Abstract
While L-type voltage-dependent calcium channels have long been considered the predominant source of calcium for myogenic constriction, recent studies of both cerebral and systemic circulations have provided evidence for the prominent expression of other members of the voltage-dependent calcium channel family, in particular the low voltage activated T-type channels. Although physiological studies have not supported the involvement of a classical low voltage activated, T-type channel in vascular function, evidence is accumulating that points to the involvement of a non-L-type, high voltage activated channel with sensitivity to T-type channel antagonists. We propose that this may arise due to expression of a T-type channel splice variant with unique biophysical characteristics resulting in a more depolarised profile. Expression of these channels in smooth muscle cells would broaden the voltage range over which sustained calcium influx occurs, while expression of T-type channels in endothelial cells could provide a feedback mechanism to prevent excessive vasoconstriction. Perturbation of this balance during pathophysiological conditions by upregulation of channel expression and endothelial dysfunction could contribute to vasospastic conditions and therapy-refractory hypertension.

Ivana Kuo (left) completed her PhD with Caryl Hill in 2010 and is currently undertaking postdoctoral research with Barbara Ehrlich at Yale University, Connecticut. Steffi Wölfle (centre) joined Caryl Hill's group in 2008 after a PhD with Cor de Wit at the University of Lübeck, Germany and postdoctoral period at the Johns Hopkins University in Baltimore, Maryland, with Rick Rivers. Caryl Hill (right) obtained her PhD from the University of Melbourne with Geoffrey Burnstock and studied at University College London and the Australian National University, becoming Professor in 2003. Together they share a common interest in the mechanisms underlying vascular coordination and the regulation of vascular tone.
Small arteries and arterioles are the main determinants of peripheral resistance and therefore importantly control blood pressure. At normal intraluminal pressures, these vessels reside in a state of partial constriction which provides the capacity to dilate or constrict in response to regional or systemic demand. Enhanced vascular smooth muscle contractility increases peripheral resistance and can contribute to hypertension.
Vasoconstriction depends on an increase in the intracellular calcium concentration of vascular smooth muscle cells. This can occur through depolarisation (electromechanical coupling) or in a voltage-independent manner that largely depends on release of calcium from internal stores (pharmacomechanical coupling; Somlyo & Somlyo, 1968). The relative contribution of these two mechanisms can vary according to agonist concentration, length of stimulus and vessel type (Low et al. 1996; Xia & Duling, 1998), with calcium release from internal stores contributing more to contraction of conduit than resistance vessels (van Breemen & Saida, 1989; Low et al. 1996). Electromechanical coupling links depolarisation to contraction of vascular smooth muscle by activation of calcium influx through voltage dependent calcium channels (VDCCs), a process recruited by a number of signalling cascades, including sympathetic and stretch-induced constriction. Importantly, a reduction in electromechanical coupling, induced by either hyperpolarisation or pharmacological blockade of voltage sensitive calcium channels, will reduce smooth muscle tension resulting in reduced peripheral resistance.
High voltage activated, L-type VDCCs have long been considered to be the major source of calcium necessary for persistent vascular tone, and enhanced activity of L-type VDCCs has been linked to hypertension and cerebrovascular disease (Pesic et al. 2004). However, despite the successful use of blockers of L-type VDCCs and antagonists of the renin–angiotensin system in the treatment of hypertension, some 20–30% of patients remain refractory to therapy (Epstein, 2007), as do patients experiencing delayed aneurysmal vasospasm following subarachnoid haemorrhage (Dorhout Mees et al. 2007; Tomassoni et al. 2008). Thus, important mechanisms contributing to the development of therapy-resistant hypertension and cerebral vasospasm remain unidentified.
Over the past 10 years, evidence from renal and mesenteric circulations has demonstrated expression of other members of the VDCC superfamily, in particular, the T-type channels, in these vessels (Gustafsson et al. 2001; Hansen et al. 2001; Jensen et al. 2004; Hayashi et al. 2007). However, the role of T-type VDCCs in the maintenance of vascular tone under physiological conditions remains controversial, largely due to the argument that the currents through these channels are tiny and transient, and that their activation and inactivation profiles lie outside the range of potentials normally experienced in physiologically active vessels.
In this brief review we extend discussion of T-type channels to their expression and role in the cerebral circulation and speculate on how they could contribute to vascular tone under normal physiological conditions and during pathophysiological events such as vasospasm.
VDCC subtypes
The 10 molecular subtypes of VDCCs are traditionally divided into two main groups: high and low voltage activated (HVA and LVA) channels, reflecting their different voltage thresholds for activation. Molecular cloning studies have described 10 distinct genes that encode the pore-forming α subunit (α1) and assigned them into three major families (CaV1, 2 and 3) based on sequence similarity. The HVA channels comprise the L-type channels (CaV1.1–1.4), and the P/Q- (CaV2.1), N- (CaV2.2) and R- (CaV2.3) type channels. The LVA channels form the third family comprising the T-type channels, CaV3.1–3.3.
Comparison of the biophysical characteristics of L- and T-type channels
VDCC activity is characterised by depolarisation-dependent activation, voltage-dependent inactivation which limits ion flow through the pore despite a persistent depolarising stimulus, and hyperpolarisation-induced channel closure, or deactivation. However, LVA and HVA channels exhibit significant differences in these biophysical properties. In comparison with L-type channels, T-type channels are more sensitive to depolarisation, activate and inactivate faster, thus providing a transient rather than persistent current, and close more slowly (Perez-Reyes, 2003). However, comparisons between L- and T-type channels are often complicated by the fact that several of these biophysical characteristics vary depending on the cell system used for study and the type and concentration of charge carrier.
The two commonly used charge carriers, barium and calcium, can affect channel conductance, gating and inactivation. The conductance of barium through Cav1 and Cav2 channels, with the exception of Cav2.3, is some threefold higher than that for calcium. In contrast, the conductance of barium relative to calcium is more similar for the three T-type channel subtypes and for Cav2.3, although barium tends to have greater conductance than calcium through Cav3.2 and Cav3.3 while the reverse holds for Cav3.1 (Kaku et al. 2003; Shcheglovitov et al. 2007). Nevertheless, the conductance of L- and T-type channels is equivalent with calcium as charge carrier (9 pS for Cav1.2; 7.5 pS for Cav3.1; 9 pS for Cav3.2; 11 pS for Cav3.3; Catterall et al. 2005). Secondly, there are changes in voltage dependence which vary with charge carrier and concentration (see Table 1). Replacement of barium with calcium leads to a shift of ∼+10mV in the voltage-dependent activation and inactivation of L-type channels, but this is not seen for T-type channels during equimolar substitution (Kaku et al. 2003; Shcheglovitov et al. 2007). While changes in gating have been attributed to generalised surface charge screening by the binding of positive calcium ions to the membrane, the absence of such an effect on T-type channels suggests that such screening is dependent on a selective binding site on the channel itself (Li et al. 2010). Effects of calcium or barium on channel gating and conductance are also concentration dependent (Kaku et al. 2003), making comparison between studies, even utilising the same carrier, more complex. Finally, inactivation of L-type channels, but not T-type channels, is calcium dependent (Kaku et al. 2003; Cens et al. 2006), leading to reduction in inactivation time constants of L-type channels when tested in calcium rather than barium. This results from the binding of calcium to calmodulin, producing changes to the interactions of sites in the carboxy terminus, including an EF-hand motif (Cens et al. 2006). Calcium-dependent inactivation does not occur in T-type channels since they lack the EF hand motif (Perez-Reyes, 2003).
Table 1.
Effect of charge carrier on the biophysical characteristics of CaV1.2 and CaV3 VDCCs transfected into HEK cells
| A. Ba2+ as charge carrier (5–30 mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Activation | Inactivation | ||||||||
| Channel | Vmax (mV) | V0.5 (mV) | Slope (k) | τact (ms) | Vh (mV) | Slope (k) | τinact (ms) | [Ba2+] (mm) | References |
| CaV1.2 | 0 | −10 | 10.3 | 1−3 | −39 | ND | ND | 5 | (Liao et al. 2005) |
| +2−3 | −9 | 6 | ND | −39 | −8 | ND | 5 | (Tang et al. 2004) | |
| +9 | −4 | 7.8 | 2.3 | −28 | −13.8 | 160, 1050 | 15−20 | (Koschak et al. 2001) | |
| +10 | 5 | 9.6 | ND | −47 | −13.1 | ND | 30 | (Li et al. 2010) | |
| CaV3.1 | ∼−20 | −29 | 8.9 | 1.8 | −72 | −4.8 | 15 | 10 | (Lee et al. 1999a) |
| CaV3.2 | ∼−25 | −31 | 8.8 | 1.7 | −81 | −5.5 | 14 | 10 | (Lee et al. 1999a) |
| CaV3.3 | ∼−20 | −25 | 8.1 | 4.7 | −68 | −6.3 | 55 | 10 | (Lee et al. 1999a) |
| B. Ca2+ as charge carrier (1.25–2 mm) | |||||||||
| Activation | Inactivation | ||||||||
| Channel | Vmax (mV) | V0.5 (mV) | Slope (k) | τact (ms) | Vh (mV) | Slope (k) | τinact (ms) | [Ca2+] (mm) | References |
| CaV1.2 | 0 | −17 | 7 | ND | −50 | −9 | >250 | 2 | (Hu & Marban, 1998) |
| +20 | +18 | 14.8 | ND | −35 | −13.7 | ND | 30 | (Li et al. 2010) | |
| CaV3.1 | −30 | −46 | 4.1 | 1 | −73 | ND | 11 | 1.25 | (Klockner et al. 1999) |
| −28 | −51 | 4.6 | 1.4 | −75 | −5.4 | 17 | 2 | (Chemin et al. 2001) | |
| CaV3.2 | −30 | −46 | 3.7 | 2 | −72 | ND | 16 | 2 | (Klockner et al. 1999) |
| CaV3.3 | −30 | −44 | 4.6 | 7 | −72 | ND | 69 | 1.25 | (Klockner et al. 1999) |
| −30 | −38 | 7.6 | ND | −72 | −5.7 | 67 | 2 | (Chemin et al. 2001) | |
| ND | −45 | 7.6 | 4 | −77 | −6.0 | 76 | 2 | (Lee et al. 1999a) | |
Values taken from studies of HEK-293 cells transfected with the appropriate VDCC subtypes. V0.5 and Vh represent half-maximum voltage of activation and inactivation, respectively, k is the slope of the curve, τact and τinact are activation and inactivation time constants, respectively. ND, not determined.
These distinctly different effects of calcium versus barium on L- and T-type channels could provide a currently underutilised mechanism for probing the molecular basis of novel channel variants that will be described in the next sections.
Window currents and their physiological relevance
Differences in rates of activation, inactivation and deactivation are physiologically relevant in excitable cells such as neurons or cardiomyocytes where timed action potential firing is important (Perez-Reyes, 2003). However, a feature common to all VDCCs, and of importance to phenomena requiring sustained calcium levels, is the window current, which is defined as the range of voltages over which the steady-state activation and inactivation curves overlap (Perez-Reyes, 2003). The window current thus defines the voltage range over which the channel has the capacity to provide persistent calcium influx, as required for vascular tone.
Due to their different voltage dependencies, the window current for L-type channels occurs over membrane potentials which are substantially more depolarised than those for T-type channels (L-type, −30 mV to 0 mV; T-type, −65 mV to −45 mV; Hirano et al. 1989). Furthermore, the size of the window current is smaller through T-type than L-type channels due to differences in voltage sensitivity and degree of overlap of activation and inactivation curves, although the electrochemical driving force for calcium would be greater through classical T-type channels, which open at more hyperpolarised potentials. Surprisingly, the range of membrane potentials recorded in smooth muscle cells of pressurised vessels (−45 to −30 mV; Loutzenhiser et al. 1997; Knot & Nelson, 1998; Welsh & Segal, 1998; Siegl et al. 2005) lies between the window currents of the classical L-type and T-type channels, leading to the quandary that neither of these two channels has the voltage profile to provide the sustained calcium influx necessary for vascular tone. Nevertheless, antagonists against L-type channels have long been known to abolish nearly all myogenic tone of large cerebral arteries (Knot & Nelson, 1998).
Pharmacological antagonism
While selective toxin antagonists are available for discrimination amongst L-, N-, P/Q- and R-type VDCCs (Catterall et al. 2005), such pharmacological selectivity has not been available for identification of T-type channels. Indeed, the most commonly used T-type channel antagonist, mibefradil, has not only been shown to antagonise L-type channels, particularly at concentrations greater than 1 μm, but also to block a number of other ion channels (Nilius et al. 1997; Chouabe et al. 1998; Gomora et al. 1999; Heady et al. 2001; Moosmang et al. 2006; Yoo et al. 2008). While a putatively more selective modification of mibefradil called NNC 55-0396 has been developed (Huang et al. 2004), this compound also exhibits dual L- and T-type effects (Kuo et al. 2010). More promise is held for the R-enantiomer of efonidipine, which has been reported to selectively block T-type channels in ventricular myocytes and rat hippocampal neurons (Furukawa et al. 2004; Tanaka et al. 2004; Shin et al. 2008). The S-enantiomer is considered a dual L- and T-type inhibitor (Furukawa et al. 2004). Nevertheless, in the clinical setting, these dual L- and T-channel blockers may be useful for the treatment of conditions in which both L- and T-type channels may be involved (Ball et al. 2009). They may also be useful to differentiate between L-type and T-type channel mediated effects in experimental studies after selective pharmacological blockade of L-type channels.
Other putative T-type channel blockers include the divalent metal ion nickel (Ni2+) and the scorpion toxin, kurtoxin. Low concentrations of Ni2+ have been used successfully as a research tool to selectively block the CaV3.2 channel; the IC50 of 13 μm being considerably lower than effects on other VDCCs (Lee et al. 1999b). In contrast, kurtoxin was initially reported to selectively antagonise CaV3.1 and 3.2 currents in Xenopus oocytes (Chuang et al. 1998); however, later reports using thalamic neurons have shown partial inhibition of N- and L-type channels at the same concentrations (Sidach & Mintz, 2002). The recent development of a new T-type selective compound, TTA-A2, with reduced sensitivity for other VDCCs (Uebele et al. 2009), may hold the promise of more clearly delineating the contribution of T-type channels in future studies.
Expression and proposed role of T-type VDCCs in systemic circulations
There is no doubt that VDCCs other than L-type channels are expressed in the systemic circulation. In the renal microcirculation, P/Q- (Cav2.1), N- (CaV2.2) and T-type (Cav3.1 and 3.2) channels have been described in addition to L-type (Cav1.2) channels (Hansen et al. 2001; Hayashi et al. 2007) and the differential distribution of L-type and T-type VDCCs in afferent and efferent arterioles has been suggested to control the complex process of glomerular filtration (Feng et al. 2004; Hayashi et al. 2005). Interestingly, a link between intracellular stores and T-type channels has also been suggested since angiotensin II-induced constriction of efferent arterioles due to calcium release from IP3 receptors was inhibited by the T-type channel antagonist mibefradil (Hayashi et al. 2005). While this effect should be confirmed with more selective T-type antagonists, such an association between T-type channels and intracellular stores could also serve to amplify calcium influx via the T-type channel window current. Due to the small size of the afferent and efferent arterioles, patch-clamp electrophysiological studies categorising VDCC currents of constituent smooth muscle cells have not been reported, although a heterogeneous population of cells containing L and T-type currents has been described in larger renal arcuate and interlobar arteries (Gordienko et al. 1994).
Expression of the T-type channels CaV3.1 and CaV3.2 has also been documented in the mesenteric circulation. In large mesenteric arteries, both L-type and T-type channels are expressed (Gustafsson et al. 2001; Ball et al. 2009), while in terminal mesenteric arterioles, T-type channels have been detected without expression of L-, N-, P/Q- or R-type channels (Morita et al. 1999; Gustafsson et al. 2001; Jensen et al. 2004) and physiological responses found to be unresponsive to selective blockade of L-, N-, P/Q- or R-type VDCCs (Morita et al. 1999; Gustafsson et al. 2001; Jensen et al. 2004; Braunstein et al. 2009). Yet surprisingly, in smooth muscle cells isolated from these terminal arterioles, transient HVA, but not LVA, currents were evoked and these currents, although less prominent, were also seen in larger vessels (see Table 2; Morita et al. 1999; Gustafsson et al. 2001; Jensen et al. 2004). These currents were sensitive to T-type channel antagonists, did not show calcium-dependent inactivation and the conductance to barium, although stated to be twice that of calcium, was reminiscent of the more modest effect seen in CaV3.2 and CaV3.3 channels when data for equimolar ion substitution was considered (Morita et al. 1999, 2002).
Table 2.
Comparison of calcium currents recorded from vascular smooth muscle cells
| Activation | Inactivation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Channel | Vmax (mV) | V0.5 (mV) | Slope (k) | τact (ms) | Vh (mV) | Slope (k) | τinact (ms) | Charge carrier (mm) | References |
| Dog basilar L-type | +20 | +3 | 6.5 | ND | −20 | −9 | 1244 | 10 Ba | (Nikitina et al. 2007) |
| Rat basilar L-type | +10 | −8 | 5.4 | 3.8 | ND | ND | 268 | 10 Ba | (Kuo et al. 2010) |
| Dog basilar T-type | −25 | −38 | 7 | 2.9 | −52 | −12 | 164 | 10 Ba | (Nikitina et al. 2007) |
| Mesenteric Non L-type | −10 | −11 | 11 | 4 | −52 | −6 | 28 | 5 Ba | (Morita et al. 1999) |
| Rat basilar Non L-type | +10 | −13 | 6.9 | 1.6 | −53 | −9 | 49 | 10 Ba | (Kuo et al. 2010) |
| Rat basilar L- + non L- | +10 | −9 | 6.1 | 2.5 | −29 | −11 | 269 | 10 Ba | (Kuo et al. 2010) |
Values taken from studies of currents recorded from smooth muscle cells of guinea pig mesenteric arterioles or dog or rat basilar arteries. V0.5 and Vh represent half-maximum voltage of activation and inactivation from −70 mV, respectively, k is the slope of the curve, and τact and τinact are activation and inactivation time constants, respectively. ND, not determined.
Together, these data suggested the expression of VDCCs which exhibit T-type pharmacology but have higher activation thresholds than those reported for T-type channels.
T-Type VDCCs and the cerebral circulation
In the cerebral circulation of juvenile and adult rats, mRNA for the T-type channels CaV3.1 and CaV3.2 is expressed with the L-type channel CaV1.2 and immunohistochemical studies have found protein for these T-type channels in smooth muscle cells and more weakly in endothelial cells (Navarro-Gonzalez et al. 2009; Kuo et al. 2010). These studies employed antibodies which had been raised against extracellular sequences of CaV3.1 and CaV3.2 proteins and previously tested for specificity using transfected HEK cells and Western blotting (Rodman et al. 2005). At the ultrastructural level, immunoreactivity for CaV3.1 and CaV1.2 proteins was found in smooth muscle cell membranes and cytoplasm, but was not colocalised, indicating their potential involvement in different functions (Kuo et al. 2010). In the basilar artery of the dog, CaV3.1 and CaV3.3 channels, but not CaV3.2, were identified in smooth muscle cells (Nikitina et al. 2007), suggesting some species differences in subtype expression. Physiological studies of rat cerebral vessels demonstrated a role for non-L-type VDCCs in vasoconstriction evoked by stretch or intraluminal pressure. These channels were sensitive to putative T-type channel antagonists (Navarro-Gonzalez et al. 2009; Kuo et al. 2010) with a greater prominence in smaller vessels (Fig. 1; Kuo et al. 2010), reminiscent of the non L-type channels in the mesenteric circulation. In the small vessels, responses at low pressures were entirely dependent on T-type channels, while the contribution of L-type channels increased with increasing pressure above 40 mmHg. Although this pressure-dependent effect might result from preferential binding of dihydropyridines to the inactive state of the channel, which would predominate at high rather than low pressures, it does not adequately explain the reduced efficacy of nifedipine in the smaller compared to the larger vessels, nor the apparently pressure-independent contribution of the residual, non-L-type component (Fig. 1).
Figure 1. Effect of L- and T-type blockers on myogenic tone of the basilar artery and branches.
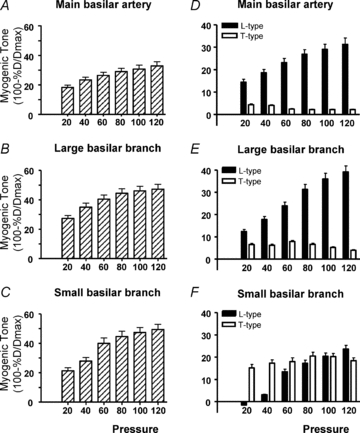
A–C, pressure-induced myogenic tone was less in the main basilar artery (∼300 μm; A) than in small (∼103 μm; C) and large (∼200 μm; B) side branches. D–F, the contribution of L-type channels to myogenic tone decreased as vessel size decreased, while the contribution of T-type channels increased. Nifedipine (1 μmol L−1) alone and in combination with mibefradil (1 μmol L−1) was used to block L- and T-type channels, respectively. Data modified from Kuo et al. 2010.
Of the other HVA channels, expression of N-type channels has been shown in the dog basilar artery. However the functional significance of these channels remains questionable, since selective antagonism was without effect on physiological responses in spite of a small reduction in HVA currents (Nikitina et al. 2007). In contrast, in the adult rat basilar and middle cerebral arteries, weak mRNA expression was found for P/Q- but not N-type channels. The R-type channel was only expressed in the middle cerebral artery where protein was confined to the adventitia, perhaps in association with the perivascular innervation (Kuo et al. 2010). Thus, taken together with the data obtained in the renal and mesenteric circulations, expression and functional contribution of these HVA channels seems to vary with vascular bed, species and vessel size.
Whole cell patch-clamp electrophysiology on isolated cerebrovascular smooth muscle cells has identified HVA currents, with characteristics typical of L-type channels (Table 2; Simard, 1991; Worley et al. 1991; Langton & Standen, 1993; Quayle et al. 1993; Wilde et al. 1994; McHugh & Beech, 1996; Nikitina et al. 2007; Kuo et al. 2010). These currents were sensitive to nanomolar concentrations of dihydropyridines, had inactivation times greater than 1 s and underwent rapid deactivation (<1 ms, Simard, 1991). The molecular identity of the HVA current seems most likely CaV1.2, given the prominent mRNA and protein expression of this subtype in these arteries (Nikitina et al. 2007; Navarro-Gonzalez et al. 2009; Kuo et al. 2010).
While LVA currents were identified in a subpopulation of smooth muscle cells in two studies (Table 2; Wilde et al. 1994; Nikitina et al. 2007) and correlated with expression of CaV3.1 and 3.3 channels (Nikitina et al. 2007), the majority of the whole cell patch-clamp studies found no evidence for the existence of a classical LVA T-type current (Simard, 1991; Worley et al. 1991; Langton & Standen, 1993; Quayle et al. 1993; McHugh & Beech, 1996; Kuo et al. 2010), in spite of the use in some studies of activation protocols from holding potentials sufficiently negative to activate an LVA channel. However, a non-L-type HVA current, which activated and inactivated faster, but deactivated slower, than L-type channels, was identified in two of these studies (Simard, 1991; Kuo et al. 2010). This variability in channel expression could have been influenced by differences in the enzymatic dissociation protocols amongst the studies, as has been reported for other ion channels (Matchkov et al. 2004; Berra-Romani et al. 2005).
The non-L-type HVA current found in one of the studies was blocked by T-type channel antagonists and exhibited biophysical properties similar to the dihydropyridine-insensitive HVA current characterised in the smooth muscle cells of terminal mesenteric arterioles (see Table 2; Kuo et al. 2010). There was also a trend towards a greater prominence of this current in smooth muscle cells isolated from smaller diameter cerebral vessels (Kuo et al. 2010). It should be noted, however, that the selectivity of dihydropyridine block for the inactive state of L-type channels could lead to changes in residual currents. This would be expected to artefactually shorten inactivation times and shift the voltage-dependent profile to more hyperpolarised potentials. However, other changes observed in time constants for activation and deactivation towards more T-type-like values could not be so readily discounted.
Evidence for a physiological role for T-type channels: gene deletion studies
Gene deletion studies might be expected to assist in providing an answer to the question of T-type channel function in the vasculature. Unfortunately the role of Cav3.1 has not yet been directly studied, although Cav3.1 knockout mice clearly demonstrate a cardiac phenotype with bradycardia and slowed atrioventricular conduction, but unchanged blood pressure (Mangoni et al. 2006). Data from CaV3.2 knockout mice point to a paradoxical role of this channel in mediating relaxation of coronary arteries, perhaps due to a functional interaction with calcium activated potassium channels (Chen et al. 2003). Blood pressure was also not altered in these mice (Chiang et al. 2009).
The role of L-type channels in blood pressure regulation has been examined through development of mice with a conditional deletion of CaV1.2 selectively in smooth muscle cells in order to avoid the embryonic lethality of global CaV1.2 deletion (Moosmang et al. 2003). Whilst blood pressure was significantly reduced in these mice and the myogenic response to intravascular pressure abolished in isolated tibialis arteries at intravascular pressures from 50 to 90 mmHg, responses were surprisingly intact at pressures below these levels (Moosmang et al. 2003). These results point to a role for additional VDCC subtypes at lower pressures, reminiscent of the data obtained in small cerebral arteries (Fig. 1; Kuo et al. 2010).
The conditional CaV1.2 knockout model was subsequently used to test whether the putative T-type channel blocker mibefradil exerted its effects on vascular tone and blood pressure via non-selective actions on L-type channels (Moosmang et al. 2006). Consistent with this proposed non-selective action, mibefradil failed to affect blood flow in an isolated hind limb preparation and the already lowered systemic blood pressure of these knockout mice (Moosmang et al. 2006). However, the authors also reported that systemic application of mibefradil induced substantial increases in heart rate, both in control mice and those lacking smooth muscle cell CaV1.2. Whilst reflex tachycardia would be expected in wild-type mice, due to peripheral vasodilatation following non-selective effects of mibefradil on vascular L-type channels, parallel effects in the smooth muscle cells of CaV1.2 knockout mice cannot be ascribed to such an L-type channel action. These data therefore suggest that, contrary to the authors’ conclusion, mibefradil does act on non-L-type channels present in the peripheral vasculature and that these channels control myogenic tone at lower pressures.
T-Type channels in the endothelium
Recent reports have described expression of CaV3.2 and also Cav3.1 channels in the endothelium of mesenteric and cerebral arteries (Braunstein et al. 2009; Kuo et al. 2010). However, such an observation is controversial since numerous patch-clamp electrophysiological studies on cultured endothelial cells from a variety of conduit vessels, including aorta (e.g. Takeda et al. 1987), pulmonary artery (e.g. Bregestovski & Ryan, 1989) and coronary artery (Daut et al. 1988), have failed to find any evidence for these voltage-dependent channels. In contrast, experiments using endothelial cells from bovine adrenal medullary capillaries did reveal three distinct VDCCs (Bossu et al. 1989, 1992a,b): a low-voltage activated, fast inactivating channel, a typical long lasting L-type channel that was sensitive to Bay K and nitrendipine, and an L-type-like channel that was not sensitive to nitrendipine. In a subsequent reinvestigation of these endothelial cells, L- and T-type channels were described, with L-type channels absent from some 35% of the cells (Vinet & Vargas, 1999).
More recent studies (Wu et al. 2003) have demonstrated that pulmonary microvascular endothelial cells express mRNA for CaV3.1 and that the voltage-dependent currents have a right-shifted activation curve, resulting in a window current that is more depolarised than typical T-type channels (−60 mV to −30 mV), and within the range of membrane potentials measured in isolated endothelial cells and those in pressurised vessels (McSherry et al. 2005; Yamamoto & Suzuki, 2005). These currents were sensitive to the T-type channel blockers flunarazine, kurtoxin, amiloride and pimozide, but with the lower sensitivity to Ni2+ of CaV3.1 channels (Wu et al. 2003). In the same studies, VDCC currents were absent from endothelial cells isolated from larger pulmonary arteries (Wu et al. 2003). These studies might therefore suggest that smaller vessels can express T-type channels in the endothelial layer, while larger conduit vessels and cultured endothelial cells do not.
Expression of T-type channels within the endothelium may have ramifications for the interpretation of the paradoxically constricted coronary arteries found in the CaV3.2 deficient mice (Chen et al. 2003). Since small (SKCa) and intermediate conductance calcium-activated potassium channels (IKCa) are expressed in the endothelium of the resistance vasculature and a link between T-type channels and SKCa channels has recently been found in dendrites of thalamic neurons (Cueni et al. 2008), it is tempting to suggest that endothelial T-type channels play a role in feedback vasodilatation of the peripheral circulation (see Fig. 2).
Figure 2. Feedforward and feedback control of vascular tone by smooth muscle and endothelial voltage dependent calcium channels (VDCCs).
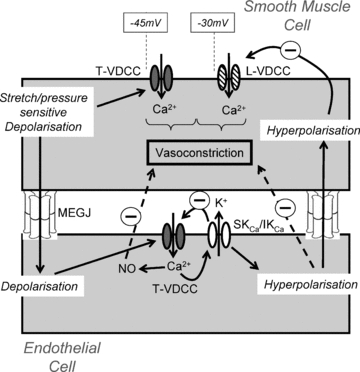
Physical distension of the vascular wall due to increasing intraluminal pressure activates mechano-sensitive channels leading to depolarisation of smooth muscle cells. At lower pressures, this depolarisation activates T-type VDCC variants (T-VDCC) leading to calcium influx and vasoconstriction. Further depolarisation activates higher threshold L-type channels (L-VDCC), augmenting vasoconstriction. Depolarisation of smooth muscle spreads electrotonically to the endothelium via myoendothelial gap junctions (MEGJs) where it activates endothelial T-type channels. Influx of calcium leads to synthesis of nitric oxide (NO) and additionally the opening of small and/or intermediate conductance potassium channels (SKCa/IKCa). The resultant hyperpolarisation, which is electrotonically transmitted back into the smooth muscle cells, along with diffusion of NO, acts as a feedback brake to excessive tonic constriction.
Modulation of T-type channel activity
Although the main electrophysiological and pharmacological characteristics of VDCCs are largely determined by the α1 pore-forming unit, interactions with the auxiliary subunits, α2δ, β and γ, can influence biophysical characteristics of the channels, as well as protein trafficking, effects of pharmacological agents and phosphorylation status (Dolphin, 2009). In the case of the HVA channels, co-assembly with the membrane-anchored extracellular subunit α2δ and the cytoplasmic β subunit is required for functional activity. In contrast, the T-type channels do not require co-assembly with these subunits for functional activity (Perez-Reyes, 2006). In fact, T-type channels lack the β subunit binding site in the I–II linker region and to date there are no studies that have shown direct binding of the T-type channel pore forming α1 subunit with α2δ and γ subunits. However, these auxiliary subunits still appear capable of modulating T-type activity. Thus in recombinant systems, when T-type channels are co-expressed with auxiliary subunits, there is increased membrane expression with α2δ (Dolphin et al. 1999), enhanced deactivation with γ2, but not γ3 or 4 (Green et al. 2001), decreased expression with γ6, but not γ4 or γ7 (Hansen et al. 2004), and accelerated opening and closing rates with α2δ and γ5 subunits (Lacinova & Klugbauer, 2004). More importantly, in mouse neuroblastoma/glioma hybrid cells, expressing endogenous LVA channels, overexpression of β2a and α2δ subunits induced a sustained high voltage activated current (Wyatt et al. 1998), while α2δ subunits shifted the steady state inactivation of Cav3.1 channels to more depolarised potentials (Hobom et al. 2000). However, it should be noted that in small mesenteric arterioles, in which L-type channels are absent but T-type channels are expressed and necessary for vasoconstriction, the β subunits, β1b, β2 and β3, are absent (Jensen et al. 2004).
Splice variants
Electrophysiological or pharmacological properties of VDCCs can also be altered by variations in mRNA sequence produced by alternative splicing of particular exons. Recent cloning studies have revealed novel splice variations of the L-type (CaV1.2) channels expressed in smooth muscle cells isolated from small cerebral arteries of the rat (Cheng et al. 2007; Liao et al. 2007; Cheng et al. 2009). Studies in HEK cells showed that the window currents of these variants were shifted by 10 mV to more hyperpolarised potentials, resulting in better overlap with the physiological range of membrane potentials found in vascular smooth muscle cells. While these L-type splice variants are therefore attractive candidates for the HVA currents identified in mesenteric and cerebral circulations, their unchanged or augmented sensitivities to dihydropyridines are remarkably inconsistent (Liao et al. 2007; Cheng et al. 2009). Indeed, the L-type splice variant with higher affinity for dihydropyridines is specifically expressed in vascular smooth muscle, while the splice variant with reduced sensitivity is expressed in cardiac myocytes (Welling et al. 1997).
Like CaV1.2, CaV3.1 and Cav3.2 channels are subject to extensive splicing with the majority of changes occurring in the linker and C-terminal regions, where channel function would be expected to be modulated (Mittman et al. 1999; Chemin et al. 2001; Emerick et al. 2006; Zhong et al. 2006). Our studies in juvenile rat basilar arteries have identified a splice variant encoding a large deletion in the I–II linker of CaV3.1 (Shcheglovitov et al. 2008; Navarro-Gonzalez et al. 2009), which resulted in increased channel expression at the membrane without change in voltage sensitivity or kinetics (Shcheglovitov et al. 2008). In contrast, alternative splicing in human Exons 25/26 in the III–IV linker region of both Cav3.1 and Cav3.2 results in shifts of the activation voltage and window currents to more depolarised potentials in recombinant systems (Chemin et al. 2001; Zhong et al. 2006). Interestingly, our unpublished experiments have identified one such variant (25 bc) to be the most prevalently expressed form in cerebral arteries (I. Y.-T. Kuo, B. K. Baillie and C. E. Hill, unpublished observations). This variant has been previously reported in developing mouse heart, where nifedipine-insensitive currents were comparatively high-voltage activated (Pignier & Potreau, 2000). Collectively, these data suggest that the 25 bc splice variant could underlie the depolarised profile of the dihydropyridine insensitive current reported in mesenteric and cerebral smooth muscle cells. The more hyperpolarised profile of this variant when expressed in HEK cells (Chemin et al. 2001) may suggest that the voltage dependence of gating of T-type channel variants can be further influenced by proteins endogenous to smooth muscle cells. While this role might be assumed by auxiliary subunits, it is also possible that distinct channels comprising particular combinations of splice variants at different sites, or with different post-translational modifications, could further change the voltage-dependent profile (Chemin et al. 2001; Zhong et al. 2006; Iftinca et al. 2007).
Splice variants of the L- and T-type channels identified in cerebral vessels would therefore result in a movement to more depolarised potentials for T-type channels and to more hyperpolarised potentials for L-type channels, leading to a better overlap of window currents with the normal physiological range of membrane potentials experienced by smooth muscle cells in pressurised vessels.
Physiological and pathophysiological relevance
Expression of both L- and T-type channel splice variants with altered voltage dependencies would widen the voltage window mediating persistent calcium current and hence the intraluminal pressure range over which sustained calcium influx could occur, an effect seen in small cerebral vessels (Fig. 1). Thus, myogenic tone at low pressures would be dominated by T-type channel variants but by L-type channel variants at higher pressures. The greater contribution of dihydropyridine insensitive T-type channels to total current observed in smaller vessels (Morita et al. 1999; Kuo et al. 2010) may thus serve to better match the capacity for sustained calcium influx at the lower intraluminal pressures experienced by smaller resistance vessels.
The apparently pressure-independent involvement of T-type channels in small cerebral vessels (Fig. 1) seems inconsistent with the restricted voltage sensitivity of a window current. One explanation could be that the voltage window encompassing the myogenic response to intraluminal pressure is in fact confined to a relatively narrow range of membrane potentials. Such an effect has indeed been demonstrated in skeletal muscle arterioles where the entire myogenic response operating from 30 to 100 mmHg was accompanied by only a 10 mV change in membrane potential from ∼−45 to −35 mV (Kotecha & Hill, 2005). Such a narrow voltage range is entirely compatible with the voltage spread of VDCC window currents. Nevertheless, conclusions regarding the role of putative T-type channels in small cerebral arteries cannot be made until the resting membrane potentials of the smooth muscle cells in these cerebral vessels are measured and compared with the voltage sensitivities of the prevalent VDCC subtypes and splice variants. Certainly the proposal that the small branches sit at more negative membrane potentials than do the main arteries in the cerebral circulation is consistent with the substantial drop in pressure which occurs across the large vessels (Faraci & Heistad, 1990), presumably serving to protect small penetrating arterioles. However, the study of skeletal muscle arterioles mentioned above (Kotecha & Hill, 2005) demonstrates that these lower pressures may not be accompanied by membrane potentials which are substantially more hyperpolarised than those recorded in vessels with myogenic tone.
As vessel size decreases, myoendothelial coupling between muscle and endothelial cell layers is increased (Hill et al. 2001). Thus mechano-sensitive channels which transpose intraluminal pressure signals into depolarisation of smooth muscle cells will also result in depolarisation of the endothelium (see Fig. 2). Subsequent activation of endothelial Cav3.1 and Cav3.2 channels could elevate endothelial calcium, increasing synthesis of nitric oxide and also leading to activation of endothelial SKCa and IKCa channels. This interaction is likely to occur in a microdomain, for example, caveolae, since depolarisation of endothelial cells is not normally associated with a global increase in calcium. The resultant diffusion of nitric oxide, coupled with the electrotonic transfer of hyperpolarisation through myoendothelial gap junctions into the smooth muscle cells, will act together to provide a feedback brake on excessive vasoconstriction (Fig. 2).
Under pathophysiological conditions, this balance is perturbed. Clinicians have struggled with the inefficacy of L-type channel antagonists to relieve potentially fatal delayed cerebral vasospasm that occurs in 30–70% of patients after subarachnoid haemorrhage. Factors contributing to this pathological constriction are multiple including a generalised reduction in nitric oxide bioavailability. More recently a role for altered expression of voltage dependent calcium channels has been recognised. Expression of L-type channels, the current target of calcium channel antagonism, has been shown to be reduced under these pathological conditions, while upregulation of both R- and T-type channels occurs in animal models of subarachnoid haemorrhage (Ishiguro et al. 2005; Nikitina et al. 2010). This possibly explains the failure of L-type channel blockade to relieve delayed vasospasm (Dorhout Mees et al. 2007; Tomassoni et al. 2008). Intriguingly, splice variations of calcium channels have been shown to be more prevalent in experimental hypertension (Tang et al. 2008).
Due to the complex control of blood pressure the successful treatment of essential hypertension remains a medical challenge and it is not surprising that no single compound can successfully reduce blood pressure in all patients. The blockade of T-type channels was previously targeted with the commercial launch of mibefradil in 1997. Unfortunately mibefradil was withdrawn from the market only a year later due to adverse drug interactions in a small group of patients, and the potential role of T-type channel antagonism in the treatment of patients with therapy-resistant hypertension was never assessed.
The future
While we have interpreted data collated here as evidence for the expression of T-type channels and their involvement in vascular function, some limitations of these studies should be reiterated. While mRNA studies have clearly identified expression of Cav3 subtypes in cerebral, renal and mesenteric vascular beds, the reliability of protein distribution data depends critically on the characterisation and specificity of the antibodies used. Confirmation of T-type channel expression in both endothelial cells and smooth muscle cells requires additional whole cell patch clamp studies in freshly isolated cells from resistance vessels. Such studies need to test the effect of different cellular isolation procedures and employ more selective antagonists. The development of new T-type channel selective compounds (Uebele et al. 2009) presents an exciting opportunity for these investigations, which should also address the involvement of Cav2 channels (N, P/Q and R), given their heterogeneous expression in different vascular beds and the availability of selective toxin antagonists.
The development of both Cav3.1 and Cav3.2 knockout mice provides an additional avenue for future experiments, although the potential for compensatory change remains a general challenge in gene deletion studies until a conditional model is available. Future studies need to determine the molecular identity of the dihydropyridine-insensitive HVA currents described in small mesenteric and cerebral arteries, and determine whether the biophysical properties of naturally occurring splice variants can be modified by auxiliary subunits or other endogenous smooth muscle cell proteins.
What is clear is that vasoconstriction in a number of small vessels cannot be prevented by classes of L-type calcium channel antagonists that are used clinically. Moreover these drugs fail to reverse cerebral vasospasm and therapy resistant hypertension or their debilitating consequences. This in itself justifies a more intensive effort to uncover the molecular identity of the channels responsible for these responses and in turn the development of more selective antagonists.
Acknowledgments
The authors acknowledge financial support from the National Health and Medical Research Council of Australia (ID 471420).
Glossary
Abbreviations
- HVA
high voltage activated
- LVA
low voltage activated
- VDCC
voltage dependent calcium channel
Author contributions
All authors contributed to the design, drafting and critical revision of the manuscript and have given their final approval for submission.
References
- Ball CJ, Wilson DP, Turner SP, Saint DA, Beltrame JF. Heterogeneity of L- and T-channels in the vasculature: rationale for the efficacy of combined L- and T-blockade. Hypertension. 2009;53:654–660. doi: 10.1161/HYPERTENSIONAHA.108.125831. [DOI] [PubMed] [Google Scholar]
- Berra-Romani R, Blaustein MP, Matteson DR. TTX-sensitive voltage-gated Na+ channels are expressed in mesenteric artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289:H137–145. doi: 10.1152/ajpheart.01156.2004. [DOI] [PubMed] [Google Scholar]
- Bossu JL, Elhamdani A, Feltz A. Voltage-dependent calcium entry in confluent bovine capillary endothelial cells. FEBS Lett. 1992a;299:239–242. doi: 10.1016/0014-5793(92)80123-x. [DOI] [PubMed] [Google Scholar]
- Bossu JL, Elhamdani A, Feltz A, Tanzi F, Aunis D, Thierse D. Voltage-gated Ca entry in isolated bovine capillary endothelial cells: evidence of a new type of BAY K 8644-sensitive channel. Pflugers Arch. 1992b;420:200–207. doi: 10.1007/BF00374991. [DOI] [PubMed] [Google Scholar]
- Bossu JL, Feltz A, Rodeau JL, Tanzi F. Voltage-dependent transient calcium currents in freshly dissociated capillary endothelial cells. FEBS Lett. 1989;255:377–380. doi: 10.1016/0014-5793(89)81126-3. [DOI] [PubMed] [Google Scholar]
- Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, Jensen LJ. The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res. 2009;46:138–151. doi: 10.1159/000151767. [DOI] [PubMed] [Google Scholar]
- Bregestovski PD, Ryan US. Voltage-gated and receptor-mediated ionic currents in the membrane of endothelial cells. J Mol Cell Cardiol. 1989;21(Suppl 1):103–108. doi: 10.1016/0022-2828(89)90844-4. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Cens T, Rousset M, Leyris JP, Fesquet P, Charnet P. Voltage- and calcium-dependent inactivation in high voltage-gated Ca2+ channels. Prog Biophys Mol Biol. 2006;90:104–117. doi: 10.1016/j.pbiomolbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P. Alternatively spliced α1G (CaV3.1) intracellular loops promote specific T-type Ca2+ channel gating properties. Biophys J. 2001;80:1238–1250. doi: 10.1016/S0006-3495(01)76100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in α1H T-type Ca2+ channels. Science. 2003;302:1416–1418. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel Ca(V)1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem. 2007;282:29211–29221. doi: 10.1074/jbc.M610623200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Pachuau J, Blaskova E, Asuncion-Chin M, Liu J, Dopico AM, Jaggar JH. Alternative splicing of Cav1.2 channel exons in smooth muscle cells of resistance-size arteries generates currents with unique electrophysiological properties. Am J Physiol Heart Circ Physiol. 2009;297:H680–688. doi: 10.1152/ajpheart.00109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CS, Huang CH, Chieng H, Chang YT, Chang D, Chen JJ, Chen YC, Chen YH, Shin HS, Campbell KP, Chen CC. The Cav3.2 T-type Ca2+ channel is required for pressure overload-induced cardiac hypertrophy in mice. Circ Res. 2009;104:522–530. doi: 10.1161/CIRCRESAHA.108.184051. [DOI] [PubMed] [Google Scholar]
- Chouabe C, Drici MD, Romey G, Barhanin J, Lazdunski M. HERG and KvLQT1/IsK, the cardiac K+ channels involved in long QT syndromes, are targets for calcium channel blockers. Mol Pharmacol. 1998;54:695–703. [PubMed] [Google Scholar]
- Chuang RS, Jaffe H, Cribbs L, Perez-Reyes E, Swartz KJ. Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat Neurosci. 1998;1:668–674. doi: 10.1038/3669. [DOI] [PubMed] [Google Scholar]
- Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, Franken P, Adelman JP, Luthi A. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- Daut J, Mehrke G, Nees S, Newman WH. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol. 1988;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Dolphin AC, Wyatt CN, Richards J, Beattie RE, Craig P, Lee JH, Cribbs LL, Volsen SG, Perez-Reyes E. The effect of α2-δ and other accessory subunits on expression and properties of the calcium channel α1G. J Physiol. 1999;519:35–45. doi: 10.1111/j.1469-7793.1999.0035o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, Van Den Bergh WM, Vermeulen M, van Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007:CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerick MC, Stein R, Kunze R, McNulty MM, Regan MR, Hanck DA, Agnew WS. Profiling the array of CaV3.1 variants from the human T-type calcium channel gene CACNA1G: alternative structures, developmental expression, and biophysical variations. Proteins. 2006;64:320–342. doi: 10.1002/prot.20877. [DOI] [PubMed] [Google Scholar]
- Epstein M. Resistant hypertension: prevalence and evolving concepts. J Clin Hypertens (Greenwich) 2007;9:2–6. doi: 10.1111/j.1524-6175.2007.06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- Feng MG, Li M, Navar LG. T-type calcium channels in the regulation of afferent and efferent arterioles in rats. Am J Physiol Renal Physiol. 2004;286:F331–337. doi: 10.1152/ajprenal.00251.2003. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Miura R, Honda M, Kamiya N, Mori Y, Takeshita S, Isshiki T, Nukada T. Identification of R(–)-isomer of efonidipine as a selective blocker of T-type Ca2+ channels. Br J Pharmacol. 2004;143:1050–1057. doi: 10.1038/sj.bjp.0705944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomora JC, Enyeart JA, Enyeart JJ. Mibefradil potently blocks ATP-activated K+ channels in adrenal cells. Mol Pharmacol. 1999;56:1192–1197. doi: 10.1124/mol.56.6.1192. [DOI] [PubMed] [Google Scholar]
- Gordienko DV, Clausen C, Goligorsky MS. Ionic currents and endothelin signaling in smooth muscle cells from rat renal resistance arteries. Am J Physiol Renal Physiol. 1994;266:F325–341. doi: 10.1152/ajprenal.1994.266.2.F325. [DOI] [PubMed] [Google Scholar]
- Green PJ, Warre R, Hayes PD, McNaughton NC, Medhurst AD, Pangalos M, Duckworth DM, Randall AD. Kinetic modification of the α1I subunit-mediated T-type Ca2+ channel by a human neuronal Ca2+ channel γ subunit. J Physiol. 2001;533:467–478. doi: 10.1111/j.1469-7793.2001.0467a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson F, Andreasen D, Salomonsson M, Jensen BL, Holstein-Rathlou N. Conducted vasoconstriction in rat mesenteric arterioles: role for dihydropyridine-insensitive Ca2+ channels. Am J Physiol Heart Circ Physiol. 2001;280:H582–590. doi: 10.1152/ajpheart.2001.280.2.H582. [DOI] [PubMed] [Google Scholar]
- Hansen JP, Chen RS, Larsen JK, Chu PJ, Janes DM, Weis KE, Best PM. Calcium channel γ6 subunits are unique modulators of low voltage-activated (Cav3.1) calcium current. J Mol Cell Cardiol. 2004;37:1147–1158. doi: 10.1016/j.yjmcc.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res. 2001;89:630–638. doi: 10.1161/hh1901.097126. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Wakino S, Homma K, Sugano N, Saruta T. Pathophysiological significance of T-type Ca2+ channels: role of T-type Ca2+ channels in renal microcirculation. J Pharmacol Sci. 2005;99:221–227. doi: 10.1254/jphs.fmj05002x6. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T. Ca2+ channel subtypes and pharmacology in the kidney. Circ Res. 2007;100:342–353. doi: 10.1161/01.RES.0000256155.31133.49. [DOI] [PubMed] [Google Scholar]
- Heady TN, Gomora JC, Macdonald TL, Perez-Reyes E. Molecular pharmacology of T-type Ca2+ channels. Jpn J Pharmacol. 2001;85:339–350. doi: 10.1254/jjp.85.339. [DOI] [PubMed] [Google Scholar]
- Hill CE, Phillips JK, Sandow SL. Heterogeneous control of blood flow amongst different vascular beds. Med Res Rev. 2001;21:1–60. doi: 10.1002/1098-1128(200101)21:1<1::aid-med1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Fozzard HA, January CT. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol Heart Circ Physiol. 1989;256:H1478–1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Hobom M, Dai S, Marais E, Lacinova L, Hofmann F, Klugbauer N. Neuronal distribution and functional characterization of the calcium channel α2δ-2 subunit. Eur J Neurosci. 2000;12:1217–1226. doi: 10.1046/j.1460-9568.2000.01009.x. [DOI] [PubMed] [Google Scholar]
- Hu H, Marban E. Isoform-specific inhibition of L-type calcium channels by dihydropyridines is independent of isoform-specific gating properties. Mol Pharmacol. 1998;53:902–907. [PubMed] [Google Scholar]
- Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS, Li M. NNC 55–0396 [(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Ther. 2004;309:193–199. doi: 10.1124/jpet.103.060814. [DOI] [PubMed] [Google Scholar]
- Iftinca M, Hamid J, Chen L, Varela D, Tadayonnejad R, Altier C, Turner RW, Zamponi GW. Regulation of T-type calcium channels by Rho-associated kinase. Nat Neurosci. 2007;10:854–860. doi: 10.1038/nn1921. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Wellman TL, Honda A, Russell SR, Tranmer BI, Wellman GC. Emergence of a R-type Ca2+ channel (CaV 2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage. Circ Res. 2005;96:419–426. doi: 10.1161/01.RES.0000157670.49936.da. [DOI] [PubMed] [Google Scholar]
- Jensen LJ, Salomonsson M, Jensen BL, Holstein-Rathlou NH. Depolarization-induced calcium influx in rat mesenteric small arterioles is mediated exclusively via mibefradil-sensitive calcium channels. Br J Pharmacol. 2004;142:709–718. doi: 10.1038/sj.bjp.0705841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku T, Lee TS, Arita M, Hadama T, Ono K. The gating and conductance properties of Cav3.2 low-voltage-activated T-type calcium channels. Jpn J Physiol. 2003;53:165–172. doi: 10.2170/jjphysiol.53.165. [DOI] [PubMed] [Google Scholar]
- Klockner U, Lee JH, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned α1 subunits, α1G, α1H and α1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci. 1999;11:4171–4178. doi: 10.1046/j.1460-9568.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508:199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. α1D (Cav1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Kotecha N, Hill MA. Myogenic contraction in rat skeletal muscle arterioles: smooth muscle membrane potential and Ca2+ signaling. Am J Physiol Heart Circ Physiol. 2005;289:H1326–1334. doi: 10.1152/ajpheart.00323.2005. [DOI] [PubMed] [Google Scholar]
- Kuo IY, Ellis A, Seymour VA, Sandow SL, Hill CE. Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries. J Cereb Blood Flow Metab. 2010;30:1226–1239. doi: 10.1038/jcbfm.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacinova L, Klugbauer N. Modulation of gating currents of the Cav3.1 calcium channel by α2δ2a and γ5 subunits. Arch Biochem Biophys. 2004;425:207–213. doi: 10.1016/j.abb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Langton PD, Standen NB. Calcium currents elicited by voltage steps and steady voltages in myocytes isolated from the rat basilar artery. J Physiol. 1993;469:535–548. doi: 10.1113/jphysiol.1993.sp019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klockner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999a;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J. 1999b;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang X, Gao G, Qu D, Yu B, Huang C, Elmslie KS, Peterson BZ. A single amino acid change in Cav1.2 channels eliminates the permeation and gating differences between Ca2+ and Ba2+ J Membr Biol. 2010;233:23–33. doi: 10.1007/s00232-009-9221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of CaV1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc Res. 2005;68:197–203. doi: 10.1016/j.cardiores.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. A smooth muscle CaV1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J Biol Chem. 2007;282:35133–35142. doi: 10.1074/jbc.M705478200. [DOI] [PubMed] [Google Scholar]
- Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: actions of angiotensin II. Am J Physiol Renal Physiol. 1997;273:F307–314. doi: 10.1152/ajprenal.1997.273.2.F307. [DOI] [PubMed] [Google Scholar]
- Low AM, Kotecha N, Neild TO, Kwan CY, Daniel EE. Relative contributions of extracellular Ca2+ and Ca2+ stores to smooth muscle contraction in arteries and arterioles of rat, guinea-pig, dog and rabbit. Clin Exp Pharmacol Physiol. 1996;23:310–316. doi: 10.1111/j.1440-1681.1996.tb02829.x. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/α1G T-type calcium channels. Circ Res. 2006;98:1422–1430. doi: 10.1161/01.RES.0000225862.14314.49. [DOI] [PubMed] [Google Scholar]
- Matchkov VV, Aalkjaer C, Nilsson H. A cyclic GMP-dependent calcium-activated chloride current in smooth-muscle cells from rat mesenteric resistance arteries. J Gen Physiol. 2004;123:121–134. doi: 10.1085/jgp.200308972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Beech DJ. Modulation of Ca2+ channel activity by ATP metabolism and internal Mg2+ in guinea-pig basilar artery smooth muscle cells. J Physiol. 1996;492:359–376. doi: 10.1113/jphysiol.1996.sp021314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSherry IN, Spitaler MM, Takano H, Dora KA. Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries. Cell Calcium. 2005;38:23–33. doi: 10.1016/j.ceca.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Mittman S, Guo J, Agnew WS. Structure and alternative splicing of the gene encoding α1G, a human brain T calcium channel α1 subunit. Neurosci Lett. 1999;274:143–146. doi: 10.1016/s0304-3940(99)00716-8. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Bruderl B, Welling A, Hofmann F. Antihypertensive effects of the putative T-type calcium channel antagonist mibefradil are mediated by the L-type calcium channel Cav1.2. Circ Res. 2006;98:105–110. doi: 10.1161/01.RES.0000197851.11031.9c. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J. 2003;22:6027–6034. doi: 10.1093/emboj/cdg583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Cousins H, Onoue H, Ito Y, Inoue R. Predominant distribution of nifedipine-insensitive, high voltage-activated Ca2+ channels in the terminal mesenteric artery of guinea pig. Circ Res. 1999;85:596–605. doi: 10.1161/01.res.85.7.596. [DOI] [PubMed] [Google Scholar]
- Morita H, Shi J, Ito Y, Inoue R. T-channel-like pharmacological properties of high voltage-activated, nifedipine-insensitive Ca2+ currents in the rat terminal mesenteric artery. Br J Pharmacol. 2002;137:467–476. doi: 10.1038/sj.bjp.0704892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Gonzalez MF, Grayson TH, Meaney KR, Cribbs LL, Hill CE. Non-L-type voltage-dependent calcium channels control vascular tone of the rat basilar artery. Clin Exp Pharmacol Physiol. 2009;36:55–66. doi: 10.1111/j.1440-1681.2008.05035.x. [DOI] [PubMed] [Google Scholar]
- Nikitina E, Kawashima A, Takahashi M, Zhang ZD, Shang X, Ai J, Macdonald RL. Alteration in voltage-dependent calcium channels in dog basilar artery after subarachnoid hemorrhage. J Neurosurg. 2010;113:870–880. doi: 10.3171/2010.2.JNS091038. [DOI] [PubMed] [Google Scholar]
- Nikitina E, Zhang ZD, Kawashima A, Jahromi BS, Bouryi VA, Takahashi M, Xie A, Macdonald RL. Voltage-dependent calcium channels of dog basilar artery. J Physiol. 2007;580:523–541. doi: 10.1113/jphysiol.2006.126128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Kamouchi M, Viana F, Voets T, Droogmans G. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca2+- and volume-activated Cl– channels in macrovascular endothelial cells. Br J Pharmacol. 1997;121:547–555. doi: 10.1038/sj.bjp.0701140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular characterization of T-type calcium channels. Cell Calcium. 2006;40:89–96. doi: 10.1016/j.ceca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- Pignier C, Potreau D. Characterization of nifedipine-resistant calcium current in neonatal rat ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H2259–2268. doi: 10.1152/ajpheart.2000.279.5.H2259. [DOI] [PubMed] [Google Scholar]
- Quayle JM, McCarron JG, Asbury JR, Nelson MT. Single calcium channels in resistance-sized cerebral arteries from rats. Am J Physiol Heart Circ Physiol. 1993;264:H470–478. doi: 10.1152/ajpheart.1993.264.2.H470. [DOI] [PubMed] [Google Scholar]
- Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, Hoedt-Miller M, Tada Y, Li KX, Cool C, Fagan K, Cribbs L. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res. 2005;96:864–872. doi: 10.1161/01.RES.0000163066.07472.ff. [DOI] [PubMed] [Google Scholar]
- Shcheglovitov A, Kostyuk P, Shuba Y. Selectivity signatures of three isoforms of recombinant T-type Ca2+ channels. Biochim Biophys Acta. 2007;1768:1406–1419. doi: 10.1016/j.bbamem.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Shcheglovitov A, Vitko I, Bidaud I, Baumgart JP, Navarro-Gonzalez MF, Grayson TH, Lory P, Hill CE, Perez-Reyes E. Alternative splicing within the I–II loop controls surface expression of T-type CaV3.1 calcium channels. FEBS Lett. 2008;582:3765–3770. doi: 10.1016/j.febslet.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MC, Kim CJ, Min BI, Ogawa S, Tanaka E, Akaike N. A selective T-type Ca2+ channel blocker R(–) efonidipine. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:411–421. doi: 10.1007/s00210-007-0239-6. [DOI] [PubMed] [Google Scholar]
- Sidach SS, Mintz IM. Kurtoxin, a gating modifier of neuronal high- and low-threshold Ca channels. J Neurosci. 2002;22:2023–2034. doi: 10.1523/JNEUROSCI.22-06-02023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- Simard JM. Calcium channel currents in isolated smooth muscle cells from the basilar artery of the guinea pig. Pflugers Arch. 1991;417:528–536. doi: 10.1007/BF00370950. [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Somlyo AP. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968;159:129–145. [PubMed] [Google Scholar]
- Takeda K, Schini V, Stoeckel H. Voltage-activated potassium, but not calcium currents in cultured bovine aortic endothelial cells. Pflugers Arch. 1987;410:385–393. doi: 10.1007/BF00586515. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Komikado C, Shimada H, Takeda K, Namekata I, Kawanishi T, Shigenobu K. The R(–)-enantiomer of efonidipine blocks T-type but not L-type calcium current in guinea pig ventricular myocardium. J Pharmacol Sci. 2004;96:499–501. doi: 10.1254/jphs.rcj04001x. [DOI] [PubMed] [Google Scholar]
- Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human L-type voltage-gated calcium channel, Cav1.2 α1 subunit. J Biol Chem. 2004;279:44335–44343. doi: 10.1074/jbc.M407023200. [DOI] [PubMed] [Google Scholar]
- Tang ZZ, Liao P, Li G, Jiang FL, Yu D, Hong X, Yong TF, Tan G, Lu S, Wang J, Soong TW. Differential splicing patterns of L-type calcium channel CaV1.2 subunit in hearts of Spontaneously Hypertensive Rats and Wistar Kyoto Rats. Biochim Biophys Acta. 2008;1783:118–130. doi: 10.1016/j.bbamcr.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Tomassoni D, Lanari A, Silvestrelli G, Traini E, Amenta F. Nimodipine and its use in cerebrovascular disease: evidence from recent preclinical and controlled clinical studies. Clin Exp Hypertens. 2008;30:744–766. doi: 10.1080/10641960802580232. [DOI] [PubMed] [Google Scholar]
- Uebele VN, Gotter AL, Nuss CE, Kraus RL, Doran SM, Garson SL, Reiss DR, Li Y, Barrow JC, Reger TS, Yang ZQ, Ballard JE, Tang C, Metzger JM, Wang SP, Koblan KS, Renger JJ. Antagonism of T-type calcium channels inhibits high-fat diet-induced weight gain in mice. J Clin Invest. 2009;119:1659–1667. doi: 10.1172/JCI36954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C, Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]
- Vinet R, Vargas FF. L- and T-type voltage-gated Ca2+ currents in adrenal medulla endothelial cells. Am J Physiol Heart Circ Physiol. 1999;276:H1313–1322. doi: 10.1152/ajpheart.1999.276.4.H1313. [DOI] [PubMed] [Google Scholar]
- Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the α1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997;81:526–532. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol Heart Circ Physiol. 1998;274:H178–186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- Wilde DW, Furspan PB, Szocik JF. Calcium current in smooth muscle cells from normotensive and genetically hypertensive rats. Hypertension. 1994;24:739–746. doi: 10.1161/01.hyp.24.6.739. [DOI] [PubMed] [Google Scholar]
- Worley JF, Quayle JM, Standen NB, Nelson MT. Regulation of single calcium channels in cerebral arteries by voltage, serotonin, and dihydropyridines. Am J Physiol Heart Circ Physiol. 1991;261:H1951–1960. doi: 10.1152/ajpheart.1991.261.6.H1951. [DOI] [PubMed] [Google Scholar]
- Wu S, Haynes J, Jr, Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T. Cav3.1 (α1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res. 2003;93:346–353. doi: 10.1161/01.RES.0000087148.75363.8F. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Page KM, Berrow NS, Brice NL, Dolphin AC. The effect of overexpression of auxiliary Ca2+ channel subunits on native Ca2+ channel currents in undifferentiated mammalian NG108-15 cells. J Physiol. 1998;510:347–360. doi: 10.1111/j.1469-7793.1998.347bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Duling BR. Patterns of excitation-contraction coupling in arterioles: dependence on time and concentration. Am J Physiol Heart Circ Physiol. 1998;274:H323–330. doi: 10.1152/ajpheart.1998.274.1.H323. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Suzuki H. Dependency of endothelial cell function on vascular smooth muscle cells in guinea-pig mesenteric arteries and arterioles. J Smooth Muscle Res. 2005;41:77–85. doi: 10.1540/jsmr.41.77. [DOI] [PubMed] [Google Scholar]
- Yoo HY, Zheng H, Nam JH, Nguyen YH, Kang TM, Earm YE, Kim SJ. Facilitation of Ca2+-activated K+ channels (IKCa1) by mibefradil in B lymphocytes. Pflugers Arch. 2008;456:549–560. doi: 10.1007/s00424-007-0438-5. [DOI] [PubMed] [Google Scholar]
- Zhong X, Liu JR, Kyle JW, Hanck DA, Agnew WS. A profile of alternative RNA splicing and transcript variation of CACNA1H, a human T-channel gene candidate for idiopathic generalized epilepsies. Hum Mol Genet. 2006;15:1497–1512. doi: 10.1093/hmg/ddl068. [DOI] [PubMed] [Google Scholar]


