Abstract
Female cancer patients who seek fertility preservation but cannot undergo ovarian stimulation and embryo preservation may consider 1) retrieval of immature oocytes followed by in vitro maturation (IVM) or 2) ovarian tissue cryopreservation followed by transplantation or in vitro follicle culture. Conventional IVM is carried out during the follicular phase of menstrual cycle. There is limited evidence demonstrating that immature oocyte retrieved during the luteal phase can mature in vitro and be fertilized to produce viable embryos. While in vitro follicle culture is successful in rodents, its application in nonhuman primates has made limited progress. The objective of this study was to investigate the competence of immature luteal-phase oocytes from baboon and to determine the effect of follicle-stimulating hormone (FSH) on baboon preantral follicle culture and oocyte maturation in vitro. Oocytes from small antral follicle cumulus-oocyte complexes (COCs) with multiple cumulus layers (42%) were more likely to resume meiosis and progress to metaphase II (MII) than oocytes with a single layer of cumulus cells or less (23% vs. 3%, respectively). Twenty-four percent of mature oocytes were successfully fertilized by intracytoplasmic sperm injection, and 25% of these developed to morula-stage embryos. Preantral follicles were encapsulated in fibrin-alginate-matrigel matrices and cultured to small antral stage in an FSH-independent manner. FSH negatively impacted follicle health by disrupting the integrity of oocyte and cumulus cells contact. Follicles grown in the absence of FSH produced MII oocytes with normal spindle structure. In conclusion, baboon luteal-phase COCs and oocytes from cultured preantral follicles can be matured in vitro. Oocyte meiotic competence correlated positively with the number of cumulus cell layers. This study clarifies the parameters of the follicle culture system in nonhuman primates and provides foundational data for future clinical development as a fertility preservation option for women with cancer.
Keywords: baboon, fibrin-alginate-matrigel, follicle, in vitro follicle growth, in vitro maturation, luteal phase
Baboon luteal-phase cumulus-oocyte complexes and oocytes from cultured preantral follicles can be matured in vitro; oocyte meiotic competence correlates positively with the number of cumulus cell layers.
INTRODUCTION
Hormone stimulation followed by oocyte aspiration, in vitro fertilization (IVF), and embryo cryopreservation is the most common approach for preserving fertility in female cancer patients prior to chemotherapy or radiation [1–5]. However, hormone stimulation requires a delay in cancer treatment, may be contraindicated in patients with hormone-sensitive malignancies, and is not an option for prepubertal girls. As the number of young cancer survivors continues to increase [6, 7], there is a need for fertility preservation options that do not require hormonal stimulation. Retrieval of immature oocytes followed by in vitro maturation (IVM) is suggested as an additional strategy for fertility preservation [8, 9]. Oocyte retrieval for IVM is commonly performed during the follicular phase of the menstrual cycle. Few data indicate whether primate immature oocytes retrieved during the luteal phase can undergo IVM and fertilization and produce viable embryos. Only a few studies describe luteal-phase-like oocyte retrieval performed during cesarean section; these studies reported that immature oocytes recovered during gynecologic surgery could be matured in vitro and fertilized [10–12]. Although a high concentration of circulating progesterone is present during pregnancy, this environment does not recapitulate the ovarian hormonal environment during the luteal phase.
Another potential alternative strategy for cancer patients involves ovarian tissue cryopreservation. Thawed tissue can be orthotopically transplanted [13–16], or immature follicles are retrieved for in vitro follicle growth, IVM, and fertilization [8, 9]. While ovarian tissue transplantation has been successful, it carries a risk of reintroducing malignant cells to the patient [17–19]. As an alternative, in vitro follicle culture techniques in rodent species are successful, and progress is being made in optimizing the system for nonhuman primates and humans.
The development of three-dimensional (3D) matrices for in vitro ovarian follicle culture is central to the success of these techniques thus far [20–22]. It is believed that 3D culture matrices may be able to maintain intercellular communication better within the follicle through the structural support of follicle architecture, specifically the transzonal projections (TZP) between the oocyte and the surrounding cumulus cells, which are necessary for oocyte growth in vitro [23, 24]. The impact of TZP maintenance on the success of IVM has been reported for mouse [25], rat [26], bovine [27], and human [12] but not in baboon. Cumulus cells also play an essential role in promoting oocyte cytoplasmic maturation that is critical for subsequent embryo development. The culture methods that maintain the cumulus-oocyte interaction may support oocyte health.
In this study, we sought to optimize the in vitro follicle culture system and IVM in nonhuman primates by using preantral follicles and cumulus-oocyte complexes (COCs) from small antral follicles isolated from luteal-phase baboon ovaries. We aimed to answer the following questions: 1) Can oocytes from luteal-phase small follicles undergo meiosis and produce viable embryos? 2) Is COC morphology an indicator of oocyte meiotic competence? 3) What criteria should in vitro follicle culture fulfill in order to produce meiotically competent nonhuman primate oocytes?
MATERIALS AND METHODS
Ovary Collection
Ovaries were obtained from six adult cycling baboons during the luteal phase, Days 7–10 postovulation (Table 1). Ovulation was detected by measuring peripheral serum levels of estradiol, beginning 7 days after the first day of menses. The day of the estradiol surge was designated Day −1, with Day 0 as the day of the ovulatory luteinizing hormone (LH) surge and Day 1 as the day of ovulation [28]. Luteal-phase ovaries were confirmed by presence of a corpus luteum. Ovaries were transported to the laboratory at room temperature within 1 h of retrieval. Experiments with animals and tissues were approved by the Animal Care and Use Committees of the University of Illinois at Chicago and Northwestern University, respectively.
TABLE 1.
Baboon information and experimental design.a
COC Isolation and Classification
Ovaries were quartered with a scalpel, and the medulla was separated from the cortex using curved scissors in MOPS-HTF medium (Cooper-Surgical, Trumbull, CT). Small antral follicles (Fig. 1) located on the border between the cortex and medulla were punctured with a 25-gauge needle and gently squeezed to release the COCs. Using a dissecting stereomicroscope, COCs were classified to three groups: 0L-COC, incomplete layer of cumulus cells; 1L-COC, one to two complete layers of cumulus cells; and ML-COC, at least three complete layers of compact cumulus cells. COC classification was performed by a single observer to ensure uniformity of COC types.
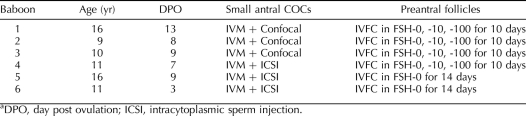
FIG. 1.
A collection of small antral follicles from a luteal-phase baboon ovary. A) Small antral follicles observed under a stereomicroscope (arrowheads). B) Gomori's trichrome-stained section of a luteal-phase baboon ovary with arrowheads indicating the location of small antral follicles on the border of the ovarian cortex and medulla. Bar = 1 mm.
Preantral Follicle Isolation
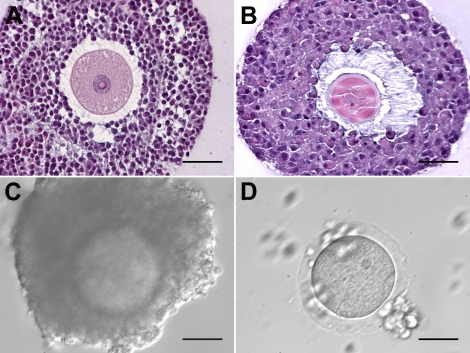
The preantral follicles were isolated as previously described with minor modifications [23]. The ovarian cortex was cut into small pieces (approximately 1–2 mm3), and tissue was enzymatically digested in αMEM (Invitrogen, Carlsbad, CA) containing 1% HSA (Irvine Scientific, Santa Ana, CA), 0.08 mg/ml Liberase Blendzyme 3 (Roche Diagnostics, Indianapolis, IN), and 0.2 mg/ml DNase (Worthington Biochemical, Lakewood, NJ) for 1 h on a shaker inside an incubator at 37°C and 5% CO2. After rinsing the cortex twice with MOPS-HTF medium, follicles were mechanically isolated using a 25-gauge needle into MOPS-HTF medium. The follicles were transferred to maintenance medium (αMEM, supplemented with 1% HSA, 100 IU/ml penicillin, and 100 μg/ml streptomycin) and placed in an incubator at 37°C and 5% CO2. Only preantral follicles (class 1 and 2) [29] that contained a clear, visible, centrally located oocyte; healthy granulosa cells; and no signs of antrum formation were encapsulated and cultured (see Fig. 7A).
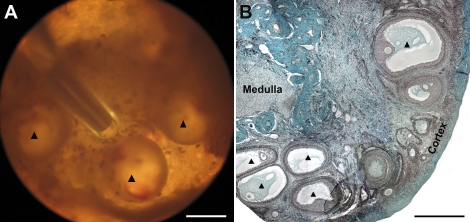
FIG. 7.
Baboon preantral follicle growth in FAM matrix and IVM of recovered oocytes. A) A preantral follicle (223 μm) isolated from the luteal-phase baboon ovarian cortex and encapsulated in FAM. B) After 14 days in culture without FSH, the follicle developed to the small antral follicle stage (667 μm). C) A compact COC was recovered from the FAM matrix for IVM. D) Cumulus cells expanded after 24 h of IVM. E, F) The oocyte resumed meiosis, reached the MII stage within 48 h, and showed normal spindle structure with the first polar body (FPB, black arrow). Bars = 100 μm (A–D), 50 μm (E), 10 μm (F).
Matrix Preparation
Sodium alginate (55–65% guluronic acid) was provided by FMC BioPolymers (Philadelphia, PA), Tisseel was kindly provided by Baxter Healthcare (BioScience Division, Westlake Village, CA), and Growth Factor Reduced BD Matrigel was purchased from BD Bioscience (BD Cat 354234, Bedford, MA). All biomaterials in this study were prepared as described previously [21, 23, 30]. Briefly, sterile alginate aliquots were reconstituted to 0.5% (w/v) in 1× PBS. Fibrinogen was reconstituted to 50 mg/ml in aprotinin (3000 KIU/ml) solution, and thrombin was reconstituted to 50 IU/ml in 50 mM CaCl2/140 mM NaCl, according to kit instructions (Baxter Healthcare). GFR-Matrigel was thawed on ice before use.
Follicle Encapsulation and Culture
In order to maximally deactivate the activity of the enzymes used for follicle isolation, preantral follicles were first embedded in 25% GFR-Matrigel for 1 h as follows [23]. GFR-Matrigel was diluted 1:3 with cold αMEM and added to a V-bottom 96-well plate. After 10 min at room temperature, individual follicles were transferred into each well, and the plate was incubated for 50 min at 37°C and 5% CO2. Follicles were then retrieved from the Matrigel using a blunt tip (Cat 2160G; Molecular BioProducts, Inc.).
The fibrin-alginate-matrigel (FAM) matrix was prepared by mixing 25 μl fibrinogen (50 mg/ml), 25 μl alginate solution (0.5%), 40 μl 1× PBS, and 10 μl GFR-Matrigel. Five to ten follicles were transferred immediately into the FAM mixture with a minimal amount of maintenance medium. Using a 10-μl pipette tip, individual follicles in 5 μl of FAM mixture were pipetted into the 50 IU/ml thrombin solution for crosslinking and gelatinization for 5 min. Fresh FAM mixture was prepared every 30 min until the encapsulation was completed. The crosslinked and gelled FAM beads, each containing a single follicle, were rinsed in maintenance medium and plated one per well in 96-well plates in 100 μl of basal culture medium: αMEM, 3 mg/ml HSA, 1 mg/ml bovine fetuin (Sigma-Aldrich, St. Louis, MO), 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium. Throughout isolation, encapsulation, and plating, follicles were maintained at 37°C and pH 7. Encapsulated follicles were cultured at 37°C in 5% CO2 up to 14 days. Every other day, half the medium (50 μl) was exchanged and stored at −80°C for use in steroid assays.
Follicle Culture in Follicle-Stimulating-Hormone-Containing Medium
In the first experimental culture phase, follicles from four baboons were randomly separated into three groups and grown for 10 days in culture medium supplemented with 0, 10, or 100 mIU/ml recombinant human follicle-stimulating hormone (FSH; NV Organon, Oss, The Netherlands). Follicles were then recovered from matrix, and oocytes underwent IVM. In the second phase, follicles from two baboons were grown for 14 days in the absence of FSH, identified in phase 1 to produce cumulus-surrounded oocytes. Follicles were then recovered, and the oocytes underwent IVM.
IVM
Oocyte maturation was carried out using an IVM kit (Cooper-Surgical) at 37°C in 5% CO2 for 46–48 h. The vendor-supplied IVM medium was supplemented with 100 mIU/ml FSH (NV Organon), 100 mIU/ml LH (Ares Serono, Randolph, MA), 1 IU/ml human chorionic gonadotropin (Sigma), 10 ng/ml epidermal growth factor (Sigma), and 5% (v/v) heat-inactivated fetal bovine serum (Invitrogen).
Ten to 15 of each class of COCs from small antral follicles underwent IVM in an Organ Tissue Culture Dish (60 × 40 mm; Falcon/BD Biosciences, San Jose, CA) containing 1 ml maturation medium covered with embryo-grade mineral oil.
In vitro cultured preantral follicles were first recovered from the FAM matrix beads by incubation in a 10 IU/ml solution of alginate lyase in prewarmed MOPS-HTF medium for 10 min. The COCs were carefully separated from the surrounding follicle using 28-gauge insulin needles, and individual COCs were transferred into a 15-μl droplet of IVM medium covered with embryo-grade mineral oil. After 46–48 h, oocytes were denuded of cumulus cells with 0.3% hyaluronidase and gentle pipetting. Oocyte state was assessed using light microscopy and characterized as follows: germinal vesicle breakdown (GVBD), if the germinal vesicle was not present; germinal vesicle (GV), if there was an intact germinal vesicle; metaphase II (MII), if a polar body was present in the perivitelline space; and degenerated, if the oocyte was fragmented or shrunken.
Intracytoplasmic Sperm Injection and Embryo Culture
Mature oocytes (MII) were inseminated by intracytoplasmic sperm injection (ICSI) with frozen-thawed baboon sperm obtained by electroejaculation from males of proven fertility housed in the colony [31]. Fertilization was evaluated 16–18 h after injection and considered normal if two pronuclei were observed. Embryos were individually cultured for 5 days in a 20-μl drop of embryo culture medium provided in the IVM kit (Cooper-Surgical) under mineral oil at 37°C in 5% CO2.
Follicle and Oocyte Measurement
During the follicle culture period, photographs of each follicle were captured using a Leica DM IL light microscope (Leica, Wetzlar, Germany) equipped with phase objectives, a heated stage, a Spot Insight 2 Megapixel Color Mosaic camera, and Spot software (Spot Diagnostic Instruments, Sterling Heights, MI). Follicle diameters were later measured using ImageJ software (National Institutes of Health, Bethesda, MD) as previously described [32, 33]. Oocyte diameters, minus the zona pellucida, were measured on Day 0, when the oocyte was enclosed in the follicle, and on Days 10 and 14, when the COC had been separated from the surrounding follicle.
Tissue Sectioning and Staining
Ovarian tissues and in vitro cultured follicles were fixed in 4% paraformaldehyde in 1× PBS overnight at 4°C. Follicles were dehydrated in ascending concentrations of ethanol (70–100%) and embedded in paraffin using an automated tissue processor (Leica, Manheim, Germany). Four micrometer sections were cut and stained with Gomori's trichrome [34] for ovarian tissues or hematoxylin and eosin for in vitro cultured follicles.
Immunostaining and Confocal Imaging
Oocytes were fixed and extracted in a microtubule-stabilizing buffer with 4% formaldehyde at 37°C for at least 30 min [35]. To visualize spindles and chromosomes, oocytes were incubated with mouse monoclonal anti-α-tubulin (1:200; Sigma) overnight at 4°C, followed by Alexa Fluor 488-conjugated rabbit-anti-mouse IgG (1:400; Molecular Probes, Eugene, OR) for 1 h at room temperature and then were mounted in VectaShield with 1 μg/ml propidium iodide (Pl; Vector Laboratories, Burlingame, CA). Images were obtained using a Leica TCS SP5X laser scanning confocal microscope (Leica, Manheim, Germany) under a 63× oil immersion objective. For each spindle, a complete Z-axis scan was collected at 0.5-μm intervals, and 3D projection was analyzed on Leica SP5 software.
Statistics
Maturation data were analyzed using one-way analysis of variance, followed by a paired t-test. Spindle data analysis was carried out with pooled data using chi-square analysis. P < 0.05 was considered statistically significant. All statistical calculations were performed using the software GraphPad Prism version 4.0.
RESULTS
In Vitro Maturation of COCs from In Vivo Small Antral Follicle
Meiotic resumption.
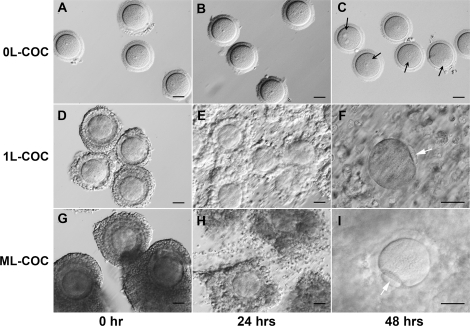
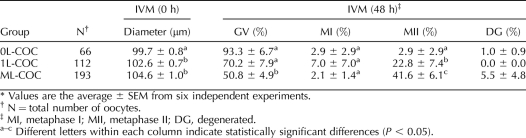
To investigate the correlation between COC morphology and oocyte meiotic competence, we collected 371 COCs from 1–2-mm small antral follicles located on the border between the ovarian cortex and medulla (Fig. 1). COCs were grouped according to the number of cumulus cell layers: 0L, 1L, or ML (Fig. 2, A, D, and G). After 48 h of IVM, the percentage of oocytes from each group that were in GV, MI, or MII stages was determined (Table 2). Most of oocytes in the 0L-COC group remained in the GV stage (Fig. 2, A–C). Cumulus cell expansion was observed after 24 h of IVM in the 1L-COC and ML-COC groups (Fig. 2, E and H), and mature oocytes (MII) were seen after 48 h (Fig. 2, F and I). Significantly more oocytes from the ML-COC group resumed meiosis and progressed to the MII stage (42%) compared with oocytes from the 0L-COC and 1L-COC groups (3% and 23%, respectively; Table 2).
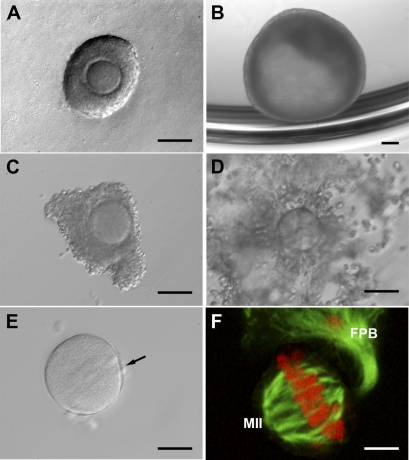
FIG. 2.
Status of small antral follicle COCs status during IVM. A–C) 0L-COC oocytes remained in the GV stage (black arrows) through 48 h of IVM. 1L-COC (D–F) and ML-COC (G–I) oocytes displayed cumulus cell mucification within 24 h and extruded the first polar body (F and I, arrows) by 48 h. Bar = 50 μm.
TABLE 2.
Oocyte status of small antral follicle at 0 and 48 h of IVM.*
Analysis of spindle apparatus of mature oocytes.
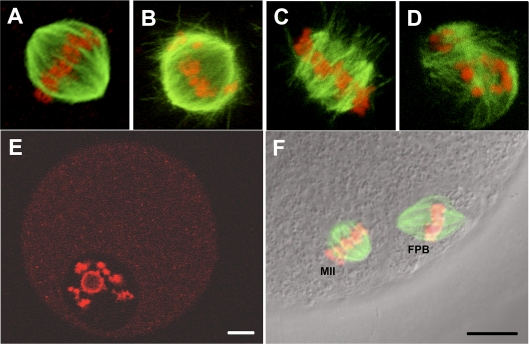
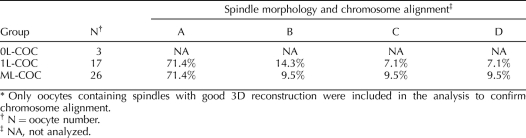
Spindle morphology and chromosome alignment of in vitro matured oocytes from small antral follicle COCs was assessed using a previously described classification system [36]. Figure 3, A–D, shows representative images from the 1L-COC and ML-COC for each of the four classifications of nuclear status: 1) bipolar spindle/aligned chromosomes show bipolar organization with pointed or flattened poles, microtubules converging at both poles, and all chromosomes present and evenly aligned at the equatorial plate (Fig. 3A); 2) bipolar spindle/nonaligned chromosomes show bipolar organization with pointed or flattened poles, microtubules meeting at both poles, and chromosomes with varying degrees of misalignment (Fig. 3B); 3) disarranged spindle/aligned chromosomes show clusters of disorganized microtubules, multipolar spindles, or spindles with microtubules not converging at one or both poles and containing closely aligned chromosomes associated with microtubules (Fig. 3C); and 4) severely disarranged or absent spindle/dispersed or absent chromosomes (Fig. 3D). No statistically significant differences were found between the 1L-COC and ML-COC groups with regard to the percentage of oocytes in each of the four categories (Table 3). All oocytes that did not resume meiosis had surrounded nucleolus (SN), in which the nucleolus is surrounded by a rim of PI-positive chromatin (Fig. 3E). MII oocyte was represented as Figure 3F. There were insufficient numbers of MII oocytes in the 0L-COC group to permit evaluation of nuclear status.
FIG. 3.
Small antral follicle oocyte spindle morphology and chromosome alignment. A) Normal bipolar spindle/aligned chromosome. B) Bipolar spindle/nonaligned chromosome. C) Disarranged spindle/aligned chromosomes. D) Severely disarranged or absent spindle/dispersed or absent chromosomes. E) GV-stage oocytes with surrounded nuclei after 48 h of IVM. F) A mature oocyte with a normal MII spindle and first polar body (FPB). DNA is stained with PI and appears red; α, β-tubulin was stained with Alexa Fluro 488 and appears green. Bar = 10 μm.
TABLE 3.
Spindle morphology and chromosome alignment in oocytes from small antral follicle COCs after 48 h of IVM.*
Fertilization and embryo development in vitro.
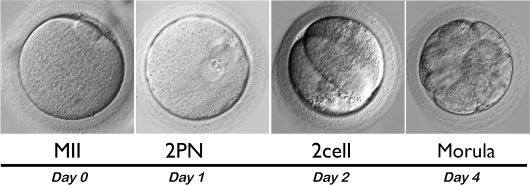
Of the 33 mature oocytes (MII) from the ML-COC group fertilized by ICSI, eight demonstrated normal fertilization with two pronuclei. Four two-cell embryos were obtained on Day 2, and of these, two developed to the morula stage by Day 4 (Fig. 4).
FIG. 4.
Characteristics of in vitro baboon embryo development. A total of 33 MII oocytes resulting from IVM of baboon small antral follicle COCs were fertilized by ICSI; by Day 1, eight had visible pronuclei (2PN); by Day 2, four embryos had reached the two-cell stage; and by Day 4, two embryos had reached the morula stage. Original magnification ×400.
In Vitro Growth and Maturation of Preantral Follicles
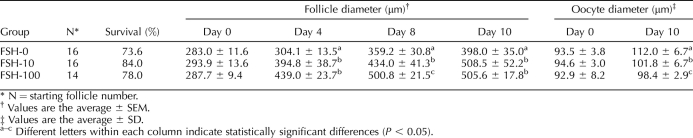
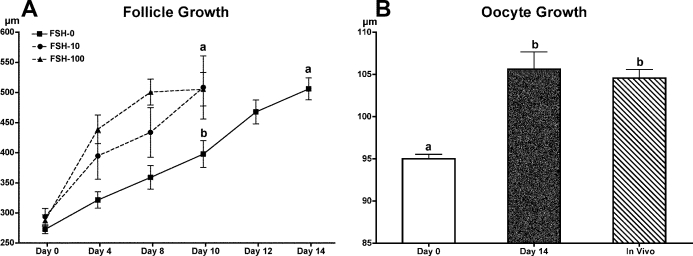
In the first experiment, 46 preantral follicles were encapsulated in FAM and cultured for 10 days in medium supplemented with 0, 10, or 100 mIU/ml FSH (Table 4). The overall survival rates of follicles among the three groups were comparable. Follicles exhibited FSH dose-dependent increases in follicle size. However, exogenous FSH negatively impacted follicle health (Fig. 5); in the presence of 100 mIU/ml FSH, granulosa cells became hypotrophic, and the oocyte lost intercellular connection with the surrounding cumulus cells (Fig. 5B). After 10 days of culture, COCs were recovered from follicles. All oocytes from the 10- and 100-mIU/ml FSH culture groups were already denuded (Fig. 5D), whereas oocytes cultured in the absence of FSH had at least one layer of cumulus cells (Fig. 5C). In addition, oocyte size was negatively correlated with FSH dose in culture (Table 4). Although IVM was performed on all the GV-intact oocytes recovered, none of the oocytes resumed meiosis after 48 h of IVM (data not shown).
TABLE 4.
Results of baboon preantral follicle culture in FAM matrices.
FIG. 5.
Characteristics of in vitro cultured preantral baboon follicles. The follicle cultured in the absence of FSH (A) had a compact COC, whereas the follicle cultured in 100 mIU/ml FSH (B) showed separation of cumulus cells from the oocyte after 10 days. A COC (C) recovered after 10 days of culture in the absence of FSH had multiple cumulus layers, compared with a denuded oocyte (D) recovered from follicles cultured in the presence of FSH. Bars = 100 μm (A, B), 50 μm (C, D).
Based on these results, 31 preantral follicles were encapsulated for the second experiment (Fig. 7A) and cultured for 14 days without FSH. With the additional 4 days of culture, follicles grew to diameters equal to those of follicles that were cultured in the presence of FSH for 10 days (Fig. 6A), and more than 50% of the follicles (17 out of 31) developed to the antral stage (Fig. 7B). In culture, oocytes grew to sizes comparable to those within small antral follicles in vivo (Fig. 6B). IVM was then carried out on 16 of the COCs, which have at least one complete layer of cumulus cells (Fig. 7C). Cumulus cells expanded within 24 h (Fig. 7D). Thirteen of the 16 oocytes exhibited GVBD within 48 h; two MII oocytes were obtained (Fig. 7E) and confirmed as having normal spindle structure and chromosome alignment by confocal microscopy (Fig. 7F).
FIG. 6.
Follicle and oocyte size during in vitro preantral follicle culture. A) Baboon preantral follicles grew continuously for 10 days in the presence of 10 or 100 mIU/ml FSH or for 14 days in the absence of FSH. B) After 14 days of culture without FSH, the average oocyte size increased from 95.0 ± 0.5 μm to 105.6 ± 2.1 μm, similar to the size of oocytes within small antral follicle COCs (in vivo; 104.6 ± 1.0 μm). Different letters indicate statistically significant differences (P < 0.05).
DISCUSSION
In this study, we first demonstrated that baboon oocytes from small antral follicles collected during the luteal phase could be matured in vitro and produce viable embryos. We also found that the meiotic competence of these oocytes was correlated to the number of surrounding cumulus cell layers. These studies provided preliminary data for the optimization of in vitro follicle culture system for nonhuman primate follicles. We subsequently demonstrated that luteal-phase baboon preantral follicles were able to reach the small antral stage after 14 days in culture, with oocytes reaching sizes similar to those from small antral follicles in vivo. The absence of FSH in the culture medium was critical to maintain a compact COC structure. For the first time in nonhuman primates, we also demonstrated that oocytes recovered from cultured follicles are able to undergo IVM and resume meiosis resulting in normal spindle structure.
Previous work in our lab and others revealed that the conditions required to support oocyte maturation in vitro are species dependent. IVM is successful in rodents [37], and immature bovine oocytes from small antral follicles (2–8 mm in diameter) are matured in vitro to produce live offspring [38]. However, the application of IVM to nonhuman primate oocytes is not currently as successful. One study showed that only 29% of baboon immature oocytes, retrieved at the time of cesarean delivery, could progress to the MII stage in vitro [39]. Based on our findings, we speculate that the low IVM rate may be the result of pooling COCs, regardless of the number of cumulus cell layers. We achieved the highest percentage of MII oocytes (41.6%) from multilayer COCs, demonstrating a strong correlation between COC morphology and oocyte meiotic competence. These data support the idea that maintaining the cumulus-oocyte connection is essential for oocyte cytoplasmic maturation. Furthermore, the relative smaller oocyte diameter in the 0L-COC group (∼99.7 μm) indicated that these oocytes might originate from less developed follicles [40, 41], and the lack of surrounding cumulus suggested that they might be from earlier atretic follicles [42]. Compared to the ML-COC group, there were fewer oocytes in the 1L-COC group that progressed to MII. Given that the 1L-COC oocytes had similar size and the same proportion of normal spindle structure and chromosome alignment as the ML-COC ones, those that did mature should have a similar developmental capacity as ML-COC oocytes [41, 43]. Unfortunately, the low number of MII-stage oocytes prevented the direct characterization of fertilization and embryo development of 1L-COC oocytes. Oocytes that did not resume meiosis had an SN, which suggested that they had reached the nuclear meiotic competence. The failure of GVBD may be due to the cytoplasmic incompetence. While a few ML-COC oocytes could be matured in vitro, fertilized, and developed to the morula stage, the overall rate was low, which could also be attributed to insufficient cytoplasmic maturation [35, 44].
In monovular species, it is generally accepted that once the dominant follicle is selected, remaining small antral follicles in the same cohort become atretic. This concept forms the basis for the conventional method of IVM, with oocyte retrievals performed during the follicular phase [41, 45]. However, this dogma is challenged by several recent studies. Two early studies reported that immature oocytes obtained from women undergoing cesarean section could be matured and fertilized in vitro [10, 12]. Because progesterone concentration is high during pregnancy, the hormonal milieu prior to delivery resembles the luteal phase of the menstrual cycle. Other investigators subsequently proved that human immature oocytes retrieved during the luteal phase could be matured in vitro [8, 10, 46], with two four-cell embryos obtained in one study [46]. In the current study, baboon oocytes collected from small antral follicles during the luteal phase could mature in vitro and produced embryos that underwent normal early development. This approach is distinct from those previously described in which COCs were retrieved from large antral follicles (<10 mm) aspirated from the ovarian surface. The ability of luteal-phase baboon small antral follicles to undergo IVM and the impact of the cumulus cells on oocyte development established two benchmarks for producing meiotically competent nonhuman primate oocytes in vitro.
Follicle culture systems are extremely successful in mice [21, 47–50] but have not yet been adapted successfully to human and nonhuman primates [51–58]. Recently, we demonstrated that rhesus monkey [22] and human follicles [23] could grow from the secondary to antral stage in an alginate-based 3D culture system. The cultured follicles contained healthy, growing oocytes that maintained TZPs with the surrounding granulosa cells [23].
Here, we took these studies an additional step forward to demonstrate the capacity of oocytes grown in vitro to reach the MII stage. We chose a semidegradable FAM matrix containing fibrin, alginate, and Matrigel for this study because it provides a dynamic mechanical environment that facilitates follicle outward expansion while maintaining follicle architecture to enhance oocyte quality [30]. Interestingly, exogenous FSH had a negative impact on baboon follicle health and morphology; this was particularly surprising, as our previous studies showed that FSH was necessary for mouse, human, and rhesus preantral follicle development in vitro [22, 23, 59]. In contrast, baboon follicles exposed to FSH in culture lost connections between oocytes and their surrounding cumulus cells and produced significantly smaller oocytes than those from follicles cultured without FSH. Baboon granulosa cell FSH receptors may be more sensitive to human recombinant FSH [60]; it is unknown whether baboon FSH receptors are more like human or rhesus monkey receptors in this respect [61]. We suspect that the premature dispersion of cumulus cells relates to FSH-induced retraction of TZP, which has been reported in the mouse model [62]. Mammalian oocyte development occurs only when tight coordination and interaction between oocyte and cumulus cells is maintained [24, 63, 64]. Thus, while FSH-stimulated follicle growth appeared normal, the retraction of TZP decreased oocyte growth and development and produced fewer oocytes with meiotic competence.
Taken together, we found that the production of meiotically competent baboon oocytes from in vitro follicle cultures could be achieved when 1) the follicle reached at least 0.5 mm, 2) the oocyte grew to 105 μm, and 3) functional cumulus-oocyte connections were maintained. These three morphological criteria could be used as a noninvasive method to determine the point at which nonhuman primate oocytes should be retrieved from the culture matrix for IVM. That said (and perhaps not unexpected), oocytes from in vitro cultured follicles progress to MII oocytes at a lower frequency than oocytes from in vivo small antral follicles. Nonetheless, to the best of our knowledge, this is the first report of primate preantral follicle culture in which MII oocytes with normal spindle structure has been derived. Future work will optimize the culture system and determine whether cultured oocytes can be fertilized and support normal embryo development.
In conclusion, our studies revealed that luteal-phase baboon follicles can be cultured in vitro to produce meiotically competent oocytes. This research represents the next step in the ongoing effort to apply in vitro follicle culture systems to the primate. In time, this technology may be applied to women with cancer who wish to preserve their fertility but who are not candidates for traditional IVF procedures.
Acknowledgments
The authors thank Mark Olson, Nichola Winston, and Patricia Mavrogianis for their assistance in the collection of baboon ovaries and semen and Stacey C. Tobin for editorial assistance.
Footnotes
Supported by grants from the National Institutes of Health to the Oncofertility Consortium (NIH RL1HD058295 and PL1EB008542) and from the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement (U54 HD 40093 to A.T.F.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
REFERENCES
- Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z. Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 2003; 18: 90 95 [DOI] [PubMed] [Google Scholar]
- Rao GD, Chian RC, Son WS, Gilbert L, Tan SL. Fertility preservation in women undergoing cancer treatment. Lancet 2004; 363: 1829 1830 [DOI] [PubMed] [Google Scholar]
- Juretzka MM, O'Hanlan KA, Katz SL, El-Danasouri I, Westphal LM. Embryo cryopreservation after diagnosis of stage IIB endometrial cancer and subsequent pregnancy in a gestational carrier. Fertil Steril 2005; 83: 1041 [DOI] [PubMed] [Google Scholar]
- Oktay K, Buyuk E, Libertella N, Akar M, Rosenwaks Z. Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol 2005; 23: 4347 4353 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol 2006; 24: 2917 2931 [DOI] [PubMed] [Google Scholar]
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med 2009; 360: 902 911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvancarova M, Samuelsen SO, Magelssen H, Fosså SD. Reproduction rates after cancer treatment: experience from the Norwegian radium hospital. J Clin Oncol 2009; 27: 334 343 [DOI] [PubMed] [Google Scholar]
- Demirtas E, Elizur SE, Holzer H, Gidoni Y, Son W-Y, Chian R-C, Tan SL. Immature oocyte retrieval in the luteal phase to preserve fertility in cancer patients. Reprod Biomed Online 2008; 17: 520 523 [DOI] [PubMed] [Google Scholar]
- Huang JYJ, Tulandi T, Holzer H, Tan SL, Chian R-C. Combining ovarian tissue cryobanking with retrieval of immature oocytes followed by in vitro maturation and vitrification: an additional strategy of fertility preservation. Fertil Steril 2008; 89: 567 572 [DOI] [PubMed] [Google Scholar]
- Cha KY, Do BR, Chi HJ, Yoon TK, Choi DH, Koo JJ, Ko JJ. viability of human follicular oocytes collected from unstimulated ovaries and matured and fertilized in vitro. Reprod Fertil Dev 1992; 4: 695 701 [Google Scholar]
- Hwang JL, Lin YH, Tsai YL. Pregnancy after immature oocyte donation and intracytoplasmic sperm injection. Fertil Steril 1997; 68: 1139 1140 [DOI] [PubMed] [Google Scholar]
- Hwang JL, Lin YH, Tsai YL. In vitro maturation and fertilization of immature oocytes: a comparative study of fertilization techniques. J Assist Reprod Genet 2000; 17: 39 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, van Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 364: 1405 1410 [DOI] [PubMed] [Google Scholar]
- Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 2005; 353: 318 321 [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist 2007; 12: 1437 1442 [DOI] [PubMed] [Google Scholar]
- Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod 2008; 23: 2266 2272 [DOI] [PubMed] [Google Scholar]
- Shaw J, Trounson A. Oncological implications in the replacement of ovarian tissue. Hum Reprod 1997; 12: 403 405 [PubMed] [Google Scholar]
- Meirow D, Ben Yehuda D, Prus D, Poliack A, Schenker JG, Rachmilewitz EA, Lewin A. Ovarian tissue banking in patients with Hodgkin's disease: is it safe? Fertil Steril 1998; 69: 996 998 [DOI] [PubMed] [Google Scholar]
- Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra'anani H, Slyusarevsky E, Amariglio N, Schiff E, Rechavi G, Nagler A, Ben Yehuda D. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod 2008; 23: 1007 1013 [DOI] [PubMed] [Google Scholar]
- Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod 2006; 75: 916 923 [DOI] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng 2006; 12: 2739 2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod 2009; 81: 587 594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell ER, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009; 24: 2531 2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SL, Shea LD, Woodruff TK. Noninvasive index of cryorecovery and growth potential for human follicles in vitro. Biol Reprod 2010; 82: 1180 1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ. A comparison between oocyte growth in coculture with granulosa cells and oocytes with granulosa cell-oocyte junctional contact maintained in vitro. J Exp Zool 1979; 209: 345 353 [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Armstrong DT. Role of cumulus cells and serum on the in vitro maturation, fertilization, and subsequent development of rat oocytes. Biol Reprod 1989; 40: 720 728 [DOI] [PubMed] [Google Scholar]
- Boni R, Cuomo A, Tosti E. Developmental potential in bovine oocytes is related to cumulus-oocyte complex grade, calcium current activity, and calcium stores. Biol Reprod 2002; 66: 836 842 [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci U S A 1999; 96: 2543 2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 1996; 17: 121 155 [DOI] [PubMed] [Google Scholar]
- Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 2009; 30: 5476 5485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer NE, McCarthy TJ, Fazleabas AT, Jeyendran RS. Assessment of semen quality in a baboon (Papio anubis) breeding colony. J Med Primatol 1992; 21: 47 48 [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007; 28: 4439 4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod 2009; 80: 432 439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Jaffe RC, Fazleabas AT. Blastocyst invasion and the stromal response in primates. Hum Reprod 1999; 14 (suppl 2): 45 55 [DOI] [PubMed] [Google Scholar]
- Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod 2002; 17: 1006 1016 [DOI] [PubMed] [Google Scholar]
- De Santis L, Coticchio G, Paynter S, Albertini D, Hutt K, Cino I, Iaccarino M, Gambardella A, Flamigni C, Borini A. Permeability of human oocytes to ethylene glycol and their survival and spindle configurations after slow cooling cryopreservation. Hum Reprod 2007; 22: 2776 2783 [DOI] [PubMed] [Google Scholar]
- Schroeder AC, Eppig JJ. The developmental capacity of mouse oocytes that matured spontaneously in vitro is normal. Dev Biol 1984; 102: 493 497 [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Brackett BG. Increased glutamine metabolism in bovine cumulus cell-enclosed and denuded oocytes after in vitro maturation with luteinizing hormone. Biol Reprod 1993; 48: 815 820 [DOI] [PubMed] [Google Scholar]
- Brzyski RG, Leland MM, Eddy CA. In vitro maturation of baboon oocytes retrieved at the time of cesarean section. Fertil Steril 1999; 71: 1153 1156 [DOI] [PubMed] [Google Scholar]
- Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev 1995; 42: 437 442 [DOI] [PubMed] [Google Scholar]
- Peluffo MC, Barrett SL, Stouffer RL, Hennebold JD, Zelinski MB. Cumulus-oocyte complexes from small antral follicles during the early follicular phase of menstrual cycles in rhesus monkeys yield oocytes that reinitiate meiosis and fertilize in vitro. Biol Reprod 2010; 83: 525 532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Loos F, Kastrop P, Van Maurik P, Van Beneden TH, Kruip TA. Heterologous cell contacts and metabolic coupling in bovine cumulus oocyte complexes. Mol Reprod Dev 1991; 28: 255 259 [DOI] [PubMed] [Google Scholar]
- Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Bovine oocyte diameter in relation to developmental competence. Theriogenology 1997; 48: 769 774 [DOI] [PubMed] [Google Scholar]
- Cavilla JL, Kennedy CR, Byskov AG, Hartshorne GM. Human immature oocytes grow during culture for IVM. Hum Reprod 2008; 23: 37 45 [DOI] [PubMed] [Google Scholar]
- Chian RC, Gulekli B, Buckett WM, Tan SL. Priming with human chorionic gonadotropin before retrieval of immature oocytes in women with infertility due to the polycystic ovary syndrome. N Engl J Med 1999; 341: 1624, 1626 [DOI] [PubMed] [Google Scholar]
- Oktay K, Demirtas E, Son W-Y, Lostritto K, Chian R-C, Tan SL. In vitro maturation of germinal vesicle oocytes recovered after premature luteinizing hormone surge: description of a novel approach to fertility preservation. Fertil Steril 2008; 89: 228.e19 e22 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 1989; 41: 268 276 [DOI] [PubMed] [Google Scholar]
- Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod 1994; 9: 527 532 [DOI] [PubMed] [Google Scholar]
- O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68: 1682 1686 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54: 197 207 [DOI] [PubMed] [Google Scholar]
- Abir R, Nitke S, Ben-Haroush A, Fisch B. In vitro maturation of human primordial ovarian follicles: clinical significance, progress in mammals, and methods for growth evaluation. Histol Histopathol 2006; 21: 887 898 [DOI] [PubMed] [Google Scholar]
- Scott JE, Zhang P, Hovatta O. Benefits of 8-bromo-guanosine 3′,5′-cyclic monophosphate (8-br-cGMP) in human ovarian cortical tissue culture. Reprod Biomed Online 2004; 8: 319 324 [DOI] [PubMed] [Google Scholar]
- Scott JE, Carlsson IB, Bavister BD, Hovatta O. Human ovarian tissue cultures: extracellular matrix composition, coating density and tissue dimensions. Reprod Biomed Online 2004; 9: 287 293 [DOI] [PubMed] [Google Scholar]
- Wright CS, Hovatta O, Margara R, Trew G, Winston RM, Franks S, Hardy K. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod 1999; 14: 1555 1562 [DOI] [PubMed] [Google Scholar]
- Hovatta O, Silye R, Abir R, Krausz T, Winston RM. Extracellular matrix improves survival of both stored and fresh human primordial and primary ovarian follicles in long-term culture. Hum Reprod 1997; 12: 1032 1036 [DOI] [PubMed] [Google Scholar]
- Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, Ben Rafael Z. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod 1999; 14: 1299 1301 [DOI] [PubMed] [Google Scholar]
- Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril 2001; 75: 141 146 [DOI] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod 2008; 23: 1151 1158 [DOI] [PubMed] [Google Scholar]
- Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod 2005; 73: 942 950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooghe TM, Mwenda JM, Hill JA. A critical review of the use and application of the baboon as a model for research in women's reproductive health. Gynecol Obstet Invest 2004; 57: 1 60 14745229 [Google Scholar]
- Nyachieo A, Spiessens C, Mwenda JM, Debrock S, D'Hooghe TM. Improving ovarian stimulation protocols for IVF in baboons: lessons from humans and rhesus monkeys. Anim Reprod Sci 2009; 110: 187 206 [DOI] [PubMed] [Google Scholar]
- Combelles CMH, Carabatsos MJ, Kumar TR, Matzuk MM, Albertini DF. Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Mol Reprod Dev 2004; 69: 347 355 [DOI] [PubMed] [Google Scholar]
- Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol 1976; 71: 680 686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol 2000; 226: 167 179 [DOI] [PubMed] [Google Scholar]