Abstract
Extinction risk varies across species and space owing to the combined and interactive effects of ecology/life history and geography. For predictive conservation science to be effective, large datasets and integrative models that quantify the relative importance of potential factors and separate rapidly changing from relatively static threat drivers are urgently required. Here, we integrate and map in space the relative and joint effects of key correlates of The International Union for Conservation of Nature-assessed extinction risk for 8700 living birds. Extinction risk varies significantly with species' broad-scale environmental niche, geographical range size, and life-history and ecological traits such as body size, developmental mode, primary diet and foraging height. Even at this broad scale, simple quantifications of past human encroachment across species' ranges emerge as key in predicting extinction risk, supporting the use of land-cover change projections for estimating future threat in an integrative setting. A final joint model explains much of the interspecific variation in extinction risk and provides a remarkably strong prediction of its observed global geography. Our approach unravels the species-level structure underlying geographical gradients in extinction risk and offers a means of disentangling static from changing components of current and future threat. This reconciliation of intrinsic and extrinsic, and of past and future extinction risk factors may offer a critical step towards a more continuous, forward-looking assessment of species' threat status based on geographically explicit environmental change projections, potentially advancing global predictive conservation science.
Keywords: birds, IUCN Red List, projected threat, future global change, structural equation models
1. Introduction
A central goal in conservation biology is to understand why some species are more vulnerable to extinction than others [1]. Uncovering the underlying threats and processes behind current species declines could improve our predictions of future declines thereby making conservation efforts more effective. The International Union for Conservation of Nature (IUCN) Red List of threatened species offers a reasonably standardized estimate of global extinction risk under past and recent conditions [2]. Broad-scale studies have demonstrated that this measure covaries with intrinsic ecological and life-history traits such as body size (e.g. [3,4]). For instance, large-bodied mammals appear to be particularly vulnerable to extinction [5]. Certain intrinsic attributes such as generation time and population growth rate (which are also associated with body size; [6]) are implicitly incorporated into threat assessments [7]. However, these attributes may not be independent of environmental conditions [8]. Separately, extrinsic environmental or geographical factors such as climatic variables and human population density may also play a critical role in species endangerment [9].
The structure of global extinction risk in species is thus characterized by complex inter-relationships among correlates [10]. But, to date, extinction risk correlates are rarely isolated, which limits our understanding of how threats vary across scale, taxa and types of potential drivers (e.g. [11]). Intrinsic traits such as species ecology and extrinsic factors such as habitat loss may also interact strongly with geographical range (and population) size to affect extinction risk [12]. Threat thus varies across species and space owing to the combined and interactive effects of a broad array of ecological/life-history (intrinsic, usually slowly evolving) and geographical (extrinsic, potentially rapidly changing) factors [13]. To avoid spurious correlations, it becomes necessary to quantify the interdependencies between threat and human encroachment, and between threat and range size while simultaneously considering the influence of other determinants of extinction risk [1].
Geographical range size has consistently emerged as the key correlate of extinction risk in mammals and amphibians [4,10,14,15]. This is true even among species that are red-listed for reasons other than having very narrow geographical distributions (i.e. criteria B; [4,7,14]). This underscores the significance of developing an explicit measure of past anthropogenic changes to geographical range area [16]. Such a quantity, e.g. human ‘encroachment’, would quantify the proportion of a geographical range that has experienced land-cover transformations because of past human activities (e.g. agriculture and urbanization). This simple measure can be extended to project species extinction risk by estimating future encroachment that could facilitate spatially explicit future conservation planning. Undoubtedly, future extinction risk will benefit as more accurate models of future land-cover transformations become available [17], and our understanding of species responses to projected change improves, e.g. through incorporation of stochastic population models [18].
As we will demonstrate here, the appreciation of joint intrinsic/extrinsic determination of species-level extinction risk also facilitates a more structured and process-based understanding of geographical gradients in average extinction risk across assemblages (species co-occurring in, e.g. a grid cell). Modelling the spatial pattern of threat as a summary response (e.g. proportion of species threatened, [19]) is popular but inference is biased by pseudoreplication and spatial autocorrelation owing to large differences in geographical range size, i.e. wide-ranging species masking the patterns of narrow-ranged ones [20]. Therefore, we argue it is critical to understand the model of threat at the species level before assessing observed assemblage threat and how it may be explained by the compound predictions of its inhabitant species [21]. This approach promotes a more mechanistic understanding of the geography of extinction risk ‘hotspots’ worldwide, and by simultaneously pinpointing species and regions, it may help integrate region- and species-focal conservation prioritization efforts.
In order to quantify and disentangle dynamic (e.g. anthropogenic activities) from largely static (e.g. body size) extinction risk factors to make future predictions, large, ideally global, datasets and integrative modelling approaches are needed. Here, we use the largest set of species to date to integrate and map in space the relative and joint effects of key correlates of endangerment and range encroachment. We assess all 8664 extant terrestrial and freshwater bird species (i.e. 90% of all birds), 10 per cent (858 species) of which are considered ‘threatened’ (IUCN Red List status of Critically Endangered, Endangered or Vulnerable; [7]). In contrast to mammals and amphibians (e.g. [4]), a global assessment of species-level extinction risk correlates in birds is currently missing (but see [11] for analysis at family level). Birds have undergone a high rate of past extinctions, particularly on islands, and breeding populations are currently exposed to threats from anthropogenic activities (e.g. land-use change) worldwide [22,23]. This grim outlook is exacerbated by the future range losses projected owing to climate and land-use change [17]. Population declines and species extinctions may have devastating ecological consequences by disrupting key ecosystem processes such as decomposition, pollination and seed dispersal [24]. Using a binary risk categorization as response, we ask the following questions: (i) what is the relative importance of intrinsic versus extrinsic predictors of extinction risk? (ii) how much extinction risk can be attributed to human encroachment of geographical ranges alone? and (iii) how successful are species-based statistical models in capturing the geographical variation, e.g. the hotspots, of extinction risk worldwide?
2. Material and methods
(a). Data
(i). Extinction risk and geographical distributions
We defined a binary extinction risk variable with species listed as Least Concern and Near Threatened as ‘Not Threatened’ and those listed as Vulnerable, Endangered and Critically Endangered as ‘Threatened’ (2008 IUCN Red List; http://www.iucnredlist.org/). We excluded species listed as Extinct, Extinct in the Wild and Data Deficient because of the unavailability of predictor variables. All marine species, newly described species, those involved in recent splits and lumps, and others for which we did not have breeding range maps or have nomenclature inconsistencies are not included. This resulted in a final list of 8664 species for our analysis (electronic supplementary material, appendix S1). For select analyses, we removed 345 species that were red-listed (Threatened) owing to observed declines in geographical range sizes (i.e. criteria B; [9]). Breeding distributions were compiled from the most accurate sources giving expert opinion range (extent of occurrence) maps [21] and are used for environmental extractions and mapping the geography of threat (see below).
(ii). Correlates of extinction risk
We assembled a database of extinction risk correlates deemed to be important in predicting bird extinction risk, population decline or sensitivity to habitat modification (table 1 and electronic supplementary material, table S1).
— Encroachment. We defined encroachment as the proportion of geographical range size transformed by past human activities (i.e. cultivated or managed, mosaics including cropland and urban areas). By overlaying the breeding range in a gridded format for each species with information on transformed habitats owing to anthropogenic activities from the Global Land Cover 2000 land-cover classification (1 km2 resolution), we quantified encroachment across all species.
— Geographical range size. We projected the world map to an equal-area grid in 0.01° resolution and calculated the true area (in km2) of each 0.01° grid cell. Range size was then obtained by summing up the area of all grid cells occupied by a species.
— Island range. We totalled the true area of ‘island’ (oceanic geological origin or distant continental/island states) grid cells that were occupied by each species and calculated island range as the proportion of a species' geographical range size.
— Intrinsic traits. Ecological and life-history traits include the following: body mass, clutch size, development mode (precocial or altricial), diet type (five primary categories) and breadth (up to seven categories), nest-cover type (non-closed or closed) and location (ground and intermediate, or high), migratory behaviour (non-migrants or migrants), activity (diurnal or nocturnal), foraging height (seven possible classes) and breadth (up to five classes) and habitat breadth (up to 25 types). Species-typical values of intrinsic traits for bird species are compiled from a variety of sources [21].
— Extrinsic factors. We used the maps of breeding ranges described above and global environmental layers to characterize broad-scale extrinsic factors. We extracted range centroid, mean actual evapo-transpiration (AET), seasonality of temperature (TempMax − TempMin), minimum elevation and potential elevation range (ElevationMax − ElevationMin) and mean human influence index (a composite measure of multiple human activities that include urban extent, population density, roads, navigable rivers and agricultural land; Last of the Wild Data v. 2 2005) across each species' range. All data extractions were performed using the ESRI Arc and Grid software (v. 9.0; [25]). For additional details and sources of correlates, see the electronic supplementary material.
Table 1.
Predictors of extinction risk—detailed SEM results. (Listed are standardized coefficients of correlates, variance explained for the three responses and other results. We analysed 8664 terrestrial and freshwater species. Coefficient in bold indicates that Z-value is significant (p < 0.05). Within-family indicates that shared ancestry is controlled for at the family level. ‘Migrant’ and ‘island’ include species that carry out altitudinal and intra- and inter-continental migration, and species that have at least 90% of their breeding range on non-continental islands, respectively. Reliability coefficient measures how well-measured each latent variable is by its indicators and should ideally be at least 0.7 (see electronic supplementary material, table S1). The strength of the coefficient generally points to more stable estimates among indicators for a latent variable (see text). Intra-class correlation measures the extent of nestedness in each model. n.a., not applicable.)
| all species |
within-family |
within-family |
||||
|---|---|---|---|---|---|---|
| cross-species | within-family | non-migrant | migrant | mainland | island | |
| correlate | ||||||
| range size | −0.58 | −0.63 | −0.54 | −0.58 | −0.61 | −0.39 |
| encroachment | 0.26 | 0.22 | 0.27 | 0.23 | 0.26 | 0.19 |
| life history | 0.24 | 0.19 | 0.32 | 0.11 | 0.20 | 0.41 |
| ecological niche | 0.15 | 0.14 | −0.02 | 0.08 | 0.12 | 0.23 |
| niche breadth | −0.15 | −0.08 | −0.19 | −0.05 | −0.25 | 0.03 |
| environmental niche | 0.22 | 0.24 | 0.17 | 0.42 | 0.38 | 0.11 |
| variance explained | ||||||
| extinction risk | 0.49 | 0.47 | 0.50 | 0.48 | 0.56 | 0.50 |
| encroachment | 0.73 | 0.67 | 0.73 | 0.54 | 0.66 | 0.75 |
| range size | 0.53 | 0.38 | 0.41 | 0.04 | 0.36 | 0.47 |
| other details | ||||||
| group size | 8664a | 6952 | 1712 | 6980 | 1684 | |
| number of threatened/non-threatened species | 858a/7806a | 747/6205 | 109/1603 | 574/6406 | 282/1402 | |
| number of free parameters | 54 | 57 | 88b | 88b | ||
| intra-class correlation | n.a. | 0.16 | 0.08b | 0.02b | ||
| reliability coefficient | ||||||
| life history | 0.86 | 0.67 | 0.67 | 0.83 | 0.69 | 0.53 |
| ecological niche | 0.88 | 0.80 | 0.80 | 0.82 | 0.81 | 0.81 |
| niche breadth | 0.01 | 0.01 | 0.08 | 0.01 | 0.29 | 0.01 |
| environmental niche | 0.88 | 0.85 | 0.78 | 0.92 | 0.85 | 0.72 |
aSimilar values are listed for different models.
bSimilar values are listed for identical models.
(b). Statistical analysis
(i). Structural equation model
We integrated our understanding of the correlates of bird extinction risk in a single-analysis framework using a multilevel structural equation model (SEM) for binary responses. SEM represents a multivariate statistical method that combines factor and path analyses to assess multiple causal pathways by analysing covariance among variables [26]. SEM is an ‘a priori’ technique that allows direct testing of hypothesized mechanisms supported by existing knowledge about expected linkages and allows theoretical multi-dimensional concepts to be characterized (via a method similar to factor analysis) by single entities or latent variables [27]. In this study, body mass, clutch size, nest type and height, and development mode are used to model the latent (unobservable) variable ‘life history’. The ‘ecological-niche’ latent variable is a combination of body mass, diet type, activity and forage height class, characterizing where, when and what the species forages on. The ‘niche-breadth’ latent variable is a combination of diet, habitat and foraging height breadths, representing the niche width of a species (generalist or specialist). Finally, the ‘environmental-niche’ latent variable is a combination of mean AET and seasonality, minimum elevation and potential elevational range, quantifying the environmental conditions observed within its geographical range. We carried out path analysis for individual correlates to examine direction and significance of their relationship with extinction risk (electronic supplementary material, table S1). The significant variables are in turn selected to load onto the respective latent variable, which are then analysed in the SEM. Each latent variable should be meaningfully represented and well measured by its correlates (table 1). Overall, SEM can test the statistical adequacy of a proposed causal model.
SEM was performed using the M-Plus program v. 5.1 [28]. For our analyses, we specified a logit model (binary response) and maximum likelihood as the estimation method, assuming that any missing data for our correlates are missing at random (electronic supplementary material, table S1). Phylogeny is a potential confounding factor in our analyses since close relatives tend to be more similar to each other than expected by chance [1]. However, because we do not have a robust dated phylogeny for birds, we partially address the phylogenetic non-independence in our dataset using taxonomy in the form of multilevel SEM [29]. Here, species are nested in families (‘within-family’; species in family clusters), where most variation for key intrinsic traits occurs [30]. Cross-species (not controlled for species relatedness) results are also provided for comparison. In addition, we repeated the within-family analysis grouping birds by (i) migratory tendency and (ii) isolation extent on islands (table 1) to see how this influenced correlates of extinction risk.
(ii). Mixed-effect model
For comparison, we also performed generalized linear mixed-effect modelling (GLMM) to identify the key correlates of extinction risks in birds with ‘family’ as a random effect (i.e. within-family). We also evaluated cross-species and within-family–order (shared ancestry at the order–family level) models (electronic supplementary material, table S2), although we focus on the within-family results. GLMM yields results that are more tractable, practical and comparable with previous studies using only generalized linear models. Further, we can corroborate the SEM results and derive model predictions that are otherwise not directly attainable in the M-Plus implementation of SEM with binary responses. By using two separate statistical techniques, we are able to provide complementary approaches to understand the drivers of threat across species and space. We calculated a series of standard goodness-of-fit measures to assess model fits (electronic supplementary material, table S2 and figure S4). Based on species range centroids, global Moran's I (0.03–0.19) and correlogram indicate minimal to negligible remaining spatial autocorrelation in model residuals (Moran's I = 0.0006–0.059; electronic supplementary material, figure S6a,b). However, unlike the SEM analyses, we did not evaluate the extinction risk correlates in birds grouped by migratory tendency or isolation extent on islands.
(iii). Geography of threat
We assessed how well species-level model predictions can explain the observed geographical gradient in average extinction risk worldwide. Linking geographical distribution data to model predictions, we generated the predicted assemblage extinction risk by averaging the species-level predicted probability of extinction risk (from the within-family mixed-effect model) across all members of an assemblage. We tested the concordance between observed (proportion of threatened species within an assemblage) and predicted assemblage extinction risks with simple regression analyses across 13 156 assemblages and space. We also evaluated the effects of species richness and average range size on the association. We further accounted for the very strong spatial autocorrelation structure in the model residuals (electronic supplementary material, figure S5c,d) by carrying out a spatial linear regression model using a bootstrapping approach. All analyses were carried out using R v. 2.8.1 [31] unless otherwise stated, while global maps were produced using ArcMap v. 9.3. See electronic supplementary material for more details on data analyses, and observed and predicted extinction risk and encroachment data for all species included in the analyses (electronic supplementary material, appendix S1).
3. Results and discussion
(a). A general structural model
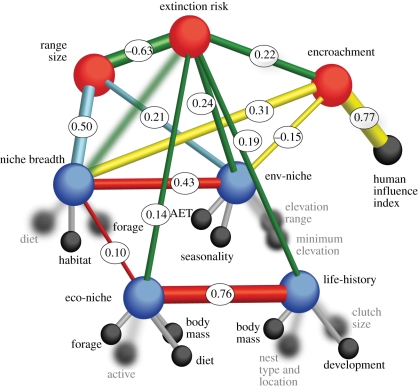
Building on previous conceptual and empirical work (e.g. [11]), we present a working model of structural relationships among core extinction risk correlates in birds (figure 1 and electronic supplementary material, table S1). We identify six main sets of drivers of binary extinction risk: encroachment, geographical range size and four latent variables (life history, ecological niche, niche breadth and environmental niche). We expect the human influence index to have an indirect effect on ‘threat’ through encroachment, i.e. the proportion of a species' geographical range that has undergone anthropogenic land-cover transformation. We use this index to predict encroachment because it is a composite measure that summarizes and captures the extent of multiple human activities (including roads). It also serves as an ‘independent’ corroboration of the degree of human impacts beyond those arising from agricultural lands and urban areas (which are quantified by the variable encroachment). Similarly, we allow for niche breadth and environmental-niche latent correlates to have indirect effects on threat via their interaction with encroachment and geographical range size. We also include additional postulated correlations among the latent variables (figure 1). We posit that this working model offers a simple, yet sound representation of the main interdependencies among core broad-scale drivers of avian extinction risk that are currently quantifiable at a global scale.
Figure 1.
Proposed and tested structural relationship between predictors of species extinction risk in birds (within-family, 8664 species) with rods representing postulated causal relationships and bubbles variables. Binary extinction risk is the main response, encroachment and range size are the secondary responses in the SEM. Encroachment measures the proportion of range size modified by humans. Life history, ecological niche (eco-niche), niche breadth and environmental niche (env-niche) are latent variables (blue) which each have several hypothesized correlates (black). Selected correlates influence bird extinction risk, population decline or sensitivity to habitat modification. Thickness of all non-grey rods increases with their relative importance. Oval labels give significant standardized partial regression coefficient. Faded bubbles and rods indicate originally hypothesized but insignificant (p > 0.05) indicators and relationships, which were excluded from the final SEM. See electronic supplementary material, table S1 for abbreviations and details.
(b). General predictors of extinction risk in birds
A SEM of bird species that accounts for shared ancestry at the family level (within-family) supports almost all of the hypothesized linkages (figure 1). All but one structural relationship is significant (electronic supplementary material, figures S1, S2 and table S1). The empirical model reveals that geographical range size, the environmental-niche latent variable, encroachment, and the life-history and ecological-niche latent variables (in decreasing order of importance) are the most significant correlates of extinction risk across 8664 bird species and explain 47 per cent of the SEM variance in threat status (figure 1 and table 1). The significant effect of ecological niche disappears in a cross-species SEM that does not consider the phylogenetic relatedness of species. The SEM variance explained in the cross-species model is larger for the secondary responses (i.e. encroachment and range size; table 1), suggesting phylogenetic conservatism in these variables. Indeed, this shows that species with shared ancestry may also be spatially clustered or occur in similar regions. Nevertheless, we acknowledge that in the absence of a comprehensive bird phylogeny, we are unable to quantify the full phylogenetic signal in bird extinction risk. These main SEM results were corroborated by a within-family mixed-effect model (figure 2; electronic supplementary material, table S2 and figure S3).
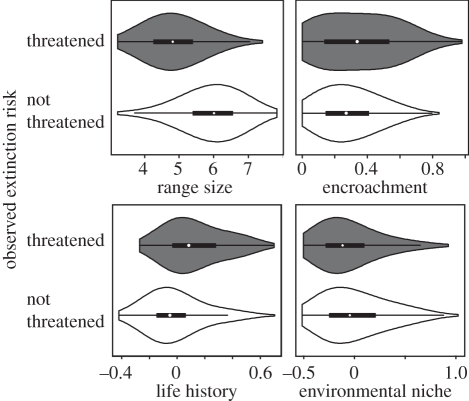
Figure 2.
Key correlates of extinction risk as identified from the main, within-family SEM model. Cross-species results are illustrated. These violin plots combine box plot and density trace (smoothed histogram of data) and embedded boxes indicate first and third quartiles, white circle the median, and horizontal lines 10th and 90th percentiles. Life history and environmental niche are latent variables whose effects are mostly driven by four predictors (electronic supplementary material, figure S1): species occupying low mean AET and high mean seasonality regions (i.e. high environmental-niche scores) are more threatened, and larger and precocial birds (i.e. high life-history scores) are more extinction prone.
Even after controlling for the covariation among correlates, range size still emerges as the strongest predictor of extinction risk, confirming previous findings in birds [12], amphibians [15] and mammals [14]. This trend is robust to the removal of the 345 species that are red-listed owing to observed declines in geographical range sizes (i.e. criteria B; electronic supplementary material, table S3). Therefore, our results include all species in order to maximize the sample size. Species with restricted range sizes are often characterized by small population sizes and high habitat specialization, so they generally are more vulnerable to extinction from stochastic, demographic and environmental processes [32]. Here, we find an effect of range size above and beyond that of niche breadth (figure 1).
Assessing the structure underlying the documented latent variables highlights a combination of putative extinction risk drivers. The effects of life-history and ecological-niche latent variables on threat are owing to large body size and precocial (i.e. young are relatively mobile, covered in feathers and independent) development mode, certain diet (vertebrate, omnivorous, plant materials or seeds compared with invertebrate) and foraging height preferences (restricted to ground and water level compared with multiple levels), respectively (electronic supplementary material, figure S1). Larger birds may be more extinction prone because of their smaller population sizes, lower reproductive rates, larger home-range requirements or have higher trophic levels exposing them to extinction threats such as hunting (e.g. [33]). The higher extinction risk in precocial birds may be owing to lack of parental protection, particularly against human-introduced nest predators in disturbed landscapes, compared with altricial species (e.g. [34]). The effect of the environmental-niche latent variable on threat is driven by AET and seasonality (i.e. species occupying low mean AET and high mean seasonality regions are more threatened; electronic supplementary material, figure S1). Their effects on extinction risk are strong even after accounting for the separate influences exerted by range size and encroachment.
Encroachment and the ensuing loss of habitat and niche space [35] has been noted as a predictor of extinction risk in numerous smaller scale studies and in quantitative analyses on forest birds [16] as well as range-restricted birds [36]. Its relative importance across all birds in explaining threat above and beyond other (covarying) intrinsic and extrinsic effects is confirmed in our structural model (figure 1). Range encroachment increases with increasing human influence index, providing independent corroboration on the degree of anthropogenic influence across all species (figure 1 and electronic supplementary material, figure S2). While estimates of encroachment will improve with more advanced satellite products, our results demonstrate the potential of remotely sensed estimates of anthropogenic impacts to assess extinction risk at a global scale and species level. Our findings validate and highlight this general approach as a rewarding and potentially transformative avenue for future threat assessments.
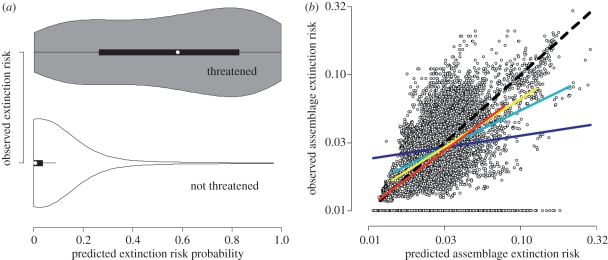
Combining the effects of all predictors, at the species level, the median predicted probability of extinction risk for threatened versus non-threatened species is 0.6 and 0, respectively (figure 3a). The within-family mixed-effect model validates our SEM approach and explains almost 44 per cent of the deviance in the data with reasonably good predictive quality (e.g. Cohen's κ = 0.64, area under the curve = 0.95; electronic supplementary material, figure S4 and table S2). Using an extinction risk probability cut-off of 0.5, we show that our model performed very well in predicting species of low conservation concern (specificity 0.98) but only reasonably well for those that are extinction prone (sensitivity 0.58; electronic supplementary material, table S2). This suggests that although our model is useful (and sensitivity increases with changes in prediction threshold), it cannot perfectly account for all predictors of global extinction risk in birds (e.g. population size). For example, threats from stochastic, demographic and environmental processes that IUCN aims to at least qualitatively take into account in its categorization are not included in our model [37]. Alternatively, the low sensitivity value for extinction-prone species appears to be common [10], and it may be owing to there being many fewer threatened species available for modelling, relative to those that are not threatened.
Figure 3.
Observed and predicted extinction risk across (a) 8664 species and (b) ca 13 000, 110 × 110 km assemblages worldwide. Predictions are based on the within-family mixed-effect model (GLMM). For details on (a), see figure 2. In (b), the dashed line indicates a 1 : 1 relationship. A different linear regression fit is illustrated for quartiles of assemblage richness. Prediction success increases with increasing richness: 10–90 species: b = 0.17, n = 3276; 91–150 species: b = 0.54, n = 3403; 151–240 species: b = 0.71, n = 3169 and 241–905 species: b = 0.83, n = 3308. Assemblages with less than 10 species are excluded. Richness per assemblage: dark blue solid line, 10–90; light blue solid line, 91–150; yellow solid line, 151–240; red solid line, 241–905.
Our integrative approach offers a framework for incorporating dynamic information such as land-cover changes along with existing, relatively static life-history and ecological correlates of extinction risk into ongoing threat assessments. With mounting interest in understanding the impacts of projected global environmental change on biodiversity [38], a modified measure that captures projected anthropogenic encroachment should be valuable and provide an alternative for making extinction predictions into the future.
Non-migratory and island birds have been suggested to have particularly high extinction risk [39,40]. For island birds (defined here as species with at least 90% of their range on oceanic geological origin or distant continental/island states), we find these assertions confirmed at the global scale, as they are disproportionately more threatened than expected (17% of island birds versus 8% of mainland birds are threatened; χ21 = 109.7, p < 0.01; table 1). Migrant species (defined here as species that performed altitudinal or continental migrations) are less threatened than expected (6% of migrants versus 11% of non-migrants are threatened; χ21 = 29.1, p < 0.01; table 1; see also [40]). As the broad ecological differences between these groups may show interactions with extinction risk correlates [5], we provide separate assessments of their extinction risk structure.
Two interesting discrepancies with the group analyses emerge. First, in migrants, the environmental-niche latent variable and encroachment (of breeding range) are stronger predictors of extinction risk than in non-migrants. This confirms suggestions that species which carry out long migrations may be particularly vulnerable to alterations of habitats or climatic conditions in their breeding range (table 1 and electronic supplementary material, table S3; [41]). Second, the effect of life-history latent variable on extinction risk is especially strong in island birds (table 1 and electronic supplementary material, table S3). Indeed, extinct native island birds generally are large and often flightless, making them susceptible to being hunted by humans and preyed upon by exotic mammalian predators [39]. Compared with mainland species, extinction risk in island birds is poorly predicted by extrinsic factors. This is consistent with the finding that human population density is a better predictor of threat level in birds in continental than in island nations [42]. This also supports observations in Pacific islands where extant endangered island species may be exposed to similar ecological pathways that lead to extinction since they possess many of the same ecological characteristics of extinct birds [43].
(c). The geography of threat and its predictors
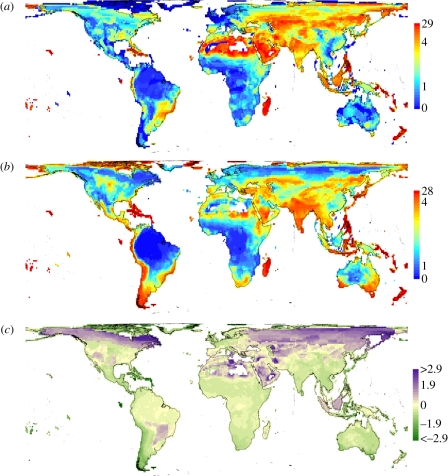
How well can this species-level assessment predict observed geographical gradients in extinction risk? Our map of observed proportion of threatened species confirms known gradients in extinction risk ([19]; figure 4a). Following the approach of Jetz et al. [21], we calculate predicted average assemblage extinction risk based on the extinction risk probabilities predicted by our within-family mixed-effect model for each species in an assemblage (or geographical grid).
Figure 4.
The geography of extinction risk based on global occurrence of species across ca 13 000 110 × 110 km equal-area assemblages. Geographical patterns of (a) observed, (b) predicted percentage of threatened species and (c) observed–predicted in units standard deviation per assemblage. The predictions in (b) are from the species-based extinction risk probabilities of the within-family GLMM. In (c), areas in purple and green shades indicate under-prediction and over-prediction of extinction risk, respectively. Assemblages with less than 10 species are excluded.
We find a positive association between predicted and observed assemblage extinction risk that is strongly mediated by assemblage richness (number of species co-occurring within a grid cell; figure 3b). As expected, average predicted assemblage risk is more variable and provides a weaker fit in low-richness regions where fewer predictions lead to a less stable average. The fits between observed and predicted extinction risks increase towards more diverse assemblages where risk is also proportionally lower (figure 3b). More interestingly, assemblage risk in low-richness areas (10–90 species) is consistently over-predicted by our model, with slopes below the 1 : 1 line (b = 0.17, tb=1 = −28.0; F1,3274 = 33.7; figure 3b). By contrast, for assemblages with greater than 240 species, the model predicts the risk level much better (e.g. b = 0.83, tb=1 = 14.7; F1,3306 = 5226; figure 3b). This interaction between risk prediction and richness in explaining assemblage risk is confirmed statistically (x = −2.86 − 0.85y + 1.04z + 0.68y : z, where x is the observed and y is the predicted proportion of threatened species and z is log (species richness); Akaike Information Criteria (AIC); AICmodel −1051; AICmodel without interaction −155; AICnull 2234) and holds even after accounting for strong spatial autocorrelation signals (see the electronic supplementary material). This may imply that additional community-level effects that our analyses ignore (e.g. species interactions such as competition; [44]) result in species in diverse communities to be more threatened than a species-level analysis would suggest. But alternatively, this pattern may simply be a consequence of the predominance of wide-ranging species in the least diverse areas where under-prediction of only a few of such species are required to drive this pattern across extended regions [20]. Partial support for this idea comes from a significant effect of average log-transformed geographical range size of species as an additional predictor of assemblage extinction risk (x = 1.68 + 3.57y − 0.33z − 0.43y : z, where z is average log range size; AICmodel − 1601; AICmodel without interaction − 1247; AICnull 2234).
In geographical terms, we find some broad concordance between the observed and predicted geography of threat in South America, Africa and East and South Asia (figure 4a,b). The residual map illustrates that regions of under- and over-prediction of assemblage extinction risks are predominantly outside the tropics (except southeast Asia) and near the poles, in areas of lower conservation concern (figure 4c). Above all, the assemblage risk predictions correspond very well with those observed in certain areas where the extinction risk is most severe (red shades, figure 4a,b; off-white, figure 4c) and where conservation actions are most urgently needed. For instance, the Andes and Himalayan mountain ranges and the Atlantic forest region are prominent hotspots of extinction risks. The mountain system hotspots, in particular, will be exacerbated under future climate change because montane bird species are projected to suffer from range contractions owing to limited dispersal and adaptive abilities, and hence higher extinction risks as a result of global warming [45]. Most of the islands (e.g. Madagascar and Polynesia), South and East Asia (e.g. India and China) are also extinction risk hotspots. This is in line with recent calls to prioritize conservation efforts on islands, particularly oceanic ones, owing to their high conservation risks, smaller land areas and high levels of plant and terrestrial vertebrate endemism richness [46].
The quantification of the detailed structure underlying extinction risk across all birds allows us to not only assess the geography of the response variable, but also that of individual predictors and thus different putative causes of cross-species and cross-space variation in extinction risk. Our map of geographical range size confirms existing gradients (electronic supplementary material, figure S5a; [47]), while areas of relatively high encroachment correspond well with intense anthropogenic footprints across the globe and, for example, are located in eastern North and Central America (including the Caribbean islands), and South and East Asia (electronic supplementary material, figure S5b). The life-history, ecological niche, environmental niche and niche-breadth mean latent scores are particularly low (blue shades) across the tropical and subtropical regions, ranging from Central and South America, West and Central Africa to southeast Asia (electronic supplementary material, figure S5c–f). These areas probably support many tropical forest species with small body mass and altricial development mode that feed on invertebrates and fruit or nectar, do not forage exclusively on the ground and around water, have relative narrow habitat breadth and occupy areas with high AET and low seasonality (electronic supplementary material, figure S1).
4. Conclusions
We set out to evaluate core causes of extinction risk in birds at the global scale in a conceptual framework that integrates life-history/ecological attributes as well as geographical environmental and anthropogenic drivers. Our findings provide support for select intrinsic determinants of extinction risk, even when we control for geographical range size, human encroachment and covariation among select latent variables. More importantly, we find that above and beyond all other factors, birds subjected to high past human encroachment (as captured by a remote-sensing-based estimate of range-wide anthropogenic land-cover transformations) are particularly likely to be threatened. This confirms, first, the usefulness of remotely sensed measures for the threat assessment of other less well-known taxa and second, the dire need to overcome the Wallacean shortfall [48,49] that currently limits such evaluations to the small portion of biodiversity with known global distributions.
A dilemma of IUCN Red List categorization to date is its reliance on past data to assess extinction risk, while ultimately seeking to predict the probability of the future survival of species [2]. This issue is exacerbated by the fact that geographical patterns of land-cover change in the past are very different from those projected for the future [50]. While exciting opportunities for the integration of metapopulation or population dynamics with forecasts of environmental change exist, often the demographic data required to parametrize them reliably for large number of species may not be available [18]. Our study provides a quantitative foundation for the potential head-on inclusion—together with the more static ecological and life-history attributes—of spatially explicit projections of encroachment in evaluations of future extinction risk. Such an assessment could be continuously refined as both geographical distribution data and land-cover projections are progressively being updated and become increasingly accurate.
We show how an integrative model combining intrinsic and extrinsic, mostly static and highly dynamic factors allows successful prediction of geographical patterns of extinction risk at the assemblage level. Our findings confirm the value of species-based approaches in understanding the geography of global extinction risk in avian assemblages. Our results highlight the need to appreciate the intricacies and inter-relationships, at the species level, among the factors that structure global extinction risk across species and space. In the context of impeding global change, measures of future extinction risk in an integrative setting are urgently needed to advance the field of predictive biodiversity science and maximize the long-term effectiveness of conservation efforts.
Acknowledgements
We are grateful to C. Sekercioglu for sharing diet data and to many others who helped in the compilation of the various databases. For help and financial support for geographical range map compilation, W.J. thanks J. Gamble, H. Lease, T. Whitaker, J. Hoyo (Lynx Ediciones), A. Richford (Academic Press, Elsevier), C. Kennedy (OUP), C. Perrins, R. Ridgely, T.-S. Ding, R. McCall, P. H. Harvey, S. Pimm and J. H. Brown. For help with additional data entry, W.J. thanks K. Fisch. We thank J. Belmaker, A. Boyer, N. Cooper, A. Davidson, D. Holway, A. Purvis, F. La Sorte and D. Woodruff and two anonymous reviewers for their feedback. This study was supported by NSF grants BCS-0648733 and DBI-0960550.
References
- 1.Purvis A., Gittleman J. L., Cowlishaw G., Mace G. M. 2000. Predicting extinction risk in declining species. Proc. R. Soc. Lond. B 267, 1947–1952 10.1098/rspb.2000.1234 (doi:10.1098/rspb.2000.1234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mace G. M., Collar N. J., Gaston K. J., Hilton-Taylor C., Akcakaya H. R., Leader-Williams N., Milner-Gulland E. J., Stuart S. N. 2008. Quantification of extinction risk: IUCN's system for classifying threatened species. Conserv. Biol. 22, 1424–1442 10.1111/j.1523-1739.2008.01044.x (doi:10.1111/j.1523-1739.2008.01044.x) [DOI] [PubMed] [Google Scholar]
- 3.Bennett P. M., Owens I. P. F. 1997. Variation in extinction risk among birds: chance or evolutionary predisposition? Proc. R. Soc. Lond. B 264, 401–408 10.1098/rspb.1997.0057 (doi:10.1098/rspb.1997.0057) [DOI] [Google Scholar]
- 4.Sodhi N. S., Bickford D., Diesmos A. C., Lee T. M., Koh L. P., Brook B. W., Sekercioglu C. H., Bradshaw C. J. A. 2008. Measuring the meltdown: drivers of global amphibian extinction and decline. PLoS ONE 3, e1636. 10.1371/journal.pone.0001636 (doi:10.1371/journal.pone.0001636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A. 2005. Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 10.1126/science.1116030 (doi:10.1126/science.1116030) [DOI] [PubMed] [Google Scholar]
- 6.McMahon T. A., Bonner J. T. 1983. On size and life. New York, NY: Scientific American Books, W. H. Freeman & Company [Google Scholar]
- 7.IUCN 2001. Red List Categories and Criteria: version 3.1. IUCN Species Survival Commission. Gland, Switzerland: IUCN [Google Scholar]
- 8.Olson V. A., Davies R. G., Orme C. D. L., Thomas G. H., Meiri S., Blackburn T. M., Gaston K. J., Owens I. P. F., Bennett P. M. 2009. Global biogeography and ecology of body size in birds. Ecol. Lett. 12, 249–259 10.1111/j.1461-0248.2009.01281.x (doi:10.1111/j.1461-0248.2009.01281.x) [DOI] [PubMed] [Google Scholar]
- 9.Cardillo M., Purvis A., Sechrest W., Gittleman J. L., Bielby J., Mace G. M. 2004. Human population density and extinction risk in the world's carnivores. PLoS Biol. 2, 909–914 10.1371/journal.pbio.0020197 (doi:10.1371/journal.pbio.0020197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson A. D., Hamilton M. J., Boyer A. G., Brown J. H., Ceballos G. 2009. Multiple ecological pathways to extinction in mammals. Proc. Natl Acad. Sci. USA 106, 10 702–10 705 10.1073/pnas.0901956106 (doi:10.1073/pnas.0901956106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owens I. P. F., Bennett P. M. 2000. Ecological basis of extinction risk in birds: habitat loss versus human persecution and introduced predators. Proc. Natl Acad. Sci. USA 97, 12 144–12 148 10.1073/pnas.200223397 (doi:10.1073/pnas.200223397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackburn T. M., Gaston K. J. 2002. Extrinsic factors and the population sizes of threatened birds. Ecol. Lett. 5, 568–576 10.1046/j.1461-0248.2002.00360.x (doi:10.1046/j.1461-0248.2002.00360.x) [DOI] [Google Scholar]
- 13.Cardillo M., Mace G. M., Gittleman J. L., Purvis A. 2006. Latent extinction risk and the future battlegrounds of mammal conservation. Proc. Natl Acad. Sci. USA 103, 4157–4161 10.1073/pnas.0510541103 (doi:10.1073/pnas.0510541103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardillo M., Mace G. M., Gittleman J. L., Jones K. E., Bielby J., Purvis A. 2008. The predictability of extinction: biological and external correlates of decline in mammals. Proc. R. Soc. B 275, 1441–1448 10.1098/rspb.2008.0179 (doi:10.1098/rspb.2008.0179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper N., Bielby J., Thomas G. H., Purvis A. 2008. Macroecology and extinction risk correlates of frogs. Glob. Ecol. Biogeogr. 17, 211–221 10.1111/j.1466-8238.2007.00355.x (doi:10.1111/j.1466-8238.2007.00355.x) [DOI] [Google Scholar]
- 16.Harris G., Pimm S. L. 2008. Range size and extinction risk in forest birds. Conserv. Biol. 22, 163–171 10.1111/j.1523-1739.2007.00798.x (doi:10.1111/j.1523-1739.2007.00798.x) [DOI] [PubMed] [Google Scholar]
- 17.Jetz W., Wilcove D. S., Dobson A. P. 2007. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 5, 1211–1219 10.1371/journal.pbio.0050157 (doi:10.1371/journal.pbio.0050157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keith D. A., Akcakaya H. R., Thuiller W., Midgley G. F., Pearson R. G., Phillips S. J., Regan H. M., Araujo M. B., Rebelo T. G. 2008. Predicting extinction risks under climate change: coupling stochastic population models with dynamic bioclimatic habitat models. Biol. Lett. 4, 560–563 10.1098/rsbl.2008.0049 (doi:10.1098/rsbl.2008.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies R. G., et al. 2006. Human impacts and the global distribution of extinction risk. Proc. R. Soc. Lond. B 273, 2127–2133 10.1098/rspb.2006.3551 (doi:10.1098/rspb.2006.3551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jetz W., Rahbek C. 2002. Geographic range size and determinants of avian species richness. Science 297, 1548–1551 10.1126/science.1072779 (doi:10.1126/science.1072779) [DOI] [PubMed] [Google Scholar]
- 21.Jetz W., Sekercioglu C. H., Bohning-Gaese K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6, 2650–2657 10.1371/journal.pbio.0060303 (doi:10.1371/journal.pbio.0060303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaston K. J., Blackburn T. M., Goldewijk K. K. 2003. Habitat conversion and global avian biodiversity loss. Proc. R. Soc. Lond. B 270, 1293–1300 10.1098/rspb.2002.2303 (doi:10.1098/rspb.2002.2303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pimm S., Raven P., Peterson A., Sekercioglu C. H., Ehrlich P. R. 2006. Human impacts on the rates of recent, present, and future bird extinctions. Proc. Natl Acad. Sci. USA 103, 10 941–10 946 10.1073/pnas.0604181103 (doi:10.1073/pnas.0604181103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekercioglu C. H., Daily G. C., Ehrlich P. R. 2004. Ecosystem consequences of bird declines. Proc. Natl Acad. Sci. USA 101, 18 042–18 047 10.1073/pnas.0408049101 (doi:10.1073/pnas.0408049101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Environmental Systems Research Institute (ESRI) 2004. ArcGIS desktop 9. Redlands, CA: Environmental Systems Research Institute [Google Scholar]
- 26.McCune B., Grace J. B. 2002. Analysis of ecological communities. Oregon, OR: MjM Software Design [Google Scholar]
- 27.Bollen K. A. 1989. Structural equations with latent variables. New York, NY: Wiley and Sons [Google Scholar]
- 28.Muthén L. K., Muthén B. O. 2007. MPlus User's Guide. Los Angeles, CA: Muthén & Muthén [Google Scholar]
- 29.Skrondal A., Rabe-Hesketh S. 2004. Generalized latent variable modeling: multilevel, longitudinal, and structural equation models. Boca Raton, FL: Chapman & Hall/CRC [Google Scholar]
- 30.Owens I. P. F., Bennett P. M. 1995. Ancient ecological diversification explains life-history variation among living birds. Proc. R. Soc. Lond. B 261, 227–232 10.1098/rspb.1995.0141 (doi:10.1098/rspb.1995.0141) [DOI] [Google Scholar]
- 31.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 32.Lande R., Engen S., Saether E. 2003. Stochastic population dynamics in ecology and conservation. Oxford Series in Ecology and Evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 33.Keane A., Brooke M. D., McGowan P. J. K. 2005. Correlates of extinction risk and hunting pressure in gamebirds (Galliformes). Biol. Conserv. 126, 216–233 10.1016/j.biocon.2005.05.011 (doi:10.1016/j.biocon.2005.05.011) [DOI] [Google Scholar]
- 34.Wilcove D. S. 1985. Nest predation in forest tracts and the decline of migratory songbirds. Ecology 66, 1211–1214 10.2307/1939174 (doi:10.2307/1939174) [DOI] [Google Scholar]
- 35.Sodhi N. S., Liow L. H., Bazzaz F. A. 2004. Avian extinctions from tropical and subtropical forests. Annu. Rev. Ecol. Evol. Syst. 35, 323–345 10.1146/annurev.ecolsys.35.112202.130209 (doi:10.1146/annurev.ecolsys.35.112202.130209) [DOI] [Google Scholar]
- 36.Scharlemann J. P. W., Balmford A., Green R. E. 2005. The level of threat to restricted-range bird species can be predicted from mapped data on land use and human population. Biol. Conserv. 123, 317–326 10.1016/j.biocon.2004.11.019 (doi:10.1016/j.biocon.2004.11.019) [DOI] [Google Scholar]
- 37.Akcakaya H. R., Stark J. D., Bridges T. S. (eds) 2008. Demographict toxicity: methods in ecological risk assessment. New York, NY: Oxford University Press [Google Scholar]
- 38.Brook B. W., Sodhi N. S., Bradshaw C. J. A. 2008. Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460 10.1016/j.tree.2008.03.011 (doi:10.1016/j.tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- 39.Blackburn T. M., Cassey P., Duncan R. P., Evans K. L., Gaston K. J. 2004. Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958 10.1126/science.1101617 (doi:10.1126/science.1101617) [DOI] [PubMed] [Google Scholar]
- 40.Green A. J. 1996. Analyses of globally threatened anatidae in relation to threats, distribution, migration patterns, and habitat use. Conserv. Biol. 10, 1435–1445 10.1046/j.1523-1739.1996.10051435.x (doi:10.1046/j.1523-1739.1996.10051435.x) [DOI] [Google Scholar]
- 41.Holmes R. T., Sherry T. W. 2001. Thirty-year bird population trends in an unfragmented temperate deciduous forest: importance of habitat change. Auk 118, 589–609 10.1642/0004-8038(2001)118[0589:TYBPTI]2.0.CO;2 (doi:10.1642/0004-8038(2001)118[0589:TYBPTI]2.0.CO;2) [DOI] [Google Scholar]
- 42.McKinney M. L. 2001. Role of human population size in raising bird and mammal threat among nations. Anim. Conserv. 4, 45–57 10.1017/S1367943001001056 (doi:10.1017/S1367943001001056) [DOI] [Google Scholar]
- 43.Boyer A. G. 2010. Consistent ecological selectivity through time in Pacific island avian extinctions. Conserv. Biol. 24, 511–519 10.1111/j.1523-1739.2009.01341.x (doi:10.1111/j.1523-1739.2009.01341.x) [DOI] [PubMed] [Google Scholar]
- 44.Ricklefs R. E. 2004. A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 7, 1–15 10.1046/j.1461-0248.2003.00554.x (doi:10.1046/j.1461-0248.2003.00554.x) [DOI] [Google Scholar]
- 45.La Sorte F. A., Jetz W. 2010. Projected range contractions of montane biodiversity under global warming. Proc. R. Soc. B 277, 3401–3410 10.1098/rspb.2010.0612 (doi:10.1098/rspb.2010.0612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kier G., Kreft H., Lee T. M., Jetz W., Ibisch P. L., Nowicki C., Mutke J., Barthlott W. 2009. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl Acad. Sci. USA 106, 9322–9327 10.1073/pnas.0810306106 (doi:10.1073/pnas.0810306106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orme C. D. L., et al. 2006. Global patterns of geographic range size in birds. PLoS Biol. 4, 1276–1283 10.1371/journal.pbio.0040208 (doi:10.1371/journal.pbio.0040208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrier S., et al. 2004. Mapping more of terrestrial biodiversity for global conservation assessment. Bioscience 54, 1101–1109 10.1641/0006-3568(2004)054[1101:MMOTBF]2.0.CO;2 (doi:10.1641/0006-3568(2004)054[1101:MMOTBF]2.0.CO;2) [DOI] [Google Scholar]
- 49.Whittaker R. J., Araujo M. B., Paul J., Ladle R. J., Watson J. E. M., Willis K. J. 2005. Conservation biogeography: assessment and prospect. Divers. Distrib. 11, 3–23 10.1111/j.1366-9516.2005.00143.x (doi:10.1111/j.1366-9516.2005.00143.x) [DOI] [Google Scholar]
- 50.Lee T. M., Jetz W. 2008. Future battlegrounds for conservation under global change. Proc. R. Soc. B 275, 1261–1270 10.1098/rspb.2007.1732 (doi:10.1098/rspb.2007.1732) [DOI] [PMC free article] [PubMed] [Google Scholar]