Abstract
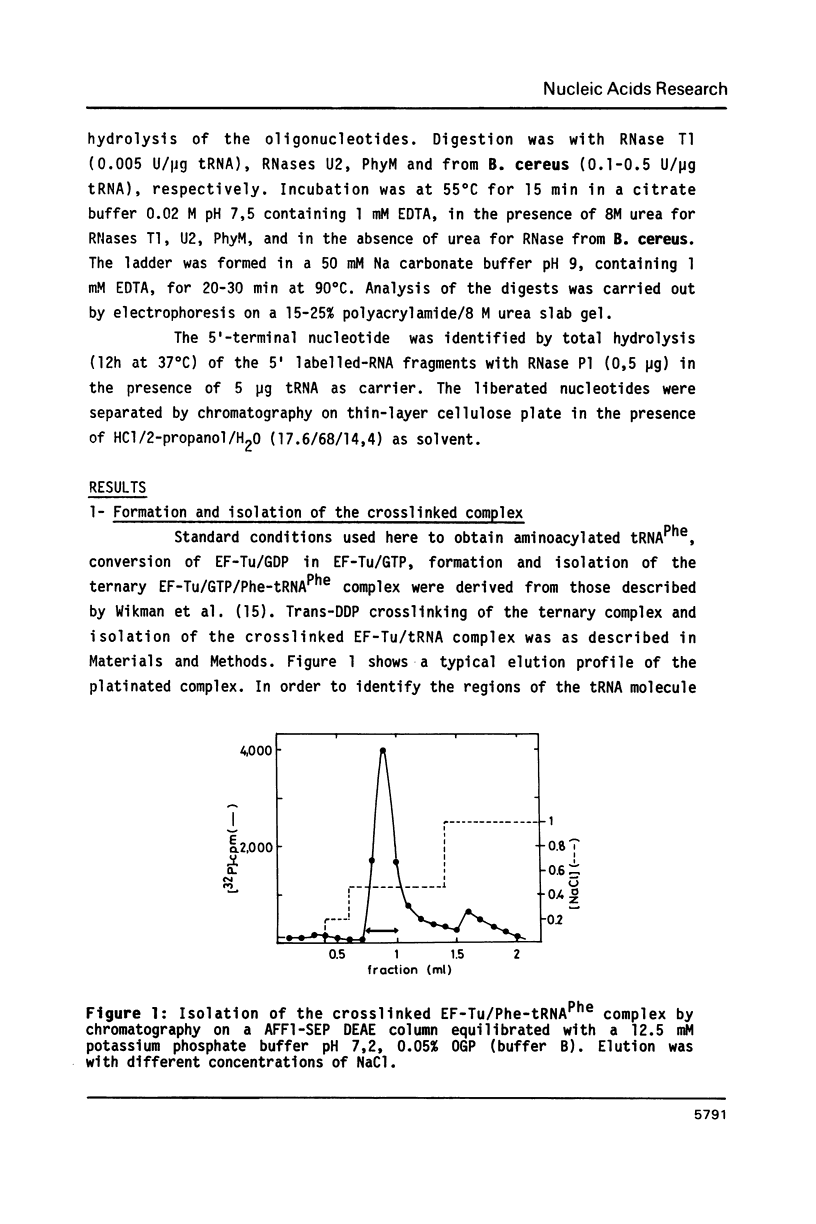
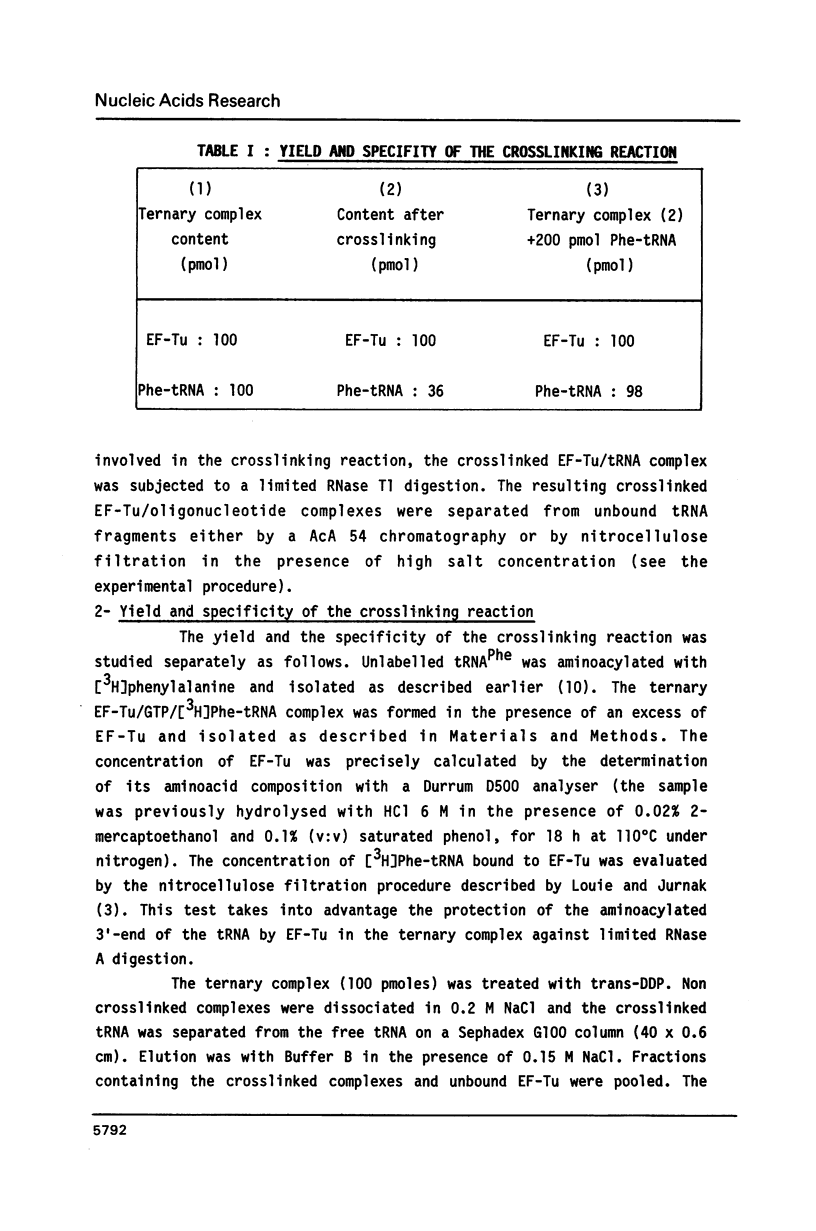
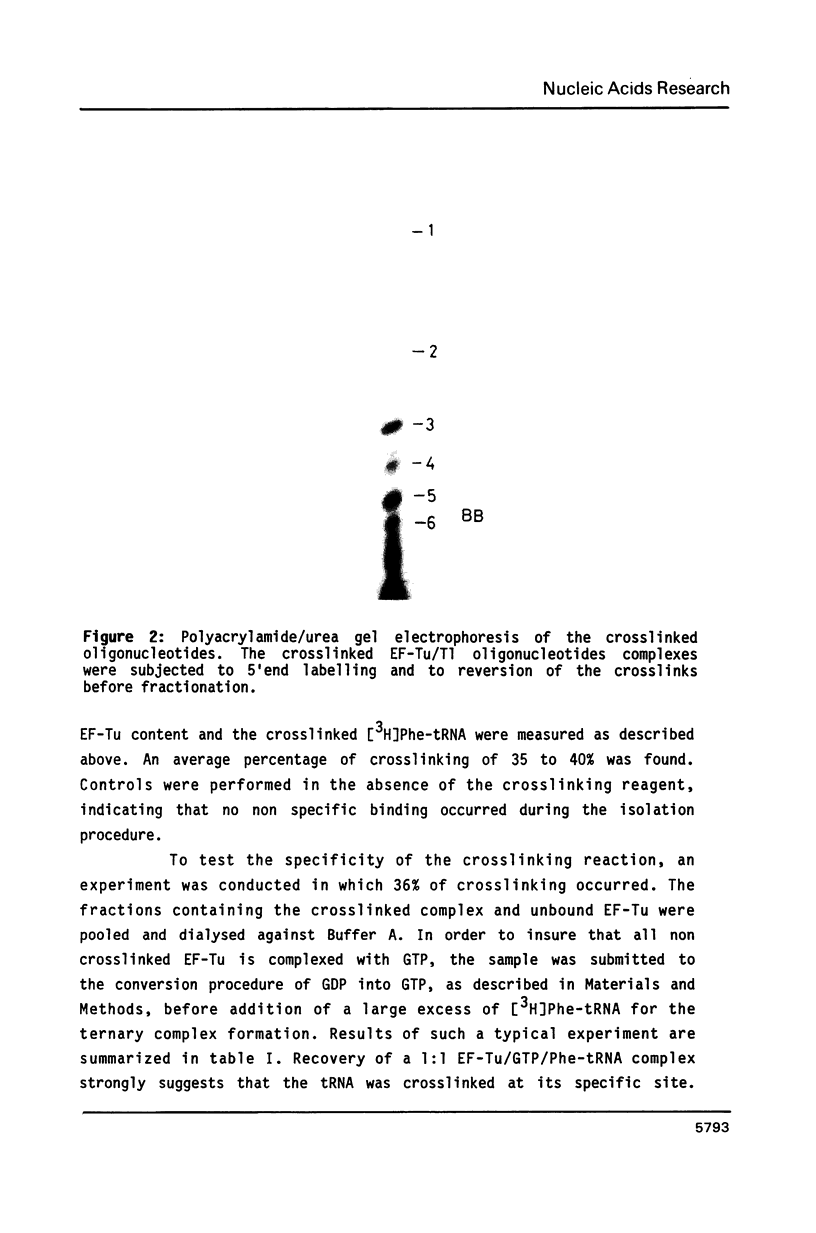
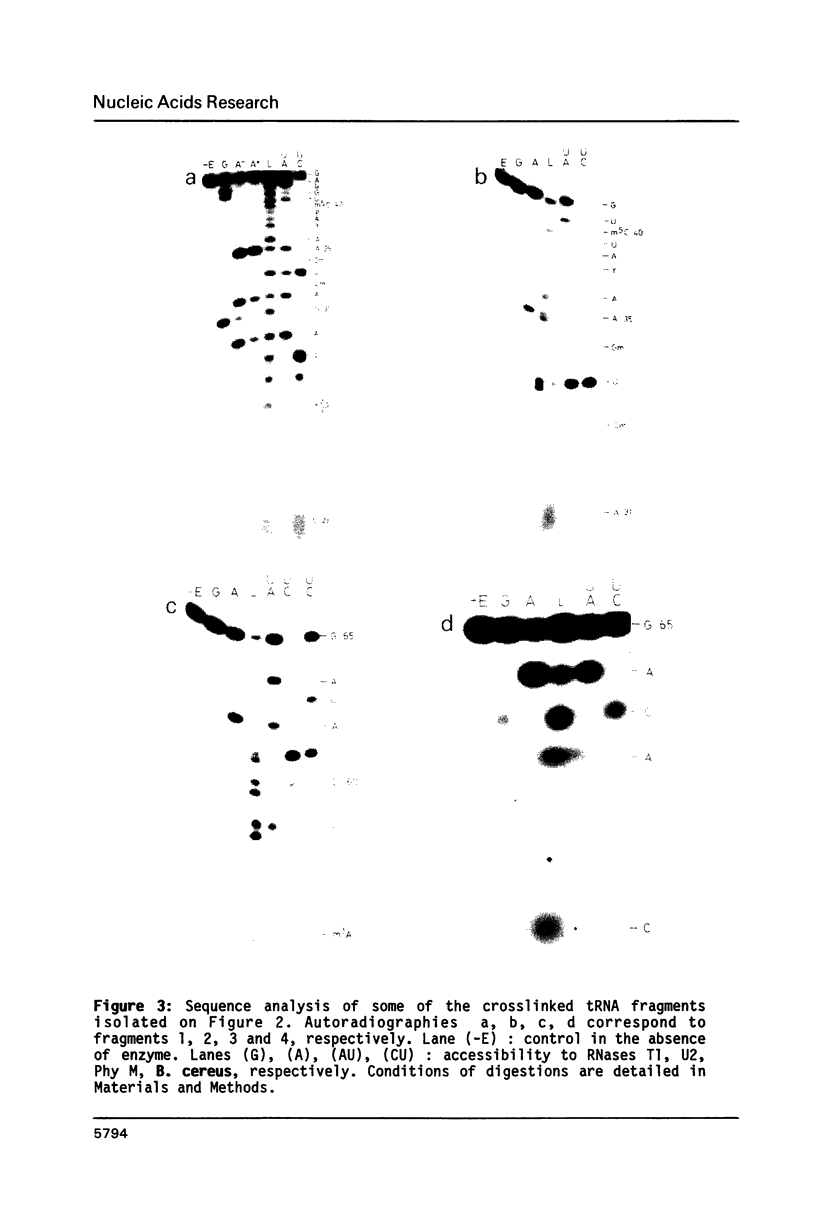
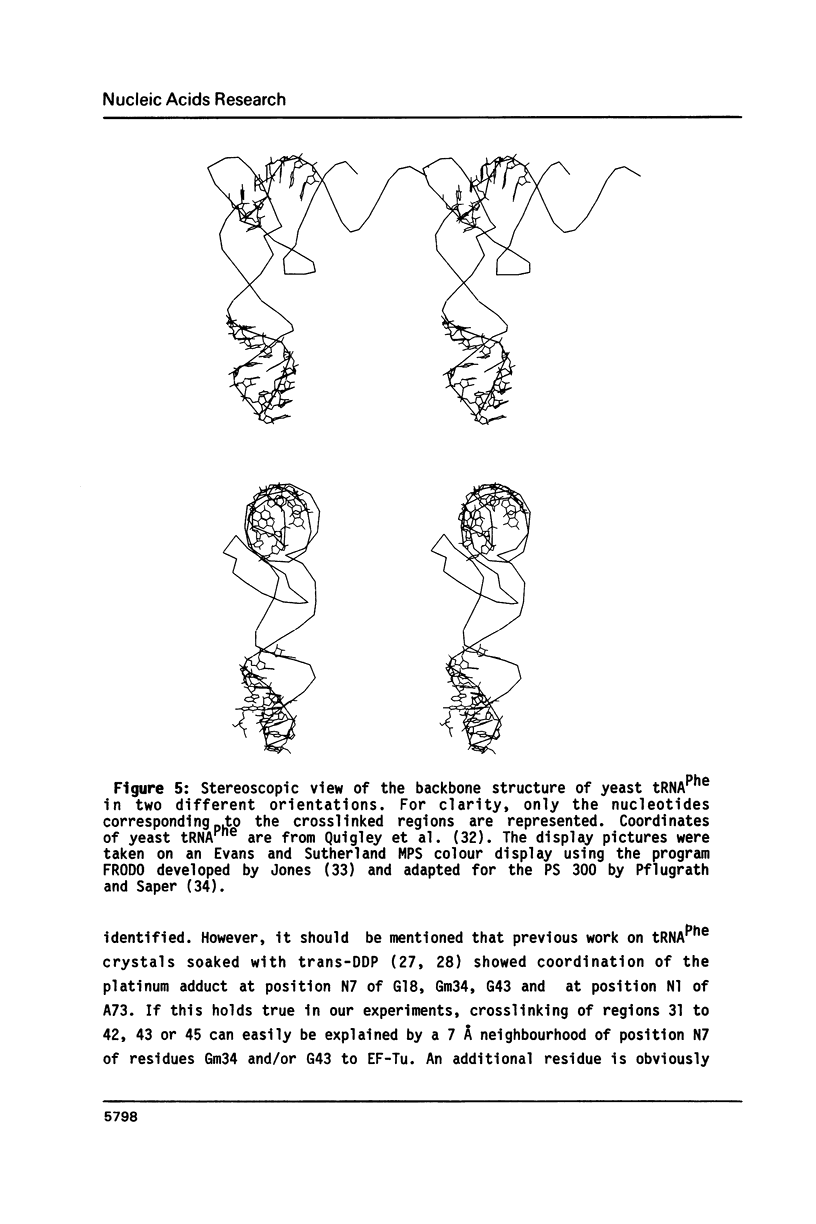
Trans-diamminedichloroplatinum (II) was used to induce reversible crosslinks between EF-Tu and Phe-tRNA(Phe) within the ternary EF-Tu/GTP/Phe-tRNA(Phe) complex. Up to 40% of the complex was specifically converted into crosslinked species. Two crosslinking sites have been unambiguously identified. The major one encompassing nucleotides 58 to 65 is located in the 3'-part of the T-stem, and the minor one encompassing nucleotides 31 to 42 includes the anticodon loop and part of the 3'-strand of the anticodon stem.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson B., Leberman R., Jacrot B., Zaccai G. Small-angle neutron scattering study of the ternary complex formed between bacterial elongation factor Tu, guanosine 5'-triphosphate, and valyl-tRNAVal. Biochemistry. 1986 Jun 17;25(12):3655–3659. doi: 10.1021/bi00360a027. [DOI] [PubMed] [Google Scholar]
- Antonsson B., Leberman R. Modification of amino groups in EF-Tu.GTP and the ternary complex EF-Tu.GTP.valyl-tRNAVal. Eur J Biochem. 1984 Jun 15;141(3):483–487. doi: 10.1111/j.1432-1033.1984.tb08218.x. [DOI] [PubMed] [Google Scholar]
- Boutorin A. S., Clark B. F., Ebel J. P., Kruse T. A., Petersen H. U., Remy P., Vassilenko S. A study of the interaction of Escherichia coli elongation factor-Tu with aminoacyl-tRNAs by partial digestion with cobra venom ribonuclease. J Mol Biol. 1981 Nov 5;152(3):593–608. doi: 10.1016/0022-2836(81)90271-0. [DOI] [PubMed] [Google Scholar]
- Dirheimer G., Ebel J. P. Fractionnement des tRNA de levure de bière par distribution en contre-courant. Bull Soc Chim Biol (Paris) 1967;49(12):1679–1687. [PubMed] [Google Scholar]
- Douthwaite S., Garrett R. A., Wagner R. Comparison of Escherichia coli tRNAPhe in the free state, in the ternary complex and in the ribosomal A and P sites by chemical probing. Eur J Biochem. 1983 Mar 15;131(2):261–269. doi: 10.1111/j.1432-1033.1983.tb07258.x. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Moine H., Mougel M., Dondon J., Grunberg-Manago M., Ebel J. P., Ehresmann B. Cross-linking of initiation factor IF3 to Escherichia coli 30S ribosomal subunit by trans-diamminedichloroplatinum(II): characterization of two cross-linking sites in 16S rRNA; a possible way of functioning for IF3. Nucleic Acids Res. 1986 Jun 25;14(12):4803–4821. doi: 10.1093/nar/14.12.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Doi T., Ikehara M., Ohtsuka E., Sprinzl M. Interaction of methionine-specific tRNAs from Escherichia coli with immobilized elongation factor Tu. FEBS Lett. 1985 Nov 11;192(1):151–154. doi: 10.1016/0014-5793(85)80062-4. [DOI] [PubMed] [Google Scholar]
- Garrett-Wheeler E., Lockard R. E., Kumar A. Mapping of psoralen cross-linked nucleotides in RNA. Nucleic Acids Res. 1984 Apr 11;12(7):3405–3423. doi: 10.1093/nar/12.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen P. K., Wikman F., Clark B. F., Hershey J. W., Uffe Petersen H. Interaction between initiator Met-tRNAfMet and elongation factor EF-Tu from E. coli. Biochimie. 1986 May;68(5):697–703. doi: 10.1016/s0300-9084(86)80163-8. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Rhodes D., Brown R. S., Klug A. A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J Mol Biol. 1977 Apr 15;111(3):315–328. doi: 10.1016/s0022-2836(77)80054-5. [DOI] [PubMed] [Google Scholar]
- Jekowsky E., Schimmel P. R., Miller D. L. Isolation, characterization and structural implications of a nuclease-digested complex of aminoacyl transfer RNA and Escherichia coli elongation factor Tu. J Mol Biol. 1977 Aug 15;114(3):451–458. doi: 10.1016/0022-2836(77)90262-5. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Gast W. H., Schulz G. E., Leberman R. Low resolution structure of partially trypsin-degraded polypeptide elongation factor, EF-TU, from Escherichia coli. J Mol Biol. 1977 Dec 25;117(4):999–1012. doi: 10.1016/s0022-2836(77)80009-0. [DOI] [PubMed] [Google Scholar]
- Kao T., Miller D. L., Abo M., Ofengand J. Formation and properties of a covalent complex between elongation factor Tu and Phe-tRNA bearing a photoaffinity probe on its 3-(3-amino-3-carboxypropyl)uridine residue. J Mol Biol. 1983 May 25;166(3):383–405. doi: 10.1016/s0022-2836(83)80091-6. [DOI] [PubMed] [Google Scholar]
- Kern D., Dietrich A., Fasiolo F., Renaud M., Giegé R., Ebel J. P. The yeast aminoacyl-tRNA synthetases. Methodology for their complete or partial purification and comparison of their relative activities under various extraction conditions. Biochimie. 1977;59(5-6):453–462. doi: 10.1016/s0300-9084(77)80050-3. [DOI] [PubMed] [Google Scholar]
- Kern D., Giegé R., Robre-Saul S., Boulanger Y., Ebel J. P. Complete purification and studies on the structural and kinetic properties of two forms of yeast valyl-tRNA synthetase. Biochimie. 1975;57(10):1167–1176. doi: 10.1016/s0300-9084(76)80579-2. [DOI] [PubMed] [Google Scholar]
- Louie A., Jurnak F. Kinetic studies of Escherichia coli elongation factor Tu-guanosine 5'-triphosphate-aminoacyl-tRNA complexes. Biochemistry. 1985 Nov 5;24(23):6433–6439. doi: 10.1021/bi00344a019. [DOI] [PubMed] [Google Scholar]
- Louie A., Ribeiro N. S., Reid B. R., Jurnak F. Relative affinities of all Escherichia coli aminoacyl-tRNAs for elongation factor Tu-GTP. J Biol Chem. 1984 Apr 25;259(8):5010–5016. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg R., Elias P., Kjems J., Bauer R. A neutron scattering study of the ternary complex EF-Tu.GTP-valyl-tRNAVal1A. J Biomol Struct Dyn. 1986 Jun;3(6):1111–1120. doi: 10.1080/07391102.1986.10508488. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Sjöberg B., Ligaarden R., Elias P. A small-angle X-ray scattering study of the complex formation between elongation factor Tu . GTP and valyl-tRNA Val I from Escherichia coli. Eur J Biochem. 1981 Jun;117(1):155–159. doi: 10.1111/j.1432-1033.1981.tb06314.x. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Urbanke C., Krauss G., Peters F., Maass G. Ternary complex formation between elongation factor Tu, GTP and aminoacyl-tRNA: an equilibrium study. Eur J Biochem. 1977 Sep;78(2):403–409. doi: 10.1111/j.1432-1033.1977.tb11752.x. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Seeman N. C., Wang A. H., Suddath F. L., Rich A. Yeast phenylalanine transfer RNA: atomic coordinates and torsion angles. Nucleic Acids Res. 1975 Dec;2(12):2329–2341. doi: 10.1093/nar/2.12.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl N., Giegé R., Ebel J. P., Ehresmann B. Effect of elongation factor Tu on the conformation of phenylalanyl-tRNAPhe. FEBS Lett. 1983 Apr 5;154(1):42–46. doi: 10.1016/0014-5793(83)80871-0. [DOI] [PubMed] [Google Scholar]
- Rubin J. R., Sabat M., Sundaralingam M. Similar binding of the carcinostatic drugs cis-[Pt(NH3)2Cl2] and [Ru(NH3)5Cl] Cl2 to tRNAphe and a comparison with the binding of the inactive trans-[Pt(NH3)2Cl2] complex - reluctance in binding to Watson-Crick base pairs within double helix. Nucleic Acids Res. 1983 Sep 24;11(18):6571–6586. doi: 10.1093/nar/11.18.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygand-Durasevic I., Kruse T. A., Clark B. F. The influence of elongation-factor-Tu . GTP and anticodon-anticodon interactions on the anticodon loop conformation of yeast tRNATyr. Eur J Biochem. 1981 May;116(1):59–65. doi: 10.1111/j.1432-1033.1981.tb05300.x. [DOI] [PubMed] [Google Scholar]
- Wikman F. P., Siboska G. E., Petersen H. U., Clark B. F. The site of interaction of aminoacyl-tRNA with elongation factor Tu. EMBO J. 1982;1(9):1095–1100. doi: 10.1002/j.1460-2075.1982.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]