERBB3 signaling (Yoo et al., 2000; Sithanandam and Anderson, 2008), and interacts with both proteins and RNAs to regulate transcription and translation (Kowalinski et al., 2007). Mice deficient for Ebp-1 are 30% smaller in size than their wild type littermates (Zhang et al., 2008). Xenopus 2G4 expression overlaps with that of Six1/Six2 (PPE, placodes, nephric mesoderm) and is detected in the cranial neural crest, branchial arches and neural tube, suggesting a role in craniofacial development.
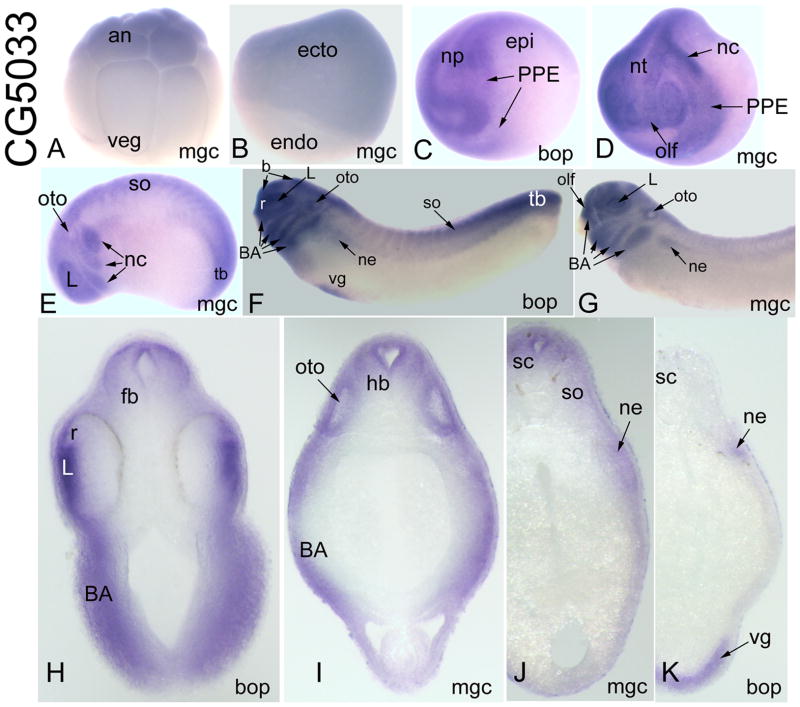
CG5033-related genes
MGC68939 and Bop-1 have very similar expression patterns (Table 2). Maternal mRNAs for both are detected in oocytes and animal blastomeres of cleavage embryos (Fig. 10A; Table 2). Both are expressed in the animal cap ectoderm of blastulas and throughout the ectoderm at gastrulation (Fig. 10B; Table 2). At early neural plate stages, they are expressed throughout the neural plate, PPE and dorsal epidermis (Fig. 10C). At late neural plate/neural tube closure stages, they are concentrated in the anterior neural plate/tube, PPE and neural crest (Fig. 10D). At early tail bud stages, staining is prominent in the anterior neural tube, lens, otocyst, migrating neural crest, somites and tail bud (Fig. 10E). At late tail bud stages, there is prominent staining in the brain, retina, placodes, branchial arches, somites, nephric mesoderm, tail bud and ventral gut (Fig. 10F–K). By larval stages there is additional staining in the hypaxial muscle precursors (Table 2).
Figure 10.
Expression of CG5033-related genes. (A) MGC68939 mRNA is detected in animal blastomeres of the16-cell embryo [Side view]. (B) It is expressed throughout the ectoderm of the gastrula, but not in the endoderm [Side view]. (C) Bop-1 is expressed throughout the neural plate, PPE and dorsal epidermis [Antero-lateral view]. (D) MGC68939 is expressed in the anterior neural tube, cranial neural crest, PPE and some placodes (e.g., olf) [Antero-lateral view]. (E) At early tail bud, MGC68939 is expressed in placodes (L, oto), the migrating neural crest, somites and tail bud [Side view]. (F) At late tail bud, Bop-1 is expressed in the brain, retina, placodes (L, oto), branchial arches, somites, nephric mesoderm, tail bud and ventral gut [Side view]. (G) At late tail bud stages, MGC68939 staining is very similar to that of Bop-1, ventral gut staining is not detected until larval stages [Side view]. Transverse sections at forebrain (H), hindbrain (I), rostral spinal (J) and more caudal spinal (K) levels demonstrate neural (fb, r, hb, sc), branchial arch, lens, otocyst, somite, nephric mesoderm and ventral gut expression.
Drosophila CG5033 is predicted to function in ribonucleoprotein binding. Vertebrate Bop-1 was first identified as a mouse gastrula cDNA clone that caused morphological changes in the developing Xenopus embryo in a high-through put screen (Chiao et al., 2005). Mouse Bop-1 is implicated in rRNA processing and ribosome biogenesis and plays a role in cell cycle progression (Strezoska et al., 2000; 2002; Rohrmoser et al., 2007); deregulation of human Bop-1 is associated with colorectal tumors (Killian et al., 2006). Xenopus CG5033-related genes are expressed in the same tissues as Six1/Six2 (PPE, placodes, somites, nephric mesoderm), and also in the cranial neural crest, branchial arches and neural tube, suggesting that they may have roles in craniofacial development.
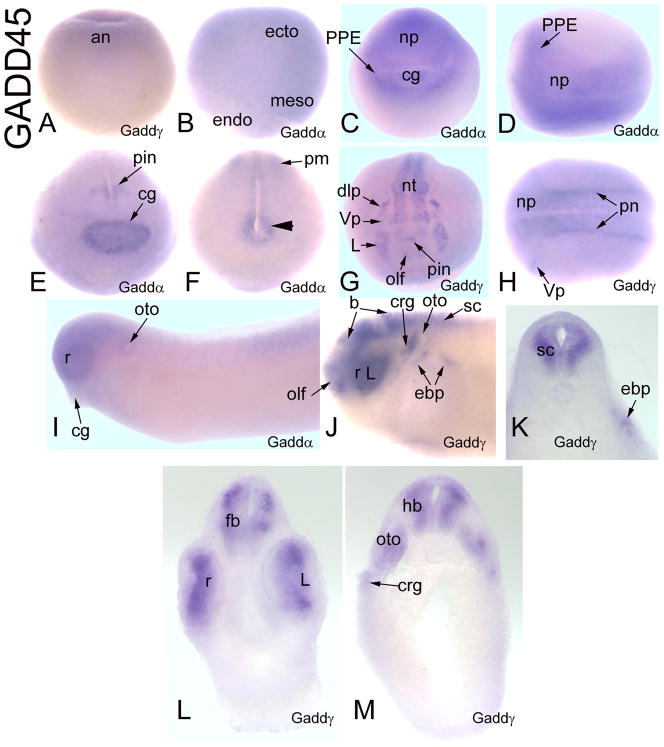
Gadd45-related genes
Xenopus Gadd45α and Gadd45γ have similar early expression patterns that diverge at neural plate stages (Table 2). Maternal mRNAs for Gadd45α or Gadd45γ are not detected. Both are weakly expressed in the animal cap ectoderm of the blastula (Fig. 11A; Table 2), throughout the embryonic ectoderm at early gastrula (Fig. 11B; Table 2) and are dorsally concentrated at late gastrulation (Table 2). At neural plate stages, Gadd45α is strongly expressed throughout the neural plate, PPE and cement gland (Fig. 11C, D). At neural tube closure, Gadd45α is strongly expressed in the cement gland, pineal, peri-cloacal mesoderm and a patch of paraxial mesoderm in the posterior trunk (Fig. 11E, F). In contrast, at neural plate stages, Gadd45γ is expressed in stripes in the neural ectoderm that correspond to the primary neurons and in the trigeminal placode (Fig. 11H; de la Calle-Mustienes et al., 2002), and at neural tube stages it stains discrete patches in the neural tube including pineal, all of the placodes (Fig. 11G) and scattered cells in the epidermis and somites (Table 2). At tail bud stages, Gadd45α staining is weak and diffuse throughout the head and anterior trunk and the cement gland staining is lost (Fig. 11I); at larval stages there is additional staining in the ventral gut (Table 2). In contrast, at tail bud and larval stages Gadd45γ is strongly expressed in the brain, retina, anterior spinal cord, and several placode derivatives (Fig. 11 J–M); expression is detected in somites at late larval stages (Table 2).
Figure 11.
Expression of Gadd45-related genes. (A) Weak expression of Gadd45γ in the animal cap ectoderm of the blastula [Side view]. (B) Gadd45α is diffusely expressed in the ectoderm and non-involuted mesoderm of the gastrula [Side view]. (C) and (D) Gadd45α is expressed throughout the neural plate, PPE and cement gland [C, Anterior view; D, Dorsal view]. At neural tube closure, Gadd45α expression in the anterior part of the embryo (E) is enhanced in the cement gland and pineal, and in the posterior part (F) in a patch of paraxial mesoderm (pm) and in mesoderm surrounding the cloaca (arrowhead). (G) At neural tube stages, Gadd45γ is expressed in the neural tube, including pineal, and multiple placodes (olf, L, Vp, dlp) [Anterior view]. (H) At neural plate Gadd45γ is expressed in stripes of primary neurons (pn) and the trigeminal placode (Vp) [Dorsal view]. (I) At larval stages, Gadd45α expression becomes weak and diffuse throughout the head and anterior trunk, and is lost from the cement gland. [Side view]. (J) At larval stages, Gadd45γ expression is confined to the brain, retina, anterior spinal cord and placode derivatives (olf, L, crg, oto, ebp) [Side view]. Transverse sections at forebrain (L), hindbrain (M), rostral spinal (K) levels demonstrate neural (fb, r, hb, sc) and placodal (L, oto, crg, ebp) expression.
In Drosophila, Gadd45 is involved in JNK signaling during very early developmental processes (Peretz et al., 2007) and in wound healing (Stramer et al., 2008). In humans and mouse, three Gadd45 proteins (α, β, γ) act to inhibit the cell cycle and promote apoptosis (Takekawa and Saito, 1998); Drosophila Gadd45 shares the highest amino acid identity with vertebrate Gadd45γ (Peretz et al., 2007). Loss of murine Gadd45α results in phenotypes similar to p53-null mice (Hollander et al., 1999); loss of murine Gadd45β interferes with activity-induced proliferation of neuronal progenitors (Ma et al., 2009); loss of murine Gadd45γ may affect the development of blood lineages (Lu et al., 2001). In Xenopus embryos, Gadd45γ reduces the number of dividing cells in the neural plate, thus promoting neuronal differentiation (de la Calle-Mustienes et al., 2002). The Xenopus tropicalis genome database annotates a Gadd45β gene (Table 1) but no cDNA clones are currently available. However, in zebrafish Gadd45β is involved in somite segmentation (Kawahara et al., 2005) and mouse Gadd45β is required for chondrocyte terminal differentiation (Ijiri et al., 2005). The Xenopus Gadd45-related genes are expressed in some of the same tissues as Six1/Six2 (PPE, placodes, somites), and also in the neural tube, suggesting that they may have roles in craniofacial development.
Conclusions
This analysis identified 33 Xenopus genes as putative orthologues of 20 of the 25 Drosophila proteins that interact with SO. Of these 33, we found that 3 are not expressed in the same tissues as Six1/Six2 (ESG2, CG33090, VhaAC39), 3 are ubiquitously expressed (RhoGEF2, Chc, RpS27) and 7 are not currently available for ISH study (Eya4, CG9083, CG1218, 2 putative CG5665 orthologues, RhoGAP93B, Gadd45β). However, the remaining Xenopus genes, representing 11 of the Drosophila SO-interacting genes, are expressed in craniofacial tissues, overlapping at least partially with the expression domains of Six1/Six2. It is notable that the Eya, Grg, Sobp, CG1135, CG7878/Ddx43, CG10576/2G4, CG5033, and Gadd45γ genes mostly closely overlapped with Six1/Six2 expression patterns in the embryonic ectoderm, PPE, placodes, somites, and nephric mesoderm. However, they all displayed additional expression domains, indicating that they likely interact with other transcription factors, including perhaps other Six family members. Also, with the exception of the Eya genes, their expression in the dorsal epidermis and PPE domains was much more diffuse that those of Six1/Six2. It also it notable that the expression domains of CG17265/ccd85c, C15 and Gadd45α overlap the least with Six1/Six2, suggesting that they may have more restricted functions in craniofacial development.
Identification of additional proteins that may interact with Six proteins as co-factors is important to fully understand the function of this family of transcription factors. This is particularly relevant because the penetrance of BO and BOR syndromes is variable, and results from Six1 heterozygous mice suggest that there are modifier genes that influence Six1 activity or function to modulate the mutant phenotype (Xu et al., 2003; Ruf et al., 2004). Because mutations in Six and Eya genes only account for about half of the BO and BOR patients, other genes are likely to be involved. Our data demonstrate that a large number of genes identified by sequence homology to putative partners of fly SO are expressed in vertebrate craniofacial tissues during development. We speculate that these may prove to encode for new Six-interacting proteins with important roles in vertebrate developmental processes, particularly in craniofacial development.
Experimental Procedures
Bioinformatics
Putative Xenopus laevis orthologues of the Drosophila genes encoding SO-interacting proteins were identified in BLAST searches using a translated nucleotide Drosophila query to search the translated nucleotide Xenopus laevis database in the NCBI GenBank. If no Xenopus laevis gene was identified, the BLAST search was repeated using the Xenopus tropicalis translated nucleotide database. If Xenopus orthologues were not identified in either of these searches, the Ensembl database was searched to determine if Drosophila-related genes are annotated in the Xenopus tropicalis genome. Amino acid sequence identity, alignment scores and E values were all considered to determine the homology between the Drosophila and Xenopus genes. If a Xenopus laevis gene was identified for the candidate SO-interacting proteins, the Xenopus tropicalis orthologue was not listed in Table 1. The exceptions are CG17265 (ccdc85c) and SOBP; in these cases the X. tropicalis sequence showed a higher amino acid sequence identity to the fly orthologues over the entire coding region than did the X. laevis sequence.
Synthesis of anti-sense RNA probes
Some plasmids were obtained from the research community (Patterson and Kreig, 1999; David et al., 2001; de la Calle-Mustienes et al., 2002; Burks et al., 2009). The other plasmids were purchased from Open BioSystems. If Xenopus laevis cDNAs were available they were used for synthesizing probes. If not, Xenopus tropicalis cDNAs were used and these are specifically identified in the text. Digoxigenin-labeled anti-sense RNA probes were synthesized as per the manufacturer’s instructions (Ambion Megascript kit).
Whole mount in situ hybridization
Xenopus laevis oocytes were surgically removed from gravid females (Sive et al., 2000). Wild type and albino Xenopus laevis embryos were obtained by natural mating of adult pairs as previously described (Moody, 2000). Embryos were staged according to Nieuwkoop and Faber (1994). Specimens were fixed and processed for whole mount in situ hybridization according to standard procedures (Sive et al., 2000). To visualize internal expression, embryos already processed for whole mount in situ hybridization were embedded in 4% agarose and sectioned at 100μm with a vibratome.
Acknowledgments
Grant Information: This work was supported in part by NIH R03 HD055321 (KMN), NSF IOS-0817902 (SAM) and NIH R01 EY1316709 (FP).
We thank Gerhard Schlosser (Eya1), Jose Gomez-Skarmeta (Gadd45γ), Ira Daar (Grg5), Paul Krieg (Hox11L2) and Betsy Pownall (Grg4) for providing plasmids. We also thank Lynne Mied and Pallavi Mhaske for technical assistance.
References
- Abe Y, Oka A, Mizuguchi M, Igarashi T, Ishikawa S, Aburatani H, Yokoyama S, Asahara H, Nagao K, Yamada M, Miyashita T. Eya4, deleted in a case with middle interhemispheric variant of Holoprosencephaly interacts with Six3 both physically and functionally. Human Mutation. 2009;30:946–955. doi: 10.1002/humu.21094. [DOI] [PubMed] [Google Scholar]
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997;15:157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Bai J, Montell D. Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development. 2002;129:5377–5388. doi: 10.1242/dev.00115. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes. I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Bernard OA, Bisson-LeConiat M, Ballerini P, Mauchauffe M, Della Valle V, Monni R, Nguyen Khac F, Mercher T, Penard-Lacronique V, Paturaud P, Gressin L, Hellig Rl, Daniel MT, Lessard M, Berger R. A new recurrent and specific cryptic translocation, t(5;14)(q35;q32), is associated with expression of the Hox11L2 gene in T acute lymphoblastic leukemia. Leukemia. 2001;15:1495–1504. doi: 10.1038/sj.leu.2402249. [DOI] [PubMed] [Google Scholar]
- Bidet Y, Jagla T, Da Ponte JP, Dastugue B, Jagla K. Modifiers of muscle and heart cell fate specification identified by gain-of-function screen in Drosophila. Mech Dev. 2003;120:991–1007. doi: 10.1016/s0925-4773(03)00182-5. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Bui QT, Gray-Board GL, Warrick JM. The Drosophila eyes absent gene directs ectopic eye formation in a pathway conserved between flies and vertebrates. Development. 1997;124:4819–4826. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, Benzer S. Multiple roles of the eyes absent gene in Drosophila. Dev Biol. 1998;196:42–57. doi: 10.1006/dbio.1997.8845. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Moody SA. Induction and specification of vertebrate ectodermal placodes: precursors of the cranial sensory organs. Biol Cell. 2005;97:303–319. doi: 10.1042/BC20040515. [DOI] [PubMed] [Google Scholar]
- Buchou T, Vernet, Blond O, Jensen HH, Pointu H, Olsen BB, Cochet C, Issinger O-G, Boldyreff B. Disruption of the regulatory β subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Bio. 2003;23:908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks PJ, Isaacs HV, Pownall ME. FGF signalling modulates transcriptional repression by Xenopus groucho-related-4. Biol Cell. 2009;101:301–308. doi: 10.1042/BC20080136. [DOI] [PubMed] [Google Scholar]
- Carpenter PB, Dunphy WG. Identification of a novel 81-kDa component of the Xenopus origin of recognition complex. J Biol Chem. 1998;273:24891–24897. doi: 10.1074/jbc.273.38.24891. [DOI] [PubMed] [Google Scholar]
- Chen Z, Montcouquiol M, Caldderon R, Jenkins NA, Copeland NG, Kelley MW, Noben-Trauth K. Jxc1/Sobp, encoding a nuclear zinc finger protein, is critical for cochlear growth, cell fate, and patterning of the Organ of Corti. J Neurosci. 2008;28:6633–6641. doi: 10.1523/JNEUROSCI.1280-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron. 1994;12:977–996. doi: 10.1016/0896-6273(94)90308-5. [DOI] [PubMed] [Google Scholar]
- Chiao E, Leonard J, Dickinson K, Baker JC. High-throughput functional screen of mouse gastrula cDNA libraries reveals new components of endoderm and mesoderm specification. Genome Res. 2005;15:44–53. doi: 10.1101/gr.2993405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury BK, Kim J, Kung HF, Li SS. Cloning and developmental expression of Xenopus cDNAs encoding the Enhancer of split groucho and related proteins. Gene. 1997;195:41–48. doi: 10.1016/s0378-1119(97)00150-9. [DOI] [PubMed] [Google Scholar]
- Christophorou NAD, Bailey AP, Hanson S, Streit A. Activation of Six1 target genes is required for sensory placode formation. Dev Biol. 2009;336:327–336. doi: 10.1016/j.ydbio.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Courey AJ, Jia S. Transcriptional repression: the long and the short of it. Genes Dev. 2001;15:2786–2796. doi: 10.1101/gad.939601. [DOI] [PubMed] [Google Scholar]
- David R, Ahrens K, Wedlich D, Schlosser G. Xenopus Eya1 demarcates all neurogenic placodes as well as migrating hypaxial muscle precursors. Mech Dev. 2001;103:189–192. doi: 10.1016/s0925-4773(01)00355-0. [DOI] [PubMed] [Google Scholar]
- Davidovic L, Bechara E, Gravel M, Jaglin XH, Tremblay S, Sik A, Bardoni B, Khandjian EW. The nuclear micospherule protein 58 is a novel RNA-binding protein that interacts with fragile X mental retardation protein in polyribosomal mRNPs from neurons. Hum Mol Genet. 2006;15:1525–1538. doi: 10.1093/hmg/ddl074. [DOI] [PubMed] [Google Scholar]
- Dear TN, Colledge WH, Carlton MB, Lavenir I, Larson T, Smith AJ, Warren AJ, Evans MJ, Sofroniew MV, Rabbitts TH. The Hox11 gene is essential for cell survival during spleen development. Development. 1995;121:2909–2915. doi: 10.1242/dev.121.9.2909. [DOI] [PubMed] [Google Scholar]
- Dehni G, Liu Y, Husain J, Stifani S. TLE expression correlates with mouse embryonic segmentation, neurogenesis, and epithelial determination. Mech Dev. 1995;53:369–381. doi: 10.1016/0925-4773(95)00452-1. [DOI] [PubMed] [Google Scholar]
- de la Calle-Mustienes E, Glavic A, Modolell J, Gomez-Skarmeta JL. Xiro homeoproteins coordinate cell cycle exit and primary neuron formation by upregulating neuronal-fate repressors and downregulating the cell-cycle inhibitor XGadd45γ. Mech Dev. 2002;119:69–80. doi: 10.1016/s0925-4773(02)00296-4. [DOI] [PubMed] [Google Scholar]
- Depreux FF, Darrow K, Conner DA, Eavey RD, Liberman MC, Seidman CE, Seidman JG. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 2008;118:471–474. doi: 10.1172/JCI32899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I, Mizuno J, Wu H, Song DH, Symes K, Seldin DC. Protein kinase CK2 is required for dorsal axis formation in Xenopus embryos. Dev Bio. 2004;274:110–124. doi: 10.1016/j.ydbio.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Dorner S, Lum L, Kim M, Paro R, Beachy PA, Green R. A genome wide screen for components of the RNAi pathway in Drosophila cultured cells. PNAS. 2006;103:11880–11885. doi: 10.1073/pnas.0605210103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve P, Bovolenta P. cSix4, a member of the six gene family of transcription factors, is expressed during placode and somite development. Mech Dev. 1999;85:161–165. doi: 10.1016/s0925-4773(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Fabrizio JJ, Boyle JM, DiNardo S. A somatic role for eyes absent (eya) and sine oculis (so) in Drosophila spermatocye development. Dev Biol. 2003;258:117–128. doi: 10.1016/s0012-1606(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Neuberg DS, Stauton J, Loh ML, Huard C, Raimondi SC, Pul CH, Downing JR, Gilliland DG, Lander ES, Golub TR, Look AT. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- Fogelgren B, Kuroyama MC, McBratney-Owen B, Spence AA, Malahn LE, Anawati MK, Cabatbat C, Alarcon VB, Marikawa Y, Lozanoff S. Misexpression of Six2 is associated with heritable frontonasal dysplasia and renal hypoplasia in 3H1 Br mice. Dev Dyn. 2008;237:1767–1779. doi: 10.1002/dvdy.21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelgren B, Yang S, Sharp IC, Huckstep OJ, Ma W, Somoponpun SJ, Carlson EC, Uyehara CFT, Lozanoff S. Deficiency in Six2 during prenatal development is associated with reduced nephron number, chronic renal failure, and hypertension in BR/+ adult mice. Am J Renal Physiol. 2009;296:F1166–F1178. doi: 10.1152/ajprenal.90550.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari H, Seo HC, Fjose A, Brandli AW. Molecular cloning and embryonic expression of Xenopus Six homeobox genes. Mech Dev. 2001;101:271–277. doi: 10.1016/s0925-4773(00)00572-4. [DOI] [PubMed] [Google Scholar]
- Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- Grifone R, Demignon J, Giordani J, Niro C, Souil E, Bertin F, Laclef C, Xu PX, Maire P. Eya1 and Eya2 proteins are required somatic myogenesis in the mouse embryo. Dev Biol. 2007;302:602–616. doi: 10.1016/j.ydbio.2006.08.059. [DOI] [PubMed] [Google Scholar]
- Guerra B, Issinger OG. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis. 1999;20:391–408. doi: 10.1002/(SICI)1522-2683(19990201)20:2<391::AID-ELPS391>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hatano M, Roberts CW, Minden M, Crist WM, Korsmeyer SJ. Deregulation of a homeobox gene, Hox11, by the t(10;14) in T cell leukemia. Science. 1991;253:79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- Hatano M, Aoki T, Dezawa M, Yusa S, Iitsuka Y, Koseki H, Taniguchi M, Tokuhisa T. A novel pathogenesis of megacolon in Ncx/Hox11L.1 deficient mice. J Clin Invest. 1997;100:795–801. doi: 10.1172/JCI119593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Reshef R, Davis RJ, Mardon G, Oliver G, Tomarev S, Lassar AB, Tabin CJ. Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 1999;15:3231–3243. doi: 10.1101/gad.13.24.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi Y, Wang Q, Liu Q, Zhang H, Sato N, Greene MI. P78/MCRS1 forms a complex with centrosomal protein Nde1and is essential for cell viability. Oncogene. 2006;25:4937–4946. doi: 10.1038/sj.onc.1209500. [DOI] [PubMed] [Google Scholar]
- Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro TA, Kim KE, Tolosa E, Ashwell JD, Rosenberg MP, Zhan Q, Fernandez-Salguero PM, Morgan WF, Fornace AJ. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–184. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- Hoskins BE, Cramer CH, Silvius D, Zou D, Raymond RM, Orten D, Kimberling WJ, Smith RJH, Weil D, Petit C, Otto EA, Xu PX, Hildebrandt F. Transcription factor SIX5 is mutated in patients with Branchio-Oto-Renal syndrome. Am J Hum Genet. 2007;80:800–804. doi: 10.1086/513322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri K, Zerbini LF, Peng H, Correa RG, Lu B, Walsh N, Zhao Y, Taniguchi N, Huang XL, Otu H, Wang H, Wang JF, Komiya S, Ducy P, Rahman MU, Flavell RA, Gravallese EM, Oettgen P, Libermann TA, Goldring MB. A novel role for GADD45beta as a mediator of MMP-13 gene expression during chondrocyte terminal differentiation. J Biol Chem. 2005;280:38544–38555. doi: 10.1074/jbc.M504202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Watanabe Y, Ohto H, Kawakami K. Molecular interaction and synergistic activation of a promoter by Six, Eya, and Dach proteins mediated through CREB binding protein. Mol Cell Biol. 2002;22:6759–6766. doi: 10.1128/MCB.22.19.6759-6766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T, Ikeda K, Sato S, Hiroshi Yajima H, Kawakami K. Differential expression of Eya1 and Eya2 during chick early embryonic development. Gene Expr Patterns. 2000;8:357–367. doi: 10.1016/j.gep.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Cook SA, Erway LC, Matthews AN, Sanford LP, Paradies NE, Friedman RA. Inner ear and kidney anomalies caused by IAP insertion in an intron of the Eya1 gene in a mouse model of BOR syndrome. Hum Mol Genet. 1999;8:645–653. doi: 10.1093/hmg/8.4.645. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Che YS, Hanaoka R, Takeda H, Dawid IB. Zebrafish GADD45beta genes are involved in somite segmentation. Proc Natl Sci. 2005;102:361–366. doi: 10.1073/pnas.0408726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Ohto H, Ikeda K, Roeder RG. Structure, function and expression of a murine homeobox protein AREC3, a homologue of Drosophila sine oculis gene product, and implication in development. Nucleic Acids Res. 1996;24:303–310. doi: 10.1093/nar/24.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes-structure and function as transcription factors and their roles in development. Bioessays. 2000;22:616–626. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kenyon KL, Li DJ, Clouser C, Tran S, Pignoni F. Fly SIX-type homeodomain proteins Sine oculis and Optix partner with different cofactors during eye development. Dev Dyn. 2005;234:497–504. doi: 10.1002/dvdy.20442. [DOI] [PubMed] [Google Scholar]
- Killian A, Sarafan-Vasseur N, Sesboue R, Le Pessot F, Blanchard F, Lamy A, Laurent M, Flaman JM, Frebourg T. Contribution of the BOP1 gene, located on 8q24, to colorectal tumorigenesis. Genes Chromosomes Cancer. 2006;45:874–881. doi: 10.1002/gcc.20351. [DOI] [PubMed] [Google Scholar]
- Knouff RA. The developmental pattern of ectodermal placodes in Rana pipiens. J Comp Neurol. 1935;62:17–71. [Google Scholar]
- Kobayashi M, Osanai H, Kawakami K, Yamamoto M. Expression of three zebrafish Six4 genes in the cranial sensory placodes and the developing somites. Mech Dev. 2000;98:151–155. doi: 10.1016/s0925-4773(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Nishikawa K, Suzuki T, Yamamoto M. The homeobox protein Six3 interacts with the Groucho corepressor and acts as a transcriptional repressor in eye and forebrain formation. Dev Biol. 2001;232:315–326. doi: 10.1006/dbio.2001.0185. [DOI] [PubMed] [Google Scholar]
- Koop KE, MacDonald LM, Lobe CG. Transcripts of Grg4, a murine groucho-related gene, are detected in adjacent tissues to other murine neurogenic gene homologues during embryonic development. Mech Dev. 1996;59:73–87. doi: 10.1016/0925-4773(96)00582-5. [DOI] [PubMed] [Google Scholar]
- Kowalinski E, Bange G, Bradatsch B, Hurt E, Wild K, Sinning I. The crystal structure of Ebp1 reveals a methionine aminopeptidase fold as binding platform for multiple interactions. FEBS Lett. 2007;581:4450–4454. doi: 10.1016/j.febslet.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Kriebel M, Muller F, Hollemann T. Xeya3 regulates survival and proliferation of neural progenitor cells within the anterior neural plate of Xenopus embryos. Dev Dyn. 2007;236:1526–1534. doi: 10.1002/dvdy.21170. [DOI] [PubMed] [Google Scholar]
- Kumar S, Deffenbacher K, Cremers CW, Van Camp G, Kimberling WJ. Brachio-oto-renal syndrome: identification of novel mutations, molecular characterization, mutation distribution and prospects for genetic testing. Genet Test. 1997;1:243–251. doi: 10.1089/gte.1997.1.243. [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev. 2003;120:669–679. doi: 10.1016/s0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Leon C, Lobe CG. Grg3, a murine Groucho-related gene, is expressed in the developing nervous system and in mesenchyme-induced epithelial structures. Dev Dyn. 1997;208:11–24. doi: 10.1002/(SICI)1097-0177(199701)208:1<11::AID-AJA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Li X, Ohgi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1–Dach–Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Lieberman SL, Ruderman JV. CK2β, which inhibits Mos function, binds to a discrete domain in the N-terminus of Mos. Dev Bio. 2004;268:271–279. doi: 10.1016/j.ydbio.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lin DY, Shih HM. Essential role of the 58-kDa microspherule protein in the modulation of Daxx-dependent transcriptional repression as revealed by nucleolar sequestration. J Biol Chem. 2002;277:25446–25456. doi: 10.1074/jbc.M200633200. [DOI] [PubMed] [Google Scholar]
- Liu YH, Jakobsen JS, Valentin G, Amarantos I, Gilmour DT, Furlong EE. A systemic analysis of Tinman function reveals Eya and JAK-STAT signaling as essential regulators of muscle development. Dev Cell. 2009;16:280–291. doi: 10.1016/j.devcel.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Logan C, Wingate RJ, McKay IJ, Lumsden A. Tlx-1 and Tlx-3 homeobox gene expression in cranial sensory ganglia and hindbrain of the chick embryo: markers of patterned connectivity. J Neurosci. 1998;18:5389–5402. doi: 10.1523/JNEUROSCI.18-14-05389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rios J, Tessmar K, Loosli F, Wittbrodt J, Bovolenta P. Six3 and Six6 activity is modulated by members of the groucho family. Development. 2003;130:185–195. doi: 10.1242/dev.00185. [DOI] [PubMed] [Google Scholar]
- Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–590. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- Lu M, Gong ZY, Shen WF, Ho AD. The tcl-3 proto-oncogene altered by chromosomal translocation in T cell leukemia codes for a homeobox protein. EMBO J. 1991;10:2905–2910. doi: 10.1002/j.1460-2075.1991.tb07840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, Flavell RA, Lu B, Ming GL, Song H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu MG, Linley AJ, Reeder SP, Badoual C, Tartour E, Rees RC, McArdle SE. HAGE, a cancer/testis antigen expressed at the protein level in a variety of cancers. Cancer Immun. 2010;10:2–8. [PMC free article] [PubMed] [Google Scholar]
- Martelange V, De Smet C, De Plaaen E, Lurquin C, Boon T. Identification on a human sarcoma of two new genes with tumor-specific expression. Cancer Res. 2000;60:3848–3855. [PubMed] [Google Scholar]
- Miyasaka H, Choudhury BK, Hou EW, Li SS. Molecular cloning and expression of mouse and human cDNA encoding AES and ESG proteins with strong similarity to Drosophila enhancer of split groucho proteins. Eur J Biochem. 1993;216:343–352. doi: 10.1111/j.1432-1033.1993.tb18151.x. [DOI] [PubMed] [Google Scholar]
- Molenaar M, Brian E, Roose J, Clevers H, Destree O. Differential expression of the Groucho-related genes 4 and 5 during early development of Xenopus laevis. Mech Dev. 2000;91:311–315. doi: 10.1016/s0925-4773(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Moody SA. Cell Lineage Analysis in Xenopus Embryos. In: Tuan RS, Lo CW, editors. Methods in Molecular Biology: Developmental Biology Protocols. Vol. 135. Humana Press; 2000. pp. 1–17. [DOI] [PubMed] [Google Scholar]
- Moody SA. Determination of pre-placodal ectoderm and sensory placodes. In: Moody SA, editor. Principles in Developmental Genetics. Elsevier; NY: 2007. pp. 590–614. [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis. Garland Publishing; 1994. [Google Scholar]
- Nybakken K, Vokes SA, Lin TY, McMahon AP, Perrimon N. A genome-wide RNA interference screen in Drosophila melanogaster cells for new components of the Hh signaling pathway. Nature Genetics. 2005;37:1323–1332. doi: 10.1038/ng1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol. 1999;19:6815–6824. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Watanabe Y, Takahashi K, Kitamura K, Tanaka A, Urase K, Momoi T, Sudo K, Sakagami J, Asano M, Iwakure Y, Kawakami K. Six4, a putative myogenin gene regulator, is not essential for mouse embryonal development. Mol Cell Biol. 2001;21:3343–3350. doi: 10.1128/MCB.21.10.3343-3350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura HO, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131:551–562. doi: 10.1242/dev.00943. [DOI] [PubMed] [Google Scholar]
- Pandur P, Moody S. Xenopus Six1 gene is expressed in neurogenic cranial placodes and maintained in the differentiating lateral lines. Mech Dev. 2000;96:253–257. doi: 10.1016/s0925-4773(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Patterson KD, Krieg PA. Hox11-family genes XHox11 and Xhox11L2 in Xenopus: XHox11L2 expression is restricted to a subset of the primary sensory neurons. Dev Dyn. 1999;214:34–43. doi: 10.1002/(SICI)1097-0177(199901)214:1<34::AID-DVDY4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Peretz G, Bakhrat A, Abdu U. Expression of the Drosophila melanogaster GADD45 homolog (CG11086) affects egg asymmetric development that is mediated by the c-jun N-terminal kinase pathway. Genetics. 2007;177:1691–1702. doi: 10.1534/genetics.107.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister M, Toth T, Thiele H, Haack B, Blin N, Zenner HP, Sziklai I, Nurnberg P, Kupka S. A 4-bp insertion in the eya-homologous region (eyaHR) of Eya4 causes hearing impairment in a Hungarian family linked to DFNA10. Mol Med. 2002;10:607–611. [PMC free article] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Pinna LA, Meggio F. Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog Cell Cycle Res. 1997;3:77–97. doi: 10.1007/978-1-4615-5371-7_7. [DOI] [PubMed] [Google Scholar]
- Raju K, Tang S, Dube ID, Kamel-Reid S, Bryce DM, Brietman ML. Characterization and developmental expression of Tlx-1, the murine homolog of Hox11. Mech Dev. 1993;44:51–64. doi: 10.1016/0925-4773(93)90016-q. [DOI] [PubMed] [Google Scholar]
- Roberts CW, Shutter JR, Korsmeyer S. Hox11 controls the genesis of the spleen. Nature. 1994;368:747–749. doi: 10.1038/368747a0. [DOI] [PubMed] [Google Scholar]
- Roberts CWM, Sonder AM, Lumsden A, Korsmeyer SJ. Developmental expression of Hox11 and specification of splenic cell fate. Am J Pathol. 1995;146:1089–1101. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Soriano J. Brachio-oto-renal syndrome. J Nephrol. 2003;16:603–605. [PubMed] [Google Scholar]
- Rohrmoser M, Holzel M, Grimm T, Malamoussi A, Harasim T, Orban M, Pfistere I, Gruber-Eber A, Kremmer E, Eick D. Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit. Mol Cell Biol. 2007;27:3682–3694. doi: 10.1128/MCB.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clever H. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–1178. doi: 10.1038/nature04209. [DOI] [PubMed] [Google Scholar]
- Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RMJ, Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, et al. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci. 2004;101:8090–8095. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Dev Genes Evol. 1999;209:399–410. doi: 10.1007/s004270050270. [DOI] [PubMed] [Google Scholar]
- Sato S, Ikeda K, Shioi G, Ochi H, Ogino H, Yajima H, Kawakami K. Conserved expression of mouse Six1 in the pre-placodal region (PPR) and identification of an enhancer for the rostral PPR. Dev Biol. 2010;344:158–171. doi: 10.1016/j.ydbio.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Dev Biol. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Awtry T, Brugmann SA, Jensen ED, Neilson K, Ruan G, Stammler A, Voelker D, Yan B, Zhang C, Klymkowsky MW, Moody SA. Eya1 and Six1 promote neurogenesis in the cranial placodes in a SoxB1-dependent fashion. Dev Biol. 2008;320:199–214. doi: 10.1016/j.ydbio.2008.05.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G, Ahrens K. Molecular anatomy of placode development in Xenopus laevis. Dev Biol. 2004;271:439–446. doi: 10.1016/j.ydbio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Northcutt RG. Development of neurogenic placodes in Xenopus laevis. J Comp Neurol. 2000;418:121–146. [PubMed] [Google Scholar]
- Schonberger J, Wang L, Shin JT, Kim SD, Depreux FS, Zhu H, Zon L, Pizard A, Kim JB, MacRae CA, Mungall AJ, Seidman JG, Seidman CE. Mutation in the transcriptional coactivator Eya4 causes dilated cardiomyopathy and sensorineural hearing loss. Nature Genetics. 2005;37:418–422. doi: 10.1038/ng1527. [DOI] [PubMed] [Google Scholar]
- Self M, Geng X, Oliver G. Six2 activity is required for the formation of the mammalian pyloric sphincter. Dev Biol. 2009;334:409–417. doi: 10.1016/j.ydbio.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serikaku MA, O’Tousa JE. sine oculis is a homeobox gene required for Drosophila visual system development. Genetics. 1994;138:1137–1150. doi: 10.1093/genetics/138.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa S, Arata A, Onimaru H, Roth KA, Brown GA, Horning S, Arata S, Okumura K, Sasazuki T, Korsmeyer S. Rnx deficiency results in congenital central hypoventilation. Nature Genet. 2000;24:287–290. doi: 10.1038/73516. [DOI] [PubMed] [Google Scholar]
- Silver SJ, Davies EL, Doyon L, Rebay I. Functional dissection of eyes absent reveals new modes of regulation within the retinal determination gene network. Mol Cell Biol. 2003;23:5989–5999. doi: 10.1128/MCB.23.17.5989-5999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sithanandam G, Anderson LM. The ERBB3 receptor in cancer and cancer gene therapy. Cancer Gene Ther. 2008;15:413–448. doi: 10.1038/cgt.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Soker T, Dalke C, Puk O, Floss T, Becker L, Bolle I, Favor J, Hans W, Holter SM, Horsch M, Kallnik M, Kling E, Moerth C, Schrewe A, Stigloher C, Topp S, Gailus-Durner V, Naton B, Beckers J, Fuchs H, Ivandic B, Klopstock T, Schulz H, Wolf E, Wurst W, Bally-Cuif L, de Angelis MH, Graw J. Pleiotropic effects in Eya3 knockout mice. BMC Dev Biol. 2008;8:118–136. doi: 10.1186/1471-213X-8-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DH, Sussman DJ, Seldin DC. Endogenous protein kinase CK2 participates in Wnt signaling in mammary epithelial cells. J Biol Chem. 2000;275:23790–23797. doi: 10.1074/jbc.M909107199. [DOI] [PubMed] [Google Scholar]
- Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci U S A. 1998;95:14220–14225. doi: 10.1073/pnas.95.24.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B, Winfield M, Shaw T, Millard TH, Woolner S, Martin P. Gene induction following wounding of the wild-type versus macrophage-deficient Drosophila embryos. EMBO Rep. 2008;9:465–471. doi: 10.1038/embor.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. Early development of the cranial sensory nervous system: from a common field to individual placodes. Dev Biol. 2004;276:1–15. doi: 10.1016/j.ydbio.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Strezoska Z, Pestov DG, Lau LF. Bop1 is a mouse WD40 repeat nucleolar protein involved in 28S and 5.8S rRNA processing and 60S ribosome biogenesis. Mol Cell Biol. 2000;20:5516–5528. doi: 10.1128/mcb.20.15.5516-5528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strezoska Z, Pestov DG, Lau F. Functional inactivation of the mouse nucleolar protein Bop1 inhibits multiple steps in pre-rRNA processing and blocks cell cycle progression. J Biol Chem. 2002;277:29617–29625. doi: 10.1074/jbc.M204381200. [DOI] [PubMed] [Google Scholar]
- Takewawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95:521–530. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- Tessmar K, Loosli F, Wittbrodt J. A screen for co-factors of Six3. Mech Dev. 2002;117:103–113. doi: 10.1016/s0925-4773(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Uchiyama K, Otsuka R, Hanaoka K. cHox11L2, a Hox11 related gene, is expressed in the peripheral nervous system and subpopulation of the spinal cord during chick development. Neurosci Letters. 1999;273:97–100. doi: 10.1016/s0304-3940(99)00637-0. [DOI] [PubMed] [Google Scholar]
- Vincent C, Kalatzis V, Abdelhak S, Chaib H, Compain S, Helias J, Vaneecloo FM, Petit C. BOR and BO syndromes are allelic defects of EYA1. Eur J Hum Genet. 1997;5:242–246. [PubMed] [Google Scholar]
- Wayne S, Robertson NG, DeClau F, Chen N, Verhoeven K, Prasad S, Tranebjarg L, Morton CC, Ryan AF, Van Camp G, Smith RJ. Mutations in the transcriptional activator Eya4 cause late-onset deafness at the DFN10 locus. Hum Mol Genet. 2001;10:195–200. doi: 10.1093/hmg/10.3.195. [DOI] [PubMed] [Google Scholar]
- Webb JF, Noden DM. Ectodermal placodes: contributions to the development of the vertebrate head. Amer Zool. 1993;33:434–447. [Google Scholar]
- Wilhelm V, Rojas P, Gatica M, Allende CC, Allende JE. Expression of the subunits of protein kinase CK2 during oogenesis in Xenopus laevis. Eur J Biochem. 1995;232:671–676. doi: 10.1111/j.1432-1033.1995.671zz.x. [DOI] [PubMed] [Google Scholar]
- Willert K, Brink M, Wodarz A, Varmus H, Nusse R. Casein kinase 2 associates with and phosphorylates disheveled. EMBO J. 1997;16:3089–3096. doi: 10.1093/emboj/16.11.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Lessor TJ, Zhang Y, Woodford N, Hamburger AW. Analysis of the expression pattern of Ebp1, an ErbB-3-binding protein. Biochem Biophys Res Comm. 2001;289:240–244. doi: 10.1006/bbrc.2001.5942. [DOI] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet. 1999;23:113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Xu PX, Cheng J, Epstein JA, Maas RL. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci. 1997;94:11974–11979. doi: 10.1073/pnas.94.22.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–3094. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Laclef C, Maire P, Maas RL, Peters H, Xu X. Eya1 is required for the morphogenesis of mammalian thymus, parathyroid and thyroid. Development. 2002;129:3033–3044. doi: 10.1242/dev.129.13.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaklichkin S, Steiner AB, Lu Q, Kessler DS. FoxD3 and Grg4 physically interact to repress transcriptional and induce mesoderm in Xenopus. J Biol Chem. 2007;282:2548–2557. doi: 10.1074/jbc.M607412200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Wang XW, Rishi AK, Lessor T, Xia XM, Gustafson TA, Hamburger AW. Interaction of the PA2G4 (EBP1) protein with ErbB-3 and regulation of this binding by heregulin. Br J Cancer. 2000;82:683–690. doi: 10.1054/bjoc.1999.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu Y, Zhou H, Lee M, Liu Z, Hassel BA, Hamburger AW. Alterations in cell growth and signaling in ErbB3 binding protein-1 (Ebp1) deficient mice. BMC Cell Biol. 2008;9:69–81. doi: 10.1186/1471-2121-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CC, Dyer MA, Uchikawa M, Kondoh H, Lagutin OV, Oliver G. Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development. 2002;129:2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]