Abstract
Background
Effects of d,l-sotalol at therapeutic concentrations (≤10 mg/L) on wavefront dynamics during ventricular fibrillation (VF) and electrophysiological heterogeneity remain unclear.
Methods and Results
By using an optical mapping system, epicardial activation patterns of VF were studied in 6 Langendorff-perfused rabbit hearts at baseline, during 10 mg/L d,l-sotalol infusion, and after washout. An additional 4 hearts, action potential duration (APD), conduction velocity, and wavelength (WL) restitutions were determined. During d,l-sotalol infusion, VF was terminated in 3 of the 6 hearts. Only one experienced a transient ventricular tachycardia (VT). d,l-Sotalol reduced the number of phase singularities (i.e., wavebreak) during VF (p<0.05). d,l-Sotalol also increased the occurrence frequency (p<0.05) and life span (p<0.05) of epicardial reentry during VF. These reentries were non-stationary in nature and did not anchor on anatomical structures. Restitution data showed that d,l-Sotalol flattened APD restitution. Furthermore, APD dispersion and spatial heterogeneity of restitutions were not enhanced by d,l-sotalol.
Conclusions
d,l-Sotalol at therapeutic concentrations decreased wavebreak and facilitated the occurrence of long-lasting non-stationary reentry during VF. However, VT rarely occurred. The related mechanisms include: (1) APD restitution flattening without enhancement of spatial heterogeneity of electrophysiological properties causes wavefront organization, and (2) WL prolongation prevents a steady anchoring of reentry.
Keywords: d,l-sotalol; reentry; ventricular fibrillation
On the basis of the ESVEM trial, d,l-sotalol has been approved for the treatment of life-threatening ventricular tachycardia (VT) and ventricular fibrillation (VF).1,2 Previous studies have shown that d,l-sotalol reduced the complexity of epicardial activation patterns and prolonged wavelength (WL) during VF in isolated rabbit hearts.3 Recently, Pak et al. reported that d,l-sotalol at therapeutic doses (≤10 mg/L) effectively terminated VF/VT by flattening action potential duration restitution (APDR) in isolated swine ventricles.4 However, the effects of d,l-sotalol at therapeutic concentrations on the wavefront characteristics of VF and the genesis of electrophysiological heterogeneity (such as APD dispersion and spatial heterogeneity of restitutions) are still not completely understood.
We have previously demonstrated that 2 types of VF exist in the same isolated rabbit heart.5 As APDR was flattened by low dose methoxyverapamil (D600), the multiple-wavelet type 1 VF was converted to VT. A further increase of D600 concentration converted VT to a slower (type 2) VF with a stationary or slow drifting mother rotor. During type 2 VF, the anatomical structure, papillary muscle (PM), always served as an anchoring site for the mother rotor.6 We hypothesized that d,l-sotalol at therapeutic concentrations, with both the effects of APDR flattening and WL prolongation, can also convert a pre-existing type 1 VF into a regular rhythm, finally leading to the termination of ventricular tachyarrhythmia. To test this hypothesis, an optical mapping system was used to record epicardial activation patterns during d,l-sotalol infusion. The aims of this study were to determine (1) whether or not acute administration of d,l-sotalol can effectively convert a pre-existing VF into VT before its termination, and (2) the wavefront characteristics of VF and the electrophysiological heterogeneity during d,l-sotalol infusion with therapeutic concentrations in isolated rabbit hearts.
Methods
This research protocol was approved by the Institutional Animal Care and Use Committee of Taichung Veterans General Hospital and followed the guidelines of American Heart Association.
Langendorff Preparation and Pseudo-ECG Recordings
Ten New Zealand white rabbits (2.7 to 4.1 kg) were studied. The hearts were excised under general anesthesia. The ascending aorta was immediately cannulated and perfused with 36.5°C Tyrode’s solution with the following composition (in mM): 125 NaCl, 4.5 KCl, 0.5 MgCl2, 24 NaHCO3, 1.8 NaH2PO4, 1.8 CaCl2, 5.5 glucose, and albumin (40 mg/L), then equilibrated with 95% O2 and 5% CO2 to maintain a pH of 7.4.7 Coronary perfusion pressure was then regulated with a flow rate of 25 to 30 mL/minute.5,6 Afterwards, the hearts were perfused and superfused in a thermostatized tissue bath made of transparent glass. The bath temperature remained at a constant value within a range of 36°C to 37°C during the experiment. A pseudo-ECG was obtained with widely spaced bipoles, one at the apex of the left ventricle (LV) and the other at the high lateral wall of the right ventricle (RV). The signals were filtered from 0.05 to 100 Hz, and were digitized by use of an AxoScope with a sampling rate of 1 kHz.5–8 The pseudo-ECG was used to determine the rhythm of the ventricles.
Optical Mappings
A single-camera optical mapping system was used and has been described previously.5,6 The hearts were stained with di-4-ANEPPS (Molecular Probes, Eugene, OR, USA). They were then excited with quasi-monochromatic light (500±40 nm) from a 250-W tungsten-halogen lamp. Fluorescent and scattered light from the heart was collected by an image-intensified charge-coupled device camera (Dalsa Inc., Waterloo, Ontario, Canada). The optical signals were gathered at 3.75-ms sampling intervals, acquired from 100×100 sites simultaneously over a 40×40-mm2 area. For each time window of recording, the optical data were acquired continuously for 2.25 seconds (i.e., 600 frames). Phase mapping was performed to evaluate the wavefront characteristics and the location and evolution of phase singularities (PSs) during VF.9 In a typical time-embedded phase portraits, the upstroke of the action potential corresponds to a phase ranging from −3/4 π to −1/4 π, roughly the light blue color (between the dark blue color and the green color) using color representation.5–8 Optically recorded voltage signals were spatiotemporally filtered to reduce noise.10 The mapped area included parts of the RV and LV anterior walls.
Study Protocols
Protocol I: Effects of d,l-Sotalol at Therapeutic Concentrations on the Wavefront Characteristics and Inducibility of VF (n=6)
A hook bipolar electrode was inserted into the RV outflow tract for pacing.5–8 We used burst pacing (cycle lengths, 75 to 100 ms; currents, 5 to 10 mA) to induce baseline VF. Baseline VF was defined as the stable VF that persisted for 5 minutes after pacing induction.5–8 Three sets of optical mapping data and corresponding pseudo-ECG recordings were obtained during baseline VF. d,l-Sotalol with a concentration of 10 mg/L was then infused for 30 minutes to observe changes in ventricular rhythm, such as VF/VT transition and VF termination. Optical and corresponding pseudo-ECG recordings were obtained every 3 to 5 minutes, including 5, 15, and 25 minutes of d,l-sotalol infusion. If the VF episode persisted without termination for 30 minutes, it was defined as no termination by d,l-sotalol. When the VF episode was successfully converted to spontaneous (sinus/idioventricular) rhythm, 3 burst pacing attempts were immediately performed to determine VF inducibility. VF inducibility was defined as the ratio of the successful VF induction instances to the number of burst pacing attempts.4,11 Successful VF induction was defined as VF persisting for at least 3 minutes after cessation of burst pacing. d,l-Sotalol was then washed out with drug-free Tyrode’s solution for 30 minutes. The VF inducibility after a 30-minute washout was again determined.
Protocol II: Restitution Curves (n=4)
To estimate conduction velocity (CV), the inverse of conduction time (CT−1, cm/s) between 2 epicardial points was measured.5 We used S1 pacing method to determine APD and CT−1 restitutions at baseline and at the end of the 30-minute infusion of different d,l-sotalol concentrations (5 and 10 mg/L) sequentially. APD and CT−1 restitutions were determined using 12 different S1 pacing cycle lengths (500, 400, 300, 250, 200, 180, 160, 150, 140, 130, 120, and 110 ms) in all 4 hearts studied.5 To minimize motion artifacts, an adjustable glass wall was used to compress and restrain hearts during pacing.
Data Analysis
Fast Fourier Transform Analysis
Fast Fourier transforms (FFTs) of pseudo-ECGs (4 seconds in duration) were used to determine the dominant frequency (DF) at 5 different time points of VF in protocol I: (1) baseline VF (no d,l-sotalol), (2) VF after 5-minute d,l-sotalol infusion, (3) VF after 15-minute d,l-sotalol infusion, (4) VF after 25-minute d,l-sotalol infusion, and (5) VF after washout (i.e., the re-induced VF after 30-minute washout).
Epicardial Activation Patterns During VF
We also evaluated the wavefront characteristics of VF at the 5 different time points mentioned above. At each time point, 2 to 3 time windows of optical recording were analyzed in each heart studied. Each time window of optical recording contained 600 phase maps (i.e. 600 frames), which were gathered every 3.75 ms for a total of 2.25 seconds.
To characterize the complexity of wavefront dynamics during VF, the number of PSs in each phase map were counted manually throughout the 600 frames in each optical recording.12–14 The average number of PSs in each phase map was then obtained. PSs were defined as sites with an ambiguous phase surrounded by pixels exhibiting a continuous phase progression from −π to +π.12–14
To evaluate the characteristics of epicardial reentry, such as life span (i.e., rotations) and trajectory of core (i.e., stationary or non-stationary), during different time points of VF, optical mapping data were displayed frame-by-frame.7 In addition, the percentage of recording time containing at least one epicardial reentry was also determined.
Construction of APD and CT−1 Restitution Curves by S1 Pacing Method
The details of constructing APD and CT−1 restitution curves have been reported elsewhere.5 Briefly, one pixel at the center of the 4 quadrants in the mapped area is used to determine the APD70 (sites a through d, see Figure 1C in reference 5).5 APD70 was the APD measured at 70% repolarization, and diastolic interval (DI) was the interval between previous APD70 point and the next initiation point of the action potential.5 An APDR curve was then constructed by plotting means of APD70 (ms) obtained from these 4 sites against different S1 pacing cycle lengths. Because CT−1 at the ventricular surface was inhomogeneous, we used the mean of CT−1 along 4 evenly distributed epicardial lines to construct CT−1 restitution (lines 1 through 4, see Figure 1B in reference 5).5 CT−1 restitution of each heart was constructed by plotting means of CT−1 along these 4 epicardial lines against different S1 pacing cycle lengths. By using the formula APD70 × CT−1 = WL (cm), WL restitutions were constructed.
Determination of Maximum Slope of APDR Curve by S1 Pacing Method
To determine the maximum slope of APDR in each heart, APDR curves of the 4 sampling pixels (a through d) were re-plotted using APD70 against the preceding DI. The maximum slope of APDR in these 4 sites was then calculated by first order exponential fitting with ORIGIN software (Microcal).5 The maximum slope of APDR in each heart was the mean of maximum APDR slopes obtained from these 4 sites at baseline and with different d,l-sotalol concentrations.
Determination of APD Dispersion
APD dispersion (ms) was defined as the difference between maximum and minimum APD70 obtained from every pixel distributed over the mapped area during S1 pacing.
Statistical Analysis
Data are presented as mean ± SD. Paired or unpaired t-tests were used to compare the results of FFT analysis and the characteristics of epicardial reentry at different time points of VF in protocol I. Paired t-tests were used to evaluate the effects of d,l-sotalol on the APD, CT−1, and WL in Protocol II (see Table). We also used ANOVA with repeated measurements to compare the results of the maximum slope of APDR, APD dispersion, and spatial heterogeneity of restitutions at baseline and d,l-sotalol (5, 10 mg/L) infusion.5 A probability value of P≤0.05 was considered significant.
Table.
Effects of d,l-sotalol on the APD, CT−1 and WL.
| d,l-sotalol, mg/L | S1 PCL, ms |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 500 | 400 | 300 | 250 | 200 | 180 | 160 | 150 | 140 | 130 | 120 | 110 | |
| APD70, (ms) | ||||||||||||
| Baseline | 162±18 | 154±15 | 147±11 | 140±13 | 120±3 | 103±14 | 98±13 | 93±12 | 88±13 | 79±10 | 78±13 | 70±11 |
| 5 | 191±12† | 185±14† | 164±10† | 149±12† | 130±8 | 118±6 | 114±20 | 103±11 | 95±14 | 101±6 | 93±0* | 80±0* |
| 10 | 223±15‡ | 213±15‡ | 197±12‡ | 182±17† | 139±10 | 133±5§ | 126±3§ | 112±1§ | 105±4§ | 102±5§ | NA | NA |
| CT−1, (cm/s) | ||||||||||||
| Baseline | 66±10 | 66±11 | 64±12 | 63±12 | 61±12 | 61±10 | 61±10 | 60±10 | 57±9 | 57±9 | 56±9 | 53±9 |
| 5 | 69±6 | 69±7 | 68±7 | 68±7 | 67±9 | 65±7 | 64±7 | 63±7 | 62±7 | 65±5 | 68±0* | 68±0* |
| 10 | 68±9 | 67±10 | 67±10 | 66±11 | 70±11 | 72±4§ | 72±4§ | 72±4§ | 69±3§ | 68±3§ | NA | NA |
| WL (cm) | ||||||||||||
| Baseline | 10.6±0.2 | 10.0±0.2 | 9.7±2.2 | 8.7±1.6 | 7.4±1.0 | 6.4±1.5 | 6.0±1.1 | 5.6±1.5 | 5.1±1.2 | 4.5±1.1 | 4.4±1.2 | 3.7±1.0 |
| 5 | 13.5±1.5† | 12.7±1.6 | 11.1±1.1 | 10.0±1.2 | 8.7±1.2 | 7.8±1.0 | 7.3±1.8 | 6.5±1.3 | 6.0±1.3 | 6.5±0.6† | 6.4±0* | 5.5±0* |
| 10 | 15.0±1.0‡ | 14.2±0.9‡ | 13.2±1.6‡ | 11.8±0.8† | 9.8±1.8† | 9.6±0.2§ | 9.1±0.6§ | 8.2±0.5§ | 7.4±0.5§ | 7.1±0.4§ | NA | NA |
NA indicates data not available. PCL indicates pacing cycle length.
data from heart #3 of protocol II without statistical comparison.
data from hearts #3 and #4 of protocol II without statistical comparison.
p<0.05,
p<0.01, by paired t test when compared with baseline.
Results
Protocol I
VF/VT Transition and VF Termination During d,l-Sotalol Infusion
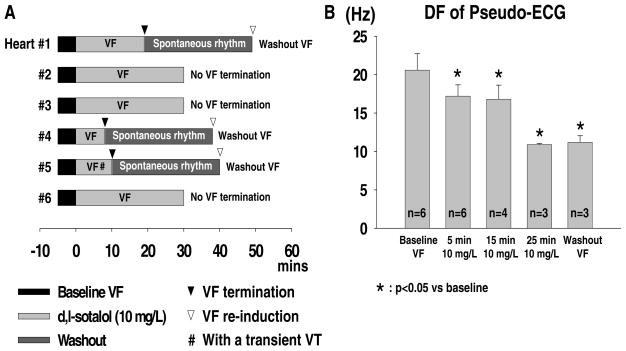
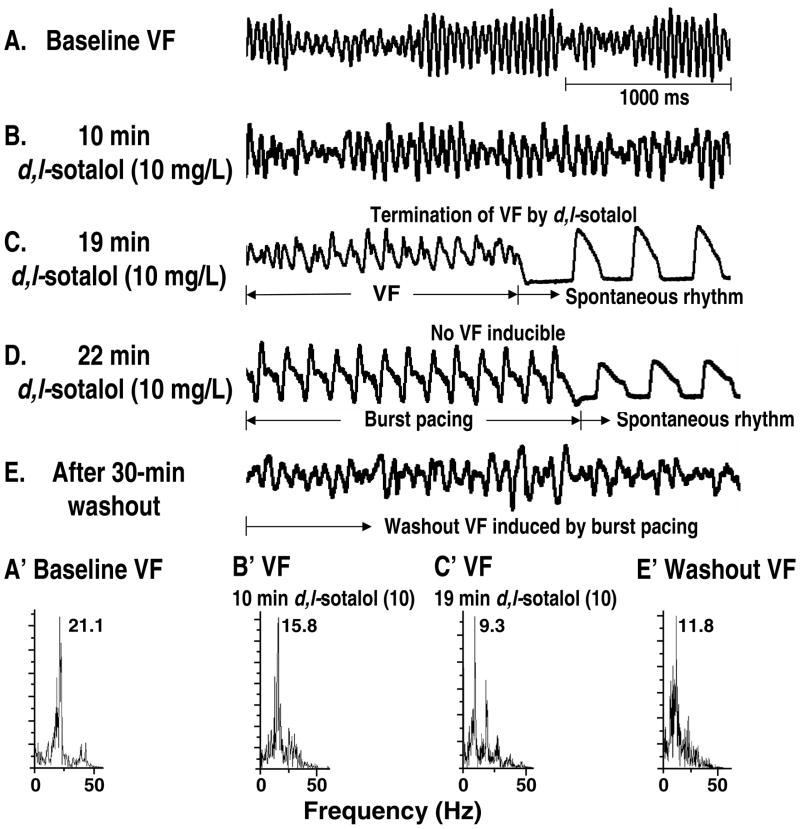
Baseline VF was successfully induced by burst pacing in all 6 hearts studied. With 10 mg/L d,l-sotalol infusion, VF was eventually terminated in 3 (hearts #1, #4, and #5). The mean elapsed time for VF termination was 12.3±5.9 minutes (ranging from 8 to 19 minutes). Two out of these 3 hearts showed abrupt VF termination without a transition to VT. Only the remaining one (heart #5) showed a transient VT (3 minutes in duration) 1 minute after the initiation of d,l-sotalol infusion. This transient VT episode was then followed by a 6-minute VF, which subsequently terminated. The VF inducibility was 0% in all 3 hearts immediately after VF termination. However, the VF inducibility rose to 100% in each of these 3 hearts after the 30-minute washout. Figure 1A summarized effects of 10mg/L d,l-sotalol on VF/VT transition and VF termination with respect to elapsed time. FFT analyses of pseudo-ECG showed that d,l-sotalol significantly reduced mean DF even during the washout period (20.6±2.1, 17.2±1.5*, 16.8±1.8*, 10.9±0.2*, and 11.2±0.9* Hz for baseline, 5-minute, 15-minute, 25-minute d,l-sotalol infusion, and washout, respectively; * indicating p<0.05 when compared with baseline) (see Figure 1B). Figure 2 is a clear example, showing effects of d,l-sotalol on pseudo-ECG and DF in VF. With the infusion of 10 mg/L d,l-sotalol, the DF of pseudo-ECG progressively decreased from 21.1 (baseline, Figures 2A and 2A′) to 15.8 Hz (10-minute d,l-sotalol infusion, Figures 2B and 2B′). Nineteen minutes after the onset of d,l-sotalol infusion, this VF episode abruptly terminated with a DF of 9.3 Hz (Figures 2C and 2C′). Burst pacing immediately after VF termination failed to induce any VF episode (Figure 2D). After washout, burst pacing again easily induced VF with a corresponding DF of 11.8 Hz (Figures 2E and 2E′).
Figure 1.
A, effect of 10 mg/L d,l-sotalol on VF/VT transition and VF termination with respect to elapsed time in all 6 hearts of protocol I. B, effect of 10 mg/L d,l-sotalol on the mean DF of pseudo-ECG in protocol I. “n” indicates the number of hearts studied.
Figure 2.
An example showing effects of d,l-sotalol on pseudo-ECG and DF in VF (data from heart #1). A through E, pseudo-ECG of VF at baseline (A), 10 mg/L d,l-sotalol infusion (B), VF termination (C), VF inducibility test (D), and washout period (E). A′ to E′, DF for corresponding pseudo-ECG tracings in A through E. See text for details.
Epicardial Activation Patterns During VF
Forty-seven time windows of optical recording obtained at 5 different time points of VF were analyzed: (1) baseline VF, 13 windows from 6 hearts; (2) VF after 5-minute d,l-sotalol infusion, 14 windows from 6 hearts; (3) VF after 15-minute d,l-sotalol infusion, 8 windows from 4 hearts; (4) VF after 25-minute d,l-sotalol infusion, 6 windows from 3 hearts; and (5) VF after washout, 6 windows from 3 hearts. In these windows, a total of 149 runs of epicardial reentry (24, 63, 25, 30, and 7 runs for baseline, 5-minute, 15-minute, 25-minute d,l-sotalol infusion, and washout, respectively) were identified and studied.
Number of PSs
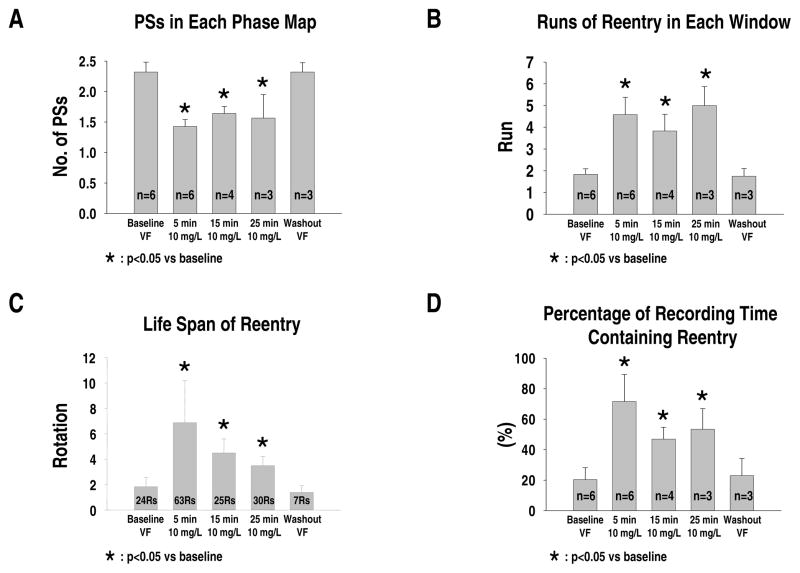
At baseline VF, optical mapping showed multiple wandering wavelets and short-lived reentry. The average number of PSs in each phase map was 2.3±0.2. After d,l-sotalol infusion, the epicardial activation pattern became more organized with the occurrence of long-lasting non-stationary reentry. The average number of PSs in each phase map significantly decreased during d,l-sotalol infusion (1.4±0.1*, 1.6±0.1*, and 1.6±0.3* for 5-minute, 15-minute, and 25-minute d,l-sotalol infusion, respectively; * indicating p<0.05 when compared with baseline). After washout, optical mapping again showed multiple wandering and short-lived wavelets with an average PS number of 2.3±0.1 (p=NS) (see Figure 3A).
Figure 3.
Effects of d,l-sotalol on wavefront characteristics during different time points of VF in protocol I. A, average number of PSs in each phase map. B, runs of epicardial reentry observed in each recording time window. C, life span (in rotations) of epicardial reentry. D, percentage of recording time containing at least one epicardial reentry. “n” indicates the number of hearts studied. “Rs” indicates runs of reentry analyzed. See text for details.
Occurrence of Long-lasting Non-stationary Reentry
Compared to the baseline (1.8±0.3 runs/time window), infusion of d,l-sotalol significantly increased the occurrence frequency of epicardial reentry in each time window of optical recording (4.6±0.8*, 3.8±0.8*, and 5.0±0.9* runs/time window for 5-minute, 15-minute, and 25-minute d,l-sotalol infusion, respectively; * indicating p<0.05 when compared with baseline). During washout period, the frequency of epicardial reentry returned to the baseline level (1.8±0.4 runs/time window, p=NS) (see Figure 3B).
The life spans (rotations) of these reentries were also significantly increased by d,l-sotalol (1.8±0.7, 6.9±3.3*, 4.5±1.1*, 3.5±0.7*, and 1.4±0.5 rotations for baseline, 5-minute, 15-minute, 25-minute d,l-sotalol infusion, and washout, respectively; * indicating p<0.05 when compared with baseline) (see Figure 3C). Similarly, the percentage of recording time containing at least one reentry was significantly higher during d,l-sotalol infusion (20±8%, 72±18%*, 47±8%*, 53±13%* for baseline, 5-minute, 15-minute, and 25-minute d,l-sotalol infusion, respectively; * indicating p<0.05 when compared with baseline). After washout, this percentage again returned to the baseline level (23±11%, p=NS) (see Figure 3D).
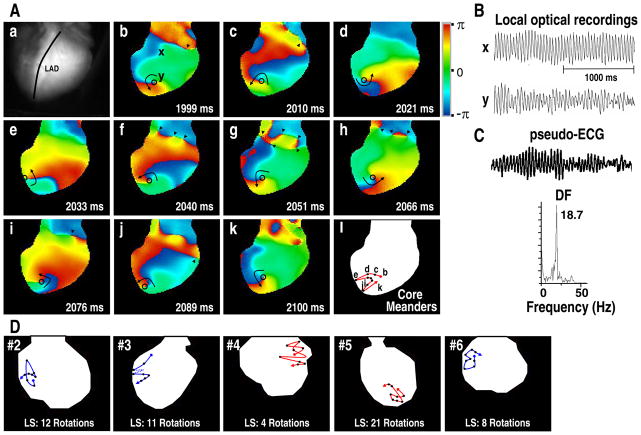
With d,l-sotalol infusion, these reentries were non-stationary in nature without anchoring on anatomical structures. They always drifted in and out of the mapped area. Figure 4 shows typical examples of organized reentry with a drifting core (one from each heart of protocol I).
Figure 4.
Examples of non-stationary reentry during VF with 10 mg/L d,l-sotalol infusion. A through C, a drifting reentry recorded 7 minutes after the onset of d,l-sotalol infusion (data from heart #1). This run of reentry had a life span of 36 rotations, lasting from 578 to 2250 ms. Aa, mapped area. LAD indicates left anterior descending coronary artery. Ab–Ak: these phase maps show that this reentry first drifted to the border of mapped area (Ae), again into the RV (Af), and finally to the interventricular septum (Ag to Ak). Black circles indicate the core of reentry. Arrowheads indicate PSs. Al shows the trajectory of core, demonstrating a meandering nature. B, local optical tracings recorded from sites x and y (see panel Ab). C, the simultaneous pseudo-ECG and corresponding DF of 18.7 Hz. D, another 5 examples of the trajectory of core of non-stationary reentry (one from hearts #2–6, respectively). LS indicates the life span of reentry. Red colored trajectory indicates counterclockwise rotation. Blue colored trajectory indicates clockwise rotation.
Protocol II
Effects of d,l-Sotalol on APD, CT−1, and WL Restitutions
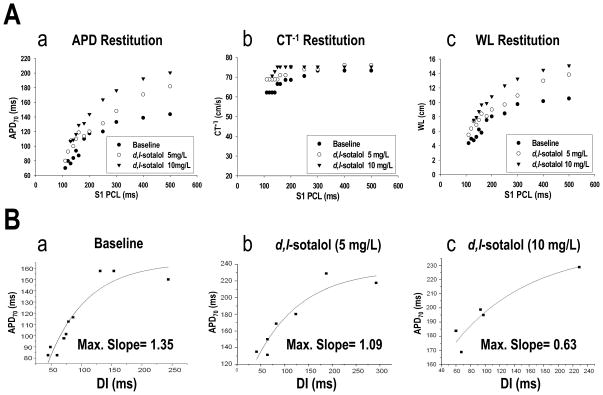
Effects of d,l-sotalol on APD, CT−1, and WL restitutions in the 4 hearts studied were similar and are summarized in Table. d,l-Sotalol progressively lengthened APD70 with increasing concentrations throughout all pacing cycle lengths. However, there was no significant effect of d,l-sotalol on the CV (estimated by CT−1) with increasing concentrations in all pacing cycle lengths. Therefore, the infusion of d,l-sotalol led to the prolongation of WL, especially at the concentration of 10 mg/L (see Table). Figure 5 is an example, showing effects of 5 and 10 mg/L d,l-sotalol on APD, CT−1, and WL restitutions. Increasing d,l-sotalol concentrations from 5 to 10 mg/L progressively prolonged APD70, shifting the APDR curve upwards (Figure 5Aa). However, the CT−1 restitution curve remained flat despite 5 and 10 mg/L d,l-sotalol infusions (Figure 5Ab). The WL restitution curve, therefore, subsequently shifted upwards as a result of APD prolongation effect with increasing concentrations of d,l-sotalol (Figure 5Ac).
Figure 5.
A, effects of d,l-sotalol on APD, CT−1 and WL restitutions. Data were obtained from heart #3 of protocol II. B, effects of d,l-sotalol on the maximum slope of APDR. Data were obtained from site b of heart #4 of protocol II. See text for details.
Using the S1 pacing method, the maximum slope of APDR decreased progressively with increasing d,l-sotalol concentrations (1.36±0.26, 1.03±0.06, and 0.90±0.16 for baseline, 5, and 10 mg/L d,l-sotalol infusion, respectively; p=0.015). Figure 5Ba–c shows an example.
Effects of d,l-Sotalol on APD Dispersion and Spatial Heterogeneity of Restitutions
APD Dispersion
In all 4 hearts studied, APD70 prolonged progressively with increasing d,l-sotalol concentrations. However, d,l-sotalol had no significant effect on spatial dispersion of APD70. APD70 dispersion at S1 pacing cycle length of 300 ms were 52±6, 56±11, and 55±8 ms for baseline, 5, and 10 mg/L of d,l-sotalol infusion, respectively (p=0.735). Similarly, APD70 dispersion at S1 pacing cycle length of 250 ms were 64±10, 59±6, and 61±5 ms for baseline, 5, and 10 mg/L of d,l-sotalol infusion, respectively (p=0.666).
APD Restitution
At baseline, the maximum slope of APDR was similar among the 4 recording sites (site a, 1.11±0.49; b, 1.52±0.72; c, 1.50±0.28; and d, 1.32±0.15; p=0.592). This heterogeneity remained insignificant with 5 mg/L (site a, 1.15±0.04; b, 1.25±0.45; c, 0.81±0.45; and d, 0.93±0.18; p=0.277) and 10 mg/L (site a, 0.90±0.52; b, 0.91±0.22; c, 0.67±0.14; and d, 1.10±0.25; p=0.341) d,l-sotalol infusion.
CT−1 Restitution
“Maximum CT−1 reduction” was used to estimate the heterogeneity of CT−1 restitution (cm/s).5 It was defined as the difference in CT−1 at the longest and the shortest S1 pacing cycle lengths (see Figure 6B in reference 5).5 There was no significant difference of maximum CT−1 reduction along the 4 different lines at baseline (line 1, 17±8; line 2, 10±7; line 3, 8±4; line 4, 7±3 cm/s; p=0.101). Heterogeneity remained insignificant with 5 mg/L (line 1, 13±6; line 2, 8±6; line 3, 8±2; line 4, 8±6 cm/s; p=0.466) and 10 mg/L (line 1, 4±5; line 2, 3±3; line 3, 5±4; line 4, 10±6 cm/s; p=0.164) d,l-sotalol infusion.
Discussion
This study has the following major findings: (1) d,l-sotalol at therapeutic concentrations flattened the APDR, decreased the PS number (i.e., wavebreak), and facilitated the occurrence of long-lasting non-stationary reentry, therefore reducing the complexity of VF activation. However, VT rarely occurred. (2) d,l-sotalol prolonged APD70 and WL without enhancing APD dispersion and spatial heterogeneity of restitutions.
Complexity of VF Activation Reduced by d,l-Sotalol
PS shown in phase maps has been employed as a valid alternate of wavebreak, which serves as a source of VF.12–14 In this study, therapeutic concentrations (10 mg/L) of d,l-sotalol significantly decreased the average number of PSs in each phase map. This indicates that d,l-sotalol effectively reduced wavebreak during VF. Also, administration of d,l-sotalol facilitated the occurrence of long-lasting reentry and increased the percentage of recording time containing organized reentry during VF, leading to the reduction of wavefront complexity. Several possible mechanisms may contribute to this anti-fibrillatory effect.
(1) APD and WL Prolongation
The WL hypothesis posits that reentry excitation is only possible if the WL of the propagating wave is shorter than the reentry path length.15,16 As shown in this study (see Table), d,l-sotalol progressively lengthened APD70 and WL with increasing concentrations. Therefore, the same-sized cardiac tissue could support fewer reentrant circuits than those at baseline, leading to the reduced complexity of VF activation and the cessation of VF.
(2) APD Restitution Flattening
A steep APDR predisposes spiral waves of VF to break up into multiple wavelets and facilitates the maintenance of VF.17 By modifying the APDR characteristics, drugs that can flatten the APDR exhibit anti-fibrillatory activity.18,19 However, previous experimental studies provide conflicting evidence about the effects of sotalol on APDR.4,20,21 This diversity may be related in part to different animal species,4,20 concentrations of d,l-sotalol used,4 isomers of sotalol (d-sotalol or d,l-sotalol),4,20,22 and different cardiac tissues (atrium or ventricle).4,20,23 Pak et al. have demonstrated that β-blocker significantly reduced VF cycle length and flattened the APDR.11 The β-adrenergic blocking activity of racemic sotalol is almost entirely derived from l-isomer.24–26 Therefore, d,l-sotalol may have a greater APDR flattening effect than d-sotalol.4,20
It has been reported by Pak et al in isolated swine RV tissues that d,l-sotalol at therapeutic doses (≤10 mg/L) flattened the APDR.4 However, a higher concentration (20 mg/L) adversely steepened the APDR and enhanced VF inducibility.4 Similar to their findings, the present study showed that use of d,l-sotalol (5 to 10 mg/L) in isolated rabbit ventricles also decreased the maximum slope of APDR. APDR flattening by d,l-sotalol per se may decrease the break up of spiral waves and enhance the occurrence of long-lasting organized reentry, thereby reducing the complexity of VF activation.
(3) No Enhancement of APD Dispersion and Spatial Heterogeneity of Restitutions
It is well known that d-sotalol increases the transmural QT dispersion, mimicking the HERG defect in long QT 2 syndrome, and predisposes the ventricular substrate to the formation of VF.27,28 However, d,l-sotalol at therapeutic concentrations has not been reported to accentuate APD dispersion in both canine atria29 and rabbit ventricles.30 Consistent with these findings, in this study, the use of 5 and 10 mg/L d,l-sotalol did not increase the spatial dispersion of APD. Furthermore, spatial heterogeneity of APD and CT−1 restitutions was not enhanced by d,l-sotalol.
Presence of Long-lasting Non-stationary Reentry Before VF Termination
To the best of our knowledge, the wavefront characteristics of VF during d,l-sotalol infusion have not been explored using an optical mapping system. A novel finding of this study is that d,l-sotalol at therapeutic concentrations facilitates the occurrence of long-lasting non-stationary reentry during VF. This finding was different from that reported by Chorro et al.3 using a similar concentration (20 μmol, 6.18 mg/L) of d,l-sotalol and an electrode mapping system with a relatively small mapped area. Inthe latter report, d,l-sotalol produced no significant variation in the life span (consecutive rotations) of reentry when compared with control.3
We have reported that both D600 and propranolol can flatten the APDR and convert a multiple-wavelet VF into a slower focal-source VF (i.e., type 2 VF) in isolated rabbit hearts.6,11 During type 2 VF, the mother rotor always anchored on the PM.6 In this study with the same animal model, d,l-sotalol also enhanced the generation of epicardial reentry during VF. However, instead of anchoring on the PM, these reentries drifted before VF termination. Yamazaki et al.31,32 also reported that nifekalant, a class III anti-arrhythmic drug that depresses Ikr, caused a pre-existing reentry to meander drastically. The presence of non-stationary reentry can be partly explained by the WL hypothesis.15,16 As the WL was prolonged by d,l-sotalol, the tip of the reentrant wavefront had to move in a much wider pattern to maintain a sufficient excitable gap, resulting in a meandering behavior. On the other hand, as the WL was shortened by D600,6 the reentry could anchor on the PM stably with an adequate excitable gap in the same-sized ventricular tissue, leading to the formation of either VT or type 2 VF.
In a 2-dimensional layer of rabbit ventricular myocardium with nifekalant infusion,31 meandering reentrant wavefronts can be extinguished by two mechanisms: (1) collision of the drifting reentry with an anatomical boundary, and (2) trapping of the reentry tip in a region surrounded by refractory tissue. Although we did not exactly record the activations immediately before VF termination in this study, it is possible that the above two mechanisms may contribute to the termination of VF during d,l-sotalol infusion.
Limitations
Firstly, 30-minute washout data showed that the DF of VF was still significantly lower when compared with baseline. This finding suggests that a 30-minute washout period may not be sufficient to completely eliminate the d,l-sotalol effect from rabbit ventricular tissues. Secondly, it is unclear whether or not the findings of this study are applicable to VF in larger and/or diseased ventricles since both geometry and tissue mass influence the dynamics of spiral reentry. Finally, this study employed only therapeutic concentrations (≤10 mg/L) of d,l-sotalol. The effects of higher concentrations (>10 mg/L) on wavefront dynamics of VF deserve further investigation.
Acknowledgments
This study was supported in part by the National Science Council Grant 96-2628-B-010-035-MY2, Taipei, Taiwan, by Yen Tjing Ling Medical Foundation, by the Taichung Veterans General Hospital Grants TCVGH-963105C (Dr. Wu), TCVGH-FCU 958215 (Dr. Horng), Taichung, Taiwan, and by an Established Investigatorship Award (Dr. Lin), American Heart Association, Dallas, TX, USA.
We thank Robin Tsai and Kai-Yuan Cheng for experimental assistance. We also thank Dr. Jiunn-Lee Lin (National Taiwan University Hospital, Taipei, Taiwan) to provide the intravenous form of d,l-sotalol for this study.
Footnotes
Conflict of Interest Disclosure
None
References
- 1.Mason JW. A comparison of electrophysiologic testing with Holter monitoring to predict antiarrhythmic-drug efficacy for ventricular tachyarrhythmias. Electrophysiologic Study versus Electrocardiographic Monitoring Investigators. N Engl J Med. 1993;329:445–51. doi: 10.1056/NEJM199308123290701. [DOI] [PubMed] [Google Scholar]
- 2.Mason JW. A comparison of seven antiarrhythmic drugs in patients with ventricular tachyarrhythmias. Electrophysiologic Study versus Electrocardiographic Monitoring Investigators. N Engl J Med. 1993;329:452–8. doi: 10.1056/NEJM199308123290702. [DOI] [PubMed] [Google Scholar]
- 3.Chorro FJ, Canoves J, Guerrero J, Mainar L, Sanchis J, Such L, et al. Alteration of ventricular fibrillation by flecainide, verapamil, and sotalol: an experimental study. Circulation. 2000;101:1606–15. doi: 10.1161/01.cir.101.13.1606. [DOI] [PubMed] [Google Scholar]
- 4.Pak HN, Kim YH, Hwang GS, Lee SJ, Lee HS, Lim HE, et al. Antifibrillatory and Proarrhythmic Effects of d,l-Sotalol Mediated by the Action Potential Duration Restitution Kinetics. Korean Circulation J. 2005;35:282–289. [Google Scholar]
- 5.Wu TJ, Lin SF, Weiss JN, Ting CT, Chen PS. Two types of ventricular fibrillation in isolated rabbit hearts: importance of excitability and action potential duration restitution. Circulation. 2002;106:1859–66. doi: 10.1161/01.cir.0000031334.49170.fb. [DOI] [PubMed] [Google Scholar]
- 6.Wu TJ, Lin SF, Baher A, Qu Z, Garfinkel A, Weiss JN, et al. Mother rotors and the mechanisms of D600-induced type 2 ventricular fibrillation. Circulation. 2004;110:2110–18. doi: 10.1161/01.CIR.0000143834.51102.91. [DOI] [PubMed] [Google Scholar]
- 7.Wu TJ, Lin SF, Hsieh YC, Ting CT, Chen PS. Ventricular fibrillation during no-flow global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2006;17:1112–20. doi: 10.1111/j.1540-8167.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 8.Wu TJ, Lin SF, Hsieh YC, Chen PS, Ting CT. Early recurrence of ventricular fibrillation after successful defibrillation during prolonged global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2008;19:203–10. doi: 10.1111/j.1540-8167.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 9.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–8. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 10.Valderrabano M, Lee MH, Ohara T, Lai AC, Fishbein MC, Lin SF, et al. Dynamics of intramural and transmural reentry during ventricular fibrillation in isolated swine ventricles. Circ Res. 2001;88:839–48. doi: 10.1161/hh0801.089259. [DOI] [PubMed] [Google Scholar]
- 11.Pak HN, Oh YS, Liu YB, Wu TJ, Karagueuzian HS, Lin SF, et al. Catheter ablation of ventricular fibrillation in rabbit ventricles treated with beta-blockers. Circulation. 2003;108:3149–56. doi: 10.1161/01.CIR.0000104563.12408.12. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi H, Lin SF, Chen PS. Preshock phase singularity and the outcome of ventricular defibrillation. Heart Rhythm. 2007;4:927–34. doi: 10.1016/j.hrthm.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 13.Liu YB, Peter A, Lamp ST, Weiss JN, Chen PS, Lin SF. Spatiotemporal correlation between phase singularities and wavebreaks during ventricular fibrillation. J Cardiovasc Electrophysiol. 2003;14:1103–9. doi: 10.1046/j.1540-8167.2003.03218.x. [DOI] [PubMed] [Google Scholar]
- 14.Valderrabano M, Chen PS, Lin SF. Spatial distribution of phase singularities in ventricular fibrillation. Circulation. 2003;108:354–9. doi: 10.1161/01.CIR.0000080322.67408.B4. [DOI] [PubMed] [Google Scholar]
- 15.Mines GR. On dynamic equilibrium in the heart. J Physiol. 1913;46:349–83. doi: 10.1113/jphysiol.1913.sp001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rensma PL, Allessie MA, Lammers WJ, Bonke FI, Schalij MJ. Length of excitation wave and susceptibility to reentrant atrial arrhythmias in normal conscious dogs. Circ Res. 1988;62:395–410. doi: 10.1161/01.res.62.2.395. [DOI] [PubMed] [Google Scholar]
- 17.Weiss JN, Garfinkel A, Karagueuzian HS, Qu Z, Chen P-S. Chaos and the transition to ventricular fibrillation: A new approach to antiarrhythmic drug evaluation. Circulation. 1999;99:2819–26. doi: 10.1161/01.cir.99.21.2819. [DOI] [PubMed] [Google Scholar]
- 18.Riccio ML, Koller ML, Gilmour RF., Jr Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circ Res. 1999;84:955–63. doi: 10.1161/01.res.84.8.955. [DOI] [PubMed] [Google Scholar]
- 19.Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci USA. 2000;97:6061–6. doi: 10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fei H, Frame LH. d-Sotalol terminates reentry by two mechanisms with different dependence on the duration of the excitable gap. J Pharmacol Exp Ther. 1996;277:174–85. [PubMed] [Google Scholar]
- 21.Qu Z, Weiss JN. Effects of Na(+) and K(+) channel blockade on vulnerability to and termination of fibrillation in simulated normal cardiac tissue. Am J Physiol Heart Circ Physiol. 2005;289:H1692–H1701. doi: 10.1152/ajpheart.00241.2005. [DOI] [PubMed] [Google Scholar]
- 22.Reiter MJ, Zetelaki Z, Kirchhof CJ, Boersma L, Allessie MA. Interaction of acute ventricular dilatation and d-sotalol during sustained reentrant ventricular tachycardia around a fixed obstacle. Circulation. 1994;89:423–31. doi: 10.1161/01.cir.89.1.423. [DOI] [PubMed] [Google Scholar]
- 23.Boyden PA, Graziano JN. Activation mapping of reentry around an anatomical barrier in the canine atrium: observations during the action of the class III agent, d-sotalol. J Cardiovasc Electrophysiol. 1993;4:266–79. doi: 10.1111/j.1540-8167.1993.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 24.Kato R, Ikeda N, Yabek SM, Kannan R, Singh BN. Electrophysiologic effects of the levo- and dextrorotatory isomers of sotalol in isolated cardiac muscle and their in vivo pharmacokinetics. J Am Coll Cardiol. 1986;7:116–25. doi: 10.1016/s0735-1097(86)80268-6. [DOI] [PubMed] [Google Scholar]
- 25.Singh BN. Historical development of the concept of controlling cardiac arrhythmias by lengthening repolarization: particular reference to sotalol. Am J Cardiol. 1990;65:3A–11A. doi: 10.1016/0002-9149(90)90195-7. [DOI] [PubMed] [Google Scholar]
- 26.Singh BN. Electrophysiologic basis for the antiarrhythmic actions of sotalol and comparison with other agents. Am J Cardiol. 1993;72:8A–18A. doi: 10.1016/0002-9149(93)90020-d. [DOI] [PubMed] [Google Scholar]
- 27.Benson AP, Aslanidi OV, Zhang H, Holden AV. The canine virtual ventricular wall: A platform for dissecting pharmacological effects on propagation and arrhythmogenesis. Prog Biophys Mol Biol. 2008;96:187–208. doi: 10.1016/j.pbiomolbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu W, Antzelevitch C. Effects of a K(+) channel opener to reduce transmural dispersion of repolarization and prevent torsade de pointes in LQT1, LQT2, and LQT3 models of the long-QT syndrome. Circulation. 2000;102:706–12. doi: 10.1161/01.cir.102.6.706. [DOI] [PubMed] [Google Scholar]
- 29.Nattel S, Bourne G, Talajic M. Insights into mechanisms of antiarrhythmic drug action from experimental models of atrial fibrillation. J Cardiovasc Electrophysiol. 1997;8:469–80. doi: 10.1111/j.1540-8167.1997.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhof P, Milberg P, Eckardt L, Breithardt G, Haverkamp W. Effect of sotalol and acute ventricular dilatation on action potential duration and dispersion of repolarization after defibrillation shocks. J Cardiovasc Pharmacol. 2003;41:640–8. doi: 10.1097/00005344-200304000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Yamazaki M, Honjo H, Nakagawa H, Ishiguro YS, Okuno Y, Amino M, et al. Mechanisms of destabilization and early termination of spiral wave reentry in the ventricle by a class III antiarrhythmic agent, nifekalant. Am J Physiol Heart Circ Physiol. 2007;292:H539–H548. doi: 10.1152/ajpheart.00640.2006. [DOI] [PubMed] [Google Scholar]
- 32.Kodama I, Honjo H, Yamazaki M, Nakagawa H, Ishiguro Y, Okuno Y, et al. Optical imaging of spiral waves: pharmacological modification of spiral-type excitations in a 2-dimensional layer of ventricular myocardium. J Electrocardiol. 2005;38:126–30. doi: 10.1016/j.jelectrocard.2005.06.025. [DOI] [PubMed] [Google Scholar]