Abstract
During erythroid development the embryonic ε-globin gene becomes silenced as erythropoiesis shifts from the yolk sac to the fetal liver where γ-globin gene expression predominates. Previous studies have shown that the ε-globin gene is autonomously silenced through promoter proximal cis-acting sequences in adult erythroid cells. We have shown a role for the methylcytosine binding domain protein 2 (MBD2) in the developmental silencing of the avian embryonic ρ-globin and human fetal γ-globin genes. To determine the roles of MBD2 and DNA methylation in human ε-globin gene silencing, transgenic mice containing all sequences extending from the 5′ hypersensitive site 5 (HS5) of the β-globin locus LCR to the human γ-globin gene promoter were generated. These mice show correct developmental expression and autonomous silencing of the transgene. Either the absence of MBD2 or treatment with the DNA methyltransferase inhibitor 5-azacytidine increases ε-globin transgene expression by 15–20 fold in adult mice. Adult mice containing the entire human β-globin locus also show an increase in expression of both the ε-globin gene transgene and endogenous εY and βH1 genes in the absence of MBD2. These results indicate the human ε-globin gene is subject to multilayered silencing mediated in part by MBD2.
Keywords: GLOBIN GENE, MBD2, DNA METHYLATION, EMBRYONIC GENE SILENCING, RED CELL
Introduction
The genes of the human β-globin locus are located on chromosome 11 in the order of their expression during development: 5′ ε, γ, δ, and β 3′. During development, a sequential switch occurs whereby the respective 5′ globin gene becomes silent and the adjacent downstream gene becomes transcriptionally active. The exact mechanisms of this process are not yet fully understood, however it has been shown to involve multiple interactions between cis elements, erythroid specific trans factors, ubiquitous trans factors, and epigenetic signals [41,42,49,54]. Mice transgenic for human β-type globin genes have provided much insight into the mechanism(s) of developmental globin gene switching. Mice containing the entire β-globin locus as a yeast artificial chromosome (β-YAC) transgene show correct developmental expression and silencing of human globin genes [13,37]. In addition, mice transgenic for smaller β-globin gene locus constructs show similar developmental regulation [51]. These transgenic studies have led to the concept of both competitive and autonomous developmental silencing of β-type globin genes. High level expression of globin genes is mediated by a complex enhancer locus located 5′ of the human ε-globin gene termed the locus control region (LCR). In the competition model, the β-type globin genes compete for LCR enhancer activity with one gene being highly expressed at the expense of the others. According to this model, in the absence of an alternative globin gene, the less competitive gene will still be expressed throughout development. The human β-globin gene is believed to be silenced during embryonic and fetal stage erythropoiesis primarily if not exclusively by this mechanism [9,10,44]. According to the autonomous silencing model, a given β-type globin gene would not be expressed outside of its correct developmental stage even in the absence of other globin genes in the locus. The predominance of published evidence suggests that the human ε-globin gene is regulated in this manner. In the case of mice transgenic for only an ε-globin gene in a construct containing a so-called “mini-LCR” consisting of the major hypersensitive sites of the locus control region, the transgene is significantly but not completely silenced in the absence of other globin genes and it thus has been assumed to be autonomously silenced [39]. The fetal γ-globin gene has been shown to be regulated by a combination of autonomous silencing and competition for the LCR, such that transgenic mice with the γ-globin gene and the LCR in the absence of other globin gene exhibit decreased expression of the transgene during the transition from fetal to adult development, but it is not as completely silenced as the ε-globin gene [2,9,10]. Recently the BCL11A gene product has been shown to exert a major effect on γ-globin developmental gene silencing in embryonic β-YAC transgenic mice [43].
The autonomous silencing of the ε-globin gene is mediated by several different known factors binding to disparate sites near the coding sequences of the gene. Both GATA-1 and YY1 have been shown to bind to distinct regions of the ε-globin gene 5′ flanking sequences and to mediate transcriptional repression [38]. In addition, a complex was identified and shown to bind to two inverted direct repeats in the region of the CCAAT box. These repeats contain a short motif analogous to DR-1 binding sites for non-steroid nuclear hormone receptors [53]. Mutation of these repeats leads to expression of the ε-globin gene in adult β-YAC transgenic mice [53]. A complex binding to this site, termed DRED, was found to contain the nuclear orphan receptors TR2 and TR4 [52]. More recent studies using transgenic β-YAC constructs have demonstrated that either additional flanking sequences of the ε-globin gene or competition for the LCR from downstream globin genes may contribute to its developmental silencing [30]. Thus, it appears that multiple factors contribute to the autonomous silencing of the human ε-globin gene during development in transgenic mice.
Vertebrate globin genes also have been shown to be regulated in part by DNA methylation. The first reports of an inverse correlation between transcription status and DNA methylation were from studies of globin genes in various species [26,27,45,55]. The compound 5-azacytidine inhibits the enzyme DNA methyl transferase-1 and leads to a decrease in DNA methylation levels. Treatment with 5-azacytidine induces the expression of silenced embryonic and fetal β-type globin genes in many different model systems as well as in human patients [3,7,15,22,32]. A family of proteins known as methylcytosine binding domain (MBD) proteins binds to densely methylated DNA and recruits transcriptional co-repressor complexes that include histone deactylases [11,18,29]. One member of this family, methylcytosine binding domain protein 2 (MBD2), has been shown to bind to a densely methylated embryonic ρ-globin construct in vitro as a large complex containing histone deactylase 1 and chromatin remodeling proteins including Mi-2, MTA-1 and RbAp48 [20,48] and it also binds in vivo at the developmentally silenced and methylated ρ-globin gene in adult erythroid cells [20]. Loss of MBD2 results in failure of complete silencing of the human fetal γ- and avian embryonic ρ-globin genes in β-YAC transgenic mice and stably-transfected MEL cells, respectively [19,40]. Short chain fatty acids, compounds known to inhibit histone deactylases, among other functions, have been shown to induce the expression of embryonic and fetal β-type globin genes [5,6,15,23,25,33–35,50]. Thus, in addition to known trans-acting factors that mediate autonomous and competitive silencing, epigenetic modifications contribute to vertebrate globin gene silencing.
Because of the extremely large gradient of silencing of the embryonic globin genes in adult erythroid cells of vertebrates, it has been postulated that multiple, perhaps redundant mechanisms may exist to enforce this process. Herein we present evidence of a role for DNA methylation and MBD2 in mediating human ε-globin silencing in adult transgenic mice in the presence or absence of competing fetal γ and adult β-globin genes. Transgenic mice containing sequences 5′ of HS5 of the LCR and extending 8 kb downstream of the ε-globin gene polyA addition site correctly silence the transgene during erythroid development. When adult transgenic mice containing these constructs are treated with 5-azacytidine they express the normally silenced ε-globin transgene at a 15–20 fold increased level compared to untreated animals. Furthermore, adult human ε-globin gene transgenic mice null for MBD2 also express the transgene at 15–20 fold higher levels than MBD2 wild type controls. Adult β-YAC transgenic mice containing the entire β-globin locus treated with 5-azacytidine or null for MBD2 also express significantly increased but relatively lower absolute levels of ε-globin mRNA. Thus, these findings confirm a role for MBD2 in the methylation mediated silencing of embryonic β-type globin genes of more than one vertebrate species.
Materials and Methods
Generation of Transgenic Mice
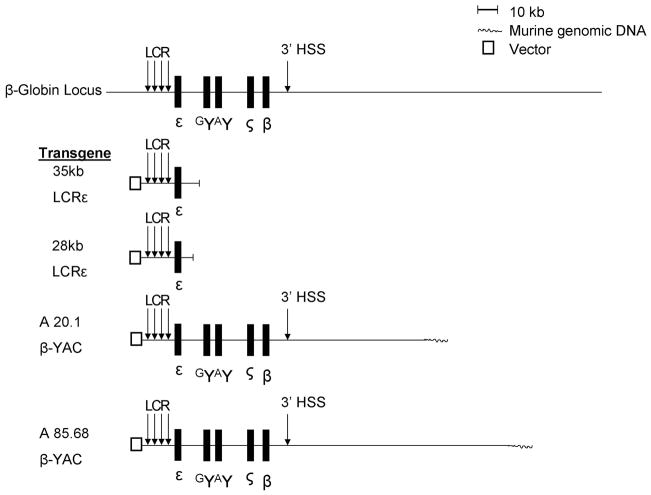
Transgenic mice were generated using two different constructs derived from the cosmid cosLCRε (a generous gift from Frank Grosveld) that contains all the sequence from 5′ of HS5 of the LCR through 12 kb 3′ of the human ε-globin gene polyA site [51] (Figure 1). A 35 kb construct was excised from cosLCRε by Nae I resulting in a 3′ sequence extending to position 33069 in GenBank access no. U01317.1 approximately 1kb upstream from exon 1 of the Gγ-globin gene. A 28 kb construct was generated by digesting cosLCRε with NotI and BstBI to release a fragment containing 4 kb of the 3′ sequence, extending to position 25351 of the GenBank sequence database access no. U01317.1which is located ~8.5 kb 5′ upstream from exon 1 of the human G γ-globin gene. The 35kb construct was run on low-melting agarose and purified with gelase and phenol/chloroform. The 28kb construct was run on a low-melting 0.6% agarose gel and purified using the Bio 101 Gene Clean Spin Kit (Qbiogene, Irvine, California). Constructs were then injected into fertilized eggs and implanted into pseudopregnant mothers to generate transgenic mice. Founders were determined by performing PCR on tail snipped DNA. A total of four independent lines were established. One line of the 28kb constructs had a single copy integration in the Y-chromosome and baseline expression of the human ε-globin gene in this line was too low to reliably measure by real-time pcr. Whether this was due to the fact that there was a single copy integration, or due to a previously described y-chromosome inactivation mechanism {{1687 De Bonis, M.L. 2006}} was not determined. β-YAC mice were a generous gift from Dr. Karin Gaensler. The A20 and A85 lines were used in this work [12,13].
Figure 1. Map of the transgenic β-globin constructs.
The 35kb LCRε and 28 kb LCRε constructs contain sequences beginning 5′ upstream of the β-globin Locus Control Region and extending to the 3′ positions shown relative to the entire locus. The coordinates for the A20.1 and A85.68 β-YAC transgenes have been published [12].
β-YAC, 28kb and 35 kb LCR-ε transgenic mice were bred with MBD2−/− (knock-out) mice to generate hemizygous transgenic mice. These mice were bred with MBD2−/− mice to generate compound transgenic β-YAC, 35kb LCRε and 28 kb LCRε/MBD2−/− mice. Mice were screened for the presence of the transgene and the absence of MBD2 by PCR with tail snip DNA. Copy number for each LCR-ε transgenic line was determined by Southern blot and qPCR. Expression levels of the human ε-globin gene were linearly correlated with copy number. All mice used for gene expression analyses were highly outbred crosses among FVB, C57BL6 and BALB/C strains to minimize inbred strain specific genetic modifier effects.
Expression Analysis of Transgenic Mouse Erythroid Cells
For adult erythroid cell analyses peripheral blood was collected from tail veins. RNA was isolated using Trizol (Invitrogen) per the manufacturer’s protocol.
The developmental regulation of the ε-globin transgene in mice was determined by analyzing expression of the respective transgene at different developmental stages. Timed matings were performed and RNA was extracted from 10.5 dpc yolk sacs, 16.5 dpc fetal livers, and peripheral blood from adult anemic mice.
For each expression analysis, RNA was reverse transcribed using iScript (Bio-Rad) and quantitated by using either Taqman or SYBR green chemistry on an Applied Biosystems 7300Real Time PCR system.
RNA levels from each independent transgenic line were corrected for gene copy number. At least 4 mice from each independent line were assayed for expression and results were analyzed by student’s t-test. The numbers of mice from each of the three independent LCRε lines were balanced in the analysis and at least 8 biologic repeats were performed for each experimental condition. As previously published for γ-globin RNA analyses the two βYAC lines had essentially equivalent expression levels. All reported results were significant at p<0.001 or greater level.
Probes and primers for real-time PCR to detect gene expression level:
-
Human Epsilon RT PCR probe and primers:
Fwd primer: 5′ GCC TTT GCT AAG CTG AGT GAG
Probe: 5′/56Fam TCA AGC TCC TGG GTA ACG TGA TGG TGA
Rev Primer: 5′ TTG CCA AAG TGA GTA GCC AGA A
-
Mouse Glycophorin A RT PCR probe and primers:
Fwd primer: 5′ CTG AAG TGT CTG CTG CGT TTG
Probe: 5′/56Fam AGA ACA GCC TGT CTC ACC ACA CAT TGG A
Rev Primer: 5′ TTG AAT TGG TGA CGG CAT TC
-
Mouse Cyclophilin A RT primers:
Fwd primer: 5′ AGC ATA CAG GTC CTG GCA TCT TGT
Rev Primer: 5′ CAA AGA CCA CAT GCT TGC CAT CCA
-
Probe and primers for mouse βH1:
Fwd primer: 5′ AGAAGCTGGTGATTGGAGTG
Probe: 5′-/56-Fam ATGGTACTTGTGGGACAGAGCATTGG
Rev Primer: 5′ TCATAGACACATGGGATTGCC
-
Probe and primers for mouse εY:
Fwd primer: 5′ TGCTGACTGCTTTTGGAGAG
Probe: 5′/56Fam TGCACTGTGACAAGCTACATGTGGA
Rev Primer: ACCAGCACATTACCCAAGAG
5 Azacytidine Treatment of Mice
Mice were treated for two days with intraperitoneal injection of 1-acetyl-2-phenylhydrazine (10 mg/mL, Sigma, St. Louis, Missouri) at a dose of 0.4 mg/10g. On the third day, mice were treated with 5-azacytidine (0.5 mg/mL, Sigma, St. Louis, Missouri) at a dose of 2 mg/kg for five days via intraperitoneal injection.
Chromatin Immunoprecipitation (ChIP) Assays
Mice were treated for 2 days with 1-acetyl-2-phenylhydrazine. On the fifth day, spleens were harvested and gently brushed into single-cell suspension in ice-cold RPMI containing 2% FBS and 5mM butyrate, PMSF, aprotinin, leupeptin, and pepstatin. Protease inhibitors were included in all steps until the final wash. Cells were then passaged through a 70μM nylon filter to remove debris. Cells were spun and washed with ice-cold PBS containing 2% FBS. Cells were then resuspended in room temperature PBS/FBS. Formaldehyde was added drop wise to a final concentration of 1%, and cells were cross linked for 10 min at room temperature. The reaction was terminated by adding glycine to 0.125 M and incubating at room temperature for 5 min. Cells were washed twice with PBS/FBS. Cells were resuspended in SDS/lysis buffer at a concentration of 25–30 mg/ml and incubated on ice for 10 min. Chromatin was sonicated eight times for 20s to achieve an average DNA fragment size of 700 bp. Twenty-five milligrams of spleen erythroblast chromatin was used per IP with anti-MBD2 (Upstate), and sheep IgG (Upstate). The remainder of the procedure was performed as described previously with slight modifications [40]. Protein G salmon sperm agarose beads (Upstate) were used and were washed three times with low-salt buffer and one time with medium-salt buffer (250 mM NaCl). The relative content of ε-globin gene and control GATA 2 gene DNA in fractions immunoprecipitated with MBD2 antibody versus non-specific IgG was determined by Real-time PCR using SYBR green chemistry as described previously [40]. Each assay was repeated at least 4 times on independent biologic samples and results of the qPCR assays were analyzed by student’s t-test. The sequences of the primers are as follows:
-
Human Epsilon RT primers:
Fwd primer: 5′ AGG ACA GAC AGG CAA GCA AG
Rev Primer: 5′ AGG GGA CAC TGG CTA CTT TG
-
Mouse GATA2 RT primers:
Fwd primer: 5′ TCC ATC CAG CAG CTT TAG GAA
Rev Primer: 5′ GGG TTC GAA GCC ACT CCA A
Results
DNA Methylation and MBD2 Contribute to Silencing of the Human ε-globin Gene
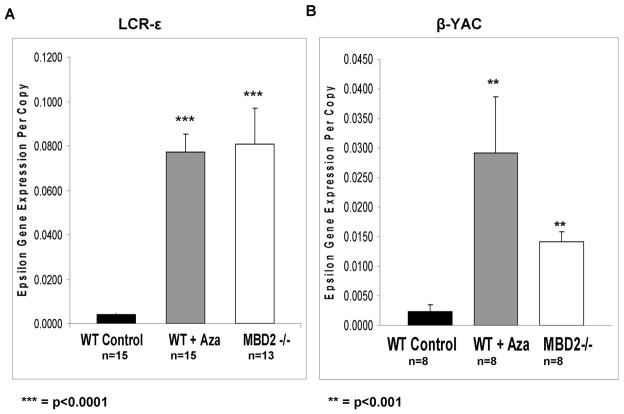
The role of DNA methylation in regulating globin gene expression has been described in a variety of model systems, and in patients [7,15,22,31,32]. In previously published studies of mice with constructs containing a “mini-LCR” sequence and truncated 3′ ε-globin gene flanking sequence, the ε-globin transgene was highly expressed in primitive erythroblasts of the 10.5 dpc yolk sac and largely silent in the definitive cells of the 16.5 dpc fetal liver [39]. We sought to determine whether 5-azacytidine could activate the silenced human embryonic globin gene in the context of 28kb and 35kb LCRε transgenic mice containing all of the sequences extending from the LCR to a site upstream of the γ-globin genes. Two independent transgenic lines of a 28kb LCRε construct and another independent transgenic line of a 35kb LCRε construct that contained an additional 7kb of 3′ downstream sequence extending to the promoter of the γG gene were generated and analyzed (Figure 1). These constructs with intact LCR and upstream ε-globin gene sequences were chosen because of evidence that sequences in the LCR outside of the “mini-LCR” hypersensitive sites contribute to enhancer activity [28]. Mice made anemic via 1-acetyl-2-phenylhydrazine treatment expressed barely detectable levels of RNA from the transgene as determined by quantitative real-time PCR. However, after a five day treatment regimen with 5-azacytidine, adult 28kb and 35kb LCRε transgenic mice express the human ε-globin gene at a level 15–20 fold higher than in untreated control mice (Figure 2A).
Figure 2. Expression of the human ε-globin gene in LCRε and β-YAC transgenic mice.
(A) LCR-ε transgenic mice. Shaded bars represent expression of ε-globin RNA in adult erythroid cells from adult transgenic LCR ε mice wild type controls, wild type treated with 5-azacytidine, or mice homozygous for knockout of MBD2(MBD2−/−) as indicated. Expression was normalized to mouse glycophorin A RNA levels and gene copy number in each experiment. (B) β-YAC transgenic mice. The expression levels shown from β-YAC mice were normalized to mouse glycophorin A RNA levels and gene copy number with the treatment and genotype indicated below each bar. The MBD2 −/− expression data show a 5 fold higher level of human ε-globin expression in LCRε transgenic mice relative to β-YAC transgenic mice (p<0.01). The number of independent erythroid samples assayed and p values determined by student’s t-test are shown in each panel.
Loss of MBD2 leads to expression of the γ-globin gene in adult β-YAC transgenic mice at nearly the same level as in wild-type adult β-YAC mice treated with 5-azacytidine, suggesting that the silencing effects of methylation are predominantly mediated through MBD2 [40]. Therefore, we sought to determine what impact MBD2 had on maintaining repression of the human ε-globin gene in 28 kb LCRε and 35 kb LCRε transgenic mice. Mice null for MBD2 were bred with mice containing either the 28 kb or 35 kb LCRε transgene. The resulting hemizygous mice were bred with MBD2 null mice again to generate transgenic homozygous MDB2 knock-out mice. Adult mice were made anemic with phenylhydrazine treatment to increase erythropoiesis. Blood was collected from the tail vein and analyzed by real time RT-PCR. The hematologic parameters for these highly outbred mice did not differ between wild type and MBD2 knockout backgrounds as previously reported {{85 Rupon, J.W. 2006}} Adult transgenic mice null for MBD2 express the transgene at ~15–20 fold levels higher than wild type transgenic mice, essentially the same level observed in wild type transgenic mice treated with 5-azacytidine (Figure 2A). In β-YAC transgenic mice, loss of MBD2 produced lower levels of human ε-globin mRNA compared to LCRε mice, which was due in part to the single copy of the β-YAC in these mice compared to multiple copies in the LCRε transgenic lines. Interestingly, the level of expression among the different LCRε transgenic lines varied in a linear relationship to copy number (data not shown). However, even when corrected for copy number, the level of ε-globin expression increase in β-YAC mice in an MBD2 knockout background was significantly less than in the LCR-ε transgenic lines. (Figure 2B). These results indicate that DNA methylation and MBD2 contribute to autonomous silencing of the human ε-globin gene in the presence or absence of the other β-type globin genes and their associated downstream sequences, and suggest a 3 to5 fold effect of LCR competition on silencing, since the LCRε transgenes contain the same upstream and downstream flanking sequences extending to the G γ gene as are in the β-YAC.
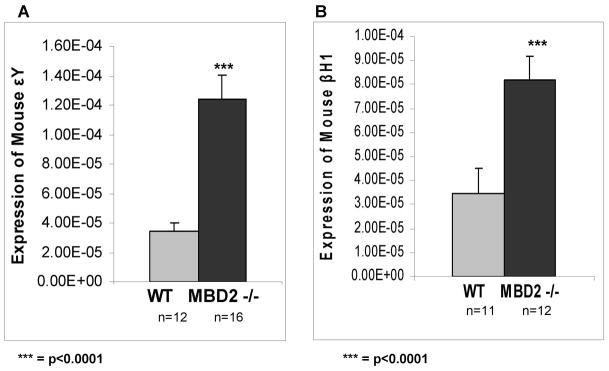
MBD2 Contributes to Silencing of the Endogenous Embryonic Mouse εY and βH1 Genes
In order to determine if there is a role for MBD2 in developmental silencing of the endogenous mouse embryonic βH1 and εY globin genes, the level of transcripts from these genes in wild type and MBD2 knockout mice were assayed by qPCR. As shown in Figure 3, the levels of both εY and βH1 were elevated in adult red cells of MBD2 knockout mice compared to wild type controls. The increased expression is somewhat more pronounced for εY (~4 fold) compared to βH1 (~2.5 fold). While the overall level of expression of these genes in MBD2 knockout adult mice remains extremely low, these results point to a contribution of MBD2 in their developmental silencing within the context of normal endogenous chromatin.
Figure 3. Expression of endogenous mouse embryonic βH1 and εY genes in wild type and MBD2 knockout adult mice.
Light shaded bars show expression levels relative to mouse glycophorin A in wild type adult red cells and dark solid bars show corresponding levels in red cells from adult MBD2 knockout mice. All relative levels were normalized to endogenous glycophorin A gene expression. The number of mice assayed in each experimental group is indicated below each bar.
MBD2 Affects Embryonic Developmental Silencing of the Transgenic Human ε-globin Gene
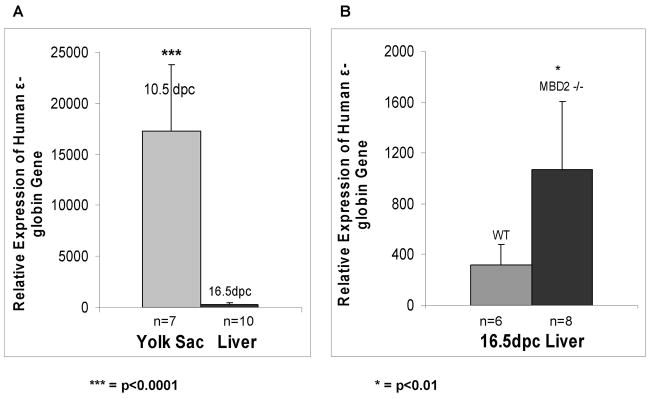
To determine whether the 28 kb and 35 kb LCRε constructs which contain an intact LCR (Figure 1) are autonomously silenced during embryonic development and to explore the impact of MBD2 on silencing, timed matings were performed to analyze expression of the transgenic human ε-globin and the endogenous εY globin gene in 10.5 dpc yolk sacs and 16.5 dpc fetal livers and adult peripheral blood. All timed matings were performed by breeding a transgene positive male with a non-transgenic female. The presence of a vaginal plug was designated 0.5 dpc. Positive embryos were identified by screening placentas for the presence or absence of the transgene by PCR. RNA was isolated from transgene positive yolk sacs and fetal livers from 28 kb LCRε and 35 kb LCRε mice. The amount of human ε-globin RNA was determined by quantitative real-time PCR. As shown in Figure 4, the transgene is highly expressed in 10.5 dpc yolk sac and is significantly silenced (~ 50 fold) by 16.5 dpc. The degree of silencing at dpc 16.5 is about 3–4 fold less in the MBD2 knockout mice. Thus, both the 28 kb and 35 kb LCRε constructs show correct developmental silencing of the transgene in concert with the endogenous embryonic genes confirming that these constructs contain all of the sequences necessary for autonomous silencing, and MBD2 appears to be required for full silencing. Furthermore transcripts from the transgene were detected at a much lower level in the peripheral blood of anemic adult mice (Figure 2).
Figure 4. Delayed silencing of the transgenic human ε-globin gene in MBD2 knockout mice.
RNA from 10.5 dpc yolk sac and 16.5 dpc fetal liver cells was extracted and assayed by real time pcr and normalized to cyclophilin gene expression. (A) The light shaded bar shows expression of the ε-globin gene in dpc 10.5 yolk sac and the dark shaded bar shows expression in dpc 16.5 fetal liver in wild type LCRε transgenic mice as indicated. (B) Expression levels of ε-globin gene expression in 16.5dpc wild-type and MBD2−/− embryonic liver cells.
MBD2 Does Not Bind Directly to the ε-globin Locus in LCRε Transgenic Mouse Erythroblasts
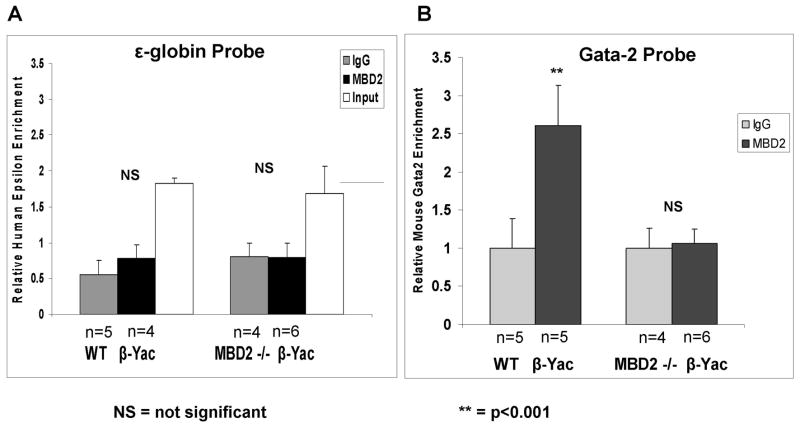
Previous studies have shown that while MBD2 contributes to γ-globin gene silencing in β-YAC transgenic mice, it does not bind proximal to the γ-globin gene sequences [40]. We have postulated that this may be due either an indirect effect of MBD2 binding outside of the β-globin locus or at a distant site in the locus. In order to determine if MBD2 binds at or near the human ε-globin gene in LCRε transgenic mice to mediate silencing, chromatin immunoprecipitation studies were carried out with anemic mouse splenic erythroblasts which demonstrate very strong ε-globin gene silencing that is partially relieved in the MBD2 knockout background. As shown in Figure 5A, there is no enrichment of ε-globin sequences in chromatin pulled down with anti-MBD2 anti-sera despite a clear enrichment in control GATA-2 sequences in the same chromatin fractions (Figure 5B). Thus it appears that, as in the case of the γ-globin gene, MBD2 mediates its silencing effects without binding directly at the ε-globin gene.
Figure 5. Chromatin Immunoprecipitation (ChIP) assay for MBD2 binding to the human ε-globin gene promoter region.
(A) ChIP assays with anti-sera for MBD2 and control IgG were performed on adult splenic erythroblasts from wild type or MBD2 homozygous knockout mice transgenic for the β-YAC construct, and immunoprecipitated ε-globin sequences were quantitated by qPCR. Input DNA was assayed to validate primers. (B) ChIP assays showing a positive control using the same MBD2 anti-sera and control IgG used in the experiments in Panel A but with qPCR probes for GATA 2.
Discussion
Transgenic mice harboring constructs containing the intact sequence extending from HS5 of the LCR through 7 kb 3′downstream of the human ε-globin gene polyA site correctly express and silence the transgene during development. Similar to other transgenic mice harboring an ε-globin gene and a “mini-LCR”, the LCRε mice generated here using a construct with an intact LCR contain all necessary cis elements for strong autonomous silencing but in addition contain all of the distal upstream sequences extending through LCR HS5 as well as all of the downstream sequences extending to but not including the Gγ promoter (Figure 1, see Materials and Methods) [39]. Many factors have been shown to bind to the human ε-globin promoter to mediate transcriptional repressor activity [36,38,52,53]. Previous studies have shown DNA methylation plays a major role in the developmental regulation of the chicken embryonic ρ-globin gene and similarly the baboon ε-globin gene [14,15,20,46–48]. A recent report has shown that the DNA methylation inhibitor decitibine induces increased expression of both the embryonic ε- and fetal γ-globin genes in non-anemic baboons [1]. Here, we show that inhibition of DNA methylation leads to a similar level of increased expression of a silenced human ε-globin transgene in adult mice. Furthermore, loss of the methyl CpG binding protein, MBD2, leads to an equivalent increase in expression of the ε-globin transgene in adult mice, suggesting that the repressive effect of DNA methylation is mediated largely by MBD2 and its associated co-repressor complex, as is the case of the human γ-globin gene [40]. These data demonstrate that MBD2 contributes significantly to developmental silencing of the human ε-globin gene.
Adult transgenic mice harboring the entire β-globin locus as a yeast artificial chromosome express the γ-globin gene after treatment with 5-azacytidine [40]. This had also been shown previously in a transgenic mouse containing a different β-YAC transgene integrated elsewhere in the mouse genome [32]. However, in that work done by Pace et al, expression of the human ε-globin gene was not measured. Interestingly, we find that when treated with 5-azacytidine, adult β-YAC transgenic mice do express very low levels of human ε-globin as measured by quantitative real-time PCR. Importantly treatment with 5-azacytidine led to an 15–20-fold increase in human ε-globin expression in adult LCRε transgenic mice that contain intact β-locus sequences extending from the LCR to the Gγ promoter (Figure 2). Some recent studies have provided evidence that 5-azacytidine induced increase in γ-globin gene expression in primary adult human CD34+ progenitor derived erythroid cells is not due to changes in DNA methylation proximal to the γ-globin genes [24]. However, studies in baboons have demonstrated that while induction of the endogenous γ-globin gene in cultured primary erythroid cells by decitibine (2-deoxy-5-azacytidine), another DNA methylase inhibitor, is not associated with changes in methylation of the γ-globin gene promoter, demethylation is seen in vivo in bone marrow erythroid cells of decitibine treated animals [4].
In this report we show that loss of MBD2 results in an equivalent increase in ε-globin gene RNA as 5-azacytidine treatment, similar to previous studies of the γ-globin gene. Also similar to the γ-globin gene, MBD2 was not found to bind near known regulatory sequences of the silenced transgenic ε-globin gene promoter in adult mice. This result is not unexpected since neither the human γ- or ε-globin gene regions contain any CpG rich sequences of the type that are present in all published instances in which MBD2 has been shown to bind in vivo, such as reported in the case of the avian embryonic ρ-globin gene [20]. To date MBD2 has not been reported to act at a long distance though a strictly cis-acting mechanism.
The data reported here support a model in which, in addition to the previously described autonomous silencing mechanisms mediated by promoter proximal sequences, the human ε-globin gene is also regulated in adult erythroid cells by DNA methylation most likely through MBD2. These data also show for the first time that silencing of the endogenous mouse εY and βH1 genes is mediated in part by MBD2. It has been observed that a large 20kb region encompassing the εY and βH1 genes is hypomethylated in vivo only in primitive erythroid cells in which these genes are expressed [17]. These data are consistent with the observed effect of MBD2 on endogenous εY and βH1 silencing in the context of the entire mouse β-globin locus. The fact that MBD has been shown to bind in vivo only at methylated CpG rich sequences suggests that MBD2 may act through binding at a site or sites outside of the proximal sequences of these relatively CpG sparse genes, as is the case for the human ε- and γ-globin genes. The lower magnitude of the effect of MBD2 on the endogenous mouse β-globin locus compared to the transgenic human locus could be due to several reasons including the presence of additional cis-acting silencer elements in the former but lacking in the human β-YAC construct, the presence of two competing embryonic β-type genes in the mouse locus, or intrinsic differences in the regulation of the mouse locus, such as binding of other methylcytosine binding domain proteins as part of the silencing mechanism.
Conclusions
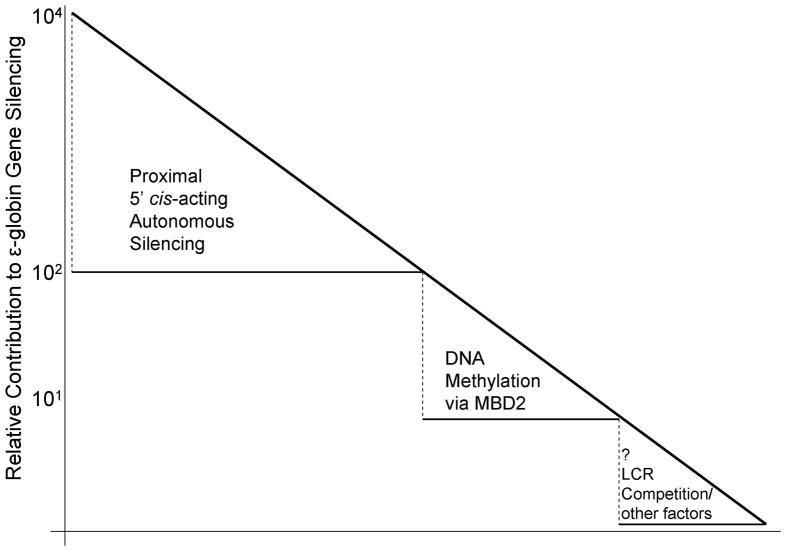
The results here support the existence of a multilayered developmental silencing process for the human embryonic ε-globin gene. As illustrated in Figure 7 the greatest amount of repression appears to be mediated by well described cis-acting sequences located proximal to the ε-globin gene promoter and their associated trans-acting factors. Mutation of these sequences in transgenic mice leads to expression of the human ε-globin gene in adult transgenic mice; however the level of expression appears to be ~1% of yolk sac expression levels [30,52]. Recently the BCL11A gene product has been shown to be an extremely potent mediator of γ-globin gene developmental silencing in β-YAC transgenic mice [41,43]. Knockout of BCL11A results in a much greater increase in endogenous embryonic mouse εY and βH1 gene expression in definitive erythroid cells than MBD2 knockout as observed in the present studies. BCL11A knockout resulted in a much lesser increase in human ε-globin gene expression than for the γ-globin gene in β-YAC transgenic mice, reinforcing the notion that additional silencing mechanisms, likely including LCR competition, are operative in the human locus [43]. Our results indicate that MBD2 contributes to human ε-globin gene silencing in adult transgenic mice, thus documenting another layer of the developmental silencing process for the human ε-globin gene. This result is consistent with previous studies showing that treatment of baboons with 5-azacytidine or decitibine leads to trace levels of ε-globin chains and demethylation of the promoter sequences of the ε-globin gene [1,8,21]. While the contribution of MBD2 is somewhat lesser in magnitude than autonomous silencing through the combined promoter proximal cis-sequences, it contributes another 15–20 fold silencing, which is comparable to each of the individual proximal silencer elements. Finally, the data presented here suggest a smaller but significant (p<0.01) 5-fold level of silencing of the ε-globin gene contributed by competition for the LCR, consistent with a previous report by Navas, et al [30]. While none of the known silencing mechanisms individually account for more than about one percent of the total silencing effect, together these mechanisms combine to produce nearly four orders of magnitude of silencing of the human ε-globin gene in adult transgenic mice, which is similar to the level of silencing of this gene in adult humans (Figure 7).
The intricate multilayered process for ε-globin silencing is important to consider when contemplating pharmacological induction of expression of this gene as a potential strategy to treat patients with hemoglobinopathies. Previous studies have shown that embryonic ε-globin expression can substitute for fetal or adult β-type globin gene expression and potently inhibit sickling in an adult sickle cell mouse model [16]. To effectively increase ε-globin expression to therapeutic levels (~10% of β-globin expression), multiple layers of silencing would have to be overcome. Any effective therapeutic agents would likely have to take into account two or more of the known silencing mechanisms to adequately induce ε-globin mRNA to a level sufficient to ameliorate the pathophysiologic processes in patients with β-globin gene disorders.
Figure 6. Graphic model illustrating contribution of MBD2 to the multiple layers of silencing of the ε-globin gene in adult erythroid cells.
The approximate fold contribution of each illustrated mechanism to the four log level of repression is indicated on the y-axis.
Acknowledgments
The authors thank Dr. Adrian Bird for providing the MBD2 knockout mice and Dr. Karin Gaensler for providing the β-YAC transgenic mice used in these studies and Dr. Jolene Windle, Director of the Transgenic Mouse and Gene Knockout Core at Massey Cancer Center for producing the LCR-ε mice. We also thank Sherida Davis-Bryan for assistance in manuscript preparation and Dr. Joyce Lloyd for critical comments. This work was supported by National Institutes of Health grant DK29902 to Gordon D. Ginder and CA016059 to Massey Cancer Center.
Footnotes
Contributors
Contribution: SZW, JWR, MG and SL performed experiments; JWR, GDG and SZW analyzed results and made figures; JWR and GDG designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeremy W. Rupon, Email: ruponj@email.chop.edu.
Shou Zhen Wang, Email: szwang@vcu.edu.
Merlin Gnanapragasam, Email: gnanapragamn@vcu.edu.
Stefanos Labropoulos, Email: slabropoulos@yahoo.com.
Gordon D. Ginder, Email: gdginder@vcu.edu.
References
- 1.Akpan I, Banzon V, Ibanez V, et al. Decitabine Increases Fetal Hemoglobin in P. Anubis by Increasing gamma-globin Gene Transcription. Exp Hematol. 2010 doi: 10.1016/j.exphem.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behringer RR, Ryan TM, Palmiter RD, Brinster RL, Townes TM. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990;4:380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- 3.Charache S, Dover GJSK, et al. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci USA. 1983;80:4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chin J, Singh M, Banzon V, et al. Transcriptional activation of the gamma-globin gene in baboons treated with decitabine and in cultured erythroid progenitor cells involves different mechanisms. Exp Hematol. 2009;37:1131–1142. doi: 10.1016/j.exphem.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constantoulakis P, Knitter G, Stamatoyannopoulos G. On the induction of fetal hemoglobin by butyrates: in vivo and in vitro studies with sodium butyrate and comparison of combination treatments with 5-AzaC and AraC. Blood. 1989;74:1963–1971. [PubMed] [Google Scholar]
- 6.Dempsey NJ, Ojalvo LS, Wu DW, Little JA. Induction of an embryonic globin gene promoter by short-chain fatty acids. Blood. 2003;102:4214–4222. doi: 10.1182/blood-2002-12-3766. [DOI] [PubMed] [Google Scholar]
- 7.DeSimone J, Heller P, Hall L, Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci USA. 1982;79:4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSimone J, Schroeder WA, Shelton JB, et al. Detection of an epsilon chain in baboons after treatment with 5-azacytidine. Hemoglobin. 1985;9:217–226. doi: 10.3109/03630268508999199. [DOI] [PubMed] [Google Scholar]
- 9.Dillon N, Grosveld F. Human gamma-globin genes silenced independently of other genes in the beta-globin locus. Nature. 1991;350:252–254. doi: 10.1038/350252a0. [DOI] [PubMed] [Google Scholar]
- 10.Enver T, Raich N, Ebens A, et al. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990;344:309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- 11.Feng Q, Zhang Y. The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes. Genes Dev; Genes Dev. 2001;15:827–832. doi: 10.1101/gad.876201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaensler KM, Burmeister M, Brownstein BH, Taillon-Miller P, Myers RM. Physical mapping of yeast artificial chromosomes containing sequences from the human beta-globin gene region. Genomics. 1991;10:976–984. doi: 10.1016/0888-7543(91)90188-k. [DOI] [PubMed] [Google Scholar]
- 13.Gaensler KM, Kitamura M, Kan YW. Germ-line transmission and developmental regulation of a 150-kb yeast artificial chromosome containing the human beta-globin locus in transgenic mice. Proc Natl Acad Sci USA. 1993;90:11381–11385. doi: 10.1073/pnas.90.23.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginder GD, Gnanapragasam MN, Mian OY. The role of the epigenetic signal, DNA methylation, in gene regulation during erythroid development. Curr Top Dev Biol; Curr Top Dev Biol. 2008;82:85–116. doi: 10.1016/S0070-2153(07)00004-X. [DOI] [PubMed] [Google Scholar]
- 15.Ginder GD, Whitters MJ, Pohlman JK. Activation of a chicken embryonic globin gene in adult erythroid cells by 5-azacytidine and sodium butyrate. Proc Natl Acad Sci USA. 1984;81:3954–3958. doi: 10.1073/pnas.81.13.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Z, Russell JE. A human embryonic hemoglobin inhibits Hb S polymerization in vitro and restores a normal phenotype to mouse models of sickle cell disease. Proc Natl Acad Sci USA. 2002;99:10635–10640. doi: 10.1073/pnas.162269099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu M, Mabaera R, Lowrey CH, Martin DI, Fiering S. CpG Hypomethylation in a Large Domain Encompassing the Embryonic {beta}-Like Globin Genes in Primitive Erythrocytes. Mol Cell Biol. 2007;27:5047–5054. doi: 10.1128/MCB.02234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones PL, Veenstra GJ, Wade PA, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 19.Kransdorf EP. Purification and Characterization of a Methyl-DNA Binding Complex from Primary Erythroid Cells. Virginia Commonwealth University; 2004. [Google Scholar]
- 20.Kransdorf EP, Wang SZ, Zhu SZ, et al. MBD2 is a critical component of a methyl cytosine-binding protein complex isolated from primary erythroid cells. Blood; Blood. 2006;108:2836–2845. doi: 10.1182/blood-2006-04-016394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavelle D, Chin J, Vaitkus K, et al. Oral decitabine reactivates expression of the methylated gamma-globin gene in Papio anubis. Am J Hematol. 2007;82:981–985. doi: 10.1002/ajh.21020. [DOI] [PubMed] [Google Scholar]
- 22.Ley TJ, DeSimone J, Anagnou NP, et al. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982;307:1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- 23.Little JA, Dempsey NJ, Tuchman M, Ginder GD. Metabolic persistence of fetal hemoglobin. Blood. 1995;85:1712–1718. [PubMed] [Google Scholar]
- 24.Mabaera R, Greene MR, Richardson CA, et al. Neither DNA hypomethylation nor changes in the kinetics of erythroid differentiation explain 5-azacytidine’s ability to induce human fetal hemoglobin. Blood. 2008;111:411–420. doi: 10.1182/blood-2007-06-093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marianna P, Kollia P, Akel S, et al. Valproic acid, trichostatin and their combination with hemin preferentially enhance gamma-globin gene expression in human erythroid liquid cultures. Haematologica. 2001;86:700–705. [PubMed] [Google Scholar]
- 26.Mavilio F, Giampaolo A, Care A, et al. Molecular mechanisms of human hemoglobin switching: selective undermethylation and expression of globin genes in embryonic, fetal, and adult erythroblasts. Proc Natl Acad Sci USA. 1983;80:6907–6911. doi: 10.1073/pnas.80.22.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGhee JD, Ginder GD. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature; Nature. 1979;280:419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- 28.Molete JM, Petrykowska H, Bouhassira EE, et al. Sequences flanking hypersensitive sites of the beta-globin locus control region are required for synergistic enhancement. Mol Cell Biol. 2001;21:2969–2980. doi: 10.1128/MCB.21.9.2969-2980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nan X, Ng HH, Johnson CA, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature; Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 30.Navas PA, Li Q, Peterson KR, Stamatoyannopoulos G. Investigations of a human embryonic globin gene silencing element using YAC transgenic mice. Exp Biol Med(Maywood) 2006;231:328–334. doi: 10.1177/153537020623100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nienhuis AW, Ley TJ, Humphries RK, Young NS, Dover G. Pharmacological manipulation of fetal hemoglobin synthesis in patients with severe beta-thalassemia. Ann N Y Acad Sci. 1985;445:198–211. doi: 10.1111/j.1749-6632.1985.tb17189.x. [DOI] [PubMed] [Google Scholar]
- 32.Pace B, Li Q, Peterson K, Stamatoyannopoulos G. alpha-Amino butyric acid cannot reactivate the silenced gamma gene of the beta locus YAC transgenic mouse. Blood. 1994;84:4344–4353. [PubMed] [Google Scholar]
- 33.Pace BS, White GL, Dover GJ, et al. Short-chain fatty acid derivatives induce fetal globin expression and erythropoiesis in vivo. Blood. 2002;100:4640–4648. doi: 10.1182/blood-2002-02-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrine SP, Ginder GD, Faller DV, et al. A short-term trial of butyrate to stimulate fetal-globin-gene expression in the beta-globin disorders. N Engl J Med; N Engl J Med. 1993;328:81–86. doi: 10.1056/NEJM199301143280202. [DOI] [PubMed] [Google Scholar]
- 35.Perrine SP, Miller BA, Greene MF, et al. Butryic acid analogues augment gamma globin gene expression in neonatal erythroid progenitors. Biochem Biophys Res Commun. 1987;148:694–700. doi: 10.1016/0006-291x(87)90932-6. [DOI] [PubMed] [Google Scholar]
- 36.Peters B, Merezhinskaya N, Diffley JF, Noguchi CT. Protein-DNA interactions in the epsilon-globin gene silencer. J Biol Chem. 1993;268:3430–3437. [PubMed] [Google Scholar]
- 37.Peterson KR, Zitnik G, Huxley C, et al. Use of yeast artificial chromosomes (YACs) for studying control of gene expression: correct regulation of the genes of a human beta-globin locus YAC following transfer to mouse erythroleukemia cell lines. Proc Natl Acad Sci USA. 1993;90:11207–11211. doi: 10.1073/pnas.90.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raich N, Clegg CH, Grofti J, Romeo PH, Stamatoyannopoulos G. GATA1 and YY1 are developmental repressors of the human epsilon-globin gene. EMBO J. 1995;14:801–809. doi: 10.1002/j.1460-2075.1995.tb07058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raich N, Enver T, Nakamoto B, et al. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990;250:1147–1149. doi: 10.1126/science.2251502. [DOI] [PubMed] [Google Scholar]
- 40.Rupon JW, Wang SZ, Gaensler K, Lloyd J, Ginder GD. Methyl binding domain protein 2 mediates {gamma}-globin gene silencing in adult human betaYAC transgenic mice. Proc Natl Acad Sci USA. 2006;103:6617–6622. doi: 10.1073/pnas.0509322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 42.Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. Br J Haematol. 2010 doi: 10.1111/j.1365-2141.2010.08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sankaran VG, Xu J, Ragoczy T, et al. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460:1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sargent TG, Buller AM, Teachey DT, McCanna KS, Lloyd JA. The gamma-globin promoter has a major role in competitive inhibition of beta-globin gene expression in early erythroid development. DNA Cell Biol. 1999;18:293–303. doi: 10.1089/104454999315358. [DOI] [PubMed] [Google Scholar]
- 45.Shen CK, Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci USA; Proc Natl Acad Sci USA. 1980;77:6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singal R, Ferris R, Little JA, Wang SZ, Ginder GD. Methylation of the minimal promoter of an embryonic globin gene silences transcription in primary erythroid cells. Proc Natl Acad Sci USA. 1997;94:13724–13729. doi: 10.1073/pnas.94.25.13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singal R, vanWert JM, Ferdinand L., Jr Methylation of alpha-type embryonic globin gene alpha pi represses transcription in primary erythroid cells. Blood. 2002;100:4217–4222. doi: 10.1182/blood-2002-02-0457. [DOI] [PubMed] [Google Scholar]
- 48.Singal R, Wang SZ, Sargent T, Zhu SZ, Ginder GD. Methylation of promoter proximal-transcribed sequences of an embryonic globin gene inhibits transcription in primary erythroid cells and promotes formation of a cell type-specific methyl cytosine binding complex. J Biol Chem. 2002;277:1897–1905. doi: 10.1074/jbc.M105580200. [DOI] [PubMed] [Google Scholar]
- 49.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stamatoyannopoulos G, Blau CA, Nakamoto B, et al. Fetal hemoglobin induction by acetate, a product of butyrate catabolism. Blood. 1994;84:3198–3204. [PubMed] [Google Scholar]
- 51.Strouboulis J, Dillon N, Grosveld F. Developmental regulation of a complete 70-kb human beta-globin locus in transgenic mice. Genes Dev. 1992;6:1857–1864. doi: 10.1101/gad.6.10.1857. [DOI] [PubMed] [Google Scholar]
- 52.Tanabe O, Katsuoka F, Campbell A, et al. An emryonic/fetal beta-type globin gene repressor contains a nuclear receptor TR2/TR4 heterodimer. EMBO J. 2002;21:3434–3442. doi: 10.1093/emboj/cdf340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanimoto K, Liu Q, Grosveld F, Bungert J, Engel JD. Context-dependent EKLF responsiveness defines the developmental specificity of the human epsilon-globin gene in erythroid cells of YAC transgenic mice. Genes Dev. 2000;14:2778–2794. doi: 10.1101/gad.822500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet. 2009;18:R216–23. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Ploeg LH, Flavell RA. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980;19:947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]