Abstract
Chemical exchange saturation transfer (CEST) and spin-locking (SL) experiments were both able to probe the exchange process between protons of non-equivalent chemical environments. To compare the characteristics of the CEST and SL approaches in the study of chemical exchange effects, we performed CEST and SL experiments at varied pH and concentrated metabolites with exchangeable amide, amine, and hydroxyl protons at 9.4 T. Our results show that: i) On-resonance SL is most sensitive to chemical exchanges in the intermediate exchange regime and is able to detect hydroxyl and amine protons on a millimolar concentration scale. Off-resonance SL and CEST approaches are sensitive to slow-exchanging protons when an optimal SL or saturation pulse power matches the exchanging rate, respectively. ii) Offset frequency-dependent SL and CEST spectra are very similar, and can be explained well with an SL model recently developed by Trott and Palmer. iii) The exchange rate and population of metabolite protons can be determined from offset-dependent SL or CEST spectra or from on-resonance SL relaxation dispersion measurements. iv) The asymmetry of the magnetization transfer ratio (MTRasym) is highly dependent on the choice of saturation pulse power. In the intermediate exchange regime, MTRasym becomes complicated and should be interpreted with care.
Keywords: Chemical exchange, spin locking, CEST, asymmetric population approximation, MTR asymmetry
Introduction
Recently, there has been an increasing number of in vivo studies that have utilized the chemical exchange (CE) effect to probe the tissue microenvironment and provide novel imaging contrasts that are not available from conventional MRI techniques. Most of these studies adopted either a chemical exchange saturation transfer (CEST) or a spin-locking (SL) approach to detect contrast in tissue pH or the population of labile protons, which have a Larmor frequency different from water. Ideally, a CE-sensitive imaging contrast should have good sensitivity and vary monotonically with pH and linearly with labile proton concentration. The CE contrast is determined by many parameters, such as the exchange rate between water and labile protons (kex), the difference in their Larmor frequencies (δ), the populations of the exchangeable protons, water T1, and the magnetic field strength (B0), etc. The CE effect in MRI is also highly sensitive to a ratio of kex to δ. kex/δ, which indicates the chemical exchange kinetics, is usually divided into three regimes: slow (kex/δ≪1), intermediate (kex/δ ∼ 1), and fast exchange (kex/δ≫1). CEST techniques are mostly applied at the slow or slow to intermediate exchange regime (1,2), while the CE is often assumed to occur at the fast exchange regime for SL applications (3,4).
In CEST studies that are based upon endogenous contrast, selective off-resonance irradiation of labile protons of protein or peptide side chains attenuates the water signal via exchange between these labile protons and bulk water. The signal intensity as a function of irradiation frequency, often referred to as the Z-spectrum, can be expressed by the magnetization transfer ratio (MTR):
| [1] |
where Ω is the frequency offset with respect to water. In practice, the conventional non-CE magnetization transfer effect and direct water saturation (or the so-called spillover effect) also affect the Z-spectrum, and these effects are assumed to be symmetrical around the water resonance frequency. To minimize these non-CE contributions, CEST contrast in MRI is usually extracted from two images— one acquired with off-resonance irradiation on the targeted labile proton and the other as a control with opposite offset frequency from the water (5). The normalized differential image, usually referred to as the asymmetry of MTR (MTRasym), is described as
| [2] |
which is sensitive to the CE effect. Previous endogenous CEST contrast is mostly based on protons in slow-exchanging regimes and has been applied in many pathological studies. For example, the amide proton transfer (APT) approach, which is based upon the exchange between amide protons of protein side chains and water, has been utilized to study tumor or stroke (5-7). At neutral pH, amide protons typically have a chemical shift of around 3.5 ppm (1400 Hz or 8800 rad/s at 9.4 T) from water, and the exchange rate with water proton is on the order of 100 s-1 (8). Recently, endogenous CEST contrast has been also observed on faster-exchanging protons, where, for example, hydroxyl-based CEST approaches were reported to provide information on the concentration of glycogen as well as glycosaminoglycans (9,10). These hydroxyl protons have chemical shifts of 1-3 ppm from water and exchange rates on the order of 700-15000 s-1 (1,8); thus, the exchange is close to the intermediate regime for 3 T (1 ppm = 128 Hz or 802 rad/s) or even 9.4 T.
The CE effect can also be studied by an SL approach, where water magnetization is first flipped away from the Z-axis and then spin-locked by either an on- or off-resonance B1 radiofrequency (RF) pulse. During the applied spin-locking pulse, the water magnetization decays with the spin-lattice relaxation time in the rotating frame (T1ρ), which is sensitive to molecular fluctations with a frequency that is close to the Rabi frequency of the SL pulse, ω1, SL (= γB1, SL). SL contrast has been utilized to characterize cartilage degradation (11-13), tumors (14-17), stroke (18,19), and neurodegenerative diseases (20,21). The T1ρ dependence on ω1, SL, referred to as the T1ρ dispersion, has also been applied in pathological studies (16,19,22). It was reported in protein phantoms that the CE effect contributes significantly to the T1ρ dispersion in the ω1, SL range below a few kHz (11,23). Previous SL studies of CE effects were often explained by theoretical models with fast exchange approximation (3,4). This assumption has hindered the application of SL approaches to slow- and intermediate-exchange protons, which are widely present in biological tissues. Recently, Trott and Palmer proposed a theoretical description to explain the CE contribution to the relaxation rate R1ρ (= 1/T1ρ) when the populations of two exchanging proton pools are highly unequal (24). Under such asymmetric population (AP) approximation, the expression of CE contribution to R1ρ can be simplified and applied beyond the fast exchange limit (24). The AP assumption holds for most in vivo chemical exchange applications, because water is the dominant pool; thus, the Trott and Palmer model may be applicable to in vivo SL studies.
The aims of this work are: i) to examine the characteristics of SL and CEST contrast for chemical exchanges in the slow-, intemediate-, and fast-exchange regimes, and ii) to explain experimental data with Trott and Palmer's AP model. On-resonance R1ρ dispersion, offset-dependent SL spectra, and CEST Z-spectra measurements were performed at varied pH and concentrated metabolite phantoms with typical exchangeable proton groups found in vivo, including amide, hydroxyl, and amine protons.
Theoretical backgrounds
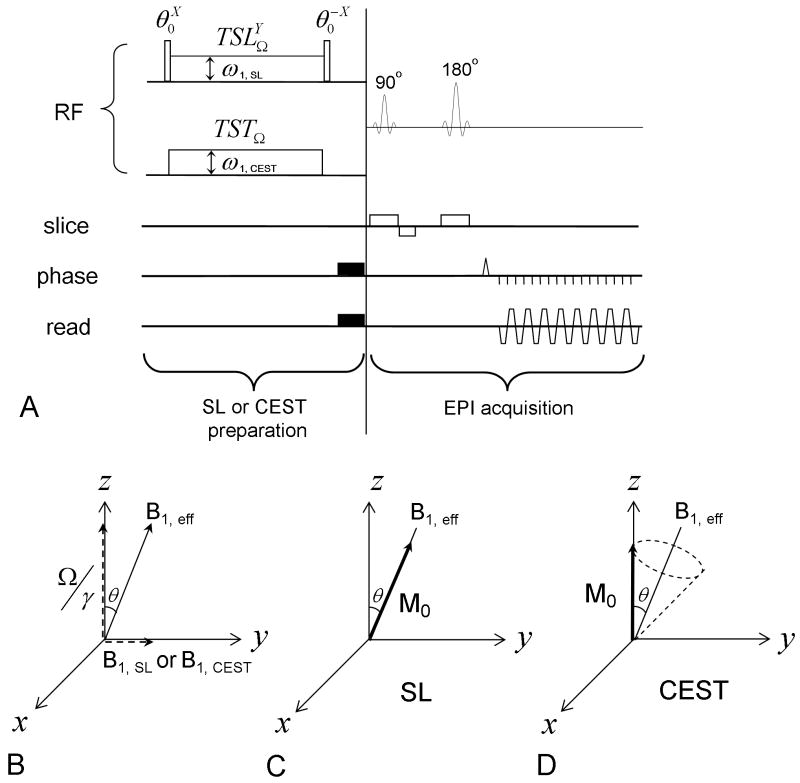
The pulse sequence for an SL experiment is illustrated in Fig. 1A, where the super- and sub-scripts of an RF pulse denote its phase and transmitter frequency, respectively. The spin-locking pulse has a Rabi frequency (SL frequency) ω1, SL and is applied on the Y-axis at a frequency offset Ω; thus, in the rotating frame, the effective SL field (Fig. 1B). To achieve SL, the water magnetization is first flipped by the θ degree pulse to the Y-Z plane, then spin-locked by B1, eff for duration of TSL, and then flipped back to the Z-axis for imaging. During TSL, the water magnetization is locked at an angle θ = arctan(ω1,SL / Ω) from the Z-axis and decays with R1ρ, the spin-lattice relaxation rate in the rotating frame (Fig. 1C). Provided that the spin relaxation is dominated by single-exponential decay, R1ρ can generally be expressed as:
Fig. 1.
(A) The pulse sequence diagram used for the SL and CEST experiments contains an SL or CEST preparation pulse and a spin-echo EPI acquisition. The super- and sub-scripts of an RF pulse denote its phase and transmitter frequency, respectively. For SL, the water magnetization is first flipped by a hard pulse and then locked by an SL pulse with a Rabi frequency of ω1, SL (=γB1, SL) and a duration of TSL. The hard pulse following the SL pulse flips the magnetization back to the Z-axis. For CEST, the saturation pulse has a Rabi frequency of ω1, CEST and a duration of TST (saturation time). (B) With an off-resonance B1, SL or B1, CEST pulse applied at the frequency offset Ω, the water magnetization in the rotating frame experiences an effective B1, eff that has an angle θ with the Z-axis. (C) In an SL experiment, the water magnetization M0 was flipped to the B1, eff direction and was spin-locked by B1, eff. (D) In a CEST experiment, the magnetization precesses around B1, eff.
| [3] |
where R1 is the longitudinal relaxation rate of water and R2 is the intrinsic water transverse relaxation rate in the absence of chemical exchange. A special case is when Ω = 0 and θ = 90°, which is the on-resonance SL. For two-site exchange between A and B with different magnetic environments (representing water and labile non-water protons, respectively), the population (p) of each site and the exchange rate constant (k) satisfy pA kA = pB kB. Using population-averaged values of R1 and R2 for protons in the two sites and assuming that the populations of the two sites are highly asymmetric (pA ≫ pB), the Bloch-McConnell equation can be solved and the chemical exchange-related relaxation rate in an SL experiment, with Ω and ω1, SL as experimental variables, can be written as (24)
| [4] |
where δ is the chemical shift of the labile proton relative to water, kex = kA + kB is the exchange rate between the two proton pools, and pA ≈ 1 is assumed. Note that the frequency offset is expressed relative to the Larmor frequency of water, and some notations are different from those in the original reference of Trott and Palmer (Ω and kex correspond to ωrf and k, respectively). Rex reaches a peak at Ω = δ. The parameters of interest, pB, kex, and δ, can be obtained by fitting Rex with Eq. [4]. To this end, two SL approaches are adapted; change in ω1,SL with fixed Ω, and change in Ω with fixed ω1,SL.
SL measurements as a function of ω1,SL can be performed at Ω = 0 (on-resonance SL) or Ω = δ. For on-resonance SL (Ω = 0),
| [5] |
The SL relaxation rate is
| [6] |
The on-resonance R1ρ dispersion data can be fitted to ω1, SL to obtain pB and kex in addition to R2 and δ. If δ is known, kex can also be inferred from the linewidth of the Rex(ω1, SL) Lorentzian-shaped curve (Rex vs. ω1, SL plot): full width at half maximum . In the case of kex/δB≪1, however, pB and kex cannot be separately determined from on-resonance R1ρ dispersion. Another SL offset frequency of particular interest is the Larmor frequency of the labile proton B (Ω = δ), for which
| [7] |
SL experiments can also be performed as a function of offset frequency (Ω) with a fixed ω1, SL, similar to a CEST Z-spectrum. The magnetization at a spin-locking time (TSL), with repetition time → ∞, is
| [8] |
When TSL is sufficiently long, the magnetization reaches steady state. An SL ratio (SLR) can be described, similar to MTR of CEST studies (see Eq. [1]), as
| [9] |
Ω-dependent SLR spectrum will be referred to as the SL Z-spectrum for comparison with the CEST Z-spectrum. Similar to MTRasym (see Eq. [2]), the CE-related contrast can be obtained from the asymmetry of the SLR; i.e., the normalized differential signal acquired from opposite frequency offsets with respect to water:
| [10] |
In off-resonance SL studies with varying Ω, Rex can be obtained by rearranging Eq. [9] from an SL Z-spectrum:
| [11] |
Ω-dependent Rex can be used for fitting kex and pB from Eq. [4]. The exchange rate kex can also be inferred from the linewidth of the Rex Lorentzian-shaped curve (Rex vs. Ω-plot): . To compare CEST results with the SL approach, an effective Rex, CEST may be constructed from the CEST Z-spectra, similar to Eq. [11]:
| [12] |
If Rex, CEST (Ω) is similar to Rex(Ω) (at ω1, CEST = ω1, SL), kex can be inferred from FWHM of the Rex, CEST vs. Ω plot and also determined using Eq. [4].
Materials and Methods
MR experiments of metabolite phantoms
All MR experiments were performed at room temperature on a 9.4T/31-cm magnet (Magnex, UK), interfaced to a Unity INOVA console (Varian). The actively shielded 12-cm-diameter gradient insert (Magnex, UK) operates at a maximum gradient strength of 40 gauss/cm and a rise time of 120 μs. A 3.8-cm-diameter volume coil (Rapid Biomedical, Ohio) was used for excitation and reception. Metabolite solution (see below) was transferred into a 9-mm I.D. syringe, and three or four syringes were inserted together into the coil for imaging. Magnetic field homogeneity was optimized by localized shimming over a ∼20 × 20 × 6 mm3 volume to yield a water spectral linewidth that was typically 10 Hz or less. The imaging parameters were: a field of view = 24 mm × 24 mm, matrix size = 64 × 64, and slice thickness = 5 mm. Before the SL and CEST experiments, a T1 map was obtained using an inversion-recovery sequence. In addition, the B1 field was also mapped for calibration of the transmit power (25). With our volume coil, the B1 map showed fairly good spatial homogeneity: the variation of B1 was less than 10% across all pixels within the samples (data not shown).
For SL and CEST experiments, the chemical exchange contrast was first generated by the SL or CEST preparation (Fig. 1A); then the residue magnetizations in the X-Y plane were dephased with crushing gradients; and finally, images were acquired with a spin-echo echo-planar imaging (EPI) technique using an echo time (TE) of 42 ms. For on-resonance R1ρ dispersion experiments, SL was either achieved with the sequence shown in Fig. 1A for Ω = 0 or with an adiabatic SL pulse sequence (25); the results were highly similar and are not distinguished here. R1ρ dispersion was measured for 10 ω1, SL values of approximately 1110, 1570, 2220, 3140, 4440, 6280, 8880, 12560, 17760, and 25120 rad/s. At each ω1, SL, 14 TSL values, ranging between 0 and 330 ms, were acquired with a repetition time (TR) of 8 s and a TE of 42 ms. For CEST and SL Z-spectra measurements, images were collected within ±10 ppm of the water resonance, with the Rabi frequency of a 4-s SL or CEST saturation pulse (ω1, SL or ω1, CEST) = ∼1100 rad/s, and the repetition time was 18 s. At each offset frequency, the SL flip angle θ was adjusted according to θ = arctan(ω1,SL / Ω). For the calculation of SLR and MTR, control M0 images were acquired at the offset frequencies of ±300 ppm.
Three sets of MRI phantom experiments were performed:
Experiment I: on-resonance R1ρ dispersion and CEST studies of nicotinamide and glucose with different concentrations. To evaluate whether SL and CEST contrast is sensitive to chemical exchanges in the slow- and intermediate-exchange regimes and to labile proton concentrations, 20, 50, 100, and 200 mM nicotinamide (Nic) and glucose (Glc) were dissolved in 1× phosphate-buffered saline (PBS) and titrated to pH of 7.4. As described in Introduction, the amide and hydroxyl protons are expected to be in the slow- and intermediate-exchange regimes, respectively. On-resonance R1ρ dispersions and CEST Z-spectra were obtained.
Experiment II: on-resonance R1ρ dispersion and CEST studies of glutamate with various pH values. To systematically study the exchange rate dependence of SL and CEST measurements, 50 mM glutamate (Glu) was dissolved in PBS and titrated to pH values of 3.1, 3.8, 4.5, 5.2, 5.9, 6.4, 6.9, 7.4, 7.9, 8.4, 9.1, and 9.8. The chemical shift between the amine (−NH2) proton and water is 3.0 ppm (2). On-resonance R1ρ dispersions and CEST Z-spectra were obtained.
Experiment III: SL and CEST Z-spectra of nicotinamide and glucose with different pH values. To compare the Z-spectra of SL and CEST, 100 mM Nic was dissolved in PBS and titrated to pH values of 7.4, 7.8, and 8.4, and 100 mM Glc was dissolved in PBS and titrated to pH values of 5.6, and 7.0. SL and CEST Z-spectra were obtained at ω1 of ∼1100 rad/s, and on-resonance R1ρ dispersions were also measured with varying ω1, SL.
Data analysis and numerical simulations
For each ω1, SL, on-resonance R1ρ maps were calculated by pixel-wise fitting of multi-TSL data to monoexponential signal decay with respect to TSL. One 5 × 5 mm2 region of interest was selected for each sample, where all data were averaged. The CEST and off-resonance SL contrasts were estimated by calculating MTRasym and SLRasym using Eqs. [2] and [10], respectively. To obtain kex, pB, and R2, the on-resonance R1ρ dispersion data were fitted to Eq. [6], assuming a chemical shift of 1.2 ppm for glucose hydroxyl protons and 3.0 ppm for glutamate amine protons (2), respectively. Glucose hydroxyl protons have more than one CEST peak (9); for simplicity, we used only one chemical shift for data fitting in this work. Note that δ is expressed in rad/s unit for the fitting of on-resonance R1ρ dispersion data to match with kex and ω1 but is expressed in ppm units for CEST or SL Z-spectra, following the literature.
Experiment I
For glucose, the chemical exchanging parameters (kex, δ, pB, and R2), determined from on-resonance R1ρ dispersions, were used to simulate SLRasym using Eqs. [8] to [10] for comparing with the experimental MTRasym, and an effective Rex, CEST was constructed from the CEST Z-spectra using Eq. [12] with measured R1 and fitted R2. To simulate SLRasym of Nic, a δ of 3.4 ppm (8545 rad/s) and kex of 100 s-1 were assumed (8), and Rex, CEST was constructed using measured R1 and assumed R2 (see results). Then, the FWHM was obtained from fitting Rex, CEST to a Lorenztian lineshape, excluding data points close to the water resonance frequency (see results below).
Experiment II
In all Glu pH phantoms, pB should be constant, while kex is varied. Note that kex and pB cannot be determined separately from on-resonance R1ρ dispersion data for samples when kex/δ ≪1. Thus, pB of Glu were first fitted with a δ of 3.0 ppm (7540 rad/s) from pH phantoms that gave the largest R1ρ dispersions (averaged from pH = 6.9, 7.4, and 7.9 samples, see results below). Then, kex was determined with a fixed pB for all pH phantoms. Similar to the data processing of Experiment I, SLRasym was simulated and the linewidth of the Rex, CEST was calculated. To study the dependence on the chemical exchanging kinetics, on- and off-resonance R1ρ (Ω = 0 and δ) were also simulated with δ = 3.0 ppm, pB = 0.0014, R1 = 0.35 s-1, and R2 = 0.5 s-1 as a function of kex/δ for a few selected values of ω1, SL.
Experiment III
SL Z-spectra and SLRasym were directly compared with the CEST Z-spectra and MTRasym.
Results
Experiment I: R1ρ and CEST effects of amide and hydroxyl protons
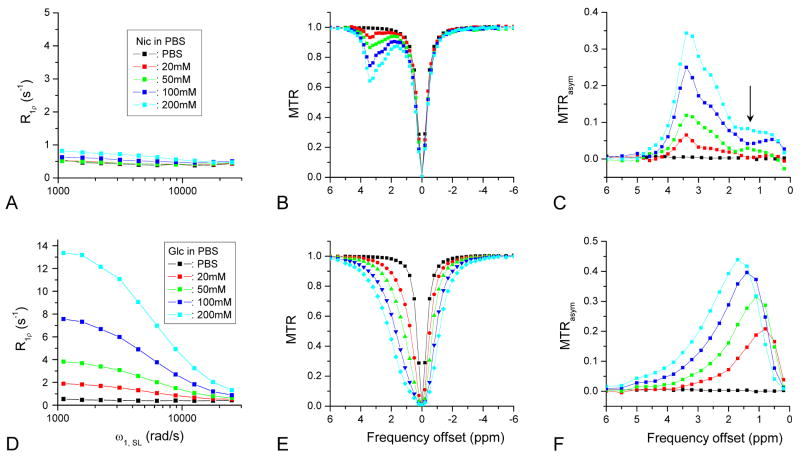
Fig. 2 shows the on-resonance R1ρ dispersions, CEST Z-spectra, and MTRasym for Nic (Figs. 2A-C) and Glc (Figs. 2D-F) phantoms with varying concentrations. As a control, the PBS solution was used (black squares), and no CE-related R1ρ dispersion or MTRasym was observed. For Nic samples with slow-exchanging amide protons, the R1ρ dispersion is very small in the whole ω1, SL range (Fig. 2A). In contrast, the CEST effect is apparent at the left side of the Z-spectra, where the MR signal dips at 3.4 ppm, more significantly with increasing Nic concentration (Fig. 2B). The spectra on the right side with negative frequency offset are independent of Nic concentration and overlap well with PBS, indicating minimal chemical exchanging effects. MTRasym spectra had an increasing peak at 3.4 ppm with concentration (Fig. 2C) but was not symmetric around the peak. There is a plateau region in the 0.5–2 ppm range (arrow), which was also reported in a previous CEST study for amide protons (26).
Fig. 2.
On-resonance R1ρ dispersion, CEST Z-spectra, and the MTRasym lineshapes for Nico (A-C) and Glc (D-F) samples with varied concentrations in PBS. The data of a pure PBS sample are also shown for comparison (black data points). For Nic with slow-exchanging amide protons, the R1ρ dispersion is small (A), but the CEST contrast is significant (B), and a well-defined MTRasym peak appears at around 3.4 ppm for all concentrations (C). A plateau is observed in MTRasym in the frequency offset range of 0.5 ppm to 2 ppm (black arrow). For Glc with faster-exchanging hydroxyl protons, the R1ρ dispersion is large and increases linearly with Glc concentration (D). The CEST Z-spectra (E) appear much broader compared to the Nic samples. The MTRasym peak offset shifts, and the peak magnitude shows a non-linear dependence with concentration (F).
Unlike Nic, Glc samples with a faster exchanging hydroxyl group show large R1ρ dispersions, where R1ρ decreases with the SL frequency ω1, SL (Fig. 2D). R1ρ at each SL frequency increases almost linearly with Glc concentration. The signal drops in the CEST Z-spectra become very broad, and the exchange effect extends to negative frequency offsets, where the signals of Glc samples are much lower as compared to PBS (Fig. 2E). Since the exchanging effect presents on both sides of the water resonance, some CEST contrast would be sacrificed when two MTR signals of opposite frequency offset are subtracted for MTRasym. The peak of MTRasym spectra shifts towards a larger frequency offset with increasing Glc concentration (Fig. 2F).
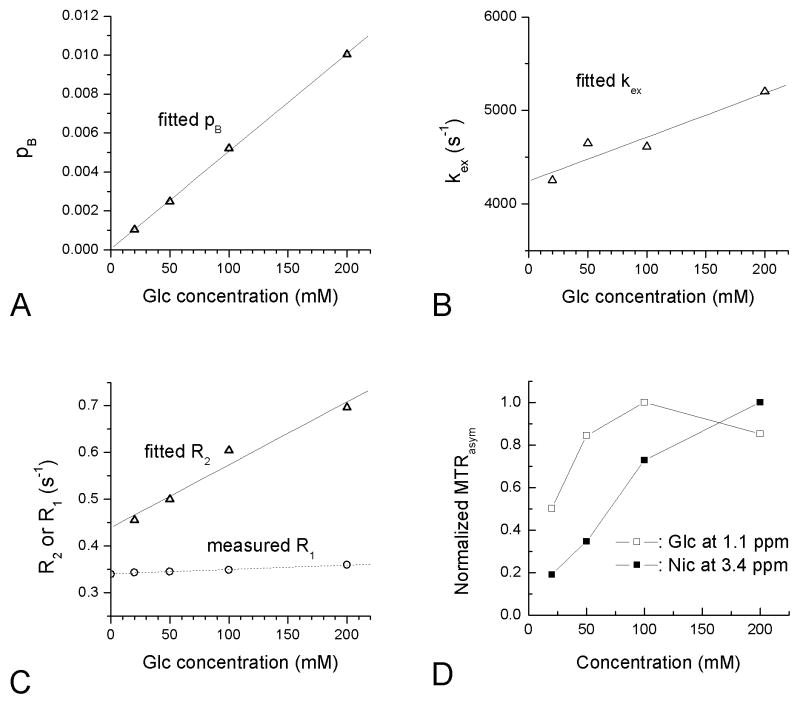
In same metabolite phantoms with different concentrations, we expect a linear increase in pB with concentration but a constant kex. Since large on-resonance R1ρ dispersions were only observed in the Glc samples, pB, kex, and R2 of glucose were obtained by fitting R1ρ dispersion data to Eq. [6] with a fixed δ of 1.2 ppm (3016 rad/s) for glucose hydroxyl groups (see Fig. 3A-C). The fitted pB is proportional to Glc concentration (r2=0.9994) (Fig. 3A). The fitted kex and R2 increase slightly with Glc concentration, probably due to the simplification of using a single chemical shift in our data fitting (Fig. 3B-C). It has been reported in a recent CEST study that the OH groups of Glc have three chemical exchange peaks with different frequency offsets (1 to 3 ppm from water) (9). Note that the measured R1 is almost independent of the Glc concentration (Fig. 3C). For Glc samples in the intermediate-exchanging regime, the peak intensity of MTRasym at 1.1 ppm does not monotonically increase with concentration (Fig. 3D). In contrast, for Nic samples in the slow-exchanging regime, the peak magnitude of MTRasym at 3.4 ppm increases with concentration in a nearly linear manner.
Fig. 3.
(A-C) Fitted results of the on-resonance R1ρ dispersion data as a function of Glc concentration, assuming δ = 1.2 ppm (3016 rad/s). The fitted pB is proportional to the Glc concentraion (A). Fitted kex (B) and R2 (C) increase with concentration, whereas the measured R1 only increases weakly with concentration (C). (D) For Nic and Glc, the MTRasym peaks, obtained at 3.4 ppm for Nic and 1.1 ppm for Glc, were normalized. MTRasym does not increase linearly with metabolite concentrations, especially for Glc.
Experiment II: SL and CEST at varying chemical exchange rate by changing pH
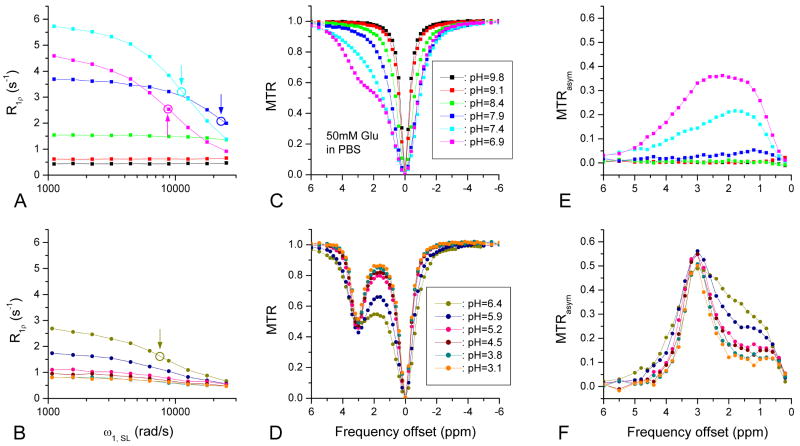
The chemical exchange rate between amine (−NH2) protons and water was systematically varied by changing pH values in 50-mM Glu samples. At lower pH, a slower exchange rate between two proton pools is expected. Significant on-resonance R1ρ dispersion was observed for samples with intermediate pH values (5.9 ≤ pH ≤ 7.9); the R1ρ dispersion peaked at a pH of ∼7.4 but was small for both very high and low pH values (Fig. 4A-B). The half widths of R1ρ dispersion decreased with pH values (arrows in Fig. 4A-B). In the CEST experiments, the Z-spectra of Glu samples with pH = 9.1 and 9.8 were narrow and symmetric around the water frequency (Ω = 0) (Fig. 4C), similar to the PBS data in Fig. 2E. When pH decreased, Z-spectra became broader initially, increased the asymmetry around the water frequency (pH = 8.4 − 7.4, Fig. 4C), and then had an increased dip at 3.0 ppm (Fig. 4D). MTRasym spectra were broad at pH = 7.4 and 6.9, and its peak shifted to 3.0 ppm for pH ≤ 6.4. The shapes of the MTRasym spectra were similar for pH ≤ 5.2 but not symmetric around the 3-ppm peak (Fig. 4E-F).
Fig. 4.
On-resonance R1ρ dispersion (A and B), CEST Z-spectra (C and D), and MTRasym lineshapes (E and F) for 50-mM Glu samples with varied pH. R1ρ dispersion is large for intermediate pH values but small for very high or low pH. The half width of the R1ρ dispersion decreases with pH (circles and arrows). The CEST Z-spectra are narrow and symmetric for high pH values (9.1 and 9.8); becomes broad and increasingly asymmetric for intermediate pH values; and shows a sharp dip at 3.0 ppm for low pH values. For pH ≤ 5.9, the MTRasym peaks at 3.0 ppm and the peak magnitude only reduces slightly with pH. For higher pHs, MTRasym lineshape becomes broad and the peak shifts to a smaller frequency offset and decreases in magnitude.
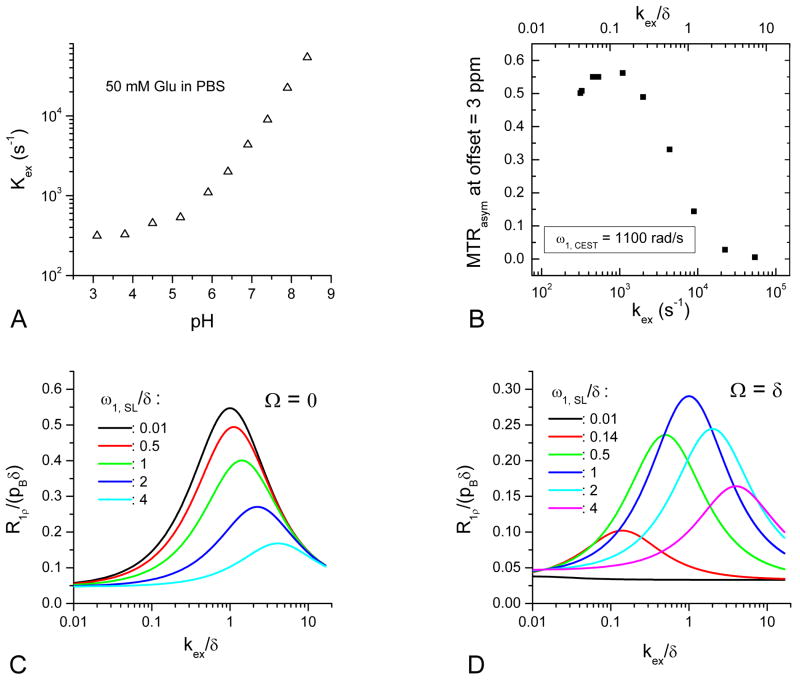
Fig. 5A shows the kex of Glu with pH ≤ 8.4, determined by fitting on-resonance R1ρ dispersions with a pB of 0.00135 ± 0.0001 (n = 3), obtained from pH = 6.9, 7.4, and 7.9 data. As expected in a base-catalyzed exchange process, kex decreases with pH, similar to recent CEST measurements of amide protons (26). Given a δ of 3 ppm (7540 rad/s), pH = 6.4 to 7.9 samples can be roughly ascribed to the intermediate-exchange regime, whereas samples with pH ≤ 5.9 and pH ≥ 8.4 are in the slow- and fast-exchange regimes, respectively. These results indicate that on-resonance R1ρ dispersion is most sensitive to the intermediate-exchange regime but much less to fast and slow exchanges (see Fig. 4A and 4B). In contrast, CEST with a relatively low ω1, CEST is sensitive to slow to intermediate exchanges but more to slow exchanges (see Fig. 4C and 4D).
Fig. 5.
(A) Fitted kex as a function of pH for 50-mM Glu samples, assuming δ = 7540 rad/s (3 ppm). (B) For Glu samples with varying pHs, the MTRasym at frequency offset of 3 ppm is plotted as a function of the fitted kex, which shows a peak around kex / δ ∼ 0.1 at the slow-exchange regime. The peak kex is ∼1100 s-1 and matches with the frequency of the applied saturation power (1100 rad/s). (C and D) Simulation of on- and off-resonance (R1ρ/pBδ) as a function of kex/δ for selected ω1, SL/δ values, assuming δ = 3 ppm, pB = 0.0014, R1 = 0.35 s-1, and R2 = 0.5 s-1. On-resonance R1ρ can only be tuned to the intermediate chemical exchange process with a small ω1, SL and a faster exchange with a higher ω1, SL (C). In contrast, off-resonance (Ω = δ) R1ρ can be tuned to slow, intermediate, and fast exchanges with small, intermediate, and large ω1, SL values, respectively (D).
The kex values obtained from on-resonance SL were plotted against the MTRasym of 3 ppm (Fig. 5B). MTRasym is maximal at a kex/δ of ∼0.1 (in the slow exchange regime) and at a kex of ∼1100 s-1 (pH = 5.9), which matches well with the Rabi frequency of the applied saturation pulse ω1, CEST (∼1100 rad/s). To compare the characteristics of on- and off-resonance SL, R1ρ on the resonance of water (Ω = 0) and labile proton (Ω = δ) were simulated as a function of kex/δ at a few selected ω1, SL (Fig. 5C and 5D), with assumptions of R1 = 0.35 s-1, R2 = 0.5 s-1, δ = 3.0 ppm, and pB = 0.0014. Although different parameters can change R1ρ values, the features of R1ρ vs. kex/δ curves remain. Note that R1ρ and ω1, SL were scaled by pBδ and δ, respectively. For on-resonance SL (Fig. 5C), the R1ρ peak starts from the intermediate-exchange regime for very small ω1, SL and shifts to faster exchanges with increasing ω1, SL. Thus, on-resonance SL is less sensitive to slow chemical exchanges as compared to intermediate exchanges. For off-resonance SL with Ω = δ (Fig. 5D), R1ρ can be made to be sensitive to different kex values by variation of ω1, SL, and the peaks appear at kex = ω1, SL. The maximum R1ρ is reached at an intermediate exchange domain with an intermediate SL frequency (ω1, SL = kex = δ). This simulation can be understood as a tuning of R1ρ to certain kex values when there is a wide distribution of kex values. In contrast to on-resonance SL, off-resonance R1ρ with a small ω1, SL can be tuned to slow exchanges, where a faster CE contribution is suppressed. For example, for our Glu data with ω1, SL = 1100 rad/s (which were obtained from Glu with a pH of 5.9), δ = 3 ppm (7540 rad/s), and ω1, SL/δ = 0.14, the peak of the R1ρ curve (solid black) appears at kex/δ ∼ 0.14 in the slow-exchange regime.
Experiment III: Similarity of SL and CEST Z-spectra
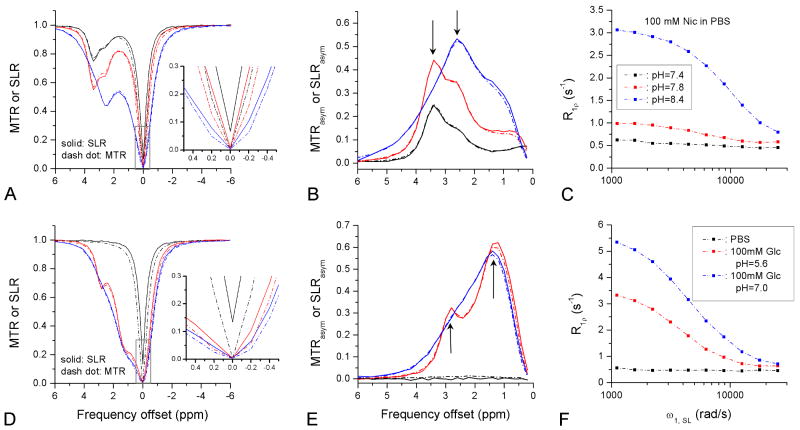
The SL Z-spectra (solid lines in Fig. 6A and D) of the Nic, Glc, and PBS samples were compared with the corresponding CEST Z-spectra (dashed lines). These two spectra match very well except at small frequency offsets (see Insets). SLR at a given offset is always higher than MTR. For example, for Nic at pH = 7.4 (6A) and PBS (6D), 7% and 13% of the MR signal remained after a 4-s on-resonance SL pulse (Ω = 0), respectively, while the CEST signals were zero due to the direct water saturation effect. The MTRasym and the SLRasym spectra (Figs. 6B and 6E) also show high similarity. The difference between MTRasym and SLRasym spectra close to 0 ppm was small, indicating that the subtraction of MTR between opposite offset frequencies is indeed an effective approach to cancel the majority of the spillover effect in CEST Z-spectra. In the SLRasym and MTRasym of Nic samples, there is a shift of the peak from 3.4 ppm to 2.6 ppm (arrows) with increasing pH from 7.8 to 8.4. In both the SLRasym and MTRasym spectra of the Glc samples, more than one −OH peak can be discerned (for pH = 5.6, arrows), similar to a previous report (9). To compare with off-resonance SL and CEST data, on-resonance R1ρ dispersions were plotted in Figs. 6C and 6F. The R1ρ dispersion in Nic and Glc increases significantly with pH due to an increase of kex from slow- to intermediate-exchange regimes.
Fig. 6.
SL Z-spectra (solid) and CEST Z-spectra (dashed), the SLRasym (solid) and MTRasym (dashed) lineshapes, and on-resonance R1ρ dispersions for Nic (A-C) and Glc (D-F) samples with varied pH in PBS. SL and CEST spectra match well for large frequency offsets, and a small difference is observed when close to the water resonance (A and D). Insets: the enlarged SL and CEST Z-spectra show that the CEST signals are smaller than those of SL due to direct water saturation. SLRasym and MTRasym also match well for all samples (B and E), and more than one peak is detected for both Nic and Glc (arrows). On-resonance R1ρ dispersions of both Nic and Glc are very sensitive to pH (C and F).
Simulated SLRasym spectra vs. experimental MTRasym spectra
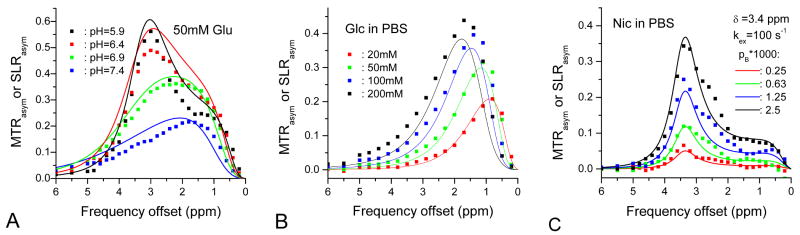
The similarity in the SL and CEST Z-spectra suggests that the experimental CEST data may be explained by Trott and Palmer's SL model. SLRasym values (lines) were simulated with the parameters obtained from on-resonance R1ρ dispersions for Glu and Glc (Fig. 7A and 7B) or assumed values for Nic (Fig. 7C) and compared with experimental CEST MTRasym data (data points). The Nic on-resonance R1ρ dispersion data cannot be fitted robustly due to its low sensitivity (see Fig. 2A). Overall, the match between simulated SLRasym and experimental MTRasym is very good, indicating that the CEST Z-spectra can be explained by the SL model.
Fig. 7.
Simulated SLRasym spectra (line) are compared with the experimental MTRasym (squares) for 50-mM Glu samples with varied pH (A) and for Glc (B) and Nic (C) samples with varied concentrations. In (A) and (B), the parameters used for the simulation of SLRasym were obtained by the fitting of on-resonance R1ρ dispersion. In (C), kex of 100 s-1 and δB of 3.4 ppm (8545 rad/s) were assumed for Nic samples.
Effective Rex obtained from CEST Z-spectra
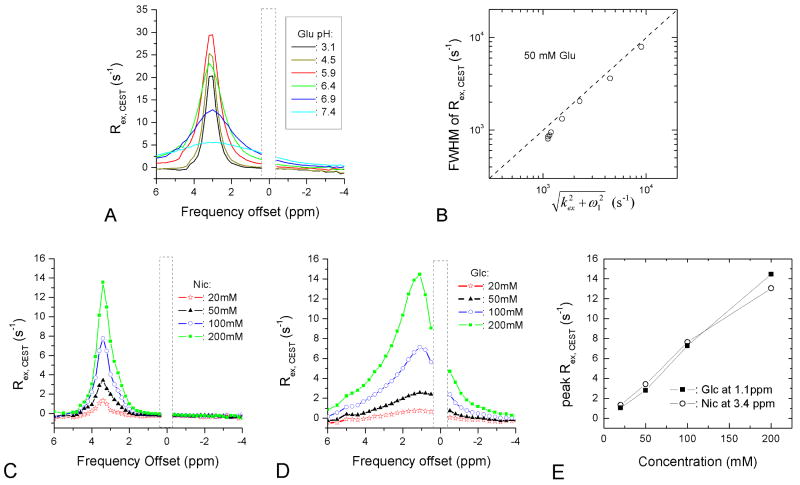
On-resonance R1ρ dispersion provides one way of characterizing the chemical exchange process that is well suited for the intermediate-exchange regime. However, it is difficult to apply to a slow-exchange regime because of reduced sensitivity, and it is also difficult to distinguish from multiple exchanging sites with different chemical shifts. Alternatively, Rex can be obtained from SL Z-spectra (Eq. [11]) or CEST Z-spectra (Eq. [12]). Unlike CEST Z-spectra (see Fig. 4C-D), the Rex, CEST of Glu samples with varied pH showed a peak at 3 ppm (Fig. 8A). The data close to water resonance were not reliable because of the direct water saturation effect and thus were excluded. The broadening of the Rex, CEST curve is sensitive to exchange rates; the FWHM of the Lorentzian shape is highly correlated with (Fig. 8B), where kex is the fitted exchange rate from on-resonance R1ρ dispersion data (Fig. 5A) and the applied ω1, CEST = 1100 rad/s. This indicates that kex can be obtained from CEST Z-spectra (more accurately Rex, CEST).
Fig. 8.
(A) Effective Rex, CEST, defined in Eq. [12], was calculated from the CEST Z-spectra of 50-mM Glu samples with pH between 3.1 and 7.4. The linewidth of Rex, CEST decreases with pH, and the peak of Rex, CEST is reached for the pH = 5.9 sample. The data at frequency offsets close to zero were excluded because of the direct water saturation effect. (B) The linewidths of Rex, CEST in (A) were fairly close to those fit from the on-resonance R1ρ dispersion data. Effective Rex, CEST was also calculated for Nic (C) and Glc (D) samples with four concentrations. The peak of Rex, CEST increases linearly with metabolite concentration (E).
When the linewidth of Rex, CEST is constant (i.e., kex constant), the peak amplitude of Rex is proportional to the labile proton population. Fig. 8C-D shows the Rex, CEST converted from Nic and Glc CEST Z-spectra data with four concentrations (see Fig. 2B and 2E). Although R2 has not been calculated for Nic samples, on-resonance Rex is minimal when ω1, SL ≫ δ and pBkex <1 s-1 (see Eq. 5 and Fig. 7C). Therefore, R2 can be approximated well with the measured R1ρ at a large ω1, SL. The averaged R1ρ is 0.48 ± 0.02 s-1 (n = 4) at ω1, SL = 25120 rad/s (Fig. 2A), so we used R2 = 0.5 s-1 for simplicity. The Rex, CEST values of Nic and Glc show a peak at 3.4 ppm and 1.1 ppm, respectively. The peak magnitude of Rex, CEST increases with concentration almost linearly for both Nic and Glc (Fig. 8E). The averaged FWHM of Rex, CEST for the four Glc is 4272 ± 628 s-1, and consequently, kex is estimated to be 4147 s-1 for a ω1, CEST of 1100 rad/s, slightly smaller than kex = 4680 ± 390 s-1 (n = 4, from Fig. 3B) obtained from the on-resonance R1ρ dispersion data. Nic samples give an averaged Rex, CEST FWHM of 1156 ± 125 s-1, which is not much larger than the applied ω1, CEST, indicating that kex is very small. To accurately determine slow kex, it is necessary to use a small ω1, CEST, similar to or less than kex.
Discussion
Both on- and off-resonance SL approaches can be applied to studies of chemical exchange. While on- and off-resonance SL is sensitive to intermediate exchanges, off-resonance SL can also be tuned to slow exchanges by adjusting ω1, SL (Fig. 5C and 5D). At high magnetic fields, such as 9.4 T, the on-resonance SL is more sensitive to hydroxyl and amine proton exchanges than amide protons, whereas off-resonance SL experiments with a low irradiation power are more sensitive to amide protons. Hence, the parameters of the SL technique, such as the SL pulse power and SL frequency offset, can be adjusted to provide optimal contrast and probe information of the tissue microenvironment for specific applications. When multiple exchangeable protons exist, such as in vivo, it would be difficult to determine the source of a CE contrast in on-resonance SL. Off-resonance SL experiments may be selective to certain types of exchanging protons within the slow-exchange domain, such as the amide protons, by locking the water magnetization on that specific Larmor frequency. However, the interpretation of the observed CE contrast should remain cautious, because other intermediate- or fast-exchanging protons (even if with a different Larmor frequency) can still contribute, due to their broad Rex spectrum.
Since off-resonance SL is similar to CEST, CEST spectra can be used to measure slow- to intermediate-exchange processes and can be explain approximately with the SL theoretical model. This is plausible, because both techniques measure the same chemical exchange phenomena, with slightly different experimental approaches (Fig. 1A). During the long off-resonance RF pulse common to both approaches, the water magnetization experiences an effective B1, tilted at an angle θ = arctan(ω1 / Ω) from the B0 direction. With the SL technique, water magnetization is first flipped to and then locked to the B1, eff direction. In a CEST experiment, without the initial flip, the magnetization along the B1, eff direction relaxes with a time constant T1ρ, and the component perpendicular to the B1, eff oscillates and decays with a time constant T2ρ (27). Thus, a CEST experiment can be considered an off-resonance SL with imperfect spin-locking: the water spins are pseudo-locked to B1, eff, precessing on the surface of a cone with a half angle of θ (Fig. 1D). Such a pseudo-SL can be a good approximation as long as θ is very small; i.e., ω1, CEST ≪ Ω. Thus, to study the chemical exchange effects, the SL technique is more versatile and can be applied to a frequency offset close to water and also for on-resonance cases.
SL and CEST results of simple metabolite phantoms can be explained well using Trott and Palmer's AP model. Previous SL models mostly assumed a fast exchange limit hence could not be applied to slower-exchanging protons. The exchange-related relaxation rate under fast exchange approximation is (24):
| [13] |
Thus, pB and δ cannot be determined separately, so the application is further limited. One simplification taken in Trott and Palmer's model is to use population-averaged values of R1 and R2 for protons of the two exchanging sites and ignore their differences, which may affect the accuracy in the estimation of kex and pB if such differences are significant. Nevertheless, this SL model is quite useful and can be applied to slow-, intermediate-, and fast-exchanging regimes, enabling quantification of CE parameters. The AP model is also compatible with current CEST models. For example, if the SL pulse is applied on the labile proton (Ω = δ), under the conditions kex≪ ω1, SL and R2 ≪ Rex, the steady state solution equation [9] can be simplified to:
| [14] |
which is equivalent to the steady state solution obtained from the CEST experiment (Eq. 23 in (28)). From Eq. [9], one can also find that
| [15] |
If the SL pulse is applied on the labile proton and under the assumption R2 ≪ Rex, the equation above can be converted to
| [16] |
which is identical to the omega-plot equation derived by Dixon et al. (29).
To quantify the concentration of labile non-water protons or the pH of a tissue microenvironment in conventional CEST approaches, McMahon et al. (26) and Sun (30) performed CEST experiments with several different ω1, CEST values and fit the experimental results to the CEST model with a number of assumed parameters. McMahon et al. (26) also proposed to measure the MTR as a function of saturation time and fit to theoretical models. Dixon et al. proposed another method to measure the exchange rate and labile proton population. From Eq. [16], a plot of Moffset / (M0 − Moffset) at a labile proton frequency vs. gives the kex for the X-intercept and the product of kex and pB for the slope. The frequency offset of the targeted labile proton should be known in all these methods.
Our results show that the effective relaxation rate Rex, CEST(Ω), converted from the CEST Z-spectra data, is well suited for the characterization of chemical exchanges in slow and intermediate regimes. Because a complete Z-spectrum is used for data fitting, a priori knowledge of frequency offset of the labile proton is unnecessary. Rex, CEST is proportional to the labile proton population in both slow- and intermediate-exchange regimes, and Rex, CEST peak intensity increases with labile proton concentration. The linewidth of Rex, CEST is closely related to exchange rates and, consequently, pH. Note that for in vivo applications, confounding effects such as magnetization transfer effects from large solid-like macromolecules, also affect the Z-spectra; hence, the extraction and analysis of Rex become much more complicated.
The asymmetrical MTR analysis from the CEST Z-spectra provides a convenient measure of chemical exchange contrast and has been proven to be successful in the slow-exchange regime, but it should be noted that MTRasym is not a monotonic function of kex or pH; for example, it can increase or decrease with kex depending on the choice of saturation pulse power. Under our condition, MTRasym peaks at kex = ω1, CEST; therefore with decreasing kex; MTRasym will decrease for kex < ω1, CEST but increase for kex > ω1, CEST. Thus, the saturation pulse power should be carefully chosen if MTRasym is used as a biomarker to detect in vivo pH changes. A similar issue has also been pointed out in a previous CEST study with numerical simulations (26). In the intermediate-exchange regime, the interpretation of MTRasym is highly complicated. i) The peak offset of MTRasym shifts with varying labile proton concentrations and pHs, making it hard to interpret the data. ii) Because MTRasym is essentially a measure of imaging contrast, it cannot be higher than 100% (9). With increasing concentrations of labile protons, MTRasym does not increase linearly in the slow-exchange regime, but this problem becomes more severe in the intermediate-exchange regime, where it can even decrease at small frequency offsets. iii) If the CEST Z-spectrum is broad and the CE contrast extends to negative offset frequencies beyond the water resonance frequency (see Fig. 2E), the subtraction method for the MTRasym may lead to a significant loss of sensitivity, especially at smaller frequency offsets (see Fig 2E vs. 2F).
One difficulty of in vivo applications of endogenous chemical exchange contrast is its limited sensitivity. The reported MTRasym of amide proton transfer at 3.5 ppm is about 2% for 1.5 T and 4% for 3 T (5,31). To enhance the chemical exchange sensitivity, a larger exchange rate, a larger difference in the Lamor frequencies of exchanging protons, and a higher magnetic field are favorable. Based on our results, 1 mM glucose and glutamate can contribute up to an on-resonance R1ρ of 0.07 and ∼0.1 s-1, repesctively. With an SL B1 of a few hundred Hz and a continuous wave spin-locking pulse length of 50 ms (close to the the T1ρ of brain cortical tissue at 9.4 T (25,32)), this relaxation rate would translate to a signal change of 0.35-0.5%, which could be well detectable by many in vivo experiments.
Conclusions
To compare the characteristics of on- and off-resonance SL and CEST experiments, metabolite phantoms were studied in the slow-, intermediate-, and fast-exchanging regimes and with varied concentrations. The off-resonance SL approach exhibits similar results as the CEST experiment when the direct water saturation effect is small. On-resonance SL is mostly sensitive to intermediate proton exchanges, whereas off-resonance SL and CEST experiments can be tuned to slow-exchanging protons using a low-power SL or saturation pulse. SL and CEST data can be explained well using Trott and Palmer's model with asymmetric population approximation. From the CEST Z-spectra, an effective exchange relaxation rate, Rex, can be constructed and can be used to quantitatively characterize the chemical exchanging process. The conventional parameter MTRasym provides an easy measure of chemical exchange contrast, but unlike Rex, it is not a monotonic function of exchange rate (and pH); its application in the intermediate-exchange regime becomes problematic.
Acknowledgments
We thank Kristy Hendrich for maintaining the 9.4 T system. This work is supported by NIH grants EB008717, EB003324, EB003375, and NS44589.
References
- 1.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) Journal of Magnetic Resonance. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 2.Zhou JY, van Zijl PCM. Chemical exchange saturation transfer imaging and spectroscopy. Prog Nucl Magn Reson Spectrosc. 2006;48(2-3):109–136. [Google Scholar]
- 3.Davis DG, Perlman ME, London RE. Direct Measurements of the Dissociation-Rate Constant for Inhibitor-Enzyme Complexes Via the T-1-Rho and T-2 (CPMG) Methods. Journal of Magnetic Resonance Series B. 1994;104(3):266–275. doi: 10.1006/jmrb.1994.1084. [DOI] [PubMed] [Google Scholar]
- 4.Fischer MWF, Majumdar A, Zuiderweg ERP. Protein NMR relaxation: theory, applications and outlook. patent Part 3-4. 1998 Nov 2;
- 5.Zhou JY, Payen JF, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 6.Sun PZ, Zhou JY, Sun WY, Huang J, van Zijl PCM. Detection of the ischemic penumbra using pH-weighted MRI. Journal of Cerebral Blood Flow and Metabolism. 2007;27(6):1129–1136. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 7.Jokivarsi KT, Grohn HI, Grohn OH, Kauppinen RA. Proton transfer ratio, lactate, and intracellular pH in acute cerebral ischemia. Magnetic Resonance in Medicine. 2007;57(4):647–653. doi: 10.1002/mrm.21181. [DOI] [PubMed] [Google Scholar]
- 8.Liepinsh E, Otting G. Proton exchange rates from amino acid side chains - Implications for image contrast. Magnetic Resonance in Medicine. 1996;35(1):30–42. doi: 10.1002/mrm.1910350106. [DOI] [PubMed] [Google Scholar]
- 9.van Zijl PCM, Jones CK, Ren J, Malloy CR, Sherry AD. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST) Proceedings of the National Academy of Sciences of the United States of America. 2007;104(11):4359–4364. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duvvuri U, Goldberg AD, Kranz JK, Hoang L, Reddy R, Wehrli FW, Wand AJ, Englander SW, Leigh JS. Water magnetic relaxation dispersion in biological systems: The contribution of proton exchange and implications for the noninvasive detection of cartilage degradation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12479–12484. doi: 10.1073/pnas.221471898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regatte RR, Akella SVS, Borthakur A, Kneeland JB, Reddy R. Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: Comparison of T2 and T1 rho. Academic Radiology. 2002;9(12):1388–1394. doi: 10.1016/s1076-6332(03)80666-9. [DOI] [PubMed] [Google Scholar]
- 13.Akella SVS, Regatte RR, Borthakur A, Kneeland JB, Leigh JS, Reddy R. T1 rho MR Imaging of the human wrist in vivo. Academic Radiology. 2003;10(6):614–619. doi: 10.1016/s1076-6332(03)80079-x. [DOI] [PubMed] [Google Scholar]
- 14.Santyr GE, Henkelman RM, Bronskill MJ. Spin locking for magnetic resonance imaging with application to human breast. Mag Reson Med. 1989;12:25–37. doi: 10.1002/mrm.1910120104. [DOI] [PubMed] [Google Scholar]
- 15.Markkola AT, Aronen HJ, Paavonen T, Hopsu E, Sipila LM, Tanttu JI, Sepponen RE. Spin lock and magnetization transfer imaging of head and neck tumors. Radiology. 1996;200(2):369–375. doi: 10.1148/radiology.200.2.8685328. [DOI] [PubMed] [Google Scholar]
- 16.Markkola AT, Aronen HJ, Paavonen T, Hopsu E, Sipila LM, Tanttu JI, Sepponen RE. T1 rho dispersion imaging of head and neck tumors: A comparison to spin lock and magnetization transfer techniques. Journal of Magnetic Resonance Imaging. 1997;7(5):873–879. doi: 10.1002/jmri.1880070516. [DOI] [PubMed] [Google Scholar]
- 17.Poptani H, Duvvuri U, Miller CG, Mancuso A, Charagundla S, Fraser NW, Glickson JD, Leigh JS, Reddy R. T1(rho) imaging of murine brain tumors at 4 T. Academic Radiology. 2001;8(1):42–47. doi: 10.1016/S1076-6332(03)80742-0. [DOI] [PubMed] [Google Scholar]
- 18.Grohn OHJ, Lukkarinen JA, Silvennoinen MJ, Pitkanen A, van Zijl PCM, Kauppinen RA. Quantitative magnetic resonance imaging assessment of cerebral ischemia in rat using on-resonance T-1 in the rotating frame. Magnetic Resonance in Medicine. 1999;42(2):268–276. doi: 10.1002/(sici)1522-2594(199908)42:2<268::aid-mrm8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Kettunen MI, Kauppinen RA, Grohn OHJ. Dispersion of cerebral on-resonance T-1 in the rotating frame (T-1p) in global ischaemia. Applied Magnetic Resonance. 2005;29(1):89–106. [Google Scholar]
- 20.Borthakur A, Gur T, Wheaton AJ, Corbo M, Trojanowski JQ, Lee VMY, Reddy R. In vivo measurement of plaque burden in a mouse model of Alzheimer's disease. Journal of Magnetic Resonance Imaging. 2006;24(5):1011–1017. doi: 10.1002/jmri.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michaeli S, Oz G, Sorce DJ, Garwood M, Ugurbil K, Majestic S, Tuite P. Assessment of brain iron and neuronal integrity in patients with Parkinson's disease using novel MRI contrasts. Movement Disorders. 2007;22(3):334–340. doi: 10.1002/mds.21227. [DOI] [PubMed] [Google Scholar]
- 22.Grohn OHJ, Kettunen MI, Makela HI, Penttonen M, Pitkanen A, Lukkarinen JA, Kauppinen RA. Early detection of irreversible cerebral ischemia in the rat using dispersion of the magnetic resonance imaging relaxation time, T-1p. Journal of Cerebral Blood Flow and Metabolism. 2000;20(10):1457–1466. doi: 10.1097/00004647-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Makela HI, Grohn OHJ, Kettunen MI, Kauppinen RA. Proton exchange as a relaxation mechanism for T-1 in the rotating frame in native and immobilized protein solutions. Biochemical and Biophysical Research Communications. 2001;289(4):813–818. doi: 10.1006/bbrc.2001.6058. [DOI] [PubMed] [Google Scholar]
- 24.Trott O, Palmer AG. R-1 rho relaxation outside of the fast-exchange limit. Journal of Magnetic Resonance. 2002;154(1):157–160. doi: 10.1006/jmre.2001.2466. [DOI] [PubMed] [Google Scholar]
- 25.Jin T, Kim SG. Change of the cerebrospinal fluid volume during brain activation investigated by T1ρ-weighted fMRI. Neuroimag. 2010;51(4):1378–1383. doi: 10.1016/j.neuroimage.2010.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon MT, Gilad AA, Zhou JY, Sun PZ, Bulte JWM, van Zijl PCM. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): pH calibration for poly-L-lysine and a starburst dendrimer. Magnetic Resonance in Medicine. 2006;55(4):836–847. doi: 10.1002/mrm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. Nmr in Biomedicine. 2001;14(2):57–64. doi: 10.1002/nbm.683. [DOI] [PubMed] [Google Scholar]
- 28.Woessner DE, Zhang SR, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magnetic Resonance in Medicine. 2005;53(4):790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 29.Dixon WT, Ren JM, Lubag AJM, Ratnakar J, Vinogradov E, Hancu I, Lenkinski RE, Sherry AD. A Concentration-Independent Method to Measure Exchange Rates in PARACEST Agents. Magnetic Resonance in Medicine. 2010;63(3):625–632. doi: 10.1002/mrm.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun PZ. Simultaneous determination of labile proton concentration and exchange rate utilizing optimal RF power: Radio frequency power (RFP) dependence of chemical exchange saturation transfer (CEST) MRI. Journal of Magnetic Resonance. 2010;202(2):155–161. doi: 10.1016/j.jmr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun PZ, Benner T, Kumar A, Sorensen AG. Investigation of optimizing and translating pH-sensitive pulsed-chemical exchange saturation transfer (CEST) imaging to a 3T clinical scanner. Magnetic Resonance in Medicine. 2008;60(4):834–841. doi: 10.1002/mrm.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makela HI, De Vita E, Grohn OHJ, Kettunen MI, Kavec M, Lythgoe M, Garwood M, Ordidge R, Kauppinen RA. B-0 dependence of the on-resonance longitudinal relaxation time in the rotating frame (T-1p) in protein phantoms and rat brain in vivo. Magnetic Resonance in Medicine. 2004;51(1):4–8. doi: 10.1002/mrm.10669. [DOI] [PubMed] [Google Scholar]