Abstract
BACKGROUND: Early insulin treatment is considered more beneficial than anti-diabetic medication with sulphonylureas, because the latter may exert negative effects on beta-cell function, while the former may help preserve it. In a previous study, we found that C-peptide response was increased in the insulin-treated group, whereas it was decreased in the glibenclamide group. However, it was not certain whether the advantage remained in the longer term. AIM: In this study, we tested whether early insulin treatment is more beneficial than glibenclamide against a 6-year follow-up perspective. METHODS: We designed a randomized clinical trial in subjects with newly diagnosed type 2 diabetes. Glucagon stimulatory tests, measuring C-peptide and islet amyloid polypeptide (IAPP), were performed after 2, and 3, days of temporary insulin and glibenclamide withdrawal. RESULTS: 18 subjects initially randomized to glibenclamide, and 16 randomized to two daily injections of insulin, participated in end-of-study investigations. C-peptide response to glucagon deteriorated (p < 0.01 vs. baseline) in initially glibenclamide-treated patients (n = 18), but not in insulin-treated patients (p < 0.05 for difference between groups, after 2 days of treatment withdrawal). The IAPP response to glucagon declined in the glibenclamide group (p < 0.001), but not in insulin-treated subjects (p = 0.05 for difference between groups). CONCLUSIONS: Early insulin treatment preserves beta-cell secretory function better than glibenclamide even in a 6-year perspective.
Keywords: type 2 diabetes, beta-cell function, insulin secretion, sulphonylurea, islet amyloid polypeptide
Abbreviations: BMI - body mass index; CV - coefficient of variation; EDTA - ethylenediaminetetraacetic acid; GADA - glutamic acid decarboxylase 65 antibody; GLP-1 - glucagon-like peptide 1; HbA1c - glycated hemoglobin; HOMA-IR - homeostasis model assessment of insulin resistance; HPLC - high performance liquid chromatography; IA-2A - islet cell antigen 2 (also called tyrosine phosphatase-like protein); IAPP - islet amyloid polypeptide; ICA - islet cell autoantibody; KIE - kallikrein inactivator units; NGSP - National Glycohemoglobin Standardization Program; NPH - neutral protamine hagedorn; NYHA III-IV - New York Heart Association class III-IV (classification grade for the severity of heart failure symptoms); RIA - radioimmunoassay; SEM - standard error of mean; SU - sulphonylureas
Introduction
Beta-cell function in type 2 diabetes is known to decline with time. We [1], and others [2, 3], have proposed that demands for increased insulin secretion, imposed by chronic hyperglycemia and insulin resistance, is a primary negative factor behind the demise of beta-cells (the "overworked beta-cell" hypothesis). Such a negative influence may be mediated by islet inflammation [4], hypersecretion of islet amyloid polypeptide (IAPP), followed by amyloid deposition [5, 6], and/or by other mechanisms.
The "overworked beta-cell" hypothesis predicts that in the long run sulphonylureas (SU), which enhance endogenous insulin secretion, could exert negative effects on beta-cell function. Also, the hypothesis considers that insulin treatment can preserve beta-cell function by inducing a relative beta-cell rest. To test this notion, we designed a randomized study to compare SU (glibenclamide) and insulin treatment in recent onset type 2 diabetes. We have already reported results at 2 [7] and 4 [8] years, after the same interventions. In the previous studies, we found that C-peptide response was increased in the insulin-treated group, whereas it was decreased in the glibenclamide group. At the end of the second year, HbA1c had deteriorated in the glibenclamide group, but not in the insulin-treated group. After 4 years, we found that beta-cell function deteriorated in both groups, but that the deterioration was faster in the glibenclamide group.
We now report outcomes after more than 6 years of treatment. We aimed to investigate whether the beneficial effects of insulin treatment early after diagnosis of type 2 diabetes vs. glibenclamide on beta-cell function, is long-lasting. This follow-up study confirms the beneficial effect of significantly better C-peptide and IAPP responses in the insulin group.
Patients and methods
Patients
Women and men, 35 to 70 years of age, with type 2 diabetes, diagnosed <2 years, were asked to take part in the study. Inclusion criteria were fasting blood glucose concentration between 7.0 and 12.0 mmol/l during screening at one occasion, and treatment by diet alone for at least one month. Exclusion criteria included:
- pharmacological treatment for diabetes for more than 6 months,
- low fasting plasma C-peptide concentrations (<0.2 nmol/l),
- ketonuria (more than trace amounts),
- BMI > 35 kg/m2,
- plasma creatinine >150 µmol/l,
- severe retinopathy (proliferative or pre-proliferative),
- severe cardiac disease (NYHA III-IV),
- positivity for islet antibodies (ICA, GADA, or IA-2A).
Forty-nine patients were eligible for randomization. Six hospital-based diabetic centers in Sweden participated in the study. The ethics committee at the Karolinska Institute approved the study protocol. All patients gave their informed consent before participating.
Experimental design
Patients were randomly assigned to monotherapy with glibenclamide, or insulin. Both treatment groups were instructed to perform home glucose monitoring. Results were discussed with a doctor or nurse during the scheduled visits.
Treatment with glibenclamide was started at a dose of 1.75 mg once daily. The dose was adjusted by steps of 1.75 or 3.5 mg, taking into account results of home glucose monitoring, and aiming at HbA1c levels within target levels, i.e. ≤1% above the upper normal HbA1c level of 6.2% (according to NGSP). Insulin was administered twice daily as pre-mixed insulin, i.e. a combination of 30% soluble and 70% NPH insulin (Mixtard 30/70; Novo Nordisk, Copenhagen, Denmark). The starting dose of insulin was 0.25U/kg/24h. Two thirds of the daily dose was given before breakfast, and one third before supper. Insulin doses were adjusted as follows:
1. Increase of total dose by 10% if mean 24-h capillary blood glucose (home glucose monitoring) above 12 mmol/l.
2. Decrease of total dose by 10% if mean capillary blood glucose at home glucose monitoring was <6 mmol/l.
3. Decrease of individual dose by 10% if blood glucose <4.0 mmol/l at a time-point 2 h or later after the last dose.
Fasting blood samples were secured at study start, and then every third month during the first two years of the study. Thereafter, blood samples were taken semi-annually. The main outcome was the assessment of beta-cell function, as tested after stimulation by glucagon. Duplicate glucagon tests were performed annually on two consecutive days (see below). To nullify the influence of anti-diabetic treatment, we withheld glibenclamide, or insulin, for two days before the first day of testing, and for an additional 24 h before the second day of testing.
The glucagon test was carried out at 8 a.m. after a 10-h fast. Blood samples were taken immediately prior to an intravenous injection of 1 mg glucagon (Novo Nordisk, Copenhagen, Denmark), and 6 min thereafter. Samples for glucose determinations were collected in tubes with fluoride and heparin. Samples for C-peptide and IAPP were collected in tubes with EDTA with addition of aprotinin 10,000 KIE/ml 0.1 ml/ml whole blood. Glucose concentrations were measured immediately. Samples for HbA1c analysis were taken as capillary blood samples on filter paper [9], and sent to the Department of Clinical Chemistry, Malmö University Hospital, Malmö, Sweden, for assay. Other samples were frozen and kept at -70°C until assayed.
Retinopathy
Fundus photography was performed at the patient's local hospital. The photographs were assessed centrally [10].
Assays
Antibodies (used for exclusions) were assayed, as described in Borg et al. [11, 12]. HbA1c was determined by HPLC [13], using reference values of 3.90-5.30%. C-peptide was measured by RIA (Euro-Diagnostica, Malmö, Sweden). The lowest detectable concentration was 0.05 nmol/l, intra-assay variation was 5%, and total variation (sum of intra- and inter-assay variation) 7%. Cross-reactivity with proinsulin was 41%. Kits from Linco Research, St. Charles, MO, USA, were used to assay proinsulin, insulin, and IAPP. The cross-reactivity of the insulin assay with proinsulin was <0.2 %. Inter- and intra-assay coefficients of variation (CV) for proinsulin were 1.5%, and 1.5%, respectively. For insulin, the inter-assay CV was 2.9%, and the intra-assay CV was 3.8%. For IAPP, the inter-assay CV was 11.9%, and the intra-assay CV was 2.6%.
Drop-outs during the study (Figure {f1})
26 patients were randomly assigned to glibenclamide, and 23 to insulin treatment. 18 patients in the SU group, and 16 patients in the insulin group, continued to the end of study period.
Two patients assigned to insulin died early in the study. The first of these died in the first year, following coronary artery bypass surgery. The second death was from gastric carcinoma in the second year of the study. Five years into the study, one patient assigned to glibenclamide died of liver carcinoma.
Twelve other patients (7 on glibenclamide and 5 on insulin) were lost from the study. Four left in the first year (2 in the SU and 2 in the insulin group). Another two left during the second year (one in each group), followed by two more in the third year (both in the SU group). In the fourth year, another insulin group patient left. In the final years of the study, two left in the fifth year (one from each group), followed by one patient in the SU group in the sixth year.
The reasons for departure from the study were given as follows. Five from the glibenclamide group left for personal reasons. In one case, this was due to relocation out of the region. The other two drop-outs in the glibenclamide group were for medical reasons. One left due to incompatibility with glibenclamide (even the lowest possible dose was leading to hypoglycemia). The second departure was for medical reasons unrelated to the treatment. In the insulin-treated group, four patients left the study for personal reasons, and one for medical reasons unrelated to the treatment.
SU failures
Seven patients in the glibenclamide-treated group discontinued glibenclamide as they needed insulin to control their diabetes (2 after one year, one after 2.5 years, one after 3 years, 2 after 4 years, and one after 5.5 years of treatment). The guideline, for perceived need for insulin treatment, was HbA1c consistently >3% above the upper reference limit (6.2%) from consecutive measurements.
Statistical analysis
Asymmetrically distributed data were logarithmically transformed. Paired, and unpaired, t-tests were used to evaluate differences within, and between, groups over time. When needed, Wilcoxon paired, and Mann Whitney, tests were used to evaluate differences within, and between, groups. A probability of p < 0.05 (two-sided) was considered significant. All statistical analyses were performed with STATISTICA 8.0 (StatSoft, Inc., Tulsa, OK, USA). Data are presented as proportions, mean ± SEM, or geometric mean (SE range).
Results
Baseline characteristics
There were no significant differences at baseline, between patients allocated to glibenclamide and those allocated to insulin treatment (Table 1).
Table 1. Baseline characteristics of the study participants.
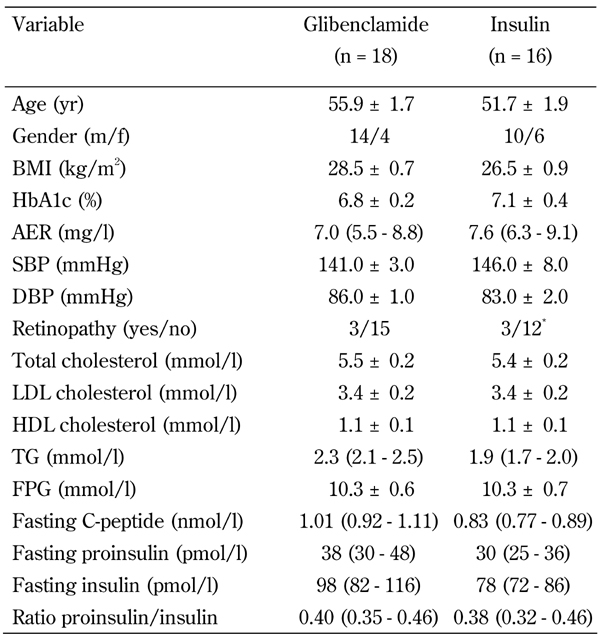
Legend: Data are presented as mean ± SEM, proportions, or geometric mean (SE range). BMI: body mass index. AER: albumin excretion rate. SBP: systolic blood pressure. DBP: diastolic blood pressure. LDL: low density lipoprotein. HDL: high density lipoprotein. TG: Triglycerides. FP: fasting plasma glucose. * 4 eyes.
Follow-up times
The follow-up time for the SU group, including failures, was 6.09 ± 0.36 yr, and for the insulin group 6.75 ± 0.18 yr.
Dosages of glibenclamide and insulin
After the first study year, the SU group received 3.5 ± 0.7 mg/day (range 0.875-10.5 mg/day) of glibenclamide. At the end of study (or at time of failure on glibenclamide), the dosage had increased to 6.0 ± 0.8 mg/day. In the insulin group, the insulin dose had increased from 20.1 ± 1.9 U insulin/day after 1 year, to 33.4 ± 4.1 U insulin/day at the end of study. The increase in dosage with time was significant (<0.05 for both glibenclamide and insulin).
Other medications during the study
Only insulin, or glibenclamide, treatments were allowed. However, many patients were on concomitant medication for treatment of hypertension and dyslipidemia. At baseline, five patients in the SU group, and one in the insulin group, were treated with beta-blocking drugs, and/or angiotensin-converting enzyme inhibitors, and angiotensin-receptor blockers. By the end of the study, the number of patients receiving these treatment had increased to 8, and 9, in the respective groups. During the study, the use of lipid-lowering drugs in the form of statins increased from two to ten patients in the SU group, and from zero to ten in the insulin group.
HbA1c
Changes in HbA1c from baseline to end of study was not significant, i.e. by -0.47 ± 0.33, in the SU group (failures included), and by -0.85 ± 0.51%, in the insulin group.
Body weight
Body weight change from baseline to end of study in the SU group was +1.5 ± 1.1 kg (not significant), and in the insulin group +3.1 ± 1.1 kg (p < 0.01). The difference in weight development between groups was not significant. In both groups the weight increase was limited to the first 2 years of intervention [8].
Short-term withdrawal of treatment had no effects on glucose levels
Fasting plasma glucose concentrations did not differ between the groups at days of testing. There were no significant increases noted between day one and day two of testing in the groups. At baseline, values were showing 0.15 ± 0.26 mmol/l in the SU group, and 0.21 ± 0.21 mmol/l in the insulin group. At end of study, values increased to 0.19 ± 0.21 mmol/l in the SU group, and 0.25 ± 0.31 mmol/l in the insulin group.
Fasting levels of insulin and proinsulin
Fasting levels of insulin and proinsulin did not change significantly. Following the design of temporary withdrawal of treatment, the recorded changes (i.e. results obtained at end of study minus baseline) of serum insulin on the first day of testing was -53.4 ± 53.4 pmol/l in the SU group, and 0.6 ± 8.3 pmol/l in the insulin group. The respective measurements on the second day of testing were -55.7 ± 56.0 pmol/l and 8.7 ± 9.0 pmol/l.
The change of proinsulin measured on the first day of testing was -20.0 ± 18.3 pmol/l in the SU group, and -3.9 ± 4.0 pmol/l in the insulin group. The respective measurements on the second day of testing were -22.4 ± 16.5 pmol/l and -2.1 ± 4.0 pmol/l. The ratio between proinsulin and insulin did not change in either group, from baseline to end of the study.
Glucagon tests: C-peptide and IAPP
The decrease in the C-peptide response to glucagon during the study was more pronounced in the SU group, compared with the insulin group, during the first day of testing. (p < 0.05; Figure 2A). Accordingly, C-peptide response to glucagon declined significantly within the SU group (p = 0.009 for day one, and p = 0.006 for day two of testing); but did not change significantly with time in the insulin group (Figure 2B).
Figure 2.
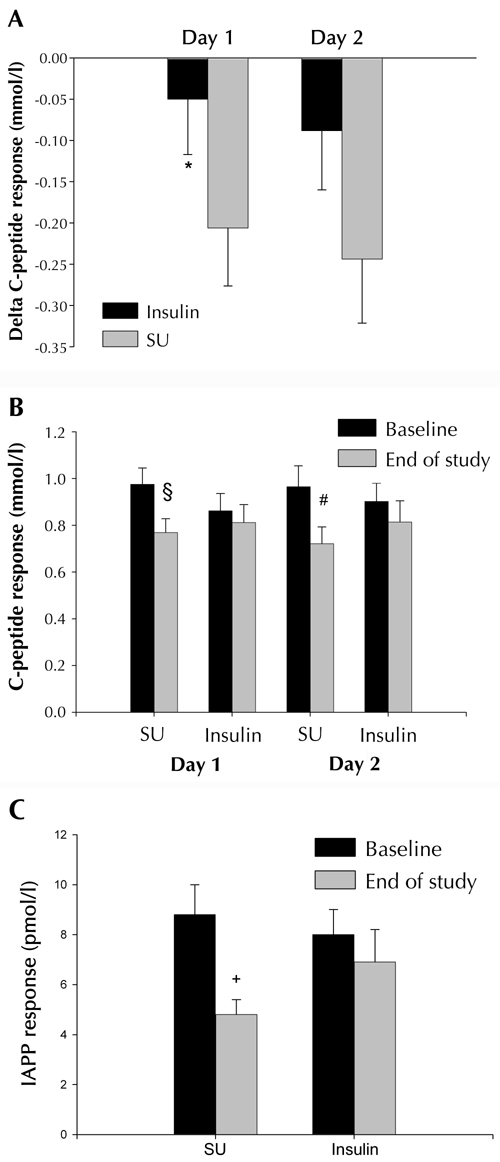
Delta C-peptide responses to glucagon (A) baseline vs. end-of-study, between the insulin-treated group (Insulin, n = 16), and the glibenclamide-treated group (SU, n = 18). Data are mean ± SEM. * p < 0.05 vs. SU. C-peptide (B) and IAPP (C) responses to glucagon in the glibenclamide-treated group (SU, n = 18), and the insulin-treated group (Insulin, n = 16), at baseline and at the end of the study. Data are mean ± SEM. § p = 0.009, # p = 0.006, + p < 0.001 for effects within groups vs. baseline.
Homeostatic assessment of insulin resistance (HOMA-IR) was the same between the treatment groups at baseline, and at the end of the study (data not shown). The results on the C-peptide response to glucagon did not change when adjusted for HOMA-IR (results not shown).
There was a strong tendency for a different development of IAPP responses between the treatment groups (p = 0.05). This tendency remained even when excluding patients who failed on glibenclamide treatment (p = 0.05). Hence, the IAPP response to glucagon declined in the SU group, whereas, in the insulin group it remained similar from baseline to the end of the study (Figure 2C).
Discussion
This study provides evidence that newly diagnosed subjects with type 2 diabetes on monotherapy with glibenclamide are more prone to deterioration of insulin secretion than subjects randomized to early insulin treatment. These findings extend our previous observations [7, 8]. Now, we have provided evidence for long-term negative effects from glibenclamide, as compared to insulin treatment. This result does not exclude the possibility that some deterioration of insulin secretion with time may also occur in insulin-treated patients [8].
We found a positive effect of insulin vs. glibenclamide treatment on glucagon-stimulated C-peptide secretion, even at more than 6 years from baseline (limited to the first day of testing). This is a remarkable finding, given that the results in the SU group included measurements from six of seven failure patients, who had switched to insulin for a mean of 3 years before end-of-study testing. These results suggest that a "window of opportunity" for beneficial effects of insulin treatment occurs early in the course of the disease.
Also, we found a decline in the IAPP response to glucagon in the glibenclamide group, but not in the insulin group. This finding may seem at odds with previous reports that SU increases IAPP secretion relative to insulin secretion [14]. However, in contrast to others, we tested the IAPP and C-peptide responses after temporary omission of SU and insulin. While the clinical implications of our finding are not entirely clear, it highlights the notion that SU vs. insulin treatment leads to profound and long-lasting differences in beta-cell function.
Was it possible to predict who would fail on glibenclamide treatment? Groop et al. reported that secondary failures on sulfonylurea treatment were partly determined by hepatic and peripheral insulin resistance [15]. Our data is compatible with this notion. Failures on SU treatment in our study tended to be phenotypically more insulin resistant, as observed by non-significantly higher age, BMI, and fasting C-peptide at baseline (data not shown). However, we could not detect any correlation between age (the strongest of the aforementioned tendencies) and deterioration of insulin secretion in the study population taken as a whole.
The small number of participants is an obvious limitation of our study. However, small numbers would primarily increase the risk of false negative results (type 2 error) and would be less likely to influence the positive effects (significant differences between treatments) that we observe. As an index of external validity, we note that with regard to failure rates, our study population is similar to larger ones. Seven of the glibenclamide-treated patients failed on treatment during the study, giving a failure rate of almost 40% after 6 years. Several previous studies found similar failure rates [16]. Interestingly, we noted that the majority of gibenclamide-treated patients, i.e. non-failures, retained acceptable glucose control.
At study start, glibenclamide was indeed first choice of pharmaceutical treatment in type 2 diabetes in Sweden. It could be argued now that this is no longer the case. However, the purpose of the study was more general and mechanistic. We wanted to compare a therapy that chronically enhances insulin secretion, against one that promotes beta-cell rest. It would be interesting to learn whether insulin treatment compared with state-of-the art enhancers on insulin secretion acting through GLP-1 receptors would arrive at results similar to ours.
Metformin is currently the drug of choice for initial pharmacological treatment of type 2 diabetes. In the Diabetes Prevention Program, it has been shown to benefit preservation of glucose tolerance [17]. In relation to the "overworked beta-cell" hypothesis, metformin may, by reducing insulin resistance, relieve demands for insulin secretion and thereby indirectly improve beta-cell function.
An asset of our study is the design feature of temporary withdrawal of insulin and glibenclamide treatments, two and three days, before testing. Also, evaluation of insulin secretion was more robust due to patients returning for an annual glucagon test, on two consecutive days. To the best of our knowledge, no previous study has compared insulin treatment with an insulin enhancer using a similar design and long follow-up time.
In summary, this study supports the hypothesis that alleviating demands on insulin secretion by insulin treatment has beneficial effects on beta-cell function.
Figure 1. Study design.
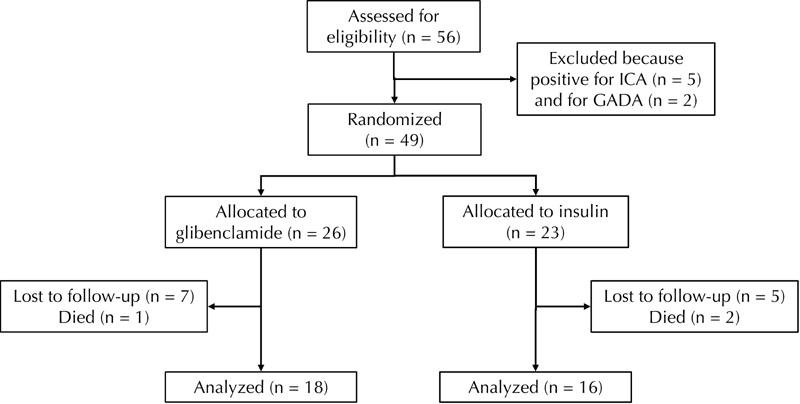
The figure shows the number of patients included into the study after assessment, the number allocated to one of the groups (glibenclamide or insulin), and the number of patients lost for follow-up during the 6-yr study period.
Acknowledgments
This study was supported by Novo Nordisk (Sweden), the Swedish Diabetes Association, the Swedish Medical Research Council (72X-14531), the Albert Påhlsson Foundation Research Funds, the Malmö University Hospital, and funds from the Karolinska Institute. We also thank Yvonne Strömberg for expert measurements of insulin, proinsulin, C-peptide, and IAPP, Gudrun Andersson, Tuula Saarinen, Annica Clark, Yvonne Strömberg, Ann Radelius, Lena Bengtsson, Christina Rosborn, Marianne Berglund, Stina Nilsson, Helena Brogren, and Ulla Aghede for dedicated assistance in the care of the patients. We are grateful to Prof. Göran Sundkvist for his important contributions to the study.
References
- 1.Grill V, Björklund A. Overstimulation and beta-cell function. Diabetes. 2001;50(Suppl 1):S122–S124. doi: 10.2337/diabetes.50.2007.s122. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan TA. Pancreatic beta-cell loss and preservation in type 2 diabetes. Clin Ther. 2003;25(Suppl B):B32–B46. doi: 10.1016/s0149-2918(03)80241-2. [DOI] [PubMed] [Google Scholar]
- 3.Fridlyand LE, Philipson LH. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells? Diabetes. 2004;53:1942–1948. doi: 10.2337/diabetes.53.8.1942. [DOI] [PubMed] [Google Scholar]
- 4.Donath MY, Schumann DM, Faulenbach M, Ellingsgard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–S164. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA. From the triumvirate to ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes. 2009;58:773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritzel RA, Meier JJ, Lin CY, Veldhuis JD, Butler PC. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes. 2007;56:65–71. doi: 10.2337/db06-0734. [DOI] [PubMed] [Google Scholar]
- 7.Alvarsson M, Sundkvist G, Lager I, Henricsson M, Berntorp K, Fernqvist-Forbes E, Steen L, Westermark G, Westermark P, Örn T. et al. Beneficial effects of insulin versus sulphoylurea on insulin secretion and metabolic control in recently diagnosed type diabetic patients. Diabetes Care. 2003;26:2231–2237. doi: 10.2337/diacare.26.8.2231. [DOI] [PubMed] [Google Scholar]
- 8.Alvarsson M, Sundkvist G, Lager I, Berntorp K, Fernqvist-Forbes E, Steen L, Örn T, Holberg MA, Kirksaether M, Grill V. Effects of insulin vs. glibenclamide in recently diagnosed patients with type 2 diabetes: a 4-year follow-up. Diabetes Obes Metab. 2008;10:421–429. doi: 10.1111/j.1463-1326.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 9.Jeppsson JO, Jerntorp P, Almer LO, Persson R, Ekberg G, Sundkvist G. Capillary blood on filter paper for determination of HbA1c by ion exchange chromatography. Diabetes Care. 1996;19:142–145. doi: 10.2337/diacare.19.2.142. [DOI] [PubMed] [Google Scholar]
- 10.Henricsson M, Nyström L, Blohme G, Östman J, Kullberg C, Svensson M, Schölin A, Arnqvist HJ, Björk E, Bolinder J. et al. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS) Diabetes Care. 2003;26:349–354. doi: 10.2337/diacare.26.2.349. [DOI] [PubMed] [Google Scholar]
- 11.Borg H, Fernlund P, Sundkvist G. Protein tyrosine phosphatase-like protein IA2-antibodies plus glutamic acid decarboxylase 65 antibodies (GADA) indicates autoimmunity as frequently as islet cell antibodies assay in children with recently diagnosed diabetes mellitus. Clin Chem. 1997;43:2358–2363. [PubMed] [Google Scholar]
- 12.Borg H, Fernlund P, Sundkvist G. Measurement of antibodies against glutamic acid decarboxylase 65 (GADA): two new 125I assays compared with 35S GAD 65-ligand binding assay. Clin Chem. 1997;43(5):779–785. [PubMed] [Google Scholar]
- 13.Jeppsson JO, Jerntorp P, Sundkvist G, Englund H, Nylund V. Measurement of hemoglobin A1c by a new liquid-chromatographic assay: methodology, clinical utility, and relation to glucose tolerance evaluated. Clin Chem. 1986;32:1867–1872. [PubMed] [Google Scholar]
- 14.Rachman J, Payne MJ, Levy JC, Barrow BA, Holman RR, Turner RC. Changes in amylin and amylin-like peptide concentrations and beta-cell function in response to sulfonylurea or insulin therapy in NIDDM. Diabetes Care. 1998;21:810–816. doi: 10.2337/diacare.21.5.810. [DOI] [PubMed] [Google Scholar]
- 15.Groop L, Schalin C, Franssila-Kallunki A, Widen E, Ekstrand A, Eriksson J. Characteristics of non-insulin-dependent diabetic patients with secondary failure to oral antidiabetic therapy. Am J Med. 1989;87:183–190. doi: 10.1016/s0002-9343(89)80695-3. [DOI] [PubMed] [Google Scholar]
- 16.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 17.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]


