Abstract
Oligoribonucleotides containing a 5′-phosphorothiolate linkage have provided effective tools to study the mechanisms of RNA catalysis, allowing resolution of kinetic ambiguity associated with mechanistic dissection and providing a strategy to establish linkage between catalysis and specific functional groups. However, challenges associated with their synthesis have limited wider application of these modified nucleic acids. Here, we describe a general semisynthetic strategy to obtain these oligoribonucleotides reliably and relatively efficiently. The approach begins with the chemical synthesis of an RNA dinucleotide containing the 5′-phosphorothiolate linkage, with the adjacent 2′-hydroxyl group protected as the photolabile 2′-O-o-nitrobenzyl or 2′-O-α-methyl-o-nitrobenzyl derivative. Enzymatic ligation of the 2′-protected dinucleotide to transcribed or chemically synthesized 5′ and 3′ flanking RNAs yields the full-length oligoribonucleotide. The photolabile protecting group increases the chemical stability of these highly activated oligoribonucleotides during synthesis and long-term storage but is easily removed with UV irradiation under neutral conditions, allowing immediate use of the modified RNA in biochemical experiments.
INTRODUCTION
Phosphorothioate-containing oligonucleotides, in which sulfur substitutes for one or both of the non-bridging oxygens within a specific phosphodiester linkage, have found numerous applications in diverse fields ranging from enzymology (1–3) to therapeutics (4–7). Their widespread use depends in part on their relative ease of synthesis—phosphorothioate linkages are introduced via a sulfurization step that can be programmed into automated solid phase oligonucleotide synthesizers (8,9). In contrast, oligonucleotides in which sulfur substitutes for a specific 5′ or 3′ bridging oxygen, or phosphorothiolates, have presented a more difficult synthetic challenge, requiring alterations to the sugar moiety itself (10). While phosphorothiolate oligonucleotides have not been investigated as thoroughly as their non-bridging counterparts, significant interest in their synthesis has grown in recent years as new applications have emerged (see Supplementary Tables S1 and S2). The 3′-phosphorothiolate, in particular, has helped to elucidate the mechanisms of several RNA (11–18) and protein (19–25) enzymes involved in chemical manipulation of nucleic acids. This linkage has also facilitated conformational studies of oligonucleotides because it shifts the conformational equilibrium of the ribose sugar to favor the 3′-endo over the 2′-endo configuration (26–28). The 3′-phosphorothiolate may also form the chemical basis for a DNA sequencing method involving silver nitrate-mediated cleavage of the sulfur linkage (29).
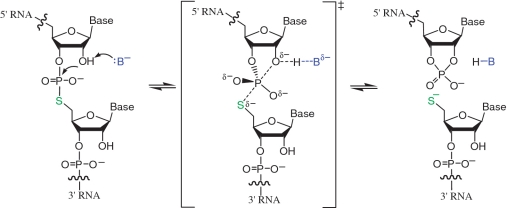
Oligonucleotides containing a single 5′ bridging sulfur atom at either DNA/DNA (d[B1psB2]) (30–34), RNA/DNA (r[B1]psd[B2]) (35,36) or RNA/RNA (r[B1]psr[B2]) (37) linkages have been synthesized and used as mechanistic tools (see Supplementary Table S2). Oligodeoxyribonucleotides containing 5′-phosphorothiolates (ps) are suicide substrates for type I DNA topoisomerases (38–40). Within oligoribonucleotides, 5′-phosphorothiolate linkages are highly activated and undergo cleavage via attack of the adjacent 2′-hydroxyl group much more rapidly than the corresponding 5′-oxygen species (35,41,42) (Figure 1). In contrast to the reaction of the natural phosphodiester linkage, cleavage of a 5′-phosphorothiolate linkage within RNA generally exhibits less susceptibility to acid-assisted catalysis because the 5′ sulfur itself is an excellent leaving group. The dinucleotide UpsU has been investigated as a substrate for RNase A and phosphodiesterases (41,42). Oligoribonucleotides containing 5′-phosphorothiolate linkages have been used successfully to investigate the mechanisms of several nucleolytic ribozymes including the hammerhead (36,37,43,44), HDV (45) and VS (46) ribozymes.
Figure 1.
Mechanism of ribozyme-mediated cleavage and ligation of RNA oligonucleotides containing a 5′-phosphorothiolate linkage.
Substrates used for hammerhead ribozyme reactions have included an all-RNA oligonucleotide containing CpsC (37) and DNA oligonucleotides containing a 5′-phosphorothiolate that immediately follows a cytidine ribonucleotide, CpsdA (35) or CpsdC (36). Synthesis of the all-RNA oligonucleotide containing CpsC employed solid phase synthesis with 2′-O-TBS-cytidine and 5′-thiocytidine phosphoramidites, but few details were reported concerning experimental procedure and characterization (37). Synthesis of the CpsdA-containing DNA oligonucleotide involved solid phase synthesis using 2′-O-(1-(2-chloroethoxy)ethyl)-cytidine and 5′-S-trityl-2′-deoxyadenosine phosphoramidites (35). To construct the CpsdC-containing DNA oligonucleotide, Thomas and Perrin used a semisynthetic approach, preparing by solid phase synthesis a DNA oligonucleotide terminating with a 5′-thiophosphorylated 2′-deoxycytidine residue and coupling this via T4 DNA ligase to an acceptor oligodeoxyribonucleotide bearing a 3′-terminal cytidine residue (36). To study the HDV ribozyme, Das and Piccirilli introduced a CpsdG linkage into an RNA substrate by using 2′-O-(o-nitrobenzyl)cytidine and 5′-S-trityldeoxyguanosine phosphoramidites (45). These examples notwithstanding, all of the solid phase strategies for synthesizing 5′-phosphorothiolates reported in the literature to date give very low yields. Moreover, many attempts by us to use solid phase strategies to synthesize RNA oligonucleotides bearing GpsA RNA linkages yielded no detectable product (Schemes S1–S4). Additional challenges associated with the lability and long-term storage of 5′-phosphorothiolate-modified oligoribonucleotides have limited their wider use as mechanistic probes in nucleic acid enzymology.
Here, we report a general semisynthetic approach to the construction of these highly activated RNA oligonucleotides. The method begins with the synthesis of an RNA dinucleotide containing a 2′-O-(o-nitrobenzyl)nucleoside linked to a second ribonucleoside via a 5′-phosphorothiolate linkage (Base1-[2′-O-(o-nitrobenzyl)]-ps-Base2). The o-nitrobenzyl protecting group prevents the 2′-hydroxyl group adjacent to the 5′-phosphorothiolate linkage from promoting cleavage prematurely, and is readily removed with UV light under neutral conditions prior to biochemical studies (45–47). The dinucleotide is then phosphorylated enzymatically and ligated to 5′ and 3′ flanking RNAs to produce a full-length 5′-phosphorothiolate oligoribonucleotide (Supplementary Figure S1). To establish proof-of-concept for this approach, we synthesized four 5′-phosphorothiolate-linked RNA dinucleotides and used two of these to construct oligoribonucleotides corresponding to substrates for the VS and HDV ribozymes.
MATERIALS AND METHODS
N2-Isobutyryl-2′-O-(o-nitrobenzyl)guanosine (2a)
Compound 2a was prepared according to a literature procedure (48). Under argon N2-isobutyrylguanosine 1 (1.72 g, 4.86 mmol) was treated with NaH (292 mg, 95%, 11.6 mmol) in N,N-dimethyl formamide (40 ml) at 0°C. After hydrogen gas generation ceased (about 40 min), o-nitrobenzyl bromide (1.58 g, 7.30 mmol) was added, and the mixture was stirred for 5 h at 0°C. The reaction was quenched with EtOH and neutralized with 1 N HCl. The mixture was evaporated, and the residue was purified by silica gel chromatography, eluting with 4–6% methanol in dichloromethane to give the product 2a (48) as a yellow foam (0.760 g, 32% yield). 1H NMR (DMSO-d6) δ 12.07 (brs, 1H, disappeared with D2O), 11.65 (s, 1H, disappeared with D2O), 8.23 (s, 1H), 7.99 (d, 1H, J = 10.0 Hz), 7.65 (d, 1H, J = 10.0 Hz), 7.51 (m, 1H, J = 5.0 Hz, 1H), 6.01 (d, 1H, J = 7.0 Hz), 5.41 (d, 1H, J = 5.0 Hz disappeared with D2O), 5.11 (t, 1H, J = 5.5 Hz, disappeared with D2O), 5.02 (d, 1H, J = 14.5 Hz), 4.87 (d, 1H, J = 14.5 Hz), 4.52 (m, 1H), 4.39 (m, 1H), 4.00 (m, 1H), 3.54–3.63 (m, 2H), 2.71 (m, 1H), 1.10 (d, 6H); 13C NMR (DMSO-d6) δ 180.7, 155.5, 149.3, 148.4, 147.7, 138.2, 134.1, 133.8, 129.6, 129.3, 124.9, 120.4, 86.7, 85.1, 82.5, 69.4, 68.7, 61.6, 35.3, 19.3, 19.2.
N2-Isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine (2b)
Compound 2b was prepared from 1 (1.72 g, 4.86 mmol), NaH (292 mg, 95%, 11.6 mmol) and α-methyl-o-nitrobenzyl bromide (1.68 g, 7.3 mmol) as described for 2a. Silica gel chromatography (4–6% methanol in dichloromethane) of the residue yielded 636 mg (26% yield) of 2b as a yellow foam. 1H NMR (DMSO-d6) δ 12.11 (s, 1H, disappeared with D2O), 11.60 (s, 1H, disappeared with D2O), 8.21 (s, 1H), 7.93–7.88 (m, 2H), 7.77 (m, 1H), 7.54 (m, 1H), 5.99 (d, 1H, J = 5.9 Hz), 5.31 (d, 1H, J = 5.0 Hz, disappeared with D2O), 5.02–4.98 (m, 2H, one H disappeared with D2O), 4.33 (t, 1H, J = 5.0 Hz), 4.10 (m, 1H), 3.90 (m, 1H), 3.57 (m, 1H), 3.47 (m, 1H), 2.78 (m, 1H), 1.34 (d, 3H, J = 6.2 Hz), 1.14 (d, 6H, J = 6.8 Hz); High resolution mass spectra calcd for C22H27N6O8 [MH+ ] 503.1885, found 503.1888.
5′-O-(Dimethoxytrityl)-N2-isobutyryl-2′-O-(o-nitrobenzyl) guanosine (3a)
To a solution of 2a (192 mg, 0.393 mmol) in dry pyridine (4.0 ml) was added dimethoxytrityl chloride (DMTrCl) (400 mg, 1.18 mmol). After the mixture was stirred at room temperature overnight under argon, methanol (2 ml) was added to the reaction mixture. The solvent was removed, and the residue was purified by silica gel chromatography, eluting with 2% MeOH in CH2Cl2 with 0.5% Et3N to give compound 3a (206 mg, 66% yield). 1H NMR (CDCl3) δ 12.02 (brs, 1H), 8.61 (brs, 1H), 8.01 (d, 1H, J = 8.0 Hz), 7.87(s, 1H), 7.67–7.18 (m, 12H), 6.80 (m, 4H), 6.01 (d, 1H, J = 3.5 Hz), 5.37 (d, 1H, J = 15.0 Hz), 5.22 (d, 1H, J = 15.0 Hz), 4.62 (t, 1H, J = 4.5 Hz), 4.55 (m, 1H), 4.27 (m, 1H), 3.77 (s, 6H), 3.55 (d, 1H, J = 10.5 Hz), 3.35 (m, 1H), 3.21 (brs, 1H), 2.29 (m, 1H), 1.12 (d, 3H, J = 7.0 Hz), 1.01 (d, 3H, J = 7.0 Hz); 13C NMR (DMSO-d6) δ 178.8, 158.6, 155.5, 147.5, 147.4, 146.9, 144.6, 137.5, 135.8, 135.6, 134.1, 133.8, 130.0, 129.9, 128.6, 128.5, 128.0, 127.9, 127.0, 124.7, 121.9, 113.2, 113.1, 87.0, 86.4, 83.6, 82.3, 69.4, 69.3, 63.1, 55.2, 36.2, 18.7, 18.6; High resolution mass spectra calcd for C42H43N6O10 [MH+] 791.3035, found 791.3041.
5′-O-(Dimethoxytrityl)-N2-isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine (3b)
Compound 3b was prepared from 2b (307 mg, 0.61 mmol) and DMTrCl (621 mg, 1.83 mmol) as described for 3a. Silica gel chromatography (1–2% methanol in dichloromethane containing 0.5% triethylamine) of the residue yielded 345 mg (70% yield) of 3b as a yellow foam. 1H NMR (CDCl3) δ 12.04 (s, 1H), 8.57 (s, 1H), 7.92–7.70 (m, 3H), 7.47–7.19 (m, 10H), 6.82–6.79 (m, 4 H), 6.01 (d, 1H, J = 1.2 Hz), 5.77 (m, 1H), 4.33–4.26 (m, 2H), 3.91 (m, 1H), 3.770 (s, 3H), 3.767 (s, 3H), 3.57–3.45 (m, 2H), 2.72 (d, J = 8.3 Hz, 1H), 2.60 (m, 1H), 1.66 (d, 3H, J = 6.3 Hz), 1.27 (d, 3H, J = 6.9 Hz), 1.21 (d, 3H, J = 6.8 Hz); 13C NMR (CDCl3) δ 178.8, 158.5, 155.3, 147.8, 147.6, 147.0, 144.4, 139.0, 136.6, 135.6, 135.5, 134.7, 130.0, 128.8, 128.0, 127.9, 127.0, 124.6, 121.9, 113.2, 87.6, 86.6, 82.7, 80.4, 72.4, 69.1, 62.5, 55.2, 36.5, 24.1, 19,2, 18.4; High resolution mass spectra calcd for C43H45N6O10 [MH+] 805.3192, found 805.3209.
N6-Benzoyl-5′-O-(dimethoxytrityl)-2′-O-(o-nitrobenzyl)adenosine (6a)
Compound 6a was prepared from 2′-O-(o-nitrobenzyl)-N6-benzoyladenosine (5a) (47) (974 mg, 1.92 mmol) and DMTrCl (1.95 g, 5.76 mmol) according to a literature procedure (47). Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the residue yielded 6a (47) (1.31 g, 84% yield) as a yellow foam. 1H NMR (CDCl3) δ 8.50 (s, 1H), 8.24 (s, 1H), 8.00 (dd, 1H, J = 1.1, 8.1 Hz), 7.84 (m, 4H), 7.56-7.17 (m, 13H), 6.80-6.77 (m, 4H), 6.21 (d, 1H, J = 4.4 Hz), 5.19 (d, 1H, J = 13.8 Hz), 5.10 (d, 1H, J = 13.8 Hz), 4.79 (t, 1H, J = 4.7 Hz), 4.51 (m, 1H), 4.27 (m, 1H), 3.76 (s, 6 H), 3.51 (m, 1H), 3.40 (m, 1H), 2.64 (d, 1H, J = 5.7 Hz).
2′-O-(o-Nitrobenzyl)-N4-phenoxyacetylcytidine (5b)
2′-O-(o-Nitrobenzyl)cytidine (4b) (49) (3.58 g, 9.46 mmol) was suspended in dry pyridine (3 × 100 ml) and dried by co-evaporation under vacuum. The residue was resuspended in dry pyridine (180 ml) under argon and chlorotrimethylsilane (9.0 ml) was added. The mixture was stirred at room temperature for 45 min. At 0°C a solution of phenoxyacetyl chloride (2.00 ml, 14.4 mmol) and 1,2,4-triazole (0.980 g, 14.3 mmol) in pyridine/acetonitrile (120 ml, 1:1) was added. The mixture was stirred overnight at 55°C. The reaction mixture was cooled to room temperature, and the reaction was quenched by addition of H2O (10.0 ml). After stirring for 5 min, concentrated aqueous NH4OH (6.5 ml) was added at 0°C, and the mixture was stirred for 30 min. The solvent was removed, and the residue was purified by silica gel chromatography, eluting with 3–5% MeOH in CH2Cl2 to give 3.44 g (71% yield) of 2′-O-(o-nitrobenzyl)-N4-phenoxyacetylcytidine (5b) as a yellow foam. 1H NMR (DMSO-d6) δ 11.04 (s, 1H), 8.51 (d, 1H, J = 7.5 Hz), 8.08 (dd, 1H, J = 1.3, 8.2 Hz), 7.94 (d, 1H, J = 7.7 Hz), 7.77 (m, 1H), 7.57 (m, 1H), 7.35–7.25 (m, 2H), 7.11 (d, 1H, J = 7.5 Hz), 6.96 (m, 1H), 6.92 (m, 2H), 5.94 (d, 1H, J = 2.4 Hz), 5.34 (d, 1H, J = 6.7 Hz), 5.25 (t, 1H, J = 5.0 Hz), 5.15 (s, 2H), 4.83 (s, 2H), 4.12 (m, 1H), 4.15 (s, 1H), 4.03–3.95 (m, 3H), 3.79 (m, 1H), 3.64 (m, 1H); High resolution mass spectra calcd for C24H25N4O9 [MH+] 513.1622, found 513.1621.
5′-O-(Dimethoxytrityl)-2′-O-(o-nitrobenzyl)-N4-phenoxyacetylcytidine (6b)
Compound 6b was prepared from 5b (1.24 g, 2.42 mmol) and DMTrCl (2.46 g, 7.26 mmol) as described for 3a. Silica gel chromatography (1% methanol in dichloromethane containing 0.5% triethylamine) of the residue yielded 1.67 g (86% yield) of 6b as a yellow foam. 1H NMR (DMSO-d6) δ 11.03 (s, 1H), 8.34 (d, 1H, J = 7.5 Hz), 8.08 (d, 1H, J = 8.2 Hz), 7.98 (d, 1H, J = 7.7 Hz), 7.76 (m, 1H), 7.57 (m, 1H), 7.40–6.85 (m, 19H), 5.94 (s, 1H), 5.40 (d, 1H, J = 7.3 Hz), 5.21 (dd, 2H, J = 15.2, 25.4 Hz), 4.82 (s, 2H), 4.35 (m, 1H), 4.15 (s, 1H), 4.01 (d, 1H, J = 4.8 Hz), 3.70 (s, 3H), 3.69 (s, 3H), 3.42–3.36 (m, 2H); High resolution mass spectra calcd for C45H43N4O11 [MH+] 815.2928, found 815.2930.
5′-O-Dimethoxytrityl-N2-isobutyryl-2′-O-(o-nitrobenzyl)guanosine-3′-H-phosphonate (7a)
To the solution of compound 3a (168 mg, 0.212 mmol) in pyridine (5 ml), diphenyl phosphite (149 mg, 0.626 mmol) was added. After 15 min (TLC analysis), the reaction was quenched by the addition of water/triethylamine (2 ml, 1:1 v/v), and the resulting mixture was stirred for 15 min. The solvent was evaporated, and the residue was partitioned between dichloromethane (50 ml) and 5% aqueous NaHCO3 (20 ml). The organic layer was washed two additional times with 5% aqueous NaHCO3 (20 ml) and subsequently dried over Na2SO4. Following removal of the solvent by evaporation under vacuum, the resulting residue was purified by silica gel chromatography, eluting with 0–10% methanol in dichloromethane containing 5% of triethylamine to give compound 7a (167 mg, 93% yield) as a yellow foam. 31P NMR (CD3CN) δ 4.07; High resolution mass spectra calcd for C42H42N6O12P [M-] 853.2598, found 853.2588.
5′-O-Dimethoxytrityl-N2-isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine-3′-H-phosphonate (7b)
Compound 7b was prepared from 3b (125 mg, 0.16 mmol), and diphenyl phosphite (113 mg, 0.47 mmol) as described for 7a. Silica gel chromatography (0–10% methanol in dichloromethane containing 5% of triethylamine) of the residue yielded 97 mg (72% yield) of 7b as a yellow foam. 31P NMR (CD3CN) δ 1.92; High resolution mass spectra calcd for C43H44N6O12P [M-] 867.2755, found 867.2762.
N6-Benzoyl-5′-O-dimethoxytrityl-2′-O-(o-nitrobenzyl) adenosine-3′-H-phosphonate (7c)
Compound 7c was prepared from 6a (340 mg, 0.42 mmol) and diphenyl phosphite (294 mg, 1.26 mmol) as described for 7a. Silica gel chromatography (0–10% methanol in dichloromethane containing 5% of triethylamine) of the residue yielded 250 mg (68% yield) of 7c as a yellow foam. 31P NMR (CD3CN): δ 2.76; High resolution mass spectra calcd for C45H40N6O11P [M-] 871.2493, found 871.2477.
5′-O-Dimethoxytrityl-2′-O-(o-nitrobenzyl)-N4-phenoxyacetylcytidine-3′-H-phosphonate (7d)
Compound 7d was prepared from 6b (65 mg, 0.080 mmol) and diphenyl phosphite (56 mg, 0.23 mmol) as described for 7a. Silica gel chromatography (0–10% methanol in dichloromethane containing 5% of triethylamine) of the residue yielded 42 mg (60% yield) of 7d as a yellow foam. 31P NMR (CD3CN): δ 2.59; High resolution mass spectra calcd for C45H40N6O11P [M-] 877.2486, found 877.2482.
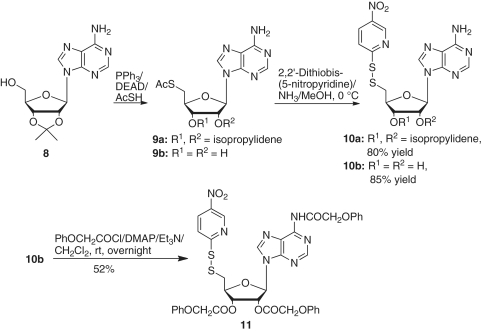
5′-Deoxy-2′,3′-O-isopropylidene-5′-(5-nitropyridinyl-2-disulfanyl)adenosine (10a)
A solution of 5′-acetylthio-5′-deoxy-2′,3′-O-isopropylideneadenosine (9a) (50) (365 mg, 1.0 mmol) in a mixed solvent of methanol (10 ml) and dichloromethane (5 ml) was saturated with ammonia at 0°C for 30 min. 2,2′-Dithiobis(5-nitropyridine) (620 g, 2.0 mmol) was then added, and the mixture was kept at 4°C for 48 h. The solvent was removed, and the residue was isolated by silica gel chromatography, eluting with 3–5% methanol in chloroform to give 10a (383 mg, 80% yield). 1H NMR (CDCl3/TMS) δ 9.21 (d, 1H, J = 2.8 Hz), 8.29 (dd, 1H, J = 2.8, 9.0 Hz), 8.22 (s, 1H), 7.91 (s, 1H), 7.72 (d, 1H, J = 9.0 Hz), 6.56 (brs, 2H), 6.09 (d, 1H, J = 1.6 Hz), 5.63 (dd, 1H, J = 1.6, 6.4 Hz), 5.16 (dd, 1H, J = 2.8, 6.4 Hz), 4.52 (m, 1H), 3.24 (d, 1H, J = 7.2 Hz), 1.59 (s, 3H), 1.40 (s, 3H); 13C NMR (CDCl3) δ 167.9, 155.8, 152.8, 148.6, 144.9, 141.8, 140.4, 131.3, 120.0, 119.0, 114.2, 91.3, 86.4, 84.0, 83.9, 41.1, 26.8, 25.1; High resolution mass spectra calcd for C18H20N7O5S2 [MH+] 478.0967, found 478.0966.
5′-Deoxy-5′-(5-nitropyridinyl-2-disulfanyl)adenosine (10b)
A solution of 5′-acetylthio-5′-deoxyadenosine (9b) (50) (0.96 g, 2.95 mmol) in methanol (3.0 ml) was saturated with ammonia at 0°C for 1 h and then kept at 0°C for additional 1 h. Thin layer chromatography showed that the reaction was complete. 2,2′-Dithiobis(5-nitropyridine) (1.66 g, 5.35 mmol) was then added, and the mixture was stirred at room temperature for 2 h. The solvent was removed and the residue was isolated by silica gel chromatography, eluting with 5% methanol in chloroform to recover the excess 2,2′-dithiobis(5-nitropyridine), followed by elution with 10% methanol in chloroform to give 10b (1.091 g, 85% yield). 1H NMR (DMSO-d6) δ 9.19 (d, 1H, J = 2.4 Hz), 8.49 (dd, 1H, J = 2.4, 8.9 Hz), 8.35 (s, 1H), 8.10 (s, 1H), 8.00 (d, 1H, J = 8.9 Hz), 7.29 (brs, 2H), 5.87 (d, 1H, J = 5.8 Hz), 5.54 (d, 1H, J = 6.1 Hz), 5.42 (d, 1H, J = 4.8 Hz), 4.83 (m, 1H), 4.19 (m, 1H), 4.09 (m, 1H); 13C NMR (DMSO-d6) δ 167.4, 156.1, 152.6, 149.3, 144.7, 142.1, 140.2, 132.4, 119.5, 119.3, 87.7, 82.3, 72.7, 72.4, 41.5; High resolution mass spectra calcd for C15H16N7O5S2 [MH+] 438.0654, found 438.0660.
5′-Deoxy-5′-(5-nitropyridinyl-2-disulfanyl)-2′,3′-O, N6-triphenoxyacetyladenosine (11)
To a solution of 10b (190 mg, 0.434 mmol) and 4-dimethylaminopyridine (213 mg, 1.74 mmol) in dichloromethane (10 ml), phenoxyacetyl chloride (0.20 ml, 1.43 mmol) was added. The reaction mixture was stirred at room temperature overnight. The solvent was removed, and the residue was isolated by silica gel chromatography, eluting with 2% methanol in chloroform to give 11 (190 mg, 52% yield). 1H NMR (CDCl3/TMS) δ 9.70 (s, 1H), 9.19 (d, 1H, J = 2.6 Hz), 8.70 (s, 1H), 8.27 (dd, 1H, J = 2.6, 8.9 Hz), 8.14 (s, 1H), 7.73 (d, 1H, J = 8.9 Hz), 7.35–7.15 (m, 6H), 7.05–6.85 (m, 7H), 6.78 (m, 2H), 6.38 (t, 1H, J = 5.5 Hz), 6.0 (d, 1H, J = 5.5 Hz), 5.18 (dd, 1H, J = 4.2, 5.2 Hz), 4.94 (brs, 2H), 4.69–4.45 (m, 5H), 3.42 (dd, 1H, J = 7.6, 14.2 Hz), 3.34 (dd, 1H, J = 5.0, 14.2 Hz); 13C NMR (CDCl3) δ 168.0, 167.8, 167.2, 157.3, 157.2, 157.0, 152.5, 151.0, 148.6, 145.0, 142.8, 142.0, 131.4, 129.7, 129.6, 129.5, 129.4, 123.1, 122.2, 122.0, 119.4, 114.7, 114.4, 114.3, 86.6, 80.8, 73.1, 72.6, 68.1, 64.7, 64.6, 40.9; High resolution mass spectra calcd for C39H34N7O11S2 [MH+] 840.1747, found 840.1749.
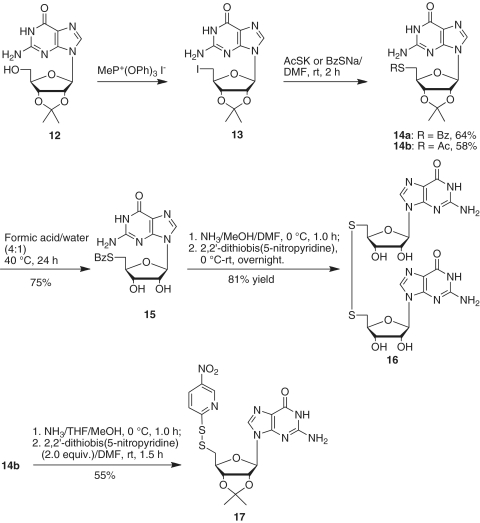
5′-Benzoylthio-5′-deoxy-2′,3′-O-isopropylideneguanosine (14a)
To a solution of 5′-deoxy-5′-iodo-2′,3′-O-isopropylideneguanosine (13) (51) (0.812 g, 1.87 mmol) in anhydrous N,N-dimethyl formamide (15 ml) was added sodium thiobenzoate (0.901 g, 5.62 mmol). The mixture was stirred at room temperature for 2 h. Thin layer chromatography showed that the reaction was complete. The solvent was removed, and the residue was partitioned between dichloromethane and saturated aqueous sodium bicarbonate. The aqueous layer was extracted with dichloromethane, and the organic layers were combined, washed with brine and dried over anhydrous magnesium sulfate. The solvent was removed, and the residue was purified by silica gel chromatography, eluting with 5% methanol in chloroform to give 14a (0.531 g, 64% yield). 1H NMR (DMSO-d6) δ 10.81 (brs, 1H), 7.88 (m, 3H), 7.66 (t, 1H, J = 7.4 Hz), 7.52 (t, 2H, J = 7.7 Hz), 6.60 (brs, 2H), 5.98 (d, 1H, J = 1.9 Hz), 5.29 (dd, 1H, J = 1.9, 6.3 Hz), 5.08 (dd, 1H, J = 3.3, 6.2 Hz), 4.16 (m, 1H), 3.42 (dd, 1H, J = 6.8, 13.7 Hz), 3.30 (dd, 1H, J = 7.0, 13.7 Hz), 1.45 (s, 3H), 1.28 (s, 3H); 13C NMR (DMSO-d6) δ 191.0, 157.3, 154.0, 150.9, 136.9, 136.3, 134.5, 129.5, 127.2, 117.2, 113.8, 88.9, 85.6, 84.1, 83.4, 31.2, 27.2, 25.5; High resolution mass spectra calcd for C20H22N5O5S [MH+] 444.1342, found 444.1343.
5′-Acetylthio-5′-deoxy-2′,3′-O-isopropylideneguanosine (14b)
According to the procedure for the synthesis of 14a, 5′-deoxy-5′-iodo-2′,3′-O-isopropylideneguanosine (13) (51) (1.545 g, 3.57 mmol) was reacted with potassium thioacetate (1.22 g, 10.7 mmol) in anhydrous N,N-dimethyl formamide (50 ml) at room temperature for 2 h. After solvent was removed, the residue was isolated by silica gel chromatography, eluting with 5% methanol in chloroform to give 14b (0.796 g, 58% yield). 1H NMR (DMSO-d6) δ 10.80 (brs, 1H), 7.85 (s, 1H), 6.57 (brs, 2H), 5.93 (brs, 1H), 5.25 (m, 1H), 4.96 (m, 1H), 4.04 (m, 1H), 3.17 (m, 1H), 3.07 (m, 1H), 2.29 (s, 3H), 1.44 (s, 3H), 1.26 (s, 3H); 13C NMR (DMSO-d6) δ 195.5, 157.3, 154.0, 150.9, 136.9, 117.1, 113.8, 88.8, 85.5, 84.0, 83.3, 31.3, 30.8, 27.2, 25.5; High resolution mass spectra calcd for C15H20N5O5S [MH+] 382.1185, found 382.1183.
5′-Benzoylthio-5′-deoxyguanosine (15)
To a flask containing 14a (0.628 g, 1.42 mmol), formic acid (8.0 ml) and water (2.0 ml) were added. After the mixture was stirred at 40°C for 24 h, the solvent was evaporated under reduced pressure, and the traces of formic acid were removed by coevaporating five times with absolute ethanol. The obtained powder was purified by silica gel column chromatography, eluting with 15–30% methanol in chloroform to give 15 (0.433 g, 75% yield). 1H NMR (DMSO-d6) δ 10.90 (brs, 1H), 7.91 (m, 3H), 7.68 (t, 1H, J = 7.2 Hz), 7.54 (m, 2H, J = 7.5 Hz), 6.58 (brs, 2H), 5.69 (d, 1H, J = 6.0 Hz), 5.57 (brs, 1H), 5.40 (brs, 1H), 4.62 (m, 1H), 4.08 (m, 1H), 3.97 (m, 1H), 3.51 (dd, 1H, J = 5.5, 13.8 Hz), 3.38 (m, 1H); 13C NMR (DMSO-d6) δ 190.7, 157.0, 153.9, 151.5, 136.2, 135.9, 134.1, 129.2, 127.0, 116.8, 86.6, 82.7, 72.7, 31.3; High resolution mass spectra calcd for C17H18N5O5S [MH+] 404.1029, found 404.1039.
5′-Deoxy-5′-thioguanosine disulfide (16)
A solution of 5′-benzoylthio-5′-deoxyguanosine (15) in methanol (2.5 ml) and N,N-dimethyl formamide (2.5 ml) was saturated with ammonia and stirred at 0°C for 30 min. The mixture was then kept at 0°C for 1 h. To the resulting solution, 2,2′-dithiobis(5-nitropyridine) (77 mg, 0.25 mmol) was added, and the mixture was stirred at room temperature overnight. The solvent was removed by vacuum, and the residue was precipitated in chloroform. The solid was filtered off and rinsed with chloroform and methanol to give a yellow solid 16 (30 mg, 81% yield). 1H NMR (DMSO-d6) δ 7.89 (s, 1H), 6.50 (brs, 2H), 5.69 (d, 1H, J = 6.0 Hz), 5.51 (brs, 1H), 5.33 (brs, 1H), 4.59 (m, 1H), 4.08 (m, 1H), 4.11–4.04 (m, 2H), 3.11 (dd, 1H, J = 5.7, 14.0 Hz), 3.02 (dd, 1H, J = 7.1, 14.0 Hz); 13C NMR (DMSO-d6) δ 156.9, 153.7, 151.5, 136.0, 116.9, 86.5, 82.6, 72.6, 72.5, 40.1; High resolution mass spectra calcd for C20H25N10O8S2 [MH+] 597.1298, found 597.1302.
5′-Deoxy-2′,3′-O-isopropylidene-5′-(5-nitropyridinyl-2-disulfanyl)guanosine (17)
A solution of 14b (100 mg, 0.26 mmol) in tetrahydrofuran (5.0 ml) and CH3OH (5.0 ml) was saturated with ammonia at 0°C for 30 min, and the mixture was kept at 0°C for additional 30 min. After the solvent was removed, the residue was dried over vacuum for 30 min. The residue was then dissolved in N,N-dimethyl formamide (5.0 ml). To the resulting solution, 2,2′-dithiobis(5-nitropyridine) (161 mg, 0.52 mmol) was added. The reaction mixture was stirred at room temperature for 1.5 h. The solvent was removed, and the residue was isolated by silica gel chromatography, eluting with 5% methanol in chloroform to give 17 (71 mg, 55% yield). 1H NMR (DMSO-d6) δ 10.95 (brs, 1H), 9.15 (d, 1H, J = 2.8 Hz), 8.48 (dd, 1H, J = 2.8, 9.2 Hz), 8.23 (s, 1H), 7.88 (s, 1H), 6.77 (brs, 2H), 6.05 (s, 1H), 5.33 (d, 1H, J = 6.0 Hz), 5.15 (m, 1H), 4.32 (m, 1H), 3.32 (dd, 1H, J = 6.0, 14.0 Hz), 3.22 (dd, 1H, J = 8.4, 14.0 Hz), 1.46 (s, 3H), 1.29 (s, 3H); 13C NMR (DMSO-d6) δ 167.0, 155.7, 154.1, 149.7, 144.8, 142.2, 136.8, 132.4, 119.5, 114.6, 113.1, 89.8, 86.3, 83.8, 83.4, 40.5, 26.8, 25.2; High resolution mass spectra calcd for C18H20N7O6S2 [MH+] 494.0917, found 494.0919.
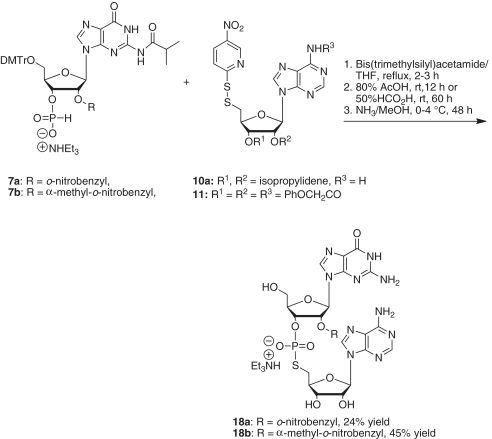
G-[2′-O-(o-nitrobenzyl)]-ps-A (18a)
Under argon, N,O-bis-(trimethylsilyl)acetamide (0.36 ml, 1.5 mmol) was added to a solution of 5′-O-DMTr-N2-isobutyryl-2′-O-(o-nitrobenzyl)guanosine 3′-H-phosphonate (7a) (63 mg, 0.066 mmol) and 5′-deoxy-5′-(5-nitropyridinyl-2-disulfanyl)-2′,3′-O,N6-triphenoxyacetyladenosine (11) (86 mg, 0.10 mmol) in anhydrous tetrahydrofuran (10 ml). The mixture was stirred under reflux for 3 h. The solvent was removed, and the residue was then treated with 80% acetic acid (10 ml) at room temperature for 12 h. The excess acetic acid was removed, and the residue was dissolved in methanol (10 ml). The resulting solution was saturated with ammonia at 0°C for 30 min and kept at 0–4°C for 48 h. The solvent was removed, and the residue was dissolved in water (10 ml). The resulting aqueous solution was washed with chloroform (3 × 10 ml), and the aqueous phase was evaporated. The remaining residue was purified by silica gel chromatography, eluting with acetonitrile/water/triethylamine (85:15:1) to give the desired dinucleotide G-[2′-O-(o-nitrobenzyl)]-ps-A (18a) (68 mg, 24% yield). 1H NMR (D2O) δ 8.23 (s, 1H), 7.85 (s, 1H), 7.59 (d, 1H, J = 8.0 Hz), 7.53 (s, 1H), 7.30 (m, 1H), 7.21 (m, 1H), 7.00 (d, 1H, J = 7.4 Hz), 5.86 (d, 1H, J = 5.3 Hz), 5.53 (d, 1H, J = 8.1 Hz); 31P NMR (CDCl3) δ 23.1; High resolution mass spectra calcd for C27H29N11O12PS [M−] 762.1456, found 762.1448.
G[2′-O-(α-methyl-o-nitrobenzyl)]-ps-A (18b)
A mixture of 5′-O-DMTr-N2-isobutyryl-2′-O-(α-methyl-o-nitrobenzyl)guanosine 3′-H-phosphonate (7b) (43 mg, 0.044 mmol), 5′-deoxy-2′,3′-O-isopropylidene-5′-(5-nitropyridinyl-2-disulfanyl)adenosine (10a) (32 mg, 0.067 mmol) and N,O-bis-(trimethylsilyl)acetamide (0.27 ml, 1.0 mmol) in tetrahydrofuran (5.0 ml) was heated to reflux for 2 h. The solvent was removed, and the residue was treated with 50% formic acid (5.0 ml) at room temperature for 60 h. The excess acid was removed under vacuum by rotary evaporation, and the residue was dissolved in methanol (10 ml). The solution was saturated with ammonia at 0°C for 30 min and kept at 0–4°C for 48 h. The solvent was evaporated, and the residue was partitioned between water (10 ml) and chloroform (10 ml). The aqueous layer was separated and washed with chloroform (2 × 10 ml). The aqueous phase was evaporated. The residue was purified by silica gel chromatography, eluting with acetonitrile/water/triethylamine (90:10:1 v/v/v) to give the desired dinucleotide G-[2′-O-(α-methyl-o-nitrobenzyl)]-ps-A (18b) (17.5 mg, 45% yield). 1H NMR (D2O) δ 8.09 (brs, 1H), 7.84 (brs, 1H), 7.64 (s, 1H), 7.50 (m, 1H), 7.44 (m, 1H), 7.28 (m, 1H), 7.11 (m, 1H), 5.76 (brs, 1H), 5.66 (brs, 1H); 13P NMR (CDCl3) δ 23.0; High resolution mass spectra calcd for C28H31N11O12PS [M−] 776.1612, found 776.1598.
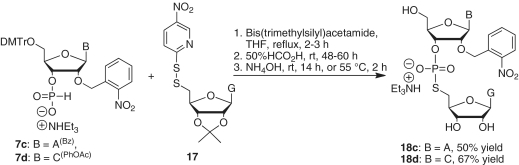
A[2′-O-(o-nitrobenzyl)]-ps-G (18c)
Under argon, N,O-bis-(trimethylsilyl)acetamide (0.17 ml, 0.69 mmol) was added to a solution of N6-benzoyl-5′-O-DMTr-2′-O-(o-nitrobenzyl)adenosine 3′-H-phosphonate (7c) (22 mg, 0.023 mmol) and 5′-deoxy-2′,3′-O-isopropylidene-5′-(5-nitropyridinyl-2-disulfanyl)guanosine (17) (14 mg, 0.028 mmol) in anhydrous tetrahydrofuran (5.0 ml). The mixture was stirred under reflux for 3 h. The solvent was removed, and the residue was treated with 50% formic acid (5.0 ml) at room temperature for 60 h. After evaporation of the mixture under vacuum, the remaining residue was treated with a mixture of NH4OH/MeOH (3:1, v/v) (3.0 ml) at 55°C in a sealed vial for 2 h. After cooling the mixture, the solvent was removed, and the residue was dissolved in water (10 ml) and washed with chloroform (3 × 10 ml). The aqueous phase was evaporated, and the residue was purified by silica gel chromatography, eluting with acetonitrile/water/triethylamine (84:15:1 v/v/v) to give the desired dinucleotide A-[2′-O-(o-nitrobenzyl)]-ps-G (18c) (10 mg, 50% yield). 1H NMR (D2O) δ 8.84 (s, 1H), 8.203 (s, 1H), 8.198 (s, 1H), 7.58 (d, 1H, J = 8.2 Hz), 7.34-7.20 (m, 3H), 5.89 (d, 1H, J = 7.6 Hz), 5.82 (d, 1H, J = 4.3 Hz); 31P NMR (CDCl3) d 22.7; High resolution mass spectra calcd for C27H29N11O12PS [M-] 762.1456, found 762.1455.
C[2′-O-(o-nitrobenzyl)]-ps-G (18d)
According to the procedure described above for the preparation of A[2′-O-(o-nitrobenzyl)]-ps-G (18c), C[2′-O-(o-nitrobenzyl)]-ps-G (18d) was prepared by the reaction of 5′-O-DMTr-2′-O-(o-nitrobenzyl)-N4-phenoxyacetylcytidine 3′-H-phosphonate (7d) (40 mg, 0.041 mmol) with 5′-deoxy-2′,3′-O-isopropylidene-5′-(5-nitropyridinyl-2-disulfanyl)guanosine (17) (24 mg, 0.049 mmol) in tetrahydrofuran (10 ml) in the presence of N,O-bis-(trimethylsilyl)acetamide (0.30 ml, 1.2 mmol). After evaporation of the reaction mixture under vacuum, the residue was treated with a mixture of NH4OH/MeOH (3.0 ml, 3:1 v/v) at room temperature for 14 h. The solvent was removed, and the residue was dissolved in water (10 ml) and washed with chloroform (3 × 10 ml). The aqueous phase was evaporated, and the crude product was purified by silica gel chromatography, eluting with acetonitrile/water/triethylamine (90:10:1 v/v/v) to give the desired dinucleotide C-[2′-O-(o-nitrobenzyl)]-ps-G (18d) (23 mg, 67% yield). 1H NMR (D2O) δ 8.19 (s, 1H), 7.84 (m, 1H), 7.75 (d, 1H, J = 7.9 Hz), 7.47 (m, 1H), 7.38 (m, 2H), 5.99 (d, 1H, J = 7.9 Hz), 5.83 (d, 1H, J = 5.4 Hz), 5.65 (d, 1H, J = 5.1 Hz); 31P NMR (CDCl3) d 22.7; High resolution mass spectra calcd for C26H29N9O13PS [M-] 738.1343, found 738.1342.
CONSTRUCTION OF THE FULL-LENGTH VS RIBOZYME SUBSTRATE
5′-phosphorylation
All reactions involving 2′-O-o-NBn protected species were performed in the dark to minimize premature photodeprotection. 5′-phosphorylation was accomplished by incubating 160 nmol of dinucleotide (G2′-O-o-NBnA5′-X, X = O or S) with 200 U of T4 PNK and 10 mM ATP in 1 × kinase buffer (50 mM Tris–HCl, pH 7.5, 10 mM MgCl2, 5 mM dithiothreitol) in a 200-µl reaction volume for 23–27 h at 37°C. Following phenol/chloroform/isoamyl alcohol (PCI) extraction, the 5′-phosphorylated dinucleotide was purified by passing the reaction mixture over a SepPak cartridge (Waters) according to the manufacturer’s instructions and evaporating to dryness on a speedvac. Phosphorylation was confirmed via reversed-phase high pressure liquid chromatography (C18 column, 90% A/10% B → 70% A/30% B over 30 min, solvent A = 0.1 M TEAA pH 7, solvent B = acetonitrile).
RNA ligase-mediated ligation
The 9-nt RNA 5′-GCG GAU UGC-3′ was purchased from Dharmacon and deprotected according to the manufacturer’s instructions. In a 30-µl reaction volume, 10 nmol each of 9-nt RNA and dinucleotide were combined with 20 U of T4 RNA ligase 1 (New England Biolabs) and 10% DMSO in 1× T4 RNA ligase 1 buffer (50 mM Tris–HCl, pH 7.8, 10 mM MgCl2, 1 mM ATP, 10 mM dithiothreitol). The reaction was incubated at 37°C for 1.5 h, extracted with PCI, precipitated with ethanol in the presence of glycogen and fractionated via 20% denaturing polyacrylamide gel electrophoresis (dPAGE). The band corresponding to the ligated 11-nt RNA was visualized by UV shadowing (as briefly as possible to minimize photodeprotection), excised, eluted into TE at pH 7.5 at 4°C overnight, extracted with PCI, precipitated with ethanol in the presence of glycogen and stored as a pellet at −20°C.
DNA ligase-mediated ligation
The 18-nt RNA 5′-pAGG GCG UCG UCG CCC CGA-3′ was purchased from Dharmacon and deprotected according to the manufacturer’s instructions. The 29-nt DNA splint 5′-TCG GGG CGA CGA CGC CCT TCG CAA TCC GC-3′ was purchased from IDT. In a 60-µl volume, 2 nmol each of splint and the 11- and 18-nt RNAs were combined with TE at pH 7.5. The mixture was heated at 90°C for 3 min and incubated at 25°C for 30 min. Ligation was initiated by adding 20 µl of 10× DNA ligase buffer [1× DNA ligase buffer: 40 mM Tris–HCl, pH 7.5, 10 mM MgCl2, 10 mM dithiothreitol, 1 mM ATP, 0.05 mg/ml bovine serum albumin], 2 nmol of T4 DNA ligase (prepared in-house) and water up to a final reaction volume of 200 µl. Following a 4-h incubation at 37°C, the ligation reaction was extracted with PCI, precipitated with ethanol in the presence of glycogen and fractionated on 20% dPAGE as described for the 11-nt ligated RNA. The band corresponding to the full-length, 29-nt VS ribozyme substrate was excised and processed in the manner described above for the 11-nt ligated RNA.
5′-LABELING, PHOTODEPROTECTION AND CHARACTERIZATION
Approximately 20 pmol VS substrate were 5′ labeled with 32P by a 30-min incubation at 37°C with an equimolar amount of radioactively labeled γ-32P ATP in the presence of 1× kinase buffer and 10 U of T4 PNK in a 10-µl reaction volume. The radiolabeled VS substrate was purified via 20% dPAGE and eluted into TE pH 7.5 as described above. The purified 5′-labeled VS substrate was resuspended in water to an activity of ∼105 cpm/µl and stored at −20°C.
To remove the 2′-O-o-NBn protecting group, 1 µl labeled RNA was combined with 80 mM Na-MOPS pH 6 in a 10-µl volume in a 0.6 ml centrifuge tube. The mixture at the bottom of the tube was suspended at the focal point of a 100-W UV lamp (365 nm) and irradiated for 5 min. Alkaline hydrolysis reactions contained 1 µl of radiolabeled RNA (pre- or post-photodeprotection) and 9 µl of 50 mM NaHCO3. Following a 15-min incubation at 90°C, 10 µl of stop solution were added (8 M urea, 50 mM ethylenediaminetetraacetic acid, 0.02% bromphenol blue/xylene cyanol) and the reaction mixture was fractionated on 20% dPAGE. RNase T1 reactions contained 1 µl of radiolabeled RNA and 1 U of RNase T1 (Roche) in TE (pH 7.5) in a 10-µl reaction volume. Following a 4-min incubation at 37°C, 10 µl of stop solution were added, and the reaction mixture was fractionated on 20% dPAGE.
For silver nitrate cleavage reactions, radiolabeled RNA (∼6000 cpm) was combined with 2 mM silver nitrate in a 20 µl reaction volume and incubated in the dark at room temperature for 30–90 min. Subsequently, 0.6 µl of 100 mM dithiothreitol was added (3 mM dithiothreitol final concentration) and the mixture was centrifuged at 14 000 rpm for 3 min. To 10 µl of the resulting supernatant were added 8 µl of stop solution, and approximately half of this mixture was fractioned on 20% dPAGE.
SYNTHESIS OF HDV RNA SUBSTRATE 27 FROM DINUCLEOTIDE 18d
RNA ligase-mediated ligation to synthesize 5′-CUC UUC2′-X G5′-Y (25)
Dinucleotide (80 nmol; either wild-type, C2′-O-o-NBnG5′-O or 5′-S, C2′-O-o-NBnG5′-S, 18d) was phosphorylated by incubation with 50 U of T4 polynucleotide kinase (PNK) and 10 mM ATP in 1× kinase buffer (70 mM Tris–HCl, pH 7.6, 10 mM MgCl2, 5 mM dithiothreitol) in a 50 μl reaction volume at 37°C overnight. The reaction mixture was worked up by PCI and ether extraction of the aqueous layers. The phosphorylated dinucleotide (24) was purified on a C18 reversed-phase high pressure liquid chromatography column (Vydac 201SP54) (0–30% acetonitrile/100–70% 0.1 M triethylammonium acetate, pH 7.0, over 30 min) and evaporated to dryness before further use. The 5-nt RNA 5′-CUCUU-3′ (40 nmol) and phosphorylated dinucleotide 24 (pC2′-XG5′-Y) (20 nmol) were incubated together with 200 U of T4 RNA ligase (NEB), 10 µl DMSO, and 1× T4 RNA ligase buffer (50 mM Tris–HCl, pH 7.78, 10 mM MgCl2, 10 mM dithiothreitol, 1 mM ATP) in a 100 μl reaction volume at 37°C for 6 h. The reaction mixture was worked up by PCI and ether extraction of the aqueous layers. The ligated product was purified by high pressure liquid chromatography on a C18 reversed-phase column (Vydac 201SP54) (6% acetonitrile/94% 0.1 M triethylammonium acetate, pH 7.0, for 5 min; then 6–16% acetonitrile/94–84% 0.1 M triethylammonium acetate, pH 7.0, over 30 min) and evaporated to dryness before further use.
DNA ligase-mediated ligation to synthesize 5′-pCUC UUC2′-X G5′-YGG UCG GC-3′ (27)
Prior to the ligation step, 5′-CUC UUC2′-X G5′-Y-3′ (20 pmol), synthesized as above, was radioactively 5′-32P labeled by incubation with 3 U of T4 PNK and 25 pmol of γ-32P ATP (Perkin-Elmer) for 30 min at 37°C. The reaction mixture was then extracted with PCI and precipitated with ethanol in the presence of glycogen. In the hybridization step prior to ligation, 5′-32P-CUC UUC2′-X G5′-Y-3′ (approximately 20 pmol), unlabeled 5′ phosphorylated donor oligonucleotide 5′-pGGU CGG C-3′ (500 pmol), and the 14-nt DNA splint 5′-GCC GAC CCG AAG AG-3′ (500 pmol) were denatured in the presence of 1 mM Tris–HCl at pH 7.5/0.1 mM ethylenediaminetetraacetic acid, pH 8.0, in a 4.5 μl reaction volume at 90°C for 2 min and then chilled on ice. NaCl was added to give a final concentration of 100 mM (reaction volume 5 μl), and the sample was allowed to hybridize at 4°C for 2–4 h. Ligation was initiated by adding DNA ligase buffer (1×: 1 mM MgCl2, 66 mM Tris–HCl, pH 7.5, 10 mM dithiothreitol, 66 μM ATP), T4 DNA ligase (170 pmol) and RNase inhibitor (Ambion Inc.; 6 U) in a 10 μl final reaction volume, and incubating at 15–20°C overnight. The DNA splint was removed by treatment with RQ1 RNase-free DNase (Promega, 10 U) for 3 h at 37°C. The reaction mixture was then PCI extracted and ethanol precipitated in the presence of glycogen, and the ligation product was purified by gel electrophoresis on a 20% denaturing polyacrylamide gel.
Analysis of HDV RNA substrates
Alkaline hydrolysis reactions contained 5′ radioactively 5′-32P labeled RNA (∼2000 cpm) and 10 mM NaHCO3, pH 9, in a 10-µl reaction volume. Samples were heated at 90°C for 15 min and chilled on ice before adding 8 µl of stop solution (90% formamide, 10 mM ethylenediaminetetraacetic acid, pH 8, 0.01% bromophenol blue/xylene cyanol). For RNase T1 sequencing ladders, radioactively 5′-32P labeled RNA (2000 cpm) was combined with 5 M urea, 20 mM sodium citrate, pH 5, and 1 U of RNase T1 (Roche) in a final reaction volume of 10 µl. After a 15-min incubation at 37°C, 8 µl of stop solution was added. UV deprotection was accomplished by placing 4 µl of radioactively 5′-32P labeled RNA into the bottom of a 0.6-ml tube and exposing the sample to UV light (UVP; 365 nm, 100 watts) for 4 min, as described previously (45). Half of each reaction mixture was loaded on a denaturing 20–25% polyacrylamide/7M urea gel to separate cleaved products from uncleaved substrate, and bands were visualized on a PhosphorImager (GE Healthcare) with ImageQuant software (Molecular Dynamics).
RESULTS AND DISCUSSION
Before embarking on the semisynthetic approach described here, we attempted to prepare RNA oligonucleotides containing G-[2′-O-(o-nitrobenzyl)]-ps-A linkages by solid phase synthesis. We prepared suitably protected phosphoramidite derivatives of 5′-mercaptoadenosine and 2′-O-(o-nitrobenzyl)]-guanosine. Solid phase synthesis using these components gave none of the desired oligonucleotide, however (Scheme S1). Multiple attempts at in situ generation of a 5′-thiophosphoramidite on the solid support followed by coupling to the 3′-OH of a suitably protected 2′-O-(o-nitrobenzyl)]-guanosine derivative (Schemes S2–S4) also yielded no desired product. We believe that three factors adversely affect the solid-phase synthesis: (i) incomplete detritylation by AgNO3 (mass spectra detected a significant amount of 5′-TrS oligonucleotide); (ii) inefficient coupling of the 5′-SH to the 3′-ribonucleoside phosphoramidites (5′-HS oligonucleotide and its disulfide were isolated and confirmed by MS); and (iii) possible degradation at the 5′-S phosphorothiolate ester linkage during postsynthetic deprotection steps [from both base and fluoride treatment (52)].
Synthesis of the modified RNA dinucleotides Base1-[2′-O-(o-nitrobenzyl)]-ps-Base2
In 1995, Liu and Reese reported the synthesis of the RNA dinucleotide UpsU containing a 5′-phosphorothiolate linkage via the reaction of 2′-O-THP-5′-O-(9-phenylxanthen-9-yl)uridine 3′-H-phosphonate with 2′,3′-O-diacetyl-5′-deoxy-5′-(2-nitrophenyl-1-disulfanyl)uridine in the presence of chlorotrimethylsilane and triethylamine (41). The reaction intermediate was first treated with ammonia in methanol (1:1) and then with 2% acetic acid in water to give UpsU (41). This dinucleotide was found to be much more susceptible to cleavage than UpU under neutral and mildly basic conditions (at pH 7 and 30°C, t1/2 = 13 h; at pH 8 and 30°C, t1/2 = 2.2 h) (41). The UpsU dinucleotide was also synthesized by Thomson et al. (42) via the reaction of 5′-iodo-5′-deoxyuridine with 2′-O-(tert-butyldimethylsilyl)uridine 3′-phosphorothioate. In 1996, Weinstein et al. (20) reported the synthesis of the 3′-phosphorothiolate-linked RNA dinucleotide inosine-spU (IspU) from 9-(3-deoxy-3-iodo-β-D-xylofuranosyl)hypoxanthine, with installation of the phosphorothiolate via an Arbusov reaction and protection of the ribose 2′-hydroxyl group with a silyl ether. Weinstein et al. (20) also reported that base-catalyzed cleavage of IspU was accelerated ∼2000-fold relative to that of IpU. Since 5′- and 3′-phosphorothiolate-linked dinucleotides are more susceptible to cleavage than their phosphate-linked counterparts, we chose to synthesize 2′-protected RNA dinucleotides of the form Base1-[2′-O-(o-nitrobenzyl)]-ps-Base2 via an Arbusov reaction. The o-nitrobenzyl group, or groups containing this moiety, prevent premature hydrolysis of the 5′-phosphorothiolate linkage until they are removed by irradiation with UV light (>320 nm wavelength). The removal of the photolabile groups involves abstraction of a hydrogen from the α-methylene carbon by the UV-excited nitro group, followed by rearrangement to o-nitrosobenzaldehyde and the deprotected alcohol (53–56) (Supplementary Figure S2). We then used the novel dinucleotides as building blocks for the construction of full-length RNA oligonucleotides containing 5′-phosphorothiolate linkages.
Synthesis of 2′-O-(o-nitrobenzyl)nucleoside 3′-H-phosphonates
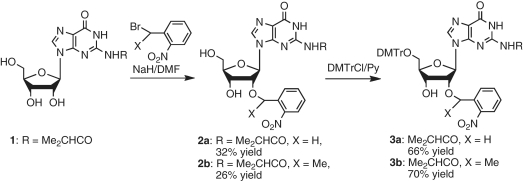
The VS, anti-genomic HDV and glmS ribozymes catalyze the cleavage of substrates containing GpA, CpG or ApG linkages, respectively. To prepare the corresponding 5′-phosphorothiolate-linked dinucleotides, we synthesized 5′-DMTr-2′-O-(o-nitrobenzyl)guanosine (3a) and 5′-DMTr-2′-O-(α-methyl-o-nitrobenzyl)guanosine (3b) from N2-isobutyrylguanosine (1) in two steps via 2′-O-(o-nitrobenzyl) and 5′-O-(DMTr) protection (Scheme 1). We prepared compound 3b using α-methyl-o-nitrobenzyl as a protecting group instead of o-nitrobenzyl because α-methyl-o-nitrobenzyl ether reportedly is more photolabile than the o-nitrobenzyl ether (57,58).
Scheme 1.
Synthesis of 5′-O-DMTr-2′-O-(o-nitrobenzyl) guanosines.
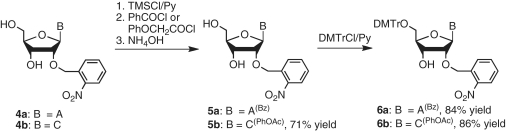
The corresponding 5′-DMTr-2′-O-(o-nitrobenzyl)adenosine derivative (6a) (47) and 5′-DMTr-2′-O-(o-nitrobenzyl)cytidine derivative (6b) were prepared from 2′-O-(o-nitrobenzyl)adenosine (4a) (47) and 2′-O-(o-nitrobenzyl)cytidine (4b) (49) in two steps via nucleobase acylation and dimethoxytritylation of the 5′-hydroxyl group (Scheme 2). The 2′-O-(o-nitrobenzyl) nucleoside derivatives 3a, 3b, 6a and 6b were then converted into the corresponding 3′-H-phosphonate derivatives 7a–d in good yields (Scheme 3).
Scheme 2.
Synthesis of 5′-O-DMTr-2′-O-(o-nitrobenzyl) adenosine and cytidine.
Scheme 3.
Synthesis of 2′-O-(o-nitrobenzyl)nucleoside 3′-H-phosphonates.
Synthesis of 5′-deoxy-5′-(5-nitropyridinyl-2-disulfanyl) nucleosides
The 5′-S-acetyl-5′-thioadenosine derivatives 9a and 9b were prepared from 2′,3′-isopropylideneadenosine (8) according to Pignot’s procedure (50). Reactions of 9a or 9b with 2,2′-dithiobis(5-nitropyridine) in an ammonia-saturated methanol solution at 0°C yielded the corresponding 5′-deoxy-5′-(5-nitropyridinyl-2-disulfanyl)adenosine derivatives (10a and b) in 80 and 85% yields, respectively. The unprotected 5′-disulfide adenosine derivative 10b was converted to the corresponding N6,2′,3′-O-triphenoxyacetyladenosine derivative 11 in 52% yield (Scheme 4).
Scheme 4.
Synthesis of 5′-deoxy-5′-(5-nitropyridinyl-2-disulfanyl) adenosine derivative.
Attempts to use Pignot’s procedure to prepare the 5′-S-acetyl-5′-thioguanosine derivative (14b) were not successful. The reactions of 2′,3′-isopropylideneguanosine (12) with AcSH in the presence of diethyl azodicarboxylate and triphenylphosphorane resulted in either a complex product mixture or the recovery of the starting material. Instead we prepared 14a and 14b from 5′-deoxy-5′-iodo-2′,3′-O-isopropylideneguanosine (51) (13) by reaction with potassium thioacetate or sodium thiobenzoate in N,N-dimethyl formamide at room temperature for 2 h (Scheme 5). Removal of the isopropylidene group from 14a using 80% formic acid gave 5′-S-benzoyl-5′-thioguanosine (15) in 75% yield. Attempts to convert 15 to the corresponding nitropyridinyl disulfide derivative using the same conditions as for the coversion of adenosine derivative 9b into 10b gave predominantly the dimer 16 in 81% yield. Only a small amount (∼10%) of the desired 5′-deoxy-5′-(5-nitropyridinyl-2-disulfanyl)guanosine derivative was detected by mass spectrometry. In contrast, we found that under similar conditions 14b was converted into 2′,3′-O-isopropylidene-5′-deoxy-5′-(5-nitropyridinyl-2-disulfanyl)guanosine 17 in 55% yield (Scheme 5).
Scheme 5.
Synthesis of 5′-deoxy-5′-(5-nitropyridinyl-2-disulfanyl) guanosine derivative.
Synthesis of RNA dinucleotides containing a 5′-S-phosphorothiolate linkage via an Arbusov reaction
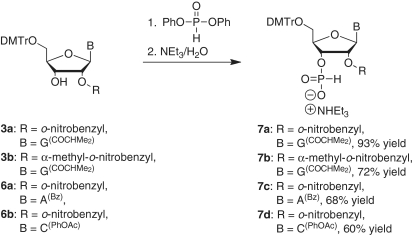
The reaction of 7a with 11 was achieved by the addition of excess bis(trimethylsilyl)acetamide in refluxing tetrahydrofuran (Scheme 6). The reaction product, which was first treated with 80% acetic acid and then with ammonia in methanol for 48 h, gave the desired dinucleotide G[2′-O-(o-nitrobenzyl)]-ps-A (18a) in 24% yield. Similarly, we prepared G[2′-O-(α-methyl-o-nitrobenzyl)]-ps-A (18b) in 45% yield from 7b and 10a. The coupling product was treated with 50% formic acid for 60 h to remove the isopropylidene group (Scheme 6). Additionally, in this reaction we found that protection of the amino group of 10a was not required. We then prepared A[2′-O-(o-nitrobenzyl)]-ps-G (18c) from 7c and 17 in 50% yield, and C[2′-O-(o-nitrobenzyl)]-ps-G (18d) from 7d and 17 in 67% yield (Scheme 7). The progress of each reaction step was monitored with 31P NMR. As expected, we found that the dinuleotides were stable under acidic conditions (50% formic acid, room temperature, 60 h), but decomposed slightly following treatment with base (NH3/MeOH or NH4OH).
Scheme 6.
Synthesis of G[2′-O-(o-nitrobenzyl)]-ps-A and G[2′-O-(α-methyl-o-nitrobenzyl)]-ps-A.
Scheme 7.
Synthesis of A[2′-O-(o-nitrobenzyl)]-ps-G and C[2′-O-(o-nitrobenzyl)]-ps-G.
Incorporation of 5′-phosphorothiolate-linked dinucleotides into RNA oligonucleotides
Construction of RNA oligonucleotides incorporating the photoprotected 5′-phosphorothiolate dinucleotide was accomplished following the scheme outlined in Supplementary Figure S1. Briefly, this involved enzymatic phosphorylation of the dinucleotide followed by successive enzymatic ligations to the 5′ and 3′ flanking RNAs.
Construction of a full-length VS ribozyme substrate
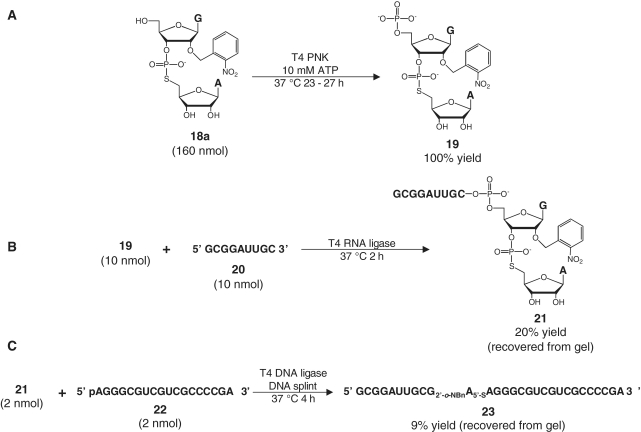
The 5′-hydroxyl group of the dinucleotide G[2′-O-(o-nitrobenzyl)]-ps-A (18a) was phosphorylated by treatment with T4 polynucleotide kinase (T4 PNK) in the presence of ATP for 23–27 h at 37°C (Figure 2A). Phosphorylation was confirmed by reversed-phase high pressure liquid chromatography, which showed that the phosphorylated dinucleotide 19 eluted about 3 min earlier than the starting material (data not shown). The high pressure liquid chromatography traces also showed that little, if any, starting material remained.
Figure 2.
Ligation scheme for constructing a 29-nt VS ribozyme substrate 23. (A) Phosphorylation of dinucleotide 18a with T4 PNK and ATP. (B) T4 RNA ligase-mediated ligation of phosphorylated dinucleotide 19 with 5′ flanking oligonucleotide 20 to yield 21. (C) Splint- and T4 DNA ligase-mediated ligation of 21 and 18-nt oligonucleotide 22 to yield full-length VS ribozyme substrate 23.
The phosphorylated dinucleotide was then ligated enzymatically to flanking RNA oligonucleotides via two sequential ligation reactions. In the first ligation step, the phosphorylated dinucleotide (19) was incubated with the 9-nt RNA 5′ GCG GAU UGC 3′ 20 in the presence of T4 RNA ligase and ATP (Figure 2B). T4 RNA ligase catalyzes the ligation of an oligonucleotide bearing a 5′ phosphate group (the donor) to a second oligonucleotide bearing a free 3′-OH group (the acceptor). This strategy usually requires the donor oligonucleotide to have a 3′ phosphate group to preclude multiple additions of the donor to the 3′-end of the ligated product (59). However, in our ligation reactions we observed an umambiguous major band corresponding to the 11-nt product 21, with few higher order ligation products (data not shown).
Once 21 had been isolated by gel purification, we ligated it to the 18-nt RNA 5′ AGG GCG UCG UCG CCC CGA 3′ 22 by using T4 DNA ligase and a complementary DNA splint (60) (Figure 2C). Ligation of 21 to 22 was less efficient than ligation of an all-oxygen version of 21 to 22 (data not shown). However, an unambiguous product band for the full-length phosphorothiolate substrate 23 was discerned and excised from the purification gel.
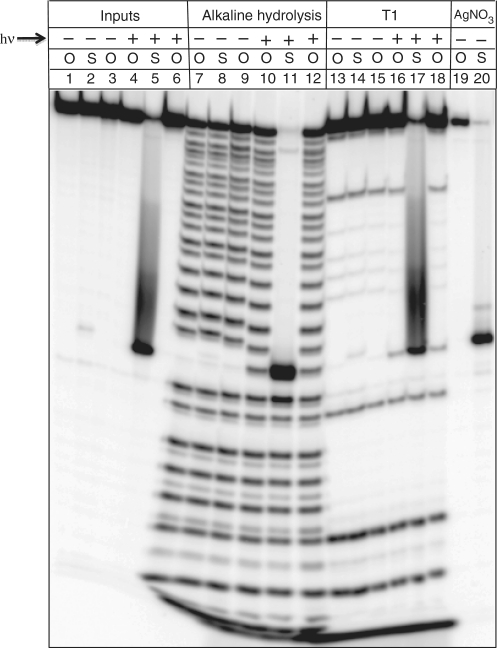
For further characterization, both phosphorothiolate substrate 23 and an all-oxygen substrate constructed in the same manner described above were radioactively 5′-32P labeled using T4 PNK and γ-32P ATP. Alkaline hydrolysis and RNase T1 ladders confirmed the expected sequence of 23, while treatment with silver nitrate confirmed the position of the sulfur linkage (Figure 3, lane 20). A gap in the alkaline hydrolysis and RNase T1 ladders was observed at the position of the nucleotide bearing the photoprotected 2′-hydroxyl group (Figure 3, lanes 7–9 and 13–15). Pre-treatment of the sample with UV light restored cleavage at this site by both base and RNase T1 (lanes 10–12 and 16–18). The oligonucleotides 21 and 23 were purified by denaturing polyacrylamide gel electrophoresis, and identities were confirmed by MALDI-TOF MS (Supplementary Figures S3 and S4).
Figure 3.
Characterization of radioactively 5′-32P labeled 29-nt VS substrate 23. The gel compares ligated substrates containing a 5′-oxygen (O) or a 5′-sulfur (S) at the cleavage site. The samples in the last three lanes of the input, alkaline hydrolysis and RNase T1 sections were irradiated with UV light (hν) for 5 min before loading (inputs) or before exposing the sample to alkaline conditions or RNase T1. Samples treated with silver nitrate are shown at the far right of the gel.
Construction of a full-length HDV ribozyme substrate
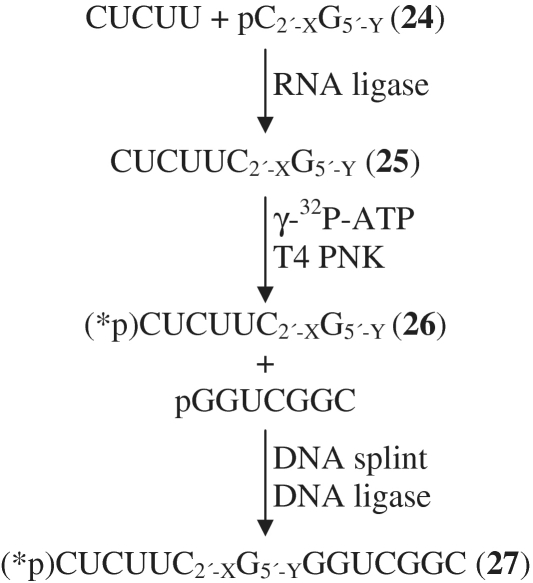
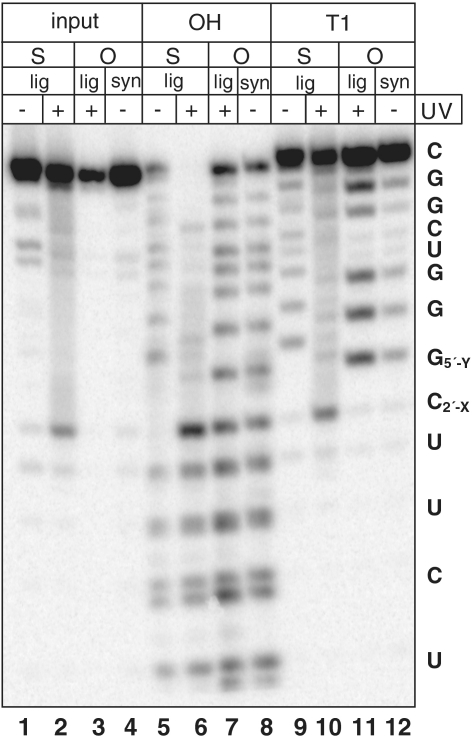
We then adapted this new two-ligation approach to synthesize a 14-nt RNA substrate for the HDV ribozyme containing both a 5′ bridging phosphorothiolate and a protected 2′ hydroxyl group at the cleavage site (Figure 4). Following 5′-phosphorylation of C[2′-O-(o-nitrobenzyl)]-ps-G (18d) (shown as C2′-XG5′-Y in Figure 4), the first RNA ligase-mediated step proceeded under conditions similar to those for the synthesis of the VS ribozyme substrate, and the ligation product (25) was purified by reversed-phase high pressure liquid chromatography (Supplementary Figure S5). However, likely due to the less favorable hybridization of the DNA splint to the short (7-nt) RNA oligonucleotides required for this substrate, the second ligation step occurs less efficiently. The ligated full-length substrate (27) was only observed if the 7-nt phosphorothiolate RNA was radiolabeled prior to the second ligation step. The full-length 14-nt RNA substrate (27) was then purified and characterized. Unmodified substrates synthesized either by ligation or by solid-phase synthesis behaved similarly to each other when subjected to alkaline hydrolysis and RNase T1 digestion (Figure 5, lanes 7, 8, 11 and 12). Alkaline hydrolysis and RNase T1 ladders confirmed the expected sequence of 27 (lanes 5 and 9). A gap in the alkaline hydrolysis ladder was observed at the position of the nucleotide bearing the photoprotected 2′-hydroxyl group (Figure 5, lane 5). Like VS ribozyme substrate 23, pre-treatment of the sample with UV light restored cleavage at this site by base (Figure 5, lanes 2, 6 and 10). Thus, the two-step ligation-based approach to synthesizing ribozyme substrates from modified dinucleotides was extended successfully to the shorter 14-nt HDV ribozyme substrate.
Figure 4.
Synthesis of HDV ribozyme substrate (27) from the dinucleotide C[2′-O-(o-nitrobenzyl)]-ps-G (C2′-XG5′-Y) (18d) by a two-step ligation-based approach.
Figure 5.
Biochemical characterization of the second step ligation products for the HDV ribozyme substrate. Columns labeled ‘O’ contain wild-type substrate, while columns labeled ‘S’ contain 2′-O-o-NO2-Bn/5′-S substrate. Substrates are labeled according to whether they were generated by the two-step ligation method (‘lig’) or by chemical synthesis (‘syn’). Lanes labeled ‘OH’ contain alkaline hydrolysis sequencing ladders, and lanes labeled ‘T1’ contain RNase T1 sequencing ladders. Samples exposed to UV light (100 W, 365 nm) for 4 min before analysis are labeled with a ‘+’ in the row labeled ‘UV’. Nucleotide sequences are indicated at the right of the gel. X = OH, O-o-NO2-Bn; Y = O, S.
In summary, we have established a reliable semisynthetic approach to construct oligoribonucleotides containing a 5′-phosphorothiolate linkage. The approach begins with synthesis of the desired dinucleotide linked by the 5′-phosphorothiolate and photocaged as an o-nitrobenzyl ether derivative at the adjacent 2′-hydroxyl group (Base1-[2′-O-(o-nitrobenzyl)]-ps-Base2). Enzymatic phosphorylation followed by consecutive ligations to upstream and downstream oligoribonucleotides effectively incorporates the phosphorothiolate dinucleotide into longer oligoribonucleotides. The photolabile o-nitrobenzyl group, removed readily via UV irradiation, stabilizes the labile 5′-phosphorothiolate linkage, facilitating handling and storage of the modified oligoribonucleotide and enhancing signal-to-noise during experiments. We anticipate that this semisynthetic strategy will provide access to oligoribonucleotides containing any 5′-phosphorothiolate dinucleotide, permitting their more widespread use in mechanistic investigations and other biological applications.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
This work was supported in part by the Howard Hughes Medical Institute; National Institutes of Health (grant numbers 1R56AI081987-01, 1R01AI081987-01A1 to J.A.P.); and Cancer Research UK to D.M.J.L. J.K.F. and S.C.K. were also supported by the University of Chicago Medical Scientist Training Program (5 T32 GM07281). Funding for open access charge: 1R01AI081987-01A1.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We thank Dr Catherine Goodman for insightful suggestions. We also thank K. Sundaram, N. Suslov and N. Tuttle for helpful discussions and critical comments on the manuscript.
REFERENCES
- 1.Burgers PM, Eckstein F, Hunneman DH. Stereochemistry of hydrolysis by snake venom phosphodiesterase. J. Biol. Chem. 1979;254:7476–7478. [PubMed] [Google Scholar]
- 2.Moore MJ, Sharp PA. Evidence for two active sites in the spliceosome provided by stereochemistry of pre-mRNA splicing. Nature. 1993;365:364–368. doi: 10.1038/365364a0. [DOI] [PubMed] [Google Scholar]
- 3.Gordon PM, Piccirilli JA. Metal ion coordination by the AGC triad in domain 5 contributes to group II intron catalysis. Nat. Struct. Biol. 2001;8:893–898. doi: 10.1038/nsb1001-893. [DOI] [PubMed] [Google Scholar]
- 4.Akhtar S, Kole R, Juliano RL. Stability of antisense DNA oligodeoxynucleotide analogs in cellular extracts and sera. Life Sci. 1991;49:1793–1801. doi: 10.1016/0024-3205(91)90480-y. [DOI] [PubMed] [Google Scholar]
- 5.Hoke GD, Draper K, Freier SM, Gonzalez C, Driver VB, Zounes MC, Ecker DJ. Effects of phosphorothioate capping on antisense oligonucleotide stability, hybridization and antiviral efficacy versus herpes simplex virus infection. Nucleic Acids Res. 1991;19:5743–5748. doi: 10.1093/nar/19.20.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein CA, Cheng YC. Antisense oligonucleotides as therapeutic agents–is the bullet really magical? Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- 7.Marshall WS, Caruthers MH. Phosphorodithioate DNA as a potential therapeutic drug. Science. 1993;259:1564–1570. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- 8.Iyer RP, Phillips LR, Egan W, Regan JB, Beaucage SL. The automated synthesis of sulfur-containing oligodeoxyribonucleotides using 3H-1,2-benzodithiol-3-one 1,1-dioxide as a sulfur-transfer reagent. J. Org. Chem. 1990;55:4693–4699. [Google Scholar]
- 9.Stec WJ, Uznanski B, Wilk A, Hirschbein BL, Fearon KL, Bergot BJ. Bis(O,O-diisopropoxy phosphinothioyl) disulfide - a highly efficient sulfurizing reagent for cost-effective synthesis of oligo(nucleoside phosphorothioate)s. Tetrahedron Lett. 1993;34:5317–5320. [Google Scholar]
- 10.Gaynor JW, Cosstick R. Synthesis, properties and application of nucleic acids containing phosphorothiolate linkages. Curr. Org. Chem. 2008;12:291–308. [Google Scholar]
- 11.Piccirilli JA, Vyle JS, Caruthers MH, Cech TR. Metal ion catalysis in the Tetrahymena ribozyme reaction. Nature. 1993;361:85–88. doi: 10.1038/361085a0. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein LB, Jones BCNM, Cosstick R, Cech TR. A second catalytic metal ion in a group I ribozyme. Nature. 1997;388:805–808. doi: 10.1038/42076. [DOI] [PubMed] [Google Scholar]
- 13.Sontheimer EJ, Sun S, Piccirilli JA. Metal ion catalysis during splicing of premessenger RNA. Nature. 1997;388:801–805. doi: 10.1038/42068. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida A, Sun S, Piccirilli JA. A new metal ion interaction in the Tetrahymena ribozyme reaction revealed by double sulfur substitution. Nat. Struct. Biol. 1999;6:318–321. doi: 10.1038/7551. [DOI] [PubMed] [Google Scholar]
- 15.Sontheimer EJ, Gordon PM, Piccirilli JA. Metal ion catalysis during group II intron self-splicing: parallels with the spliceosome. Genes Dev. 1999;13:1729–1741. doi: 10.1101/gad.13.13.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon PM, Sontheimer EJ, Piccirilli JA. Kinetic characterization of the second step of group II intron splicing: role of metal ions and the cleavage site 2′-OH in catalysis. Biochemistry. 2000;39:12939–12952. doi: 10.1021/bi001089o. [DOI] [PubMed] [Google Scholar]
- 17.Shan S, Yoshida A, Sun S, Piccirilli JA, Herschlag D. Three metal ions at the active site of the Tetrahymena group I ribozyme. Proc. Natl Acad. Sci. USA. 1999;96:12299–12304. doi: 10.1073/pnas.96.22.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon PM, Sontheimer EJ, Piccirilli JA. Metal ion catalysis during the exon-ligation step of nuclear pre-mRNA splicing: extending the parallels between the spliceosome and group II introns. RNA. 2000;6:199–205. doi: 10.1017/s1355838200992069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murata T, Iwai S, Ohtsuka E. Synthesis of a dodecadeoxyribooligonucleotide containing a 3′-thio analog of thymidine photodimer. Heterocycles. 1992;33:529–531. [Google Scholar]
- 20.Weinstein LB, Earnshaw DJ, Cosstick R, Cech TR. Synthesis and characterization of an RNA dinucleotide containing a 3′-S-phosphorothiolate linkage. J. Am. Chem. Soc. 1996;118:10341–10350. [Google Scholar]
- 21.Liu XH, Reese CB. 3′-Thiouridylyl-(3′→5′)-uridine. Tetrahedron Lett. 1996;37:925–928. [Google Scholar]
- 22.Curley JF, Joyce CM, Piccirilli JA. Functional evidence that the 3′-5′ exonuclease domain of Escherichia coli DNA polymerase I employs a divalent metal ion in leaving group stabilization. J. Am. Chem. Soc. 1997;119:12691–12692. [Google Scholar]
- 23.Shah R, Cosstick R, West SC. The RuvC protein dimer resolves Holliday junctions by a dual incision mechanism that involves base-specific contacts. EMBO J. 1997;16:1464–1472. doi: 10.1093/emboj/16.6.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott SL, Brazier J, Cosstick R, Connolly BA. Mechanism of the Escherichia coli DNA T:G-mismatch endonuclease (Vsr protein) probed with thiophosphate-containing oligodeoxynucleotides. J. Mol. Biol. 2005;353:692–703. doi: 10.1016/j.jmb.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Perez B, Lucas M, Cooke LA, Vyle JS, de la Cruz F, Moncalian G. Analysis of DNA processing reactions in bacterial conjugation by using suicide oligonucleotides. EMBO J. 2007;26:3847–3857. doi: 10.1038/sj.emboj.7601806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beevers APG, Witch EM, Jones BCNM, Cosstick R, Arnold JRP, Fisher J. Conformational analysis of 3′-S-PO3-linked ribo- and deoxyribodinucleoside monophosphates. Magn. Reson. Chem. 1999;37:814–820. [Google Scholar]
- 27.Beevers AP, Fettes KJ, O'Neil IA, Roberts SM, Arnold JR, Cosstick R, Fisher J. Probing the effect of a 3′-S-phosphorothiolatelink on the conformation of a DNA:RNA hybrid; implications for antisense drug design. Chem. Commun. 2002:1458–1459. doi: 10.1039/b203582k. [DOI] [PubMed] [Google Scholar]
- 28.Beevers AP, Fettes KJ, Sabbagh G, Murad FK, Arnold JR, Cosstick R, Fisher J. NMR and UV studies of 3′-S-phosphorothiolate modified DNA in a DNA : RNA hybrid dodecamer duplex; implications for antisense drug design. Org. Biomol. Chem. 2004;2:114–119. doi: 10.1039/b311923h. [DOI] [PubMed] [Google Scholar]
- 29.Douglas SR, McKernan KJ. USA patent no. 2010 Patent 7645866 B2. [Google Scholar]
- 30.Michelson AM. Polynucleotides. Part IV. Synthesis of Oligonucleotide Analogues Substituted in the Sugar Portion. J. Chem. Soc. 1962:979–982. [Google Scholar]
- 31.Cook AF. Nucleoside S-alkyl phosphorothioates. IV. Synthesis of nucleoside phosphorothioate monoesters. J. Am. Chem. Soc. 1970;92:190–195. [Google Scholar]
- 32.Chladek S, Nagyvary J. Nucleophilic reactions of some nucleoside phosphorothioates. J. Am. Chem. Soc. 1972;94:2079–2085. doi: 10.1021/ja00761a047. [DOI] [PubMed] [Google Scholar]
- 33.Rybakov VN, Rivkin MI, Kumarev VP. Some substrate properties of analogues of oligothymidylates with p-s-C5′ bonds. Nucleic Acids Res. 1981;9:189–201. doi: 10.1093/nar/9.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jahn-Hofmann K, Engels JW. Efficient solid phase synthesis of cleavable oligodeoxynucleotides based on a novel strategy for the synthesis of 5′-S-(4,4′-dimethoxytrityl)-2′-deoxy-5′-thionucleoside phosphoramidites. Helv. Chim. Acta. 2004;87:2812–2828. [Google Scholar]
- 35.Kuimelis RG, McLaughlin LW. Cleavage properties of an oligonucleotide containing a bridged internucleotide 5′-phosphorothioate RNA linkage. Nucleic Acids Res. 1995;23:4753–4760. doi: 10.1093/nar/23.23.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas JM, Perrin DM. Probing general acid catalysis in the hammerhead ribozyme. J. Am. Chem. Soc. 2009;131:1135–1143. doi: 10.1021/ja807790e. [DOI] [PubMed] [Google Scholar]
- 37.Zhou DM, Usman N, Wincott FE, MatulicAdamic J, Orita M, Zhang LH, Komiyama M, Kumar PKR, Taira K. Evidence for the rate-limiting departure of the 5′-oxygen in nonenzymatic and hammerhead ribozyme-catalyzed reactions. J. Am. Chem. Soc. 1996;118:5862–5866. [Google Scholar]
- 38.Burgin AB, Jr, Huizenga BN, Nash HA. A novel suicide substrate for DNA topoisomerases and site-specific recombinases. Nucleic Acids Res. 1995;23:2973–2979. doi: 10.1093/nar/23.15.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgin AB, Jr, Nash HA. Suicide substrates reveal properties of the homology-dependent steps during integrative recombination of bacteriophage lambda. Curr. Biol. 1995;5:1312–1321. doi: 10.1016/s0960-9822(95)00258-2. [DOI] [PubMed] [Google Scholar]
- 40.Henningfeld KA, Arslan T, Hecht SM. Alteration of DNA primary structure by DNA topoisomerase I. Isolation of the covalent topoisomerase I-DNA binary complex in enzymatically competent form. J. Am. Chem. Soc. 1996;118:11701–11714. [Google Scholar]
- 41.Liu XH, Reese CB. Uridylyl-(3′→5′)-(5′-thiouridine) - an exceptionally base-labile di-ribonucleoside phosphate analog. Tetrahedron Lett. 1995;36:3413–3416. [Google Scholar]
- 42.Thomson JB, Patel BK, Jimenez V, Eckart K, Eckstein F. Synthesis and properties of diuridine phosphate analogues containing thio and amino modifications. J. Org. Chem. 1996;61:6273–6281. doi: 10.1021/jo960795l. [DOI] [PubMed] [Google Scholar]
- 43.Kuimelis RG, Mclaughlin LW. Hammerhead ribozyme-mediated cleavage of a substrate-analog containing an internucleotidic bridging 5′-phosphorothioate - implications for the cleavage mechanism and the catalytic role of the metal cofactor. J. Am. Chem. Soc. 1995;117:11019–11020. [Google Scholar]
- 44.Kuimelis RG, McLaughlin LW. Application of a 5′-bridging phosphorothioate to probe divalent metal and hammerhead ribozyme mediated RNA cleavage. Bioorg. Med. Chem. 1997;5:1051–1061. doi: 10.1016/s0968-0896(97)00041-2. [DOI] [PubMed] [Google Scholar]
- 45.Das SR, Piccirilli JA. General acid catalysis by the hepatitis delta virus ribozyme. Nat. Chem. Biol. 2005;1:45–52. doi: 10.1038/nchembio703. [DOI] [PubMed] [Google Scholar]
- 46.Wilson TJ, Li NS, Lu J, Frederiksen JK, Piccirilli JA, Lilley DM. Nucleobase-mediated general acid-base catalysis in the Varkud satellite ribozyme. Proc. Natl Acad. Sci. USA. 2010;107:11751–11756. doi: 10.1073/pnas.1004255107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaulk SG, MacMillan AM. Caged RNA: photo-control of a ribozyme reaction. Nucleic Acids Res. 1998;26:3173–3178. doi: 10.1093/nar/26.13.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohtsuka E, Tanaka S, Ikehara M. Studies of Transfer Ribonucleic-Acids and Related Compounds. XVIII. Photolabile 2′-Ether of Guanosine as an Intermediate for Oligonucleotide Synthesis. Synthesis-Stuttgart. 1977:453–454. [Google Scholar]
- 49.Ohtsuka E, Tanaka S, Ikehara M. Studies on transfer ribonucleic-acids and related compounds. XVI. Synthesis of ribooligonucleotides using a photosensitive o-nitrobenzyl protection for the 2′-hydroxyl group. Chem. Pharm. Bull. 1977;25:949–959. [Google Scholar]
- 50.Pignot M, Pljevaljcic G, Weinhold E. Efficient synthesis of S-adenosyl-L-homocysteine natural product analogues and their use to elucidate the structural determinant for cofactor binding of the DNA methyltransferase M center dot HhaI. Eur. J. Org. Chem. 2000:549–555. [Google Scholar]
- 51.Zhang BL, Cui ZY, Sun LL. Synthesis of 5′-deoxy-5′-thioguanosine-5′-monophosphorothioate and its incorporation into RNA 5′-termini. Org. Lett. 2001;3:275–278. doi: 10.1021/ol006916s. [DOI] [PubMed] [Google Scholar]
- 52.Sund C, Chattopadhyaya J. Intramolecular and intermolecular nucleophilic phosphorus sulfur bond-cleavage - the reaction of fluoride-ion with O-aryl-O,S-dialkylphosphorothioates, and the degradation of phosphorothioate linkage in di-ribonucleotides by the vicinal 2′-hydroxyl group. Tetrahedron. 1989;45:7523–7544. [Google Scholar]
- 53.Barltrop JA, Plant PJ, Schofield P. Photosensitive protective groups. Chem. Commun. 1966:822–823. [Google Scholar]
- 54.Patchornik A, Amit B, Woodward RB. Photosensitive Protecting Groups. J. Am. Chem. Soc. 1970;92:6333–6335. [Google Scholar]
- 55.Pillai VNR. Photo-removable protecting groups in organic-synthesis. Synthesis-Stuttgart. 1980:1–26. [Google Scholar]
- 56.Hasan A, Stengele KP, Giegrich H, Cornwell P, Isham KR, Sachleben RA, Pfleiderer W, Foote RS. Photolabile protecting groups for nucleosides: Synthesis and photodeprotection rates. Tetrahedron. 1997;53:4247–4264. [Google Scholar]
- 57.Hobartner C, Silverman SK. Modulation of RNA tertiary folding by incorporation of caged nucleotides. Angew. Chem. Int. Ed. Engl. 2005;44:7305–7309. doi: 10.1002/anie.200502928. [DOI] [PubMed] [Google Scholar]
- 58.Wenter P, Furtig B, Hainard A, Schwalbe H, Pitsch S. Kinetics of photoinduced RNA refolding by real-time NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2005;44:2600–2603. doi: 10.1002/anie.200462724. [DOI] [PubMed] [Google Scholar]
- 59.England TE, Uhlenbeck OC. Enzymatic oligoribonucleotide synthesis with T4 RNA ligase. Biochemistry. 1978;17:2069–2076. doi: 10.1021/bi00604a008. [DOI] [PubMed] [Google Scholar]
- 60.Moore MJ, Sharp PA. Site-specific modification of pre-mRNA: the 2′-hydroxyl groups at the splice sites. Science. 1992;256:992–997. doi: 10.1126/science.1589782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.