Abstract
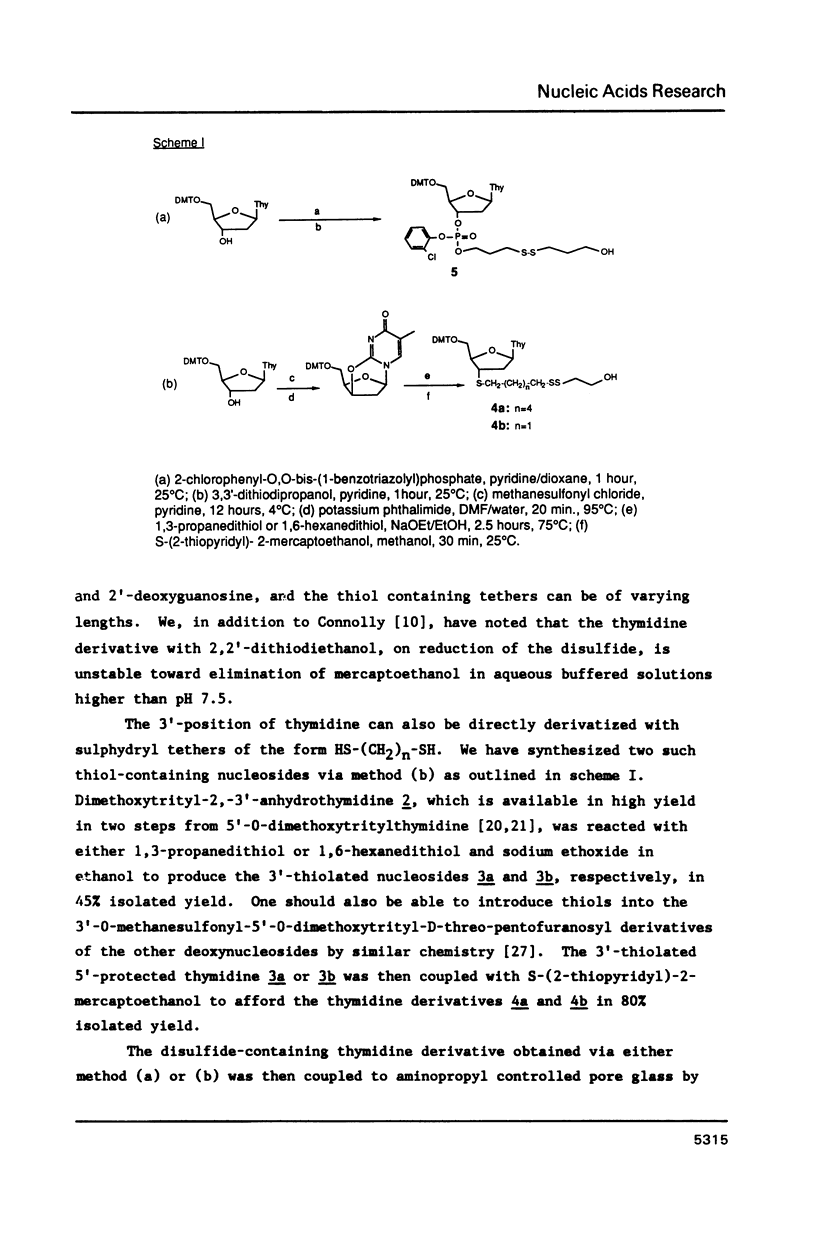
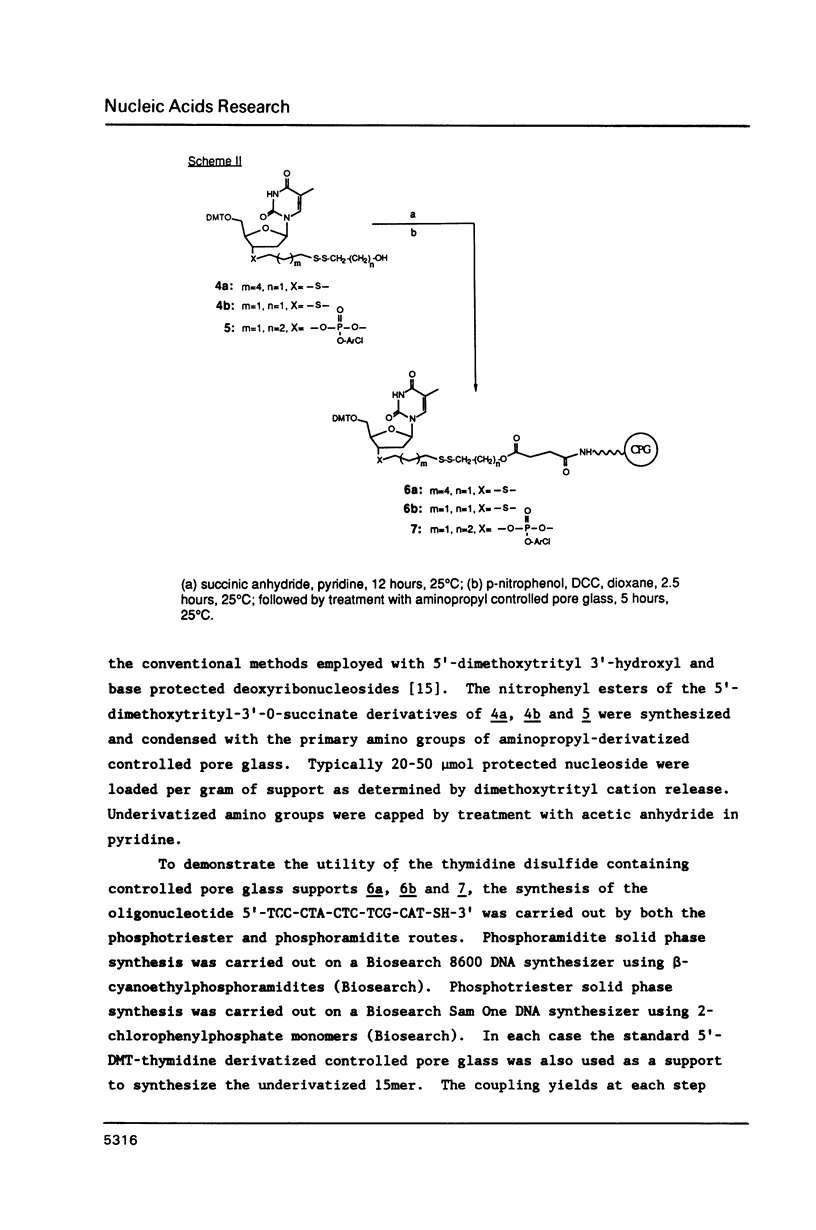
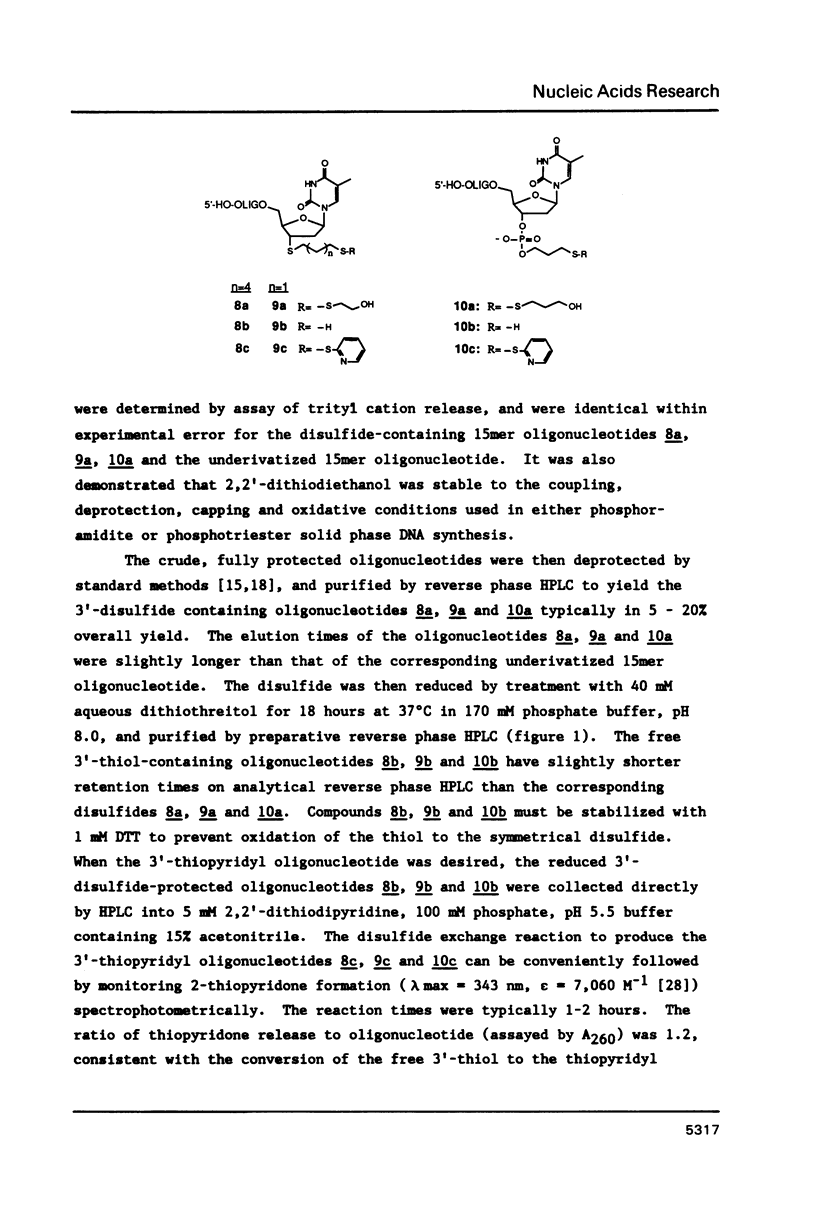
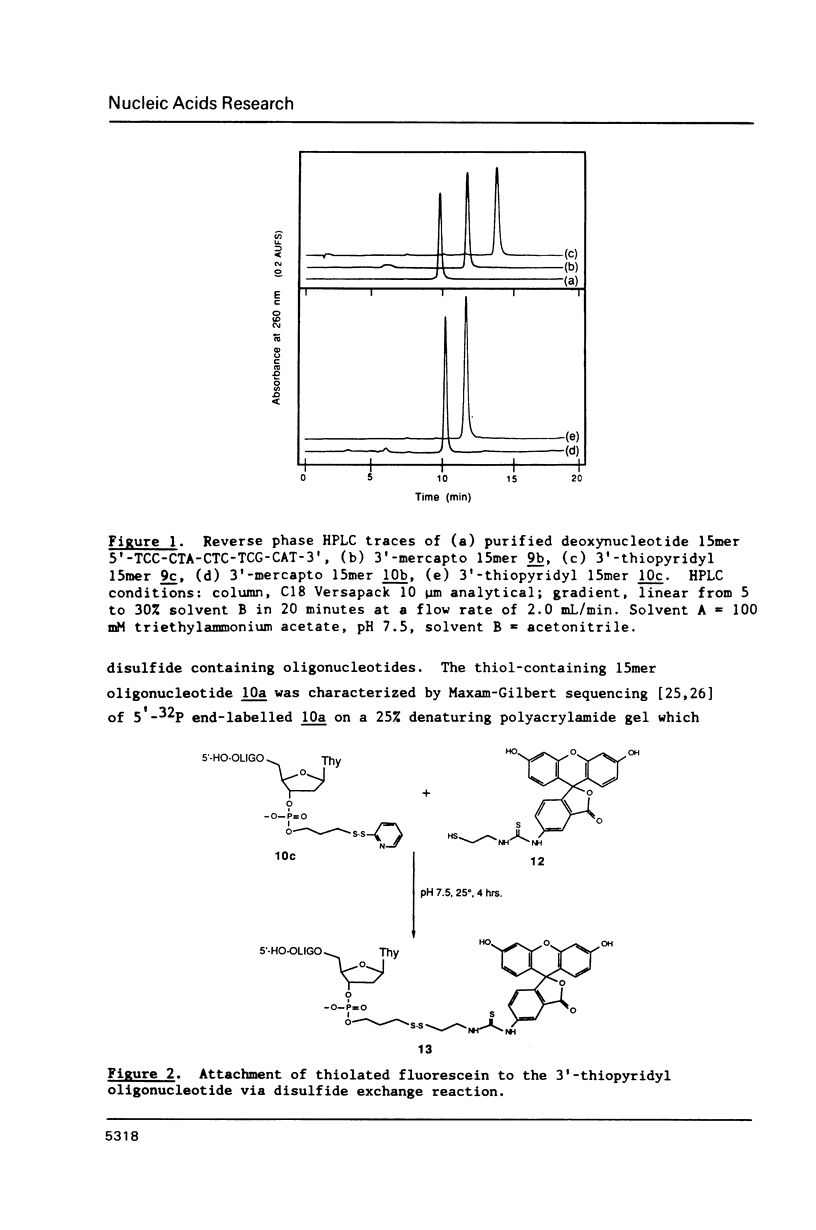
Methodology is described for the synthesis of DNA oligomers containing a free 3'-thiol group which can be selectively crosslinked with a wide variety of probes. This chemistry is compatible with both phosphotriester and phosphoramidite solid phase chemistry. Moreover, the sulphydryl group is introduced into the 3'-nucleoside solid support linkage prior to oligonucleotide synthesis. Consequently, no additional coupling steps are required after oligonucleotide synthesis, and isolation of the 3'-thiol oligonucleotide requires only one additional deprotection step. Cross-linking of the thiol-containing oligonucleotide to a fluorescent probe was carried out with high selectivity, in high yield, and under mild conditions.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Christodoulou C., Gait M. J. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Aug 11;14(15):6227–6245. doi: 10.1093/nar/14.15.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet A., Kawashima E. H. Biotin-labeled synthetic oligodeoxyribonucleotides: chemical synthesis and uses as hybridization probes. Nucleic Acids Res. 1985 Mar 11;13(5):1529–1541. doi: 10.1093/nar/13.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Detection of specific DNA sequences with short biotin-labeled probes. DNA. 1985 Aug;4(4):327–331. doi: 10.1089/dna.1985.4.327. [DOI] [PubMed] [Google Scholar]
- Chu B. C., Wahl G. M., Orgel L. E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983 Sep 24;11(18):6513–6529. doi: 10.1093/nar/11.18.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A., Rider P. Chemical synthesis of oligonucleotides containing a free sulphydryl group and subsequent attachment of thiol specific probes. Nucleic Acids Res. 1985 Jun 25;13(12):4485–4502. doi: 10.1093/nar/13.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosstick R., McLaughlin L. W., Eckstein F. Fluorescent labelling of tRNA and oligodeoxynucleotides using T4 RNA ligase. Nucleic Acids Res. 1984 Feb 24;12(4):1791–1810. doi: 10.1093/nar/12.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassetti D. R., Murray J. F., Jr Determination of sulfhydryl groups with 2,2'- or 4,4'-dithiodipyridine. Arch Biochem Biophys. 1967 Mar;119(1):41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- Hampton A., Kappler F., Chawla R. R. Design of species- or isozyme-specific enzyme inhibitors. 1. Effect of thymidine substituents on affinity for the thymidine site of hamster cytoplasmic thymidine kinase. J Med Chem. 1979 Jun;22(6):621–631. doi: 10.1021/jm00192a005. [DOI] [PubMed] [Google Scholar]
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Kempe T., Sundquist W. I., Chow F., Hu S. L. Chemical and enzymatic biotin-labeling of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Jan 11;13(1):45–57. doi: 10.1093/nar/13.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. S., Mancini W. R. Synthesis and antineoplastic activity of 3'-azido and 3'-amino analogues of pyrimidine deoxyribonucleoside. J Med Chem. 1983 Apr;26(4):544–548. doi: 10.1021/jm00358a016. [DOI] [PubMed] [Google Scholar]
- Murasugi A., Wallace R. B. Biotin-labeled oligonucleotides: enzymatic synthesis and use as hybridization probes. DNA. 1984 Jun;3(3):269–277. doi: 10.1089/dna.1.1984.3.269. [DOI] [PubMed] [Google Scholar]
- Richardson R. W., Gumport R. I. Biotin and fluorescent labeling of RNA using T4 RNA ligase. Nucleic Acids Res. 1983 Sep 24;11(18):6167–6184. doi: 10.1093/nar/11.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Fung S., Hunkapiller M. W., Hunkapiller T. J., Hood L. E. The synthesis of oligonucleotides containing an aliphatic amino group at the 5' terminus: synthesis of fluorescent DNA primers for use in DNA sequence analysis. Nucleic Acids Res. 1985 Apr 11;13(7):2399–2412. doi: 10.1093/nar/13.7.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter L., Jablonski J. A., Ramachandran K. L. A simple and efficient procedure for the synthesis of 5'-aminoalkyl oligodeoxynucleotides. Nucleic Acids Res. 1986 Oct 24;14(20):7985–7994. doi: 10.1093/nar/14.20.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]


