Abstract
In animal models of HIV-associated nephropathy, the expression of HIV regulatory genes in epithelial cells is sufficient to cause disease, but how the CD4-negative epithelial cells come to express HIV genes is unknown. Here, we co-cultured T cells infected with fluorescently tagged HIV with renal tubular epithelial cells and observed efficient virus transfer between these cells. The quantity of HIV transferred was much greater than that achieved by exposure to large amounts of cell-free virus and occurred without a requirement for CD4 or Env. The transfer required stable cell–cell adhesion, which could be blocked by sulfated polysaccharides or poly-anionic compounds. We found that the internalization of virus could lead to de novo synthesis of viral protein from incoming viral RNAs even in the presence of a reverse transcriptase inhibitor. These results illustrate an interaction between infected T cells and nonimmune cells, supporting the presence of virological synapses between HIV-harboring T cells and renal tubular epithelial cells, allowing viral uptake and gene expression in epithelial cells.
HIV-associated nephropathy (HIVAN) is a disease characterized by decreased renal function and active viral replication in the kidney. Renal biopsy shows glomerular sclerosis with varying degrees of collapse, tubular epithelial cell degeneration, interstitial fibrosis, and immune cell infiltration.1 In transgenic mouse models of HIVAN, expression of viral genes is sufficient to produce glomerulosclerosis and microcystic tubule disease typical of the human disease.2 In particular, expression of the HIV proteins, Nef or Vpr, can cause HIVAN in mice. Expression of HIV nef induces podocyte dedifferentiation and proliferation.3–5 HIV vpr contributes to renal pathology by causing G2 arrest and inhibiting cytokinesis in tubular cells, which leads to cellular hypertrophy and apoptosis.6
HIV-1 RNA and proviral DNA have been detected in renal epithelial cells in biopsy samples from HIVAN patients. Phylogenetic comparison of gp120 sequences from kidney epithelia to those from peripheral blood provides evidence for tissue-specific evolution.7,8 These data show that viral replication occurs in the kidney, which could serve as a tissue reservoir for HIV-1.
Generally epithelial cells are inefficient targets for HIV infection, because they usually lack the expression of CD4 and CCR5, which mediate HIV-1 entry into CD4 T cells.7,9,10 The C-type lectin receptor DEC-205 can mediate viral internalization, but without mediating productive infection.11 The frequent presence of interstitial infiltrating leukocytes in HIVAN renal biopsies suggests that infected T cells may participate in viral spread within the tissue.
Studies of HIV infection in renal cells have thus far focused on inoculation of cells with cell-free virus where low levels of infection can be observed.12 Recent reports indicated that cell–cell contact can mediate transfer of HIV into recipient cells with a much greater efficiency than cell-free HIV.13,14 In models of extralymphoid HIV interactions, virus transfer is also described from infected T cells to epithelial cells lining the intestinal,15,16 vaginal,17 or oral18 epithelia. Because most epithelial cells do not express CD4, T-cell to epithelial cell virus transfer likely involves distinct CD4-independent mechanisms. Interactions between HIV-infected lymphocytes and intestinal epithelial cells implicate CD4-independent mechanisms of virus uptake.15
Because HIV-infected infiltrating leukocytes are present in HIVAN biopsies,19 we hypothesized that renal tubular epithelial cells may acquire viral particles and/or gene products from infiltrating, HIV-1–infected leukocytes via direct cell–cell contact. We report here that co-cultivation of HIV-infected T cells with noninfected renal tubular epithelial cells results in the massive transfer of viral material to the renal epithelial cells through a CD4- and Env-independent mechanism. Sulfated proteoglycans can interrupt the intercellular interactions and subsequent viral transfer. Furthermore, exposure of epithelial cells to cell-associated HIV generated high levels of HIV early gene expression. Interactions of infected T cells with renal epithelia may be relevant to HIVAN pathogenesis.
RESULTS
HIV-1 Transfer between Primary T Cells and Primary Human Renal Tubular Epithelial Cells
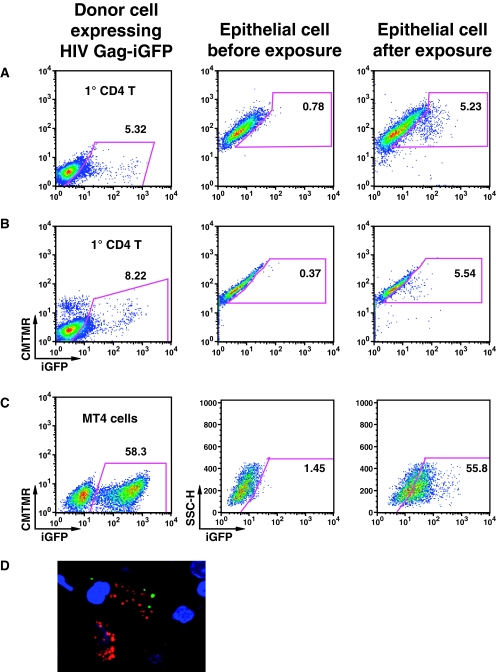
Given the proximity of infected leukocytes and renal epithelia in HIVAN tissue biopsies, we studied the ability of HIV-1 to be transferred from infected T cells to a monolayer of renal epithelial cells. To monitor transfer of HIV from cell to cell, we used an infectious molecular clone of HIV, HIV-1 Gag-iGFP, which carries a genetic insertion of the green fluorescence protein (GFP) in the structural protein Gag.20 The intense fluorescence labeling of the viral particles allows a highly sensitive detection of viral transfer between cells. Primary CD4+ T cells were infected with HIV Gag-iGFP pseudovirions to synchronously infect 5 to 10% of the cells (Figure 1, A and B). These infected cells were co-cultured with primary human renal cortical epithelial cells (HRCEpCs) from normal human donors or with MS114 cells, which are primary renal tubule cells derived from a pediatric HIVAN renal biopsy (M. J. Ross, unpublished data). Target epithelial cells were labeled with Cell Tracker orange CMTMR to distinguish them from donor cells. After 3 hours, the T cells were removed, and the adherent epithelial cells were treated with trypsin EDTA to detach the cells and remove surface-bound virions. Flow cytometry showed that 5% of the primary tubule cells had taken up GFP-laden virus particles (Figure 1, A and B). Similar levels of viral transfer were observed in cells from both sources. GFP-positive epithelial cells were flow sorted and examined by confocal microscopy to exclude the possibility that the fluorescence was caused by doublets or cells that had internalized entire lymphocytes. We observed that the internalized HIV Gag-iGFP was present in punctate, cytoplasmic vesicular structures of heterogeneous size (Figure 1D). The HPT-1b cells are a conditionally transformed renal epithelial cell line21 and showed a strong acquisition of virus in 56% of the cells following co-culture with highly infected, HIV-Gag-iGFP–expressing MT4 cells (Figure 1C). The high rate of acquisition of fluorescence virus in the Hpt-1b cells was in proportion to the high rate of infection of the MT4 donor T-cell line.
Figure 1.
Co-culture of HIV Gag-iGFP infected primary CD4 T cells or MT4 cells with primary renal tubular epithelial cells results in transfer of viral materials into the epithelial cell. Transfer of fluorescence virus into (A) MS114 primary epithelial cells from a HIVAN patient, (B) Primary HRCEpC, and (C) Hpt1b renal tubular cells. (D) MS114 cells were imaged by confocal microscope after virus transfer (DAPI, blue; HIV Gag-iGFP, green; CMTMR, red).
Cell-to-Cell Transfer is Cell Type–Specific
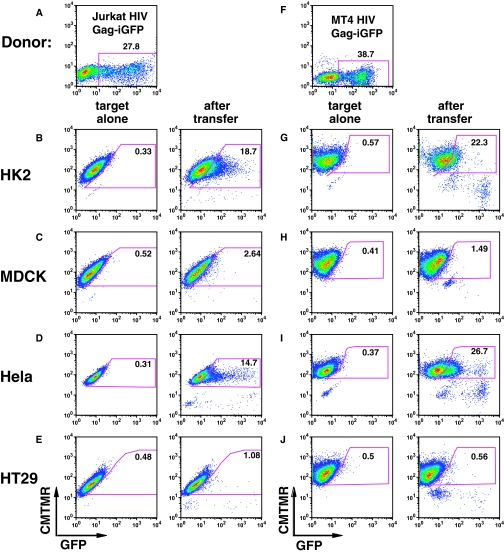
We tested several different epithelial cell lines to determine which had the ability to engage in cell-to-cell transfer with HIV-infected T-cell lines. HK-2 (human renal proximal tubular), MDCK (Madin Darby canine kidney), HeLa (human cervical carcinoma), or HT29 (human intestinal epithelial) cells were co-cultured for 3 hours with HIV-Gag-iGFP–transfected Jurkat T cells (Figure 2A). HK-2 cells exhibited a strong fluorescence shift in 18.7% indicative of efficient viral transfer (Figure 2B). In contrast, viral uptake by MDCK cells was inefficient (Figure 2C). Viral uptake was observed in the cervical carcinoma cell line, HeLa (Figure 2D) but not in HT29 cells (Figure 2E). We observed a similar pattern of viral transfer when HIV-infected MT4 cells (Figure 2F) were used as donor cells (Figure 2, G–J). An immortalized human podocyte line did not show significant uptake from HIV-infected T cells (Supplemental Figure 1). We conclude that the transfer of HIV from infected T cells into epithelial cells is epithelial cell type–specific and not unique to renal tubular cells.
Figure 2.
HIV-1 transfer occurs between HIV-expressing T cells and some epithelial cell lines. (A) HIV Gag-iGFP–transfected Jurkat cells were used as donor cells to epithelial cells below. (B) HK-2 cells were examined before (left) and after (right) 3-hour co-culture with HIV Gag-iGFP–nucleofected Jurkat cells. (C) MDCK cells, (D) HeLa cells, and (E) HT29 cells as target cells with HIV Gag-iGFP–nucleofected Jurkat cells. (F) HIV Gag-iGFP–infected MT4 cells were used as donor cells to the epithelial cells below. (G) HK-2 cells exposed to HIV Gag-iGFP–nucleofected Jurkat cells. (H) MDCK cells, (I) HeLa cells, or (J) HT29 cells as target cells with MT4 cell donors. Epithelial cells were labeled with CMTMR orange and grown in a 24-well plate overnight. Following co-culture for 3 hours at 37°C, cells were washed three times with PBS, detached with trypsin-EDTA treatment at 37°C for 5 to 8 minutes, fixed with 4% PFA, and analyzed by flow cytometry. Numbers within the gate show the percentage of target cells acquiring GFP signal from the donor cells.
Kinetics and Dose Dependency of HIV-1 Transfer into HK-2 Cell Line
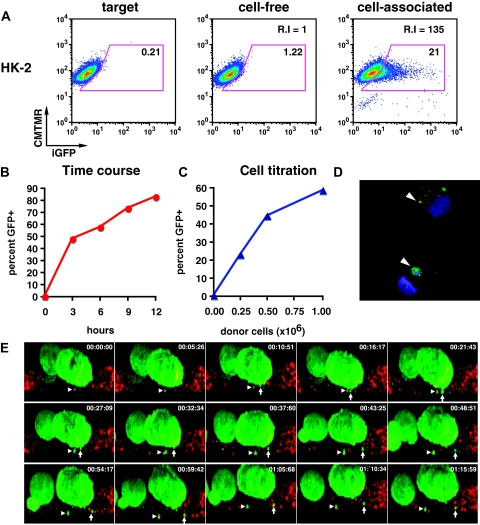
HK-2 cells have previously been studied as a model system for understanding pathologic changes in HIVAN.6,22,23 We therefore examined how HK-2 cells internalize cell-free virus versus cell-associated HIV Gag-iGFP. Exposure of HK-2 cells to a high concentration of cell-free virus (50 ng/ml) resulted in a small fluorescence shift in 1.2% of the exposed cells (Figure 3A). This large viral input was 100-fold greater than is typically produced in Jurkat donor cells over a 3-hour period (data not shown). In contrast, exposure of HK-2 cells to cell-associated HIV Gag-iGFP expressed in Jurkat T cells, 21% of the epithelial cells acquired trypsin-resistant fluorescence. A relative fluorescence index measured the amount of fluorescence transferred into the target cell population as a function of both the percentage of GFP-positive cells and their relative fluorescence intensity.13 This relative fluorescence index was 135-fold greater than cell-free virus in HK-2 cells. Control experiments where cells were separated by transwell membranes resulted in no transfer of fluorescence virus to the HK-2 cells and indicated that transfer requires direct cell contact between epithelial cells and infected T cells (data not shown).
Figure 3.
Cell-associated virus acquisition by renal tubular epithelial cells is more efficient than cell-free viral uptake. (A) HK-2 cells before (left) and after exposure to cell-free HIV Gag-iGFP (middle) or cell-associated HIV Gag-iGFP (right). (B) Time course of T cell to HK-2 cell viral transfer at 3, 6, 9, and 12 hours of co-culture. (C) Titration of donor cells (0, 0.25 × 106, 0.5 × 106, and 1 × 106) resulted in a dose-dependent transfer of fluorescence. (D) Confocal images of flow-sorted HK-2 cells after virus transfer. DAPI staining of nuclei (blue); HIV Gag-iGFP (green); arrowhead, GFP-positive viral material. (E) Live imaging of viral transfer from HIV Gag-iGFP expressing Jurkat (green) to HK-2 (CMTMR-labeled, red). Selected XZ-projection from time lapse images for about 1 hour and 15 minutes. Arrow and arrowhead indicate two viral puncta emerging from donor cell and entry into target cells.
The viral transfer between HIV Gag-iGFP–infected T-cell line, MT4, and target HK-2 increased over time with 50% acquiring GFP at 3 hours to >80% at 12 hours (Figure 3B). The relatively high fraction of cells internalizing HIV when co-cultured with infected MT4 cells again correlated with the high infection efficiency of these cells (data not shown). Viral uptake was dependent on donor-to-target cell ratio increasing from 23 to 43 to 58% at ratios of 1:1, 2:1, and 4:1 (Figure 3C). After cell–cell transfer, confocal images of the FACS-sorted HK-2 cells showed punctate, vesicular cytoplasmic structures of heterogeneous size (Figure 3D). Although most of the cells contained small, fluorescence puncta, occasional larger fluorescence accumulations were also observed (Figure 3D, arrowheads).
Using live confocal imaging, we followed the interactions between infected T cells and epithelial cells, acquiring a 3D image stack every 5 minutes. HIV Gag-iGFP–expressing donor cells stably bound to target cells and the movement of fluorescence puncta was observed to associate with the target cells over time (Figure 3E). In one example, the directional movements of two viral puncta suggest a role for active cellular motors (Figure 3E, Supplemental Movie).
HIV Transfer into HK-2 Cells Does Not Require Env
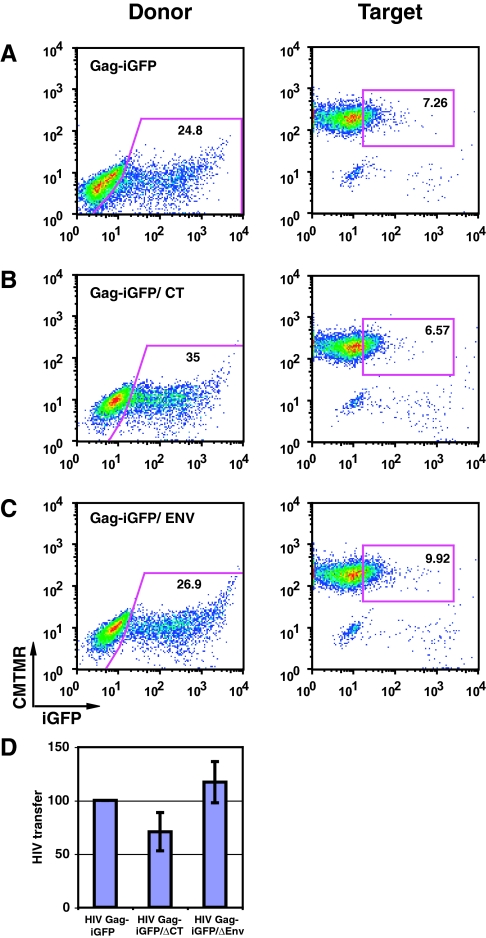
Viral transfer assays using two mutant viral constructs13 carrying a deletion of the cytoplasmic tail of Env, HIV Gag-iGFP ΔCT, or a frame shift mutation that eliminates Env expression, HIV Gag-iGFP ΔEnv, tested the role of the viral glycoprotein in cell–cell HIV transfer (Figure 4, B and C). The mutant constructs expressed comparable, wild-type levels of Gag-iGFP fluorescence (Figure 4A). When normalized to transfection efficiency (Figure 4D), the mutants transferred similar amounts of virus to target cells, showing that Env-mediated mechanisms are not required for T cell-to-epithelial cell transfer.
Figure 4.
Env is not required for viral uptake into target epithelial cells. (A) HIV Gag-iGFP transfer between Jurkat cells and HK-2 cells. (B) HIV Gag-iGFP/ΔCT in donor cells. (C) HIV Gag-iGFP ΔEnv, Env deficient virus, in donor cells. (D) HIV transfer normalized to HIV expression in donor cells is plotted for each of the mutants. Error bars represent SD from the mean of three independent experiments.
Sulfated Polysaccharides Inhibit T Cell-to-Epithelial Transfer of HIV
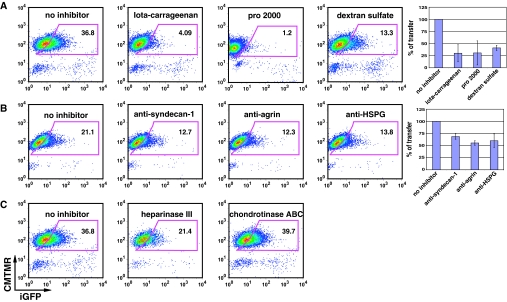
Previous studies have shown important roles for sulfated polysaccharides in viral transmission between T cells and epithelial cells.24 Iota carrageenan, a seaweed-derived sulfated polysaccharide that blocks the attachment of HIV-1–infected lymphocytes to epithelia,24 inhibited viral transfer from Jurkat to HK-2 cell by 89% (Figure 5A). The negatively charged sulfated polymer, dextran sulfate (molecular weight > 500,000) decreased transfer by 64% (Figure 5A). Another sulfonated polymer, Pro 2000, was an effective inhibitor of virus transfer (Figure 5A).
Figure 5.
Inhibition of virus transfer between Jurkat HIV Gag-iGFP and HK-2 cells. (A) Affect of charged polymers Iota carageenan (100 μg/ml), pro 2000 (20 μg/ml), or Dextran sulfate (25 μg/ml) on viral transfer. (B) Affect of antibodies against HSPG and specific HSPG molecules anti-agrin (100 μg/ml) or anti-syndecan 1 (50 μg/ml). (C) Enzymatic treatment of cells with heparinase (10 IU/ml) or chondroitinase (50 U/ml). Donor and target cells as described in Figure 2A were pretreated with or without inhibitors for 45 minutes. HIV transfer was carried out by co-culture donor and target cells for 3 hours at 37°C in the presence or absence of inhibitors.
Heparan sulfate proteoglycans (HSPG), such as agrin or syndecans, can bind to HIV through their heparan sulfate (HS) polysaccharide chains. For example, syndecans serve as attachment receptors for HIV-1 on macrophages (syndecan-1)25 and dendritic cells (syndecan-3).26 We found that anti-agrin (100 μg/ml) and anti-syndecan-1 (50 μg/ml) monoclonal antibodies blocked approximately 50% of the virus transfer (Figure 5B). Enzymatic removal of cell surface heparan sulfate with heparinase inhibited transfer by nearly 50%, whereas treatment with chondroitinase had no effect (Figure 5C). Together, these results suggest a role for heparan sulfate proteoglycan in the cell-to-cell exchange of HIV.
HIV-1 Gene Expression in HK-2 Cells after Virus Transfer
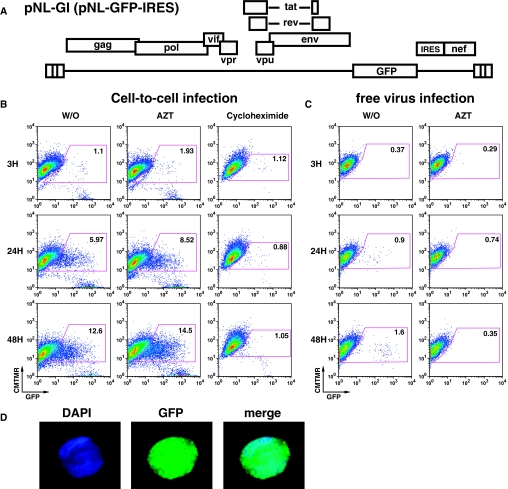
To determine whether viral genes can be expressed after transfer, we used a GFP-expressing HIV reporter virus, HIV NL-GI, which expresses GFP in place of the early gene nef27 (Figure 6A). The NL-GI viral particles are not fluorescently labeled, and as a result, co-culture of NL-GI–expressing donor cells with the HK-2 target cells did not result in transfer of fluorescence signal at 3 hours (Figure 6B), but when the target cells were cultured for 24 hours, 6% of the target cells expressed high levels of GFP (Figure 6B), and by 48 hours, the level of GFP-positive epithelial cells increased to about 13% (Figure 6B). Surprisingly, GFP expression was not blocked by azidothymidine (AZT), indicating that the viral gene expression did not require new proviral synthesis. In addition, GFP expression was not blocked by another reverse transcriptase (RT) inhibitor (tenofovir), a protease inhibitor (indinavir), or an integrase inhibitor (raltegravir) (data not shown). GFP expression in HK-2 cells was blocked in the presence of the translation inhibitor cycloheximide, indicating a requirement for active protein synthesis (Figure 6B). HK2 cell morphology and growth was not affected by cycloheximide (data not shown). When the HK2 cells were exposed to a large amount of cell-free NL-GI virus (50 ng/ml), a small fraction (1.6%) of the cells reproducibly expressed GFP in an AZT-sensitive manner (Figure 6C). This shows that cell-free infection of CD4-negative cells can occur with low efficiency, and this route of viral gene expression requires reverse transcription of the entering virus.
Figure 6.
HIV directed GFP expression in HK-2 cells after coculture with Jurkat T cells expressing HIV-NL-GI. (A) NL-GI viral construct carries GFP in place of nef and restores nef expression with an internal ribosome entry site (IRES). (B) Time course of HIV-associated GFP expression in epithelial cells at 3, 24, and 48 hours after exposure of cell-associated NL-GI for 3 hours. Target HK-2 cells were cultured for 48 hours in the presence of AZT (100 uM) or cycloheximide (2.5 μg/ml). Number indicated in each gate is the percentage of HK-2 cells expressing GFP. (C) Infection of HK-2 cells with large excess of cell-free HIV NL-GI (50 ng/ml capsid antigen, p24) results in low level infection that is sensitive to AZT. (D) Flow sorted GFP-expressing HK-2 cells examined by confocal microscopy show a diffuse GFP staining pattern and not a punctate endosomal pattern. DAPI staining of cell nuclei (blue), GFP expression (green), and merged (right).
NL-GI–infected target cells expressed a diffuse GFP pattern indicative of de novo GFP expression (Figure 6D). This pattern of GFP contrasts with the punctate pattern obtained after the uptake of fluorescence viral materials (Figures 1 and 3). This diffuse GFP localization and cycloheximide sensitivity imply de novo translation in the epithelial cell, possibly from a transferred viral mRNA. Intracellular staining for the viral protein nef showed enhanced anti-Nef staining in cells that were also expressing high levels of virally driven GFP (Supplemental Figure S2).
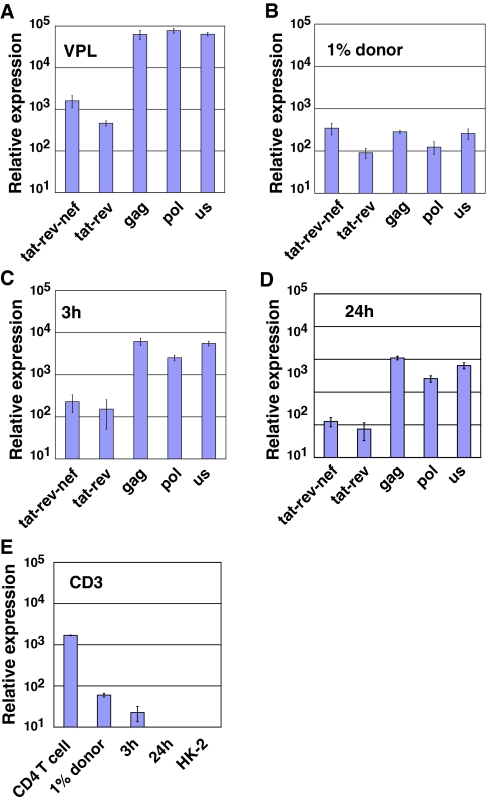
We next examined which viral RNAs are transferred into epithelial cells through direct contact with HIV-infected leukocytes. If the transfer of RNAs was caused by the exchange of cellular contents or nonspecific uptake of dead cell debris, the RNAs in the cell should resemble those in the donor T cell. If, however, the RNA transfer is restricted to viral particles, the RNAs should be enriched for unspliced genomic RNAs typically found within virus particles. Quantitative real-time PCR analyses were performed on viral RNA purified from flow sorted HK-2 epithelial cells after they had been co-cultured with HIV-expressing Jurkat cells. Three independent quantitative PCR assays (us, gag, pol), recognizing the unspliced viral RNA, and two PCR-assays for multi-spliced viral RNAs (tat-rev-nef and tat-rev), were measured along with the reference gene, tubulin.28 Reference RNA from a mixture of pelleted virus particles and uninfected HK-2 cells yielded an unspliced to spliced (U:S) ratio at about 50:1 (Figure 7A). RNA from HIV-expressing Jurkat T cells yielded a U:S ratio of nearly 1:1, showing balanced pool of unspliced and multispliced RNA within the donor cells (Figure 7B). In the flow sorted target HK-2 cells, the HIV RNA profile resembled that of virus particles with U:S ratio of 50:1, consistent with the uptake and internalization of virus particles at 3 and 24 hours (Figure 7, C and D). T-cell contamination in epithelial cell samples, as determined by quantitative real-time PCR for CD3, was undetectable in the purified epithelial cells (Figure 7E). These results argue that the epithelial cells had acquired viral RNAs and not simply randomly acquired RNA by cell fusion, phagocytosis, or contamination.
Figure 7.
Transfer of particle-associated viral RNAs into HK-2 cells. Quantitative RT-PCR for two spliced and three unspliced forms of HIV RNAs was used to evaluate the viral RNA species transferred into HK-2 cells at 3 and 24 hours after co-culture with infected T cells. (A) Viral RNA profile from virion-associated RNAs from pelleted HIV mixed with uninfected HK-2 cells. (B) Viral RNAs from 1% mixture of HIV infected Jurkat cells and HK-2 cells. (C) Viral RNAs from cells sorted after 3 hours. (D) Viral RNAs from cells sorted after 24 hours. (E) Quantitative RT PCR for CD3 genes (CD3 ε and γ chain) to determine the level of T-cell contamination in the 3- and 24-hour sorted cell populations.
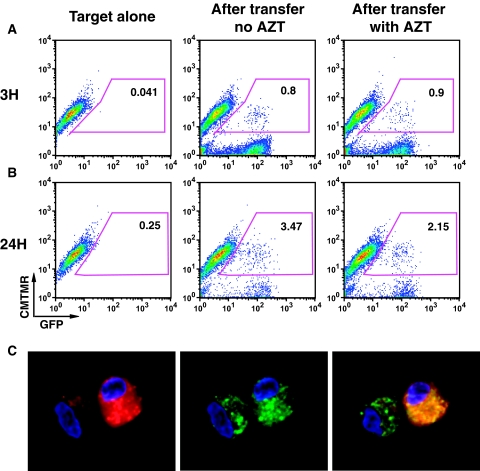
In the HK2 cells, HIV-driven GFP expression was not inhibited by AZT, other viral reverse transcriptase, or integrase antagonists. We then tested whether HIV-driven GFP expression occurred after viral transfer to primary epithelial cells. Three hours after exposure of primary renal tubular epithelial cells to CD4 T cells infected with NL-GI, fluorescence was detected in 0.8% of the target cells, indicative of uptake of fluorescence viral material (Figure 8A). After removing the donor cells and culturing for an additional 24 hours, about 3.5% of target cells became GFP positive (Figure 8B), even in the presence of AZT. Confocal microscopy of the flow sorted cells showed that the Cell Tracker–labeled epithelial cells contained both diffuse and punctate dots of GFP in their cytoplasm indicative of GFP expression and possible internalization of debris from infected cells (Figure 8C). These data indicate that a virus that expresses GFP from the nef coding region can be expressed in primary human epithelial cells at 24 hours after cell–cell co-culture.
Figure 8.
Exposure of primary tubular cells to primary T cells infected with HIV NL-GI results in GFP expression in epithelial cells at 24 hours. (A) FACS profile of GFP fluorescence in epithelial cells after 3 hours of co-culture. (B) FACS profile after 24 hours of co-culture. (C) Flow sorted GFP-expressing primary renal epithelial cells examined by confocal microscopy show both diffused and punctate dots of GFP in the cytoplasm. DAPI staining of cell nuclei (blue), GFP expression (green), and CMTMR (red).
DISCUSSION
In this study, we used an in vitro co-culture system to model the interstitial contacts between infected infiltrating lymphocytes and renal tubular epithelial cells that occur in kidneys of patients with HIVAN.19 We found that direct cell–cell contact between infected T cells and renal tubular cells can mediate the transfer of large quantities of virus into a trypsin-resistant, internal compartment in CD4-negative renal epithelial cells. T cell-to-epithelial cell viral transfer occurred with high efficiency from primary T cells to primary renal epithelial cells and between the model cell lines Jurkat/MT4 and HK-2/HPT-1b. All tubular epithelial cells tested, but not all epithelial cell lines or podocytes, exhibited this phenotype, suggesting cell type specificity. The high levels of virus transferred into epithelial cells were unexpected and suggest a novel mechanism by which infected interstitial T cells could cause pathology in epithelial cells.
The amount of virus transferred into cells was time dependent and increased with increasing numbers of donor cells. The HIV-associated fluorescence achieved was much higher than that which could be achieved with high concentrations of cell-free virus. Using the HK2 cell as a model system, RNA profiling of the viral material after cell–cell contact confirmed that the interaction between cells results in the exchange of virus and not transfer of the contents of the infiltrating donor cell or nonspecific uptake of cellular debris. Our reporter virus experiments suggest that viral genes can be expressed after viral transfer. This is particularly relevant because, in HIVAN animal models, the expression of a single viral gene such as nef is sufficient to cause disease. Within a kidney carrying HIV-infected infiltrating cells, it is possible that this mechanism could result in viral gene expression in epithelial cells, leading to epithelial cell dysfunction. We also found that infection with high levels of cell-free virus may lead to low but detectable infection of epithelial cells, indicating that there may be alternative pathways that lead to viral gene expression in epithelial cells. The expression of viral genes in epithelial cells may also enhance the recruitment of additional lymphocytes, thereby potentiating a positive feedback cycle where viral transfer enhances recruitment of inflammatory cells.29
The route of entry of the viral RNA into the cell is still unclear. The internalization into what seem to be endocytic compartments does not require Env or CD4, so it is likely to use nonclassical receptors. It is clear from the RNA profiling of viral RNAs in the target cell that the transfer of materials is specific to a subset of viral components, strongly suggesting that it is not influenced by a process whereby tubular cells absorb apoptotic or necrotic cell debris. Additional studies are needed to understand the process of viral uptake.
Because previous studies have shown that peripheral blood mononuclear cells can transfer CCR5 to CCR5-negative cells,30 we examined whether HIV may be transferred from infected to uninfected cells through an exosome-like pathway. Using fluorescent dye–labeled microparticles from infected T cells, we found that the HIV infection status did not alter microparticle release or uptake by HK-2 cells, suggesting that the process was not dependent on microparticle transfer.
The viral transfer from T cell to epithelial cell can be inhibited with sulfated/sulfonated polymers, iota-carageenan, PRO2000, or dextran sulfate, all of which have been shown to inhibit HIV binding to cells.31–35 However, the interactions here seem independent of Env, suggesting that some pathogenic mechanisms can occur in the absence of canonical viral receptor interactions. Because heparan sulfate proteoglycan molecules also play important roles in cell adhesion, it may be that these compounds act by disrupting cell–cell interactions mediated by HSPGs. Although many HSPGs could be involved based on the inhibitory effects of the anti-HSPG antibody and heparinase, the results with monoclonal antibodies against the HSPGs syndecan-1 or agrin suggest a more specific role for these molecules. Agrin and syndecan have both been implicated in HIV trans-infection of T cells through virus-carrying epithelial cells.36,37
This study suggested that HIV can induce pathology in nonlymphoid target organs by the transfer of virions through CD4-independent mechanisms. It was particularly surprising that we observed viral gene expression accompanying viral internalization into the epithelial cells even in the presence of reverse transcriptase, protease, or integrase inhibitors. However, we did observe that some level of infection by cell-free virus of CD4-negative epithelial cells was sensitive to AZT. The extent to which viral gene expression in epithelial cells is driven by fully integrated proviruses in vivo is still unknown, and the decay of viral RNA signals after exposure to cell-associated HIV suggests that active replication is very inefficient. Given the presence of infiltrating infected cells in HIVAN biopsies, these data show novel mechanisms whereby renal cell pathology may be induced by cell contact with infected T cells.
CONCISE METHODS
Cells and Tissue Culture
The CD4 T-cell lines Jurkat and MT4 and the human renal proximal epithelial cell line HK-2 were obtained from American Type Culture Collection. Jurkat cells were maintained in RPMI 1640 with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FBS. Cells were passaged regularly and were maintained at a density of <5 × 105/ml. HK-2 cells were cultured in Keratinocyte-SFM (Invitrogen) supplemented with 2.5 mg/ml bovine pituitary extract and 0.25 g/ml recombinant EGF (Invitrogen). The medium was changed every 2 to 3 days. HRCEpCs (#C-12660; PromoCell) were cultured in renal epithelial cell growth medium 2 (#C-26030; PromoCell). MS114, a nontransformed primary cell originated from a child with HIVAN, was cultured as described previously.21 The HPT-1b cell line is a clonal cell line derived from the previously reported HPT-1 cell line by limiting dilution. HPT-1b cells are conditionally immortalized with the temperature-sensitive SV40 large T antigen. HPT-1b cells were expanded at 33°C until they reached 80% confluence and subsequently cultured at 37°C for 2 weeks to induce T-antigen degradation and cellular differentiation.
HIV Infection and Nucleofection of Donor T Cells
HIV-expressing donor cells were obtained by infecting MT4 cells with HIV Gag-iGFP virus or by infecting PHA activated primary CD4 T cells using VSV-G pseudotyped HIV Gag-iGFP virus. Infectious virus was produced in 293T cells by transfection.13 One million MT4 or CD4 T cells were infected with 25 ng of free virus. Expression of HIV Gag-iGFP was monitored by flow cytometry. At 48 hours after infection, infected donor cells were washed and resuspended in culture medium at a density of 2 × 106/ml. To introduce HIV into Jurkat cells, HIV-1 proviral constructs were transfected into Jurkat cells using Amaxa nucleofection (Amaxa Biosystems). Briefly, 3 μg of endotoxin-free HIV-1 proviral plasmids was nucleofected into 5 × 106 Jurkat cells by using Cell Line Nucleofector Kit V, program S-18. Nucleofected Jurkat cells were enriched 24 hours later by centrifugation on a Ficoll-Hypaque density gradient. Enriched cells were cultured for another 24 hours and were used as donors in all experiments described hereafter.
Viral Transfer and Inhibition Assay
T cell to epithelial virus transfer was carried out in a co-culture system. The target cells were labeled with 2 μM of CellTracker orange CMTMR fluorescent dye (Molecular Probes) at 37°C for 30 minutes, as described in manufacturer's instructions. Dye-labeled target cells were washed with PBS, and 2.5 × 105 cells were seeded in a 24-well plate, cultured overnight, and rinsed again with PBS before co-culture with donor cells. Unless specified, a total of 1 × 106 donor cells (HIV-infected MT4, primary CD4 T cell, or HIV-transfected Jurkat) were added to the target epithelial monolayer and co-cultured for 3 hours. When specified in text, the amount of donor cells or the co-culture time was varied. The co-culture was terminated by removing the donor cells with three PBS washes. Adherent cells were detached by trypsin-EDTA treatment at 37°C for 10 minutes and washed once before being fixed with 2% paraformaldehyde (PFA) at room temperature for 10 minutes or at 4°C overnight. Fixed cells were washed and resuspended in PBS followed by flow cytometry analysis using BD FACScan or FACSCaliber. Transfer of virus (HIV Gag-iGFP) was analyzed with FlowJo software and calculated as the percentage of target cells that acquired the Gag-iGFP signals from donor cells. To quantify the viral transfer into the population of recipient epithelial cells, we calculated the relative fluorescence transfer index.13 For the inhibition studies, we used Jurkat transfected with HIV Gag-iGFP as donors and HK-2 as targets. Both donor and target cells were pretreated with indicated inhibitors or reagents at 37°C for 30 to 45 minutes, followed by co-culture at 37°C for 3 hours in the presence or absence of inhibitors listed as follows: iota carrageenan, dextran sulfate (molecular weight > 500,000), heparinase III, and chondrotinase ABC, purchased from Sigma-Aldrich (St Louis, MO); PRO 2000 (sulfonated polymer), obtained from Indevus Pharmaceuticals (Lexington, MA); inhibitory monoclonal antibodies against Agrin, Syndecan-1, purchased from Santa Cruz Biotech (Santa Cruz, CA); and monoclonal antibody against HSPG, purchased from Thermo Fisher Scientific.
HIV-Specific Gene Expression in Target Cells after Virus Transfer
To verify the infection of HIV in HK-2 cells, a reporter GFP-expressing HIV virus, HIV NL-GI, was used in the transfer assay.38 Donor cells expressing HIV NL-GI were co-cultured with target HK-2 cells for 3 hours and were removed with extensive wash with PBS. Target HK-2 cells were cultured in the presence or absence of cycloheximide (protein synthesis inhibitor) or AZT (HIV reverse transcriptase inhibitor). GFP expression in target cells was measured by flow cytometry at 3, 24, and 48 hours after co-culture. Only the CMTMR-labeled HK-2 population was gated for analysis. To characterize the viral RNA profile in the cultured target HK-2 cells, quantitative RT-PCR was used to detect the unspliced or multiple-spliced forms of viral RNA. Primer sets for unspliced form (gag, pol, and us), multiple-spliced form (tat-rev-nef and tat-rev), and housekeep gene (tubulin) were used. To avoid the contamination of carryover donor cells, we purified target HK-2 cells using flow sorting by gating the CMTMR-labeled HK-2 population (GFP+ or GFP−) with exclusion of doublets. Cells were briefly fixed with 1% of PFA before flow sorting, and RNA was extracted using RecoverAll Total Nucleic Acid Isolation, according to the manufacturer's instructions (#1975; Ambion). DNA was removed by digestion with DNAse for 30 minutes at room temperature. Quantitative RT-PCR was performed using ABI7900 Real-Time PCR machine. Level of HIV-specific RNA was normalized to the expression of the cytoskeletal gene, tubulin.
Confocal and Live Imaging
Live imaging was carried out in a sealed, gas-permeable microchamber (Ibidi Biosciences). Donor cells (Jurkat expressing HIV Gag-iGFP) were layered on top of the CMTMR-labeled HK-2 monolayer grew on Ibidi chamber, at the ratio donor:target of 4. The chamber was placed on a Zeiss Axiovert 200 microscope fitted with a laser-scanning confocal microscope 510 META detector. Differential interference contrast imaging and confocal green (for GFP) and red (for CMTMR) fluorescence were acquired in a multitrack configuration to avoid cross-talk between fluorescence channels. Images were recorded at 5-minutes intervals continuously for 3 hours. To visualize the pattern of HIV Gag-iGFP green virus particles within the target HK-2 or the expression of the reporter HIV NL-GI in the target HK-2 cells, the flow sorted HK-2 cells (either after virus transfer or virus gene expression) were washed and mounted on coverslips, and an image was taken as described before.13 Confocal images were analyzed with the Volocity or Image J software packages.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
The work was supported by NIH Grant AI074420-02 and a Burroughs Wellcome Fund Investigator Award to B.K.C. and Grant P01DK056492 to P.E.K. Imaging was supported by MSSM-Microscopy Shared Resource Facility Grants NIH-NCI 5R24 CA095823-04, NSF DBI-9724504, and NIH S10RR09145-01.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “HIV-1 Entry into Renal Epithelia,” on pages 399–401.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Wyatt CM, Rosenstiel PE, Klotman PE: HIV-associated nephropathy. Contrib Nephrol 159: 151–161, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE: Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest 100: 84–92, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sunamoto M, Husain M, He JC, Schwartz EJ, Klotman PE: Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int 64: 1695–1701, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Husain M, D'Agati VD, He JC, Klotman ME, Klotman PE: HIV-1 Nef induces dedifferentiation of podocytes in vivo: A characteristic feature of HIVAN. Aids 19: 1975–1980, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Lu TC, He JC, Wang ZH, Feng X, Fukumi-Tominaga T, Chen N, Xu J, Iyengar R, Klotman PE: HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem 283: 8173–8182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosenstiel PE, Gruosso T, Letourneau AM, Chan JJ, Leblanc A, Husain M, Najfeld V, Planelles V, D'Agati VD, Klotman ME, Klotman PE: HIV-1 Vpr inhibits cytokinesis in human proximal tubule cells. Kidney Int 74: 1049–1058, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marras D, Bruggeman LA, Gao F, Tanji N, Mansukhani MM, Cara A, Ross MD, Gusella GL, Benson G, D'Agati VD, Hahn BH, Klotman ME, Klotman PE: Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med 8: 522–526, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Tanji N, Ross MD, Tanji K, Bruggeman LA, Markowitz GS, Klotman PE, D'Agati VD: Detection and localization of HIV-1 DNA in renal tissues by in situ polymerase chain reaction. Histol Histopathol 21: 393–401, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Conaldi PG, Biancone L, Bottelli A, Wade-Evans A, Racusen LC, Boccellino M, Orlandi V, Serra C, Camussi G, Toniolo A: HIV-1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J Clin Invest 102: 2041–2049, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eitner F, Cui Y, Hudkins KL, Stokes MB, Segerer S, Mack M, Lewis PL, Abraham AA, Schlondorff D, Gallo G, Kimmel PL, Alpers CE: Chemokine receptor CCR5 and CXCR4 expression in HIV-associated kidney disease. J Am Soc Nephrol 11: 856–867, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Hatsukari I, Singh P, Hitosugi N, Messmer D, Valderrama E, Teichberg S, Chaung W, Gross E, Schmidtmayerova H, Singhal PC: DEC-205-mediated internalization of HIV-1 results in the establishment of silent infection in renal tubular cells. J Am Soc Nephrol 18: 780–787, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Ray PE, Liu XH, Henry D, Dye L, III, Xu L, Orenstein JM, Schuztbank TE: Infection of human primary renal epithelial cells with HIV-1 from children with HIV-associated nephropathy. Kidney Int 53: 1217–1229, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Chen P, Hubner W, Spinelli MA, Chen BK: Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol 81: 12582–12595, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK: Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science 323: 1743–1747, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phillips DM, Bourinbaiar AS: Mechanism of HIV spread from lymphocytes to epithelia. Virology 186: 261–273, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Bomsel M: Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med 3: 42–47, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Tan X, Phillips DM: Cell-mediated infection of cervix derived epithelial cells with primary isolates of human immunodeficiency virus. Arch Virol 141: 1177–1189, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Moore JS, Rahemtulla F, Kent LW, Hall SD, Ikizler MR, Wright PF, Nguyen HH, Jackson S: Oral epithelial cells are susceptible to cell-free and cell-associated HIV-1 infection in vitro. Virology 313: 343–353, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, Burns GC, D'Agati VD, Winston JA, Klotman ME, Klotman PE: Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol 11: 2079–2087, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Hubner W, Chen P, Del Portillo A, Liu Y, Gordon RE, Chen BK: Sequence of human immunodeficiency virus type 1 (HIV-1) Gag localization and oligomerization monitored with live confocal imaging of a replication-competent, fluorescently tagged HIV-1. J Virol 81: 12596–12607, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ross MJ, Wosnitzer MS, Ross MD, Granelli B, Gusella GL, Husain M, Kaufman L, Vasievich M, D'Agati VD, Wilson PD, Klotman ME, Klotman PE: Role of ubiquitin-like protein FAT10 in epithelial apoptosis in renal disease. J Am Soc Nephrol 17: 996–1004, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Snyder A, Alsauskas Z, Gong P, Rosenstiel PE, Klotman ME, Klotman PE, Ross MJ: FAT10: a novel mediator of Vpr-induced apoptosis in human immunodeficiency virus-associated nephropathy. J Virol 83: 11983–11988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenstiel PE, Chan J, Snyder A, Planelles V, D'Agati VD, Klotman PE, Klotman ME: HIV-1 Vpr activates the DNA damage response in renal tubule epithelial cells. AIDS 23: 2054–2056, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pearce-Pratt R, Phillips DM: Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of human immunodeficiency virus-1. Biol Reprod 54: 173–182, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA: Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol 75: 9187–9200, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P: Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc Natl Acad Sci USA 104: 19464–19469, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D: The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10: 661–671, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Fang J, Acheampong E, Dave R, Wang F, Mukhtar M, Pomerantz RJ: The RNA helicase DDX1 is involved in restricted HIV-1 Rev function in human astrocytes. Virology 336: 299–307, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Ross MJ, Fan C, Ross MD, Chu TH, Shi Y, Kaufman L, Zhang W, Klotman ME, Klotman PE: HIV-1 infection initiates an inflammatory cascade in human renal tubular epithelial cells. J AIDS 42: 1–11, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, Plachy J, Stangassinger M, Erfle V, Schlondorff D: Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat Med 6: 769–775, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Lynch G, Low L, Li S, Sloane A, Adams S, Parish C, Kemp B, Cunningham AL: Sulfated polyanions prevent HIV infection of lymphocytes by disruption of the CD4-gp120 interaction, but do not inhibit monocyte infection. J Leukoc Biol 56: 266–272, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Mitsuya H, Looney DJ, Kuno S, Ueno R, Wong-Staal F, Broder S: Dextran sulfate suppression of viruses in the HIV family: Inhibition of virion binding to CD4+ cells. Science 240: 646–649, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Moulard M, Lortat-Jacob H, Mondor I, Roca G, Wyatt R, Sodroski J, Zhao L, Olson W, Kwong PD, Sattentau QJ: Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J Virol 74: 1948–1960, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sachdev DD, Zerhouni-Layachi B, Ortigoza M, Profy AT, Tuen M, Hioe CE, Klotman ME: The differential binding and activity of PRO 2000 against diverse HIV-1 envelopes. J AIDS 51: 125–129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rusconi S, Moonis M, Merrill DP, Pallai PV, Neidhardt EA, Singh SK, Willis KJ, Osburne MS, Profy AT, Jenson JC, Hirsch MS: Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob Agents Chemother 40: 234–236, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alfsen A, Yu H, Magerus-Chatinet A, Schmitt A, Bomsel M: HIV-1-infected blood mononuclear cells form an integrin- and agrin-dependent viral synapse to induce efficient HIV-1 transcytosis across epithelial cell monolayer. Mol Biol Cell 16: 4267–4279, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bobardt MD, Chatterji U, Selvarajah S, Van der Schueren B, David G, Kahn B, Gallay PA: Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol 81: 395–405, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen BK, Gandhi RT, Baltimore D: CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J Virol 70: 6044–6053, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.