Epithelial GPR30 is a negative regulator of ERα-dependent uterine growth in response to E2 via inhibition of stromal ERK1/2 and Erα phosphorylation signaling.
Abstract
Although estradiol-17β (E2)-regulated early and late phase uterine responses have been well defined, the molecular mechanisms linking the phases remain poorly understood. We have previously shown that E2-regulated early signals mediate cross talk with estrogen receptor (ER)-α to elicit uterine late growth responses. G protein-coupled receptor (GPR30) has been implicated in early nongenomic signaling mediated by E2, although its role in E2-dependent uterine biology is unclear. Using selective activation of GPR30 by G-1, we show here a new function of GPR30 in regulating early signaling events, including the inhibition of ERK1/2 and ERα (Ser118) phosphorylation signals and perturbation of growth regulation under the direction of E2 in the mouse uterus. We observed that GPR30 primarily localizes in the uterine epithelial cells, and its activation alters gene expression and mediates inhibition of ERK1/2 and ERα (Ser118) phosphorylation signals in the stromal compartment, suggesting a paracrine signaling is involved. Importantly, viral-driven manipulation of GPR30 or pharmacological inhibition of ERK1/2 activation effectively alters E2-dependent uterine growth responses. Overall, GPR30 is a negative regulator of ERα-dependent uterine growth in response to E2. Our work has uncovered a novel GPR30-regulated inhibitory event, which may be physiologically relevant in both normal and pathological situations to negatively balance ERα-dependent uterine growth regulatory functions induced by E2.
Estradiol-17β (E2) normally exerts cellular growth and differentiation as well as a variety of other functions in different target tissues (1–3), which are primarily mediated via estrogen receptors (ERs). The receptors, ERα and ERβ, are members of the superfamily of nuclear receptors that normally function as ligand-inducible transcription factors (4, 5). The uterus is a major target of E2 for various functions during the reproductive cycle and pregnancy (6). In mice, uterine effects of E2 are considered to be biphasic in nature. The early (phase I) responses that occur within 6 h are characterized by increased water imbibition, macromolecular uptake, and alteration of vascular permeability, whereas the late (phase II) responses that occur between 18 and 30 h normally cause increased DNA synthesis and proliferation of epithelial cells (1, 6). The molecular relationship between these two phases is ill defined, although recent studies suggest that they are coordinately controlled via ER-dependent and ER-independent mechanisms (7–10). There is a large body of evidence based on physiological, pharmacological, and genetic studies that demonstrates that E2 can elicit a variety of early signaling systems that do not require classical signaling via ERs in the uterus (7, 9–14). Our long-standing hypothesis is that estrogen-dependent early nonclassical responders participate in a concerted manner to ultimately control the ERα-mediated functions conducive for the induction of late uterine growth responses (11, 12, 15).
In general, E2-regulated early effects involve rapid (<30 min) activation of intracellular signaling pathways. Interestingly, these responses do not require RNA or protein synthesis but instead are attributed to estrogen signaling through the plasma membrane. There are reports that estrogens, without displaying any ER-binding capacity, exhibit mitogenic action in the uterus, presumably by increasing cAMP levels and protein kinase A activity (16, 17). Protein kinase C can also modulate uterine ER levels, and protein kinase C inhibitors can reduce E2-induced mitogenic actions (18). This implies that membrane-bound receptors acting via protein kinases can increase the expression of the same genes activated by nuclear estrogen receptors. This provides a basis for the concept that other nonnuclear receptors interact with E2 or its mimics. Although the presence of a membrane ER has been postulated for more than 3 decades (19), and a recent wide array of studies in many different cell types primarily demonstrate the nature and some function of membrane ER (20–23), the subject remains poorly understood in respect of the physiological context. The molecular identity of the membrane ER has been proposed to be a full-length classical ER translocated to specialized structures in the plasma membrane (20). In addition, the existence of other estrogen binding membrane proteins, including maxi-K channel and G protein-coupled receptor 30 (GPR30), has been reported (21–23). However, the physiological significance of these membrane receptors in uterine biology remains unclear.
GPR30 has been suggested to be a nonclassical estrogen binding receptor that mediates various rapid intracellular signaling pathways, including signaling via activation of ERK1/2 MAPKs, phosphatidylinositol 3-kinase, Akt, or increases of cAMP levels or calcium mobilization (22–25). Based on cell culture studies, it has been widely documented that activation of GPR30 by E2 leads to the alteration of estrogen-regulated gene actions and enhancement of cellular proliferation (26–29). In contrast, studies from GPR30 knockout mice appear to imply that GPR30's role in uterine biology is minimal for estrogenic growth regulation (30–33). Additionally, G-1, a selective agonist for GPR30, causes activation of the early intracellular signaling mainly in cell-based studies (34). However, in mouse uterine studies, it was unclear whether G-1's proliferation action mimics that of E2 (35, 36). It is clear that there is a need to reevaluate the role of GPR30 in uterine physiological responses.
Our long-term goal was to define a molecular relationship between the phase I and phase II estrogenic responses in the uterus. In this study, our objective was to test whether GPR30 has any effects on uterine estrogen signaling. GPR30's activation was undertaken via G-1, either individually or in combination with E2, to evaluate GPR30's roles in two-phase responses. Based on dose-dependent studies in mice, we show that G-1 is capable of inducing epithelial activation of GPR30 to enhance negative regulatory actions in the stroma, including inhibition of ERK1/2 and ERα (Ser118) phosphorylation signals, which are essential for the onset of E2-regulated uterine epithelial cell proliferation. Viral-driven overexpression of GPR30 and pharmacological inhibition of ERK1/2 activation strongly confirms these findings.
Materials and Methods
Animals, injections, and tissue processing
Adult CD-1 (Charles River Laboratories, Wilmington, MA) female mice were housed in our institutional animal care facility according to National Institutes of Health and institutional guidelines for laboratory animals. The animal experimental procedures were approved by the IACUC. Animal injections and tissue processing are described in detail in the Supplemental Methods, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
3H-thymidine incorporation
Frozen tissue sections were directly subjected to autoradiography for the analysis of 3H-thymidine incorporation, as previously described (37).
Antibodies and other reagents
Details of this section are given in the Supplemental Methods.
Immunohistochemistry
This protocol was followed as previously described (15, 38). Details are given in the Supplemental Methods.
Western blotting
This procedure was conducted as previously described (38).
Probes and Northern blot hybridization
Detail description about probes is given in the Supplemental Methods. The procedures for Northern blot hybridization and stripping of probes were previously described (7).
Recombinant adenoviral plasmids and preparation of viral particles
The replication-defective adenoviral virus particles were generated as we previously described (39). Details of the vector constructs are given in the Supplemental Methods.
In vivo delivery of adenovirus in the mouse uterus
This was performed as previously described (11, 12, 15, 40). Virus injection procedures are given in the Supplemental Methods.
Results
A GPR30 selective agonist, G-1, negatively influences E2-dependent uterine late growth responses in adult ovariectomized mice
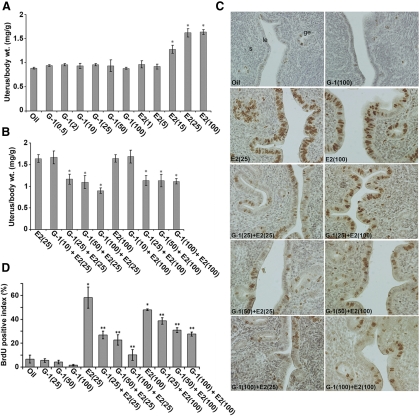
To evaluate the role of GPR30 in uterine estrogen signaling, we first analyzed the effects after the selective activation of GPR30 by its agonist G-1 in the uteri of ovariectomized mice. An injection of G-1 at doses ranging from 0.5 to 100 μg/mouse, given either for 6-h (Supplemental Fig. 1) or 24-h (Fig. 1A) treatments, did not show any significant alteration of uterine wet weight responses at either time point when compared with the control (vehicle treated). In contrast, an injection of E2 at doses of 15–100 ng/mouse or greater caused a significant increase in uterine weights after both 6 h (Supplemental Fig. 1) and 24 h (Fig. 1A). These results suggest that G-1 does not possess any estrogen-like activity in respect to the early and late uterine effects, which is consistent with the reported literature (35). To determine whether the addition of G-1 before the E2 injection alters E2-dependent uterine effects, we injected G-1 at doses of 10–100 μg/mouse 30 min before E2 injection (at two responsive doses, 25 or 100 ng/mouse) and analyzed the uterine wet weights at 6 and 24 h after the last injection. We found that an injection of G-1, at doses of 25–100 μg/mouse or greater, significantly inhibited E2-dependent late uterine wet weight response (Fig. 1B), whereas the early response did not show any alteration by such treatments (Supplemental Fig. 1). Furthermore, the addition of G-1 at doses ranging from 0.5 to 100 μg/mouse, together with suboptimal doses of E2 at 1 or 5 ng/mouse, did not reveal any increase in uterine weights for the early and late effects (data not shown). These results also imply that G-1 does not possess any augmenting effects with E2.
Fig. 1.
G-1 by itself is not stimulatory but can antagonize E2-induced uterine growth responses. Dosage-dependent studies were conducted in adult ovariectomized mice after sc injections of oil (vehicle control), G-1 (0.5, 2, 10, 25, 50, or 100 μg/mouse), E2 (1, 5, 15, 25, or 100 ng/mouse) (A), or G-1 (10, 25, 50, or 100 μg/mouse) 30 min before an injection of E2 (25 ng/mouse) or E2 (100 ng/mouse) (B). Mice were killed 24 h after the last injection. Uterine wet weights from five to 10 mice for each group were analyzed. The error bars represent ses. *, Values are statistically different compared with the vehicle control (P < 0.01, ANOVA followed by Newman-Keul's multiple range test). C, G-1 inhibits E2-induced uterine epithelial cell proliferation. Adult ovariectomized mice were injected with different agents as described above, and BrdU was injected 2 h before the animals were killed. Formaldehyde-fixed paraffin-embedded tissue sections were stained for BrdU incorporation as described in Materials and Methods. Representative tissue sections are shown and reddish brown nuclear deposits indicate the sites of positive immunostaining. Pictures were taken at ×200. le, Luminal epithelium; ge, glandular epithelium; s, stroma cells. D, Quantitation of BrdU-positive cells. The percentage of BrdU labeling index was determined for the luminal and glandular epithelial cells after counting at least 500 cells per animal on consecutive fields. The data presented after the analysis of at least five to 10 mice for each group. The error bars represent ses. *, Values are statistically different (P < 0.01) against the oil (control). **, Values are statistically different (P < 0.05) against E2 (25 ng/mouse or 100 ng/mouse) based on ANOVA followed by Newman-Keul's multiple range test.
We then examined cell proliferation using multiple cell cycle phase-specific markers including 5-bromo-2′-deoxyuridine (BrdU) (S phase), phosphorylated histone H3 (pHH3) (G2-M phases), and Ki67 (G1-S-G2-M phases). Our analyses of proliferation as judged by BrdU incorporation (Fig. 1, C and D) and pHH3 staining (Supplemental Fig. 2) were consistent with our earlier results. However, the expression of Ki67 was inconsistent with the above results (Supplemental Fig. 2), suggesting that Ki67 may not be an appropriate marker for the assessment of proliferation under the above conditions. Overall, these results indicate that G-1 by itself is not estrogenic, but it can inhibit E2-dependent uterine growth responses in mice.
E2 negatively influences uterine GPR30 levels, whereas G-1 is ineffective both by itself and in combination with E2
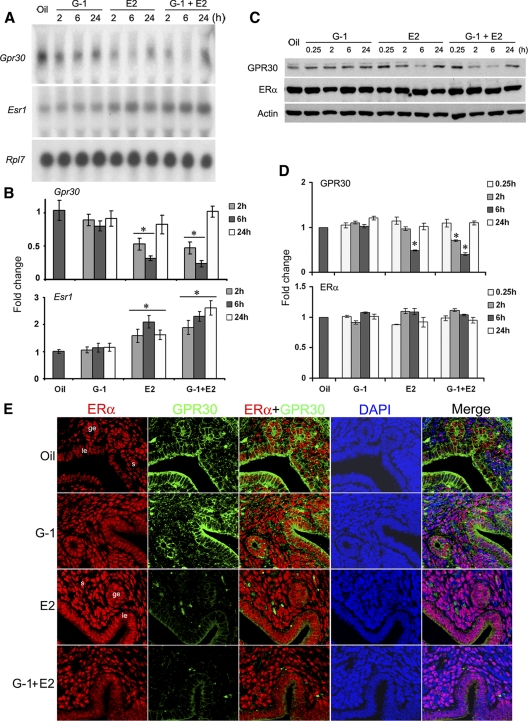
The inhibition of E2-induced uterine epithelial cell proliferation by G-1 encouraged us to analyze the expression of GPR30 and ERα receptors in the uterus under the conditions described above. Ovariectomized mice received an injection of G-1 (100 μg/mouse), E2 (25 ng/mouse), or G-1 plus E2 at the same doses as described above and were killed at the indicated times to analyze the expression by Northern blot hybridization and Western blotting experiments (Fig. 2, A–D). G-1 by itself was unable to alter either ERα or GPR30 mRNAs or protein levels, compared with the control. In contrast, both GPR30 mRNA and protein levels were significantly reduced during the early phase by E2 or G-1 plus E2 treatments (Fig. 2, A–D). In the case of ERα, the injections of E2 or E2 plus G-1 resulted in significant increases of mRNAs, without influencing the protein levels (Fig. 2, A–D), suggesting mRNA and protein turnovers may be differently controlled. These studies revealed that uterine GPR30 remains repressed by E2 during the early phase, whereas G-1 appeared to have no effect.
Fig. 2.
Uterine expression of GPR30 is suppressed by E2 treatment. Adult ovariectomized mice were given a single injection of G-1 (100 μg/mouse), E2 (25 ng/mouse), or the same doses of G-1 plus E2 and killed at the indicated times. Mice injected with oil and killed after 6 h served as vehicle controls. Temporal effects of uterine mRNA (A) and protein (C) levels for the expression of Esr1 and Gpr30 were analyzed by Northern and Western blotting techniques, respectively. These experiments were repeated four times with independent samples, and a representative blot is presented. As previously reported by us (9, 10), the quantitation of mRNA transcripts was achieved by direct analysis of radioactivity bound to the hybridized bands using a radioimage analysis system (Ambis Systems, San Diego, CA). The quantitation of protein levels from the Western blots was determined by densitometric scanning. Fold changes in mRNA (B) or protein (D) levels were calculated against oil and normalized by Rpl7 or actin levels, respectively. The error bars represent ses. *, Values are statistically different (P < 0.05, ANOVA followed by Newman-Keul's multiple range test) against the oil-treated group. E, GPR30 is colocalized with ERα in uterine epithelial cells. Adult ovariectomized mice were treated as above and killed after 6 h. Formalin-fixed, paraffin-embedded uterine sections were incubated with primary antibodies for ERα and GPR30 followed by incubation of secondary antibodies tagged with Texas-red (red) and Cy2 (green), respectively. Nuclei were stained by 4′,6′-diamidino-2-phenylindole (DAPI) (blue). Merged images are shown with overlapping colors. No immunostaining was noted when sections were incubated with preimmune serum instead of primary antibody (data not shown). Pictures were taken at ×200. le, Luminal epithelium; ge, glandular epithelium; s, stroma cells. These experiments were repeated at least three times with similar results.
GPR30 colocalizes with ERα in uterine epithelial cells
We next examined uterine cell-specific expression of these two proteins under the above conditions using confocal microscopy. Our results show that ERα primarily localized in the nuclei of both uterine epithelial and stromal cells and remained unchanged after above treatments by 2 h (data not shown) or 6 h (Fig. 2E). In contrast, distinct signal intensity for GPR30 expression was primarily detected in the plasma membrane of luminal and glandular epithelial cells following the treatments of oil or G-1 for 6 h (Fig. 2E). However, consistent with the Western blotting results, injections of E2 alone or combination of E2 plus G-1 for 6 h resulted in a marked reduction in the signal intensity for GPR30 (Fig. 2E). Similar results were also observed after the above treatments by 2 h (data not shown). Collectively these observations show that GPR30 mainly colocalizes with ERα in uterine epithelial cells but is not influenced by time or treatments.
G-1 mediated activation of GPR30 modulates E2-dependent uterine genes
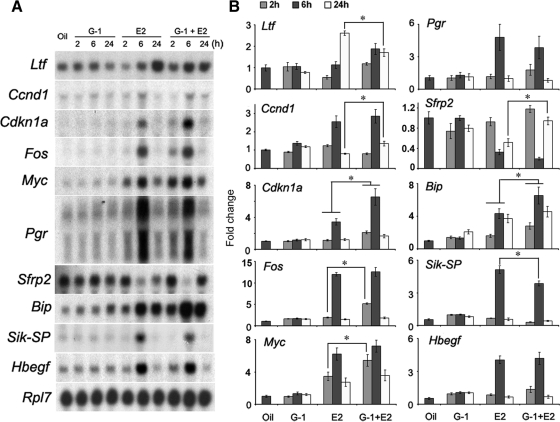
Because G-1 effectively inhibits E2-dependent uterine epithelial cell proliferation, we wanted to examine whether this ligand also alters uterine gene expression under the direction of E2. Ovariectomized mice were given an injection of oil, G-1 (100 μg/mouse), E2 (25 ng/mouse), or G-1 plus E2 at the same doses described in Materials and Methods, and uterine tissues were collected at the indicated times after the last injection. Our analysis of expression included several E2-regulated uterine genes that are known to be regulated via ERα (Ltf, Ccnd1, Cdkn1a, Fos, Myc, and Pgr) (8–10, 12, 41–44), several known not to involve ER (Sfrp2, Bip, and Sik-SP) (7), and some yet-to-be-classified pathways (HB-EGF) (24, 45). The Northern blot hybridization analysis revealed that E2, as opposed to G-1, indeed altered uterine gene expression when compared with the control group (Fig. 3). In contrast, an injection of G-1 before E2 primarily inhibited E2-dependent up-regulation of expression for Ltf and Sik-SP at 24 and 6 h, respectively, whereas that of Fos, Myc, Cdkn1a, Bip, and Ccnd1 was further enhanced primarily at 2, 2, 6, 6, and 24 h, respectively (Fig. 3). In case of Sfrp-2, E2-dependent suppression of expression was opposed by G-1 plus E2 at 24 h (Fig. 3). In addition, in case of Pgr and Hbegf, E2-stimulated expression appeared to be unaffected by G-1 plus E2 (Fig. 3). Taken together, these results imply that G-1 induces alterations of E2-dependent uterine gene expression, although G-1 by itself is not effective.
Fig. 3.
Activation of GPR30 modulates E2-regulated uterine gene expression. Adult ovariectomized mice were treated as in Fig. 2 and killed at the indicated times. Mice injected with oil and killed after 6 h served as vehicle controls. A, Northern blot hybridization for the analysis of temporal effects of the above agents on uterine mRNA expression of Ltf, cdkn1a, Ccnd1, Fos, Myc, Pgr, Sfrp2, Bip, Sik-SP, and Hbegf genes. Rpl7 was used as an internal control gene. These experiments were repeated four times with independent RNA samples, and a representative blot is presented. B, Fold changes in mRNA levels were determined as described in Fig. 2. The error bars represent ses. *, Values are statistically different (P < 0.05, ANOVA followed by Newman-Keul's multiple range test) between the compared groups.
G-1-dependent activation of GPR30 prompts modulation of E2-regulated early intracellular signaling pathways
Next, we wanted to explore whether GPR30 activation via G-1 causes any alteration of E2-induced early nongenomic signaling, such as ERK1/2 and ERα activation. Adult ovariectomized mice were treated as described above, and uterine tissues were analyzed at the indicated times. Mice injected with oil for 6 h served as a control.
ERK1/2 signaling
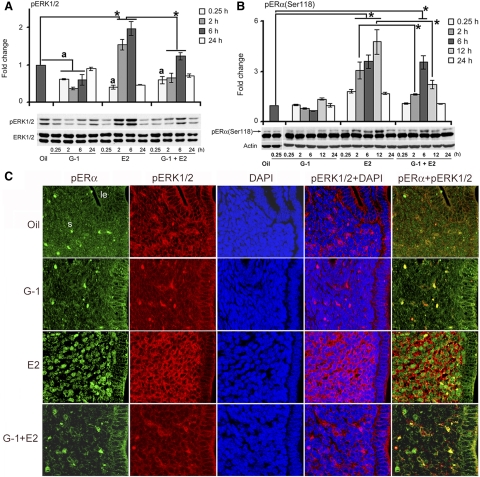
Several studies have demonstrated that E2 rapidly activates ERK1/2 phosphorylation in a number of cell-based model systems (24, 46, 47). Our analysis of ERK1/2 activation signaling in the uterus shows that an injection of G-1 alone was partially inhibitory during the early phase (between 0.25 and 6 h), compared with the control (Fig. 4A). E2 resulted in strong stimulation by 2 h that was sustained through 6 h and then declined by 24 h (Fig. 4A). In contrast, an injection of G-1 before E2 exhibited dramatic suppression of E2-stimulated activation of ERK1/2 during the 2- to 6-h period (Fig. 4A). Furthermore, it is interesting to note that there was an apparent consistent suppression of phosphorylated (p) ERK at 0.25 h after the treatments of E2 or G-1 plus E2, compared with control. In summary, GPR30 activation by G-1, either by itself or in combination with E2, leads to inhibition of uterine ERK activation during the early phase.
Fig. 4.
G-1 mediated activation of GPR30 inhibits E2-induced uterine ERK and ERα phosphorylation in the stromal compartment. Adult ovariectomized mice were treated as in Fig. 2 and killed at the indicated times. Mice injected with oil and killed after 6 h served as vehicle controls. Western blot analysis of whole uterine tissue extracts for pERK1/2 (A) and pERα (Ser118) (B). These experiments were repeated four times with independent samples, and a representative blot is presented. Fold changes in the levels of pERK1/2 and pERα (Ser118) were compared against the control, and the values were determined after normalization with ERK1/2 and actin levels, respectively. The error bars represent ses. *, Values are statistically different (P < 0.05, ANOVA followed by Newman-Keul's multiple range test) between the compared groups. a, Values are significantly different (P < 0.05, ANOVA followed by Newman-Keul's multiple range test) compared with oil treated group. C, Immunofluorescence analyses of pERK1/2 and pERα (Ser118) in uteri of ovariectomized mice treated with agents as described in Fig. 2 and analyzed at 2, 6, and 12 h after the last injection. Formalin-fixed, paraffin-embedded uterine sections were incubated with primary antibodies for pERK1/2 and pERα (Ser118) followed by incubation of secondary antibodies tagged with Cy3 (red) and Cy2 (green), respectively. Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (blue). Merged images are shown with overlapping colors. Representative pictures are shown for the 2-h-treated groups. Similar results were obtained for the 6- and 12-h-treated groups (data not shown). No immunostaining was noted when sections were incubated with preimmune serum instead of primary antibody (data not shown). Pictures were taken at ×200. le, Luminal epithelium; ge, glandular epithelium; s, stroma cells. These experiments were repeated at least three times with similar results.
ERα phosphorylation
Previous studies have shown that ERα (Ser118) phosphorylation can be induced by E2-mediated signaling through the Ras-ERK cascade and that this phosphorylated ERα critically controls the activation function 1 domain-specific transcriptional activity of ERα (48, 49) Our analysis of this ERα phosphorylation reveals that G-1 alone was ineffective in altering activation, although a slight decline in ERα phosphorylation was detected after 6 h, compared with the control (Fig. 4B). The E2 treatment, however, was strongly supportive of induced ERα phosphorylation by 2 h, sustained through 6 h, increased further by 12 h, and then declined by 24 h (Fig. 4B). Interestingly, the addition of G-1 together with E2 caused repression of E2-dependent stimulation of ERα phosphorylation, primarily by 2 and 12 h (Fig. 4B). Overall, these results suggest that GPR30 activation by G-1 induces inhibition of E2-dependent activation of ERα phosphorylation, whereas G-1 by itself is generally not effective.
GPR30 activation by G-1 elicits inhibition of E2-dependent colocalized activation of ERK1/2 and ERα (Ser118) phosphorylation in uterine stromal cells
Because the above studies implied that G-1 mediated activation of GPR30 causes inhibition of E2-induced ERK1/2 and ERα (Ser118) phosphorylation signals in the uterus, we wanted to more closely examine the cell-specific contribution to this inhibition. Using confocal analyses, as shown in Fig. 4C, we found that E2 caused induction of phosphorylated ERα (Ser118) immunolocalization mainly in the uterine stromal cell nuclei, whereas the injections of oil or G-1 alone did not reveal such stimulation. Interestingly, the inclusion of G-1 before E2 injection caused a dramatic suppression of E2-induced stromal effects (Fig. 4C). Our analysis of ERK1/2 phosphorylation revealed similar results, with the exception that E2-induced ERK1/2 phosphorylation was mainly detected in the extranuclear locations of the stromal cells (Fig. 4C). These results indicate that the E2-induced accumulation of pERα and pERK1/2 is colocalized within the stromal cells and that this effect can be neutralized by prior injection of G-1.
Activation of ERK1/2 phosphorylation critically controls uterine physiological responses to E2
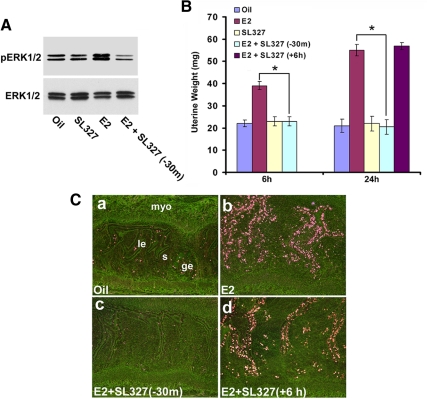
To test whether pharmacological inhibition of ERK1/2 activation influences E2-regulated early and late responses in the uterus, the selective inhibitor SL327 was used before the induction of E2-dependent effects in the ovariectomized mice. Our initial studies indeed confirmed that the prior injection of SL327 was able to induce inhibitory response for E2-dependent activation of uterine pERK1/2 activity, as judged by the Western blotting experiments (Fig. 5A). In the case of uterine weight analyses, injections of E2 caused significant increases for both the early and late phase responses over the control (oil) (Fig. 5B). An injection of SL327 30 min before E2 injection completely abrogated E2-dependent uterine responses during both the phases, whereas the inhibitor given 6 h after E2 had no inhibitory effects on E2-dependent late responses (Fig. 5B). Furthermore, the early inhibition of ERK signaling yielded similar results with respect to E2-dependent uterine epithelial cell proliferation (Fig. 5C). For example, an injection of E2 caused the induction of 3H-thymidine incorporation in uterine luminal and glandular epithelial cells by 24 h (Fig. 5C, panel b), whereas an injection of oil (Fig. 5C, panel a) or SL327 (data not shown) had minimal effects. Contrastingly, an injection of the inhibitor before E2 completely eliminated this E2-dependent late response (Fig. 5C, panel c), but the inhibitor given 6 h after E2 was not supportive of this inhibition (Fig. 5C, panel d). This suggests that ERK1/2-dependent activity crucially controls E2-dependent uterine growth regulation, primarily acting during the early phase. Taken together, these results indicate that E2-dependent ERK activation is an upstream regulatory event for E2-induced uterine growth response. Furthermore, this may provide a new avenue to dissect out the relative physiological importance of the two phases of estrogen action in uterine biology.
Fig. 5.
Pharmacological inhibition of ERK activation abrogates E2-dependent uterine responses. Adult ovariectomized mice were given injections of oil, E2 (100 ng/mouse), SL327 (160 mg/kg), or SL327 (160 mg/kg) 30 min before (−30 m) or 6 h after (+6 h) E2 (100 ng/mouse). A, Western blotting (at 6 h time point) confirms the appropriate inhibition of E2-dependent uterine pERK1/2 activation by SL327. B, Uterine wet weights were recorded at indicated times after the last injection. Uterine wet weights from five to seven mice for each group were analyzed. *, Values are statistically different between the compared groups (P < 0.05) based on ANOVA followed by Newman-Keul's multiple range test. C, Autoradiographic analysis of uterine cell proliferation by 3H-thymidine incorporation. Dark-field photomicrographs of representative tissue sections are shown. Pictures were taken at ×100. le, Luminal epithelium; ge, glandular epithelium; s, stroma; and myo, myometrium. These experiments were repeated at least three times with similar results.
Uterine manipulation of GPR30 expression by recombinant adenovirus strategy causes alteration of E2-induced cell proliferation
Because uterine GPR30 undergoes repression by E2, we hypothesize that manipulation of GPR30 levels in the mouse uterus via adenovirus-driven strategy should alter uterine growth regulation under the direction of E2. Recombinant adenovirus particles carrying GPR30 sense [rAdGPR30(S)], antisense [rAdGPR30(AS)], or the empty vector (as control) under the direction of cytomegalovirus promoter were applied in the uterus, as previously described by us (11, 12). These constructs were also equipped with a green fluorescent protein (GFP) expression system under a separate cytomegalovirus promoter. Because the GPR30 transgene, which we used in generating the adenovirus particles, is a mouse-specific gene, it is not possible to distinguish the expression levels of the GPR30 transgene vs. the native GPR30 gene in the uterus. To circumvent this problem, we analyzed the expression of virus-driven GFP. Analysis of uterine GFP by direct visualization under the immunofluorescence microscopy revealed that the expression is primarily localized in the apical or lateral surfaces of epithelial cell lining and subluminal stroma but not in the open lumen space, indicating that uterine cells indeed infected by the viruses after administration of rAdGPR30(S) (Supplemental Fig. 3) or rAdGPR30(AS) (data not shown).
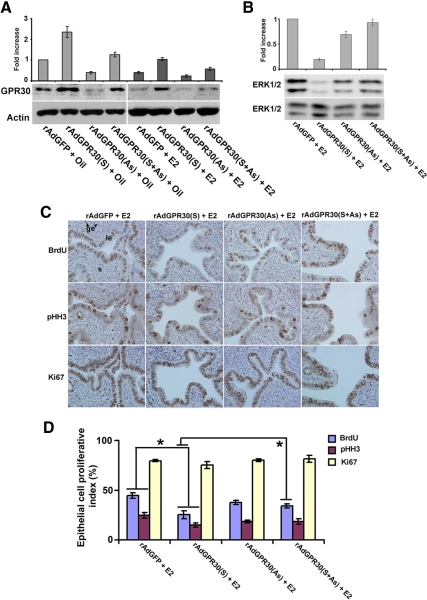
Our further analysis revealed that the rAdGPR30(S) virus was indeed capable of inducing uterine GPR30 levels compared with the levels detected by the control virus (Fig. 6A). Furthermore, this up-regulated status of expression appeared to be antagonized by the addition of the antisense virus, implying GRP30 expression can be appropriately manipulated in the mouse uterus (Fig. 6A). Because the stimulation of GPR30 interfered with the E2-dependent activity of ERK1/2, we wanted to examine whether increased uterine expression of GPR30 can induce an inhibitory influence on E2-dependent ERK activation. Indeed, our results show that forced expression of GPR30 strongly suppressed E2-induced phosphorylation of ERK1/2 (Fig. 6B). The addition of the antisense virus in conjunction with the sense virus reversed the inhibitory effect of the sense construct on ERK activation (Fig. 6B), suggesting this regulation is specifically induced by the uterine levels of GPR30. Given this effective manipulation, we examined whether this is a regulatory control of uterine cell proliferation under the direction of E2. Our initial analysis of uterine weights after virus manipulation did not reveal any significant alteration (data not shown). However, the overexpression of GPR30 caused significant inhibition of E2-stimulated uterine epithelial cell proliferation, as judged by the analysis of both BrdU and pHH3 on serial sections (Fig. 6, C and D). Furthermore, the antisense virus suppressed overexpression and showed a reversal of cell proliferation, as examined by BrdU (Fig. 6, C and D). Only Ki67 staining did not reveal any statistical differences between the above treatments (Fig. 6, C and D), again suggesting the limitation of this technique in the present study. Overall, these results demonstrate that viral-driven manipulation of GPR30 expression can effectively alter E2-dependent uterine cell proliferation.
Fig. 6.
Adenoviral vector-driven manipulation of GPR30 causes alteration of ERK activation and uterine epithelial cell proliferation induced by E2. A, Ovariectomized mice were administrated with intraluminal injections of adenoviruses empty vector (rAdGFP; control), rAdGPR30(S), AdGPR30(AS), or rAdGPR30(S+AS), and uterine tissues were collected after injections of oil or E2 (25 ng/mouse) at 6 and 24 h. This experiment used at least five mice for each group and repeated four times. Western blotting (at 6 h time point) confirms the appropriate regulation of uterine expression for GPR30 levels (A) and E2-dependent regulation of ERK1/2 activation (B). Fold changes of protein levels were determined by densitometric scanning and calculated against the control virus, and the values were normalized by either actin or ERK1/2 levels, respectively. C, Analysis of uterine epithelial cell proliferation (at 24 h time point) was examined by immunostaining with BrdU, pHH3, and Ki67. Representative tissue sections are shown, and reddish brown nuclear deposits indicate the sites of positive immunostaining. Pictures were taken at ×200. le, Luminal epithelium; ge, glandular epithelium; s, stroma cells. D, Quantitation of BrdU-, pHH3-, and Ki67-positive cells was determined for the luminal and glandular epithelial cells after counting at least 500 cells per animal on consecutive fields. The data are presented after the analysis of at least five to 10 mice for each group. The error bars represent ses. *, Values are statistically different (P < 0.05) between compared groups, based on ANOVA followed by Newman-Keul's multiple range test.
Discussion
Emerging evidence indicates that estrogen exerts nongenomic effects in target tissues and that these effects primarily involve early activation of intracellular signaling pathways (6, 7, 20). The similarity of these effects to those induced by growth factors supports the contention that a putative membrane ER is involved (8, 20). Our data provide evidence that uterine epithelial activation of GPR30 can produce an inhibitory signal, leading to the perturbation of colocalized increases of pERK1/2 and pERα phosphorylation signals in the stroma by E2. Our studies also showed that pharmacological inhibition of ERK1/2 activation via prior injection of SL327 completely abrogates both the phase I and phase II uterine responses. In contrast, an injection of SL327 6 h after E2 injection fails to alter the late responses, bolstering the idea that E2-dependent activation of early ERK1/2 phosphorylation critically prepares the uterus during the early phase. The virally driven manipulation of uterine GPR30 also supports the fact that GPR30 acts as an upstream regulator in mediating the inhibition of both ERK activation and epithelial cell proliferation under the direction of E2. Overall, estrogen-regulated early nongenomic responses mediated by ERK1/2 activation intimately associate with the late uterine growth response, whereas the early GPR30 activation signal may be a balancing act to negatively control uterine ERK and ultimately regulate uterine growth in response to E2.
Consistent with the previous report (35), G-1 (at doses ranging from 0.5 to 100 μg/mouse), as a GPR30-specific agonist without binding to ERα and ERβ (34), did not induce uterine water imbibition or late hyperplasia (Fig. 1 and Supplemental Figs. 1 and 2), suggesting that G-1-mediated activation of GPR30 is not mimicking estrogen-like activities in the mouse uterus. Some studies have shown that G-1 (at doses of 40 ng/mouse to 1 μg/mouse) was capable of inducing only a weak proliferative response (3- to 4-fold vs. 17-fold by E2), based on a singular approach using Ki67 staining (36). The reason for this discrepancy is not clearly understood. One should be cautious comparing and interpreting estrogen-regulated uterine responses obtained in different laboratories because environmental differences such as diet and habitat can alter estrogen sensitivity in the mouse uterus (50). On the other hand, our analysis of cell proliferation using different cell cycle phase-specific markers demonstrated that Ki67 is not a suitable marker to study uterine cell proliferation under the conditions described above. Furthermore, studies have shown that G-1 can induce cell cycle arrest either at G1 to S or G2 to M (51, 52) and that Ki67 staining is not appropriate for discriminating among these arrested cells (53).
Previously it was shown that G-1 (at its maximum dose of 2 μg/mouse) was unable to induce alteration of the E2 (100 ng/mouse)-dependent late response (35), which is indeed consistent with our findings at doses of 0.5–10 μg/mouse. However, our studies also revealed a new finding: that G-1 can cause inhibition of E2-induced late phase responses in a dose-dependent manner, primarily at doses of 25–100 μg/mouse or greater (Fig. 1 and Supplemental Fig. 2). These doses of G-1 were 500- to 1000-fold the molar concentration of E2, but they have been used in mice without any signs of secondary effects (35, 51). These G-1 effects appear to be specific because a GPR30-selective antagonist (G15) given 30 min before E2 did not reveal any alteration of E2-dependent uterine late wet weights (Supplemental Fig. 4A), the aspect of proliferation in respect of BrdU incorporation (Supplemental Fig. 4, B and C), and the activation of pERK (Supplemental Fig. 5A). Furthermore, G15 alone was also ineffective to alter uterine responses (Supplemental Fig. 4, A–C).
Although both E2 and G-1 are defined as GPR30 agonists, whether the downstream events mediated by these two agonists either individually or in combination are same or different is not clearly understood. Based on our studies, we have seen that G-1 prior injection is able to alter E2-mediated uterine effects. In this regard, we speculate that the preinjection of G-1 might rapidly activate intracellular signaling via GPR30 before E2 comes into action, through interaction with two existing receptors GPR30 and ERα in the uterus. The selective end point of G-1 or G15 is not clearly demonstrated, although G-1 affected some of E2's end point in the uterus. In this regard, it is worth mentioning that G-1 alone can effectively induce Akt signaling during the early phase in the uterus (data not shown). In addition, Akt phosphorylation signaling is also induced by E2, which is unaffected by prior injection of G-1 (data not shown), suggesting that selective activation by these two ligands in the uterus remains unknown.
Previous studies have established that ERα acts as the sole receptor to mediate E2-regulated physiological responses in the mouse uterus, whereas ERβ has been detected at low levels and mostly negatively regulates ERα-mediated effects (1, 54, 55). Our findings, that activation of GPR30 via G-1 exhibits inhibition of E2-induced uterine epithelial cell proliferation, provide evidence that this extranuclear ER acts as an upstream negative regulator for ERα-mediated growth. Viral vector-mediated suppression, as opposed to overexpression, of GPR30 did not reveal any exaggerated response for E2-dependent regulation of uterine cell proliferation, as well as pERK1/2 activity, compared with the control virus (Fig. 6), and these results are in agreement with the finding of GPR30 knockout mice (31). It is worth mentioning that mice lacking ERβ, a negative regulator for ERα, exhibited exaggerated responses in respect to uterine cell proliferation by E2 (55), implying that the mechanism of negative regulation by GPR30 is different from that of ERβ.
Our observation that the down-regulation of uterine GPR30 during the early phase by E2, but not by G-1, is clearly interesting and suggests that this effect may be mediated through ERα. In our study, the injection of G-1, given 30 min before E2, presumably acts in a predominant manner through the basal GPR30 and thus elicits intracellular signaling (leading to the inhibition of E2/ERα mediated growth) before E2 can effectively suppress the uterine levels of GPR30. Overall, these results suggest that G-1/GPR30 mediated inhibitory action can also be antagonized by E2/ERα action through suppression of GPR30 during the early phase. Furthermore, it can also be suggested that sustained expression of GPR30 may be detrimental for E2/ERα-dependent uterine growth. Indeed, our further studies using adenoviral vector-driven overexpression of GPR30 caused inhibition of epithelial cell proliferation by E2, compared with that of control virus (Fig. 6). A recent study similarly indicates that GPR30 undergoes down-regulation by E2 via an ERα-dependent mechanism (56). Other studies have implicated that the suppression of GPR30 may be mediated through interactions at its promoter level via forkhead box A1-independent ERα binding sites and coactivator-associated arginine methyltransferase 1 sites (57). To our knowledge, there is no other known endogenous ligand, except E2, that can bind to GPR30; thus, uterine GPR30's role remains unknown when estrogen is limited in the circulation. In this regard, the ligand-independent activity of GPR30 has not yet been documented, but one should not undermine this possibility and should require future attention. Overall, it appears that E2-mediated down-regulation of GPR30 (a negative regulator of ERα) may be necessary to amplify ERα-dependent uterine physiological responses.
The inhibition of cell proliferation by the activation of GPR30 appears to be accompanied by differential regulation of estrogen-regulated uterine gene expression (Fig. 3). For example, the increased expression of Cdkn1a (an inhibitor of cell cycle progression) with G-1 plus E2, but not with E2 alone, indicates this gene may be involved in inhibiting cell proliferation. This is consistent with the fact that G-1 can induce cell cycle arrest through up-regulation of p21 (also known as Cdkn1a), depending on the cell type (51, 56). A prior injection of G-1 also appeared to affect the E2-induced late phase response in Ltf, an estrogen-responsive gene in the mouse uterus (42, 56); to date, this gene's involvement in uterine growth regulation remains unknown. We did find that G-1 was able to reverse E2-dependent suppression of Sfrp2 during the late phase. Sfrp2 is an ER-independent estrogen-suppressive stromal gene in the mouse uterus (7). Previously we showed that overexpression of Sfrp2, an antagonist of Wnt signaling, abrogates E2-dependent uterine growth responses (11). Thus, it can be speculated that G-1-mediated reversal of Sfrp2 may inhibit E2-regulated uterine growth.
Growth factors, such as IGF-I and epidermal growth factor (EGF), activate diverse intracellular signaling pathways, including the ERK1/2, which is known as a point of convergence for growth factors and ERα-initiated activities (58). A recent global chromatin association study also establishes that ERα and MAPK pathways cooperate via colocalized recruitment of ERα and ERK2 proteins at the gene promoter levels to critically control gene transcription and cell cycle progression (59). Our experiments using the inhibitor of ERK activation show that the early responsive signals mediated via ERK are critically linked to the late-phase growth responses in the mouse uterus (Fig. 5). Our inability to induce uterine ERK1/2 activation via G-1 alone suggests that GPR30 is not involved in E2-mediated regulation. G-1 stimulated GPR30-mediated inhibition of E2-induced ERK1/2 and ERα signaling activation during the early phase could serve to inhibit uterine epithelial cell proliferation. These effects again appear to be specific because G15-mediated effects were not regulatory to E2-dependent ERK1/2 and ERα phosphorylation signals (Supplemental Fig. 5). Promoting epithelial cell proliferation with growth factors requires the presence of ERα in the mouse uterus (58), indicating cross talk can occur between kinase signaling and ERα in a concomitant fashion, which may ultimately lead to ERα phosphorylation (48). Indeed, our studies have shown that ERK activation and ERα phosphorylation signals are induced in the uterine stromal compartment in a colocalized fashion under the direction of E2 (Fig. 4). Also, G-1 mediated activation of GPR30 in the epithelial cells inhibits both of the above activation signals in the stroma (Fig. 4), suggesting that epithelial GPR30 activation elicits a signaling cross talk with the stromal compartment. The uterus is composed of heterogeneous cell types, and estrogen-dependent growth regulation has been implicated through paracrine interactions between the epithelial and stromal compartments (60). Based on tissue recombination studies, it is known that stromal ERα plays a major role in regulating E2-dependent uterine epithelial cell proliferation (60, 61). Our finding of epithelial GPR30 activation for the inhibition of ERK1/2 and ERα activation signals in stromal cells further emphasizes the importance of the stroma in mediating this novel signaling to regulate epithelial cell proliferation under the direction of E2.
GPR30 has been detected in the human uterus (62) and is reported to be overexpressed in endometrial carcinoma and cancer cells (63). It is also known that GPR30 possesses multiple beneficial and protective roles for estrogen in different organs (30, 32, 64, 65). Therefore, we believe that our study will provide a novel understanding in respect of GPR30-mediated negative control of ERα-dependent growth regulation and also help to develop alternative therapeutic strategies targeting GPR30 to combat uterine pathological conditions directed by estrogen.
Supplementary Material
Acknowledgments
We thank Erin L. Adams for editing the manuscript. We also thank Bert Vogelstein (Johns Hopkins Institution, Baltimore, MD) for providing reagents to generate recombinant adenoviral clones.
This work was supported by National Institutes of Health Grants R01 ES07814 and R01 HD56044.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- 5-Bromo-2′-deoxyuridine
- E2
- estradiol-17β
- EGF
- epidermal growth factor
- ER
- estrogen receptor
- GFP
- green fluorescent protein
- GPR30
- G protein-coupled receptor 30
- p
- phosphorylated
- pHH3
- phosphorylated histone H3
- rAdGPR30(AS)
- recombinant adenovirus particles carrying GPR30 antisense
- rAdGPR30(S)
- recombinant adenovirus particles carrying GPR30 sense.
References
- 1. Couse JF, Korach KS. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- 2. Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. 1991. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the Nurses' Health Study. N Engl J Med 325:756–762 [DOI] [PubMed] [Google Scholar]
- 3. McDonnell DP, Norris JD. 2002. Connections and regulation of the human estrogen receptor. Science 296:1642–1644 [DOI] [PubMed] [Google Scholar]
- 4. Tsai MJ, O'Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- 5. Beato M, Herrlich P, Schütz G. 1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857 [DOI] [PubMed] [Google Scholar]
- 6. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. 2004. Molecular cues to implantation. Endocr Rev 25:341–373 [DOI] [PubMed] [Google Scholar]
- 7. Das SK, Tan J, Raja S, Halder J, Paria BC, Dey SK. 2000. Estrogen targets genes involved in the protein processing, calcium homeostasis, and Wnt signaling in the mouse uterus independent of estrogen receptor-α and -β. J Biol Chem 275:28834–28842 [DOI] [PubMed] [Google Scholar]
- 8. Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. 2003. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol 17:2070–2083 [DOI] [PubMed] [Google Scholar]
- 9. Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. 1997. Estrogenic responses in estrogen receptor-α deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci USA 94:12786–12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das SK, Tan J, Johnson DC, Dey SK. 1998. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology 139:2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hou X, Tan Y, Li M, Dey SK, Das SK. 2004. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18:3035–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ray S, Hou X, Zhou HE, Wang H, Das SK. 2006. Bip is a molecular link between the phase I and phase II estrogenic responses in uterus. Mol Endocrinol 20:1825–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. 2006. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J Biol Chem 281:26683–26692 [DOI] [PubMed] [Google Scholar]
- 14. Watanabe H, Suzuki A, Kobayashi M, Takahashi E, Itamoto M, Lubahn DB, Handa H, Iguchi T. 2003. Analysis of temporal changes in the expression of estrogen-regulated genes in the uterus. J Mol Endocrinol 30:347–358 [DOI] [PubMed] [Google Scholar]
- 15. Ray S, Xu F, Wang H, Das SK. 2008. Cooperative control via lymphoid enhancer factor 1/T cell factor 3 and estrogen receptor-α for uterine gene regulation by estrogen. Mol Endocrinol 22:1125–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart PJ, Webster RA. 1983. Intrauterine injection of cholera toxin induces estrogen-like uterine growth. Biol Reprod 29:671–679 [DOI] [PubMed] [Google Scholar]
- 17. Aronica SM, Kraus WL, Katzenellenbogen BS. 1994. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci USA 91:8517–8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajkumar K. 1993. Effect of protein kinase-C inhibitor on estradiol-induced deoxyribonucleic acid synthesis in rats. Steroids 58:100–105 [DOI] [PubMed] [Google Scholar]
- 19. Pietras RJ, Szego CM. 1977. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature 265:69–72 [DOI] [PubMed] [Google Scholar]
- 20. Hammes SR, Levin ER. 2007. Extranuclear steroid receptors: nature and actions. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- 21. Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. 1999. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science 285:1929–1931 [DOI] [PubMed] [Google Scholar]
- 22. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- 23. Thomas P, Pang Y, Filardo EJ, Dong J. 2005. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- 24. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- 25. Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. 2002. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- 26. Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, Musti AM, Picard D, Andò S, Maggiolini M. 2006. 17β-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol Pharmacol 70:1414–1423 [DOI] [PubMed] [Google Scholar]
- 27. Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Andò S, Maggiolini M. 2006. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17β-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20:631–646 [DOI] [PubMed] [Google Scholar]
- 28. Albanito L, Sisci D, Aquila S, Brunelli E, Vivacqua A, Madeo A, Lappano R, Pandey DP, Picard D, Mauro L, Andò S, Maggiolini M. 2008. Epidermal growth factor induces G protein-coupled receptor 30 expression in estrogen receptor-negative breast cancer cells. Endocrinology 149:3799–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, Maggiolini M. 2007. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 [DOI] [PubMed] [Google Scholar]
- 30. Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. 2008. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 22:636–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. 2009. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80:34–41 [DOI] [PubMed] [Google Scholar]
- 32. Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. 2009. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150:687–698 [DOI] [PubMed] [Google Scholar]
- 33. Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. 2009. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology 150:1722–1730 [DOI] [PubMed] [Google Scholar]
- 34. Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. 2006. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- 35. Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. 2008. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology 149:4846–4856 [DOI] [PubMed] [Google Scholar]
- 36. Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. 2009. In vivo effects of a GPR30 antagonist. Nat Chem Biol 5:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paria BC, Lim H, Wang XN, Liehr J, Das SK, Dey SK. 1998. Coordination of differential effects of primary estrogen and catecholestrogen on two distinct targets mediates embryo implantation in the mouse. Endocrinology 139:5235–5246 [DOI] [PubMed] [Google Scholar]
- 38. Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. 2002. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev 111:99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. 2004. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol 265:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ray S, Xu F, Li P, Sanchez NS, Wang H, Das SK. 2007. Increased level of cellular Bip critically determines estrogenic potency for a xenoestrogen kepone in the mouse uterus. Endocrinology 148:4774–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- 42. Liu YH, Teng CT. 1991. Characterization of estrogen-responsive mouse lactoferrin promoter. J Biol Chem 266:21880–21885 [PubMed] [Google Scholar]
- 43. Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. 1995. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol 9:1441–1454 [DOI] [PubMed] [Google Scholar]
- 44. Kirkland JL, Murthy L, Stancel GM. 1992. Progesterone inhibits the estrogen-induced expression of c-fos messenger ribonucleic acid in the uterus. Endocrinology 130:3223–3230 [DOI] [PubMed] [Google Scholar]
- 45. Wang XN, Das SK, Damm D, Klagsbrun M, Abraham JA, Dey SK. 1994. Differential regulation of heparin-binding epidermal growth factor-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology 135:1264–1271 [DOI] [PubMed] [Google Scholar]
- 46. Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. 1996. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J 15:1292–1300 [PMC free article] [PubMed] [Google Scholar]
- 47. Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ. 2002. Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Mol Endocrinol 16:116–127 [DOI] [PubMed] [Google Scholar]
- 48. Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. 1995. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 270:1491–1494 [DOI] [PubMed] [Google Scholar]
- 49. Chen D, Riedl T, Washbrook E, Pace PE, Coombes RC, Egly JM, Ali S. 2000. Activation of estrogen receptor α by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol Cell 6:127–137 [PubMed] [Google Scholar]
- 50. Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. 2005. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA 102:9960–9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chan QK, Lam HM, Ng CF, Lee AY, Chan ES, Ng HK, Ho SM, Lau KM. 2010. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G(2) cell-cycle arrest. Cell Death Differ 17:1511–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ahola TM, Manninen T, Alkio N, Ylikomi T. 2002. G protein-coupled receptor 30 is critical for a progestin-induced growth inhibition in MCF-7 breast cancer cells. Endocrinology 143:3376–3384 [DOI] [PubMed] [Google Scholar]
- 53. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715 [PubMed] [Google Scholar]
- 54. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. 1993. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weihua Z, Saji S, Mäkinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. 2000. Estrogen receptor (ER) β, a modulator of ERα in the uterus. Proc Natl Acad Sci USA 97:5936–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ariazi EA, Brailoiu E, Yerrum S, Shupp HA, Slifker MJ, Cunliffe HE, Black MA, Donato AL, Arterburn JB, Oprea TI, Prossnitz ER, Dun NJ, Jordan VC. 2010. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res 70:1184–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lupien M, Eeckhoute J, Meyer CA, Krum SA, Rhodes DR, Liu XS, Brown M. 2009. Coactivator function defines the active estrogen receptor α cistrome. Mol Cell Biol 29:3413–3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. 2002. Requirement of estrogen receptor-α in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. J Biol Chem 277:8531–8537 [DOI] [PubMed] [Google Scholar]
- 59. Madak-Erdogan Z, Lupien M, Stossi F, Brown M, Katzenellenbogen BS. 2011. Genomic collaboration of estrogen receptor α and extracellular signal-regulated kinase 2 in regulating gene and proliferation programs. Mol Cell Biol 31:226–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. 1997. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA 94:6535–6540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Buchanan DL, Setiawan T, Lubahn DB, Taylor JA, Kurita T, Cunha GR, Cooke PS. 1999. Tissue compartment-specific estrogen receptor-α participation in the mouse uterine epithelial secretory response. Endocrinology 140:484–491 [DOI] [PubMed] [Google Scholar]
- 62. Kolkova Z, Noskova V, Ehinger A, Hansson S, Casslen B. 2010. G protein-coupled estrogen receptor 1 (GPER, GPR 30) in normal human endometrium and early pregnancy decidua. Mol Hum Reprod 16:743–751 [DOI] [PubMed] [Google Scholar]
- 63. Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. 2007. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol 196:386.e381–e389; discussion 386.e389–e311 [DOI] [PubMed] [Google Scholar]
- 64. Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, Belsham DD. 2010. Estrogen receptor α and G-protein coupled receptor 30 mediate the neuroprotective effects of 17β-estradiol in novel murine hippocampal cell models. Neuroscience 170:54–66 [DOI] [PubMed] [Google Scholar]
- 65. Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. 2010. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery 148:436–443 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.