Abstract
C-type lectin receptors encoded by the natural killer gene complex play critical roles in enabling NK cell discrimination between self and non-self. In recent years, additional genes at this locus have been identified with patterns of expression that extend to cells of the myeloid lineage where many of the encoded inhibitory receptors have equally important functions as regulators of immune homeostasis. In the present review we highlight the roles of some of these receptors including recent insights gained with regard to the identification of exogenous and endogenous ligands, mechanisms of cellular inhibition and activation, regulated expression within different cellular and immune contexts, as well as functions that include the regulation of bone homeostasis and involvement in autoimmunity.
Keywords: Inhibitory receptor, C-type lectin, Myeloid cell, Immune homeostasis
1. Introduction
1.1. C-type lectin receptors
C-type lectin receptors (CLRs) are characterised by the presence of one or more C-type lectin-like (CTLD) domains. The CTLD, referred to as a carbohydrate-recognition domain (CRD) in cases where carbohydrates are recognised, comprises a distinctive, compact protein fold arising from disulphide bridges formed between six conserved canonical cysteine residues [1] and is marked by its ability to recognise a diverse repertoire of structurally dissimilar microbe-associated or endogenous ligands. Classical CLRs constitute the largest and most diverse group and bind carbohydrates in a calcium-dependent manner. These C-type lectins harbour mannose-binding EPN (Glu-Pro-Asn) or galactose-binding QPD (Gln-Pro-Asp) triplets in their CRDs [2]. Their non-classical counterparts, while being structurally homologous, lack the residues required for calcium-dependent carbohydrate binding and are referred to as C-type lectin-like receptors (CLLRs) [3]. These receptors either use alternative mechanisms in carbohydrate recognition or recognise non-carbohydrate ligands such as proteins.
Membrane-bound CLRs were initially divided into two types: Type I CLRs (mannose receptor family) have multiple CRDs at their NH2 terminus which facilitate the binding and internalisation of glycosylated antigens by receptor-mediated endocytosis. Type I CLRs include the macrophage-mannose receptor (MMR) and DEC205, as well as selectins which mediate tethering and rolling of leukocytes on endothelial cells. Type II CLRs (asialoglycoprotein-receptor family) have a single CRD at the COOH-terminus and include hepatic asialoglycoprotein receptors (ASGPRs), macrophage lectin, DC-specific ICAM3-grabbing non-integrin (DC-SIGN), Langerin, DC-associated C-type lectin (dectin-1) and DC immunoreceptor (DCIR) [4]. More recently however, these functionally heterogeneous lectins have been divided into 17 groups based upon domain organisation and phylogeny [3].
1.2. The natural killer gene complex (NKC)
The natural killer gene complex (NKC) located on chromosome 6F3 in the mouse and on chromosome 12p13.1 in humans, is a genetic locus encoding numerous activating and inhibitory receptors originally identified based upon their predominant expression on natural killer (NK) cells [5] (Fig. 1). These receptors play critical roles in enabling NK cell discrimination between self, non-self, missing-self and induced self where they regulate the fine balance between NK cell activation and inhibition. Many of these receptors are group-II and -V C-type lectins which are also expressed on cells of the myeloid lineage including neutrophils, dendritic cells (DCs), monocytes and macrophages. In this context, they recognise endogenous and/or exogenous ligands and as such, may have roles in homeostatic regulation of the immune system. Table 1 provides a summary of selected CLRs expressed on myeloid cells.
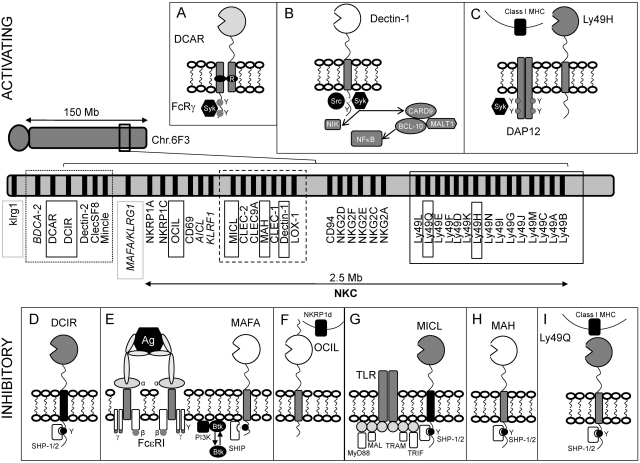
Fig. 1.
C-type lectin receptors encoded by the natural killer gene complex (NKC). The murine NKC comprises genes located on chromosome 6F3 and spans a region of approximately 2.5 Mb. In humans, its equivalent is located on chromosome 12p13.1. The dectin-1 cluster (black dashed square) comprises genes encoding group V CLRs including MICL (G), CLEC-2, CLEC-9A, MAH (H), CLEC-1, dectin-1 (B) and LOX-1. The dectin-2 cluster (black dotted square) comprises genes centromeric to the NKC which encode group II CLRs including BDCA-2, DCAR (A), DCIR (D), dectin-2, CLECSF8 and Mincle. The murine Ly49 family (black solid square) includes both activating receptors such as Ly49H (C) and inhibitory receptors such as Ly49Q (I). Genes encoding MAFA/KLRG1 (humans) and its murine orthologue (klrg1) are highlighted in solid grey squares. Names in italics represent genes present in humans but absent from the mouse NKC. Upper panels show activating receptors and bottom panels show inhibitory receptors, both in order of chromosomal localisation from left (centromeric) to right (telomeric). ITAM:  ; ITIM: ●; ITAM-like: ○. Activating receptor substrates such as Src and Syk kinases and inhibitory receptor substrates such as protein tyrosine phosphatases SHP-1,-2 and SHIP are also shown. (E) Rat MAFA inhibits the secretory response induced by IgE-mediated FcɛRI aggregation, (G) human MICL ligation suppresses TLR-induced responses within specific immune and cellular contexts.
; ITIM: ●; ITAM-like: ○. Activating receptor substrates such as Src and Syk kinases and inhibitory receptor substrates such as protein tyrosine phosphatases SHP-1,-2 and SHIP are also shown. (E) Rat MAFA inhibits the secretory response induced by IgE-mediated FcɛRI aggregation, (G) human MICL ligation suppresses TLR-induced responses within specific immune and cellular contexts.
Table 1.
Selected activating and inhibitory C-type lectin receptors expressed in myeloid cells.
| Group | CLR | Expression | Ligand/s | Signalling | References |
|---|---|---|---|---|---|
| II Calcium-dependent CRD |
BDCA-2 | pDC, Mo, MØ, Neu. | Unknown | FcRγ: activating and inhibitory. Syk, PLCγ2, BLNK, BTK | [114–116] |
| DCAR | DC, Mo, MØ, B. | Unknown | FcRγ: activating | [67] | |
| DCIR | mDCs, pDCs, Mo, MØ, B, Neu. | PRR for HIV-1 | ITIM: inhibitory. SHP-1/SHP-2 | [63,65,71] | |
| Dectin-2 | mDCs, pDCs, Mo, MØ, B, Neu. | PRR for various fungi and house dust mites | FcRγ: activating. Src kinases and Syk | [117–119] | |
| CLECS-F8 | MØ | Unknown | Unknown | [120] | |
| Mincle | mDCs, Mo, MØ | Damaged cells; PRR for Malassezia species, Mycobacteria, Candida | FcRγ: activating. SYK and CARD9 | [121–124] | |
| DC-SIGN | mDCs | PRR for numerous viral, bacterial and fungal species. E.g. M. tuberculosis and HIV-1. Endogenous ligands: ICAM-2, ICAM-3, CEACAM-1, CEA. | No motif or adaptor. Mostly activating. Src kinases, Ras, RAF1, PAKs, RHOA, LSP1, LARG. | [125–148] | |
| Langerin | LCs, dermal DC subset | PRR for HIV-1 and fungal species. Endogenous ligands: Type I pro-collagen. | Proline-rich domain. Unknown signalling function. | [149–156] | |
| MGL | mDCs, MØ | PRR for Filoviruses, Influenza virus and S. Mansoni. Endogenous ligands: CD45, gangliosides, MUC-1. | Unknown | [157–162] | |
| V Non-calcium-dependent CTLD |
MICL | mDCs, Mo, MØ, Neu. | Unknown endogenous mMICL ligands detected in several tissues. | ITIM. Inhibitory. SHP1/SHP2, ERK. | [51–56] |
| CLEC-2 | Platelets, peripheral blood neutrophils | Podoplanin, Snake venom rhodocytin, PRR for HIV-1. | ITAM-like YxxL. Activating. Syk, PLCγ2, RAC1, LAT, Vav1/3, SLP-76, Btk. | [163–173] | |
| CLEC9A | BDCA3+ DCs, Mo, B. | Necrotic cells | ITAM-like YxxL, Syk. Activating | [174–177] | |
| MAH | MØ | Unknown | ITIM, SHP-1, SHP-2 | [59] | |
| CLEC-1 | DCs | Unknown | Unknown | [163,178] | |
| Dectin-1 | mDCs, Mo, MØ, B. | PRR for M. tuberculosis and various fungal species. Recognises an endogenous ligand on T cells. | ITAM-like YxxL. Activating. Syk, PLCγ2, CARD9, Bcl10, MALT1, NIK, RAF-1. | [25–27,179–191] | |
| LOX-1 | MØ, platelets, endothelial cells, smooth muscle cells. | Scavenger receptor for oxidised LDL and red blood cells. PRR for bacterial species including E. coli and S. Aureus. | Activating. | [192–209] | |
| OCIL | MØ, DCs, Osteoblasts | NKRP1d | Inhibitory | [83,88,90,92,93] | |
| VI Calcium-dependent CRD (Classical) |
Mannose receptor | mDCs, MØ | PRR for Mycobacteria, various bacterial species, HIV-1, fungal species, allergens. Endogenous ligands: l-selectin, MUC-1. | Cdc42, ROCK1, PAKs, RHOB. | [210–217] |
| DEC205 | mDCs | Unknown | Unknown | [218–221] | |
DC, dendritic cell; pDC, plasmacytoid dendritic cell; mDC, myeloid dendritic cell; MØ, macrophage; Mo, monocyte; Neu, Neutrophil; LC, Langerhans cell; PRR, pattern recognition receptor.
In mice, NKC-encoded receptors include members of the NKRP1 and Ly49 families (Fig. 1). Independent control of Ly49 gene transcription allows for mono-allelic expression on overlapping subsets of NK cells and T-lymphocytes. Members of the Ly49 family recognize polymorphic epitopes on H-2D and H-2K Class I MHC molecules and play important roles in regulating NK cytotoxicity, where cells inappropriately expressing reduced cell surface Class I MHC or related molecules are destroyed [6]. The Ly49 family includes activating receptors such as Ly49D and Ly49H (Official name: Klra8) which associate with immunoreceptor tyrosine-based activation motif (ITAM)-bearing adaptor proteins such as DNAX Activating Protein of 12 kDa (DAP-12) (Fig. 1C). Inhibitory receptors within this family include Ly49Q which harbour immunoreceptor tyrosine-based inhibition motifs (ITIMs) in their cytoplasmic domains. ITIM tyrosine phosphorylation results in the recruitment of the protein tyrosine phosphatases SHP-1 and SHP-2, the inhibition of cytokine production, the suppression of NK cytotoxicity and the consequent prevention of self-killing of target cells by NK cells [7–9]. Furthermore, activating and inhibitory Ly49 receptors may be expressed simultaneously allowing for the selective elimination of virus-infected or transformed target cells that show lost or reduced expression of inhibitory receptor ligand but retain the expression of ligand for activating receptors [10].
The human equivalents of the Ly49 family are referred to as killer cell Ig-like receptors (KIRs) and they recognize human leukocyte antigens such as HLA-A, HLA-B and HLA-C. The human NKC also encodes group V CLRs such as CD69, CD94 and members of the NKG2 family which recognise HLA-E or ligands expressed on stressed, virally infected or tumourigenic cells [5,11] (Fig. 1). Many NKC-encoded CLR genes expressed in myeloid cells occur in two distinct clusters: The dectin-1 cluster includes genes encoding group V CLRs such as dectin-1, lectin-like receptor for low density lipoprotein-1 (LOX-1), myeloid inhibitory C-type lectin-like receptor (MICL), C-type lectin-like receptor (CLEC)-1, CLEC-2, CLEC-9A and macrophage antigen h (MAH) (CLEC-12B) [12,13] (Fig. 1). Group V CLRs are non-classical type II trans-membrane proteins harbouring a single CTLD, a stalk region of variable length and a cytoplasmic tail which may contain consensus signalling motifs. The dectin-2 cluster which occurs centromeric to the NKC includes genes encoding group II CLRs including dectin-2, CLECS-F8, Mincle, BDCA-2, DCAR and DCIR (Fig. 1). Group II CLRs are generally classical C-type lectins with similar structures to those of group V CLRs but have shorter cytoplasmic tails [3].
1.3. Activating and inhibitory receptors
CLRs may be activating or inhibitory based upon their ability to associate with signalling molecules or the presence of specific motifs in their cytoplasmic tails. Most Group II CLRs such as dectin-2, DCAR, BDCA-2 and Mincle are predicted to be activating receptors based upon the association of a positively charged residue in their trans-membrane region with adaptor proteins such as FcγR which in turn harbour ITAMs (Fig. 1A). Following ligand binding and receptor clustering, the tyrosine residues of an ITAM (YxxI/Lx(6–12)YxxI/L) are phosphorylated by Src family kinases which in turn promote the recruitment of Syk family kinases [14–17]. The initiation of a series of downstream signalling events usually culminates in the activation of various cellular responses [18–21].
Other activating CLRs such as the fungal pattern recognition receptor (PRR) dectin-1 harbour ITAM-like motifs (Fig. 1B). These motifs are defined by the presence of a dispensable membrane distal N-terminal tyrosine residing within a YxxxL/I sequence as opposed to the YxxL/I motif in a conventional ITAM [22–27].
Inhibitory CLRs exemplified by DCIR and MICL may be defined by the presence of ITIMs (I/V/L/SxYxxI/L/V) in their cytoplasmic tails. Here, receptor engagement leads to ITIM tyrosine phosphorylation by Src kinases, the recruitment and activation of protein tyrosine phosphatases such as SHP-1 and SHP-2 and the dephosphorylation of substrates regulated by immunoreceptors leading to the inhibition of cellular activation [10,28] (Fig. 1D and G).
Several examples also exist of ITIM-bearing receptors paradoxically mediating cellular activation. In such cases, the receptors may recruit novel substrates to their cytoplasmic domains or they may inhibit other inhibitory receptors in a more conventional SHP-1/2-dependent manner: the platelet co-stimulatory ITIM receptor TREM-like transcript-1 (TLT-1) recruits SHP-2 yet augments FcɛRI-mediated calcium signalling. It has been suggested that this may be mediated by a unique poly-proline-rich region in TLT-1 which could recruit SH3 domain-containing substrates [29]. Another example is that of signal regulatory protein-α (Sirp-α), a receptor belonging to the immunoglobulin superfamily (IgSF) [30–35] that is expressed on myeloid cells and neurons [36–41]. Binding of SIRP-α to its widely expressed endogenous ligand, CD47 has both inhibitory as well as activating effects. The inhibitory effects, mediated by the recruitment of SHP-1 and SHP-2 [42–46] include the inhibition of red blood cell phagocytosis by macrophages [43] and the inhibition of DC maturation and cytokine production [40]. The activating effects of this ITIM-bearing receptor may be mediated by the recruitment of JAK-2 to its cytoplasmic C-terminal tyrosine. This triggers the JAK-2/STAT, PI3K/Rac-1/NADPH oxidase/H2O2 pathways, enhancing the retention of SHP-1 and SHP-2 and abrogating their inhibitory effects [47]. In this regard, SIRP-α has been shown to play a role in the promotion of antigen-specific cytotoxic T-lymphocyte activation by DCs [41], the induction of nitric oxide and reactive oxygen species production in macrophages [47] as well as the development of Th17-driven auto-immune diseases such as contact hypersensitivity, collagen-induced arthritis and experimental autoimmune encephalomyelitis [48–50].
The present mini-review will highlight selected myeloid cell-expressed CLRs including MICL, MAH, DCIR, Ly49Q, OCIL and MAFA with an emphasis on their roles in the regulation of immune homeostasis as well as their ability to have both inhibitory and activating effects.
2. Myeloid inhibitory C type-like lectin (MICL)
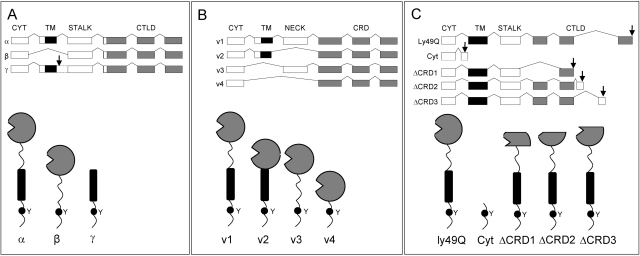
MICL (DCAL-2, CLL-1, KLRL1) (Official name: CLEC12A) has been identified independently by several groups [51–54]. It is a type II trans-membrane glycoprotein comprising an extracellular C-terminal CTLD, a stalk, trans-membrane region and an N-terminal cytoplasmic tail (Fig. 1G). The CTLD of human MICL (hMICL) shares ∼30% identity with that of other dectin-1 cluster CLLRs (Fig. 1), and 49% identity with the CTLD of murine MICL (mMICL) [54,55]. Marshall et al. [53] also reported three alternatively spliced hMICL isoforms α,β and γ (Fig. 2A). The β-isoform lacks the exon encoding the trans-membrane region while the γ-isoform has a stop codon after the exon encoding the trans-membrane region. The extracellular portion of hMICL comprises six potential N-glycosylation sites with the two most C-terminal sites of the stalk region contributing the majority of cell-specific N-glycosylation. This feature is thought to prevent its dimerisation despite the presence of two conserved cysteine residues [53,56]. In contrast, mMICL has one less stalk N-glycosylation site and has been reported to exist as a dimer.
Fig. 2.
Isoforms of inhibitory C-type lectin receptors. (A) Three alternatively spliced hMICL isoforms (α, β, γ). (B) Four different forms of alternatively spliced DCIR mRNA (v1–v4). (C) Four splice variants of the ly49q1 gene in mouse strains JF1, MSM and SV129 (Cyt, ΔCRD1, ΔCRD2, ΔCRD3). Arrows indicate translation stop codons.
At the protein level hMICL expression has been detected in the spleen and on myeloid cells including DCs, monocytes and granulocytes in human peripheral blood and bone marrow [52,54,56,57], but has been shown to be absent from blood NK cells. Furthermore, it is specifically expressed on primary acute myeloid leukaemia (AML) blasts and in a leukemic CD34 + CD38-stem cell compartment, which has highlighted its potential as a diagnostic and therapeutic target in AML [52,58]. mMICL protein appears to have a broader cellular distribution and has been found to be expressed in the spleen and on peripheral blood monocytes, neutrophils, eosinophils and basophils as well as on B-lymphocytes, bone marrow-derived DCs and thioglycolate-elicited macrophages and neutrophils. While mMICL is absent from blood NK cells, it is expressed on bone marrow NK cells [55,57].
Both MICL orthologues show a down-regulation in their expression in response to myeloid cell activation, migration to peripheral tissues and recruitment to sites of inflammation [52,53,55,56]. MICL harbours an ITIM (human: VTYADL; mouse: IVYANL) in its cytoplasmic tail which associates with SHP-1 and SHP-2 (Fig. 1G). A chimera comprising a portion of the MICL stalk, trans-membrane region and cytoplasmic tail fused with the extracellular portion of dectin-1 was able to suppress zymosan-induced TNF-α production through full-length dectin-1, supporting MICL's primary role as an inhibitory receptor [53]. A down-regulation in its expression may thus attenuate these inhibitory effects and potentiate myeloid cell activation.
Apart from its ability to inhibit activating receptors, MICL has been shown to mediate antigen uptake and presentation [53,57]. mMICL-expressing CD8+ conventional DCs (cDCs) were successfully targeted with an anti-mMICL rat monoclonal antibody and elicited robust anti-rat Ig responses in conjunction with the TLR4 agonist, LPS. Furthermore, conjugation of OVA to this monoclonal antibody induced the proliferation of OVA-specific T-lymphocytes [57].
In immature DCs and in the absence of TLR agonists, cross-linking of hMICL induced tyrosine phosphorylation, ERK and p38MAPK activation, an up-regulation of CCR7 expression and the induction of IL-6 and IL-10 production [54]. However, in the presence of TLR agonists or T-lymphocyte-dependent CD40L signalling hMICL ligation appeared to have different effects on DCs. Here, TLR-induced IL-12 expression and the induction of Th1 cells was suppressed by hMICL cross-linking while in response to CD40L signalling, IL-12 production and Th1 polarization was enhanced by hMICL ligation [54]. Similar antibody-mediated cross-linking approaches had no effects on primary murine leukocyte responses [55]. This, together with differences in the ability of hMICL and mMICL to dimerise as well as the broader cellular distribution of mMICL suggests that these orthologues may play different roles in vivo as homeostatic receptors.
As an orphan receptor, the ligand/s of MICL are as yet unknown. Using an Fc-mMICL fusion protein together with a BWZ.36 reporter cell system, Pyz et al. [55] detected putative ligand expression in several tissues including bone marrow, thymus, heart, spleen and kidney. Such a broad expression of ligands suggests a possible role for mMICL as a regulator of immune homeostasis where it may interact with endogenous ligands in the blood or at sites of immune privilege where MICL-expressing myeloid cells are not normally activated [53,55].
3. Macrophage antigen H (MAH)
Macrophage antigen H (MAH) (Official name: CLEC12B) was identified based on a search for homology with the NK cell receptor NKG2D (36% similarity) [59]. MAH is located within the dectin-1 cluster of the NKC and within this cluster it shares the highest homology with MICL (Clec-12A) (34% similarity) (Fig. 1H). Human MAH (hMAH) is a type II trans-membrane glycoprotein that is expressed on in vitro differentiated macrophages. Unlike NKG2D, which is an activating receptor that harbours a charged residue in its trans-membrane region, both mouse and human MAH contain an ITIM (VTYATL) in their cytoplasmic tails. Furthermore, following receptor phosphorylation, this ITIM is able to recruit SHP-1 and SHP-2. As such, MAH triggering was not only able to inhibit NKG2D-mediated NK cell activation but could also inhibit activating signals emanating from other NK receptors such as 2B4 [59]. To date the in vivo role as well as the identity of MAH ligands remain unknown.
4. Dendritic cell immunoreceptor (DCIR)
Human DCIR (hDCIR) (CLECS-F6, LLIR) (Official name: CLEC4A) was identified based upon homology with the macrophage lectin (42%) and hepatic asialoglycoprotein receptors (ASGPR)-1 and -2 (35–37%) [60]. It is expressed on monocytes, neutrophils, macrophages, monocyte-derived DCs, myeloid DCs, plasmacytoid DCs (pDCs) and B-lymphocytes but not on NK cells [60–63]. hDCIR is a 237 amino acid glycoprotein with a single N-glycosylation site [60] (Fig. 1D). It has a calcium-binding CRD containing an EPS (Glu-Pro-Ser) motif that enables binding to galactose-containing ligands. However, to date the identity of these ligands remains unknown. Murine DCIR (mDCIR) shares 54% identity and 65% homology with its human orthologue and has two additional predicted N-glycosylation sites.
Alternate splicing results in the generation of four different forms of DCIR mRNA (v1–v4) which have been detected in several tissues and cell-types (Fig. 2B) [60,62]. In neutrophils, this includes a short form missing the neck region of 33 amino acids (v2) while in DCs, two trans-membrane deletion variants have been found (v3 and v4) [64]. The fourth (long) form of DCIR (v1) is predicted to have the ability to form functional oligomers at the cell surface, a possible requirement for efficient ligand binding and inhibitory signal transmission. The short form-encoded protein on the other hand lacks a neck region cysteine residue required for oligomerization and as such is predicted to exist as a non-functional monomer.
Like MICL, DCIR harbours a canonical ITIM (ITYAEV) in its cytoplasmic tail that recruits phosphorylated SHP-1 and non-phosphorylated SHP-2. In support of its role as an inhibitory receptor, Kanazawa et al. [61] showed that a chimeric receptor comprising the cytoplasmic tail of mDCIR and the extracellular portion of FcγRIIB, was able to inhibit protein tyrosine phosphorylation and Ca2+ mobilisation following colligation with the B-cell receptor (BCR) and that this was dependent upon an intact tyrosine within the ITIM of DCIR.
DCIR may modulate immune responses by exhibiting inhibitory cross-talk with other receptors. In the case of human pDCs, antibody-mediated cross-linking of DCIR resulted in its clathrin-dependent internalisation and trafficking to endosomal compartments where it inhibited TLR9-induced TNFα and IFNγ production [63]. Furthermore, in human monocyte-derived DC's, antibody-mediated cross-linking resulted in a similar internalisation of DCIR and the inhibition of TLR 8-induced TNFα and IL-12 production [65]. Internalised DCIR was also able to present antigens to T-lymphocytes [63] and in this regard Klechevsky et al. [66] recently showed that in human DCs, DCIR mediated potent antigen cross-presentation and the induction of antigen-specific CD8+ T lymphocyte immunity that was augmented with TLR 7/8 agonists.
DCIR may form part of an inhibitory-activating receptor pair without a requirement for receptor-mediated endocytosis. The dendritic cell activating receptor (DCAR) (Official name: clec4b1) shares a 91% amino acid sequence identity with DCIR in its CRD suggesting that the two receptors may recognise similar or even identical ligands. As an activating receptor DCAR transmits signals via an association between a charged arginine in its trans-membrane region and the ITAM-containing adaptor FcRγ (Fig. 1A) [67]. These activating signals may be inhibited via the ITIM of DCIR [68].
Cellular activation and inflammation may be achieved by reducing the ability of DCIR to engage ligand or by decreasing its surface expression. In DC's DCIR surface expression was down-regulated in response to signals inducing DC maturation [60]. Similarly, in pDCs, TLR9-induced maturation reduced DCIR expression [63]. In neutrophils, pro-inflammatory stimuli such as LPS, TNF-α and IL-1α mediated their effects by blocking the inhibitory effect of DCIR through a reduction in its surface expression [62]. Similarly, IL-3, IL-4, IL-13 and GM-CSF induced cellular activation and consequent inflammation by promoting an accumulation of mRNA encoding shorter non-functional DCIR which competes for translation with that encoding the long form of DCIR (Fig. 2B), reducing its surface expression and its ability to engage ligands and transmit inhibitory signals [62].
In light of its likely role as an inhibitory CLR, the possible in vivo functions of DCIR have been investigated in DCIR knockout mice. Here, DCIR deficiency was associated with the spontaneous development of a late onset disease resulting in joint abnormalities. Histologically, this was characterised by enthesitis and sialadenitis as well as elevated levels of auto-antibodies. Moreover, these animals showed increased populations of DCs and activated CD4+ T cells. Young DCIR−/− mice were susceptible to collagen-induced arthritis marked by excessive DC expansion in the lymph nodes, an increase in cytokines IL-4, IL-10 and IL-17 as well as increased IgG1 and IgG3 production. In support of the role of excessive DCIR−/− DC expansion in collagen-induced arthritis, the authors also reported enhanced DCIR−/− bone marrow-derived DC (BMDC) proliferation in response to GM-CSF and enhanced STAT-5 phosphorylation suggesting that DCIR negatively regulated DC expansion and GM-CSF signalling [69]. In human studies, rheumatoid arthritis has been associated with the widespread and abundant expression of DCIR in NK cells, CD4+ and CD8+ T cells, monocytes, B cells, DCs and granulocytes suggesting that synovial inflammation induces DCIR expression. Furthermore, DCIR+ T-cells in synovial fluid were activated and found with a greater abundance as compared with peripheral blood. However, the function of DCIR within this T lymphocyte population remains as yet unknown [70].
In addition to the recognition of endogenous ligands, DCIR also binds exogenous ligands. The neck region has been shown to play a key role in the ability of DCIR to act as an HIV-1 attachment factor to DCs where the efficient oligomerisation mediated by this region enables multivalent recognition of HIV-1 gp120 by the DCIR CRD. DCIR facilitates viral capture and CD4+ T-lymphocyte trans-infection by promoting increased interactions between HIV-1 gp120 and CD4 and/or mediating viral endocytosis into non-degradative endosomes permitting the intracellular storage of intact virions. Successful and productive de novo virus production in DCs may also lead to cis-infection of CD4+ T-lymphocytes [71].
5. Ly49Q
The gene encoding Ly49Q (Official name: Klra17) was originally cloned using RNA from fetal liver mononuclear cells. It encodes a 273 amino acid type II membrane glycoprotein harbouring five potential N-glycosylation sites in its extracellular region (Fig. 1I). Like other Ly49 family members, the CTLD of Ly49Q lacks the residues required for calcium binding or recognition of galactose- or mannose-containing carbohydrates. As an inhibitory receptor, the cytoplasmic domain of Ly49Q harbours a canonical ITIM (VxYxxV) and its recruitment of SHP-1 and SHP-2 is essential for signal transduction [72]. The lack of a cytoplasmic internalisation motif makes it unlikely that Ly49Q plays a role in antigen uptake and internalisation.
At least 3 alleles of Ly49Q (ly49q1a, ly49q1b, ly49q1c) have been identified in different mouse strains. These alleles were found to harbour 4 amino acid variations in their stalk regions and 3 variations in their CTLDs. All of the resultant proteins were shown to be expressed at the cell surface. Furthermore, in mouse strains JF1, MSM and SV129, the ly49q1 gene was reported to comprise three additional exons as well as the potential to generate four splice variants, none of which however were shown to be stably expressed (Fig. 2C) [73].
Unlike most receptors within the Ly49 family which are expressed exclusively as disulphide-linked dimers on T lymphocytes and NK cells, Ly49Q is uniquely absent from NK cells but is instead predominantly expressed as a dimer or oligomer on immature bone marrow Gr-1+ myeloid cell precursors and immature monocytes. Ly49Q expression decreases upon monocyte maturation where it disappears from peripheral blood monocytes and reappears following their activation in the periphery. In certain DC maturational stages, Ly49Q expression is up-regulated by IFNα or IFNγ suggesting its potential role in anti-viral immune responses. In GM-CSF-induced bone marrow derived myeloid DCs, Ly49Q expression decreases upon differentiation while in pDCs, Ly49Q expression increases upon maturation [74].
In macrophages Ly49Q expression is up-regulated by IFNγ. In these cells, antibody-mediated Ly49Q engagement and ITIM-dependent signalling results in actin cytoskeleton reorganization, polarization, cell adhesion and spreading, a process which may permit rapid cell migration leading to enhanced surveillance and ingestion of pathogens in inflamed tissues [72]. Ly49Q has similar effects in neutrophils where it may act as an inhibitory receptor in the steady state and as an activating receptor in the presence of chemo-attractant stimuli.
In the steady state, SHP-1 recruitment by the ITIM of Ly49Q inhibits PI3 and src kinases and suppresses the formation of focal adhesion complexes, inhibiting the inappropriate adhesion and spreading of neutrophils. In the presence of chemo-attractant stimuli, Ly49Q is endocytosed where it plays a role in the spatiotemporal regulation of membrane rafts and raft-associated signalling molecules. This is associated with raft internalisation and redistribution where SHP-2 recruitment to membrane lipid raft compartments containing Ly49Q results in rapid neutrophil polarization and consequent infiltration of neutrophils into extravascular tissues. In this regard, it has been postulated that as one of the more ancient members of the Ly49 family, the regulation of membrane lipid dynamics by Ly49Q in more primitive phagocytes has involved cis-interactions with class I MHC ligands and that this has been followed by the evolutionarily more recent development of trans-interactions between these ligands and Ly49 members expressed on NK cells [75].
The maturation-dependent expression of Ly49Q is influenced by β2 microglobulin-associated Class I MHC-like molecules in the periphery which suggests that like other Ly49 members, the likely ligand for Ly49Q is a class I MHC or a related β2 microglobulin-associated molecule. H-2Kb has been identified as a high affinity class I MHC cis-ligand of Ly49Q [76]. Clusters of this ligand on activated B lymphocytes were able to up-regulate the expression of co-stimulatory molecules on pDCs and induce their maturation [77]. Counter-intuitively, Ly49q-H2Kb interactions positively regulated TLR signals and subsequent cytokine production in pDCs. These interactions were not only required for IFNα secretion by pDCs but also for the production of cytokines including IFNα and IL-12p70 in response to TLR-7 and -9 stimulation.
As described previously for neutrophils under conditions of chemo-attractant stimulation, Ly49Q affects TLR9 signalling in pDCs through the spatiotemporal regulation of membrane trafficking. Here it plays a critical role in the development of tubular endolysosomes during intracellular trafficking of TLR9 and its ligand, CpG. This is achieved by the internalisation of Ly49Q in cis with its class I MHC ligand, a process dependent upon the ITIM-mediated recruitment of SHP-1 and SHP-2 [78]. The mechanism by which this so-called “inhibitory” CLR increases IFNα production in response to TLR-7 and -9 agonists remains unknown although it may be occurring in the presence of inhibitory DAP-12 coupled-receptors in pDCs or the Ly49Q ITIM may be acting as an ITAM under these conditions [79].
Recently, the role of Ly49Q in osteoclast development (osteoclastogenesis) has been investigated [80]. RANKL (Receptor activator of NF-κB ligand) stimulation of bone marrow-derived monocyte-macrophage precursor cells resulted in selective Ly49Q induction. Following short hairpin RNA knockdown of Ly49Q, there was a significant impairment of osteoclastogenesis in vitro as well as a significant reduction in the formation of RANKL-induced tartrate-resistance acid phosphatase (TRAP)-positive multinucleated cells and reduced expression of osteoclast-specific genes [80]. In this context it has been suggested that Ly49Q promotes osteoclastogenesis by inhibiting an inhibitory receptor. Here, it may compete for SHP-1 association with another paired ITIM-bearing receptor, immunoglobulin-like receptor B (PIR-B), which is a negative regulator of osteoclast differentiation [80]. This highlights one of the mechanisms by which ITIM-harbouring receptors can activate cellular responses. A mouse Ly49Q knock-out had no effects on bone volume, osteoclast differentiation or function suggesting that a compensatory mechanism may exist for Ly49Q deficiency in vivo [80]. Interestingly, recent data demonstrating the ability of human monocyte-derived osteoclasts to function as antigen-presenting cells and to activate T-lymphocytes may point to additional roles for Ly49Q in this respect and merits further investigation [81]. While Ly49Q may function as a positive regulator of osteoclast differentiation, another C-type-like lectin-like NK receptor, osteoclast inhibitory lectin (OCIL) has been identified as an inhibitor of osteoclast development.
6. Osteoclast inhibitory lectin (OCIL)
OCIL (Official name: CLEC2D) is also referred to as C-type lectin-related molecule-b (clr-b) and lectin-like transcript-1 (LLT-1). Murine OCIL (mOCIL) is a 207 amino acid type II membrane-bound C-type lectin-like NK receptor belonging to a family of osteoclast formation inhibitors (Fig. 1F) [82–84]. Other members of this family include OCILrP1, OCILrP2 and OCILrP2b and like OCIL are encoded by genes within the murine NKC on chromosome 6 (Fig. 1). These C-type lectins are evolutionarily related and appear to have arisen as a result of gene duplication as is the case for receptors within the Ly49 and NKRP1 families [82,83,85].
The extracellular domains of the OCIL family, in particular the CTLDs, are well conserved and display a high degree of amino acid sequence identity, sharing a similar structure and biological function [5,85]. Furthermore, the CTLD of OCIL shares 36% homology with that of another group V C-type lectin within the NKC, CD69 [82]. In both murine and human OCIL, the CTLD lacks the residues required for calcium-dependent carbohydrate recognition. However, OCIL has been shown to bind with a high affinity to large sulphated glycosaminoglycans [86]. In addition to the CTLD, the extracellular domain includes a neck region and a C-terminal extension as well as three potential N-glycosylation sites and five conserved cysteine residues [82]. While the trans-membrane region of OCIL lacks charged residues required for an association with adaptor proteins and the short cytoplasmic tail shows an absence of consensus signalling motifs, the cytoplasmic domain does include a casein kinase II (CKII) phosphorylation site while in the case of mOCILrP1 there are two protein kinase C (PKC) phosphorylation sites [83].
With respect to its roles in osteoblast differentiation and osteoclast development, OCIL expression mirrors that of RANKL and both proteins appear to occupy the same osteoblast membrane compartments. OCIL expression is regulated by hormones and cytokines active in bone including retinoic acid, IL-1α, IL-11, calcitriol and parathyroid hormone (PTH). While RANKL is an established promoter of osteoclastogenesis, OCIL and other family members inhibit osteoclast formation primarily in the proliferative phase and in a manner that is neither dependent on osteoprotegerin (OPG) nor on the ability of OCIL to bind sulphated glycosaminoglycans [83]. Unlike Ly49Q, where knockout studies showed functional redundancy in the ability to promote osteoclastogenesis, Kartsogiannis et al. [87] found that OCIL-deficient mice, while showing no apparent defect in immune function, displayed phenotypic abnormalities in bone physiology. This was characterised by increased osteoclastogenesis and reduced bone formation confirming the role of OCIL as a negative regulator of bone homeostasis. In addition to this, and independent of its effects on osteoclasts, OCIL also inhibits the differentiation of osteoblasts and adipocytes [88].
The human homologue of OCIL is encoded by a gene on chromosome 12 and is a 191 amino acid type II membrane protein displaying a 53% identity with rat OCIL and mOCIL. Its expression is similarly up-regulated as that of its murine counterpart and it shows comparable biological effects on osteoclastogenesis [84]. In this regard, an association has been demonstrated between a single nucleotide polymorphism, generating an Asn19Lys substitution and bone mineral density in older women [89].
Apart from its expression on osteoblasts, OCIL is also expressed in chondrocytes, extraskeletal tissues, DCs, lymphocyte and macrophage populations [82,83,90,91]. With regard to its expression on immune cells, NKRP1d, an NKC-encoded, ITIM-bearing NK cell-associated C-type lectin, has been identified as an OCIL ligand [90,92]. Binding of OCIL to this inhibitory receptor suppresses NK cell-mediated killing of target cells. In support of this, Aust et al. [93] demonstrated that NKRP1d+ NK cells, while readily killing target cells expressing low levels of OCIL, were unable to kill transfected cells expressing high OCIL Levels. It has been suggested that this may represent a parallel means of missing-self recognition by regulating NK cell activation following NKRP1d binding to OCIL on potential macrophage, DC or tumour targets [90,92].
7. Mast cell function-associated antigen (MAFA)
Mast cell function-associated antigen (MAFA) (Official name: Klrg1) is a highly glycosylated 188 amino acid type II membrane glycoprotein originally identified in the rat where its expression is restricted to mast cells and basophils as a monomer or disulphide-linked homodimer [94–97].
MAFA has been shown to inhibit the secretory response induced by IgE-mediated aggregation of the activating receptor FcɛRI in rat RBL-2H3 mast cells (Fig. 1E) [94]. This secretory response, following aggregated FcɛRI signalling from membrane lipid raft microenvironments, is characterised by the release of de novo synthesised cytokines and granular mediators such as histamine.
The MAFA extracellular domain comprises 11 conserved cysteine residues which form intra-chain disulphide linkages to generate a CTLD that displays significant homology with those of other C-type lectins including the NK receptors CD94, Ly49A, NKG2D and CD69 [95,96]. However, unlike these receptors, MAFA does not bind class I MHC ligands although it is able to bind mannose-terminated glycans [98,99]. MAFA comprises a short cytoplasmic tail which harbours an ITIM (SIYSTL) that differs at the Y-2 position from the canonical ITIM sequences present in most other inhibitory receptors. The cytoplasmic tail also includes a PAAP motif that is able to bind SH3-domain-containing proteins such as the protein tyrosine kinase Lyn, the recruitment of which is an important step in ITIM phosphorylation [100,101]. Antibody-mediated MAFA clustering induced ITIM tyrosine phosphorylation and the recruitment of the protein tyrosine phosphatases SHP-2 and SHIP but not SHP-1 as is the case with many other ITIM-bearing receptors [100,102]. SHIP is the principal mediator of MAFA's inhibitory function where the hydrolysis of PIP3, the decrease in PLC-γ activity, and the inhibition of transient intracellular calcium elevation, suppressed the secretory response to FcɛRI activation [100,103]. Notably, unlike other inhibitory receptors, MAFA-mediated inhibition does not require co-clustering with FcɛRI although such co-clustering potentiated its inhibitory capacity [104,105]. In this regard, it has been shown that MAFA functions within membrane lipid raft microdomains and in close proximity to FcɛRI [106–108]. Other functions of MAFA include an involvement in mast cell adhesion [95,96,109] and the inhibition of mast cell proliferation [102].
The mouse homologue of MAFA, also known as killer cell lectin-like receptor G1 (Klrg1) is encoded by a gene centromeric to and outside the NKC on chromosome 6 [110] (Fig. 1). Unlike rat MAFA, it is absent from mast cells but is expressed on a subset of memory T cells in naïve mice. Furthermore, klrg1 expression was induced on CD8+ T-lymphocytes during viral and parasitic infection and on CD4+ T-lymphocytes during parasitic infection [111]. Cytokine activation of NK cells also induced Klrg1 expression where it may inhibit NK cell effector functions [111,112]. The human MAFA-like receptor (MAFA-L) (KLRG1) is expressed on peripheral blood NK cells but unlike its rat counterpart, it may inhibit responses to receptors other than FcɛRI [113].
8. Conclusions
In ensuring the critical discrimination between self and non-self as well as the balance between immune activation and inhibition, the natural killer gene complex has evolved to encode numerous activating and inhibitory C-type lectin receptors with patterns of expression that extend to several myeloid cell populations. In the present review we have highlighted some of the important and increasingly diverse and complex roles of inhibitory C-type lectin receptors in these cell populations. The ability of these receptors to paradoxically activate cellular responses under certain circumstances underscores their versatility in response to alterations in receptor ligation, cellular compartmentalisation, receptor co-localisation and the ability of ITIMs to recruit both inhibitory proteins and to modulate activating molecules. Knock-down and knock-out studies have provided valuable insights into the functioning of these receptors in vivo including the extent of their functional redundancy. Efforts to similarly elucidate the in vivo functions of other CLRs such as MICL and MAH as well as attempts at identifying their endogenous and/or exogenous ligands promises to increase our understanding of their roles as regulators of immune homeostasis.
Acknowledgements
The authors wish to thank the Wellcome Trust U.K. for its generous financial support.
References
- 1.Drickamer K. C-type lectin-like domains. Curr Opin Struct Biol. 1999;9:585–590. doi: 10.1016/s0959-440x(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 2.Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1992;360:183–186. doi: 10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- 3.Zelensky A.N., Gready J.E. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 4.Geijtenbeek T.B., Gringhuis S.I. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama W.M., Plougastel B.F. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–316. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 6.Scarpellino L., Oeschger F., Guillaume P., Coudert J.D., Levy F., Leclercq G. Interactions of Ly49 family receptors with MHC class I ligands in trans and cis. J Immunol. 2007;178:1277–1284. doi: 10.4049/jimmunol.178.3.1277. [DOI] [PubMed] [Google Scholar]
- 7.Lanier L.L. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 8.Takei F., McQueen K.L., Maeda M., Wilhelm B.T., Lohwasser S., Lian R.H. Ly49 and CD94/NKG2: developmentally regulated expression and evolution. Immunol Rev. 2001;181:90–103. doi: 10.1034/j.1600-065x.2001.1810107.x. [DOI] [PubMed] [Google Scholar]
- 9.Anderson S.K., Ortaldo J.R., McVicar D.W. The ever-expanding Ly49 gene family: repertoire and signaling. Immunol Rev. 2001;181:79–89. doi: 10.1034/j.1600-065x.2001.1810106.x. [DOI] [PubMed] [Google Scholar]
- 10.Ravetch J.V., Lanier L.L. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 11.Radaev S., Sun P.D. Structure and function of natural killer cell surface receptors. Annu Rev Biophys Biomol Struct. 2003;32:93–114. doi: 10.1146/annurev.biophys.32.110601.142347. [DOI] [PubMed] [Google Scholar]
- 12.Hofer E., Sobanov Y., Brostjan C., Lehrach H., Duchler M. The centromeric part of the human natural killer (NK) receptor complex: lectin-like receptor genes expressed in NK, dendritic and endothelial cells. Immunol Rev. 2001;181:5–19. doi: 10.1034/j.1600-065x.2001.1810101.x. [DOI] [PubMed] [Google Scholar]
- 13.Sobanov Y, Bernreiter A., Derdak S., Mechtcheriakova D., Schweighofer B., Duchler M. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur J Immunol. 2001;31:3493–3503. doi: 10.1002/1521-4141(200112)31:12<3493::aid-immu3493>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Shaw A.S., Gauen L.K., Zhu Y. Interactions of TCR tyrosine based activation motifs with tyrosine kinases. Semin Immunol. 1995;7:13–20. doi: 10.1016/1044-5323(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 15.Isakov N. Immunoreceptor tyrosine-based activation motif (ITAM), a unique module linking antigen and Fc receptors to their signaling cascades. J Leukoc Biol. 1997;61:6–16. doi: 10.1002/jlb.61.1.6. [DOI] [PubMed] [Google Scholar]
- 16.Barrow A.D, Trowsdale J. You say ITAM and I say ITIM, let's call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol. 2006;36:1646–1653. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 17.Underhill D.M., Goodridge H.S. The many faces of ITAMs. Trends Immunol. 2007;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Pitcher L.A., van Oers N.S. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Dal Porto J.M., Gauld S.B., Merrell K.T., Mills D., Pugh-Bernard A.E., Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Rivera J., Gilfillan A.M. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 21.Mocsai A., Abram C.L., Jakus Z., Hu Y., Lanier L.L., Lowell C.A. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown G.D., Herre J., Williams D.L., Willment J.A., Marshall A.S., Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gantner B.N., Simmons R.M., Canavera S.J., Akira S., Underhill D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herre J., Willment J.A., Gordon S., Brown G.D. The role of Dectin-1 in antifungal immunity. Crit Rev Immunol. 2004;24:193–203. doi: 10.1615/critrevimmunol.v24.i3.30. [DOI] [PubMed] [Google Scholar]
- 25.Rogers N.C., Slack E.C., Edwards A.D., Nolte M.A., Schulz O., Schweighoffer E. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Underhill D.M., Rossnagle E., Lowell C.A., Simmons R.M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown G.D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 28.Veillette A., Latour S., Davidson D. Negative regulation of immunoreceptor signaling. Annu Rev Immunol. 2002;20:669–707. doi: 10.1146/annurev.immunol.20.081501.130710. [DOI] [PubMed] [Google Scholar]
- 29.Barrow A.D., Astoul E., Floto A., Brooke G., Relou I.A., Jennings N.S. Cutting edge: TREM-like transcript-1, a platelet immunoreceptor tyrosine-based inhibition motif encoding costimulatory immunoreceptor that enhances, rather than inhibits, calcium signaling via SHP-2. J Immunol. 2004;172:5838–5842. doi: 10.4049/jimmunol.172.10.5838. [DOI] [PubMed] [Google Scholar]
- 30.Fujioka Y, Matozaki T., Noguchi T., Iwamatsu A., Yamao T., Takahashi N. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharitonenkov A., Chen Z., Sures I., Wang H., Schilling J., Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 32.Mi Z.P., Jiang P., Weng W.L., Lindberg F.P., Narayanan V., Lagenaur C.F. Expression of a synapse-associated membrane protein, P84/SHPS-1, and its ligand, IAP/CD47, in mouse retina. J Comp Neurol. 2000;416:335–344. [PubMed] [Google Scholar]
- 33.Ohnishi H., Yamada M., Kubota M., Hatanaka H., Sano S. Tyrosine phosphorylation and association of BIT with SHP-2 induced by neurotrophins. J Neurochem. 1999;72:1402–1408. doi: 10.1046/j.1471-4159.1999.721402.x. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg T.K., van Beek E.M., Buhring H.J., Colonna M., Hamaguchi M., Howard C.J. A nomenclature for signal regulatory protein family members. J Immunol. 2005;175:7788–7789. doi: 10.4049/jimmunol.175.12.7788. [DOI] [PubMed] [Google Scholar]
- 35.Matozaki T., Murata Y., Okazawa H., Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Adams S., van der Laan L.J., Vernon-Wilson E., Renardel de Lavalette C., Dopp E.A., Dijkstra C.D. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol. 1998;161:1853–1859. [PubMed] [Google Scholar]
- 37.Veillette A., Thibaudeau E., Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem. 1998;273:22719–22728. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- 38.Seiffert M., Cant C., Chen Z., Rappold I., Brugger W., Kanz L. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94:3633–3643. [PubMed] [Google Scholar]
- 39.Jiang P, Lagenaur C.F., Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 40.Latour S., Tanaka H., Demeure C., Mateo V., Rubio M., Brown E.J. Bidirectional negative regulation of human T and dendritic cells by CD47 and its cognate receptor signal-regulator protein-alpha: down-regulation of IL-12 responsiveness and inhibition of dendritic cell activation. J Immunol. 2001;167:2547–2554. doi: 10.4049/jimmunol.167.5.2547. [DOI] [PubMed] [Google Scholar]
- 41.Seiffert M., Brossart P., Cant C., Cella M., Colonna M., Brugger W. Signal-regulatory protein alpha (SIRPalpha) but not SIRPbeta is involved in T-cell activation, binds to CD47 with high affinity, and is expressed on immature CD34(+)CD38(−) hematopoietic cells. Blood. 2001;97:2741–2749. doi: 10.1182/blood.v97.9.2741. [DOI] [PubMed] [Google Scholar]
- 42.Takada T, Matozaki T., Takeda H., Fukunaga K., Noguchi T., Fujioka Y. Roles of the complex formation of SHPS-1 with SHP-2 in insulin-stimulated mitogen-activated protein kinase activation. J Biol Chem. 1998;273:9234–9242. doi: 10.1074/jbc.273.15.9234. [DOI] [PubMed] [Google Scholar]
- 43.Oldenborg P.A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 44.Okazawa H., Motegi S., Ohyama N., Ohnishi H., Tomizawa T., Kaneko Y. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol. 2005;174:2004–2011. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 45.Johansen M.L., Brown E.J. Dual regulation of SIRPalpha phosphorylation by integrins and CD47. J Biol Chem. 2007;282:24219–24230. doi: 10.1074/jbc.M701565200. [DOI] [PubMed] [Google Scholar]
- 46.Murata T., Ohnishi H., Okazawa H., Murata Y., Kusakari S., Hayashi Y. CD47 promotes neuronal development through Src- and FRG/Vav2-mediated activation of Rac and Cdc42. J Neurosci. 2006;26:12397–12407. doi: 10.1523/JNEUROSCI.3981-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alblas J., Honing H., de Lavalette C.R., Brown M.H., Dijkstra C.D., van den Berg T.K. Signal regulatory protein alpha ligation induces macrophage nitric oxide production through JAK/STAT- and phosphatidylinositol 3-kinase/Rac1/NAPDH oxidase/H2O2-dependent pathways. Mol Cell Biol. 2005;25:7181–7192. doi: 10.1128/MCB.25.16.7181-7192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomizawa T., Kaneko Y., Saito Y., Ohnishi H., Okajo J., Okuzawa C. Resistance to experimental autoimmune encephalomyelitis and impaired T cell priming by dendritic cells in Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 mutant mice. J Immunol. 2007;179:869–877. doi: 10.4049/jimmunol.179.2.869. [DOI] [PubMed] [Google Scholar]
- 49.Okuzawa C., Kaneko Y., Murata Y., Miyake A., Saito Y., Okajo J. Resistance to collagen-induced arthritis in SHPS-1 mutant mice. Biochem Biophys Res Commun. 2008;371:561–566. doi: 10.1016/j.bbrc.2008.04.124. [DOI] [PubMed] [Google Scholar]
- 50.Motegi S., Okazawa H., Murata Y., Kanazawa Y., Saito Y., Kobayashi H. Essential roles of SHPS-1 in induction of contact hypersensitivity of skin. Immunol Lett. 2008;121:52–60. doi: 10.1016/j.imlet.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Han Y., Zhang M., Li N., Chen T., Zhang Y., Wan T. KLRL1, a novel killer cell lectinlike receptor, inhibits natural killer cell cytotoxicity. Blood. 2004;104:2858–2866. doi: 10.1182/blood-2004-03-0878. [DOI] [PubMed] [Google Scholar]
- 52.Bakker A.B, van den Oudenrijn S., Bakker A.Q., Feller N., van Meijer M., Bia J.A. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64:8443–8450. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- 53.Marshall A.S., Willment J.A., Lin H.H., Williams D.L., Gordon S., Brown G.D. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem. 2004;279:14792–14802. doi: 10.1074/jbc.M313127200. [DOI] [PubMed] [Google Scholar]
- 54.Chen C.H., Floyd H., Olson N.E., Magaletti D., Li C., Draves K. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood. 2006;107:1459–1467. doi: 10.1182/blood-2005-08-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pyz E., Huysamen C., Marshall A.S., Gordon S., Taylor P.R., Brown G.D. Characterisation of murine MICL (CLEC12A) and evidence for an endogenous ligand. Eur J Immunol. 2008;38:1157–1163. doi: 10.1002/eji.200738057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marshall A.S., Willment J.A., Pyz E., Dennehy K.M., Reid D.M., Dri P. Human MICL (CLEC12A) is differentially glycosylated and is down-regulated following cellular activation. Eur J Immunol. 2006;36:2159–2169. doi: 10.1002/eji.200535628. [DOI] [PubMed] [Google Scholar]
- 57.Lahoud M.H., Proietto A.I., Ahmet F., Kitsoulis S., Eidsmo L., Wu L. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol. 2009;182:7587–7594. doi: 10.4049/jimmunol.0900464. [DOI] [PubMed] [Google Scholar]
- 58.van Rhenen A., van Dongen G.A., Kelder A., Rombouts E.J., Feller N., Moshaver B. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmann S.C., Schellack C., Textor S., Konold S., Schmitz D., Cerwenka A. Identification of CLEC12B, an inhibitory receptor on myeloid cells. J Biol Chem. 2007;282:22370–22375. doi: 10.1074/jbc.M704250200. [DOI] [PubMed] [Google Scholar]
- 60.Bates E.E, Fournier N., Garcia E., Valladeau J., Durand I., Pin J.J. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J Immunol. 1999;163:1973–1983. [PubMed] [Google Scholar]
- 61.Kanazawa N., Okazaki T., Nishimura H., Tashiro K., Inaba K., Miyachi Y. DCIR acts as an inhibitory receptor depending on its immunoreceptor tyrosine-based inhibitory motif. J Invest Dermatol. 2002;118:261–266. doi: 10.1046/j.0022-202x.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 62.Richard M., Veilleux P., Rouleau M., Paquin R., Beaulieu A.D. The expression pattern of the ITIM-bearing lectin CLECSF6 in neutrophils suggests a key role in the control of inflammation. J Leukoc Biol. 2002;71:871–880. [PubMed] [Google Scholar]
- 63.Meyer-Wentrup F., Benitez-Ribas D., Tacken P.J., Punt C.J., Figdor C.G., de Vries I.J. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–4253. doi: 10.1182/blood-2007-03-081398. [DOI] [PubMed] [Google Scholar]
- 64.Huang X., Yuan Z., Chen G., Zhang M., Zhang W., Yu Y. Cloning and characterization of a novel ITIM containing lectin-like immunoreceptor LLIR and its two transmembrane region deletion variants. Biochem Biophys Res Commun. 2001;281:131–140. doi: 10.1006/bbrc.2001.4322. [DOI] [PubMed] [Google Scholar]
- 65.Meyer-Wentrup F., Cambi A., Joosten B., Looman M.W., de Vries I.J., Figdor C.G. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol. 2009;85:518–525. doi: 10.1189/jlb.0608352. [DOI] [PubMed] [Google Scholar]
- 66.Klechevsky E., Flamar A.L., Cao Y., Blanck J.P., Liu M., O’Bar A. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010 doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanazawa N., Tashiro K., Inaba K., Miyachi Y. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor gamma chain. J Biol Chem. 2003;278:32645–32652. doi: 10.1074/jbc.M304226200. [DOI] [PubMed] [Google Scholar]
- 68.Kanazawa N. Dendritic cell immunoreceptors: C-type lectin receptors for pattern-recognition and signaling on antigen-presenting cells. J Dermatol Sci. 2007;45:77–86. doi: 10.1016/j.jdermsci.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Fujikado N., Saijo S., Yonezawa T., Shimamori K., Ishii A., Sugai S. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. 2008;14:176–180. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- 70.Eklow C., Makrygiannakis D., Backdahl L., Padyukov L., Ulfgren A.K., Lorentzen J.C. Cellular distribution of the C-type II lectin dendritic cell immunoreceptor (DCIR) and its expression in the rheumatic joint: identification of a subpopulation of DCIR+ T cells. Ann Rheum Dis. 2008;67:1742–1749. doi: 10.1136/ard.2007.076976. [DOI] [PubMed] [Google Scholar]
- 71.Lambert A.A., Gilbert C., Richard M., Beaulieu A.D., Tremblay M.J. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112:1299–1307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toyama-Sorimachi N., Tsujimura Y., Maruya M., Onoda A., Kubota T., Koyasu S. Ly49Q, a member of the Ly49 family that is selectively expressed on myeloid lineage cells and involved in regulation of cytoskeletal architecture. Proc Natl Acad Sci USA. 2004;101:1016–1021. doi: 10.1073/pnas.0305400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toyama-Sorimachi N, Omatsu Y., Onoda A., Tsujimura Y., Iyoda T., Kikuchi-Maki A. Inhibitory NK receptor Ly49Q is expressed on subsets of dendritic cells in a cellular maturation- and cytokine stimulation-dependent manner. J Immunol. 2005;174:4621–4629. doi: 10.4049/jimmunol.174.8.4621. [DOI] [PubMed] [Google Scholar]
- 74.Kamogawa-Schifter Y., Ohkawa J., Namiki S., Arai N., Arai K., Liu Y. Ly49Q defines 2 pDC subsets in mice. Blood. 2005;105:2787–2792. doi: 10.1182/blood-2004-09-3388. [DOI] [PubMed] [Google Scholar]
- 75.Sasawatari S., Yoshizaki M., Taya C., Tazawa A., Furuyama-Tanaka K., Yonekawa H. The Ly49Q receptor plays a crucial role in neutrophil polarization and migration by regulating raft trafficking. Immunity. 2010;32:200–213. doi: 10.1016/j.immuni.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 76.Tai L.H., Goulet M.L., Belanger S., Troke A.D., St-Laurent A.G., Mesci A. Recognition of H-2K(b) by Ly49Q suggests a role for class Ia MHC regulation of plasmacytoid dendritic cell function. Mol Immunol. 2007;44:2638–2646. doi: 10.1016/j.molimm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Toma-Hirano M., Namiki S., Shibata Y., Ishida K., Arase H., Miyatake S. Ly49Q ligand expressed by activated B cells induces plasmacytoid DC maturation. Eur J Immunol. 2009;39:1344–1352. doi: 10.1002/eji.200838363. [DOI] [PubMed] [Google Scholar]
- 78.Yoshizaki M., Tazawa A., Kasumi E., Sasawatari S., Itoh K., Dohi T. Spatiotemporal regulation of intracellular trafficking of Toll-like receptor 9 by an inhibitory receptor, Ly49Q. Blood. 2009;114:1518–1527. doi: 10.1182/blood-2008-12-192344. [DOI] [PubMed] [Google Scholar]
- 79.Tai L.H., Goulet M.L., Belanger S., Toyama-Sorimachi N., Fodil-Cornu N., Vidal S.M. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J Exp Med. 2008;205:3187–3199. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayashi M., Nakashima T., Kodama T., Makrigiannis A.P., Toyama-Sorimachi N., Takayanagi H. Ly49Q, an ITIM-bearing NK receptor, positively regulates osteoclast differentiation. Biochem Biophys Res Commun. 2010;393:432–438. doi: 10.1016/j.bbrc.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Li H, Hong S., Qian J., Zheng Y., Yang J., Yi Q. Cross talk between the bone and immune systems: osteoclasts function as antigen-presenting cells and activate CD4+ and CD8+ T cells. Blood. 2010;116:210–217. doi: 10.1182/blood-2009-11-255026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou H., Kartsogiannis V., Hu Y.S., Elliott J., Quinn J.M., McKinstry W.J. A novel osteoblast-derived C-type lectin that inhibits osteoclast formation. J Biol Chem. 2001;276:14916–14923. doi: 10.1074/jbc.M011554200. [DOI] [PubMed] [Google Scholar]
- 83.Zhou H., Kartsogiannis V., Quinn J.M., Ly C., Gange C., Elliott J. Osteoclast inhibitory lectin, a family of new osteoclast inhibitors. J Biol Chem. 2002;277:48808–48815. doi: 10.1074/jbc.M209059200. [DOI] [PubMed] [Google Scholar]
- 84.Hu Y.S, Zhou H., Myers D., Quinn J.M., Atkins G.J., Ly C. Isolation of a human homolog of osteoclast inhibitory lectin that inhibits the formation and function of osteoclasts. J Bone Miner Res. 2004;19:89–99. doi: 10.1359/JBMR.0301215. [DOI] [PubMed] [Google Scholar]
- 85.Plougastel B., Dubbelde C., Yokoyama W.M. Cloning of Clr, a new family of lectin-like genes localized between mouse Nkrp1a and Cd69. Immunogenetics. 2001;53:209–214. doi: 10.1007/s002510100319. [DOI] [PubMed] [Google Scholar]
- 86.Gange C.T, Quinn J.M., Zhou H., Kartsogiannis V., Gillespie M.T., Ng K.W. Characterization of sugar binding by osteoclast inhibitory lectin. J Biol Chem. 2004;279:29043–29049. doi: 10.1074/jbc.M312518200. [DOI] [PubMed] [Google Scholar]
- 87.Kartsogiannis V., Sims N.A., Quinn J.M., Ly C., Cipetic M., Poulton I.J. Osteoclast inhibitory lectin, an immune cell product that is required for normal bone physiology in vivo. J Biol Chem. 2008;283:30850–30860. doi: 10.1074/jbc.M801761200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakamura A, Ly C., Cipetic M., Sims N.A., Vieusseux J., Kartsogiannis V. Osteoclast inhibitory lectin (OCIL) inhibits osteoblast differentiation and function in vitro. Bone. 2007;40:305–315. doi: 10.1016/j.bone.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Pineda B., Laporta P., Cano A., Garcia-Perez M.A. The Asn19Lys substitution in the osteoclast inhibitory lectin (OCIL) gene is associated with a reduction of bone mineral density in postmenopausal women. Calcif Tissue Int. 2008;82:348–353. doi: 10.1007/s00223-008-9135-4. [DOI] [PubMed] [Google Scholar]
- 90.Iizuka K., Naidenko O.V., Plougastel B.F., Fremont D.H., Yokoyama W.M. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat Immunol. 2003;4:801–807. doi: 10.1038/ni954. [DOI] [PubMed] [Google Scholar]
- 91.Katsu Y., Lubahn D.B., Iguchi T. Expression of a novel C-type lectin in the mouse vagina. Endocrinology. 2003;144:2597–2605. doi: 10.1210/en.2002-220980. [DOI] [PubMed] [Google Scholar]
- 92.Carlyle J.R., Jamieson A.M., Gasser S., Clingan C.S., Arase H., Raulet D.H. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc Natl Acad Sci USA. 2004;101:3527–3532. doi: 10.1073/pnas.0308304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aust J.G., Gays F., Mickiewicz K.M., Buchanan E., Brooks C.G. The expression and function of the NKRP1 receptor family in C57BL/6 mice. J Immunol. 2009;183:106–116. doi: 10.4049/jimmunol.0804281. [DOI] [PubMed] [Google Scholar]
- 94.Ortega E., Schweitzer-Stenner R., Pecht I. Possible orientational constraints determine secretory signals induced by aggregation of IgE receptors on mast cells. EMBO J. 1988;7:4101–4109. doi: 10.1002/j.1460-2075.1988.tb03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guthmann M.D., Tal M., Pecht I. A secretion inhibitory signal transduction molecule on mast cells is another C-type lectin. Proc Natl Acad Sci USA. 1995;92:9397–9401. doi: 10.1073/pnas.92.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guthmann M.D., Tal M., Pecht I. A new member of the C-type lectin family is a modulator of the mast cell secretory response. Int Arch Allergy Immunol. 1995;107:82–86. doi: 10.1159/000236938. [DOI] [PubMed] [Google Scholar]
- 97.Bocek P., Jr., Guthmann M.D., Pecht I. Analysis of the genes encoding the mast cell function-associated antigen and its alternatively spliced transcripts. J Immunol. 1997;158:3235–3243. [PubMed] [Google Scholar]
- 98.Corral L., Hanke T., Vance R.E., Cado D., Raulet D.H. NK cell expression of the killer cell lectin-like receptor G1 (KLRG1), the mouse homolog of MAFA, is modulated by MHC class I molecules. Eur J Immunol. 2000;30:920–930. doi: 10.1002/1521-4141(200003)30:3<920::AID-IMMU920>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 99.Binsack R, Pecht I. The mast cell function-associated antigen exhibits saccharide binding capacity. Eur J Immunol. 1997;27:2557–2561. doi: 10.1002/eji.1830271014. [DOI] [PubMed] [Google Scholar]
- 100.Xu R., Abramson J., Fridkin M., Pecht I. SH2 domain-containing inositol polyphosphate 5’-phosphatase is the main mediator of the inhibitory action of the mast cell function-associated antigen. J Immunol. 2001;167:6394–6402. doi: 10.4049/jimmunol.167.11.6394. [DOI] [PubMed] [Google Scholar]
- 101.Xu R., Pecht I. The protein tyrosine kinase syk activity is reduced by clustering the mast cell function-associated antigen. Eur J Immunol. 2001;31:1571–1581. doi: 10.1002/1521-4141(200105)31:5<1571::AID-IMMU1571>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 102.Abramson J., Pecht I. Clustering the mast cell function-associated antigen (MAFA) leads to tyrosine phosphorylation of p62Dok and SHIP and affects RBL-2H3 cell cycle. Immunol Lett. 2002;82:23–28. doi: 10.1016/s0165-2478(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 103.Abramson J., Licht A., Pecht I. Selective inhibition of the Fc epsilon RI-induced de novo synthesis of mediators by an inhibitory receptor. EMBO J. 2006;25:323–334. doi: 10.1038/sj.emboj.7600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Licht A., Pecht I., Schweitzer-Stenner R. Regulation of mast cells’ secretory response by co-clustering the Type 1 Fcepsilon receptor with the mast cell function-associated antigen. Eur J Immunol. 2005;35:1621–1633. doi: 10.1002/eji.200425964. [DOI] [PubMed] [Google Scholar]
- 105.Licht A., Abramson J., Pecht I. Co-clustering activating and inhibitory receptors: impact at varying expression levels of the latter. Immunol Lett. 2006;104:166–170. doi: 10.1016/j.imlet.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 106.Song J., Hagen G., Smith S.M., Roess D.A., Pecht I., Barisas B.G. Interactions of the mast cell function-associated antigen with the type I Fcepsilon receptor. Mol Immunol. 2002;38:1315–1321. doi: 10.1016/s0161-5890(02)00081-0. [DOI] [PubMed] [Google Scholar]
- 107.Song J., Hagen G.M., Roess D.A., Pecht I., Barisas B.G. The mast cell function-associated antigen and its interactions with the type I Fcepsilon receptor. Biochemistry. 2002;41:881–889. doi: 10.1021/bi011566i. [DOI] [PubMed] [Google Scholar]
- 108.Barisas B.G., Smith S.M., Liu J., Song J., Hagen G.M., Pecht I. Compartmentalization of the Type I Fc epsilon receptor and MAFA on mast cell membranes. Biophys Chem. 2007;126:209–217. doi: 10.1016/j.bpc.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 109.Cohen-Dayag A., Schneider H., Pecht I. Variants of the mucosal mast cell line (RBL-2H3) deficient in a functional membrane glycoprotein. Immunobiology. 1992;185:124–149. doi: 10.1016/S0171-2985(11)80636-4. [DOI] [PubMed] [Google Scholar]
- 110.Voehringer D., Kaufmann M., Pircher H. Genomic structure, alternative splicing, and physical mapping of the killer cell lectin-like receptor G1 gene (KLRG1), the mouse homologue of MAFA. Immunogenetics. 2001;52:206–211. doi: 10.1007/s002510000282. [DOI] [PubMed] [Google Scholar]
- 111.Robbins S.H, Terrizzi S.C., Sydora B.C., Mikayama T., Brossay L. Differential regulation of killer cell lectin-like receptor G1 expression on T cells. J Immunol. 2003;170:5876–5885. doi: 10.4049/jimmunol.170.12.5876. [DOI] [PubMed] [Google Scholar]
- 112.Robbins S.H., Nguyen K.B., Takahashi N., Mikayama T., Biron C.A., Brossay L. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- 113.Butcher S., Arney K.L., Cook G.P. MAFA-L, an ITIM-containing receptor encoded by the human NK cell gene complex and expressed by basophils and NK cells. Eur J Immunol. 1998;28:3755–3762. doi: 10.1002/(SICI)1521-4141(199811)28:11<3755::AID-IMMU3755>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 114.Cao W, Zhang L., Rosen D.B., Bover L., Watanabe G., Bao M. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rock J., Schneider E., Grun J.R., Grutzkau A., Kuppers R., Schmitz J. CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLCgamma2. Eur J Immunol. 2007;37:3564–3575. doi: 10.1002/eji.200737711. [DOI] [PubMed] [Google Scholar]
- 116.Dzionek A., Sohma Y., Nagafune J., Cella M., Colonna M., Facchetti F. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sato K, Yang X.L., Yudate T., Chung J.S., Wu J., Luby-Phelps K. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
- 118.Barrett N.A., Maekawa A., Rahman O.M., Austen K.F., Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McGreal E.P., Rosas M., Brown G.D., Zamze S., Wong S.Y., Gordon S. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology. 2006;16:422–430. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 120.Arce I., Martinez-Munoz L., Roda-Navarro P., Fernandez-Ruiz E. The human C-type lectin CLECSF8 is a novel monocyte/macrophage endocytic receptor. Eur J Immunol. 2004;34:210–220. doi: 10.1002/eji.200324230. [DOI] [PubMed] [Google Scholar]
- 121.Yamasaki S., Ishikawa E., Sakuma M., Hara H., Ogata K., Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 122.Yamasaki S., Matsumoto M., Takeuchi O., Matsuzawa T., Ishikawa E., Sakuma M. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci USA. 2009;106:1897–1902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schoenen H., Bodendorfer B., Hitchens K., Manzanero S., Werninghaus K., Nimmerjahn F. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bugarcic A., Hitchens K., Beckhouse A.G., Wells C.A., Ashman R.B., Blanchard H. Human and mouse macrophage-inducible C-type lectin (Mincle) bind Candida albicans. Glycobiology. 2008;18:679–685. doi: 10.1093/glycob/cwn046. [DOI] [PubMed] [Google Scholar]
- 125.Gringhuis S.I., den Dunnen J., Litjens M., van Het Hof B., van Kooyk Y., Geijtenbeek T.B. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 126.Geijtenbeek T.B., Krooshoop D.J., Bleijs D.A., van Vliet S.J., van Duijnhoven G.C., Grabovsky V. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353–357. doi: 10.1038/79815. [DOI] [PubMed] [Google Scholar]
- 127.Geijtenbeek T.B., Kwon D.S., Torensma R., van Vliet S.J., van Duijnhoven G.C., Middel J. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 128.Geijtenbeek T.B, Torensma R., van Vliet S.J., van Duijnhoven G.C., Adema G.J., van Kooyk Y. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 129.Geijtenbeek T.B., van Kooyk Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. APMIS. 2003;111:698–714. doi: 10.1034/j.1600-0463.2003.11107803.x. [DOI] [PubMed] [Google Scholar]
- 130.Geijtenbeek T.B., van Kooyk Y. DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Curr Top Microbiol Immunol. 2003;276:31–54. doi: 10.1007/978-3-662-06508-2_2. [DOI] [PubMed] [Google Scholar]
- 131.Geijtenbeek T.B., Van Vliet S.J., Koppel E.A., Sanchez-Hernandez M., Vandenbroucke-Grauls C.M., Appelmelk B. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hodges A., Sharrocks K., Edelmann M., Baban D., Moris A., Schwartz O. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 133.Engering A., Geijtenbeek T.B., van Vliet S.J., Wijers M., van Liempt E., Demaurex N. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 134.Engering A., Van Vliet S.J., Geijtenbeek T.B., Van Kooyk Y. Subset of DC-SIGN(+) dendritic cells in human blood transmits HIV-1 to T lymphocytes. Blood. 2002;100:1780–1786. doi: 10.1182/blood-2001-12-0179. [DOI] [PubMed] [Google Scholar]
- 135.Appelmelk B.J., van Die I., van Vliet S.J., Vandenbroucke-Grauls C.M., Geijtenbeek T.B., van Kooyk Y. Cutting edge: carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 136.Tassaneetrithep B., Burgess T.H., Granelli-Piperno A., Trumpfheller C., Finke J., Sun W. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bergman M.P., Engering A., Smits H.H., van Vliet S.J., van Bodegraven A.A., Wirth H.P. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J Exp Med. 2004;200:979–990. doi: 10.1084/jem.20041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marzi A., Gramberg T., Simmons G., Moller P., Rennekamp A.J., Krumbiegel M. DC-SIGN and DC-SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J Virol. 2004;78:12090–12095. doi: 10.1128/JVI.78.21.12090-12095.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smits H.H., Engering A., van der Kleij D., de Jong E.C., Schipper K., van Capel T.M. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 140.van Gisbergen K.P., Aarnoudse C.A., Meijer G.A., Geijtenbeek T.B., van Kooyk Y. Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res. 2005;65:5935–5944. doi: 10.1158/0008-5472.CAN-04-4140. [DOI] [PubMed] [Google Scholar]
- 141.van Gisbergen K.P., Ludwig I.S., Geijtenbeek T.B., van Kooyk Y. Interactions of DC-SIGN with Mac-1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett. 2005;579:6159–6168. doi: 10.1016/j.febslet.2005.09.089. [DOI] [PubMed] [Google Scholar]
- 142.van Gisbergen K.P., Sanchez-Hernandez M., Geijtenbeek T.B., van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koppel E.A., Saeland E., de Cooker D.J., van Kooyk Y., Geijtenbeek T.B. DC-SIGN specifically recognizes Streptococcus pneumoniae serotypes 3 and 14. Immunobiology. 2005;210:203–210. doi: 10.1016/j.imbio.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 144.Caparros E., Munoz P., Sierra-Filardi E., Serrano-Gomez D., Puig-Kroger A., Rodriguez-Fernandez J.L. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 145.Smith A.L., Ganesh L., Leung K., Jongstra-Bilen J., Jongstra J., Nabel G.J. Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells. J Exp Med. 2007;204:421–430. doi: 10.1084/jem.20061604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Witte L., de Vries R.D., van der Vlist M., Yuksel S., Litjens M., de Swart R.L. DC-SIGN and CD150 have distinct roles in transmission of measles virus from dendritic cells to T-lymphocytes. PLoS Pathog. 2008;4:e1000049. doi: 10.1371/journal.ppat.1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mikulak J., Teichberg S., Arora S., Kumar D., Yadav A., Salhan D. DC-specific ICAM-3-Grabbing Nonintegrin Mediates Internalization of HIV-1 into Human Podocytes. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00629.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Driessen N.N., Ummels R., Maaskant J.J., Gurcha S.S., Besra G.S., Ainge G.D. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect Immun. 2009;77:4538–4547. doi: 10.1128/IAI.01256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mc Dermott R., Ziylan U., Spehner D., Bausinger H., Lipsker D., Mommaas M. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol Biol Cell. 2002;13:317–335. doi: 10.1091/mbc.01-06-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Turville S, Wilkinson J., Cameron P., Dable J., Cunningham A.L. The role of dendritic cell C-type lectin receptors in HIV pathogenesis. J Leukoc Biol. 2003;74:710–718. doi: 10.1189/jlb.0503208. [DOI] [PubMed] [Google Scholar]
- 151.Stambach N.S., Taylor M.E. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology. 2003;13:401–410. doi: 10.1093/glycob/cwg045. [DOI] [PubMed] [Google Scholar]
- 152.Hunger R.E, Sieling P.A., Ochoa M.T., Sugaya M., Burdick A.E., Rea T.H. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701–708. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tada Y., Riedl E., Lowenthal M.S., Liotta L.A., Briner D.M., Crouch E.C. Identification and characterization of endogenous Langerin ligands in murine extracellular matrix. J Invest Dermatol. 2006;126:1549–1558. doi: 10.1038/sj.jid.5700283. [DOI] [PubMed] [Google Scholar]
- 154.de Witte L., Nabatov A., Pion M., Fluitsma D., de Jong M.A., de Gruijl T. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367–371. doi: 10.1038/nm1541. [DOI] [PubMed] [Google Scholar]
- 155.Merad M., Ginhoux F., Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 156.de Jong M.A, Vriend L.E., Theelen B., Taylor M.E., Fluitsma D., Boekhout T. C-type lectin Langerin is a beta-glucan receptor on human Langerhans cells that recognizes opportunistic and pathogenic fungi. Mol Immunol. 2010;47:1216–1225. doi: 10.1016/j.molimm.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tsuiji M., Fujimori M., Ohashi Y., Higashi N., Onami T.M., Hedrick S.M. Molecular cloning and characterization of a novel mouse macrophage C-type lectin, mMGL2, which has a distinct carbohydrate specificity from mMGL1. J Biol Chem. 2002;277:28892–28901. doi: 10.1074/jbc.M203774200. [DOI] [PubMed] [Google Scholar]
- 158.Takada A, Fujioka K., Tsuiji M., Morikawa A., Higashi N., Ebihara H. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J Virol. 2004;78:2943–2947. doi: 10.1128/JVI.78.6.2943-2947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van Vliet S.J., van Liempt E., Saeland E., Aarnoudse C.A., Appelmelk B., Irimura T. Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol. 2005;17:661–669. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 160.van Vliet S.J., Gringhuis S.I., Geijtenbeek T.B., van Kooyk Y. Regulation of effector T cells by antigen-presenting cells via interaction of the C-type lectin MGL with CD45. Nat Immunol. 2006;7:1200–1208. doi: 10.1038/ni1390. [DOI] [PubMed] [Google Scholar]
- 161.van Vliet S.J., Saeland E., van Kooyk Y. Sweet preferences of MGL: carbohydrate specificity and function. Trends Immunol. 2008;29:83–90. doi: 10.1016/j.it.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 162.Upham J.P., Pickett D., Irimura T., Anders E.M., Reading P.C. Macrophage receptors for influenza A virus: role of the macrophage galactose-type lectin and mannose receptor in viral entry. J Virol. 2010;84:3730–3737. doi: 10.1128/JVI.02148-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Colonna M., Samaridis J., Angman L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J Immunol. 2000;30:697–704. doi: 10.1002/1521-4141(200002)30:2<697::AID-IMMU697>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 164.Suzuki-Inoue K, Fuller G.L., Garcia A., Eble J.A., Pohlmann S., Inoue O. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 165.Suzuki-Inoue K., Kato Y., Inoue O., Kaneko M.K., Mishima K., Yatomi Y. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282:25993–26001. doi: 10.1074/jbc.M702327200. [DOI] [PubMed] [Google Scholar]
- 166.Chaipan C, Soilleux E.J., Simpson P., Hofmann H., Gramberg T., Marzi A. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J Virol. 2006;80:8951–8960. doi: 10.1128/JVI.00136-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fuller G.L., Williams J.A., Tomlinson M.G., Eble J.A., Hanna S.L., Pohlmann S. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pleines I, Elvers M., Strehl A., Pozgajova M., Varga-Szabo D., May F. Rac1 is essential for phospholipase C-gamma2 activation in platelets. Pflugers Arch. 2009;457:1173–1185. doi: 10.1007/s00424-008-0573-7. [DOI] [PubMed] [Google Scholar]
- 169.Kerrigan A.M., Dennehy K.M., Mourao-Sa D., Faro-Trindade I., Willment J.A., Taylor P.R. CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils. J Immunol. 2009;182:4150–4157. doi: 10.4049/jimmunol.0802808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Christou C.M., Pearce A.C., Watson A.A., Mistry A.R., Pollitt A.Y., Fenton-May A.E. Renal cells activate the platelet receptor CLEC-2 through podoplanin. Biochem J. 2008;411:133–140. doi: 10.1042/BJ20071216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kato Y., Kaneko M.K., Kunita A., Ito H., Kameyama A., Ogasawara S. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008;99:54–61. doi: 10.1111/j.1349-7006.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.May F., Hagedorn I., Pleines I., Bender M., Vogtle T., Eble J. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114:3464–3472. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 173.Chaipan C., Steffen I., Tsegaye T.S., Bertram S., Glowacka I., Kato Y. Incorporation of podoplanin into HIV released from HEK-293T cells, but not PBMC, is required for efficient binding to the attachment factor CLEC-2. Retrovirology. 2010;7:47. doi: 10.1186/1742-4690-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sancho D, Mourao-Sa D., Joffre O.P., Schulz O., Rogers N.C., Pennington D.J. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Caminschi I., Proietto A.I., Ahmet F., Kitsoulis S., Shin Teh J., Lo J.C. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huysamen C., Willment J.A., Dennehy K.M., Brown G.D. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–16701. doi: 10.1074/jbc.M709923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sancho D., Joffre O.P., Keller A.M., Rogers N.C., Martinez D., Hernanz-Falcon P. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thebault P., Lhermite N., Tilly G., Le Texier L., Quillard T., Heslan M. The C-type lectin-like receptor CLEC-1, expressed by myeloid cells and endothelial cells, is up-regulated by immunoregulatory mediators and moderates T cell activation. J Immunol. 2009;183:3099–3108. doi: 10.4049/jimmunol.0803767. [DOI] [PubMed] [Google Scholar]
- 179.Herre J, Gordon S., Brown G.D. Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol Immunol. 2004;40:869–876. doi: 10.1016/j.molimm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 180.Gross O., Gewies A., Finger K., Schafer M., Sparwasser T., Peschel C. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 181.Dillon S., Agrawal S., Banerjee K., Letterio J., Denning T.L., Oswald-Richter K. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Saijo S, Fujikado N., Furuta T., Chung S.H., Kotaki H., Seki K. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 183.Taylor P.R., Tsoni S.V., Willment J.A., Dennehy K.M., Rosas M., Findon H. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.LeibundGut-Landmann S., Gross O., Robinson M.J., Osorio F., Slack E.C., Tsoni S.V. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 185.Gringhuis S.I., den Dunnen J., Litjens M., van der Vlist M., Wevers B., Bruijns S.C. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009;10:203–213. doi: 10.1038/ni.1692. [DOI] [PubMed] [Google Scholar]
- 186.Sorgi C.A., Secatto A., Fontanari C., Turato W.M., Belanger C., de Medeiros A.I. Histoplasma capsulatum cell wall {beta}-glucan induces lipid body formation through CD18, TLR2, and dectin-1 receptors: correlation with leukotriene B4 generation and role in HIV-1 infection. J Immunol. 2009;182:4025–4035. doi: 10.4049/jimmunol.0801795. [DOI] [PubMed] [Google Scholar]
- 187.Xu S, Huo J., Gunawan M., Su I.H., Lam K.P. Activated dectin-1 localizes to lipid raft microdomains for signaling and activation of phagocytosis and cytokine production in dendritic cells. J Biol Chem. 2009;284:22005–22011. doi: 10.1074/jbc.M109.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu S., Huo J., Lee K.G., Kurosaki T., Lam K.P. Phospholipase Cgamma2 is critical for Dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J Biol Chem. 2009;284:7038–7046. doi: 10.1074/jbc.M806650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lee H.M., Yuk J.M., Shin D.M., Jo E.K. Dectin-1 is inducible and plays an essential role for mycobacteria-induced innate immune responses in airway epithelial cells. J Clin Immunol. 2009;29:795–805. doi: 10.1007/s10875-009-9319-3. [DOI] [PubMed] [Google Scholar]
- 190.Schorey J.S., Lawrence C. The pattern recognition receptor Dectin-1: from fungi to mycobacteria. Curr Drug Targets. 2008;9:123–129. doi: 10.2174/138945008783502430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yadav M., Schorey J.S. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sawamura T., Kume N., Aoyama T., Moriwaki H., Hoshikawa H., Aiba Y. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 193.Yoshida H., Kondratenko N., Green S., Steinberg D., Quehenberger O. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem J. 1998;334(Pt 1):9–13. doi: 10.1042/bj3340009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Murase T., Kume N., Korenaga R., Ando J., Sawamura T., Masaki T. Fluid shear stress transcriptionally induces lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:328–333. doi: 10.1161/01.res.83.3.328. [DOI] [PubMed] [Google Scholar]
- 195.Kume N., Murase T., Moriwaki H., Aoyama T., Sawamura T., Masaki T. Inducible expression of lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:322–327. doi: 10.1161/01.res.83.3.322. [DOI] [PubMed] [Google Scholar]
- 196.Moriwaki H., Kume N., Kataoka H., Murase T., Nishi E., Sawamura T. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-alpha. FEBS Lett. 1998;440:29–32. doi: 10.1016/s0014-5793(98)01414-8. [DOI] [PubMed] [Google Scholar]
- 197.Moriwaki H., Kume N., Sawamura T., Aoyama T., Hoshikawa H., Ochi H. Ligand specificity of LOX-1, a novel endothelial receptor for oxidized low density lipoprotein. Arterioscler Thromb Vasc Biol. 1998;18:1541–1547. doi: 10.1161/01.atv.18.10.1541. [DOI] [PubMed] [Google Scholar]
- 198.Mehta J.L, Li D.Y. Identification and autoregulation of receptor for OX-LDL in cultured human coronary artery endothelial cells. Biochem Biophys Res Commun. 1998;248:511–514. doi: 10.1006/bbrc.1998.9004. [DOI] [PubMed] [Google Scholar]
- 199.Oka K., Sawamura T., Kikuta K., Itokawa S., Kume N., Kita T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Draude G., Hrboticky N., Lorenz R.L. The expression of the lectin-like oxidized low-density lipoprotein receptor (LOX-1) on human vascular smooth muscle cells and monocytes and its down-regulation by lovastatin. Biochem Pharmacol. 1999;57:383–386. doi: 10.1016/s0006-2952(98)00313-x. [DOI] [PubMed] [Google Scholar]
- 201.Kakutani M., Masaki T., Sawamura T. A platelet-endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1. Proc Natl Acad Sci USA. 2000;97:360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chen M., Narumiya S., Masaki T., Sawamura T. Conserved C-terminal residues within the lectin-like domain of LOX-1 are essential for oxidized low-density-lipoprotein binding. Biochem J. 2001;355:289–296. doi: 10.1042/0264-6021:3550289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li D.Y., Chen H.J., Mehta J.L. Statins inhibit oxidized-LDL-mediated LOX-1 expression, uptake of oxidized-LDL and reduction in PKB phosphorylation. Cardiovasc Res. 2001;52:130–135. doi: 10.1016/s0008-6363(01)00371-6. [DOI] [PubMed] [Google Scholar]
- 204.Kataoka H, Kume N., Miyamoto S., Minami M., Morimoto M., Hayashida K. Oxidized LDL modulates Bax/Bcl-2 through the lectinlike Ox-LDL receptor-1 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:955–960. doi: 10.1161/01.atv.21.6.955. [DOI] [PubMed] [Google Scholar]
- 205.Nagase M., Ando K., Nagase T., Kaname S., Sawamura T., Fujita T. Redox-sensitive regulation of lox-1 gene expression in vascular endothelium. Biochem Biophys Res Commun. 2001;281:720–725. doi: 10.1006/bbrc.2001.4374. [DOI] [PubMed] [Google Scholar]
- 206.Shimaoka T., Kume N., Minami M., Hayashida K., Sawamura T., Kita T. Lectin-like oxidized low density lipoprotein receptor-1 (LOX-1) supports cell adhesion to fibronectin. FEBS Lett. 2001;504:65–68. doi: 10.1016/s0014-5793(01)02774-0. [DOI] [PubMed] [Google Scholar]
- 207.Shimaoka T., Kume N., Minami M., Hayashida K., Sawamura T., Kita T. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J Immunol. 2001;166:5108–5114. doi: 10.4049/jimmunol.166.8.5108. [DOI] [PubMed] [Google Scholar]
- 208.Chen M., Masaki T., Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95:89–100. doi: 10.1016/s0163-7258(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 209.Parlato S, Romagnoli G., Spadaro F., Canini I., Sirabella P., Borghi P. LOX-1 as a natural IFN-alpha-mediated signal for apoptotic cell uptake and antigen presentation in dendritic cells. Blood. 2010;115:1554–1563. doi: 10.1182/blood-2009-07-234468. [DOI] [PubMed] [Google Scholar]
- 210.Chieppa M., Bianchi G., Doni A., Del Prete A., Sironi M., Laskarin G. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–4560. doi: 10.4049/jimmunol.171.9.4552. [DOI] [PubMed] [Google Scholar]
- 211.Zhang J., Tachado S.D., Patel N., Zhu J., Imrich A., Manfruelli P. Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. J Leukoc Biol. 2005;78:665–674. doi: 10.1189/jlb.1204699. [DOI] [PubMed] [Google Scholar]
- 212.Zhang J., Zhu J., Bu X., Cushion M., Kinane T.B., Avraham H. Cdc42 and RhoB activation are required for mannose receptor-mediated phagocytosis by human alveolar macrophages. Mol Biol Cell. 2005;16:824–834. doi: 10.1091/mbc.E04-06-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Miller J.L., de Wet B.J., Martinez-Pomares L., Radcliffe C.M., Dwek R.A., Rudd P.M. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog. 2008;4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gazi U., Martinez-Pomares L. Influence of the mannose receptor in host immune responses. Immunobiology. 2009;214:554–561. doi: 10.1016/j.imbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 215.Royer P.J., Emara M., Yang C., Al-Ghouleh A., Tighe P., Jones N. The mannose receptor mediates the uptake of diverse native allergens by dendritic cells and determines allergen-induced T cell polarization through modulation of IDO activity. J Immunol. 2010;185:1522–1531. doi: 10.4049/jimmunol.1000774. [DOI] [PubMed] [Google Scholar]
- 216.Diaz-Silvestre H., Espinosa-Cueto P., Sanchez-Gonzalez A., Esparza-Ceron M.A., Pereira-Suarez A.L., Bernal-Fernandez G. The 19-kDa antigen of Mycobacterium tuberculosis is a major adhesin that binds the mannose receptor of THP-1 monocytic cells and promotes phagocytosis of mycobacteria. Microb Pathog. 2005;39:97–107. doi: 10.1016/j.micpath.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 217.Lai J., Bernhard O.K., Turville S.G., Harman A.N., Wilkinson J., Cunningham A.L. Oligomerization of the macrophage mannose receptor enhances gp120-mediated binding of HIV-1. J Biol Chem. 2009;284:11027–11038. doi: 10.1074/jbc.M809698200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mahnke K., Guo M., Lee S., Sepulveda H., Swain S.L., Nussenzweig M. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–684. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Howard M.J, Isacke C.M. The C-type lectin receptor Endo180 displays internalization and recycling properties distinct from other members of the mannose receptor family. J Biol Chem. 2002;277:32320–32331. doi: 10.1074/jbc.M203631200. [DOI] [PubMed] [Google Scholar]
- 220.Bonifaz L., Bonnyay D., Mahnke K., Rivera M., Nussenzweig M.C., Steinman R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bonifaz L.C., Bonnyay D.P., Charalambous A., Darguste D.I., Fujii S., Soares H. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]