Abstract
Paternal behavior greatly affects the survival, social development, and cognitive development of infants. Nevertheless, little research has been done to assess how paternal experience modifies the behavioral characteristics of fathers, including fear and stress responses to a novel environment. We investigated long-term behavioral and physiologic effects of parental experience in mice (Peromyscus californicus) and how this response activates the hypothalamic–pituitary–adrenal axis (as measured by corticosterone and dehydroepiandrosterone [DHEA] levels) and interacts with anxiety-related behaviors. Three groups of adult males were tested—fathers exposed to pups, virgins exposed to pups, and virgins never exposed to pups—in 2 environments designed to elicit anxiety response: an open field with a novel object placed in the center and a closed cage containing a sample of a component of fox feces. Behavioral responses were measured by using traditional methods (duration and frequency) and behavioral-chain sequences. Results indicated that paternal experience significantly modifies a male mouse's behavioral and physiologic responses to stress-provoking stimuli. Compared with inexperienced male mice, experienced male mice had a significant decrease in the occurrence of incomplete behavioral chains during the exposure to the novel object, an index of reduced stress. Further, even moderate pup exposure induced behavioral modifications in virgin male mice. These behavioral responses were correlated with changes in corticosterone and DHEA levels. Together, these data provide evidence that interactions between male mice and offspring may have mutually beneficial long-term behavioral and physiologic effects.
Abbreviations: DHEA:CORT, dehydroepiandrosterone:corticosterone ratio; MDS, multidimensional scaling; PEV, pup-exposed virgin; TMT, 2,5-dihydro-2,4,5,-trimethylthiazoline
Parental care plays a central role in the somatic and psychologic development of offspring in mammals.23,50,57 The offspring's early developmental environment is closely tied to subsequent behavioral propensities; indeed, focusing on social interactions, early dysfunctions in mother–infant interactions have been linked to the emergence of seemingly maladaptive responses during adulthood, including depression, anxiety, and various psychiatric disorders.6,43,49,60 Human parents display a wide range of parenting skills, and recent scientific literature reveals experiential, environmental, and social factors affecting maternal behavior.41,73 In the past 2 decades, research has elucidated the fundamental components of the maternal response,50,51 pointing out not only the crucial role played by the quality of maternal care in infant development,8,26,27 but also how the process of pregnancy, parturition, and close interaction with the infants during lactation can have long-term effects on the brain and behavioral characteristics of the mothers.35 Although these cited studies elucidated the role of the mother in parent–child interactions, we still know relatively little about the physiologic correlates of variation in paternal behavior.
Extreme variability in paternal responses is observed across mammalian species; whereas approximately 95% of mammalian species do not exhibit paternal responses, the remaining species play an integral role in raising offspring.37,71 Focusing on rodents, this variability can be observed in closely related species of the same genus, such as the paternal prairie vole (Microtus ochrogaster) and the nonpaternal montane vole (M. montanus).65 Many controversial arguments have been raised in regard to human paternal behavior; however, there is little doubt that humans are characterized by extreme behavioral flexibility.52 Consequently, research exploring the long-term effects of father–infant interactions on the neurobiologic and behavioral responses of male caregivers is necessary to build an informed picture of the complexities of paternal–offspring interactions.19,33
Although less extreme than the effects in females, animal models clearly demonstrate that males experience hormonal alterations associated with copulation, pair-bonding, and paternal care.36 Using the paternal model, investigators have been able to identify specific factors accompanying varying degrees of paternal responsiveness, such as distinct patterns of vasopressin receptor binding sites.74 Marmosets (genus: Callithrix) and cotton-top tamarins (genus: Saguinus), New World monkeys characterized by extensive involvement of the father in the care of the newborns,75 exhibit behavioral and physiologic modifications in response to the interaction with the newborns.47,76 Further, an interesting interaction between reproductive experience and physiologic changes has been identified in these monkeys. Paternally experienced cotton-top tamarin fathers exhibited significant elevations in prolactin and glucocorticoids at the midpoint of their mate's pregnancy compared with those of male monkeys encountering their mate's first pregnancy.3
Stress responses typically are measured by assessing levels of glucocorticoids (cortisol and corticosterone), corticotrophin-releasing hormone, neuropeptide Y, and norepinephrine. There is little research regarding dehydroepiandrosterone (DHEA) and stress, although compelling evidence exists indicating that this hormone plays a crucial role in the stress response and coping mechanisms.14 DHEA has been shown to be released parallel to cortisol during physical stress,14,24 to protect the body against the negative effects of prolonged exposure to glucocorticoids.46 Research has demonstrated that DHEA can act centrally to decrease glucocorticoid-induced neuronal death in the hippocampus and to promote neurogenesis in the dentate gyrus of the hippocampus and sensory dorsal root ganglion neurons.55,68 In humans, increased levels of DHEA have been correlated with reduced mental illness symptomology and better task performance in patients suffering from posttraumatic stress disorder as well as more accurate spatial navigation in an underwater scuba-diving task.44,59,68 Furthermore, the ratio between DHEA and cortisol has been found to be a reliable index of neuroprotection.44,55,68 Specifically, increased corticosteroid levels can be adaptive in some situations: corticosteroids, for example, facilitate both the storage of novel information as well as the extinction of behavior that is no longer adaptive or relevant in a particular situation.16,53 At the same time, the organism needs to be protected by the negative effects of high corticosterone levels; consequently, the emerging theory from recent stress research suggests that elevated DHEA concentrations (among other chemicals able to counteract stress), as compared with corticosterone concentrations, can indeed confer an advantage in demanding environments.8,25,26,27,69,70
Detailed analyses of stress-induced behavioral responses are not often used in neurobiologic research.67 When behavioral responses to stress have been investigated in rodents, dependent variables, such as latency, duration, and frequency of behaviors (for example, freezing, exploring, grooming, and time spent interacting with a several kinds of novel objects), have been reported.67 These general measures lack the sensitivity to detect relevant trends, especially when interactions exist among multiple rapidly changing variables.30,31 The common and widespread use of averaged measures taken at specific time intervals is useful for collecting ‘snapshot’ summaries of behavior, but they fail to untangle more subtle variations in behavior.4,5 In the present work, we similarly used behavioral sequences to improve our ability to detect disrupted behavior due to stress of fear responses. The patterned structure of rodent behavior makes it attractive to neurobehavioral stress research, given that stress can evoke ‘displacement’ behavior that can be monitored by the frequency of interruptions and transitions.39 Grooming, in particular, normally proceeds in a cephalocaudal direction and consists of several consecutive stages, including licking the paws, washing movements over the head, fur licking, and tail or genitals cleaning.29 When these behavioral microstructures do not follow the normal pattern, or are interrupted frequently, it indicates an increase in stress or anxiety.30,31
Because the complex behavioral repertoire comprising parental care constitutes an expensive metabolic and genetic investment pivotal to species survival,13 energy expenditure during the stress response becomes a key issue for parents. Previous studies have also found that, when confronted with a stressful stimulus, maternal rats display fewer stress-related behaviors than do nonmaternal rats.22,58 In biparental species, neurobiologic alterations likely accompany various altered behavioral strategies associated with added parental responsibilities. If paternal care exists in some species because it increases the chance of infant survival,20 it is expected that males, as well as females, have resolved some of the evolutionary problems related to the increased complexity of providing for offspring while remaining vigilant and active in a dangerous environment. As a consequence, repeated exposure to pups likely should be reflected in many aspects of paternal life, including fear responses, activation of the hypothalamic–pituitary–adrenal axis, and anxiety in a novel environment.12
In the present work, we assessed the influence of parental experience on specific behavioral strategies and neuroendocrine stress responses of adult male California mice (Peromyscus californicus). Male mice with varying paternal experience (that is, biological fathers, foster fathers, and pup-naïve virgins) were exposed to unfamiliar pups for 3 d. Subsequently, behavioral and hormonal stress responsiveness was assessed in 2 experiments. In the first test, mice were introduced to a novel open field with a novel object located in the center. The following day, mice were exposed acutely to 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), a component of fox feces widely used to stimulate fear response in mice.18 Corticosterone and DHEA were measured noninvasively by collecting fecal samples. Behavioral responses were collected by using both traditional methods (duration, frequency, latency) and advanced behavioral measures, such as chain sequences, to improve our ability to detect disrupted behavior due to stress of fear responses. On the basis of prior work with maternal rodents,42,58 we hypothesized that, compared with inexperienced male mice, experienced fathers (both biological and foster fathers) would express diminished anxiety-related behaviors and activation of the hypothalamic–pituitary–adrenal axis in the form of an increased ratio between DHEA and corticosterone values (DHEA:CORT ratio).
Materials and Methods
Animals.
Adult male California mice (Peromyscus californicus; n = 24; age, 5 to 10 mo) originally purchased from Peromyscus Genetic Stock Center (University of South Carolina, Columbia, SC) were used in this study. Adult male mice were organized in 3 experimental groups on the basis of their reproductive experience. Male mice with parental experience (‘dads,’ n = 8) and those without parental experience (pup-exposed virgins [PEV], n = 8) were exposed to pups for 3 consecutive days before the behavioral tests. Parental experience was defined as having raised at least one offspring; all dads had 1 or 2 litters prior to the experiment. A third group of adult mice (virgins, n = 8) were never exposed to pups and therefore served as the control group. Dads were significantly older than PEV and virgins (mean, 279 d compared with 154 d; t22 = 6.98; P < 0.001). Consequently, because several studies demonstrated that age can be a factor in paternal behavior in mice,64 we used age as a covariate in all following analyses.
On arrival to the laboratory, mice in all groups were housed under the same conditions (pair-housed in 29 cm × 18 cm × 12.5 cm wire-lidded cages with filter tops). Cages were lined with aspen bedding (Harlan Teklad, Madison, WI); food (no. 2018, Harlan Teklad) and water were provided ad libitum. The animals were maintained on a 16:8-h light:dark schedule, with lights on at 0700 h as recommended by the breeder. Animals were maintained in accordance with policies and guidelines of the Randolph–Macon College Institutional Animal Care and Use Committee (IACUC approval no. 07-01).
Procedures.
Dads and PEV were exposed to unfamiliar pups for 3 consecutive days before the behavioral tests. The same pup was never used more than twice daily; specifically, if the same pup was used for multiple tests, the pup was returned to its home cage and parents for at least 10 min before the next 10-min test. Dads and PEV were placed daily in an experimental cage (46 cm × 24 cm × 16 cm) with clean bedding and, after a 10-min habituation period, mice were exposed to a single unrelated alien-conspecific pup for 10 min. The pups used in the pup-exposure protocol were housed with both parents, and these ‘donor families’ were not involved in the experiment in any other way. Pups were approximately 3 to 8 d of age. Adult male mice housed together were exposed to pups from the same donor family, and donor families remained consistent throughout the exposure period. To provide additional pup-related stimuli in the form of olfactory stimulation for the PEV, each day a cotton nest pad was wiped on the fur of the pup's mother and placed in the male mouse's cage until the next test session. Pilot data from our laboratory have shown that this method decreases attacks on pups. Control animals were exposed to the same experimental manipulations as the other groups except for pup exposure.
The 10-min pup exposure sessions were sufficient to reliably elicit paternal behavior. In each pup-exposure session, the following dependent measures were observed: grooming or licking the pup; retrieving or crouching over the pup; approach or sniffing the pup; stretch attends or freezing responses; and number of attacks (Table 1). If an attack occurred, the experimenter immediately tapped the top of the cage to startle the male mouse and disrupt the contact. The noise produced was similar to that produced during normal opening and closing of cages, a sound familiar to the animals and sufficient to change their activity. After an attack, the session was terminated for that day; the pup was examined for any injuries and returned to the donor family cage. Intensity of the attack was quantified in terms of both the duration and the persistence of the attack, on a scale between 1 (light) to 3 (severe). A composed index based on both the number and intensity of attacks was calculated by using the formula A = [N (severe attacks) × 3] + [N (mild attacks) × 2] + N (light attacks). If the male mouse directed aggressive responses toward a pup a second time, the session again was terminated, and all future exposures were conducted with the pup placed in a protective enclosure inside the exposure cage. This small plastic container had holes bored around it, allowing the male mouse to experience pup-related stimuli (for example, visual, olfactory, and auditory stimuli); a restriction of this scenario was that grooming and crouching responses could no longer be assessed.
Table 1.
Frequency of parental behavior in parenting-experienced (dads) and -inexperienced (PEV) male mice during pup-exposure
| Dads | PEV | |
| Licking/grooming | 18 | 14 |
| Crouching/retrieving | 12 | 9 |
| Sniffing /approaching | 3 | 6 |
| Freezing /stretch attends | 6 | 9 |
| Attack | 2 | 7a |
Significantly (P < 0.05) difference compared with value for dads.
Approximately 26 h after the third and final pup exposure, all subjects were tested in the open field containing a novel object (a wooden hollow half-log; Creatures and Critters Pet Refuge, Mac's Pet Products, Richmond, VA) in the center (Figure 1). The open-field apparatus was 122 cm × 91 cm × 51 cm and consisted of a white linoleum floor and walls. The novel object was selected to simulate an ecologically relevant object. Activity in the open field was recorded (model DCR-SX41, Handycam, Sony, San Jose, CA). Mice were left in the open field containing the novel object for 5 min. After approximately 12 h, fecal samples were collected from all mice to monitor the physiologic response to the open field with novel object.
Figure 1.

Picture of the novel object, a hollow wooden half-log, located in the center of the open field.
The following day, at approximately 1400, mice were introduced into a novel cage (29 cm × 18 cm × 12.5 cm) with a filter-top lid; in the middle of one end of the cage a piece of filter paper was taped (approximately 25 mm × 25 mm) saturated with 30 µL 2,5-dihydro-2,4,5-trimethylthiazoline (TMT; Phero Tech, Delta, Canada), a component of fox feces widely used to stimulate fear response in mice.18 Mice were exposed to TMT for 3 min, and activity during TMT exposure was recorded (model DCR-SX41, Handycam, Sony).
Behavioral responses.
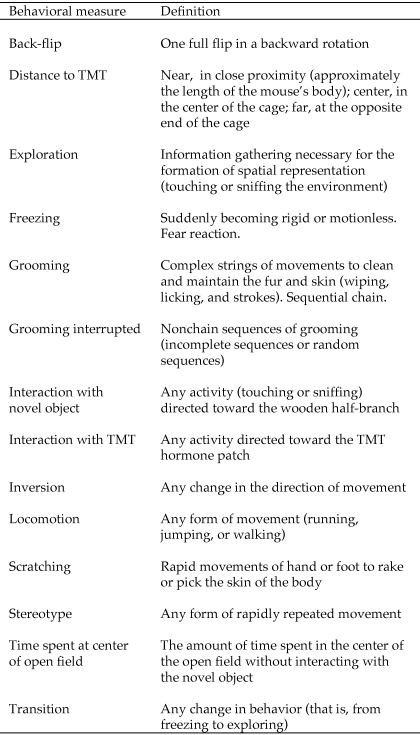
During the novel-object open-field test, the following behavioral responses were recorded: frequency, duration, and direction of locomotion (walking, running, jumping); frequency, duration, and latency of freezing; exploration; scratching; grooming; interaction with the novel object; time spent in the center of the open-field (but not in contact with the novel object); and frequency of back-flips (Figure 2). In addition to these traditional behavioral measures, we recorded behavioral sequences and transitions for movements and grooming. We calculated the numbers of interrupted grooming sequences (either an incomplete chain or a chain diverging from the typical cephalocaudal structure), inversions (that is, changes in directions while moving, such as walking from clockwise to counterclockwise), stereotypic movements, and behavioral transitions (for example, from running to freezing).
Figure 2.
Ethogram of the behavioral measures.
During acute exposure of mice to TMT, we recorded the following behavioral responses (Figure 2): latency and duration of freezing; latency and duration of exploration; latency and duration of grooming; number of jumps and back-flips; and number of approaches to the TMT patch. We also recorded the spatial location of the subject in relation to the TMT patch: when the mouse was in close proximity (that is, within approximately the body length of the mouse), the location was recorded as ‘near;’ when the mouse was in the middle of the cage floor, we recorded the location as ‘center;’ and when the mouse was at end of the cage opposite the TMT patch, the location was recorded as ‘far.’
Physiologic responses.
To assess the physiologic responses to pup exposure and the novel-object open-field test, we measured corticosterone and DHEA metabolized in excreta by using fresh fecal samples collected approximately 12 h after the end of the pup exposure (collection 1) and 12 h after the open-field test (collection 2). Previous research has identified 12 h as the optimal time point at which to collect metabolized steroid hormones.7 Mice were isolated briefly (no mouse needed more than 10 min for sample collection), and fecal samples (0.1 g each) were collected and frozen unmixed in sealed containers at −70 °C until assaying. A total of 48 samples was collected and saved for corticosterone and DHEA extraction and assay procedures.
Before extraction, previously collected fecal samples were thawed at room temperature and placed in a glass tube with 1 mL 100% methanol.9,62 The contents of the tube were mixed rapidly (Vortex Genie 2, Scientific Industries, Bohemia, NY) for approximately 30 s and the tube centrifuged for 10 min at 1000 g. A transfer pipette was used to transfer the sample to a glass test tube (13 × 100 mm). The final step of the extraction procedures was to dilute the sample in methanol in assay buffer (1:20; Correlate Enzyme ImmunoAssay Kit, Assay Designs, Ann Arbor, MI). Assay procedures were completed by using the materials and protocols provided with the kit. Results were read at 405 nm on an automated microplate reader (model ELx800, Bio-Tek, Richmond, VA) and manufacturer-supplied software (Kcjunior version 1.3, Bio-Tek).
Statistical analysis.
For each dependent variable, univariate ANOVA was used to determine the effects of reproductive experience (dads, PEV, and virgins) on each dependent measure. The Tukey test was used for post hoc comparisons (α = 0.05). Because age was a potential confounding factor, we used it as a covariate in all ANOVA. All statistical processing was completed by using SPSS 16.0 (SPSS, Chicago, IL).
Multidimensional scaling (MDS) analysis was conducted in order to provide a model of independent associations among the variables. We preferred MDS to other multivariate techniques because it does not require data that are multinormally distributed, a condition rarely satisfied when dealing with behavioral measures. MDS is a data-reduction technique used to uncover a ‘hidden structure’ to a set of data.40 MDS refers to graphical models that provide a spatial representation of the similarity structure of variables. By using correlations, the relationships (that is, proximities) among variables can be displayed graphically in a ‘map.’ The closer 2 variables are on the map, the more highly correlated they are.
Results
Behavioral and physiologic responses to pup-exposure.
Adult male California mice that were biological fathers (‘dads’) and those that had not sired a litter but had been exposed to pups according to our protocol (that is, PEV) both exhibited spontaneous paternal behavior for unfamiliar pups (retrieving, crouching over, and licking the pup). Six mice (4 PEV and 2 dads) attacked the pups at some point during the exposure period and therefore were categorized as inadequate parents. Only the number of attacks differed between dads and PEV (t14 = 2.45, P = 0.04; Table 2). Subjects characterized by inadequate paternal behavior had shorter durations of grooming during the behavioral test (t14= 2.19, P = 0.046). They also tended to spend more time (t14= 2.03, P = 0.062) interacting with the object in the center of the open-field, although this difference was not significant at the 0.05-level (Figure 3). We found a significant correlation between the composed attack index (number and intensity of dangerous behaviors toward the pups) and the number of interrupted grooming sequences (r = 0.57, P = 0.020). After pup exposure, dads had higher levels of corticosterone (F2,20 = 4.54, P = 0.03, Figure 4 A) and DHEA (F2,20 = 4.13, P = 0.05, Figure 4 B) than did PEV and virgins, whereas PEV had a higher DHEA:CORT ratio (F2,20 = 3.99, P = 0.05, Figure 4 C).
Table 2.
Traditional behavioral measures in the open field by group (dads, PEV, and virgin controls)
| Behavioral Measure | F(2,21) | P |
| Back-flips | 0.22 | 0.76 |
| Exploration | 0.73 | 0.46 |
| Freezing | 0.37 | 0.69 |
| Grooming | 0.91 | 0.42 |
| Interaction with novel object | 0.11 | 0.89 |
| Locomotion | 1.99 | 0.16 |
| Scratching | 1.05 | 0.39 |
| Time at the center of the open field | 1.68 | 0.21 |
Figure 3.
Differences in time (min; mean ± SEM) spent grooming and exploring the novel object in the open-field test between male mice showing adequate parental behavior during exposure to unfamiliar pups (Adequate) and males attacking the pups during the exposure (Inadequate).
Figure 4.
Physiologic response to the pup-exposure training of male mice grouped by parental experience (dads, pup-exposed virgins [PEV], and control virgins). Dads showed significantly higher levels of (A) corticosterone and (B) DHEA, but (C) PEV had the highest DHEA: CORT ratios.
Behavioral and physiologic responses to the novel-object open-field test.
None of the traditional behavioral measures showed a significant effect of reproductive experience. Freezing, movements, exploration of the open field, interaction with the novel object, and grooming (both latency and duration) were not significantly different among the 3 experimental groups (all P values > 0.15; Table 2).
Interestingly, significant effects of the 3 paternal experience levels occurred among all the measures of structured behavior. The number of interrupted grooming sequences decreased as paternal experience increased (mean interruptions per session: virgins, 8.12; PEV, 4.75; dads, 1.38; F2,20 = 5.21, P = 0.01; Figure 5 A). The number of behavioral transitions showed a similar trend (average number per session: virgins, 380; PEV, 302; dads, 282; F2,20 = 4.92, P = 0.02; Figure 5 B). The number of inversions in direction was highest in virgins as well (mean inversions per session: virgins, 16.88; PEV, 6.88; dads, 5.62; F2,20 = 4.08, P = 0.05; Figure 5 C). Finally, the ratio between running clockwise and counterclockwise was significantly different among the 3 groups (% running clockwise: virgins, 67%; PEV, 38%; dads, 55%; F2,20 = 6.22, P = 0.01; Figure 5 D). No significant differences in corticosterone and DHEA levels after the novel-object open-field test were observed among the 3 reproductive groups (P > 0.13 for all comparisons).
Figure 5.
Behavioral response to the open-field test of male mice grouped by parental experience (dads, pup-exposed virgins [PEV], and control virgins). (A) Dads showed significantly lower levels of grooming interruptions, whereas both PEV and dads were significantly lower than control virgins in the number of (B) behavioral transitions and (C) inversions in direction. (D) PEV showed the lowest percentage of clockwise movement.
Behavioral and physiologic responses to TMT exposure.
None of the behavioral measures recorded during acute exposure to TMT differed significantly among the 3 experimental groups (P > 0.15 for all comparisons). All mice spent significantly more time far from the TMT patch than close to it (F2,42 = 7.08, P = 0.002; Figure 6 A), thus confirming that TMT was an aversive stimulus. To further demonstrate the aversiveness of TMT, we compared the time spent freezing in the cage containing TMT with that during the novel-object open-field test to assess the differential fear response in the 2 separate tests. Mice in the TMT cage froze nearly 10 times more often per unit time (F1,21 = 16.6, P < 0.001; Figure 6 B). All 3 groups had similar corticosterone and DHEA levels after TMT exposure (P > 0.20 for all comparisons)
Figure 6.
Behavioral response to the TMT test. All animals, regardless of their parental experience, showed significant aversion for the TMT stimulus (**, P = 0.002). (A) Animals spent significantly more time away from the TMT patch than in any other location. (B) Animals froze significantly more during the TMT test than during the OF test (**, P < 0.001).
Multidimensional scaling.
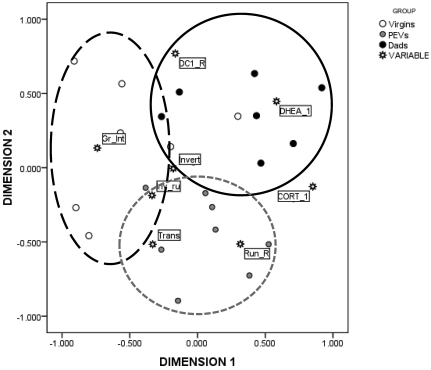
The MDS technique generated the map shown in Figure 7. The Kruskal stress index determined an s-stress value of 0.11, indicating a good fit between the dimensions and mapped distances. The R2 value designated that 91% of the variance was explained by the data. Based on the 2 dimensions, the responses to the 2 stressful conditions were divided into 3 major clusters, indicated in the figure by circles. Circle 1 (in the upper-right quadrant) represents animals that displayed higher physiologic responses to the novel-object open-field test (cluster 1, characterized by association with DHEA, corticosterone, and their ratio). The second group, in the lower quadrant, was characterized by mice displaying displacement activities (behavioral responses; circle 2, characterized by several measures of interrupted behavioral chains, such as behavioral transitions). The third group, in the left quadrant (circle 3), was characterized by grooming interrupted. Figure 7 also illustrates how the subjects, grouped by their reproductive experience, were mapped according to the 2 dimensions. Dads are clustered together with high physiologic responses, as indicated in the figures by black dots, whereas PEV (gray dots) clustered with a variety of behavioral responses. In contrast, control virgins (white dots) were characterized by high levels of interrupted grooming sequences, a seemingly poor adaptive strategy to cope with the anxiety provoked by the novel-object open-field test.
Figure 7.
Multidimensional scaling map of the association between the behavioral and physiologic responses during the open-field test. Dimensions represent linear combinations of the original variables which are not correlated; therefore they are useful to order the original variables in the map along a continuum. Dimensions are thought to ‘explain’ the perceived similarity between variables. In our dataset, dimension 1 represents the distance between physiologic responses (corticosterone [CORT_1] and DHEA [DHEA_1]) and grooming interrupted (Gr_Int). Circles on the map are arbitrary and are only useful to focus on the interpretation given according to the relative distances. Criteria to draw circles included maximizing the number of subjects in the same group per circles while minimizing the area of overlapping among different circles. In the proposed interpretation, Dads are clustered together with high physiologic responses (circle 1, black dots), whereas PEV are clustered with a variety of behavioral responses (circle 2, gray dots). In contrast, control virgins are characterized by high levels of interrupted grooming sequences (Circle 3, white dots), a seemingly less adaptive strategy to cope with the anxiety provoked by the open-field test. DC_R, DHEA:CORT ratio; CORT_1, corticosterone levels; DHEA_1, DHEA levels; Gr_Int, grooming interrupted; Invert, inverted running; Inv_ru, changes of direction; Run_R, running clockwise; Trans, behavioral transitions.
Discussion
The current study indicates that reproductive experience can have long-term effects on the behavioral and physiologic propensities of males in biparental species such as the California mouse (P. californicus). As expected, when confronted with the challenge of exploring a new environment containing a novel object (the novel-object open-field test), male mice with paternal experience (dads) exhibited fewer disrupted behavioral chains (grooming, locomotion, and behavioral transitions) than did pup-naïve virgins. Dads also were characterized by an increased physiologic response, although this response did not affect behavior during the novel-object open-field test.
The data presented here also indicated that even moderate experience with pups (that is, exposure for few minutes for 3 consecutive days) alters both the behavioral and physiologic responses of male mice to stress. Although the number of interrupted grooming chains, a reliable index of stress in rodents,4,28-31 was lower in dads than PEV, there was a significant difference between PEV and control virgins as well. Providing further evidence of the neurobiologic effect of the short period of pup exposure, the remaining measures of behavioral disruptions (changes in direction and behavioral transitions) were not significantly different between Dads and PEV; however, in both groups they were significantly lower than values in virgins with no paternal experience. Further, considering that the ratio between DHEA and cortisol has been found to be a reliable index of neuroprotection,44 the observation that PEV had the highest DHEA:CORT ratio after pup exposure, significantly higher than those for dads and virgin controls, was a notable result. We speculate that in biparental species, males confronted with unfamiliar pups for the first time react with a significant shift of their allostatic regulation, necessary to evaluate accurately the new stimuli offered by pups. From an evolutionary point of view, this mechanism could be helpful in preparing new fathers to react positively to the newborns by avoiding aversive stimuli associated with stressors, such as a novel object or environment, consequently helping to regulate the onset of paternal behavior necessary for proper care of offspring.
Recent evidence suggests that higher activation of the hypothalamic–pituitary–adrenal axis can facilitate learning in demanding environments;26,27,70 specifically, adrenal stress hormones released in response to acute stress may yield memory-enhancing effects when released after learning and impairing effects at memory retrieval.63 These data suggest that in biparental species fathers have an integral role in shaping offspring development through infant-directed behaviors such as huddling, licking, grooming, and retrieving, a role for which fathers must be prepared physiologically, likely facilitated by sensitive allostatic adjustments.10 In evolutionary terms, this strategy would be adaptive considering that P. californicus males invest considerable amount of time and energy in the care of offspring. Clearly, the stress response, including anxiety and fear responses, consists of generally adaptive behaviors that determine survival in many environmental situations.66 Considering the connections among health, stress, and reproductive fitness, it is important to continue to understand how parental experience can modify males’ physiologic and behavioral characteristics as reproductive stakes are increased with the addition of offspring. Even if it is adaptive to release high levels of glucocorticoids and maintain freezing behavior in the short term for individual animals,61 this option may be too costly to be maintained for a prolonged time.34 Our data indicate that parental experience, and the surrounding events, can provide opportunities to increase both the physiologic and behavioral response efficiency.
Assessing the complex interconnections among the behavioral and physiologic measures recorded in the current study, multidimensional scaling confirmed a clear separation among the 3 parental experience groups in terms of the behavioral and physiologic responses to the experimental paradigm, thus providing supplementary information in support of the overall conclusion of this and other investigations: parental experience may alter neural and physiologic central mechanisms linked to more efficient and adaptive responses of males in challenging environments. To map all of the variables into a desired space (2 dimensional or greater), a certain lack of fit, the ‘s-stress’ (ranging from 0 [perfect fit] to 1 [worst possible fit]), has to be accepted. The aim of MDS is to find a map of the variables that minimizes the s-stress for a given number of dimensions. The number of dimensions can be likened to the number of latent underlying factors in the dataset. Therefore, when choosing the number of dimensions to represent the data, one must consider: 1) the number of variables in the model; 2) the lack of fit (s-stress value), given the number of dimensions; 3) the index of fit of the model (R2 value), and 4) the interpretability of the dimensions.40 The first point addresses the fact that for each dimension of the data, there should be approximately 4 variables entered into the model. Thus, for a 2-dimensional map, approximately 8 variables should be used. The second point addresses how well the MDS map actually fits the data. Stress values below 0.15 typically are deemed acceptable. The third point addresses the variance accounted for within the model. As is the case with any regression analysis, one must consider the amount of variance being accounted for. Typically, R2 values of 0.8 or higher are desirable. Finally, one must pick a solution based on interpretability of the dimensions. Parsimony is crucial to interpreting the map generated from any given dataset. MDS analysis is a technique that provides additional information about the ‘structure’ of a dataset, which is not possible with standard parametric statistical techniques.
Given that dads were grouped together with higher levels of physiologic activation in response to the novel environment and object (the novel-object open-field test), it is plausible to speculate that parental experience facilitates and accelerates the processes of discrimination between adaptive responses to fear stimulation mediated by physiologic responses. Accordingly, dads appear to be able to discriminate quickly between a severe threat (TMT stimulus) and a less threatening situation (novel environment in the novel-object open-field test). Therefore, these results support the hypothesis that parental experience enables animals to assess stressful environments and respond in more adaptive ways than those of males with no or limited parental experience.
This interpretation also was confirmed by the observation that adequate and inadequate parents exhibited significantly different amounts of self-grooming after pup-exposure. Further, the intensity of inadequate behavior was correlated with the frequency of disrupted grooming chains. In several species, including primates and rodents, the quality of maternal behavior is related to self-grooming during pregnancy and lactation; specifically, better maternal care is correlated with increased self-grooming.6,7,8,29 This increase in completed chains of grooming behavior can be interpreted as an indicator of the change in the internal status of pregnant females, both in psychologic and physiologic terms, ultimately leading to a focus on the care of the newborn. In fact, in many instances after delivery, we observed a shifting from self-grooming to the grooming of offspring.8 Self-grooming during the perinatal period therefore might be seen as a way to improve the quality of care toward an infant by means of a shift from the care of oneself to the care of the offspring's body, at least during the very early stages of life, when progeny are always in contact with the caretaker.8 The present study, for the first time, indicates that a similar mechanism could be in effect for males as well.
In addition, our data point toward the importance of collecting behavioral chain sequences when studying the behavioral stress response of mammals. Traditionally, little attention has been directed to the description and measurements of the behavioral changes that are elicited by stressful situations.67 When behavioral responses to stressful stimuli have been investigated in rodents, latency, duration, and frequency of behaviors like freezing, exploring, grooming, and time spent interacting with several kinds of novel objects have been reported.67 A limitation of this approach is that the reciprocal interaction between complex systems, such as physiologic status and behavioral outcome, is difficult to describe by using conventional methods.31 Time series of behavioral sequences can be analyzed in terms of the variations due to random phenomena or from the deterministic interactions of several variables. Behavioral time series during stressful conditions, although they often appear erratic, reveal precise spectra. In other words, they present characteristics that could better represent the physiologic status of the animal.1,2 A case in point is represented by grooming. In rodents, grooming is an important part of the behavioral repertoire, representing a complex hierarchically ordered cephalocaudal sequence of patterns sensitive to a variety of stimuli.48 Alterations in grooming microstructure in different test situations can be a reliable indicator of stress and anxiety in rats.29 Another recent study has found that parental experience had a significant influence on the behavior of Mongolian gerbil pups, including self-grooming.56 Long-range correlation in biologic systems is adaptive because it serves as an organizing principle for highly complex, nonlinear processes and it avoids restricting the functional response of an organism to highly periodic behavior.11,21 Other studies have identified that scale invariant temporal fluctuations in respiratory intervals in animals,32 the heart rate of healthy individuals,38,45 neuronal discharges during sleep,72 and even in human activity.15 For these reasons, modeling the interaction between behavioral and physiologic time series can provide a complementary approach to the study of the behavioral responses to stress. We found that the number of interrupted grooming sequences decreased as paternal experience increased. The number of behavioral transitions and inversions in direction showed similar trends. Considering that rodents frequently stop when they are learning a new environment,4 this difference could be related to an enhanced efficiency with which reproductive experienced animals process new information in novel environments. Finally, the ratio between running clockwise and counterclockwise was significantly different among the 3 groups. Ratios closer to the theoretical 50% indicate a more homogenous and systematic exploration of the environment, and the higher the departure from this rate, the more chaotic the learning process.
A major difference between the effects of maternal compared with paternal experience that emerged from the present study is that whereas maternal experience can reshape all aspects of the female behavioral repertoire, including the fear response,58 the fear response of all male mice in our study, regardless of their parental experience, was augmented strongly by the acute exposure to TMT, a chemical isolated from red fox feces. Male mice in all of the 3 parental experience groups showed extreme aversion for the TMT stimulus. Time spent freezing, a commonly used indicator of fear,17 was almost 10 times higher than that of the same animals in the novel-object open-field test. The current study is the first to examine Peromyscus response to TMT; however, we anticipated that fathers may have an enhanced response, in that suppression of motor activity during TMT exposure was more prominent in reproductive male meadow voles (M. pennsylvanicus) than nonreproductive males or females.54 As reviewed elsewhere,18 stronger responses to TMT are seen in small, enclosed chambers, such as the one used in the current study. Perhaps a more subtle fear stimulus would facilitate assessment of potential differences in fear behavior between groups. However, we surmise from this study that although parental experience can modify how quickly males become familiar with a new environment in absence of a specific threat, when confronted with a predator-like stimulus, dads, PEV, and control virgins all react similarly. The difference between the fear responses of males and females could be viewed in terms of the different investment in offspring between the 2 sexes. Even if paternity in biparental species could be confirmed, it is clear that females, from an evolutionary and biologic point of view, invest more energy and time than do males, and this difference might be reflected in the amount of neural and behavioral reshaping due to reproduction.
Future research should address 3 main limitations in the present study. Dads were significantly older than were both PEV and virgins (these 2 groups did not differ in age). Studies have found that paternal age can be a factor in both paternal behavior and infant development in mice.64 To address this important issue, we used age as covariate in a very conservative effort to minimize any age effect. Moreover, because deficits in social and paternal behavior are expected in older parents, this potential confounding factor would have worked against our predictions, thereby making the results we obtained even stronger. A second important issue was due to potential additional stress for male mice that attacked pups and then were startled by the experimenter to disrupt the attack. As detailed in the Materials and Methods, we tried to minimize this effect, but clearly it was beyond our control to make this experience equitable among all groups, given that some mice attacked more than others. Even tapping all cages the same number of times would not have recreated the same effects of startling the male and removing the pup, because these events would have created fear responses toward the pups or interrupted nurturing social contact. A third potential was related to individual differences among pups. Although we rotated pups and made sure that they were rested before introducing them in the apparatus, potential interindividual differences might have had affected our results. However, any individual differences between pups would have existed for both pup-exposed groups.
In light of the behavioral and hormonal differences among the 3 reproductive groups of adult male California mice in the present study, we conclude that paternal experience significantly modifies a male mouse's behavioral and hormonal repertoire in response to stress-provoking stimuli but does not affect the male fear response (to TMT, in this case) itself. According to the results, even a moderate exposure to pups can induce behavioral and physiologic modifications in male mice. Specifically, using nontraditional, highly structured behavioral measures increased the ability to discriminate changes in the behavioral repertoire of male mice exposed to stressful stimuli. This information adds to previous research findings demonstrating the marked improvements in learning and memory ability, attenuated stress-response, and survival strategies of maternal animals,6,43,49,60 thereby highlighting the critical role of reproductive experience in both sexes. Future research is necessary to further address specific changes in neural circuits responsible for the regulation of paternal behavior.
Acknowledgments
This research was funded by grant no. 0723341 (to KGL) from the National Science Foundation. We are also grateful for contributions provided by the Schapiro Undergraduate Research Fellowship program and the Psychology Department at Randolph–Macon College.
References
- 1.Alados CL, Huffman MA. 2000. Fractal long-range correlations in behavioural sequences of wild chimpanzees: a non-invasive analytical tool for the evaluation of health. Ethology 106:105–116 [Google Scholar]
- 2.Alados CL, Weber DN. 1999. Lead effects on the predictability of reproductive behavior in fathead minnows (Pimephales promelas): a mathematical model. Environ Toxicol Chem 18:2392–2399 [DOI] [PubMed] [Google Scholar]
- 3.Almond RE, Ziegler TE, Snowdon CT. 2008. Changes in prolactin and glucocorticoid levels in cotton-top tamarin fathers during their mate's pregnancy: the effect of infants and paternal experience. Am J Primatol 70:560–565 [DOI] [PubMed] [Google Scholar]
- 4.Avni R, Tzvaigrach Y, Eilam D. 2008. Exploration and navigation in the blind mole rat (Spalax ehrenbergi): global calibration as a primer of spatial representation. J Exp Biol 211:2817–2826 [DOI] [PubMed] [Google Scholar]
- 5.Bakeman R, Gottman JM. 1997. Observing interaction: an introduction to sequential analysis. Cambridge (UK): Cambridge University Press [Google Scholar]
- 6.Bardi M, French JA, Ramirez SM, Brent L. 2004. The role of the endocrine system in baboon maternal behavior. Biol Psychiatry 55:724–732 [DOI] [PubMed] [Google Scholar]
- 7.Bardi M, Hampton JE, Lambert KG. 2010. Fecal dehydroepiandrosterone (DHEA) immunoreactivity as a noninvasive index of circulating DHEA activity in young male laboratory rats. Comp Med 60:455–460 [PMC free article] [PubMed] [Google Scholar]
- 8.Bardi M, Shimizu K, Barrett GM, Borgognini-Tarli SM, Huffman MA. 2003. Peripartum cortisol levels and mother–infant interactions in Japanese macaques. Am J Phys Anthropol 120:298–304 [DOI] [PubMed] [Google Scholar]
- 9.Barrett GM, Shimizu K, Bardi M, Mori A. 2002. Fecal testosterone immunoreactivity as a non-invasive index of functional testosterone dynamics in male Japanese macaques (Macaca fuscata). Primates 43:29–39 [DOI] [PubMed] [Google Scholar]
- 10.Becker EA, Moore BM, Auger C, Marler CA. 2010. Paternal behavior increases testosterone levels in offspring of the California mouse. Horm Behav 58:385–389 [DOI] [PubMed] [Google Scholar]
- 11.Buldyrev SV, Goldberger AL, Havlin S, Mantegna RN, Matsa ME, Peng CK, Simons M, Stanley HE. 1995. Long-range correlation properties of coding and noncoding DNA sequences: GenBank analysis. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 51:5084–5091 [DOI] [PubMed] [Google Scholar]
- 12.Byrnes EM, Lee JO, Bridges RS. 2007. Alterations in GABAA receptor α2 subunit mRNA expression following reproductive experience in rat. Neuroendocrinology 85:148–156 [DOI] [PubMed] [Google Scholar]
- 13.Cameron NM, Shahrokh D, Del Corpo A, Dhir SK, Szyf M, Champagne FA, Meaney MJ. 2008. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J Neuroendocrinol 20:795–801 [DOI] [PubMed] [Google Scholar]
- 14.Charney DS. 2004. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry 161:195–216 [DOI] [PubMed] [Google Scholar]
- 15.Chern CM, Kuo TB, Sheng WY, Wong WJ, Luk YO, Hsu LC, Hu HH. 1999. Spectral analysis of arterial blood pressure and cerebral blood flow velocity during supine rest and orthostasis. J Cereb Blood Flow Metab 19:1136–1141 [DOI] [PubMed] [Google Scholar]
- 16.de Kloet ER, Oitzl MS, Joëls M. 1999. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci 22:422–426 [DOI] [PubMed] [Google Scholar]
- 17.Fanselow MS, Helmstetter FJ. 1988. Conditional analgesia, defensive freezing, and benzodiazepines. Behav Neurosci 102:233–243 [DOI] [PubMed] [Google Scholar]
- 18.Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. 2005. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev 29:1145–1156 [DOI] [PubMed] [Google Scholar]
- 19.Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. 2010. Oxytocin and the development of parenting in humans. Biol Psychiatr 68:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubernick DJ, Teferi T. 2000. Adaptive significance of male parental care in a monogamous mammal. Proc Biol Sci 267:147–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havlin S, Buldyrev SV, Bunde A, Goldberger AL, Ivanov PCh, Peng CK, Stanley HE. 1999. Scaling in nature: from DNA through heartbeats to weather. Physica A 273:46–69 [DOI] [PubMed] [Google Scholar]
- 22.Hawley DF, Bardi M, Everette AM, Higgins TJ, Tu KM, Kinsley CH, Lambert KG. 2010. Neurobiological constituents of active, passive, and variable coping strategies in rats: integration of regional brain neuropeptide Y levels and cardiovascular responses. Stress 13:172–183 [DOI] [PubMed] [Google Scholar]
- 23.Hrdy SB. 1999. Mother nature: a history of mothers, infants, and natural selection. New York (NY): Ballantine; [DOI] [PubMed] [Google Scholar]
- 24.Izawa S, Sugaya N, Shirotsuki K, Yamada KC, Ogawa N, Ouchi Y, Nagano Y, Suzuki K, Nomura S. 2008. Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biol Psychol 79:294–298 [DOI] [PubMed] [Google Scholar]
- 25.Joëls M, Baram TZ. 2009. The neurosymphony of stress. Nat Rev Neurosci 10:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joëls M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, Verkuyl M, Lucassen PJ, Krugers HJ. 2004. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress 7:221–231 [DOI] [PubMed] [Google Scholar]
- 27.Joëls M, Krugers H, Karst H. 2008. Stress-induced changes in hippocampal function. Prog Brain Res 167:3–15 [DOI] [PubMed] [Google Scholar]
- 28.Kaffman A, Meaney MJ. 2007. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry 48:224–244 [DOI] [PubMed] [Google Scholar]
- 29.Kalueff AV, La Porte JL, Bergner CL. 2010. Neurobiology of grooming behavior. Cambridge (MA): Cambridge University Press [Google Scholar]
- 30.Kalueff AV, Tuohimaa P. 2004. Grooming analysis algorithm for neurobehavioural stress research. Brain Res Brain Res Protoc 13:151–158 [DOI] [PubMed] [Google Scholar]
- 31.Kalueff AV, Tuohimaa P. 2005. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods 143:169–177 [DOI] [PubMed] [Google Scholar]
- 32.Kawahara K, Yamauchi Y, Nakazono Y, Miyamoto Y. 1989. Spectral analysis on low-frequency fluctuation in respiratory rhythm in the decerebrate cat. Biol Cybern 61:265–270 [DOI] [PubMed] [Google Scholar]
- 33.Kentner AC, Abizaid A, Bielajew C. 2010. Modeling dad: animal models of paternal behavior. Neurosci Biobehav Rev 34:438–451 [DOI] [PubMed] [Google Scholar]
- 34.Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G. 2008. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Arch Sex Behav 37:43–56 [DOI] [PubMed] [Google Scholar]
- 35.Kinsley CH, Lambert KG. 2006. The maternal brain. Sci Am 294:72–79 [DOI] [PubMed] [Google Scholar]
- 36.Kinsley CH, Lambert KG. 2008. Reproduction-induced neuroplasticity: natural behavioural and neuronal alterations associated with the production and care of offspring. J Neuroendocrinol 20:515–525 [DOI] [PubMed] [Google Scholar]
- 37.Kleiman DG. 1981. An ethological problem. Science 212:155–156 [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi M, Musha T. 1982. 1/f fluctuation of heartbeat period. IEEE Trans Biomed Eng 29:456–457 [DOI] [PubMed] [Google Scholar]
- 39.Komorowska J, Pellis SM. 2004. Regulatory mechanisms underlying novelty-induced grooming in the laboratory rat. Behav Processes 67:287–293 [DOI] [PubMed] [Google Scholar]
- 40.Kruskal JB, Wish M. 1978. Multidimensional scaling. London (UK): Sage [Google Scholar]
- 41.Leckman JF, Herman AE. 2002. Maternal behavior and developmental psychopathology. Biol Psychiatry 51:27–53 [DOI] [PubMed] [Google Scholar]
- 42.Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG. 2005. Maternal experience produces long-lasting behavioral modifications in the rat. Behav Neurosci 119:1084–1096 [DOI] [PubMed] [Google Scholar]
- 43.Lupien SJ, King S, Meaney MJ, McEwen BS. 2000. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biol Psychiatry 48:976–980 [DOI] [PubMed] [Google Scholar]
- 44.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. 2009. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol 30:65–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meesmann M, Grüneis F, Flachenecker P, Kniffki KD. 1993. A new method for analysis of heart rate variability: counting statistics of 1/f fluctuations. Biol Cybern 68:299–306 [DOI] [PubMed] [Google Scholar]
- 46.Morgan CA 3rd, Rasmusson A, Pietrzak RH, Coric V, Southwick SM. 2009. Relationships among plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate, cortisol, symptoms of dissociation, and objective performance in humans exposed to underwater navigation stress. Biol Psychiatr 66:334–340 [DOI] [PubMed] [Google Scholar]
- 47.Mota MT, Sousa MB. 2000. Prolactin levels of fathers and helpers related to alloparental care in common marmosets, Callithrix jacchus. Folia Primatol (Basel) 71:22–26 [DOI] [PubMed] [Google Scholar]
- 48.Munn NL. 1950. Handbook of psychological research on the rat. Boston (MA): Cambridge University Press [Google Scholar]
- 49.Murray L, Creswell C, Cooper PJ. 2009. The development of anxiety disorders in childhood: an integrative review. Psychol Med 39:1413–1423 [DOI] [PubMed] [Google Scholar]
- 50.Numan M, Insel TR. 2003. The neurobiology of parental behavior. New York (NY): Springer [Google Scholar]
- 51.Numan M, Stolzenberg DS. 2009. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol 30:46–64 [DOI] [PubMed] [Google Scholar]
- 52.Nyström K, Ohrling K. 2004. Parenthood experiences during the child's first year: literature review. J Adv Nurs 46:319–330 [DOI] [PubMed] [Google Scholar]
- 53.Oitzl MS, Champagne DL, van der Veen R, de Kloet ER. 2010. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci Biobehav Rev 34:853–866 [DOI] [PubMed] [Google Scholar]
- 54.Perrot-Sinal T, Ossenkopp KP, Kavaliers M. 2000. Influence of natural stressor (predator odor) on locomotor activity in the meadow vole (Microtus pennsylvanicus): modulation by sex, reproductive condition, and gonadal hormones. Psychoneuroendocrinology 25:259–276 [DOI] [PubMed] [Google Scholar]
- 55.Pinnock SB, Lazic SE, Wong HT, Wong IHW, Herbert J. 2009. Synergistic effects of dehydroepiandrosterone and fluoxetine on proliferation of progenitor cells in the dentate gyrus of the adult male rat. Neuroscience 158:1644–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piovanotti MRA, Vieira ML. 2004. Presence of the father and parental experience have differentiated effects on pup development in Mongolian gerbils (Meriones unguiculatus). Behav Processes 66:107–117 [DOI] [PubMed] [Google Scholar]
- 57.Pryce CR, Martin RD, Skuse D. 1995. Motherhood in human and nonhuman primates. Basel (Switzerland): Karger [Google Scholar]
- 58.Rasmusson AM, Vasek J, Lipschitz DS, Volvoda D, Mustone ME, Shi Q, Gudmundsen G, Morgan CA, Wolfe J, Charney DS. 2004. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology 29:1546–1557 [DOI] [PubMed] [Google Scholar]
- 59.Rima BN, Bardi M, Friedenberg JM, Christon LM, Karelina KE, Lambert KG, Kinsley CH. 2009. Reproductive experience and the response of female Sprague–Dawley rats to fear and stress. Comp Med 59:437–443 [PMC free article] [PubMed] [Google Scholar]
- 60.Sanchez MM, Ladd CO, Plotsky PM. 2001. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol 13:419–449 [DOI] [PubMed] [Google Scholar]
- 61.Sapolsky RM. 2003. Stress and plasticity in the limbic system. Neurochem Res 28:1735–1742 1026021307833 [DOI] [PubMed] [Google Scholar]
- 62.Shideler SE, Ortuno AM, Moran FM, Moorman EA, Lasley BL. 1993. Simple extraction and enzyme immunoassays for estrogen and progesterone metabolites in the feces of Macaca fascicularis during nonconceptive and conceptive ovarian cycles. Biol Reprod 48:1290–1298 [DOI] [PubMed] [Google Scholar]
- 63.Smeets T, Otgaar H, Candel I, Wolf OT. 2008. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology 33:1378–1386 [DOI] [PubMed] [Google Scholar]
- 64.Smith RG, Kember RL, Mill J, Fernandes C, Schalkwyk LC, Buxbaum JD, Reichenberg A. 2009. Advancing paternal age is associated with deficits in social and exploratory behaviors in the offspring: a mouse model. PLoS ONE 4:e8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stribley JM, Carter CS. 1999. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci USA 96:12601–12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi LK, Nakashima BR, Hong H, Watanabe K. 2005. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev 29:1157–1167 [DOI] [PubMed] [Google Scholar]
- 67.Troisi A. 2002. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress 5:47–54 [DOI] [PubMed] [Google Scholar]
- 68.Ulmann L, Rodeau JL, Danoux L, Contet-Audonneau JL, Pauly G, Schlichter R. 2009. Dehydroepiandrosterone and neurotrophins favor axonal growth in a sensory neuron-keratinocyte coculture model. Neuroscience 159:514–525 [DOI] [PubMed] [Google Scholar]
- 69.van Niekerk JK, Huppert FA, Herbert J. 2001. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of 3 months of DHEA supplementation. Psychoneuroendocrinology 26:591–612 [DOI] [PubMed] [Google Scholar]
- 70.Wemm S, Koone T, Blough ER, Mewaldt S, Bardi M. 2010. The role of DHEA in relation to problem solving and academic performance. Biol Psychol 85:53–61 [DOI] [PubMed] [Google Scholar]
- 71.Woodroffe R, Vincent A. 1994. Mother's little helpers: patterns of male care in mammals. Trends Ecol Evol 9:294–297 [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto M, Nakahama H, Shima K, Kodama T, Mushiake H. 1986. Markov-dependency and spectral analyses on spike-counts in mesencephalic reticular neurons during sleep and attentive states. Brain Res 366:279–289 [DOI] [PubMed] [Google Scholar]
- 73.Young KA, Liu Y, Wang Z. 2008. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol 148:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young LJ, Winslow JT, Nilsen R, Insel TR. 1997. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav Neurosci 111:599–605 [DOI] [PubMed] [Google Scholar]
- 75.Zahed SR, Kurian AV, Snowdon CT. 2010. Social dynamics and individual plasticity of infant care behavior in cooperatively breeding cotton-top tamarins. Am J Primatol 72:296–306 [DOI] [PubMed] [Google Scholar]
- 76.Ziegler TE, Prudom SL, Zahed SR. 2009. Variations in male parenting behavior and physiology in the common marmoset. Am J Hum Biol 21:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]