Abstract
Certain behavioral features of buprenorphine, including a bell-shaped curve for antinociception and attenuation of alcohol consumption, are thought to be mediated by activation of nociceptin/orphanin FQ peptide (NOP) receptors, despite moderate affinity and low efficacy at NOP receptors. We hypothesized that ligands with buprenorphine's physical properties, but possessing increased NOP receptor affinity and efficacy, would improve the profile as a drug abuse medication and reduce addiction liability. Using this strategy, we designed several compounds with universally high affinity, i.e., less than 10 nM at μ, δ, κ, and NOP receptors. Among these, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028) has high affinity at all opioid receptors and increased NOP receptor efficacy in vitro in the [35S]GTPγS binding assay, however, while still being a partial agonist. In vivo, BU08028 was evaluated in an acute thermal antinociception assay, for its ability to induce conditioned place preference (CPP), and for its effect on cocaine-induced CPP. BU08028 is a very potent long-lasting analgesic. It produces an increase in locomotor activity and a significant CPP. As a pretreatment to cocaine, BU08028 does not alter cocaine CPP but causes a further increase in cocaine-induced locomotor activity. The analgesic, rewarding, and stimulant effects are probably caused by μ receptor stimulation. It is likely that with BU08028, a partial agonist at both NOP and μ receptors, μ-mediated activity overpowers NOP-mediated effects. Thus, it is possible that a different buprenorphine analog that is a universal high-affinity opioid ligand but with “full agonist” activity at NOP may counteract traditional opioid-mediated effects such as antinociception and reward.
Introduction
Buprenorphine has been a successful analgesic and drug abuse medication. It is a potent long-lasting partial agonist with reduced addiction liability, relative to morphine (Jasinski et al., 1978), and is currently used for opiate maintenance as an alternative to methadone. Although it has reduced addiction liability, buprenorphine has been classified a schedule III compound by the Drug Enforcement Agency. Animal models of abuse liability of buprenorphine have been somewhat contradictory; with some experiments suggesting that buprenorphine is rewarding, and others suggesting that it is not (Kosten et al., 1991; Rowlett et al., 1994). Regardless of its own rewarding potential, buprenorphine administration diminishes cocaine self-administration in both animal models and people (Mello et al., 1989; Montoya et al., 2004), a feature attributed to the μ partial agonist component of its profile (Mello et al., 1993).
Buprenorphine can also display an unusual dose-response relationship. In antinociception experiments, it can have an inverted U-shaped dose-response curve, with high doses inducing a decrease in antinociceptive activity (Lutfy et al., 2003). A similar phenomenon has been reported when measuring the ability of buprenorphine to modify ethanol consumption in rats. Buprenorphine increases alcohol consumption at low doses but attenuates consumption at higher doses (Ciccocioppo et al., 2007). In both cases, the high dose reduction in antinociception and alcohol consumption is attributed to activation of nociceptin/orphanin FQ peptide (NOP) receptors (formerly known as ORL1), thereby diminishing the μ-mediated antinociceptive activity and μ-mediated increase in alcohol consumption. This is despite the fact that the affinity of buprenorphine for NOP receptors is modest, and its efficacy at this receptor is extremely low (Spagnolo et al., 2008; Khroyan et al., 2009).
The NOP receptor belongs to the opioid family and binds the endogenous ligand nociception/orphanin FQ (N/OFQ). N/OFQ, through NOP-receptor activation, has been shown to have antiopiate properties in the brain (Meunier et al., 1995; Reinscheid et al., 1995) and is able to block the reward induced by morphine (Murphy et al., 1999), cocaine (Kotlińska et al., 2002; Sakoori and Murphy, 2004), ethanol (Ciccocioppo et al., 1999; Kuzmin et al., 2003), and methamphetamine (Zhao et al., 2003). N/OFQ, when administered intracerebroventricularly, also has been shown to block development of tolerance to morphine (Lutfy et al., 2001). These studies, as well as our previous work (Toll et al., 2009), suggest that a compound that activates both the μ-opioid and NOP receptors might be an ideal candidate for a nonaddicting analgesic with reduced tolerance development and may also have potential efficacy for the treatment of drug addiction. However, the optimum profile of μ and NOP receptor affinity and/or efficacy for an optimum balance of analgesia to antagonism, reward to reward attenuation, and locomotor activation to sedation for a useful therapeutic profile cannot be predicted. We have demonstrated that such mixed NOP/μ compounds can be developed with significant antinociceptive activity and greatly reduced reward, but to attenuate reward in a traditional NOP receptor nonopioid small-molecule pharmacophore (see Zaveri et al., 2005), the NOP component must overshadow the μ component with respect to affinity and/or efficacy (Khroyan et al., 2007; Toll et al., 2009). This is not the case with buprenorphine, in which the μ affinity is far greater than the NOP receptor affinity. If beneficial actions of buprenorphine are caused by NOP receptor activation, it seems reasonable that a buprenorphine analog with higher affinity and efficacy at NOP would have an in vivo profile preferable to that of buprenorphine itself with reduced reward and reduced tolerance development. We applied this hypothesis to the buprenorphine class of compounds and have designed buprenorphine analogs that have higher NOP affinity and efficacy. Here, we describe the in vitro profile and the behavioral effects of a buprenorphine analog, (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol (BU08028), the first “universal opioid ligand” with high affinity at all four opioid family receptors.
Materials and Methods
Drugs
BU08028 (Fig. 1) was prepared using the standard techniques for orvinol synthesis (Bentley and Hardy, 1967; Grundt et al., 2003). Treatment of N-cyclopropylmethyldihydronorthevinone with freshly prepared t-pentyl magnesium bromide and subsequent 3-O-demethylation using sodium propane thiolate gave BU08028, which was converted to its hydrochloride salt. For behavioral experiments, BU08028 was dissolved in 1 to 2% dimethyl sulfoxide and 0.5% aqueous hydroxypropylcellulose. Morphine sulfate (Eli Lilly & Co., Indianapolis, IN), and buprenorphine HCl (drug supply from the National Institute on Drug Abuse, Bethesda, MD) were dissolved in water. Drugs were injected in a volume of 0.1 ml/25g s.c.. Controls received 0.1 ml/25 g of the appropriate vehicle.
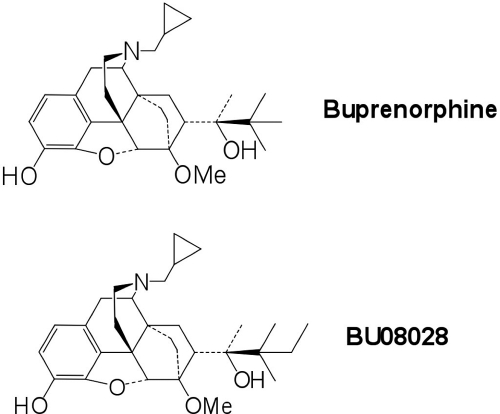
Fig. 1.
Chemical structures of buprenorphine (top) and BU08028 (bottom).
Animals
Male ICR mice weighing 25 to 30 g at the start of the experiment were used. Animals were group-housed under standard laboratory conditions and kept on a 12:12-h day/night cycle (lights on at 7:00 AM). Animals were handled for 3 to 4 days before the experiments were conducted. On behavioral test days, animals were transported to the testing room and acclimated to the environment for 1 h. Mice were maintained in accordance with the guidelines of SRI International and the National Research Council's Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003).
Experimental Procedures
In Vitro Characterization.
Cell culture.
All receptors were individually expressed in Chinese hamster ovary cells stably transfected with human receptor cDNA developed in our laboratory. Receptor expression levels were 1.2, 1.6, 1.8, and 3.7 pmol per milligram of protein for the NOP, μ, κ, and δ opioid receptors, respectively. The cells were grown in Dulbecco's modified Eagle medium with 10% fetal bovine serum in the presence of 0.4 mg/ml G418 (Geneticin) and 0.1% penicillin/streptomycin in 100-mm plastic culture dishes. For binding assays, the cells were scraped off the plate at confluence.
Receptor binding.
Binding to cell membranes was conducted in a 96-well format as described previously (Dooley et al., 1997; Toll et al., 1998). Cells were removed from the plates by scraping with a rubber policeman, homogenized in 50 mM Tris pH 7.5, using a Polytron homogenizer (Brinkmann Instruments, Westbury, NY), then centrifuged once and washed by an additional centrifugation at 27,000g for 15 min. The pellet was resuspended in Tris, and the suspension was incubated with [3H]DAMGO (51 Ci/mmol, 1.6 nM), [3H]Cl-DPDPE (42 Ci/mmol, 1.4 nM), [3H] N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide (U69593) (41.7 Ci/mmol, 1.9 nM), or [3H]N/OFQ (120 Ci/mmol, 0.2 nM) for binding to, μ, δ, κ, and NOP receptors, respectively. Nonspecific binding was determined with 1 μM unlabeled DAMGO, DPDPE, ethylketocyclazocine, and N/OFQ, respectively. Total volume of incubation was 1.0 ml, and samples were incubated for 60 min at 25°C. The amount of protein in the binding assay was 15 μg. The reaction was terminated by filtration using a Tomtec 96 harvester (Tomtec, Orange, CT) through glass fiber filters. Bound radioactivity was counted on a β-plate liquid scintillation counter (PerkinElmer Life and Analytical Sciences, Waltham, MA) and expressed in cpm. IC50 values were determined using at least six concentrations of each peptide analog and calculated using Prism (GraphPad Software, Inc., San Diego, CA). Ki values were determined by the method of Cheng and Prusoff (1973).
[35S]GTPγS binding.
[35S]GTPγS binding was conducted basically as described by Traynor and Nahorski (1995). Cells were scraped from tissue culture dishes into 20 mM HEPES and 1 mM EDTA, then centrifuged at 500g for 10 min. Cells were resuspended in this buffer and homogenized using a Polytron homogenizer. The homogenate was centrifuged at 27,000g for 15 min, and the pellet was resuspended in buffer A containing 20 mM HEPES, 10 mM MgCl2, and 100 mM NaCl, pH 7.4. The suspension was recentrifuged at 27,000g and suspended once more in buffer A. For the binding assay, membranes (8–15 μg protein) were incubated with [35S]GTPγS (50 pM), GDP (10 μM), and the appropriate compound in a total volume of 1.0 ml for 60 min at 25°C. Samples were filtered over glass fiber filters and counted as described for the binding assays. Statistical analysis was conducted using the program Prism.
In Vivo Characterization
Assessment of Thermal Nociception.
Tail-flick assay.
Acute nociception was assessed using the tail-flick assay with an analgesia instrument (Stoelting, Wood Dale, IL) that uses radiant heat. This instrument is equipped with an automatic quantification of tail-flick latency and a 15-s cutoff to prevent damage to the animal's tail. During testing, the focused beam of light was applied to the lower half of the animal's tail, and tail-flick latency was recorded. Baseline values for tail-flick latency were determined before drug administration in each animal. The mean basal tail-flick latency was 4.32 ± 0.12 S.E.M.
After baseline measures, animals received a subcutaneous injection of their assigned dose of drug and were tested for tail-flick latencies at 30, 60, 120, and 240 min postinjection. The animals were then taken back to their home room and retested 24 and 48 h after the injection. Controls received an injection of vehicle before testing.
Drug regimen and acute testing.
Separate groups of animals (n = 8–14/group) received subcutaneous injections of BU08028 (0.3–30 mg/kg). Because BU08028 is structurally related to buprenorphine, a separate group of animals received subcutaneous injections of buprenorphine (0.1–10 mg/kg). As positive controls, separate groups of animals received injections of morphine (10.0 mg/kg). After assessment of baseline values for tail-flick latency, animals received an injection of their assigned dose/drug and were tested following the paradigm described above. A group of animals also served as vehicle controls.
In follow-up experiments, we examined whether BU08028-induced antinociception was altered after coadministration with the opiate antagonist naloxone (1 mg/kg) or the NOP antagonist (5S,7S)-7-{[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl}-1-methyl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-ol (SB612111) (10 mg/kg). Doses of the antagonists were chosen based on our previous experiments.
Drug regimen and tolerance testing.
To assess tolerance development, separate groups of animals (n = 8–9/group) received subcutaneous injections of vehicle, morphine (10 mg/kg), BU08028 (10 mg/kg), or buprenorphine (10 mg/kg) once daily for 9 days. The 10 mg/kg dose of BU08028 was chosen because it produced a near-maximal effect 1 h after injection. The 10 mg/kg dose of morphine was chosen because it produced similar levels of antinociception as 10 mg/kg BU08028. Finally, 10 mg/kg buprenorphine was used because it produced the highest level of antinociception. We have used this regimen previously (Khroyan et al., 2007). Animals received daily injections of their respective drug, and on days 1, 2, 4, and 9 they were tested for tail-flick latency 1 h after their injection using the equipment and methods described above. The mean ± S.E.M. basal tail-flick latencies were consistent across test days. The 1-h time point was chosen because it captured the maximal effects of BU08028, morphine, and buprenorphine.
Statistical analyses.
Antinociception (%MPE) was quantified by the following formula: %MPE = 100 × [(test latency − baseline latency)/(15 − baseline latency)]. If the animal did not respond before the 15-s cutoff, the animal was assigned a score of 100%. Behavioral results were analyzed using repeated-measures ANOVAs with drug treatment as between-group variables and time after drug injection (30, 60, 120, and 240 min and 24 and 48 h for acute injection) and drug injection day (1, 2, 4, and 9 for tolerance experiments) as repeated measures followed by Student Newman-Keuls post hoc tests where appropriate. The level of significance was set at P < 0.05.
Assessment of Acute Rewarding Effects using the Place Conditioning Paradigm
Place Conditioning Apparatus.
The apparatus consisted of rectangular Plexiglas chambers divided into two distinct equal-sized compartments (19 × 22.8 × 18 cm high; Lafayette Instruments, Lafayette, IN). One compartment had cedar-scented bedding underneath a bar grid floor, and all walls but the front walls were black. The other compartment had pine-scented bedding beneath a mesh floor and all walls but the front wall were white. The front walls were transparent so that the animal's behavior could be monitored. A removable partition divided the two compartments. During conditioning, the compartments were divided by a solid partition. On the place conditioning (PC) test day, the solid partition was replaced with a partition that had an opening, allowing the animal free access to both compartments. The apparatus is unbiased, because untreated animals do not show a preference for one compartment over the other (see Figs. 6 and 7).
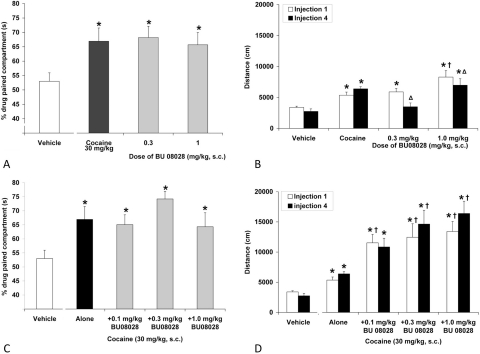
Fig. 6.
Effects of BU08028 on PC (A) and global activity (B) and the effects of buprenorphine on PC (C) and global activity (D). Data are mean (± S.E.M.) percentage of time spent in drug-paired compartment (A and C) or activity (cm) after the first drug injection (B and D). *, significant difference from vehicle control. †, significant difference from morphine alone (P < 0.05).
Fig. 7.
Effects of BU08028 on PC (A), global activity (B), cocaine-induced CPP (C), and cocaine-induced activity (D). Data are mean (± S.E.M.) percentage of time spent in drug-paired compartment (A and C) or activity (cm) after the first and last drug injections (B and D). *, significant difference from vehicle control. ▵, significant difference from first injection. †, significant difference from cocaine alone. P < 0.05.
PC Training.
Because of the long-lasting antinociceptive activity of BU08028, we initially used a single-trial PC paradigm with conditioning sessions separated by 48 h to allow the drug to have limited behavioral actions. A conditioning trial was composed of two sessions conducted over 3 consecutive days. During the drug session, animals received a subcutaneous injection of their respective drug and were confined to one of the compartments for 60 min. During the vehicle session, animals received an injection of vehicle and were confined to the alternate compartment for 60 min. The particular compartment paired with the drug and the order of the drug versus the saline session was counterbalanced across groups. Although this single-trial PC paradigm was used to prevent BU08028's rewarding effects from being present during the vehicle training session, it should be noted that the rewarding and antinociceptive effects of a drug may not have the same onset and duration.
Assessment of Global Activity.
During conditioning, overall activity of the animals after acute drug injection was recorded. These data were captured by the Spontaneous Motor Recording and Tracking software system (Panlab, Barcelona, Spain), a color image-capturing system that works in real time and tracks all the movements of the animal, for a given amount of time, via a video camera connected to the computer. This system tracks and records all behavior that results in any movement/positional changes. We term this as “global activity,” because it encompasses all movement detected including fine movement, movement caused by rearing, grooming, sniffing, and locomotor activity.
PC Test Day.
Forty eight hours after the last conditioning session, animals were given access to both compartments simultaneously for 15 min, and the amount of time animals spent in each compartment was recorded.
Drug Regimen.
Animals were assigned to groups (n = 8/group) receiving subcutaneous injections of BU08028 (1–10 mg/kg), buprenorphine (1–10 mg/kg), or morphine (15 mg/kg) alone. During the drug session, animals were immediately placed in the drug-paired compartment for 60 min after their subcutaneous injection. A group of mice received vehicle in both compartments and served as controls.
Statistical Analyses.
Global activity was analyzed using a one-way ANOVA with drug as the between-subject measure. For the PC test day data, the percentage of time animals spent in their drug-paired compartment was analyzed using one-way ANOVAs, and significant effects were further analyzed with post hoc tests. A conditioned place preference (CPP) was evident if the animals spent significantly more time in their drug-paired compartment relative to control animals. The level of significance was set at P < 0.05.
Effect of Repeat Administration of BU08028 on Reward and Cocaine-Induced CPP
Testing and Drug Regimen.
To assess whether repeated injections of BU08028 alone would also induce CPP and alter cocaine-induced CPP, we used a more traditional PC conditioning regimen. The same experimental apparatus and methods as described above were used except for the following: Conditioning trials were composed of two sessions conducted over 2 consecutive days. The drug session and vehicle session were 24 h apart and animals were confined to their respective compartments for 30 min. These two sessions were repeated over 8 consecutive days such that animals received four drug sessions and four vehicle sessions. Global activity was assessed after the first and fourth drug injection using the methods described above. Because increases in global activity produced by one injection of BU08028 had disappeared within 24 h and were similar to controls (data not shown), we opted to conduct the drug and vehicle sessions over 2 consecutive days as opposed to every other day as performed in the acute PC experiment described above.
Animals were assigned to groups (n = 6–9/group) receiving subcutaneous injections of either vehicle, BU08028 (0.3–1 mg/kg) alone, cocaine (30 mg/kg) alone, or cocaine coadministered with BU08028 (0.1–1 mg/kg). On drug days, animals received their injection of drug (or vehicle) and were immediately placed in the compartment for 30 min. The PC test day was conducted 24 h after the last conditioning session.
Statistical Analyses.
Global activity was analyzed using a repeated ANOVA with drug as the between-subject measure and injection day (first versus fourth) as the repeated measure. PC data were analyzed in the same way as described above.
Results
Receptor and [35S]GTPγS Binding.
BU08028 (Fig. 1) is an orvinol with structural similarity to buprenorphine. As seen in Table 1, BU08028 has a Ki of less than 10 nM at each receptor in the opioid receptor family, making it the first universally high-affinity opioid ligand. Affinity is highest at the δ receptor (Ki = 1.6 nM), but it has a Ki within a factor of 5 at all four opioid receptors: NOP, μ, δ, and κ. More importantly, even though its structure is very similar to that of buprenorphine, its affinity at NOP is almost 10-fold higher than that of buprenorphine itself (Table 1). In vitro functional activity, as determined by [35S]GTPγS binding (Table 2), indicated that BU08028 has virtually no efficacy at δ and κ receptors. At μ receptors, the functional activity is very similar to buprenorphine. It is slightly more potent, with an EC50 of 6.03 nM, but it is a weak partial agonist with only 21% stimulation relative to DAMGO, very similar to that of buprenorphine. At NOP, the EC50 is 43.5 nM, approximately six times more potent than buprenorphine, and has significantly higher efficacy showing 44% stimulation, compared with buprenorphine, which stimulates only in the range of 15%. These results suggest that the behavioral actions produced by BU08028 administration should have significantly more NOP character than buprenorphine.
TABLE 1.
Binding affinities Ki (nM) of BU08028, compared with buprenorphine, morphine, and other prototypical agonists at NOP and opioid receptors
Data show mean ± S.D. for at least two experiments conducted in triplicate. SR16435 data are from Khroyan et al., 2007.
| Receptor Binding Ki ± S.D. |
||||
|---|---|---|---|---|
| NOP | μ | δ | κ | |
| nM | ||||
| Buprenorphine | 77.4 ± 16.1 | 1.52 ± 0.8 | 6.13 ± 0.4 | 2.50 ± 1.2 |
| N/OFQ | 0.08 ± 0.03 | 133 ± 30 | >10,000 | 247 ± 3.4 |
| DAMGO | >10,000 | 1.59 ± 0.17 | 300 ± 58.6 | 305 ± 46 |
| DPDPE | >10,000 | 503 ± 10.0 | 1.24 ± 0.09 | >10,000 |
| U69593 | >10,000 | >10,000 | >10,000 | 1.62 ± 0.26 |
| Etorphine | 56.9 ± 2.61 | 0.33 ± 0.05 | 1.54 ± 0.6 | 0.22 ± 0.05 |
| SR16435 | 7.49 ± 1.31 | 2.70 ± 0.1 | >1,000 | 31.7 ± 4.8 |
| BU08028 | 8.46 ± 1.31 | 2.14 ± 0.79 | 1.59 ± 0.28 | 5.63 ± 1.30 |
TABLE 2.
Stimulation of [35S]GTPγS binding by BU08028, compared with buprenorphine, morphine, and other prototypical agonists at NOP and opioid receptors
Data show mean ± S.D. for at least two experiments conducted in triplicate. SR16435 data are from Khroyan et al., 2007.
| NOP |
μ |
δ |
κ |
|||||
|---|---|---|---|---|---|---|---|---|
| EC50 | %Stimulated | EC50 (nM) | %Stimulated | EC50 | %Stimulated | EC50 | %Stimulated | |
| nM | nM | nM | nM | |||||
| Buprenorphine | 251 ± 94 | 15.5 ± 5.8 | 10.2 ± 2.2 | 28.7 ± 1.0 | >10,000 | >10,000 | ||
| N/OFQ | 8.11 ± 1.4 | 100 | ||||||
| DAMGO | 35.3 ± 0.5 | 100 | ||||||
| DPDPE | 6.90 ± 0.4 | 100 | ||||||
| U69593 | 78.5 ± 8.8 | 100 | ||||||
| SR16435 | 28.7 ± 0.6 | 45.0 ± 5.1 | 2.73 ± 0.01 | 29.5 ± 10 | N.D. | >10,000 | ||
| BU08028 | 78.6 ± 49 | 48 ± 13 | 6.03 ± 2.1 | 21.1 ± 8.7 | —* | 10.8 ± 6.8 | >10,000 | |
N.D., not determined.
Stimulation was too low to accurately determine EC50 value.
Acute Effects of BU08028 on Tail-Flick Latency: Comparison with Morphine and Buprenorphine.
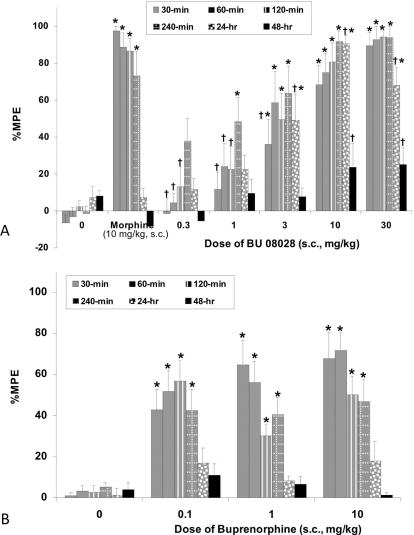
The effect of BU08028 on tail-flick latency is shown in Fig. 2A. Initial experiments demonstrated that BU08028 has a long-lasting effect on tail-flick latency, which was therefore assessed over a 2-day period. The overall ANOVA indicated that there was a significant interaction effect (F30,235 = 5.4; P < 0.05). The positive control morphine produced the anticipated increase in %MPE at the earlier time points of 30, 60, 120, and 240 min postinjection. Morphine antinociception was not evident at the 24- and 48-h time points. BU08028, at 3, 10, and 30 mg/kg, produced a dose-dependent increase in %MPE that was significantly different from vehicle controls up to 24 h postinjection (Fig. 2A). BU08028 was longer-lasting, but also slower-acting than morphine, but by 240 min postinjection all the doses of BU08028 (1–30 mg/kg) that were antinociceptive produced a similar increase in %MPE as morphine.
Fig. 2.
Acute thermal antinociceptive effect of BU08028 (A) and buprenorphine (B) alone in the tail-flick assay. Data are mean %MPE (± S.E.M.). *, significant difference from vehicle control (P < 0.05). †, significant difference from morphine alone (P < 0.05).
To compare the two drugs, we also examined the acute effects of buprenorphine using the same procedure as with BU08028. The effect of buprenorphine on tail-flick latency is shown in Fig. 2B. The overall ANOVA indicated that there was a significant interaction effect (F15,160 = 6.23; P < 0.05). The doses of 0.1 to 10 mg/kg produced a significant increase in %MPE at the 30- to 240-min time points relative to vehicle controls. However, these doses of buprenorphine did not produce an increase in %MPE at 24 and 48 h postinjection. Thus, although buprenorphine produced antinociceptive effects at lower doses relative to BU08028, its effects were neither as great nor as long-lasting as BU08028. As described previously, buprenorphine-induced antinociception produced a very shallow dose-response curve and never reached maximal tail-flick latency.
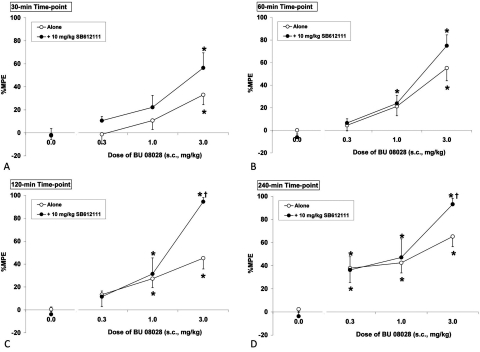
Enhancement of BU08028-Induced Antinociception by Pretreatment with SB612111 and Reversal by Pretreatment with Naloxone.
The effect of the NOP antagonist SB612111 on BU08028-induced antinociception is shown in Fig. 3. BU08028 was tested at low and moderate doses (0.3–3.0 mg/kg) to determine whether its NOP agonist activity was attenuating its μ-mediated antinociceptive activity. The overall ANOVAs indicated a significant interaction (F9,216 = 2.22; P < 0.05), showing that depending on the postinjection time and dose of BU08028, SB612111 had a differential effect. The NOP antagonist SB612111 potentiated BU08028-induced antinociception; however, this potentiation was observed at the 3 mg/kg dose of BU08028 and was statistically significant only at 120 and 240 min postinjection (Fig. 3).
Fig. 3.
The effect of NOP antagonist SB612111 on BU08028-induced antinociception 30 min (A), 60 min (B), 120 min (C), and 240 min (D) postinjection. Data are mean %MPE (± S.E.M.). *, significant difference from vehicle control (P < 0.05). †, significant difference from BU08028 alone (P < 0.05).
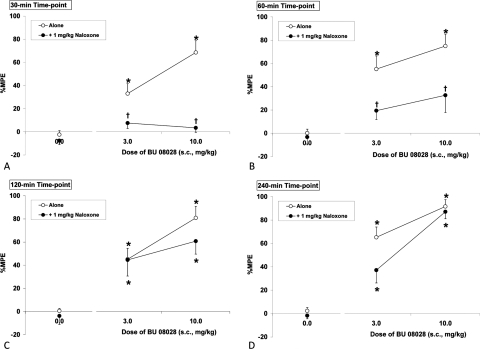
The effect of naloxone on BU08028 (3 and 10 mg/kg)-induced antinociception across time is shown in Fig. 4. The overall ANOVAs indicated a significant interaction (F6, 150 = 3.22; P < 0.05). Naloxone (1 mg/kg) attenuated BU08028-induced antinociception, indicating μ-mediated antinociceptive activity. The effects of naloxone were short-lived because attenuation of BU08028-induced antinociception was only evident at the 30- and 60-min time points, with significant antinociception returning at 2 and 4 h. Because the antagonist activity of SB612111 and naloxone are short-lived, with no differences observed beyond the 4-h time point, the 24- and 48-h data are not shown.
Fig. 4.
The effect of naloxone on BU08028-induced antinociception 30 min (A), 60 min (B), 120 min (C), and 240 min (D) postinjection. *, significant difference from vehicle control (P < 0.05). †, significant difference from BU08028 alone (P < 0.05).
Tolerance Development to Thermal Antinociception Produced by BU08028.
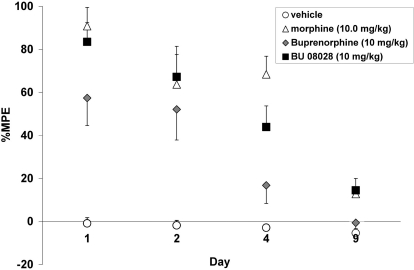
The effect of repeated administration of BU08028, buprenorphine, and morphine across 9 days is shown in Fig. 5. The overall ANOVA indicated a significant interaction (F9,87 = 3.69; P < 0.05). To examine the effects of tolerance development, levels of %MPE on test days 2, 4, and 9 were compared with test day 1. As expected, tolerance developed to the antinociceptive effects of 10 mg/kg morphine (F3,21 = 7.72; P < 0.05). Compared with day 1 morphine produced a significant reduction in antinociception by day 9. After repeated injections of BU08028 tolerance development was also evident (F3,24 = 15.50; P < 0.05). Tolerance to the antinociceptive effects of BU08028 was evident sooner than that observed with morphine, with a significant reduction in antinociception observed on days 4 and 9. Similar to BU08028, tolerance to buprenorphine-induced antinociception was evident by day 4 (F3,21 = 10.22; P < 0.05).
Fig. 5.
Tolerance development after repeated injections of 10 mg/kg BU08028, morphine, and 10 mg/kg buprenorphine. Animals received daily injections of their respective drug, and on day 1, 2, 4, and 9 they were tested for tail-flick latency 1 h after their injection. Data represent mean ± S.E.M. *, significant difference from vehicle control (P < 0.05). †, significant difference from day 1 (P < 0.05).
Acute Behavioral and Rewarding Effects of BU08028.
The effect of BU08028 on PC after one drug injection is shown in Fig. 6A. The initial PC experiment was conducted with a 48-h waiting period between drug and vehicle sessions (rather than the normal 24-h period) to avoid any interference with the CPP test caused by the long half-life of BU08028. The overall ANOVA indicated a significant main effect of dose (F4,35 = 5.34; P < 0.05). As demonstrated previously (Marquez et al., 2008), animals that received one injection of 10 mg/kg morphine during conditioning exhibited a significant CPP relative to controls. Likewise, animals that received 1 and 10 mg/kg BU08028 also exhibited a significant CPP relative to controls, whereas 3 mg/kg BU08028 showed a trend for a preference for the drug-paired compartment; however, this was not significant. Buprenorphine was also tested using the same parameters, and only the 3 mg/kg dose of buprenorphine produced a significant CPP relative to controls (Fig. 6C).
During the PC training phase, drug effects on the animals' global activity were also examined. Acute administration of morphine and BU08028 produced an increase in global activity relative to vehicle controls (F4,35 = 11.9; P < 0.05; Fig. 6B). In fact, the 3 mg/kg dose of BU08028 produced a greater increase in global activity than morphine. Buprenorphine also produced a significant increase in global activity relative to vehicle controls (Fig. 6D); however, levels of global activity were lower than that observed in animals that received BU08028.
Effects of Repeat Administration of BU08028 Alone and on Cocaine CPP.
The effect of repeated injections of BU08028 on PC is shown in Fig. 7A. The overall ANOVA indicated a significant effect (F3,19 = 3.18; P = 0.05). The 0.3 and 1.0 mg/kg doses of BU08028 produced a significant CPP, similar to cocaine (30 mg/kg) alone. During conditioning, global activity was also assessed after the first and fourth drug injection (Fig. 7B). The overall ANOVA indicated that there was a significant dose by injection day interaction (F3,19 = 16.7; P < 0.05). BU08028 (0.3–1.0 mg/kg) produced an increase in global activity after the first injection. In fact, the increase in global activity produced by 1.0 mg/kg BU08028 was higher than that observed with cocaine. After the fourth injection, the levels of global activity produced by 0.3 to 1.0 mg/kg BU08028 were attenuated relative to the first injection, indicative of tolerance. Indeed, 0.3 mg/kg was no different from controls; only the 1.0 mg/kg dose of BU08028 produced a significant increase in global activity relative to vehicle controls.
The effect of BU08028 on cocaine-induced behaviors is shown in Fig. 7, C and D. BU08028 (0.1–1.0 mg/kg) did not attenuate or potentiate cocaine-induced CPP. However, it dose-dependently potentiated cocaine-induced global activity (F4,31 = 10.56; P < 0.05; Fig. 7D). Even though cocaine alone produced an increase in global activity relative to controls. BU08028 further potentiated cocaine-induced activity after the first and fourth drug injections.
Discussion
The oripavine buprenorphine has a remarkable activity profile. It is a weak partial agonist at the μ opioid receptor and an antagonist at κ receptors. Although it is a potent analgesic, it has reduced addiction liability as well as an increased safety margin because of a ceiling effect on respiratory depression. In addition, buprenorphine is a long-lasting compound. These properties make buprenorphine useful as an analgesic and also a potential replacement for methadone in drug abuse pharmacotherapy (Lewis, 1985; Lutfy and Cowan, 2004).
One interesting phenomenon about buprenorphine is its inverted U-shaped dose-response curve for antinociceptive activity when countering an appropriate painful stimulus (Cowan et al., 1977). The inverted U-shape has been attributed to its agonist activity at NOP receptors, which presumably attenuates its μ-mediated antinociceptive activity at higher doses, despite moderate binding affinity to NOP receptors (Table 1) and very weak partial agonist activity at this receptor (Lutfy et al., 2003; Khroyan et al., 2009). Other mixed NOP/μ compounds, such as 1-(1-cyclooctylpiperidin-4-yl)-indolin-2-one) (SR14150) and 1-(1-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)piperidinl-4-yl)-indolin-2-one (SR16835), which are not in the oripavine family, but bind with greater affinity to the NOP receptor, also have μ-mediated antinociceptive activity that is attenuated by the NOP component (Khroyan et al., 2009). Likewise, buprenorphine enhances alcohol consumption at low doses, presumably through μ opioid receptors, but reduces alcohol consumption at higher doses. This reduction in alcohol consumption is blocked by the NOP receptor antagonist [Nphe1,Arg14,Lys15]nociceptin-NH2 (UFP-101), suggesting that the buprenorphine-induced reduction in alcohol consumption is caused by activation of NOP receptors (Ciccocioppo et al., 2007). An NOP-mediated reduction in CPP and self-administration of a variety of abused drugs by N/OFQ is already well documented (Murphy et al., 1999; Kotlińska et al., 2002; Ciccocioppo et al., 2003; Zhao et al., 2003). Therefore, it is possible that increasing the NOP agonist efficacy in buprenorphine, or other opioid ligands, may produce an effective nonaddicting analgesic and may be more efficacious than buprenorphine for drug abuse pharmacotherapy. The buprenorphine analog BU08028 was designed to have increased affinity and efficacy at the NOP receptors. BU08028 is the first reported “universal opioid ligand” that binds with high affinity (under 10 nM) to each of the receptors in the opioid receptor family. BU08028 is almost 10 times more potent at NOP receptors than buprenorphine and also has higher functional efficacy at NOP compared with buprenorphine in vitro (Table 2).
Because BU08028 has similar μ receptor activity to buprenorphine but increased NOP activity, we expect diminished μ-receptor-mediated effects such as antinociceptive activity, tolerance development, and reward. In acute antinociception assays, BU08028 seems less potent than buprenorphine, showing virtually no antinociceptive activity at 0.1 mg/kg and little activity at 0.3 mg/kg (Fig. 2), possibly suggesting NOP-mediated suppression of μ-mediated antinociception. However, the antinociceptive activity of BU08028 is potentiated only by the NOP receptor antagonist SB612111 at higher doses of BU08028, suggesting NOP receptor agonist activity has little or no discernable effect on antinociception at low doses of BU08028. Alternatively, the lack of antinociceptive activity at low doses might suggest that the higher logP of BU08028 is hindering its access to the central nervous system. Even though the partial agonist activity of BU08028 at μ receptors in vitro is similar to that of buprenorphine, the dose-response curve of BU08028 for antinociception is much steeper than that of buprenorphine, where the higher doses of BU08028 produced near maximal effects. As demonstrated in many reports, the dose-response curve for buprenorphine is very shallow, never approaching 100% MPE (Cowan et al., 1977; Ide et al., 2004). In addition, animals dosed with BU08028 exhibit a very profound Straub tail (T. Khroyan, unpublished observation), indicative of μ-like excitatory activity, and an obvious increase in locomotor activity, also a μ-mediated effect in mice (Cowan et al., 1977).
BU08028 produces antinociceptive effects that are longer-lasting than buprenorphine, consistent with BU08028's higher logP. BU08028 antinociception is still strongly evident 24 h postinjection with virtually 100% activity remaining at 10 mg/kg, whereas buprenorphine antinociceptive activity is no longer evident at this later time (Fig. 2). Furthermore, it was anticipated that a compound with higher NOP efficacy, similar to the mixed NOP/μ agonist SR16435, would likely have a reduced tolerance development (Khroyan et al., 2007). However, as shown in Fig. 5, administration of BU08028 over 9 days resulted in tolerance to its antinociceptive effects, similar to that seen with morphine and buprenorphine. From these experiments it is evident that BU08028 produces a very long-lasting antinociceptive effect, but tolerance to this effect develops at a similar pace compared with the parent compound buprenorphine.
Initial assessment of the rewarding effects of BU08028 was conducted using the one-trial PC paradigm with a 48-h period between drug and vehicle sessions (Fig. 6). This behavioral protocol was used to avoid any interference with the long-lasting effects of BU08028. Similar to morphine, BU08028 produced CPP. BU08028 was also directly compared with its parent compound buprenorphine using this same protocol. Buprenorphine produced an inverted U-shaped dose-response curve where the 3 mg/mg dose produced a significant CPP and 1 and 10 mg/kg did not, a phenomenon that has previously been observed in rats (Brown et al., 1991; Rowlett et al., 1994). In mice, CPP to buprenorphine has been reported after repeated injections at the 3 mg/kg dose, whereas one-trial conditioning with this dose did not result in CPP (Marquez et al., 2007, 2008). Using the more common repeated-injection PC protocol, BU08028 also produced CPP after four drug pairings, when drug sessions were not separated by 48 h (Fig. 7). Apparently, the effect of BU08028, with respect to “reward,” is not as long-lasting as its antinociceptive effect, lasting less than 24 h, being associated only with the drug-paired compartment and not interfering with the production of a CPP. 18,19-Dehydrobuprenorphine (HS 599), a didehydro derivative of buprenorphine that is also long-lasting, did not produce CPP in the traditional PC training regimen (Lattanzi et al., 2001). It is possible that, unlike BU08028, HS 599 may have longer-lasting effects that influence the development of CPP, and that the drug effects could have carried over to the vehicle session. In any case, the present findings show that BU08028 produces CPP alone and suggest that its NOP agonist activity is not attenuating its μ receptor-mediated behavior with respect to antinociception, tolerance development, or reward.
Although buprenorphine is approved for maintenance for heroin addiction, it has been considered as a potential pharmacotherapy for other abused drugs. Previous research has examined the effects of buprenorphine on cocaine-induced behaviors. Chronic buprenorphine administration can decrease cocaine intake during self-administration, drug-induced reinstatement of extinguished cocaine seeking, and cocaine-induced locomotor activity (Sorge et al., 2005; Sorge and Stewart, 2006; Placenza et al., 2008). In the PC paradigm, buprenorphine has been reported to both attenuate and potentiate cocaine-induced CPP (Brown et al., 1991; Kosten et al., 1991). These discrepant findings are probably caused by the experimental parameters including the drug regimen used. We were hoping that BU08208 would reduce cocaine-induced CPP. Not surprisingly, however, because BU08028 produced CPP, it was unable to attenuate cocaine-induced CPP.
The goal of this project was to synthesize and characterize compounds with significant NOP receptor activity to supplement μ (and potentially δ and κ) activity found in the buprenorphine scaffold. The behavioral results obtained with BU08028 can also be compared with other bifunctional μ/NOP receptor ligands that we have characterized previously. We have demonstrated previously that SR14150 and SR16435, both with high affinity and partial agonist activity at NOP and μ receptors, have antinociceptive activity that is inhibited by naloxone and potentiated by the NOP receptor antagonist SB612111 (Khroyan et al., 2009; Toll et al., 2009). SR16435, which has an in vitro profile strikingly similar to BU08028, produces antinociception and CPP, indicating that, like BU08028, the NOP component is not sufficient to block the rewarding effect of the μ component. It is interesting to note that SR16435 has one behavioral feature diametrically opposed to that of BU08028. Although BU08028 causes a large increase in locomotor activity, SR16435 does the opposite, resulting in reduced activity levels, a behavior attributed to NOP receptor activation. This difference in global activity observed with SR16435 and BU08028 is difficult to explain because these compounds have a similar in vitro profile with respect to NOP, μ, and κ receptors, but suggest once again that the μ-mediated activities are strongly represented in BU08028. Alternately, it is possible that the very weak δ component of BU08028, which is not present at all in SR16435, might contribute to the increased global activity seen in BU08028.
As we have shown previously, it is possible to overcome potential μ-, κ-, and δ-mediated behavior by increasing the relative NOP activity of a compound. For example, we have shown that SR14150, which has increased NOP affinity and is 20-fold selective for NOP over μ receptors, is not rewarding on its own (Toll et al., 2009). To go one step further, SR16835 is a full agonist at NOP receptors and a weak partial agonist at μ receptors. This compound is not analgesic on its own and is able to attenuate morphine CPP (Toll et al., 2009). Together, all of these data suggest that the balance of NOP and μ components of mixed-profile compounds such as these can have significant impact in modulating the antinociceptive and rewarding aspects of the compounds. With BU08028, which is a partial agonist at both NOP and μ receptors, μ-mediated activity masks effects that could be mediated by the NOP receptor. It is possible that a different buprenorphine analog with high affinity at all the opioid receptors but with full agonist activity at NOP may counteract other traditional opioid-mediated effects such as reward and antinociception.
Acknowledgments
We thank Rajesh Khanna for help with graphics and Lucita Jimenez for technical assistance with the experiments.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA023281, DA014026] (to L.T. and N.T.Z., respectively).
Preliminary reports of these data were presented at: Toll L, Khroyan TV, Polgar WE, Cami-Kobeci G, and Husbands SM (2009) BU08028: A novel buprenorphine-like ligand, with high affinity for NOP receptors, that has long lasting antinociceptive effects in mice. Society for Neuroscience Annual Meeting; 2009 Oct 18–21; Chicago, IL. Society for Neuroscience, Washington, DC. Polgar WE, Khroyan TV, Toll L, Cami-Kobeci G, and Husbands SM (2010) BU08028, the first high affinity universal opioid receptor family ligand. International Narcotics Research Conference; 2010 Jul 11–16; Malmo, Sweden.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.175620.
Abbreviations:
- NOP
- nociceptin/orphanin FQ peptide
- BU08028
- (2S)-2-[(5R,6R,7R,14S)-N-cyclopropylmethyl-4,5-epoxy-6,14-ethano-3-hydroxy-6-methoxymorphinan-7-yl]-3,3-dimethylpentan-2-ol
- CPP
- conditioned place preference
- PC
- place conditioning
- N/OFQ
- nociceptin/orphanin FQ
- MPE
- maximum potential effect
- ANOVA
- analysis of variance
- SB612111
- (5S,7S)-7-{[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl}-1-methyl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-ol
- SR14150
- 1-(1-cyclooctylpiperidin-4-yl)-indolin-2-one)
- SR16835
- 1-(1-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)piperidinl-4-yl)-indolin-2-one
- UFP-101
- [Nphe1,Arg14,Lys15]nociceptin-NH2
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- DPEPE
- [d-Pen2,5]enkephalin
- U69593
- N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl)-1-oxaspiro[4.5]dec-8-yl]acetamide
- HS 599
- 18,19-dehydrobuprenorphine.
Authorship Contributions
Participated in research design: Khroyan, Husbands, and Toll.
Conducted experiments: Khroyan, Polgar, and Cami-Kobeci.
Performed data analysis: Khroyan.
Wrote or contributed to the writing of the manuscript: Khroyan, Husbands, Zaveri, and Toll.
Other: Zaveri and Toll acquired funding for the research.
References
- Bentley KW, Hardy DG. (1967) Novel analgesics and molecular rearrangements in the morphine-thebaine group. 3. Alcohols of the 6,14-endo-ethenotetrahydrooripavine series and derived analogs of N-allylnormorphine and -norcodeine. J Am Chem Soc 89:3281–3292 [DOI] [PubMed] [Google Scholar]
- Brown EE, Finlay JM, Wong JT, Damsma G, Fibiger HC. (1991) Behavioral and neurochemical interactions between cocaine and buprenorphine: implications for the pharmacotherapy of cocaine abuse. J Pharmacol Exp Ther 256:119–126 [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Massi M. (2003) The nociceptin/orphanin FQ/NOP receptor system as a target for treatment of alcohol abuse: a review of recent work in alcohol-preferring rats. Physiol Behav 79:121–128 [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Rimondini R, Sommer W, Massi M, Heilig M. (2007) Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol Psychiatry 61:4–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. (1999) Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology 141:220–224 [DOI] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. (1977) Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley CT, Spaeth CG, Berzetei-Gurske IP, Craymer K, Adapa ID, Brandt SR, Houghten RA, Toll L. (1997) Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J Pharmacol Exp Ther 283:735–741 [PubMed] [Google Scholar]
- Grundt P, Martinez-Bermejo F, Lewis JW, Husbands SM. (2003) Formic acid catalyzed rearrangement of thevinols (=4,5-epoxy-3,6-dimethoxy-α,17-dimethyl-6,14-ethenomorphinan-7-methanols) and their vinylogous analogues: effects of 5β-methyl substitution. Helv Chim Acta 86:2287–2298 [Google Scholar]
- Ide S, Minami M, Satoh M, Uhl GR, Sora I, Ikeda K. (2004) Buprenorphine antinociception is abolished, but naloxone-sensitive reward is retained, in mu-opioid receptor knockout mice. Neuropsychopharmacology 29:1656–1663 [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Pevnick JS, Griffith JD. (1978) Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry 35:501–516 [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Polgar WE, Jiang F, Zaveri NT, Toll L. (2009) NOP receptor activation attenuates antinociception induced by mixed NOP/mu-opioid receptor agonists. J Pharmacol Exp Ther 331:946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Zaveri NT, Polgar WE, Orduna J, Olsen C, Jiang F, Toll L. (2007) SR 16435 [1-(1-(bicyclo[3.3.1]nonan-9-yl)piperidin-4-yl)indolin-2-one], a novel mixed nociceptin/orphanin FQ/mu-opioid receptor partial agonist: analgesic and rewarding properties in mice. J Pharmacol Exp Ther 320:934–943 [DOI] [PubMed] [Google Scholar]
- Kosten TA, Marby DW, Nestler EJ. (1991) Cocaine conditioned place preference is attenuated by chronic buprenorphine treatment. Life Sci 49:PL201–PL206 [DOI] [PubMed] [Google Scholar]
- Kotlińska J, Wichmann J, Legowska A, Rolka K, Silberring J. (2002) Orphanin FQ/nociceptin but not Ro 65–6570 inhibits the expression of cocaine-induced conditioned place preference. Behav Pharmacol 13:229–235 [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. (2003) Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther 304:310–318 [DOI] [PubMed] [Google Scholar]
- Lattanzi R, Negri L, Giannini E, Schmidhammer H, Schutz J, Improta G. (2001) HS-599: a novel long acting opioid analgesic does not induce place-preference in rats. Br J Pharmacol 134:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW. (1985) Buprenorphine. Drug Alcohol Depend 14:363–372 [DOI] [PubMed] [Google Scholar]
- Lutfy K, Cowan A. (2004) Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol 2:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, et al. (2003) Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci 23:10331–10337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Hossain SM, Khaliq I, Maidment NT. (2001) Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats. Br J Pharmacol 134:529–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Kieffer BL, Lutfy K. (2007) The μ opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology 52:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. (2008) The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology 54:564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Mendelson JH, Drieze J. (1993) Naltrexone-buprenorphine interactions: effects on cocaine self-administration. Neuropsychopharmacology 9:211–224 [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. (1989) Buprenorphine suppresses cocaine self-administration in rhesus monkeys. NIDA Res Monogr 95:333–334 [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535 [DOI] [PubMed] [Google Scholar]
- Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, Lange WR, Contoreggi C, Johnson RE, Fudala PJ. (2004) Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther 75:34–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. (1999) Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res 832:168–170 [DOI] [PubMed] [Google Scholar]
- Placenza FM, Rajabi H, Stewart J. (2008) Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology 200:347–355 [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270:792–794 [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Gibson TR, Bardo MT. (1994) Dissociation of buprenorphine-induced locomotor sensitization and conditioned place preference in rats. Pharmacol Biochem Behav 49:241–245 [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. (2004) Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology 172:129–136 [DOI] [PubMed] [Google Scholar]
- Sorge RE, Rajabi H, Stewart J. (2005) Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology 30:1681–1692 [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. (2006) The effects of chronic buprenorphine on intake of heroin and cocaine in rats and its effects on nucleus accumbens dopamine levels during self-administration. Psychopharmacology 188:28–41 [DOI] [PubMed] [Google Scholar]
- Spagnolo B, Calo G, Polgar WE, Jiang F, Olsen CM, Berzetei-Gurske I, Khroyan TV, Husbands SM, Lewis JW, Toll L, et al. (2008) Activities of mixed NOP and mu-opioid receptor ligands. Br J Pharmacol 153:609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, et al. (1998) Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr 178:440–466 [PubMed] [Google Scholar]
- Toll L, Khroyan TV, Polgar WE, Jiang F, Olsen C, Zaveri NT. (2009) Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/-μ opioid receptor ligands: implications for therapeutic applications. J Pharmacol Exp Ther 331:954–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Nahorski SR. (1995) Modulation by μ-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol Pharmacol 47:848–854 [DOI] [PubMed] [Google Scholar]
- Zaveri N, Jiang F, Olsen C, Polgar W, Toll L. (2005) Small-molecule agonists and antagonists of the opioid receptor-like receptor (ORL1, NOP): ligand-based analysis of structural factors influencing intrinsic activity at NOP. Aaps J 7:E345–E352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW. (2003) Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport 14:2383–2385 [DOI] [PubMed] [Google Scholar]