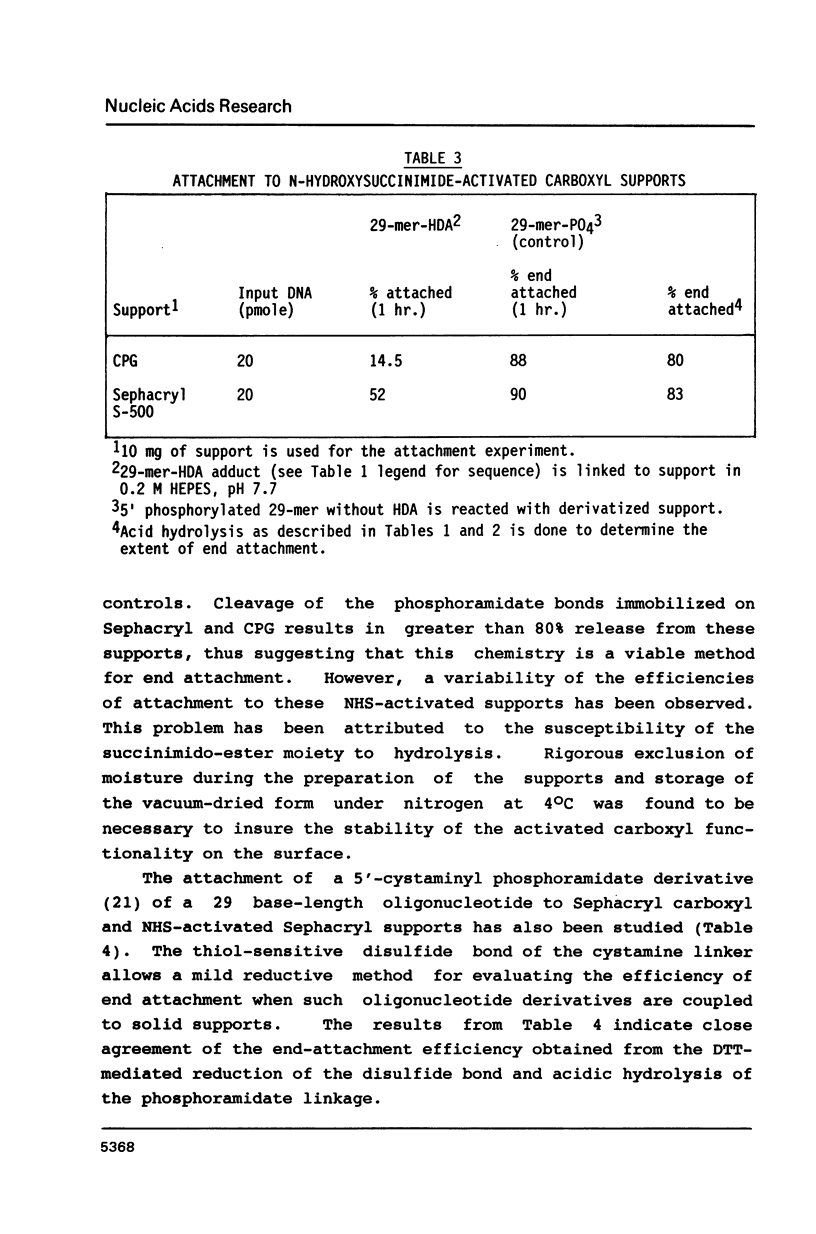
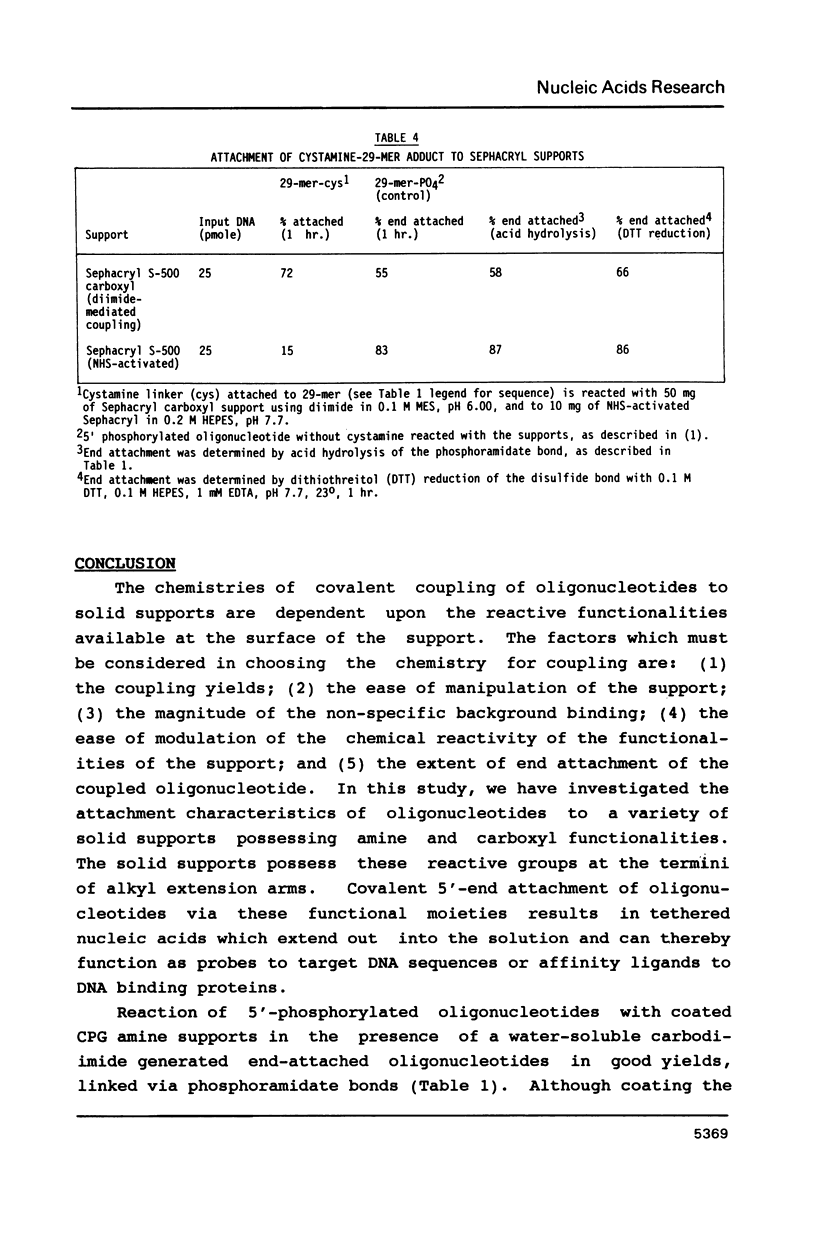
Abstract
Coupling efficiencies for the covalent attachment of oligonucleotides (17-29 bases in length) to solid supports derivatized with alkyl-amino and -carboxylic functionalities have been determined. Attachment efficiencies of 60-80% were obtained for coated long-chain alkylamino controlled pore glass (CPG) supports. Similar efficiencies of immobilization were observed for carboxyl-bearing supports, which additionally exhibited lower levels of non-covalent binding. The extent of terminally linked oligonucleotide was determined to be 50-55% of the overall attachment in the carbodiimide-mediated coupling reaction of a 5'-aminohexyl phosphoramidate derivative of a 29-mer to Sephacryl carboxyl support. While lower overall efficiencies of attachment were obtained in the reaction with Sephacryl N-hydroxysuccinimide-activated carboxyl support, greater than 80% of this coupling results in end-attached oligonucleotides.
Full text
PDF








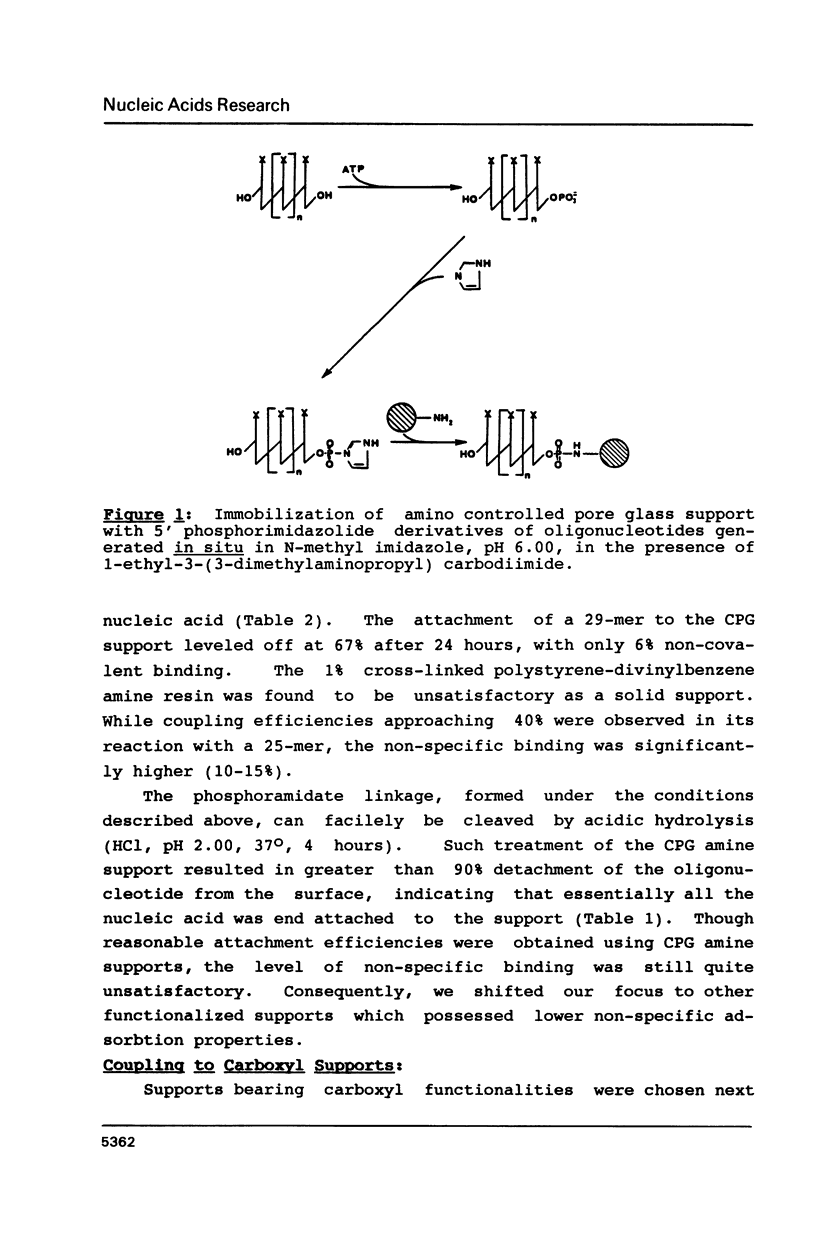
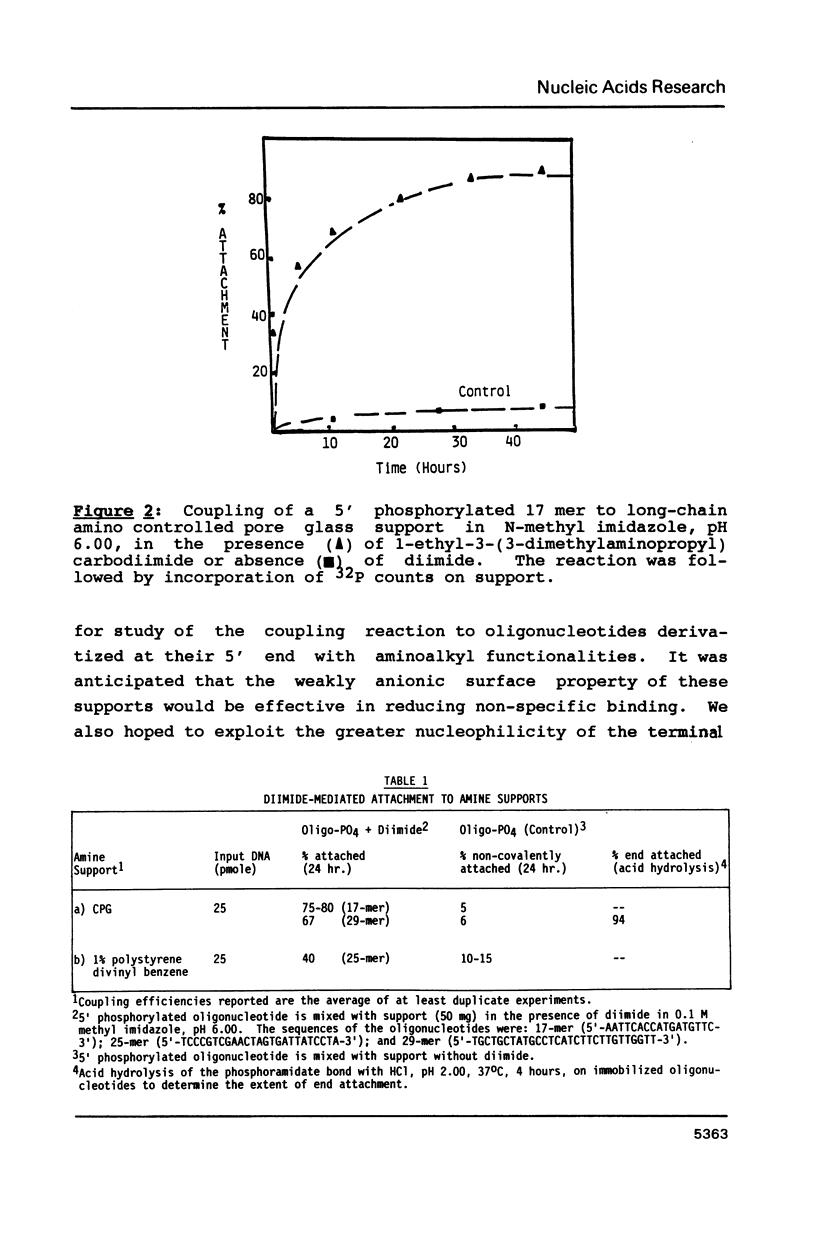
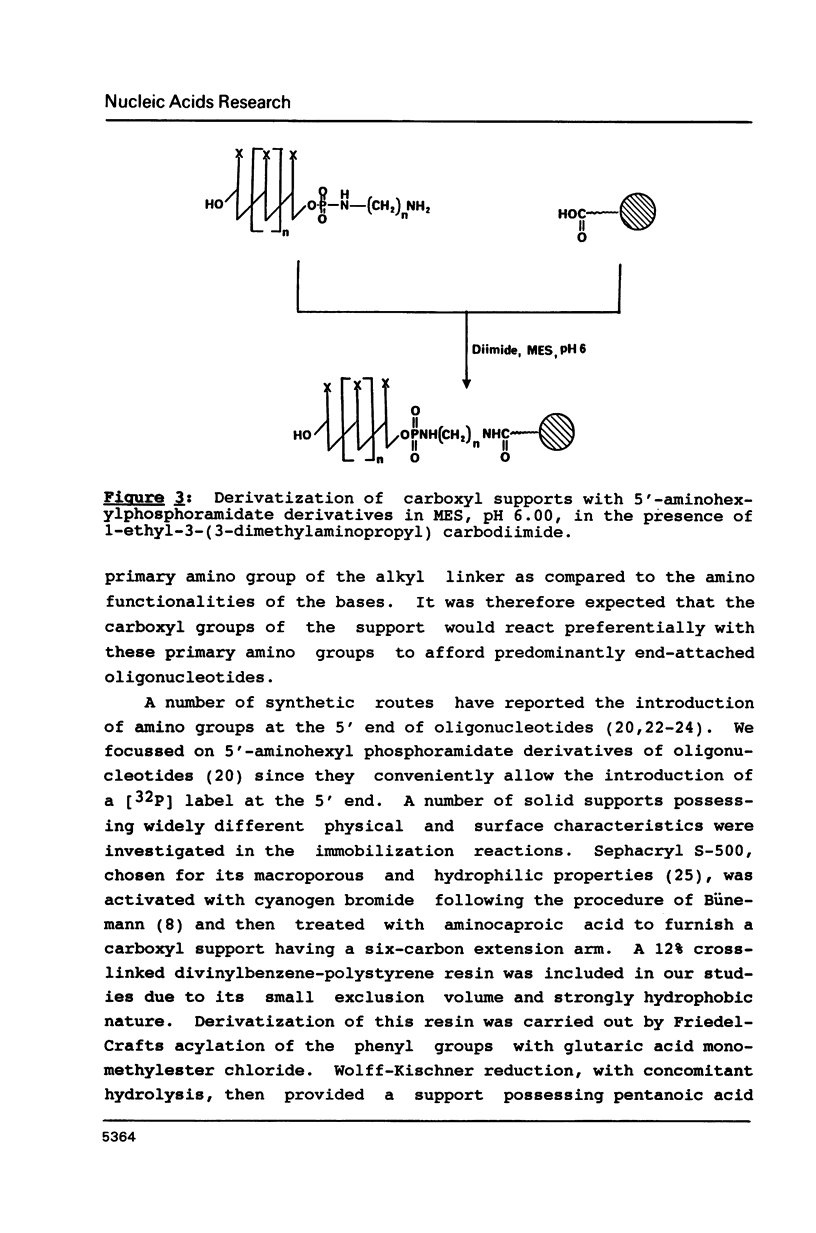

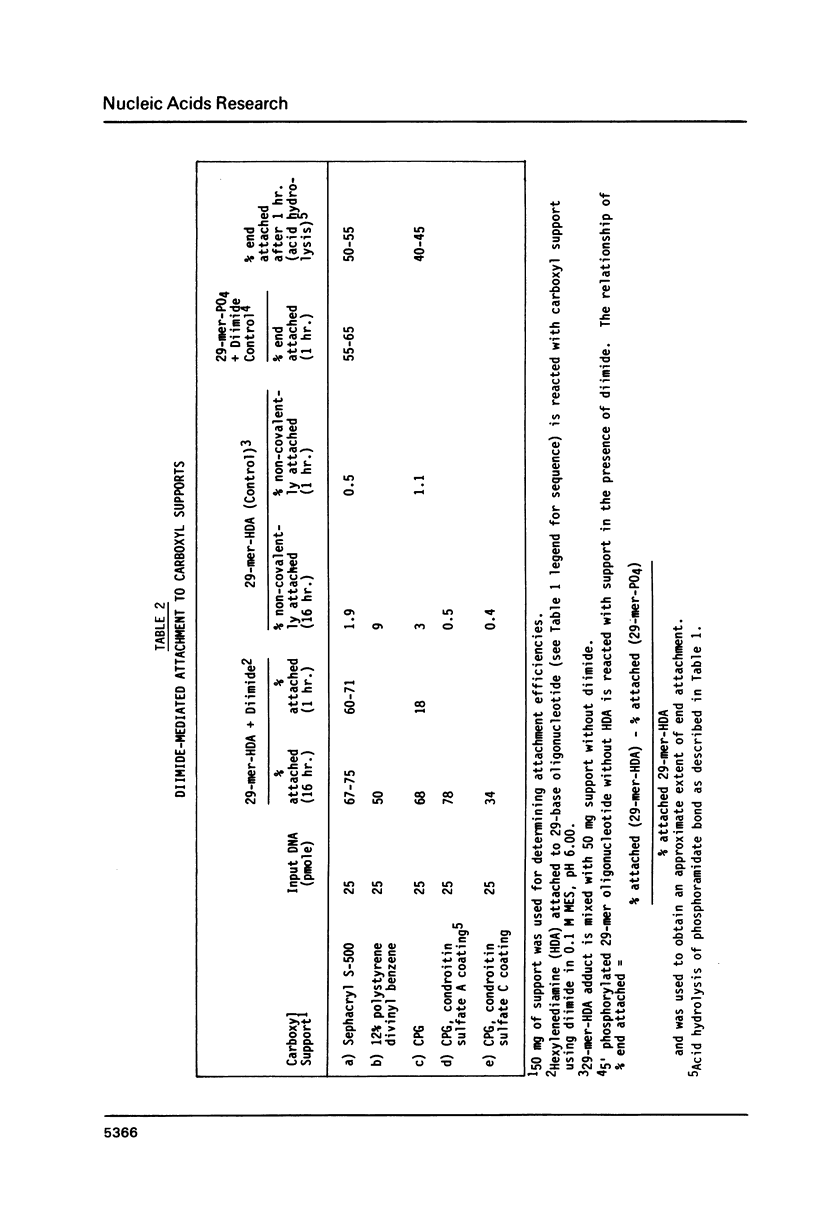




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Christodoulou C., Gait M. J. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Aug 11;14(15):6227–6245. doi: 10.1093/nar/14.15.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Jovin T. M., Bähr W., Frischauf A. M., Marquardt M. Covalent attachment of DNA to agarose. Improved synthesis and use in affinity chromatography. Eur J Biochem. 1975 Jun;54(2):411–418. doi: 10.1111/j.1432-1033.1975.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Bayer E., Breitmaier E., Jung G., Parr W. Synthese des C-terminalen Hexapeptidamids von Secretin an einem neuen festen Träger. Hoppe Seylers Z Physiol Chem. 1971 May;352(5):759–760. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bridson P. K., Orgel L. E. Catalysis of accurate poly(C)-directed synthesis of 3'-5'-linked oligoguanylates by Zn2+. J Mol Biol. 1980 Dec 25;144(4):567–577. doi: 10.1016/0022-2836(80)90337-x. [DOI] [PubMed] [Google Scholar]
- Bünemann H., Westhoff P., Herrmann R. G. Immobilization of denatured DNA to macroporous supports: I. Efficiency of different coupling procedures. Nucleic Acids Res. 1982 Nov 25;10(22):7163–7180. doi: 10.1093/nar/10.22.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet A., Kawashima E. H. Biotin-labeled synthetic oligodeoxyribonucleotides: chemical synthesis and uses as hybridization probes. Nucleic Acids Res. 1985 Mar 11;13(5):1529–1541. doi: 10.1093/nar/13.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Kramer F. R., Orgel L. E. Synthesis of an amplifiable reporter RNA for bioassays. Nucleic Acids Res. 1986 Jul 25;14(14):5591–5603. doi: 10.1093/nar/14.14.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Detection of specific DNA sequences with short biotin-labeled probes. DNA. 1985 Aug;4(4):327–331. doi: 10.1089/dna.1985.4.327. [DOI] [PubMed] [Google Scholar]
- Chu B. C., Wahl G. M., Orgel L. E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983 Sep 24;11(18):6513–6529. doi: 10.1093/nar/11.18.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A. The synthesis of oligonucleotides containing a primary amino group at the 5'-terminus. Nucleic Acids Res. 1987 Apr 10;15(7):3131–3139. doi: 10.1093/nar/15.7.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisin B. F. The monitoring of reactions in solid-phase peptide synthesis with picric acid. Anal Chim Acta. 1972 Jan;58(1):248–249. doi: 10.1016/S0003-2670(00)86882-8. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Langdale J. A., Malcolm A. D. A rapid method of gene detection using DNA bound to Sephacryl. Gene. 1985;36(3):201–210. doi: 10.1016/0378-1119(85)90175-1. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Panet A., Khorana H. G. Studies on polynucleotides. The linkage of deoxyribopolynucleotide templates to cellulose and its use in their replication. J Biol Chem. 1974 Aug 25;249(16):5213–5221. [PubMed] [Google Scholar]
- Siddell S. G. RNA hybridization to DNA coupled with cyanogen-bromide-activated sephadex. The purification of polyoma messenger RNA. Eur J Biochem. 1978 Dec;92(2):621–629. doi: 10.1111/j.1432-1033.1978.tb12785.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]


