Abstract
Pain is a dominant symptom associated with inflammatory conditions. Pharmacotherapy with opioids may be limited by poor blood-brain barrier (BBB) permeability. One approach that may improve central nervous system (CNS) delivery is to target endogenous BBB transporters such as organic anion-transporting polypeptide 1a4 (Oatp1a4). It is critical to identify and characterize biological mechanisms that enable peripheral pain/inflammation to “transmit” upstream signals and alter CNS drug transport processes. Our goal was to investigate, in vivo, BBB functional expression of Oatp1a4 in animals subjected to peripheral inflammatory pain. Inflammatory pain was induced in female Sprague-Dawley rats (200–250 g) by subcutaneous injection of 3% λ-carrageenan into the right hind paw; control animals were injected with 0.9% saline. In rat brain microvessels, Oatp1a4 expression was increased during acute pain/inflammation. Uptake of taurocholate and [d-penicillamine2,5]-enkephalin, two established Oatp substrates, was increased in animals subjected to peripheral pain, suggesting increased Oatp1a4-mediated transport. Inhibition of inflammatory pain with the anti-inflammatory drug diclofenac attenuated these changes in Oatp1a4 functional expression, suggesting that inflammation in the periphery can modulate BBB transporters. In addition, diclofenac prevented changes in the peripheral signaling cytokine transforming growth factor-β1 (TGF-β1) levels and brain microvascular TGF-β receptor expression induced by inflammatory pain. Pretreatment with the pharmacological TGF-β receptor inhibitor 4-[4-(1,3-benzodioxol-5-yl)-5-(2-pyridinyl)-1H-imidazol-2-yl]benzamide (SB431542) increased Oatp1a4 functional expression in λ-carrageenan-treated animals and saline controls, suggesting that TGF-β signaling is involved in Oatp1a4 regulation at the BBB. Our findings indicate that BBB transporters (i.e., Oatp1a4) can be targeted during drug development to improve CNS delivery of highly promising therapeutics.
Introduction
Pain is associated with multiple pathological conditions, particularly those with an inflammatory component. Pain pharmacotherapy often involves opioids, which act by binding to opioid receptors localized throughout brain, spinal cord, and peripheral nerves (Stein et al., 2003). Although opioids can provide analgesia by binding to peripheral opioid receptors, efficacious pharmacotherapy requires accumulation of such therapeutics within the CNS (Labuz et al., 2007). Novel pain treatment approaches include development of peptides that act as potent opioid receptor agonists; however, treatment with such peptides is hindered by difficulties in CNS delivery (Witt and Davis, 2006). Opioid brain uptake is highly restricted by the blood-brain barrier (BBB) existing between the brain and the systemic circulation. Structurally, the BBB is composed of a monolayer of nonfenestrated endothelial cells surrounded by pericytes and astrocytes. BBB endothelial cells are joined by tight junctions that impart a transendothelial resistance of 1500 to 2000 Ω × cm2 (Butt et al., 1990). The net result of this high transendothelial resistance is very low paracellular permeability of circulating xenobiotics to the brain.
An alternative approach for delivering opioid drugs to the brain is to target endogenous BBB transporters known to be involved in blood-to-brain xenobiotic transport. One such family of transporters are organic anion-transporting polypeptides (OATPs in humans; Oatps in rodents), a group of sodium-independent transporters classified within the larger solute carrier superfamily (Hagenbuch and Meier, 2004). OATPs/Oatps have distinct substrate preferences for amphipathic solutes (Hagenbuch and Meier, 2004). For example, studies in Xenopus laevis oocytes have shown OATP1A2 mediated uptake of peptides such as [d-penicillamine2,5]-enkephalin (DPDPE) and deltorphin II (Gao et al., 2000). Although OATP isoforms are expressed in several tissues, not all exist at the BBB. Immunofluorescence staining of human brain frontal cortex demonstrated OATP1A2 localization along microvascular endothelium (Gao et al., 2000). In rodent brain, expression of Oatp1a4 and Oatp1c1 has been reported in capillary enriched fractions and/or capillary endothelial cells (Sugiyama et al., 2003; Taogoshi et al., 2005; Westholm et al., 2009a,b). Oatp1c1 has relatively narrow substrate specificity and primarily transports thyroxine and conjugated sterols at the BBB (Westholm et al., 2009a,b). It has been proposed that Oatp1a4, a rodent homolog of OATP1A2, is the primary drug-transporting Oatp isoform expressed at the rat BBB (Hagenbuch and Meier, 2004). Using Oatp1a4(−/−) mice, Ose et al. (2010) demonstrated enhanced blood-to-brain transport of various Oatp substrates (i.e., pitavastatin, rosuvastatin, digoxin, taurocholate, ochratoxin A) compared with wild-type controls; however, the ability of Oatp1a4 to facilitate effective CNS drug delivery remains controversial.
Pathologies associated with pain can alter the BBB, an important therapeutic consideration. Our laboratory has shown, in vivo, modifications in functional BBB integrity and changes in CNS drug delivery induced by peripheral inflammatory pain (Huber et al., 2001; Hau et al., 2004; Brooks et al., 2006, 2008; Seelbach et al., 2007; Campos et al., 2008; Ronaldson et al., 2009). The critical link between inflammation in peripheral tissues and altered BBB permeability and/or transport may involve changes in serum cytokines such as transforming growth factor-β (TGF-β). TGF-β regulates BBB integrity by a precise balance mediated by two receptors, designated activin receptor-like kinase 1 (ALK1) and ALK5 (Goumans et al., 2002). Whereas the ALK1 pathway leads to increased permeability, ALK5-mediated signaling reduces vascular permeability to circulating solutes (Goumans et al., 2002). Our laboratory has shown reduced TGF-β/ALK5 signaling during pain/inflammation, leading to increased paracellular BBB permeability (Ronaldson et al., 2009). It is currently unknown whether circulating TGF-β levels and/or signaling via TGF-β/ALK5 receptors can regulate specific BBB transporters such as Oatp1a4.
To date, all studies on Oatp1a4 functional expression have been conducted in nonpathological (i.e., healthy) in vitro or in vivo model systems. Therefore, we sought to determine whether pathological insult (i.e., inflammatory pain) could modulate expression and/or activity of Oatp1a4 at the BBB and whether such changes could be exploited to enhance CNS drug delivery. In the present study, we investigated in vivo 1) Oatp1a4 protein expression in rat brain microvessels and 2) Oatp1a4 transport activity at the BBB by measuring brain uptake of [3H]taurocholate and [3H]DPDPE, two established Oatp1a4 substrates, using in situ brain perfusion. We also examined the role of TGF-β/ALK5 signaling on the regulation of Oatp1a4 functional expression at the BBB using 4-[4-(1,3-benzodioxol-5-yl)-5-(2-pyridinyl)-1H-imidazol-2-yl] benzamide (SB431542), a known pharmacological ALK5 inhibitor. All research objectives were evaluated using the well established and highly reproducible λ-carrageenan model of peripheral inflammatory pain (Morris, 2003).
Materials and Methods
Materials.
DPDPE was a generous gift from the National Institute for Drug Abuse, National Institutes of Health (Bethesda, MD) and was radiolabeled with tritium (40 Ci/mmol) by PolyPeptide Laboratories (San Diego, CA). [3H]taurocholic acid (4.6 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Rabbit polyclonal antibodies against Oatp1a4 (M-50), ALK1 (H-150), and ALK-5 (V-22) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Estrone-3-sulfate (E13S), fexofenadine, digoxin, bromosulfophthalein (BSP), the specific P-glycoprotein (P-gp) inhibitory peptide reversin 205 ([Boc-Glu(Obzl)]2-Lys-Ome), the nonsteroidal anti-inflammatory drug diclofenac, the selective ALK5 inhibitor SB431542, λ-carrageenan, sodium pentobarbital, and the murine monoclonal antiactin antibody AC40 all were purchased from Sigma-Aldrich (St. Louis, MO).
Animals and Treatments.
All animal experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and conform to National Institutes of Health guidelines. Female Sprague-Dawley rats (200–250 g) were purchased from Harlan (Indianapolis, IN), housed under standard 12-h light/12-h dark conditions, and provided with food and water ad libitum. Female rats were selected to maintain consistency with previous work (Seelbach et al., 2007; Brooks et al., 2008; Campos et al., 2008; Ronaldson et al., 2009). In addition, androgens have been shown to repress functional expression of transport proteins (Cui et al., 2009), an observation that implies that female rodents may be preferable for in vivo drug transport studies. Animals were randomly assigned to each treatment group. Rats were injected (100 μl s.c.) with 3% λ-carrageenan (m/v in 0.9% saline) or 0.9% saline (m/v) into the plantar surface of the right hind paw. For experiments designed to examine the effect of peripheral inflammation on Oatp1a4 functional expression at the BBB diclofenac [30 mg/kg (1.0 ml/kg) i.p.)], a well characterized nonsteroidal anti-inflammatory drug, was dissolved in water and injected 15 min after footpad injection. For experiments designed to evaluate the involvement of TGF-β/ALK5-mediated signaling SB431542 [1.5 mg/kg (1.0 ml/kg) i.p.], a selective ALK5 inhibitor, was dissolved in 100% dimethyl sulfoxide and administered 30 min before footpad injection. The dimethyl sulfoxide vehicle has been shown previously to have no effect on BBB integrity or solute permeability (Ronaldson et al., 2009). After 3-h exposure to λ-carrageenan or saline, animals were anesthetized with sodium pentobarbital [64.8 mg/kg (1.0 ml/kg) i.p.] and prepared for serum collection, microvessel isolation, or in situ brain perfusion.
Microvessel Isolation.
After anesthesia, rats were decapitated, and brains were removed. The meninges and choroid plexus were excised, and cerebral hemispheres were homogenized in 4 ml of buffer (103 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 15 mM HEPES, 0.1 mM EGTA, 1 mM sodium orthovanadate, 0.5 mM phenylmethylsulfonyl fluoride, pH 7.4) containing protease inhibitor (PI) cocktail (Sigma-Aldrich). At that time, 8 ml of 26% dextran (m/v) at 4°C was added to the homogenate. Samples were gently vortexed and centrifuged (5600g; 4°C) for 10 min, and the supernatant was aspirated. Pellets were resuspended in 10 ml of fresh buffer and passed through a 70-μm filter (BD Biosciences, Franklin Lakes, NJ). Filtered homogenates were pelleted by centrifugation at 3000g for 10 min. At that time, the supernatant was aspirated, and the pellet, enriched in brain microvessels, was collected.
After brain microvessels were collected, the pellet was resuspended in 500 μl of CellLytic (Sigma-Aldrich) containing PI cocktail. The samples were subjected to a 15-s homogenization every 15 min for 1 h while on ice and then centrifuged at 10,000g (30 min, 4°C). The supernatants were collected and centrifuged at 100,000g (90 min, 4°C). At that time, the supernatant was discarded, and the pellet was resuspended in phosphate-buffered saline containing PI cocktail. The protein concentration of each sample was determined using the BCA protein assay (Pierce Biotechnology, Rockford, IL) and used for Western blot analysis.
Western Blotting.
For Western blotting, 10-μg aliquots of crude membrane preparations of rat brain microvessels or 10-μg aliquots of rat liver homogenate were mixed in XT sample loading buffer containing Tris(2-carboxyethyl) phosphine and heated for 10 min at 70°C. The samples were then resolved on 10% lithium dodecylsulfate-polyacrylamide gels (Bis-Tris Criterion XT; Bio-Rad Laboratories, Hercules, CA). The gels were electrotransferred onto a polyvinylidene difluoride (PVDF) membrane, and protein transfer was verified by Ponceau S staining. The membranes were blocked for 1 h at room temperature in Superblock (Pierce Biotechnology) containing 0.05% (v/v) Tween 20. After six washes (5 min each) with Tris-buffered saline (15 mM Tris-HCl, 150 mM NaCl, pH 7.6) containing 0.05% (v/v) Tween 20, the membrane was incubated at 4°C overnight with polyclonal Oatp1a4 antibody M-50 (1:500 dilution), polyclonal ALK-1 antibody H-150 (1:500 dilution), polyclonal ALK-5 antibody V-22 (1:500 dilution), or mouse monoclonal actin antibody AC40 (1:500 dilution). After a second wash, membranes were incubated for 1 to 2 h with anti-mouse (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) or anti-rabbit (GE Healthcare) horseradish peroxidase-conjugated secondary antibodies in Superblock containing 0.05% (v/v) Tween 20 at room temperature. Protein bands were detected by enhanced chemiluminescence. Bands were quantitated and corrected for background using ImageJ densitometric software (Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, MD). Protein expression in membrane preparations were normalized to the expression of actin, which served as a loading control. Enrichment of plasma membrane in each membrane sample was confirmed by expression of Glut1, an established plasma membrane marker of brain microvascular endothelial cells (Cornford et al., 1994).
Enzyme-Linked Immunosorbent Assay Analysis.
A Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) was used to determine serum concentrations of TGF-β1 from rats treated with λ-carrageenan or saline in the presence and absence of diclofenac. A standard curve for TGF-β1 (0–2500 pg/ml) was generated using recombinant rat TGF-β1, and the assay was performed according to the manufacturer's instructions. Absorbance was read at 450 nm using a GENios microplate spectrophotometer (Tecan Systems, San Jose, CA). Absorbance was also measured at 550 nm to correct for optical imperfections in the assay plate. The concentration of serum TGF-β1 was expressed as picograms per milliliter.
In Situ Brain Perfusion.
In situ brain perfusion was performed as described previously (Seelbach et al., 2007; Ronaldson et al., 2009). Three hours after paw injection, animals were anesthetized and heparinized (10,000 U/kg i.p.). Body temperature was maintained at 37°C using a heating pad. The common carotid arteries were cannulated with silicone tubing connected to a perfusion circuit. The perfusate was erythrocyte-free modified mammalian Ringer's solution consisting of 117 mM NaCl, 4.7 mM KCl, 0.8 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 10 mM d-glucose, 3.9% (w/v) dextran (molecular weight 60,000), and 1.0 g/liter bovine serum albumin (type IV), pH 7.4, warmed to 37°C and oxygenated with 95% O2/5% CO2. Evan's blue dye (55 mg/liter) was added to the perfusate to serve as a visual marker of BBB integrity. Perfusion pressure and flow rate were maintained at 95 to 105 mm Hg and 3.1 ml/min, respectively. Both jugular veins were severed to allow for perfusate drainage. Using a slow-drive syringe pump (Harvard Apparatus Inc., Holliston, MA), [3H]taurocholic acid (1 μCi/ml; 10 μM total concentration) or [3H]DPDPE (0.5 μCi/ml; 0.013 μM total concentration) was added to the inflowing perfusion solution at a rate of 0.5 ml/min per cerebral hemisphere. For inhibition studies, animals were perfused with erythrocyte-free modified mammalian Ringer's solution containing transport inhibitor (i.e., 1.0 mM BSP, 200 μM digoxin, 100 μM E13S, 100 μM fexofenadine, 10 μM reversin 205) for 10 min before perfusion with [3H]taurocholic acid or [3H]DPDPE. HPLC analyses confirmed the stability of [3H]taurocholic acid and [3H]DPDPE in both perfusion medium and jugular vein venous outflow from in situ perfusion experiments. For these analyses, samples were prepared by the addition of an equal volume of acetonitrile and centrifugation at 13,000g for 5 min. The supernatant was removed and diluted to produce a sample with a final acetonitrile concentration of <10% before HPLC analysis.
Immediately after perfusion for the desired time (2.5, 5, 10, 15, or 20 min), rats were decapitated and brains were removed. The meninges and choroid plexus were excised and cerebral hemispheres were sectioned. TS2 tissue solubilizer (1 ml; Research Products International, Mt. Prospect, IL) was added to the tissue samples, which were allowed to solubilize for 2 days at room temperature. To eliminate chemiluminescence, 100 μl of 30% glacial acetic acid was added, along with 2.0 ml of Optiphase SuperMix liquid scintillation cocktail (PerkinElmer Life and Analytical Sciences). Radioactivity was measured using a model 1450 Liquid Scintillation and Luminescence Counter (PerkinElmer Life and Analytical Sciences). Results were reported as picomoles of radiolabeled compound per gram of brain tissue (C; pmol/g tissue), which is equal to the total amount of radioisotope in the brain [CBrain; dpm/g tissue] divided by the amount of radioisotope in the perfusate [CPerfusate; dpm/pmol]: C = CBrain/CPerfusate (1).
The brain vascular volume in rats has been previously shown to range between 6 and 9 μl/g brain tissue (Takasato et al., 1984). Because brain tissue was processed immediately after perfusion with radiolabeled substrate, all uptake values required correction for brain vascular volume. This was accomplished by subtracting the average vascular volume (i.e., 8.0 μl/g brain tissue as calculated from data reported by Takasato and colleagues) from whole-brain uptake data obtained using [3H]taurocholic acid or [3H]DPDPE.
Multiple time uptake data were best-fit to a nonlinear least-squares regression model, using the following equation, as described previously (Seelbach et al., 2007): C = (KIN/kout) × (1 − e−k(out)T) (2), where C is the concentration of drug per gram of brain tissue, T is time in minutes, KIN is the calculated uptake transfer constant, and kout is the brain efflux rate coefficient estimated. The estimated brain volume of distribution was calculated using the following equation as described previously (Seelbach et al., 2007): VBr = KIN/kout (3).
All kinetic parameters were calculated using SigmaPlot 11 graphical and statistical software (SPSS Inc., Chicago, IL).
Capillary Depletion Analysis.
Capillary depletion analysis was performed according to the method of Triguero et al. (1990) with few modifications. In brief, rats were decapitated and brains were removed immediately after in situ brain perfusion with [3H]taurocholic acid. Brain tissue was homogenized (13 strokes) using a glass homogenizer with 4 ml of ice-cold capillary depletion buffer (141 mM NaCl, 4 mM KCl, 1 mM MgSO4, 1 mM NaH2PO4, 2.8 mM CaCl2, 10 mM HEPES, 10 mM d-glucose). After homogenization, 7 ml of ice-cold 26% dextran (60,000 Da) was added to the homogenate and the sample was homogenized (three strokes). The homogenate was then centrifuged at 5400g for 15 min at 4°C. After centrifugation, brain debris was aspirated and two aliquots of supernatant were collected for measurement of radioactivity. Because [3H]taurocholic acid can leak from the capillary fraction into the brain supernatant fraction during sample preparation (Triguero et al., 1990), it is necessary to correct for this release by using a small-molecule vascular marker that does not typically cross the BBB (i.e., 14C-sucrose). We detected less than 10% of 14C-sucrose within brain supernatant in samples prepared from untreated control animals, which implies minimal leakage during tissue processing. All brain supernatant data with [3H]taurocholic acid was corrected based on these results with 14C-sucrose. The remaining supernatant was aspirated, and the pellet (i.e., capillary fraction) was resuspended in 1 ml of 1× phosphate-buffered saline, pH 7.4. Two aliquots of capillary fraction were collected for measurement of radioactivity. The amount of [3H]taurocholic acid in the capillary fraction and brain supernatant was expressed as picomoles per gram of tissue.
Statistical Analysis.
Densitometric analysis data were reported as mean ± S.D. from three separate experiments where each treatment group consisted of pooled microvessels from two animals. In situ brain perfusion data were reported as mean ± S.D. from six individual animals per treatment group. To determine statistical significance between treatment groups in ELISA and Western blot experiments, Student's t test was used for unpaired experimental data. To determine the significance of [3H]taurocholate or [3H]DPDPE accumulation, the test of repeated-measures analysis of variance and the post hoc multiple-comparison Bonferroni t test was used. A value of p < 0.05 was accepted as statistically significant.
Results
Expression of Oatp1a4 in Rat Brain Microvessels during Peripheral Inflammatory Pain.
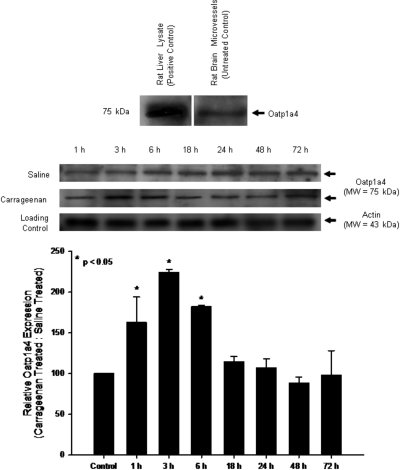
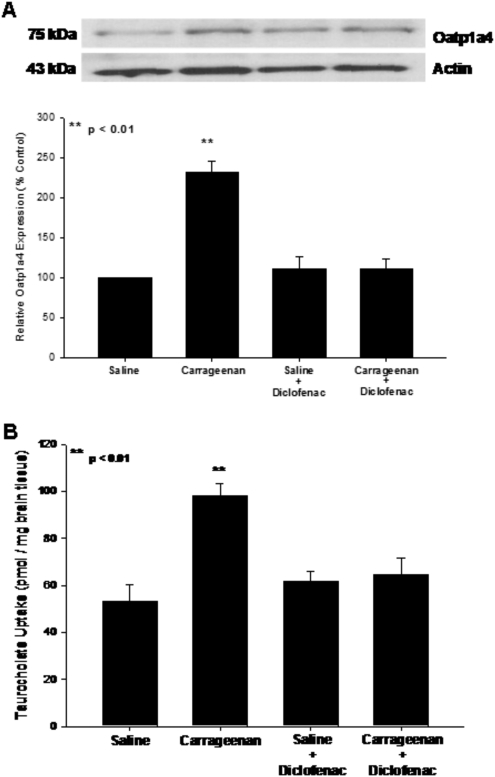
Previous data suggest that Oatp1a4 is expressed at the BBB (Gao et al., 1999; Ose et al., 2010); however, it is unknown whether brain microvascular expression of this transporter is altered by pathophysiological insult. Therefore, we examined the effect of peripheral inflammatory pain on BBB expression of Oatp1a4 in the rodent λ-carrageenan-induced inflammatory pain model (Morris, 2003). Oatp1a4 protein expression, as detected by a single band at approximately 75 kDa (Gao et al., 1999), was increased up to 2.2-fold after 3-h peripheral inflammatory pain; however, Oatp1a4 expression was not altered in animals exposed to peripheral pain conditions for longer than 18 h (Fig. 1; Table 1). Therefore, all further experiments were conducted during the acute phase of peripheral pain at the 3-h time point (i.e., the time point where we detected the maximal change in Oatp1a4 protein expression).
Fig. 1.
Expression of Oatp1a4 protein in rat brain microvessels. Top, Western blot analysis of brain microvessels isolated from rats administered 0.9% saline or 3% λ-carrageenan by subcutaneous injection into the plantar surface of the right hind paw. Crude membrane preparations (10 μg) from rat brain microvessels were resolved on a 10% lithium dodecyl sulfate-polyacrylamide gel, transferred to a PVDF membrane, and analyzed for expression of Oatp1a4 (M50 antibody; 1:500 dilution). Oatp1a4 in brain microvessels and liver lysate from naive rats is shown as a positive control. Bottom, Oatp1a4 expression in λ-carrageenan-treated animals and saline controls was determined by densitometric analysis. Results are expressed as mean ± S.D. of three separate experiments. Asterisks represent data points that are significantly different from control.
TABLE 1.
Densitometric analysis of Oatp1a4 expression in rat brain microvessels during peripheral inflammatory pain
| 1 h | 3 h | 6 h | 18 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|---|---|
| Saline: actin | 105.26 ± 2.02 | 105.48 ± 3.83 | 105.05 ± 8.00 | 102.71 ± 3.35 | 104.11 ± 6.36 | 102.60 ± 1.55 | 101.58 ± 7.16 |
| λ-Carrageenan: actin | 172.64 ± 29.97 | 236.17 ± 10.22 | 191.11 ± 12.90 | 117.21 ± 3.52 | 111.21 ± 16.80 | 90.95 ± 7.52 | 98.20 ± 25.50 |
| Relative expression (λ-carrageenan: saline) | 164.41 ± 31.38* | 223.89 ± 3.87* | 181.00 ± 1.60* | 114.25 ± 6.47 | 106.53 ± 11.40 | 88.70 ± 8.15 | 97.77 ± 31.12 |
P < 0.05 compared with rat brain microvessels isolated from untreated animals.
BBB Permeability to [3H]Taurocholate.
To determine whether increased Oatp1a4 expression corresponded to altered blood-to-brain transport activity, we measured brain uptake of [3H]taurocholate, a selective Oatp substrate (Noé et al., 1997). Several rodent Oatp isoforms are known to be involved in taurocholate transport including Oatp1a1, Oatp1a4, Oatp1a5, Oatp3a1, and Oatp4a1 (Hagenbuch and Meier, 2004). We did not detect Oatp1a1, Oatp1a5, Oatp3a1, or Oatp4a1 in our rat brain microvessel preparations (data not shown). We did, however, detect expression of Oatp1c1 in these same rat brain microvessel preparations. Oatp1c1 has narrow substrate specificity and primarily transports thyroxine and conjugated sterols at the BBB (Westholm et al., 2009a,b), implying that Oatp1c1 is unlikely to be involved in BBB taurocholate transport. Based on these data, we expect that any change in taurocholate accumulation reflects modulation of Oatp1a4 transport activity. The time course of brain [3H]taurocholate uptake (10 μM) in saline-treated animals showed increasing accumulation over the entire duration of the experiment (i.e., 20 min) (Fig. 2A). In animals subjected to peripheral inflammatory pain, a significant increase (1.8-fold; p < 0.01) in [3H]taurocholate uptake was observed compared with the uptake time course in saline controls. Multiple-time uptake data were fit using eq. 2, which provided R2 values of 0.995 (saline) and 0.991 (λ-carrageenan). Kinetic analysis of multiple-time uptake data revealed a significant increase (p < 0.01) in both KIN and kout during peripheral pain compared with saline controls (Table 2), suggesting enhancement of a selective transport process for taurocholate at the BBB. Of particular significance, the estimated brain distribution volume at steady-state (VBr) increased (1.4-fold) after 3-h inflammatory pain compared with saline controls, which implies enhanced distribution of taurocholate to the brain. HPLC analysis of venous outflow revealed a single peak (RT = 22 min) corresponding to taurocholate retention (Fig. 2A), confirming that taurocholate remained metabolically intact throughout our experiments.
Fig. 2.
Uptake of taurocholate into rat brain after λ-carrageenan-induced inflammatory pain. A, multiple time analysis of [3H]taurocholate brain uptake was measured by in situ perfusion 3 h postinjection with 0.9% saline or 3% λ-carrageenan. Taurocholate uptake increased in a nonlinear manner and was fit to a nonlinear regression model (R2 = 0.995 for saline and 0.991 for λ-carrageenan). Results are expressed as mean ± S.D. of six animals per time point per treatment group. Asterisks represent data points that are significantly different from control. Representative HPLC-radiochromatograms of inflowing perfusion fluid and jugular vein venous outflow after 20 min in situ perfusion with [ 3H]taurocholate are displayed above uptake curves. Analysis was performed on 100-μl aliquots of perfusion fluid or venous outflow. Data are expressed as relative radioactivity in dpm. B, taurocholate accumulation was measured at 3 and 48 h in animals injected with 0.9% saline or 3% λ-carrageenan. Uptake of taurocholate into brain tissue was measured after 10 min in situ perfusion. Results are expressed as mean ± S.D. of six animals per treatment group. Asterisks represent data points that are significantly different from control.
TABLE 2.
Kinetic analysis of brain taurocholate uptake during peripheral inflammatory pain
Results are expressed as mean ± S.D.
| Saline | λ-Carrageenan | |
|---|---|---|
| VBr | 97.61 ± 5.43 pmol/g | 137.26 ± 8.08 pmol/g* |
| KIN | 8.23 ± 0.17 pmol/g × min | 15.07 ± 0.18 pmol/g × min* |
| kout | 0.08 ± 0.01/min | 0.11 ± 0.01/min* |
P < 0.01.
To establish that changes in Oatp-mediated taurocholate transport at the BBB correlated with time-dependent modulation of Oatp1a4 protein expression during peripheral inflammatory pain, we measured brain uptake of taurocholate after 3- and 48-h treatment with saline or λ-carrageenan. Brain uptake of [3H]taurocholate at 3 h was significantly enhanced (p < 0.01) in animals subjected to λ-carrageenan-induced inflammatory pain compared with saline controls (Fig. 2B). In contrast, we detected no difference in [3H]taurocholate accumulation in brain tissue isolated from rats exposed to either saline or λ-carrageenan for 48 h, an observation that directly correlates with our Western blot data on Oatp1a4 expression in rat brain microvessels. Overall, these results imply that Oatp-mediated transport of [3H]taurocholate at the BBB is correlated with Oatp1a4 protein expression at the brain microvascular endothelium.
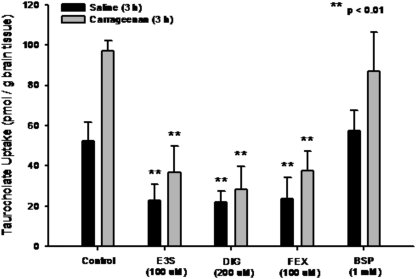
To confirm involvement of Oatp-mediated transport in taurocholate brain uptake, we performed in situ perfusion studies in saline-treated and λ-carrageenan-treated animals in the presence and absence of Oatp inhibitors (i.e., E13S, digoxin, fexofenadine, BSP). Brain uptake of [3H]taurocholate in saline-treated animals was significantly reduced (p < 0.01) in the presence of 100 μM E13S (2.2-fold), 200 μM digoxin (2.4-fold), or 100 μM fexofenadine (2.2-fold) (Fig. 3). In addition, [3H]taurocholate accumulation was significantly decreased up to 3.4-fold (p < 0.01) in animals subjected to peripheral inflammatory pain and treated with these same inhibitors. [3H]taurocholate accumulation was not altered by BSP, an organic anion that does not interact with rat Oatp1a4 (Jacquemin et al., 1994; Hagenbuch et al., 2001; van Montfoort et al., 2002). Taken together, these data confirmed involvement of Oatp1a4 in blood-to-brain transport of taurocholate.
Fig. 3.
Effect of Oatp inhibitors on the uptake of taurocholate into rat brain after 3-h λ-carrageenan-induced inflammatory pain. Taurocholate accumulation was measured in animals injected with 0.9% saline or 3% λ-carrageenan in the presence of E13S (100 μM), digoxin (DK3) (200 μM), fexofenadine (FEX) (100 μM), or bromosulfophthalein (BSP) (1 mM). Results are expressed as mean ± S.D. of six animals per treatment group. Asterisks represent data points that are significantly different from control.
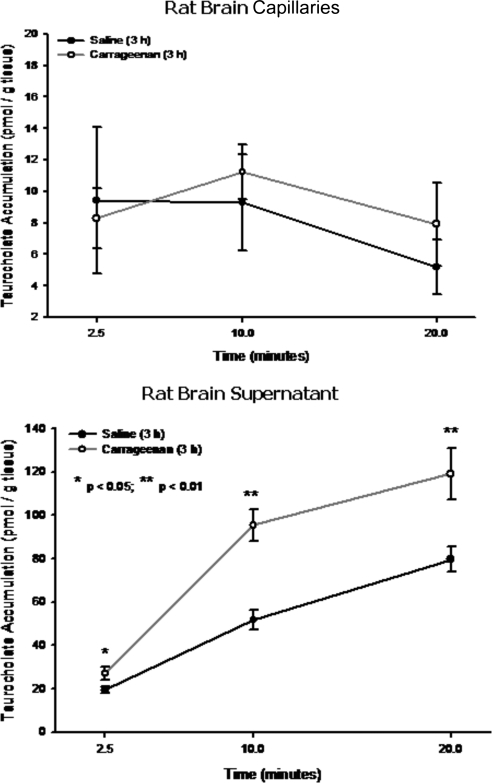
Although our results suggest increased brain taurocholate uptake during peripheral inflammatory pain, they do not indicate whether taurocholate was transported into brain parenchyma or sequestered within brain microvessels. This is a particularly important issue because parenchymal accumulation of taurocholate may imply that Oatp1a4 can be targeted in an effort to optimize CNS drug delivery. We performed capillary depletion analysis to address this question and observed no difference in taurocholate accumulation within the vascular compartment in either peripheral inflammatory pain animals or saline controls (Fig. 4). In contrast, we observed a time-dependent increase in taurocholate accumulation within brain supernatant (Fig. 4), suggesting that increased taurocholate uptake observed in our previous in situ perfusion experiments was caused by increased CNS delivery. In animals subjected to peripheral inflammatory pain, a significant increase (1.7-fold; p < 0.01) in [3H]taurocholate accumulation within brain supernatant was observed compared with saline controls, suggesting an enhancement in blood-to-brain transport of taurocholate that is mediated by Oatp1a4.
Fig. 4.
Capillary depletion analysis of taurocholate uptake in rat brain. Analysis of taurocholate accumulation in rat brain capillaries (top) and rat brain supernatant (bottom) 3 h postinjection with 0.9% saline or 3% λ-carrageenan. Results are expressed as mean ± S.D. of six animals per time point per treatment group. Asterisks represent data points that are significantly different from control.
BBB Permeability to [3H]DPDPE.
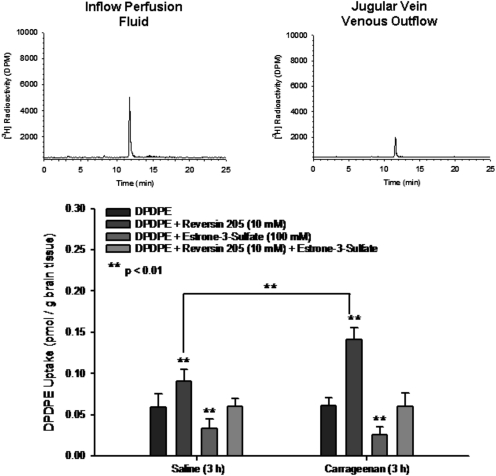
Our taurocholate data indicate that Oatp1a4 may be exploited during peripheral inflammatory pain as a means to enhance and/or optimize CNS delivery of drugs such as opioids. Effective analgesia with opioids requires that these drugs traverse the BBB and accumulate within brain parenchyma (Labuz et al., 2007). Therefore, we examined brain uptake of the opioid peptide DPDPE, a known Oatp1a4 substrate (Gao et al., 2000; Dagenais et al., 2001). We detected no difference in brain [3H]DPDPE uptake (0.013 μM) between saline-treated and λ-carrageenan-treated animals (Fig. 5). This result was not unexpected because DPDPE transport at the BBB also involves P-gp, an ATP-dependent efflux pump that is known to transport a vast array of drugs (Dagenais et al., 2001). Therefore, the Oatp1a4 component of DPDPE transport at the BBB can only be studied in the absence of P-gp efflux activity. To block P-gp-mediated DPDPE transport, we used the high-affinity P-gp inhibitor reversin 205 (Sharom et al., 1999). Reversin 205 (10 μM) significantly enhanced (p < 0.01) brain uptake of [3H]DPDPE in both saline-treated and λ-carrageenan-treated animals (Fig. 5). Of particular note, brain DPDPE accumulation was significantly greater in animals subjected to peripheral inflammatory pain compared with saline-treated animals, which is indicative of an increase in Oatp1a4-mediated DPDPE transport during peripheral inflammatory pain. The involvement of Oatp1a4 in BBB transport of DPDPE was further demonstrated using the Oatp inhibitor E13S. [3H]DPDPE uptake was decreased in the presence of 100 μM E13S in both treatment groups. We also detected no change in [3H]DPDPE accumulation in both saline- and λ-carrageenan-treated animals administered 10 μM reversin 205 and 100 μM E13S, which indicates that a considerable amount of DPDPE uptake transport at the BBB is caused by Oatp1a4. HPLC analysis of venous outflow revealed a single peak (RT = 12 min) corresponding to DPDPE (Fig. 5), which indicates the ability of this peptide to remain intact during our in situ perfusion experiments.
Fig. 5.
Uptake of DPDPE into rat brain in the presence and absence of a P-glycoprotein inhibitor and/or an Oatp inhibitor after 3-h λ-carrageenan-induced inflammatory pain. DPDPE accumulation was measured in animals injected with 0.9% saline or 3% λ-carrageenan in the presence of E13S (100 μM) and/or R205 (10 μM). Results are expressed as mean ± S.D. of six animals per treatment group. Asterisks represent data points that are significantly different from control. Representative HPLC-radiochromatograms of inflowing perfusion fluid and jugular vein venous outflow after 10 min in situ perfusion with [3H]DPDPE are displayed above the uptake graph. Analysis was performed on 100-μl aliquots of perfusion fluid or venous outflow. Data are expressed as relative radioactivity in dpm.
Comparison of [3H]DPDPE accumulation data in animals administered reversin 205 and animals receiving reversin 205 and E13S enabled an estimation of the relative contribution of Oatp1a4 to DPDPE uptake transport. In the presence of 10 μM reversin 205 and 100 μM E13S, the brain DPDPE concentration was 0.040 ± 0.010 pmol/g brain tissue in saline-treated animals; however, DPDPE accumulation was increased to 0.091 ± 0.014 pmol/g brain tissue in saline-treated animals receiving 10 μM reversin 205. This corresponds to a relative Oatp contribution of 56% under control conditions. During inflammatory pain, brain DPDPE accumulation was 0.041 ± 0.016 pmol/g brain tissue in the presence of 10 μM reversin 205 and 100 μM E13S; however, DPDPE accumulation was increased to 0.142 ± 0.014 pmol/g brain tissue in animals subjected to peripheral inflammatory pain and receiving 10 μM reversin 205. These data indicate that the relative Oatp contribution was increased to 71% in animals administered λ-carrageenan. The increased involvement of Oatp1a4 in CNS uptake of DPDPE further suggests that this transporter may be a useful target for delivery of peptide therapeutics to the brain.
Effect of Peripheral Inflammation on Oatp1a4 Functional Expression.
The above data suggest that pain/inflammation can increase functional expression of Oatp1a4 at the BBB; however, it is currently unknown how peripheral inflammation can lead to alterations in drug transport at the BBB. To determine whether inflammation in the periphery can directly affect transporter functional expression at the BBB, animals were administered the nonsteroidal anti-inflammatory drug diclofenac (30 mg/kg i.p.) 15 min after footpad injection with saline or λ-carrageenan. This dose of diclofenac significantly reduced paw swelling and reduced responsiveness to a non-noxious thermal stimulus in animals subjected to peripheral inflammatory pain (data not shown). Western blot analysis showed reduced Oatp1a4 protein expression in brain microvessels isolated from rats administered diclofenac and λ-carrageenan compared with animals receiving λ-carrageenan alone (Fig. 6A). Furthermore, [3H]taurocholate uptake in animals dosed with diclofenac after induction of inflammatory pain was significantly reduced (p < 0.01) compared with rats administered λ-carrageenan alone (Fig. 6B). Taken together, these data suggest that pharmacological blockade of peripheral inflammation may attenuate increases in Oatp1a4 functional expression during inflammatory pain and, by extension, represent a novel means for controlling CNS delivery of Oatp1a4 substrate drugs.
Fig. 6.
Effect of diclofenac, a nonsteroidal anti-inflammatory drug, on Oatp1a4 functional expression. A, Western blot analysis of microvessels isolated from rats treated with saline or λ-carrageenan in the presence and absence of diclofenac. Crude membrane preparations from rat brain microvessels (10 μg) were resolved on a 10% lithium dodecyl sulfate-polyacrylamide gel and transferred to a PVDF membrane. Samples were analyzed for expression of Oatp1a4 using the polyclonal antibody M-50 (1:500 dilution). Relative levels of Oatp1a4 were determined by densitometric analysis. Results are expressed as mean ± S.D. of three separate experiments. Asterisks represent data points that are significantly different from control. B, uptake of taurocholate into rat brain after 3-h λ-carrageenan-induced inflammatory pain in the presence and absence of diclofenac. Graph shows the concentration of taurocholate detected in rat brain tissue for the four treatment groups after injection of 3% λ-carrageenan or 0.9% saline into the plantar surface of the right hind paw. Diclofenac (30 mg/kg) was injected 15 min after footpad injection. Results are expressed as mean ± S.D. of six animals per treatment group. Asterisks represent data points that are significantly different from control.
Effect of Peripheral Inflammation on Serum TGF-β1 and Brain Microvascular Expression of TGF-β Type II Receptors.
Our laboratory has previously demonstrated that peripheral inflammatory pain induced a significant decrease in circulating TGF-β1 levels and brain microvascular expression of TGF-β/ALK5 receptors, which led to a decrease in TGF-β/ALK5 signaling and altered BBB functional integrity (Ronaldson et al., 2009). However, it is currently unknown whether peripheral inflammation directly affects circulating TGF-β1 levels. Therefore, we measured TGF-β1 serum concentrations in rats administered saline or λ-carrageenan in the presence and absence of diclofenac. ELISA analysis detected a significant decrease (p < 0.05) in serum TGF-β1 concentration in rats subjected to inflammatory pain compared with saline controls (Fig. 7), results that were consistent with our previous study (Ronaldson et al., 2009). Diclofenac treatment prevented this decrease in circulating TGF-β1.
Fig. 7.
Effect of diclofenac, a nonsteroidal anti-inflammatory drug, on TGF-β1 concentration in rat serum. Rats were treated with 0.9% saline or 3% λ-carrageenan for 3 h. Diclofenac (30 mg/kg) was injected 15 min after footpad injection. Serum was collected, and TGF-β1 concentration was determined by ELISA analysis. Data points are expressed as mean ± S.D. from nine individual rats. Asterisks represent data points that are significantly different from control.
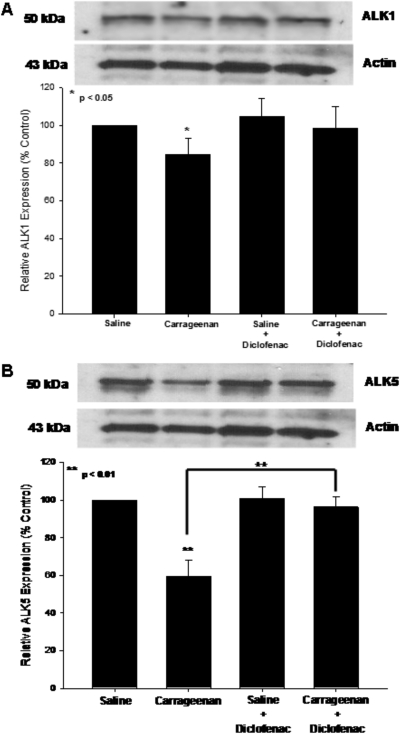
Previous studies have shown that two TGF-β type I receptors (i.e., ALK1, ALK5) are expressed at the level of the brain microvascular endothelium and contribute to the maintenance of BBB functional integrity (De Groot et al., 1999; Lebrin et al., 2005). Therefore, we examined expression of both ALK1 and ALK5 in microvessels isolated from rats subjected to 3-h peripheral inflammatory pain, in both the presence and absence of diclofenac. Western blot analysis detected single bands for ALK1 and ALK5 at 53 and 50 kDa, respectively (Fig. 8). Peripheral inflammatory pain reduced expression of both receptors compared with saline controls; however, diclofenac treatment prevented decreased expression of the ALK5 receptor only. Taken together with our ELISA results, these data indicate that peripheral inflammation may regulate TGF-β-mediated signaling at the BBB.
Fig. 8.
Effect of diclofenac, a nonsteroidal anti-inflammatory drug, on TGF-β/ALK1 and TGF-β/ALK5 receptors in rat brain microvessels. Western blot analysis of microvessels isolated from rats treated with saline or λ-carrageenan in the presence and absence of diclofenac. Crude membrane preparations from rat brain microvessels (10 μg) were resolved on a 10% lithium dodecyl sulfate-polyacrylamide gel and transferred to a PVDF membrane. Samples were analyzed for expression of TGF-β/ALK1 (A) or TGF-β/ALK5 (B) using the polyclonal antibody H-120 (1:500 dilution) for ALK1 or the polyclonal antibody V-22 (1:500 dilution) for ALK5. Relative levels of TGF-β/ALK1 and TGF-β/ALK5 were determined by densitometric analysis. Results are expressed as mean ± S.D. of three separate experiments. Asterisks represent data points that are significantly different from control.
Effect of TGF-β/ALK5 Receptor on Oatp1a4 Functional Expression.
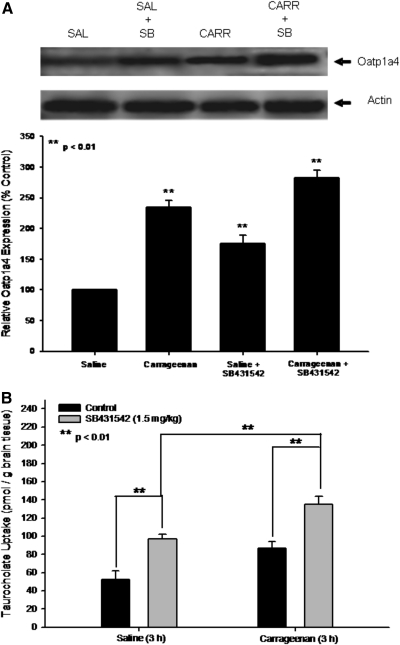
Although the above data demonstrate that peripheral inflammatory pain alters Oatp1a4 functional expression and TGF-β signaling pathway components, it is unknown whether TGF-β/ALK5 signaling can regulate BBB transport systems (i.e., Oatp1a4). Therefore, we investigated the involvement of TGF-β/ALK5 signaling in the regulation of Oatp1a4 functional expression in the presence and absence of SB431542, a selective ALK5 inhibitor, in saline-treated and λ-carrageenan-treated animals. We show that pretreatment with SB431542 before inflammatory pain significantly enhanced (p < 0.01) protein expression of Oatp1a4 (2.8-fold) to a greater degree than in rats administered λ-carrageenan alone (Fig. 9A). An increase in Oatp1a4 protein expression was also observed in animals pretreated with SB431542 before saline injection (1.9-fold), further implicating TGF-β/ALK5 signaling in Oatp1a4 protein regulation. The complexity of TGF-β/ALK5 signaling at the BBB is emphasized by increased expression of Oatp1a4 in rats treated with SB431542 and λ-carrageenan compared with animals administered SB431542 alone.
Fig. 9.
Effect of SB431542, a selective pharmacological inhibitor of TGF-β/ALK5 signaling, on Oatp1a4 functional expression. A, Western blot analysis of microvessels isolated from rats treated with saline (SAL) or λ-carrageenan (CARR) in the presence and absence of SB431542 (SB). Crude membrane preparations from rat brain microvessels (10 μg) were resolved on a 10% lithium dodecyl sulfate-polyacrylamide gel and transferred to a PVDF membrane. Samples were analyzed for expression of Oatp1a4 using the polyclonal antibody M-50 (1:500 dilution). Relative levels of Oatp1a4 were determined by densitometric analysis. Results are expressed as mean ± S.D. of three separate experiments. Asterisks represent data points that are significantly different from control. B, uptake of taurocholate into rat brain after 3-h λ-carrageenan-induced inflammatory pain in the presence and absence of SB431542. Graph shows the concentration of taurocholate detected in rat brain tissue for the four treatment groups after injection of 3% λ-carrageenan or 0.9% saline into the plantar surface of the right hind paw. SB431542 (1.5 mg/kg) was injected 30 min before footpad injection. Results are expressed as mean ± S.D. of six animals per treatment group. Asterisks represent data points that are significantly different from control.
Administration of SB431542 significantly enhanced brain uptake of [3H]taurocholate in animals treated with both saline (p < 0.01) or λ-carrageenan (p < 0.01) (Fig. 9B). In addition, accumulation of [3H]taurocholate in rats treated with SB431542 and λ-carrageenan was significantly greater (p < 0.01) than in animals administered λ-carrageenan alone. [3H]taurocholate brain uptake in rats treated with SB431542 and λ-carrageenan was significantly increased (p < 0.05) compared with animals administered SB431542 and saline, emphasizing that TGF-β/ALK5 signaling is prominently involved in the regulation of BBB taurocholate transport via Oatp1a4.
Discussion
Effective treatment of pain with opioid analgesics requires efficacious CNS concentrations in addition to effective peripheral concentrations. An alternative approach for delivering drugs to the brain is to target endogenous BBB transporters. An intriguing candidate is Oatp1a4, which is known to transport opioids (Gao et al., 2000; Dagenais et al., 2001; Ose et al., 2010). For example, murine in situ perfusion studies showed that DPDPE brain uptake was inhibited by various Oatp1a4 substrates such as digoxin, estradiol-17β-glucuronide, and fexofenadine (Dagenais et al., 2001). Studies in human embryonic kidney cells transfected with rat Oatp1a4 demonstrated saturable DPDPE uptake (Ose et al., 2010). We report for the first time increased Oatp1a4 protein expression in brain microvessels isolated from rats subjected to peripheral pain. In addition, we demonstrate that this increase in expression corresponded to enhanced Oatp1a4 functional activity. Evidence for increased Oatp1a4 transport at the BBB included 1) increased brain accumulation during peripheral inflammatory pain of the Oatp substrate taurocholate; 2) attenuation of brain taurocholate uptake by various Oatp transport inhibitors (i.e., digoxin, E13S, fexofenadine); 3) an increase in KIN for taurocholate during peripheral pain, which indicates enhanced blood-to-brain taurocholate transport; and 4) an increase in taurocholate accumulation within brain interstitial fluid but no change in taurocholate sequestration within the BBB endothelium itself. Physiologically, increased Oatp1a4 functional expression during acute inflammatory pain may contribute to enhanced blood-to-brain transport of prostaglandins. Oatp1a4 has been shown to be involved in blood-to-brain transport of the neuroprotective prostaglandin PGE1 (Taogoshi et al., 2005). Because peripheral inflammation is associated with increased neuronal apoptosis (Czapski et al., 2007) and PGE1 can prevent neuronal cell death (Kawamura et al., 1997), enhanced Oatp1a4 activity may be a particularly critical mechanism of neuroprotection by increasing CNS prostaglandin delivery during disease. In addition, we observed an increase in kout after peripheral inflammatory pain, which implies taurocholate efflux. This is a function of the Oatp1a4 transport mechanism, a process governed by the transmembrane solute gradient (Li et al., 2000). Therefore, continued increase in taurocholate accumulation within microvascular endothelium induces a change in direction of the luminal membrane concentration gradient and subsequent efflux into blood. A crucial consideration in interpretation of our data is the contribution of paracellular diffusion to brain uptake of taurocholate. Our laboratory has previously reported increased BBB permeability via the paracellular route for solutes such as morphine (Seelbach et al., 2007), codeine (Hau et al., 2004), and sucrose (Ronaldson et al., 2009) under identical peripheral pain conditions. The molecular mass of taurocholate (537.7 Da) is larger than morphine (285 Da), codeine (300 Da), or sucrose (342 Da), suggesting a lesser degree of paracellular diffusion. Because our data showed no statistical difference in taurocholate uptake between animals subjected to peripheral pain that were administered Oatp1a4 transport inhibitors and saline controls dosed with these same inhibitory compounds, we conclude that paracellular diffusion was not a significant factor in our study. We have also previously shown negligible changes in cerebral vascular blood volume during λ-carrageenan-induced inflammatory pain (Huber et al., 2001), which further implies that changes in taurocholate and/or DPDPE uptake are caused by modulation of discrete BBB transport mechanisms.
The effectiveness of Oatp1a4 as a CNS drug delivery facilitator has been a controversial subject. Although our studies with taurocholate demonstrated increased delivery to the CNS milieu by an Oatp-dependent process, it is essential to extend these studies to blood-to-brain transport of a drug that may actually require CNS delivery. Therefore, we studied BBB transport of the peptidic Oatp1a4 substrate DPDPE. In Oatp1a4(−/−) mice, Ose et al. (2010) reported no difference in blood-to-brain transport of DPDPE compared with wild-type controls; however, that study did not account for additional mechanisms involved in DPDPE transport at the BBB such as transcytosis (Egleton and Davis, 1999) and P-gp-mediated efflux (Dagenais et al., 2004). Our laboratory has previously reported increased functional expression of P-gp at the BBB in response to peripheral inflammatory pain (Seelbach et al., 2007). Although the present study demonstrated increased Oatp1a4 functional expression at the BBB in animals subjected to inflammatory pain, we did not observe any change in DPDPE brain uptake. This can be explained by considering that increased in Oatp1a4-mediated DPDPE uptake may be negated by enhanced P-gp efflux activity during peripheral inflammatory pain. Therefore, the relative contribution of Oatp1a4 to DPDPE brain uptake can be determined only in the absence of P-gp transport activity. We assessed blood-to-brain transport of DPDPE in the presence of a highly selective P-gp inhibitor (i.e., reversin 205). Although DPDPE accumulation was increased in both saline-treated and λ-carrageenan-treated animals administered reversin 205, the magnitude of DPDPE accumulation was greater under conditions of pain/inflammation. In fact, we calculated the relative contribution of Oatp1a4 to DPDPE uptake to be 71% during peripheral pain compared with 56% in saline controls. DPDPE uptake was significantly reduced in both experimental conditions by administration of E13S, which emphasizes involvement of Oatp1a4 in blood-to-brain DPDPE uptake. Furthermore, these data were particularly critical because they demonstrate that Oatp1a4 may be a viable target for delivering pharmacological agents to the brain, a result that resolves the controversy on Oatp1a4's role in CNS drug delivery.
It is paramount to identify and characterize biological mechanisms that enable peripheral pain to “transmit” signals and alter BBB drug transporters. Previously, our laboratory demonstrated that in vivo administration of a nonsteroidal anti-inflammatory drug (i.e., diclofenac) attenuated changes in functional BBB integrity that are observed during inflammatory pain (Brooks et al., 2008). That study examined the effect of diclofenac on nonspecific paracellular permeability only to sucrose and did not evaluate its effect on specific BBB transport proteins. Our study extends this work by demonstrating that blockade of peripheral inflammation with diclofenac also attenuates the increase in Oatp1a4 functional expression observed under peripheral pain conditions. The peripheral signal that leads to changes in BBB integrity and/or transport may involve alterations in serum concentrations of cytokines such as TGF-β, a critical regulator of brain microvascular homeostasis (Lebrin et al., 2005). We have reported decreased TGF-β1 concentrations in rat serum during peripheral pain, which leads to decreased BBB functional integrity (Ronaldson et al., 2009). Because diclofenac induces TGF-β synthesis in cultured J774.2 macrophages (Ayoub et al., 2009), we postulated that diclofenac administration may also prevent decreases in serum TGF-β1 observed during peripheral pain. Indeed, our current study demonstrated that diclofenac treatment maintained circulating TGF-β1 at levels similar to saline controls in animals subjected to peripheral inflammatory pain. In addition, we showed that diclofenac administration prevented down-regulation of TGF-β receptors (i.e., ALK1 and ALK5) during inflammatory pain, which we reported previously (Ronaldson et al., 2009). Taken together, these data imply that peripheral inflammation is capable of regulating (via signaling) BBB drug transport systems, an effect that may be related to changes in serum TGF-β1 concentrations and/or expression of brain microvascular TGF-β receptors.
To determine whether signaling via brain microvascular TGF-β receptors is involved in the regulation of Oatp1a4, we used SB431542, a highly selective ALK5 inhibitor (Inman et al., 2002). ALK5 propagates intracellular signals via phosphorylation of specific Smad proteins designated Smad2 and Smad3. This is distinct from other type I TGF-β receptors expressed at the BBB endothelium (i.e., ALK1), which signal through phosphorylation of Smad1, Smad5, and Smad8. Therefore, use of an exogenous compound to delineate ALK5 effects from ALK1 effects must alter phosphorylation of Smad2 and/or Smad3 only. Our previous data showed a decrease in Smad2 and Smad3 phosphorylation in animals pretreated with SB431542 before footpad injection (Ronaldson et al., 2009). This inhibitor elicited no change in Smad1/5/8 phosphorylation (Ronaldson et al., 2009), demonstrating that SB431542 can be used as a specific inhibitor of TGF-β/ALK5 signaling. Because decreased TGF-β/ALK5 signaling is associated with increased BBB permeability (Ronaldson et al., 2009), we hypothesized that this signaling mechanism may also lead to up-regulation of specific transporters involved in blood-to-brain solute transport. Indeed, we observed increased Oatp1a4 expression in animals pretreated with SB431542 before saline or λ-carrageenan injection. Furthermore, pretreatment with SB431542 before induction of peripheral inflammatory pain further increased taurocholate brain uptake, suggesting that functional changes in Oatp1a4-mediated transport are enhanced by ALK5 receptor inhibition. Although studies in immortalized mouse brain endothelial cells (MBE4) have shown involvement of ALK5-mediated signaling in regulation of P-glycoprotein (Dohgu et al., 2004), we are the first to report the regulation of a BBB drug uptake transporter by TGF-β/ALK5 signaling.
In summary, this study describes, in vivo, increased functional expression of Oatp1a4 during peripheral pain. Our data also demonstrate that peripheral inflammatory pain can directly modulate specific BBB drug transporters and provides evidence for involvement of TGF-β/ALK5 signaling in regulation of Oatp1a4 functional expression. In addition, these observations suggest that ALK5 may represent a novel target to control brain delivery of Oatp1a4 substrate drugs such as opioids. Overall, these results suggest that changes in Oatp1a4 expression and/or activity can be exploited in an effort to enhance the CNS delivery of drugs such as opioids, a class of therapeutics whose utility is limited by difficulties in effective BBB permeation.
Acknowledgments
We thank Dr. Robert Kuester and Mr. Gabriel Knudsen for excellent technical assistance with HPLC analyses.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA11271] (to T.P.D.); and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS42652] (to T.P.D.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.174151.
Abbreviations:
- CNS
- central nervous system
- ALK
- activin receptor-like kinase
- BBB
- blood-brain barrier
- BSP
- bromosulfophthalein
- DPDPE
- [d-penicillamine2,5]-enkephalin
- ELISA
- enzyme-linked immunosorbent assay
- E13S
- estrone-3-sulfate
- Oatp
- organic anion transporting polypeptide
- PI
- protease inhibitor
- PVDF
- polyvinylidene difluoride
- TGF
- transforming growth factor
- SB431542
- 4-[4-(1,3-benzodioxol-5-yl)-5-(2-pyridinyl)-1H-imidazol-2-yl]benzamide
- HPLC
- high-performance liquid chromatography
- P-gp
- P-glycoprotein.
Authorship Contributions
Participated in research design: Ronaldson and Davis.
Conducted experiments: Ronaldson, Finch, DeMarco, and Quigley.
Performed data analysis: Ronaldson, Finch, and DeMarco.
Wrote or contributed to the writing of the manuscript: Ronaldson and Davis.
Other: Davis acquired funding for the research.
References
- Ayoub SS, Botting RM, Joshi AN, Seed MP, Colville-Nash PR. (2009) Activation of macrophage peroxisome proliferator-activated receptor-γ by diclofenac results in the induction of cyclooxygenase-2 protein and the synthesis of anti-inflammatory cytokines. Mol Cell Biochem 327:101–110 [DOI] [PubMed] [Google Scholar]
- Brooks TA, Nametz N, Charles R, Davis TP. (2008) Diclofenac attenuates the regional effect of λ-carrageenan on blood-brain barrier function and cytoarchitecture. J Pharmacol Exp Ther 325:665–673 [DOI] [PubMed] [Google Scholar]
- Brooks TA, Ocheltree SM, Seelbach MJ, Charles RA, Nametz N, Egleton RD, Davis TP. (2006) Biphasic cytoarchitecture and functional changes in the BBB induced by chronic inflammatory pain. Brain Res 1120:172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ. (1990) Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 429:47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos CR, Ocheltree SM, Hom S, Egleton RD, Davis TP. (2008) Nociceptive inhibition prevents inflammatory pain induced changes in the blood-brain barrier. Brain Res 1221:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford EM, Hyman S, Swartz BE. (1994) The human brain GLUT1 glucose transporter: ultrastructural localization to the blood-brain barrier endothelia. J Cereb Blood Flow Metab 14:106–112 [DOI] [PubMed] [Google Scholar]
- Cui YJ, Cheng X, Weaver YM, Klaassen CD. (2009) Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos 37:203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapski GA, Cakala M, Chalimoniuk M, Gajkowska B, Strosznajder JB. (2007) Role of nitric oxide in the brain during lipopolysaccharide-evoked systemic inflammation. J Neurosci Res 85:1694–1703 [DOI] [PubMed] [Google Scholar]
- Dagenais C, Ducharme J, Pollack GM. (2001) Uptake and efflux of the peptidic δ-opioid receptor agonist. Neurosci Lett 301:155–158 [DOI] [PubMed] [Google Scholar]
- Dagenais C, Graff CL, Pollack GM. (2004) Variable modulation of opioid brain uptake by P-glycoprotein in mice. Biochem Pharmacol 67:269–276 [DOI] [PubMed] [Google Scholar]
- De Groot CJ, Montagne L, Barten AD, Sminia P, Van Der Valk P. (1999) Expression of transforming growth factor (TGF)-β1, -β2, and -β3 isoforms and TGF-β type I and type II receptors in multiple sclerosis lesions and human adult astrocyte cultures. J Neuropathol Exp Neurol 58:174–187 [DOI] [PubMed] [Google Scholar]
- Dohgu S, Yamauchi A, Takata F, Naito M, Tsuruo T, Higuchi S, Sawada Y, Kataoka Y. (2004) Transforming growth factor-β1 up-regulates the tight junction and P-glycoprotein of brain microvascular endothelial cells. Cell Mol Neurobiol 24:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egleton RD, Davis TP. (1999) Transport of the δ-opioid receptor agonist [d-penicillamine,2,5] enkephalin across the blood-brain barrier involves transcytosis1. J Pharm Sci 88:392–397 [DOI] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. (2000) Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther 294:73–79 [PubMed] [Google Scholar]
- Gao B, Stieger B, Noé B, Fritschy JM, Meier PJ. (1999) Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem 47:1255–1264 [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. (2002) Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J 21:1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. (2004) Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch 447:653–665 [DOI] [PubMed] [Google Scholar]
- Hagenbuch N, Reichel C, Stieger B, Cattori V, Fattinger KE, Landmann L, Meier PJ, Kullak-Ublick GA. (2001) Effect of phenobarbital on the expression of bile salt and organic anion transporters of rat liver. J Hepatol 34:881–887 [DOI] [PubMed] [Google Scholar]
- Hau VS, Huber JD, Campos CR, Davis RT, Davis TP. (2004) Effect of λ-carrageenan-induced inflammatory pain on brain uptake of codeine and antinociception. Brain Res 1018:257–264 [DOI] [PubMed] [Google Scholar]
- Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. (2001) Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol 280:H1241–H1248 [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62:65–74 [DOI] [PubMed] [Google Scholar]
- Jacquemin E, Hagenbuch B, Stieger B, Wolkoff AW, Meier PJ. (1994) Expression cloning of a rat liver Na(+)-independent organic anion transporter. Proc Natl Acad Sci USA 91:133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Akira T, Watanabe M, Kagitani Y. (1997) Prostaglandin E1 prevents apoptotic cell death in superficial dorsal horn of rat spinal cord. Neuropharmacology 36:1023–1030 [DOI] [PubMed] [Google Scholar]
- Labuz D, Mousa SA, Schäfer M, Stein C, Machelska H. (2007) Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res 1160:30–38 [DOI] [PubMed] [Google Scholar]
- Lebrin F, Deckers M, Bertolino P, Ten Dijke P. (2005) TGF-β receptor function in the endothelium. Cardiovasc Res 65:599–608 [DOI] [PubMed] [Google Scholar]
- Li L, Meier PJ, Ballatori N. (2000) Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol 58:335–340 [DOI] [PubMed] [Google Scholar]
- Morris CJ. (2003) Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol 225:115–121 [DOI] [PubMed] [Google Scholar]
- Noé B, Hagenbuch B, Stieger B, Meier PJ. (1997) Isolation of a multispecific organic anion and cardiac glycoside transporter from rat brain. Proc Natl Acad Sci USA 94:10346–10350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, Sugiyama Y. (2010) Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos 38:168–176 [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. (2009) Transforming growth factor-β signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J Cereb Blood Flow Metab 29:1084–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelbach MJ, Brooks TA, Egleton RD, Davis TP. (2007) Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem 102:1677–1690 [DOI] [PubMed] [Google Scholar]
- Sharom FJ, Yu X, Lu P, Liu R, Chu JW, Szabó K, Müller M, Hose CD, Monks A, Váradi A, et al. (1999) Interaction of the P-glycoprotein multidrug transporter (MDR1) with high affinity peptide chemosensitizers in isolated membranes, reconstituted systems, and intact cells. Biochem Pharmacol 58:571–586 [DOI] [PubMed] [Google Scholar]
- Stein C, Schäfer M, Machelska H. (2003) Attacking pain at its source: new perspectives on opioids. Nat Med 9:1003–1008 [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y. (2003) Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495 [DOI] [PubMed] [Google Scholar]
- Takasato Y, Rapoport SI, Smith QR. (1984) An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol Heart Circ Physiol 247:H484–H493 [DOI] [PubMed] [Google Scholar]
- Taogoshi T, Nomura A, Murakami T, Nagai J, Takano M. (2005) Transport of prostaglandin E1 across the blood-brain barrier in rats. J Pharm Pharmacol 57:61–66 [DOI] [PubMed] [Google Scholar]
- Triguero D, Buciak J, Pardridge WM. (1990) Capillary depletion method for quantification of blood-brain barrier transport of circulating peptides and plasma proteins. J Neurochem 54:1882–1888 [DOI] [PubMed] [Google Scholar]
- van Montfoort JE, Schmid TE, Adler ID, Meier PJ, Hagenbuch B. (2002) Functional characterization of the mouse organic-anion-transporting polypeptide 2. Biochim Biophys Acta 1564:183–188 [DOI] [PubMed] [Google Scholar]
- Westholm DE, Salo DR, Viken KJ, Rumbley JN, Anderson GW. (2009a) The blood-brain barrier thyroxine transporter organic anion-transporting polypeptide 1c1 displays atypical transport kinetics. Endocrinology 150:5153–5162 [DOI] [PubMed] [Google Scholar]
- Westholm DE, Stenehjem DD, Rumbley JN, Drewes LR, Anderson GW. (2009b) Competitive inhibition of organic anion transporting polypeptide 1c1-mediated thyroxine transport by the fenamate class of nonsteroidal antiinflammatory drugs. Endocrinology 150:1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt KA, Davis TP. (2006) CNS drug delivery: opioid peptides and the blood-brain barrier. AAPS J 8:E76–E88 [DOI] [PMC free article] [PubMed] [Google Scholar]