Abstract
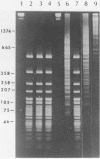
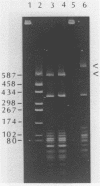
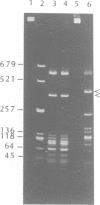
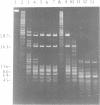
A type II restriction endonuclease, named CviJI, was isolated from a eukaryotic Chlorella-like green alga infected with the dsDNA containing virus IL-3A. CviJI is the first restriction endonuclease to recognize the sequence PuGCPy; CviJI cleaves DNA between the G and C. Methylation of the cytosine in PuGCPy sequences prevents cleavage by CviJI. CviJI cleaved DNA into smaller but defined fragments in the presence of ATP. This "star" activity was stimulated by dithiothreitol and/or S-adenosylmethionine but did not occur under conditions which favor "star" activity of other restriction endonucleases.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H., Scibienski E., Slocum H., Roulland-Dussoix D. The in vitro restriction of the replicative form of W.T. and mutant fd phage DNA. Virology. 1971 Dec;46(3):703–710. doi: 10.1016/0042-6822(71)90072-9. [DOI] [PubMed] [Google Scholar]
- Bächi B., Reiser J., Pirrotta V. Methylation and cleavage sequences of the EcoP1 restriction-modification enzyme. J Mol Biol. 1979 Feb 25;128(2):143–163. doi: 10.1016/0022-2836(79)90123-2. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Jeppesen P. G. Separation and isolation of DNA fragments using linear polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1980;65(1):305–319. doi: 10.1016/s0076-6879(80)65041-1. [DOI] [PubMed] [Google Scholar]
- Kauc L., Piekarowicz A. Purification and properties of a new restriction endonuclease from Haemophilus influenzae Rf. Eur J Biochem. 1978 Dec;92(2):417–426. doi: 10.1111/j.1432-1033.1978.tb12762.x. [DOI] [PubMed] [Google Scholar]
- Kessler C., Neumaier P. S., Wolf W. Recognition sequences of restriction endonucleases and methylases--a review. Gene. 1985;33(1):1–102. doi: 10.1016/0378-1119(85)90119-2. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Carraway M., Frey A. Z., Brown L., Arraj J. A. Insertion mutations in the dam gene of Escherichia coli K-12. Mol Gen Genet. 1983;192(1-2):288–289. doi: 10.1007/BF00327681. [DOI] [PubMed] [Google Scholar]
- McClelland M., Nelson M. The effect of site specific methylation on restriction endonuclease digestion. Nucleic Acids Res. 1985;13 (Suppl):r201–r207. doi: 10.1093/nar/13.suppl.r201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw R. A., 3rd Dideoxy DNA sequencing with end-labeled oligonucleotide primers. Anal Biochem. 1984 Dec;143(2):298–303. doi: 10.1016/0003-2697(84)90666-3. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Polisky B., Greene P., Garfin D. E., McCarthy B. J., Goodman H. M., Boyer H. W. Specificity of substrate recognition by the EcoRI restriction endonuclease. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3310–3314. doi: 10.1073/pnas.72.9.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1985;13 (Suppl):r165–r200. doi: 10.1093/nar/13.suppl.r165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Assaying of organisms for the presence of restriction endonucleases. Methods Enzymol. 1980;65(1):19–23. doi: 10.1016/s0076-6879(80)65004-6. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Burbank D. E., Meister B., Skrdla M. P., Meints R. H., Hattman S., Swinton D., Van Etten J. L. Characterization of viruses infecting a eukaryotic Chlorella-like green alga. Virology. 1986 Apr 15;150(1):170–177. doi: 10.1016/0042-6822(86)90276-x. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Schuster A. M., Meints R. H. Lytic viruses infecting a Chlorella-like alga. Virology. 1985 Jan 15;140(1):135–143. doi: 10.1016/0042-6822(85)90452-0. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Schuster A. M., Girton L., Burbank D. E., Swinton D., Hattman S. DNA methylation of viruses infecting a eukaryotic Chlorella-like green alga. Nucleic Acids Res. 1985 May 24;13(10):3471–3478. doi: 10.1093/nar/13.10.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Burbank D. E., Uher L., Rabussay D., Van Etten J. L. Restriction endonuclease activity induced by PBCV-1 virus infection of a Chlorella-like green alga. Mol Cell Biol. 1986 May;6(5):1430–1439. doi: 10.1128/mcb.6.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Van Etten J. L. DNA methyltransferase induced by PBCV-1 virus infection of a Chlorella-like green alga. Mol Cell Biol. 1986 May;6(5):1440–1445. doi: 10.1128/mcb.6.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Burbank D. E., Van Etten J. L. Restriction endonuclease activity induced by NC-1A virus infection of a Chlorella-like green alga. Nucleic Acids Res. 1986 Aug 11;14(15):6017–6030. doi: 10.1093/nar/14.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]