Abstract
Smooth muscle of the uterus stays remarkably quiescent during normal pregnancy to allow sufficient time for development of the fetus. At present the mechanisms leading to uterine quiescence during pregnancy and how the suppression of activity is relieved at term are poorly understood. Myometrial excitability is governed by ion channels, and a major hypothesis regarding the regulation of contractility during pregnancy has been that expression of certain channels is regulated by hormonal influences. We have explored the expression and function of stretch-dependent K+ (SDK) channels, which are likely to be due to TREK channels, in murine myometrial tissues and myocytes using PCR, Western blots, patch clamp, intracellular microelectrode and isometric force measurements. TREK-1 is more highly expressed than TREK-2 in myometrium, and there was no detectable expression of TRAAK. Expression of TREK-1 transcripts and protein was regulated during pregnancy and delivery. SDK channels were activated in response to negative pressure applied to patches. SDK channels were insensitive to a broad-spectrum of K+ channel blockers, including tetraethylammonium and 4-aminopyridine, and insensitive to intracellular Ca2+. SDK channels were activated by stretch and arachidonic acid and inhibited by reagents that block TREK-1 channels, l-methionine and/or methioninol. Our data suggest that uterine excitability and contractility during pregnancy is regulated by the expression of SDK/TREK-1 channels. Up-regulation of these channels stabilizes membrane potential and controls contraction during pregnancy and down-regulation of these channels induces the onset of delivery.
Non-technical summary
During pregnancy the uterus must maintain a low contractile state to permit growth of the fetus and inhibt premature delivery. We show uterine smooth muscle cells express specific potassium channels (called stretch-dependent potassium channels; TREK-1). These channels are activated by stretch, stabilize resting membrane potentials of cells at negative potentials, and reduce excitability. During pregnancy the expression of TREK-1 channels increases, and this may contribute to reduced excitability. Near the onset of labour, TREK-1 expression declines, and this may promote the transition to a contractile state. Thus, our data suggest dynamic regulation of TREK-1 channel expression in the uterus contributes to the maintenance of pregnancy.
Introduction
The uterus expands in volume dramatically during pregnancy. Distension and hypertrophy of the organ are required to allow sufficient space for the developing fetus. The non-pregnant uterus contracts in response to stretch, but mechanisms are in play during pregnancy to stabilize the excitability of myometrial muscles and maintain mechanical quiescence to allow sufficient time for the development of the fetus before it is expelled during labour (Parkington & Coleman, 1990). At term, changes occur to counteract the factors maintaining quiescence, and successful parturition requires an increase in electrical excitability of myometrial muscles and coordination of contractions between uterine muscle bundles. At present the mechanisms that facilitate uterine quiescence during pregnancy and enhance contractility at term are poorly understood, but these are important questions since premature labour is a major health problem (Challis et al. 2000).
Myometrial smooth muscle excitability is governed by ion channels, and a major hypothesis regarding the regulation of contractility during pregnancy has been that expression of certain channels is regulated by hormonal influences (Toro et al. 1990; Song et al. 1999, 2001; Knock et al. 2001; Sawada et al. 2005). Like other muscles, myometrial smooth muscle cells (MSMCs) have negative resting potentials maintained by K+ conductances (Toro et al. 1990; Wang et al. 1998). Contractile events in MSMCs are initiated by the influx of Ca2+ via CaV1.2 (L-type) Ca2+ channels (Mironneau, 1973; Ohya & Sperelakis, 1989). While there are reports of isoform switching of Ca2+ channels between non-pregnant and pregnant MSMCs and non-genomic reduction of Ca2+ currents by 17β-oestradiol (Yamamoto, 1995; Helguera et al. 2002), the consequences of these effects in terms of the delivery of Ca2+ for contraction are unknown, and it is unlikely that these changes are a primary means to regulate MSMC excitability. Many types of K+ channels are expressed by MSMCs. During pregnancy the function and composition of K+ conductances is dynamic, and changes in the types and levels of channel isoforms and subunits expressed, and changes in regulatory mechanisms have been reported (Toro et al. 1990; Anwer et al. 1993; Knock et al. 2001; Song et al. 2001; Sawada et al. 2005; Brown et al. 2007). While the expression of some K+ channels is consistent with maintenance of quiescence during pregnancy, decreases in expression of Kv4.3 would tend to enhance spontaneous excitability during pregnancy (Song et al. 2001). In other cases changes in channel expression are not mirrored by functional changes. For example, the up-regulation of large-conductance Ca2+ activated K+ (BK) currents during pregnancy is not matched with the sensitivity of uterine muscle strips to specific blockers of these channels (Aaronson et al. 2006). Thus, at the present time, there is no clear explanation for the changes in excitability during pregnancy or at the onset of labour.
In the present study we explored the expression and function of stretch-activated K+ channels in murine MSMCs. These channels, encoded by members of the two-pore K+ channel superfamily (Kcnk; Patel et al. 1998), are expressed by visceral smooth muscle organs that perform a ‘reservoir function’ and can expand in volume without large increases in luminal pressure or activation of phasic contractions (e.g. bladder; gastrointestinal tract; Ordway et al. 1995; Koh & Sanders, 2001; Park et al. 2005; Baker et al. 2008). Two-pore K+ channels are expressed in human myometrium (Bai et al. 2005; Tichenor et al. 2005; Buxton et al. 2010), but the function of these channels has not been characterized in MSMCs. We hypothesize that distension of the uterus and maintenance of quiescence during pregnancy are facilitated by stretch-activation of an outward current to counteract other stretch-dependent inward currents common to smooth muscles. Increased expression of these channels and distension of the uterus during pregnancy could enhance the membrane-stabilizing effects of this conductance, and loss of these channels near term might dramatically enhance excitability. Here we describe novel hormonal regulation of TREK-1 (Kcnk2) channels in murine myometrium and describe the function of these channels in MSMCs and intact strips of uterine muscles. We also document a dramatic reduction in channel expression near term and conclude that stretch-dependent K+ (SDK) channels contribute to the regulation of myometrial excitability during pregnancy.
Methods
Tissue and cell preparation
CD1 (2–3 months old) and ovariectomized (OVX, 2 weeks after ovariectomy) mice were killed by inhalation of isoflurane (Baxter Healthcare, Deerfield, IL, USA) followed by cervical dislocation. Uteri were cut open from the neck to the end of the uterine horns and rinsed with Krebs–Ringer bicarbonate buffer (KRB, see below). Muscle strips were used for isometric force and intracellular microelectrode recordings. The animals used for these studies were maintained, and experiments were performed, in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno.
Isolation of smooth muscle cells
Small strips of smooth muscle layer from the uterine horns were equilibrated in Ca2+-free Hanks’ solution consisting of (mm): 125 NaCl, 5.6 KCl, 15 NaHCO3, 0.36 Na2HPO4, 0.4 KH2PO4, 10 glucose, 2.0 sucrose and 10 Hepes adjusted to pH 7.2 with NaOH, for 30 min. The buffer was replaced with an enzyme solution containing (per ml) collagenase 1.3 mg, bovine serum albumin 2 mg, trypsin inhibitor 2 mg and ATP 0.27 mg. The tissue was placed in a 37°C water bath for 10–12 min without agitation. After three to four washes with Ca2+-free Hanks’ solution, the tissue was triturated through a series of three blunt pipettes of decreasing tip diameters. Dispersed cells were stored at 4°C in Ca2+-free solution. Experiments were performed at room temperature within 6 h of cell dispersion.
Patch clamp techniques
The single-channel patch clamp technique was used to record membrane currents from dissociated MSMCs. Currents were amplified with an Axopatch 200B (Molecular Devices, Sunnyvale, CA, USA). Data were digitized with either a 12-bit or a 16-bit analog to digital converter (Digidata 1322A and Digidata 1320A, respectively, Molecular Devices). Data were stored directly and digitized online using pCLAMP software (v. 9.0, Molecular Devices). The data were sampled at 5 kHz for single-channel recordings with low pass filtered at 1 kHz using an eight-pole Bessel filter. The pipette resistances were 5–8 MΩ. Single-channel recordings were performed in asymmetrical K+ (5/140 mm) solution with charybdotoxin (200 nm) in the pipette solution to block BK channels. Negative pressure was applied to patches by suction via a 1 ml syringe and pressure–volume relationships were calibrated using a pressure transducer.
Intracellular microelectrode recordings
After preparing the tissue, impalements of cells were made with glass microelectrodes having resistances of 80–120 MΩ. Transmembrane potentials were recorded with a standard electrometer (Duo 773; WPI, Sarasota, FL, USA). Data were recorded on a PC running Acknowledge 3.2.6 software (Biopac Systems, Santa Barbara, CA, USA). To allow for prolonged intracellular recordings during stretch, a small area of muscle in the plane of stretch was pinned down using micropins. Impalements were made at the margin between the stretched and pinned areas of muscle. Stretch was induced by a micromanipulator (WPI).
Isometric force measurements
Standard organ bath techniques were employed to measure the changes in force generated by uterus smooth muscle strips. One end of a smooth muscle strip was attached to a fixed mount and the opposite end to an isometric strain gauge (Fort 10, WPI) in oxygenated KRB solution maintained at 37.5 ± 0.5°C. A resting force of 4.9 mN was applied to set the muscles at optimum length, and the muscles were allowed to equilibrate for 1–2 h with constant perfusion with KRB solution. Mechanical responses were recorded on a computer running Acqknowlege 3.2.6 and measurements of the area under the curve (AUC) obtained. The AUC was determined as the integral values above the baseline of selected area for 10 min recordings (mN min). The AUC for the tissues exposed to tested drugs were compared to the AUC for tissues under control conditions, during an equivalent period of time. Bathing solutions were exchanged by switching the perfusion to the drug-containing solution.
Molecular studies
Myometrium total RNA and cDNA preparation and amplification were identical to those reported previously (Baker et al. 2008). Briefly, RNA was prepared using SNAP Total RNA isolation kit (Invitrogen, Carlsbad, CA, USA) as per the manufacturer's instructions. RNA was treated with RNAse-free DNAse I (2 units) at 37°C (New England Biolabs, Inc., Ipswich, MA, USA) prior to cDNA preparation. First strand cDNA was synthesized from each RNA using Superscript II reverse transcriptase with 500 μg μl−1 of oligo dT primers cDNA. The following PCR primers designed against murine sequences were used (the genebank accession number is given in parenthesis for the reference nucleotide sequence used): TREK-1 (Kcnk2) (accession no. NM_010607, nt 506–527 and 803–822, amplicon = 316 bp or nt 1485–1512 and 1637–1664, amplicon = 180 bp or nt 1226–1337, amplicon = 112 bp); TREK-2 (Kcnk10) (accession no. NM_029911, nt 1915–1942 and 2070–2097, amplicon = 183 bp or nt 908–1034, amplicon = 127 bp); TRAAK (Kcnk4) (accession no. NM_008431, nt 880–901 and 960–980, amplicon = 101 bp); cytoglobin (accession no. NM013607, nt 268–289 and 497–519, amplicon = 252 bp (589 bp, if genomic DNA contamination)); GAPDH (glyceraldehyde-3-phosphate dehydrogenase; accession no. NM_001001303, nt 353–371 and 505–522, amplicon = 170 bp (270 bp, if genomic DNA contamination)); and HPRT (hypoxanthinephophoribosyltransferase; accession no. NM_013556, nt 289–486, amplicon = 198 bp). Real time-PCR (RT-PCR) was performed on murine uterine smooth muscle in reproductive ages (2–3 months old). Real time quantitative PCR (qPCR) was performed using Syber Green chemistry on a SDS 7300 real-time PCR system (Applied Biosystems, Foster City, CA, USA). Regression analysis of the mean values of eight multiplex RT-PCRs for the log10 diluted cDNA was used to generate standard curves. Unknown quantities relative to the standard curve for a particular set of primers were calculated yielding transcriptional quantification of gene products relative to the endogenous standard GAPDH, cytoglobin or HPRT. The reproducibility of the assay was tested by analysis of variance comparing repeat runs of samples, and the mean values generated at individual time points were compared by Student's t test.
Western blot and immunohistochemistry
Western blot
For membrane protein extraction, murine myometrium was homogenized in lysis buffer. Membrane preparations were obtained by centrifugation (1000 g for 10 min, P1; 10,000 g for 20 min, P2; and 100,000 g for 60 min, P3) at 4°C. Protein concentrations were determined by the Bradford assay using bovine gamma globulin as standard and the protein concentration was adjusted to 1 mg ml−1. The blots were incubated with either affinity-purified anti-TREK-1 polyclonal antibodies (Alomone Laboratories, Jerusalem, Israel) or myosin heavy chain polyclonal antibody (Chemicon/Millipore). Blots were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies, visualized by ECL chemistry (Lumigen, GE Healthcare, Piscataway, NJ, USA). Densitometric analysis of the Western blot TIFF images was performed using Un-Scan-It software (Silk Scientific, Orem, UT, USA). TREK-1 protein expression in Sham and ovariectomized mice uteri was calculated from the pixel intensity values (minus background) normalized to the pixel intensity values of MHC (myosin heavy chain). Each antiserum was pre-incubated with the appropriate immunizing peptide as a control experiment to block reactivity.
TREK-1 immunohistochemistry
Distribution of TREK-1 channel proteins in murine myometrium was examined with immunohistochemistry using antibodies raised against TREK-1 (Alomone Laboratories). Tissues for immunohistochemical studies were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) for 30 min at room temperature then washed (4 × 15 min) in PBS and cryoprotected in a graded series of sucrose solutions (5, 10, 15 and 20% w/v in PBS, 1 h each). Tissues were then embedded in TissueTek embedding medium (Sakura Finetek, Torrance, CA, USA) and 20% sucrose in PBS (1 part/2 parts v/v) and rapidly frozen in isopentane pre-cooled in liquid nitrogen. Cryosections were cut using a Leica CM 3500 cryostat at a thickness of 10–15 μm and collected on vectabond coated microscope slides. Non-specific antibody binding was blocked using bovine serum albumin (BSA, 1% in PBS, Sigma) for 1 h at room temperature. Tissues were incubated with primary antibody to TREK-1 (1: 200) overnight at 4°C. After labelling with primary antibody, tissues and sections were washed for 2 h in PBS before being incubated in secondary antibody (Alexa Fluor 488-coupled goat anti-rabbit IgG) for 1 h at room temperature. Secondary antibody was obtained from Molecular Probes/Invitrogen and diluted to 1:200 in PBS. After washing with PBS, specimens were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA). For the negative control TREK-1 antibody had been pre-absorbed with its control antigen. Tissues were examined with a Zeiss LSM 510 META confocal microscope with an excitation wavelength of 488 nm. Confocal micrographs shown are digital composites of Z-series scans of several optical sections through a depth of 20–30 × 0.5 μm for full or partial thickness of the musculature. Final images were constructed with Zeiss LSM 510 META software.
Solutions and drugs
In intracellular microelectrode recordings and mechanical experiments the tissue chamber housing muscles was constantly perfused with oxygenated KRB of the following composition (mm): NaCl 118.5, KCl 4.5, MgCl2 1.2, NaHCO3 23.8, KH2PO4 1.2, dextrose 11.0, CaCl2 2.4. The pH of the KRB was 7.3–7.4 when bubbled with 97% O2–3% CO2 at 37.0 ± 0.5°C. In patch-clamp experiments smooth muscle myocytes were bathed in high K+ (HK+) solution containing (mm): 140 KCl, 0.1 EGTA, 5 Hepes adjusted to pH 7.4 with Tris. The pipette solution was a Ca2+-free physiological salt solution (MnPSS) containing (mm): 5 KCl, 135 NaCl, 2 MnCl2, 10 glucose, 1.2 MgCl2, and 10 Hepes adjusted to pH 7.4 with Tris. Charybodotoxin (ChTx, 200 nm) was included in the patch pipette to inhibit BK channels. Arachidonic acid (AA), tetraethylammonium (TEA) chloride, 4-aminopyridine (4-AP), and charybdotoxin were obtained from Sigma-Aldrich Corp. (St Louis, MO, USA). Methioninol and l-methionine were purchased from Fluka Biochemika (Buchs, Switzerland). These drugs were dissolved in test solutions directly. AA was dissolved in ethanol (10 mg ml−1) and stored under nitrogen at −20°C before experiments. The final concentration of ethanol was less than 0.1% and had no effect at this concentration.
Statistical analysis
Data are expressed as means ±s.e.m. Student's t test was used where appropriate to evaluate differences in the data. P values less than 0.05 were taken as statistically significant differences. The n values reported in the text refer to the number of recordings from the muscle strips, which is equivalent to the number of animals used unless otherwise stated.
Results
The expression of mechanosensitive 2-pore domain K+ (K2P) channels in myometrium
Molecular candidates for the SDK conductance include TREK-1, TREK-2 and TRAAK channels (Franks & Honore, 2004). We hypothesized that myometrial smooth muscles may express mechanosensitive K+ channel to help stabilize excitability of uterine muscles. RT-PCR was performed on murine uterine smooth muscle at reproductive ages (2–3 months old). Amplicons for TREK-1 and TREK-2 but not TRAAK were detected (Fig. 1A–D). The relative expression levels of TREK-1 and TREK-2 were determined by qPCR. TREK-1 transcript levels were significantly higher than TREK-2 transcript levels (Fig. 1E, 0.133 ± 0.032 vs. 0.008 ± 0.002, respectively, normalized to cytoglobin; P < 0.005, n= 6).
Figure 1. Expression of mechano-sensitive K2P channels in murine myometrium.
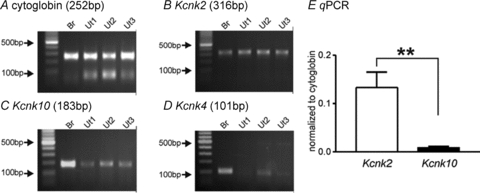
Representative agarose gels displaying amplification products from myometrium-derived RNA using gene specific primers for TREK-1 (Kcnk2), TREK-2 (Kcnk10), and TRAAK (Kcnk4). A, cytoglobin primers were used to confirm that the products generated were representative of RNA. Three independent preparations are presented. B–D, TREK-1 (B) and TREK-2 (C) but not TRAAK (D) was expressed in murine myometrium. E, qPCR revealed higher transcriptional expression of TREK-1 than TREK-2. The values are normalized to cytoglobin expression in the myometrial tissue. **P < 0.005. Br and Ut denote brain and uterine smooth muscle, respectively.
We then examined TREK-1 protein expression in murine myometrium (n= 4 animals). Figure 2A shows Masson Trichrome staining of a cryostat section of the uterus showing endometrium (EM) and myometrium (MM). After antibody labelling, examination of cross-sections by confocal microscopy showed TREK-1-like immunoreactivity (TREK-1-LI) throughout the myometrium (Fig. 2B). Higher magnification confocal micrographs of sections revealed TREK-1-LI in the plasma membrane in single myometrial myocytes (Fig. 2C). No immunoreactivity was observed in the negative control where TREK-1 antibody had been pre-absorbed with its control antigen (Fig. 2D). These experiments demonstrate expression of TREK-1 protein in murine myometrium.
Figure 2. Immunohistochemistry of TREK-1 in murine myometrium.
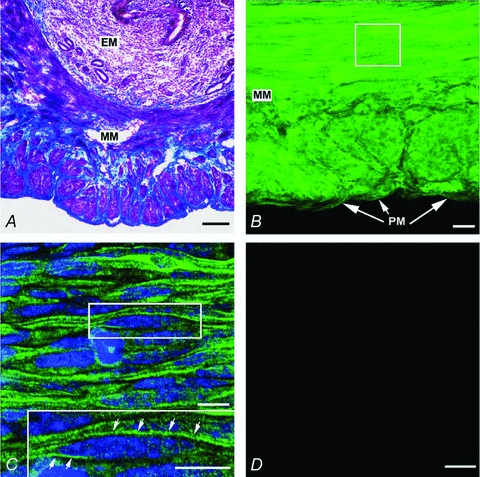
A, Masson Trichrome staining in a cryostat section revealed the histology of the murine endometrium (EM) and the myometrium (MM). Laser scanning confocal micrographs showed TREK-1-like immunoreactivity (TREK-1-LI) in cryostat sections. B, intenseTREK-1-LI immunostaining (green fluorescence) is observed throughout the myometrium (MM). C, higher magnification of the selected region in panel B showed the TREK-1-LI in plasma membrane in myocytes (see arrows in inset). D, no immunoreactivity is observed in the negative control where the TREK-1 antibody that had been pre-absorbed with its control antigen respectively. Scale bars: A and B, 100 μm; C, 20μm; inset in C, 10 μm; D, 20μm.
Mechanosensitive K+ channels in myometrial smooth muscle
Functional SDK channels in murine MSMCs were characterized with single-channel recordings. Single-channel currents evoked by negative pipette pressure were obtained in cell-attached patches of MSMCs. On-cell patches were held at 0 mV in asymmetrical K+ gradients (5/140 mm) (to exclude contamination from non-selective cation conductances) and exposed to negative pipette pressures from −20 to −40 cmH2O. The pipette solution contained charybdotoxin (200 nm) to block BK channels. Application of −40 cmH2O dramatically increased NPo (number of channels (N) × open probability (Po)) of SDK channels to 0.76 ± 0.41 from only rare openings in control (n= 17 from 25 patches). Upon application of −20 cmH2O to the same patch, NPo was slightly increased (Fig. 3A). When atmospheric pressure was restored, the open probability reversed to low levels. The channels activated under these conditions generated outward currents (i.e. K+ channels) and the amplitude of unitary currents was 2.1 ± 0.2 pA at 0 mV, which was a similar conductance (∼90 pS in symmetrical K+) to that in previous reports (Koh & Sanders, 2001; Koh et al. 2001). We tested the effects of several common K+ channel blockers on SDK channel activity of MSMCs in inside-out patches. SDK channels were strongly activated in inside-out patches due to patch excision. Applications of 4-aminopyridine (4-AP, 5 mm) and TEA (1–10 mm) to the cytoplasmic surface of patches had no significant effect on the NPo of SDK channels (Fig. 3B, n= 4 respectively). The cytoplasmic surface of the patch was also exposed to Ca2+ concentrations ranging from 10−7 to 10−6m. NPo of SDK channels was not affected by changes in cytoplasmic Ca2+ concentration (Fig. 3B, n= 4), confirming that the currents recorded were not due to an isoform of Ca2+-activated K+ channels. SDK channels in MSMCs were also tested for responses to arachidonic acid (AA), an activator of TREK-1 channels (Franks & Honore, 2004), and methioninol, a blocker of SDK channels (Park et al. 2005; Baker et al. 2008). In these experiments there were few openings of SDK channels at a holding potential of 0 mV in asymmetrical K+ gradient (5/140 mm). AA (10 μm) dramatically increased NPo of SDK channels (Fig. 3C). The amplitude of unitary currents from AA-activated channels was 2.0 ± 0.1 pA at 0 mV (n= 4). This unitary amplitude at 0 mV was identical to previously reported SDK channels in murine colonic and bladder smooth muscle cells (Koh & Sanders, 2001; Baker et al. 2008). Amplitude histograms in Fig. 3D and E were obtained from 1 min of recording under control conditions (NPo= near 0) and during exposure to AA (10 μm) (NPo= 0.45 ± 0.12, n= 4, after 1 min exposure of AA). Methioninol (1 mm) decreased NPo of AA-activated K+ channels (Fig. 3C). The effects of methioninol were reversible upon washout of the drug.
Figure 3. Properties of stretch-dependent K+ (SDK) channels in uterus myocytes.
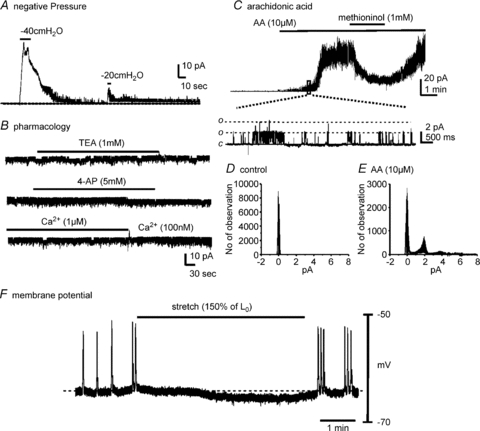
Cells were held at 0 mV in asymmetrical K+ (5/140 mm). A, application of negative pressure (−40 cmH2O or −20 cmH2O) to patch pipettes increased channel activity in cell-attached patches of murine myometrial smooth muscle cells. B, excised patches from the same cell in panel A revealed a high open probability of SDK channels. 4-Aminopyridine (4-AP), tetraethylammonium (TEA) and changes in cytosolic Ca2+ had no effect on open probability of SDK channels. C, arachidonic acid (AA) increased the open probability of SDK channels and methioninol decreased the open probability of SDK channels. c and o denote channel close and open, respectively. C, lower panel, an extended time scale from the box. D and E, all-point amplitude histograms were displayed before and after arachidonic acid (AA). F, intracellular microelectrode recording from intact murine myometrium shows that stretch (150% elongation from control length; Lo) caused hyperpolarization and cessation of spontaneous action potentials.
The functional role of SDK channels in murine myometrium was investigated by testing the effects of stretch on resting membrane potential using intracellular microelectrode recordings. Potential contamination by spontaneous neural activity was reduced by addition of tetrodotoxin (TTX, 1 μm), nitro-l-arginine (l-NA, 100 μm) and atropine (1 μm) to the KRB solution bathing the muscles. Under these conditions, stretch (150% elongation from control length; Lo) caused hyperpolarization (5.4 ± 0.7 mV, n= 5, P < 0.01) of myometrial muscle cells and decreased firing of action potentials (Fig. 3F), suggesting that stretch may activate a K+ conductance in intact muscle strips. Thus, activation of SDK channels and elicitation of hyperpolarization may stabilize myometrial muscles and reduce the generation of action potentials and contractions in response to elongation of myometrial muscle fibres.
The Effects of l-methionine on electrical and mechanical activity of myometrium
Intracellular microelectrode recordings from murine myometrial muscles were also made to test the effects of l-methionine on membrane potential. Figure 4A shows typical basal electrical activity of myometrial muscles with regular clustered action potentials. Addition of l-methionine (1 mm) in the presence of TTX depolarized muscles from −61 ± 3 mV to −53 ± 2 mV (n= 4; P < 0.01, Fig. 4A) and increased the frequency of action potentials. These data suggest that L-methionine-sensitive K+ channels (i.e. SDK/TREK-1) contribute to the resting membrane potentials and excitability of myometrium. Therefore we investigated the effects of l-methionine on myometrial contractility. The ‘contractility’ was determined from averaged area under the contraction curve (AUC) for a 10 min recording period (see Methods). l-Methionine (1 mm) increased the AUC from 43.5 ± 5.1 to 72.6 ± 7.2 mN min (Fig. 4B upper and b; n= 4; P < 0.01). We also tested the effects of l-methionine on myometrial contractility in the presence of TEA. TEA (1 mm) also increased the AUC (to 72.7 ± 8.4 mN min, Fig. 4C upper and b), and addition of l-methionine (1 mm) in the continued presence of TEA further increased AUC to 160.1 ± 12.5 mN min (n= 4; P < 0.001 compare to TEA presence, Fig. 4C upper and c). We tested the effects of methioninol (1 mm) on myometrial contractility from ovariectomized (OVX) mice and from mice at day 18 of pregnancy (p-18). Methioninol had no affect on tone or AUC (n= 5, Fig. 5A) on myometrial muscles of OVX mice. However, methioninol increased basal tone to 7.2 ± 2.9 mN (n= 4, Fig. 5B) in myometrial muscles of P-18 mice. These data suggest that TREK-1 channels play a very important role in preventing contractions at late stages of pregnancy.
Figure 4. The effects ofl-methionine on membrane potential and contractility of intact uterine muscle strips.
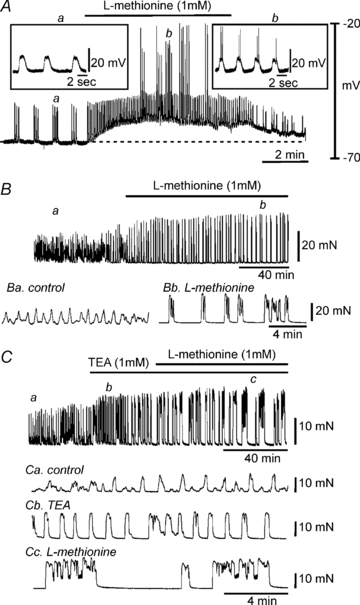
A, l-methionine (1 mm) depolarized uterine smooth muscle and induced continuous firing of action potentials. Regions a and b are shown in the insets on an expanded time scale. Dashed line denotes level of resting membrane potential under control conditions. B and C, uterine muscles displayed spontaneous contractile activity. B, addition of l-methionine (1 mm) induced large phasic contractions with increased amplitude. Regions a and b are shown below on an expanded time scale. C, tetraethylammonium (TEA) increased contractility in strips of murine uterus (upper panel and b). The application of l-methionine further increased contractility and duration of phasic contractions in the presence of TEA (upper panel and c). a, b and c are expanded from the upper panel.
Figure 5. The effects of methioninol on contractility of ovariectomized (OVX) and pregnant (p-18) myometrium.
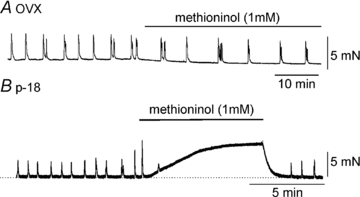
A, methioninol (1 mm) did not affect phasic contractions. B, in contrast, methioninol increased basal tone in pregnant myometrium. Dashed line denotes level of basal tone.
Dynamics of TREK-1 expression in murine myometrium
We used ovariectomized mice to test whether extreme changes in hormonal levels affect TREK-1 expression. Immunoblotting revealed expression of TREK-1 proteins in myometrium from both sham-operated and OVX mice (upper panel in Fig. 6A). TREK-1 antiserum detected a 45 kDa protein whole cell lysates in the reducing agent β-mercaptoethanol. This 45 kDa monomer corresponds to the predicted molecular weight of TREK-1. Pre-incubation of TREK-1 blocking peptide caused competitive loss of immunoreactivity of TREK-1 (middle panel in Fig. 6A). Expression of MHC was used as a control. Expression of TREK-1 in sham-operated and OVX muscles was normalized to MHC. TREK-1 was significantly decreased in OVX muscles (0.12 ± 0.02) in comparison to sham-operated controls (0.28 ± 0.02) (P < 0.001, n= 6 animals for each group, Fig. 6B). These data suggest that TREK-1 channels in the myometrium may be regulated by hormone levels.
Figure 6. Western blots of TREK-1 protein in murine non-pregnant myometrium (Sham) and ovariectomized (OVX) myometrium.
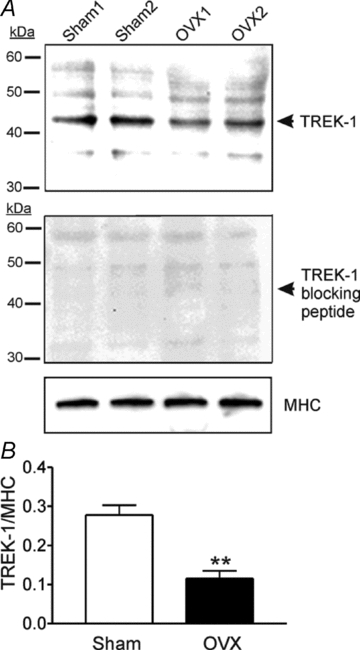
A, representative immunoblot showing protein expression of TREK-1 from the homogenates of uterus smooth muscle preparations in sham-operated and ovariectomized (OVX) mice. Upper, each lane contains 50 μg of protein. Middle, immunoblot of TREK-1 from the homogenates of uterus smooth muscle from sham-operated and OVX mice in the presence of TREK-1 blocking peptide. Lower, an immunoblot of smooth muscle myosin heavy chain (MHC). B, densitometry revealed TREK-1 expression in sham-operated and OVX myometrium. Expression ratio of TREK-1 protein was normalized to MHC expression. *P < 0.001 from 6 tissues.
Transcriptional changes in TREK-1 expression during gestation and labour
We compared the expression of TREK-1 (Kcnk2) transcript in non-pregnant, pregnant and post-partum myometrium. TREK-1 was expressed throughout pregnancy in myometrium (day 14 and day 18, Fig. 7A). The level of TREK-1 expression in 18 days of pregnancy (p-18) was significantly greater than in non-pregnant myometrium (0.20 ± 0.07 vs. 0.69 ± 0.08, respectively; P < 0.01; n= 6 animals for each group, Fig. 7B). These values were normalized to cytoglobin. We also investigated the expression of TREK-1 transcripts in murine non-pregnant (np) and postpartum (pp: within 24 h after delivery) myometrium. TREK-1 transcripts were not resolvable at the qualitative level in postpartum myometrium (Fig. 7C), but qPCR revealed decreased levels of TREK-1 transcripts in postpartum myometrium compared with non-pregnant myometrium (0.050 ± 0.02 vs. 0.28 ± 0.07, respectively. P < 0.05; n= 6 animals for each group, Fig. 7D). We also compared expression of TREK-2 (Kcnk10) in non-pregnant and pregnant (p-18) myometrium. Unlike TREK-1 expression during pregnancy (Fig. 7A, B, E and F), TREK-2 transcripts were unchanged at P-18 (Fig. 7E and G, n= 5 animals for each group). In fact TREK-2 expression is low in non-pregnant myometrium and is not dynamically regulated during pregnancy. In contrast, TREK-1 expression is dynamic during pregnancy. Activation of TREK-1 channels may help to stabilize excitability during through most of pregnancy, and decreased expression of these channels near term may facilitate the onset of labour to terminate pregnancy.
Figure 7. Transcriptional changes in TREK-1 (Kcnk2) expression in non-pregnant, pregnant and postpartum myometrium.
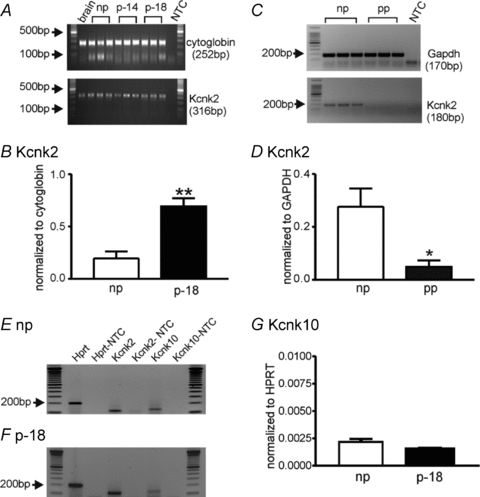
A, representative gels displaying amplification products from myometrium-derived RNA using gene specific primers for TREK-1 in non-pregnant (np), pregnant day 14 (p-14) and pregnant day 18 (p-18) myometrium. Three independent preparations are presented for each stage of gestation. Cytoglobin primers were used to confirm that the products generated were representative of RNA. Brain is included as positive control. B, real-time quantitative PCR revealed significantly increased levels of TREK-1 transcripts in pregnant day 18 (p-18) myometrium. **P < 0.01 from 6 tissues. C, representative gels displaying TREK-1 expression in murine non-pregnant (np) and postpartum (pp; within 24 h after delivery) myometrium. GAPDH primers were used to confirm that the products generated were representative of RNA. D, qPCR revealed decreased levels of TREK-1 transcripts in postpartum (pp) myometrium compared with non-pregnant (np) myometrium. *P < 0.05 from 6 animals. E and F, representative gels displaying TREK-1 (Kcnk2) and TREK-2 (Kcnk10) expression in non-pregnant (np, E) and pregnant (p-18, F) myometrium. G, qPCR showed that TREK-2 expression was not significantly different between non-pregnant (np) and pregnant (p-18) myometrium. Hypoxanthine guanine phosphoribosyl transferase (Hprt) was used as a house-keeping gene. NTC denotes non-template control.
Discussion
This study shows expression of K2P channels in murine uterus, confirming previous studies showing expression of these channels in human myometrium (Bai et al. 2005; Tichenor et al. 2005; Buxton et al. 2010). These channels regulate excitability responses to stretch in myometrial muscles. Stretch of uterine muscles resulted in net hyperpolarization, and pharmacological blockade of TREK-1 channels increased excitability. An exciting observation was that the expression of TREK-1 channels is not constant throughout pregnancy. The expression of TREK-1 increased during most of the period of pregnancy, and expression fell precipitously near term. Thus, TREK-1 channels may help stabilize membrane potential and excitability during pregnancy, and loss of these channels may increase excitability at term. Data from OVX mice suggest that hormones regulate expression of TREK-1 channels, as expression fell to low levels in OVX muscles. Hormonal regulation, possibly via oestrogen, may be responsible for increased TREK-1 expression during pregnancy and the drop in expression near term because these changes mirror the changes in oestrogen levels in pregnancy.
Non-pregnant uterus contracts in response to distension (Bani et al. 1999) so mechanisms must exist to stabilize excitability of the pregnant uterus to counteract the stretch produced by growth of the fetus. The excitability mechanisms of the uterus are under dynamic hormonal regulation, and there is extensive literature investigating changes in ion channels in pregnancy (Sanborn, 2000; Wray & Noble, 2008). In most smooth muscles, influx of Ca2+ via L-type Ca2+ channels is required for contraction, and the open probability of Ca2+ channels is mainly regulated by membrane potential. While modifications in L-type Ca2+ channel isoform occur during pregnancy which might affect Ca2+ entry (Helgura et al. 2002), the dominant regulation of excitability of the myometrium appears to occur through changes in K+ channel expression. It should be noted that the changes in K+ channel expression would occur in reverse of the frequently discussed concept of contraction-associated proteins (CAPs) that have been described as a cassette of proteins that are expressed by myometrial cells to initiate the increase in contractility at the onset of labour (Lefebvre et al. 1995). Expression of K+ channels would be expected to increase to facilitate uterine quiescence, but K+ channels would likely need to drop at term to facilitate labour.
Many types of K+ channels are expressed by MSMCs (Toro et al. 1990), and current densities due to several types of channels change during pregnancy and/or at term (Toro et al. 1990). Thus, regulation of myometrial excitability is likely to be a highly integrated response. Considerable investigation has focused on BK channels because of the abundant expression of these channels by MSMCs. Even subtle changes in expression of BK channels could profoundly affect excitability due to their large unitary conductance (Anwer et al. 1993; Perez et al. 1993). Several reports have probed gestational regulation of BK channels, and changes in Ca2+ sensitivity and responsiveness to protein kinases have been reported (Perez et al. 1993; Pérez & Toro, 1994; Zhou et al. 2000). An interesting form of regulation occurs via BK splice variant switching. Expression of BK channels with the ‘STREX’ exons adds a phosphorylation sequence to the α-subunit that switches responses to PKA-dependent phosphorylation from activation to inhibition (Tian et al. 2001). Isoform expression with STREX exons decreases during pregnancy and expression is also suppressed by oestrogen (Zhu et al. 2005). This isoform switching of BK channels might contribute to the conversion of inhibition of BK channel open probability in response to PKA and PKG in non-pregnant myometrium to activation of BK channels in pregnant myometrium (Pérez & Toro, 1994). While this mechanism could contribute to the maintenance of uterine quiescence during pregnancy, sustained down-regulation of STREX-containing BK channels, even several hours post partum, suggests that BK channels are not involved in the preparative stages of labour and actually may oppose the strengthening of contractions at term. It should also be noted that selective BK channel blocking drugs were ineffective in regulating contractility of either pregnant or non-pregnant myometrial muscles (Aaronson et al. 2006) bringing into question the importance of this conductance in regulating uterine excitability.
ATP sensitive K+ channels (KATP) are also expressed in MSMCs, and the effects of KATP agonists have been reported to increase during pregnancy and then to decline to non-pregnant levels at term (Modzelewska et al. 1998; Okawa et al. 1999; Sawada et al. 2005). KATP channels are composed of Kir6.1 and SUR2B subunits in the rat, and expression of these subunits followed the gestational changes in responses to KATP agonists. Thus, increasing KATP expression could also contribute to reduced uterine excitability during pregnancy and decreased expression near term could contribute to enhanced contractility near birth. Expression of another inward rectifier, ROMK, has also been reported to change during gestation (Lundgren et al. 1997), but nothing is known about the significance of this conductance in regulating myometrial excitability. Finally, several types of voltage-dependent K+ (Kv) channels have also been observed in MSMCs (Knock et al, 2001) and there are reports of gestational regulation of expression of Kv channels, particularly Kv4 family channels. Non-pregnant rat myometrial muscles respond vigorously to 4-AP and phrixotoxin-2, but this response disappears in pregnant muscles (Aaronson et al. 2006; Smith et al. 2007). This effect correlates with the loss of expression of Kv4.3 channel protein, which, due to voltage-dependent properties of these channels, might contribute to resting membrane potentials of MSMC. Oestrogen treatment also greatly reduces the expression of Kv4.3 channels, mimicking the down-regulation of Kv4 during pregnancy (Song et al. 2001). Loss of a prominent K+ channel, particularly one that contributes to resting potentials of MSMCs, however, seems contrary to the need to reduce excitability during pregnancy.
Smooth muscle cells express a variety of mechanosensitive ion channels that, when activated, provide net inward current under physiological conditions. These include swelling-activated Cl− channels (Dick et al. 1999), stretch-activated, non-selective cation channels (Wellner & Isenberg, 1993, 1994), Ca2+-activated Cl− channels (Ji et al. 2002) and Ca2+ channels (Farrugia et al. 1999). Activation of mechanosensitive inward currents leads to depolarization, Ca2+ entry and muscle contraction, and this cellular reflex has been referred to for many years as the ‘myogenic effect’. Many visceral smooth muscle organs have the need to avoid contractile responses as they fill because they serve a storage function during normal physiological processes. Although neural reflexes can produce ‘receptive relaxation’ in some organs (e.g. proximal stomach), visceral smooth muscle cells themselves employ mechanisms to reduce contractions in response to stretch. For example, colonic and bladder myocytes express K2P channel genes, most importantly for Kcnk2, which encodes TREK-1 channels that display stretch-dependent activation. These channels stabilize membrane potential during elongation of smooth muscle cells and tissues (Koh & Sanders, 2001; Park et al. 2005; Baker et al. 2008). Data from the present study shows that uterine muscles utilize K2P channels to limit excitability in response to stretch. TREK-1 is the dominant member of stretch-dependent K2P in MSMCs. In this study we demonstrated an increase in TREK-1 expression in pregnant myometrium and this correlated with increased basal tone in response to TREK-1 channel blockade. In contrast, TREK-1 expression was very low in myometrium of OVX mice, and TREK-1 channel blockers had no effect on tone. TREK-2 expression did not change in pregnancy. TREK-1 expression is dynamic during pregnancy, and these channels provide a conductance that increases during pregnancy to dampen excitability. This conductance falls dramatically at term to facilitate uterine excitability during labour. Regulation of TREK-1 expression appears to be controlled by female hormones, and failure to regulate this conductance could be a factor in pre-mature labour.
The present study suggests that Kcnk2 encodes SDK channels in MSMCs: (i) SDK channel openings were stimulated by negative pressure applied to patches; (ii) SDK channels were K+ channels with the same conductance as TREK-1 channels expressed in HEK293 cells (Koh et al. 2001); (iii) TREK channels and the SDK channels in MSMCs were relatively insensitive to a broad-spectrum of K+ channel blockers, including TEA and 4-AP (Koh & Sanders, 2001); (iv) the SDK conductance in MSMCs was unaffected by intracellular Ca2+, confirming currents were not due to large-conductance Ca2+ activated K+ channels, and insensitivity to intracellular Ca2+ is also a property of TREK channels (Patel et al. 1998; Koh & Sanders, 2001); (v) channels of the same conductance as those activated by stretch in MSMCs were activated by arachidonic acid, a known activator of TREK-1 channels (Patel et al. 1998; Franks & Honore, 2004); (vi) stretch and arachidonic acid-activated currents were inhibited by l-methionine and/or methioninol, reagents that block TREK-1 channels, but have no effect on other K+ conductances common to smooth muscle cells (e.g. delayed rectifiers, large and small conductance Ca2+-activated K+ channels, and KATP: see Park et al. 2005); (vii) TREK-1 was more highly expressed in MSCMs than TREK-2, and TRAAK was not expressed; (viii) expression of TREK-1 was regulated during pregnancy.
In conclusion our data support the concept that uterine excitability and contractility during pregnancy may be regulated by dynamic expression of SDK/TREK-1 channels. It is possible that increased expression of these channels stabilizes membrane potential and limits contraction during pregnancy and down-regulation of these channels contributes to facilitation of labour. More extensive studies will be required to determine which hormonal pathway is involved in regulation of TREK-1 expression.
Acknowledgments
This work has been supported by R21HD060077 from NIH/NICHD. Molecular (CORE B) and Protein expression (CORE C) Laboratoratories from the COBRE (NIH/NCRR, 5P20-RR018751) were used to produce PCR, qPCR and immunohistohemistry in this study. There is no conflict of interest to disclose.
Glossary
Abbreviations
- MSMC
myometrial smooth muscle cell
- OVX
ovariectomized
- PP
postpartum
- SDK
stretch-dependent K+
Author contributions
S.A.B., L.D. and K.S.P. carried out the intracellular and contractility recordings. K.M. performed the molecular experiments. W.C.H. obtained the immunohistochemistical data. S.D.K. performed the electrophysiological recordings. S.D.K. and K.M.S. were involved in conception, design, interpretation of data, and drafting of the manuscript. All authors approved the final manuscript.
References
- Aaronson PI, Sarwar U, Gin S, Rockenbauch U, Connolly M, Tillet A, Watson S, Liu B, Tribe RM. A role for voltage-gated, but not Ca2+-activated, K+ channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br J Pharmacol. 2006;147:815–824. doi: 10.1038/sj.bjp.0706644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwer K, Oberti C, Perez GJ, Perez-Reyes N, McDougall JK, Monga M, Sanborn BM, Stefani E, Toro L. Calcium-activated K+ channels as modulators of human myometrial contractile activity. Am J Physiol Cell Physiol. 1993;265:C976–C985. doi: 10.1152/ajpcell.1993.265.4.C976. [DOI] [PubMed] [Google Scholar]
- Bai X, Bugg GJ, Greenwood SL, Glazier JD, Sibley CP, Baker PN, Taggart MJ, Fyfe GK. Expression of TASK and TREK, two-pore domain K+ channels, in human myometrium. Reproduction. 2005;129:525–530. doi: 10.1530/rep.1.00442. [DOI] [PubMed] [Google Scholar]
- Baker SA, Hennigh GW, Britton FC, Smith TK, Koh SD. Methionine and its derivatives increase bladder excitability by inhibiting stretch-dependent K+ channels. Br J Pharmacol. 2008;153:1259–1271. doi: 10.1038/sj.bjp.0707690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bani D, Baccari MC, Nistri S, Calamai F, Bigazzi M, Sacchi TB. Relaxin up-regulates the nitric oxide biosynthetic pathway in the mouse uterus: involvement in the inhibition of myometrial contractility. Endocrin. 1999;140:4434–4441. doi: 10.1210/endo.140.10.7055. [DOI] [PubMed] [Google Scholar]
- Brown A, Cornwell T, Korniyenko I, Solodushko V, Bond CT, Adelman JP, Taylor MS. Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contraction. Am J Physiol Cell Physiol. 2007;292:C832–C840. doi: 10.1152/ajpcell.00268.2006. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Singer CA, Tichenor JN. Expression of stretch-activated two-pore potassium channels in human myometrium in pregnancy and labor. PLoS One. 2010;5:e12372. doi: 10.1371/journal.pone.0012372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Dick GM, Kong ID, Sanders KM. Effects of anion channel antagonists in canine colonic myocytes: comparative pharmacology of Cl−, Ca2+ and K+ currents. Br J Pharmacol. 1999;127:1819–1831. doi: 10.1038/sj.bjp.0702730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G, Holm AN, Rich A, Sarr MG, Szurszewski JH, Rae JL. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterol. 1999;117:900–905. doi: 10.1016/s0016-5085(99)70349-5. [DOI] [PubMed] [Google Scholar]
- Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25:601–608. doi: 10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Helguera G, Olcese R, Song M, Toro L, Stefani E. Tissue-specific regulation of Ca2+ channel protein expression by sex hormones. Biochim Biophys Acta. 2002;1569:59–66. doi: 10.1016/s0304-4165(01)00234-3. [DOI] [PubMed] [Google Scholar]
- Ji G, Barsotti RJ, Feldman ME, Kotlikoff MI. Stretch-induced calcium release in smooth muscle. J Gen Physiol. 2002;119:533–544. doi: 10.1085/jgp.20028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knock GA, Tribe RM, Hassoni AA, Aaronson PI. Modulation of potassium current characteristics in human myometrial smooth muscle by 17β-estradiol and progesterone. Biol Reprod. 2001;64:1526–1534. doi: 10.1095/biolreprod64.5.1526. [DOI] [PubMed] [Google Scholar]
- Koh SD, Monaghan K, Sergeant GP, Ro S, Walker RL, Sanders KM, Horowitz B. TREK-1 regulation by nitric oxide and CGMP dependent protein kinase: An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem. 2001;276:44338–44346. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol. 2001;533:155–163. doi: 10.1111/j.1469-7793.2001.0155b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre DL, Piersanti M, Bai XH, Chen ZQ, Lye SJ. Myometrial transcriptional regulation of the gap junction gene, connexin-43. Reprod Fertil Dev. 1995;7:603–611. doi: 10.1071/rd9950603. [DOI] [PubMed] [Google Scholar]
- Lundgren DW, Moore JJ, Chang SM, Collins PL, Chang AS. Gestational changes in the uterine expression of an inwardly rectifying K+ channel, ROMK. Proc Soc Exp Biol Med. 1997;216:57–64. doi: 10.3181/00379727-216-44156. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Excitation–contraction coupling in voltageclamped uterine muscle. J Physiol. 1973;233:127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewska B, Sipowicz MA, Saavedra JE, Keefer LK, Kostrzewska A. Involvement of K+ATP channels in nitric oxide-induced inhibition of spontaneous contractile activity of the nonpregnant human myometrium. Biochem Biophys Res Commun. 1998;253:653–657. doi: 10.1006/bbrc.1998.9844. [DOI] [PubMed] [Google Scholar]
- Ohya Y, Sperelakis N. Fast Na+ and slow Ca2+ channels in single uterine muscle cells from pregnant rats. Am J Physiol Cell Physiol. 1989;257:C408–412. doi: 10.1152/ajpcell.1989.257.2.C408. [DOI] [PubMed] [Google Scholar]
- Okawa T, Vedernikov YP, Saade GR, Longo M, Olson GL, Chwalisz K, Garfield RE. Roles of potassium channels and nitric oxide in modulation of uterine contractions in rat pregnancy. Am J Obstet Gynecol. 1999;181:649–655. doi: 10.1016/s0002-9378(99)70508-9. [DOI] [PubMed] [Google Scholar]
- Ordway RW, Petrou S, Kirber MT, Walsh JV, Jr, Singer JJ. Stretch activation of a toad smooth muscle K+ channel may be mediated by fatty acids. J Physiol. 1995;484:331–337. doi: 10.1113/jphysiol.1995.sp020668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005;144:1126–1137. doi: 10.1038/sj.bjp.0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkington HC, Coleman HA. The role of membrane potential in the control of uterine motility. In: Carsten ME, Miller JD, editors. Uterine Function: Molecular and Cellular Aspects. New York: Plenum; 1990. pp. 195–248. [Google Scholar]
- Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez GJ, Toro L. Differential modulation of large-conductance KCa channels by PKA in pregnant and nonpregnant myometrium. Am J Physiol Cell Physiol. 1994;266:C1459–C1463. doi: 10.1152/ajpcell.1994.266.5.C1459. [DOI] [PubMed] [Google Scholar]
- Perez GJ, Toro L, Erulkar SD, Stefani E. Characterization of large-conductance, calcium-activated potassium channels from human myometrium. Am J Obstet Gynecol. 1993;168:652–660. doi: 10.1016/0002-9378(93)90513-i. [DOI] [PubMed] [Google Scholar]
- Sanborn BM. Relationship of ion channel activity to control of myometrial calcium. J Soc Gynecol Investig. 2000;7:4–11. doi: 10.1016/s1071-5576(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Sawada K, Morishige K, Hashimoto K, Tasaka K, Kurachi H, Murata Y, Kurachi Y. Gestational change of K+ channel opener effect is correlated with the expression of uterine KATP channel subunits. Eur J Obstet Gynecol Reprod Biol. 2005;122:49–56. doi: 10.1016/j.ejogrb.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Smith RC, McClure MC, Smith MA, Abel PW, Bradley ME. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol. 2007;5:41–53. doi: 10.1186/1477-7827-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, Toro L, Stefani E. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem. 2001;276:31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- Song M, Zhu N, Olcese R, Barila B, Toro L, Stefani E. Hormonal control of protein expression and mRNA levels of the MaxiK channel alpha subunit in myometrium. FEBS Lett. 1999;460:427–432. doi: 10.1016/s0014-5793(99)01394-0. [DOI] [PubMed] [Google Scholar]
- Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- Tichenor JN, Hansen ET, Buxton IL. Expression of stretch-activated potassium channels in human myometrium. Proc West Pharmacol Soc. 2005;48:44–48. [PubMed] [Google Scholar]
- Toro L, Stefani E, Erulkar S. Hormonal regulation of potassium currents in single myometrial cells. Proc Natl Acad Sci U S A. 1990;87:2892–2895. doi: 10.1073/pnas.87.8.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Yoshino M, Sui JL, Wakui M, Kao PN, Kao CY. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. J Gen Physiol. 1998;112:737–756. doi: 10.1085/jgp.112.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner MC, Isenberg G. Properties of stretch-activated channels in myocytes from the guinea-pig urinary bladder. J Physiol. 1993;466:213–227. [PMC free article] [PubMed] [Google Scholar]
- Wellner MC, Isenberg G. Stretch effects on whole-cell currents of guinea-pig urinary bladder myocytes. J Physiol. 1994;480:439–448. doi: 10.1113/jphysiol.1994.sp020373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S, Noble K. Sex hormones and excitation-contraction coupling in the uterus: the effects of oestrous and hormones. J Neuroendocrinol. 2008;20:451–61. doi: 10.1111/j.1365-2826.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Effects of estrogens on Ca channels in myometrial cells isolated from pregnant rats. Am J Physiol Cell Physiol. 1995;268:C64–C69. doi: 10.1152/ajpcell.1995.268.1.C64. [DOI] [PubMed] [Google Scholar]
- Zhou XB, Wang GX, Ruth P, Hüneke B, Korth M. BKCa channel activation by membrane-associated cGMP kinase may contribute to uterine quiescence in pregnancy. Am J Physiol Cell Physiol. 2000;279:C1751–C1759. doi: 10.1152/ajpcell.2000.279.6.C1751. [DOI] [PubMed] [Google Scholar]
- Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett. 2005;579:4856–60. doi: 10.1016/j.febslet.2005.07.069. [DOI] [PubMed] [Google Scholar]


