Abstract
D-Aspartate (D-Asp) can substitute for L-Glutamate (L-Glu) at excitatory Glu receptors, and occurs as free D-Asp in the mammalian brain. D-Asp electrophysiological responses were studied as a potential correlate of aging in the California sea hare, Aplysia californica. Whole cell voltage- and current clamp measurements were made from primary neuron cultures of the pleural ganglion (PVC) and buccal ganglion S cluster (BSC) in 3 egg cohorts at sexual maturity and senescence. D-Asp activated an inward current at the hyperpolarized voltage of −70 mV, where molluscan NMDA receptors open free of constitutive block by Mg2+. Half of the cells responded to both D-Asp and L-Glu while the remainder responded only to D-Asp or L-Glu, suggesting that D-Asp activated non-Glu channels in a subpopulation of these cells. The frequency of D-Asp-induced currents and their density were significantly decreased in senescent PVC cells but not in senescent BSC cells. These changes in sensory neurons of the tail predict functional deficits that may contribute to an overall decline in reflexive movement in aged Aplysia.
Keywords: A. californica, voltage clamp, D-Asp, glutamate, agonist, NMDA
1. Introduction
L- amino acids, ubiquitous in proteins where homochirality is important for proper folding, are often assumed to be the isomers with receptor binding roles in neurotransmission. Glutamate, L-Glu, is the main excitatory neurotransmitter of the central nervous system (CNS) of vertebrates and invertebrates (Antzoulatos and Byrne, 2004; Collingridge and Lester, 1989) and is the best studied free amino acid with a neurotransmitter role. Free D-amino acids such as D-aspartate (D-Asp) and D-serine (D-Ser), however, are also are found in the brains of both vertebrates and invertebrates, including some species of Aplysia (D’Aniello et al., 1993; Furuchi and Homma, 2005; Spinelli et al., 2006; Williams et al., 2006), where their role is less clear. D-Ser and D-Asp are source material for manufacture of their left hand enantiomers L-Ser and L-Asp, respectively (Fujii and Saito, 2004). Recently D-Ser and D-Asp have been proposed as candidate neurotransmitters (reviewed in Schell, 2004; D’Aniello, 2007) based on fulfillment of a number of criteria necessary for such a role, including the machinery for synthesis and degradation near the proposed site of action, and the release from nerve terminals (Kuffler et al., 1984). Both D-Ser and D-Asp are agonists at specific Glu receptors, which include the subtypes termed N-methyl-D-Aspartate (NMDA), alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid (AMPA) and kainate receptors. All of these Glu receptors have associated ion channels that open when agonist is bound. D-Ser substitutes for glycine as a co-agonist at NMDA receptors (Fuchs et al., 2005). D-Asp, structurally similar to Glu, is hypothesized to substitute for L-Glu at excitatory Glu receptors of unidentified subtype (Miao et al., 2006). Direct electrophysiological evidence for the actions of D-Asp at Glu channels, however, is very scarce, and the identity of the site of action is not known. We are pursuing studies in the California sea hare, Aplysia californica, subsequently termed Aplysia, that support a neurotransmitter role for D-Asp.
L-Glu, D-Asp and D-Ser levels are all altered in human brains affected by Alzheimer’s Disease (Chen and Lipton, 2006; D’Aniello et al., 1998; Fisher et al., 1994). This study focused on D-Asp-induced electrophysiological responses as a further correlate of changes observed in neurotransmitter levels and their actions in the aging brain. We hypothesized that D-Asp- or L-Glu-induced ion currents, with the latter known to undergo changes in vertebrate animals associated with AD and other diseases of aging, will show differences in mature vs. senescent Aplysia. Aplysia is a convenient model for such studies due to its short lifespan and its well-studied and greatly simplified nervous system. This species of Aplysia is an annual animal with a predictable period of senescence characterized by declining responsiveness, reproduction, appetite and weight (Gerdes and Fieber, 2006), followed by death at an age of one year. These features, combined with a wide understanding of its nervous system anatomy and physiology, should make Aplysia an excellent model for determining how changes in function and gene expression in select groups of neurons are altered by the aging process.
D-Asp-activated channels are present in specific cells of the Aplysia CNS, namely in the ventral caudal neurons of the pleural ganglion (PVC) and buccal ganglion S cluster (BSC) neurons. These neuron clusters are populations of mechanoafferent sensory neurons serving principally the tail and buccal mass, respectively, and each cluster appears to exhibit homogeneous physiological responses (Walters et al., 2004). Both have been used in studies of long-term synaptic plasticity (Chin et al., 1999). In situ hybridization studies show that these cells possess NMDA receptor subunits (Ha et al., 2006), making them good candidates for investigation of possible Glu receptor-channel activation by D-Asp, a reasonable starting point for understanding the effects of D-Asp. To address this, we designed a multi-cohort, prospective study to compare electrophysiological responses in animals from peak sexual maturity and senescence.
2. Results
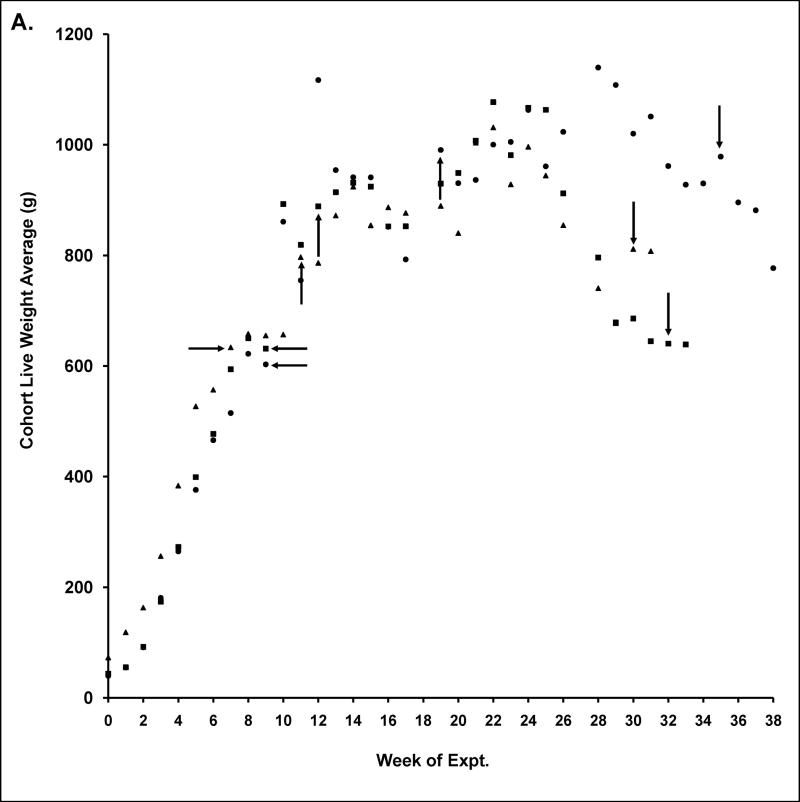
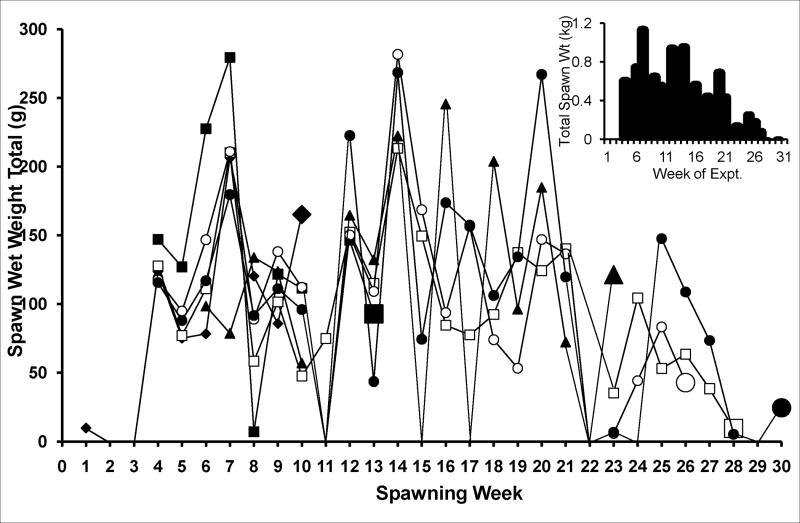
Aplysia development to sexual maturation is marked by steady growth in length and weight, followed by copulation and egg laying (Fieber, 1998; Fieber et al., 2005), as illustrated in Fig. 1. Animals achieved maximal body mass during sexual maturity, despite the investment in spawn in this hermaphroditic species (Fig. 1A). Although spawn weights produced were irregular, the period of maximal spawning began 3–9 wks after first spawning (Fig. 1B). Once the peak of body mass and spawn mass were attained, the morphological and anatomical indices of senescence were observed. These were significantly decreased body mass from peak values (Fig. 1A; 2 of 3 egg masses p≤0.05 Wilcoxon signed rank test), significantly decreased egg production (Fig. 1B; all 3 egg masses p≤0.05 Mann-Whitney U test), decreased appetite, and loss of muscle tone. Some cell morphological differences also were observed in neurons cultured from mature and senescent animals, as illustrated in Fig. 2. Cultured PVC neurons from animals at the peak of sexual maturity often elaborated axons and other processes in culture (Fig. 2A), while those cultured from senescent animals oft en lacked cell processes (Fig. 2B). In a subset of PVC cells in which both photographic data, for evidence of processes, and capacitance data, for cell size, were collected, 50% of mature (n=12), but only 19% of senescent cells (n=16) had processes. The cell body of senescent PVC cells often appeared larger in diameter than mature PVC cell bodies. For all cells in which capacitance was studied (n=112), however, capacitance measurements of mature and senescent PVC neurons were not significantly different (Table 1). BSC neurons, in contrast, elaborated processes when cultured from animals at both maturity and senescence (data not shown). In addition, senescent BSC neurons had a significantly larger capacitance (Table 1).
Fig. 1. Animal husbandry of Aplysia californica used in the study.
A. Average live weight of animals in each egg mass over the course of the study ±SE. Horizontal arrows denote first egg mass from newly sexually mature adults. Upward arrows denote time of first sacrifices at peak of maturity, while downward arrows denote first sacrifices at senescence.
B. Total weekly spawn weight in 6 cages of animals, summarizing spawn of the 30 animals in egg mass 3. Larger symbols designate when animals in that cage were sacrificed for electrophysiological experiments at maturity and senescence. Insert: total weekly spawn weight for this egg mass.
Fig. 2. Cultured PVC sensory neurons from Aplysia.
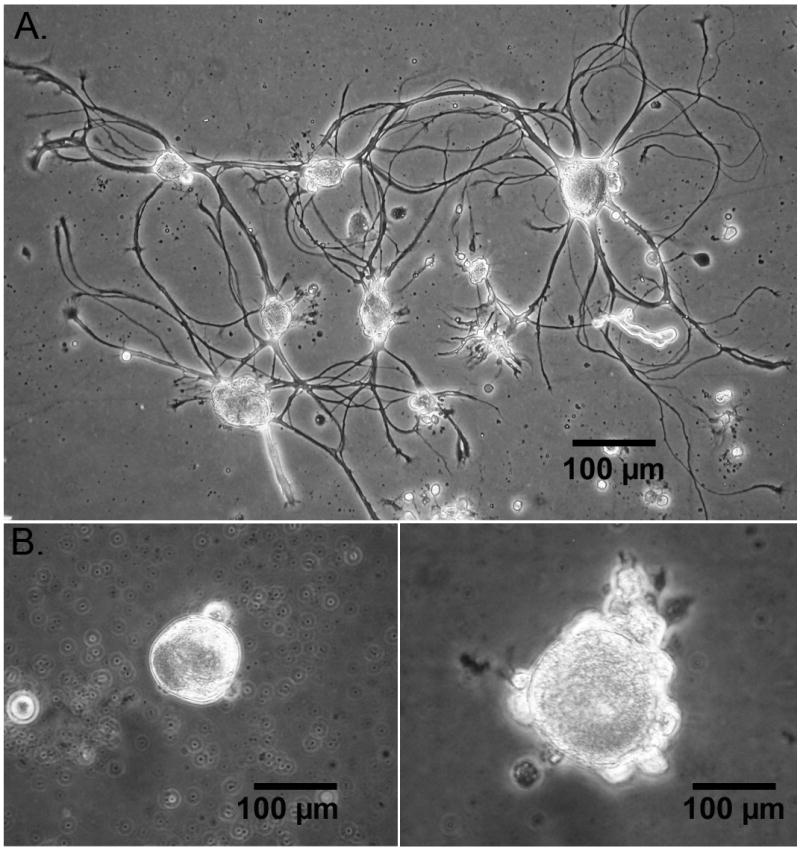
A. Cells from animal at peak of sexual maturity.
B. Cells from a senescent animal.
Table 1.
Comparison in mature and aged PVC and BSC neurons of biophysical characteristics and D–Asp-induced responses at −70 mV.
| Resting Potential (mV) | Cell Capacitance (pF) | % Cells with D-Asp Currents | D-Asp Current Density (pA/pF) | |||||
|---|---|---|---|---|---|---|---|---|
| Mature | Senescent | Mature | Senescent | Mature | Senescent | Mature | Senescent | |
| Egg mass | Pleural Ganglion Ventral-caudal Neurons | |||||||
| 1 | −13±18 (8) | −28±6.9 (33) | 124±47.7 (9) | 90.5±23.6 (38) | 100 (7) | 39.4 (38) | 1.30±0.989 (7) | 0.102±0.183 (35) |
| 2 | −28±13 (3) | −27±7.3 (17) | 71.6±26.1 (8) | 82.9±22.5 (17) | 71.4 (7) | 70.6 (17) | 1.07±1.03 (8) | 0.349±0.535 (16) |
| 3 | −23±7.3 (5) | −27±6.8 (31) | 101±42.6 (12) | 82.4±20.2 (28) | 100 (12) | 84.6 (26) | 0.958±1.05 (5) | 0.612±0.570 (25) |
| mean | −19±0.060 (16) | −27±0.0010 (81) | 100±0.100 (29) | 86.2±0.00388 (83) | 92.3 (26) | 60.4 (81) | 1.07±0.00244 (20) | 0.468±0.000949 (76) |
| Egg mass | Buccal Ganglion S Cluster Neurons | |||||||
| 1 | −33±10 (17) | −23±10 (8) | 113±13.3 (23) | 171±9.69 (21) | 65.0 (20) | 83.3 (18) | 1.63±1.34 (12) | 1.35±1.57 (14) |
| 2 | −25±7.4 (6) | −29±8.4 (13) | 77.1±31.8 (6) | 143±86.9 (16) | 66.7 (6) | 90.9 (11) | 4.65±2.44 (4) | 1.59±1.75 (10) |
| 3 | −24±4.2 (6) | −27±9.0 (5) | 87.8±39.2 (7) | 102±44.2 (10) | 87.5 (8) | 85.7 (7) | 3.93±4.37 (6) | 2.06±1.17 (7) |
| mean | −29±0.11 (29) | −25±0.013 (26) | 102±0.0838 (36) | 147±0.0396 (47) | 70.6 (34) | 86.1 (36) | 2.80±0.00589 (22) | 1.586±0.00159 (31) |
Cohort Mean values±1 standard deviation; overall Mean values±1 standard error (except for cohort mean frequency of D-Asp currents); numbers in parentheses are cell sample size
Mean values shown in boldface and underlined are significantly different between mature and senescent at p≤0.05 based on 2-sample T-test or G-test (G-test of independence (Sokal and Rohlf, 1981) used for % cells with D-Asp currents)
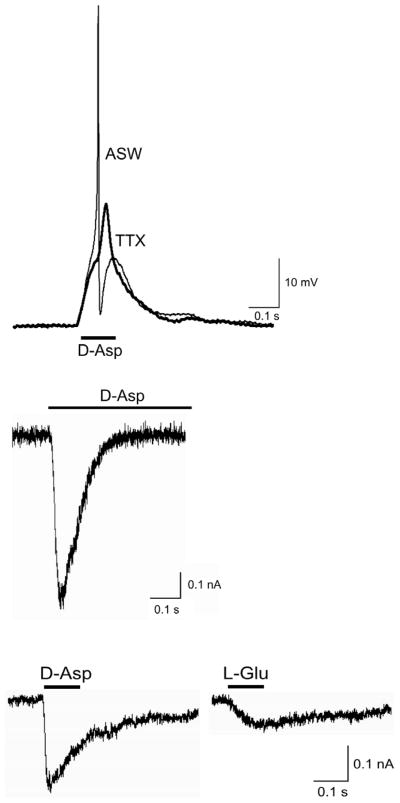
D-Asp-evoked action potentials, shown in Fig. 3A (ASW), were observed in response to either bath- or picospritzer pulse-application of 1 mM D-Asp in ASW, but not to ASW alone (not shown). No visible excitatory postsynaptic potential (epsp) preceded the impulse. When D-Asp-induced depolarizations were elicited in the presence of tetrodotoxin (TTX; 100 nM) a depolarization attributable directly to application of the agonist was observed (Fig. 3A, TTX). In many cells, this type of subthreshold depolarization was observed in response to application of D-Asp alone. This verifies that D-Asp can act as an excitatory agonist in Aplysia neurons of the pleural and buccal ganglia. A full description of this current, including its ion selectivity and pharmacological identity is in progress (Carlson and Fieber, unpublished data).
Fig. 3. Excitatory responses to bath application of D-Asp or L-Glu.
A. Current clamp responses in a PVC neuron to D-Asp in ASW (1 mM; bar) and in TTX + ASW (100 μM; bold). Resting potential −33 mV. B. Whole cell voltage clamp D-Asp-induced current in PVC neuron at −70 mV.
C. Whole cell currents in a BSC neuron at −70 mV activated consecutively by bath-applied D-Asp (1 mM) and L-Glu (1 mM).
D-Asp activated ion currents under voltage clamp appeared distinct from L-Glu receptor currents in many cells. D-Asp induced an inward current in voltage-clamped cells at the hyperpolarized potential of −70 mV that inactivated during agonist application, as shown in Fig. 3B. A majority of BSC and PVC cells from sexually mature animals responded to D-Asp with an excitatory current (Table 1). Although L-Glu also elicited an inward current at −70 mV in many of these same neurons, and L-Glu currents superficially resembled D-Asp-induced currents, as shown in Fig. 3C, not all cells respond to both agonists. For example, in PVC cells exposed alternately to D-Asp and L-Glu, approximately 50% of cells responded to both L-Glu and D-Asp (21 cells from 5 animals) while the remainder responded only to D-Asp (22 cells from 4 animals) or L-Glu (3 cells from 1 animal).
The frequency of D-Asp-induced currents was significantly different in mature and senescent PVC cells, with fewer currents in senescent cells (Table 1; p<0.001, pooled G test; comparison of egg masses using a heterogeneity G calculation indicated no significant differences between batches, allowing results to be pooled for all masses). Current density also was significantly lower in senescent PVC cells. The average resting potential was higher in senescent than in mature PVC cells. Capacitance, a measure of cell size, was significantly higher in senescent BSC neurons than in mature BSC neurons.
In contrast to variations in some D-Asp currents, there were no obvious differences in either PVC or BSC neurons with age in density of Na+ and Ca2+ channels or in the biophysical characteristics of these voltage-gated channels such as activation voltage, sensitivity to holding potential or time to inactivation. For example, fifteen mature and 15 senescent PVC neurons from animals of all 3 egg masses were compared. Mean Na+ current densities were not significantly different at 0.068±0.056 (n=15) and 0.10±0.080 (n=15) nA/pF (mean±SD), respectively, with activation at approximately −40 mV from Vh of −70 mV, and V1/2 of approximately −30 mV in both mature and senescent neurons. Mean Ca2+ current densities were not significantly different at 0.013±0.011 (n=10) and 0.015±0.012 (n=15) nA/pF (mean±SD) in mature and senescent PVC cells from all 3 masses, respectively, with activation at approximately −25 mV from Vh of −70 mV, and V1/2 of approximately −25 mV in both mature and senescent neurons.
Furthermore there were no apparent differences observed in L-Glu currents, which were less common in these neurons than D-Asp currents, as noted above.
3. Discussion
Free bulk D-Asp is abundant in the CNS of both vertebrates and invertebrates. D-Asp has been proposed to have a role in neural development, based on its abundance in neonatal rat brain that declines dramatically after birth (Sakai et al., 1998), yet persists in the adult (Schell et al., 1997). In adults, D-Asp appears to have an endocrine role, stimulating the release of hormones from the hypothalamus, pituitary, and testes (D’Aniello, 2007). Although D-Asp has not been tracked in larval or early juvenile Aplysia, free D-Asp is present in the CNS of adult Aplysia species (Zhao and Liu, 2001; Miao et al., 2005; Spinelli et al., 2006).
We have demonstrated a subpopulation of neurons in Aplysia characterized by D-Asp-activated channels that are insensitive to L-Glu. D-Asp-induced whole cell currents have been documented in Aplysia (Miao et al., 2005) and in other species (Brown et al., 2007; Gong et al., 2005); responses to L-Asp in Aplysia californica and A. dactylomela also have been documented (Carpenter et al., 1977). D-Asp has been hypothesized to act as a partial or full agonist at L-Glu receptors of the NMDA, kainate, or AMPA receptor subtypes, depending on the preparation. In support of the ability of D-Asp to act at specific Glu receptors, the relative potency of D-Asp at NMDA-like receptors has been documented (Verdoom and Dingledine, 1988). D-Asp had additional actions opposite that of an excitatory agonist, acting as an antagonist at AMPA receptors (Gong et al., 2005), and activating a Cl− conductance as a by-product of uptake by the Glu transporter (Carpenter et al., 1995; Huang et al., 2004). The idea of a receptor-channel activated by D-Asp but not L-Glu is untested thus far.
D-Asp is known to activate NMDA-like receptors (Verdoom and Dingledine, 1988), and the electrophysiological characteristics of NMDA-like receptors have been described in molluscan species (Moroz et al., 1993). Certain characteristics of the D-Asp current documented here share similarities with molluscan NMDA-like receptor currents. The D-Asp-activated current was inward at −70 mV in cultured Aplysia BSC and PVC neurons. This voltage is hyperpolarized compared to the resting potential of −40 to −55 mV observed in PVC and BSC cells in reduced preparations in which the synapse was intact (Walters et al., 1983a; Walters et al., 2004). At this voltage, current through NMDA receptors of vertebrates would be negligible due to Mg2+ block of the channel (Mayer and Westbrook, 1987). In contrast, molluscan NMDA-like receptors in both marine and freshwater species are free of constitutive block by Mg2+ (Moroz et al., 1993), and open at hyperpolarized potentials. While this may be crucial to NMDA receptor function in marine mollusks, in which the Mg2+ concentration of hemolymph is high (> 10 mM), in freshwater species the requirement for Mg2+ independent function is not as clear, since in these species hemolymph Mg2+ is lower (≤ 1 mM; Gustafson et al., 2005; Shakhmatova et al., 2006). An apparent independence from constitutive block of the channel by Mg2+ ions at the resting potential allies the channel studied here with NMDA channels in other molluscs such as Lymnaea, but sets it apart from mammalian NMDA receptors (Dingledine et al., 1999). The observation that half of Aplysia neurons from pleural ganglia had D-Asp induced currents unresponsive to L-Glu suggests that the D-Asp channel observed in many PVC cells may be unique, but closely related to molluscan NMDA channels.
Both NMDA-like and AMPA-like receptors, termed ApNR1 and ApGluR1 and ApGluR2, respectively, have been cloned from Aplysia (Ha et al., 2006; Li et al., 2009), and named for homology with vertebrate receptors in the pore forming regions (Sprengel et al., 2001). These receptors share with mammalian homologues 95% identity of the pore regions important for ion conduction and 80% identity of the ligand binding domains, suggesting they will show biophysical similarities to vertebrate receptors in these properties. In situ hybridization localized ApNR1 to neurons within most Aplysia ganglia, including PVC and BSC neurons (Ha et al., 2006). Thus it is possible that the D-Asp receptor-channel studied here is ApNR1. Because successful expression and physiological characterization of the cloned receptors has not yet occurred, due possibly to heteromultimeric composition of the native receptors (Hutton et al., 1991), characterization of native receptors in intact neurons may be the best way to identify them physiologically. A detailed characterization of D-Asp induced currents in BSC cells is in progress (Carlson and Fieber in preparation).
The tail withdrawal reflex of Aplysia consists of monosynaptic sensory-motoneuron pairs in which the sensory neuron mechanoefferent located in the PVC is believed to be directly excited by tail touch or shock (Walters et al., 1983a). This tail stimulus is transmitted to effector motoneurons in the nearby pedal ganglion that directly contract the tail. L-Glu released at the synapse causes excitation of the motoneuron. L-Glu is the only hormone known to be involved in the unmodified circuit (Antzoulatos and Byrne, 2004), because the sensory neuron is activated by electrical stimulation at the tail, and synaptic connections to either other sensory neurons in the PVC or interneurons are unlikely (Walters et al., 1983b); in this regard, PVC cells are regarded as a homogeneous population. The sensory-motoneuron synapse is, however, capable of being modulated by hormones whose actions result in facilitation (serotonin, Walters et al., 1983b) or synaptic depression of the reflex (NMDA, Walters et al., 1983b; L-Glu, FMRFamide, Xu et al., 1994; Aplysia Mytilus inhibitory peptide-related peptide, McDearmid et al., 2002), and it is possible that D-Asp-induced excitatory currents in PVC cells such as those documented here also play a modulatory role.
Without additional functional information about the synapses involving PVC cells, the higher resting potential of senescent PVC cells suggests only that these cells were healthy at the time of experiments.
Although much less is known about the function and role of BSC neurons, they are also mechanosensory cells whose axons travel to the periphery through the buccal nerves, and appear to innervate the buccal mass and perioral area (Fiore and Geppeti, 1981; Walters et al., 2004). BSC cells respond to strong physical stimulation with long-duration impulses characterized by large afterhyperpolarizations, and, sometimes, afterdischarges (Walters et al., 2004). The requirement for chemical neurotransmission of the peripheral mechanical stimulus to the BSC terminals, as well as the identity of any neurotransmitter or modulating hormone acting on sensory BSC cells is unknown. The role of the D-Asp response in BSC cells remains to be elucidated, but it may serve to modulate BSC cell function. Dopamine is the excitatory neurotransmitter of the consummatory neural feeding circuit of Aplysia (Baxter and Byrne 2006), to which BSC cells contribute based on these prior studies. Modulation of this circuit includes synaptic facilitation and depression, leaving open the possibility that neurotransmitters such as those described for modulation of tail withdrawal, and possibly, D-Asp, contribute.
Certain significant changes observed in senescent PVC and BSC neurons suggest they may be associated with a functional deficit with age, such as the decrease in D-Asp-induced current density and decreased current frequency of PVC cells derived from senescent animals. A decrease in an excitatory current like the D-Asp current could imply a decline in excitatory modulation of PVC cells with age, or a relief from inhibitory modulation for which D-Asp currents provide negative feedback. Either scenario may contribute to a change in reflexive movement in aged Aplysia. Aged Aplysia have impaired righting and tail withdrawal reflexes (Kempsell and Fieber, unpublished data), suggesting that aging-related behavioral changes may reflect changes in nervous system function and not merely muscle wasting.
The absence of changes in D-Asp-induced currents in BSC neurons with aging suggest either that no such changes occur in BSC cells, or that these cells are aging at a different rate than PVC cells (Moroz and Kohn, in press). Other changes likely had no significance for the aging phenomenon. Thus senescent BSC cells were significantly larger probably because molluscan neurons grow as the animal grows (Croll and Chiasson, 1989), but may not shrink with aging as the body does. Mature animals’ cells were measured before the growth peak, while the senescent animals’ cells were measured after the peak. No such difference in capacitance with aging was observed in PVC cells. Despite an apparently larger cell body in senescent cells, the dearth of cell processes in aged PVC cells, which ordinarily contribute to the capacitance measurement, may explain the lack of significant difference in size.
Studies have shown that brain glutamatergic receptors or their agonist affinities declined during aging in humans and rodents, and were correlated with motor deficits (Segovia et al., 2001). Although the role of D-Asp in ionotropic Glu receptor physiology and pathology is unknown, D-Asp-induced ion currents declined with aging in the Aplysia model system. Given the ease of the manipulation of the aging process in Aplysia and the large background on its nervous system function, Aplysia should be a useful model for studies on aging-related changes in brain neurotransmitter-activated ion currents.
4. Experimental Procedures
4.1. Animals
Animals were selected from three unrelated cohorts (egg masses) reared at the National Resource for Aplysia in Miami, Florida. Animals within each cohort were half- or full siblings from matings of wild–collected parents housed in pairs. Cohort ages at the beginning of the experiment were 4.75, 4.75 and 4.25 mos for egg masses 1, 2 and 3, respectively. Thirty offspring per egg mass were housed 5 per cage and fed ad lib on a mixed diet of the red algae Aghardiella sp. and Gracilaria ferox. The lifespan of animals from all 3 cohorts under these rearing conditions was approximately one year, as expected from Capo et al. (2002). The animals were weighed once per week, and upon reaching sexual maturity the egg masses in each cage were weighed daily to plot the animals’ passage through adulthood into senescence. Egg laying began at ages 6.25–6.5 months during October, within the reproductive period of wild Aplysia (Audesirk, 1979). Animals from each cohort were sampled at the peak of sexual maturity (≥3 weeks after the beginning of egg laying, Capo et al., 2003) and during senescence defined according to Gerdes and Fieber (2006). Whole cell recordings were made from 2–7 animals per cohort of BSC and PVC neurons at sexual maturity and senescence.
4.2 Electrophysiological recording
Primary cultures of PVC and BSC neurons were prepared according to the methods in Fieber (2000). Animals were anesthetized for 1 hr in a 1:1 aerated solution of isotonic MgCl2 and seawater. Cells were dissociated onto 35 × 10 mm polystyrene culture plates (Falcon) coated with poly-D-lysine, and stored at 17°C in a humidified atmosphere until used in experiments 24–72 hours later. Whole cell voltage clamp and current clamp measurements were made using glass patch electrodes containing intracellular solution consisting of (mM) 458 KCl, 2.9 CaCl2 (2 H2O), 2.5 MgCl2 (6 H2O), 5 Na2ATP, 1 EGTA, and 40 HEPES-KOH, pH 7.4. Voltage and current data were collected and whole-cell capacitance and series resistance compensations were made using Axopatch 200B or 200A clamp amplifiers, with capacitance compensation ranges of 1–100 pF and 101–1000 pF, respectively, each connected to a PC and Digidata 1200 A/D converter using pClamp software to record data and issue voltage and current commands. Control artificial seawater (ASW) contained (mM) 417 NaCl, 10 KCl, 10 CaCl2 (2 H2O), 55 MgCl2 (6 H2O), 15 HEPES-NaOH, pH 7.6, and also was the extracellular solution used to study voltage gated Na+ currents and D-Asp-induced currents. Ba-Cs- tetraethylammonimum solution consisting of (mM): 11 BaCl2, 10.45 CsCl, 460 tetraethylammonimum, 10 HEPES-CsOH, 55 MgCl2 (6 H2O), pH 7.6 was used to study Ba2+ through voltage gated Ca2+ channels. These solutions were flowed onto cells during recording via a gravity-fed sewer pipe system that dispensed solution from a 1 μl micropipette near the cell. Solutions containing agonist (e.g. D-Asp in ASW) were briefly applied at 2 min intervals via sewer pipe or via micropipette attached to a picospritzer powered by a stream of N2 adjustable for force and duration. All reagents were from Sigma-Aldrich.
4.3 Statistical analysis
Significant differences were at p≤0.05 using Mann-Whitney U test, Wilcoxon signed rank test, G-test, T-test or ANOVA with Sheffe post hoc test.
Acknowledgments
Funded by NIH P40RR01029, the Korein Foundation, and a University of Miami Fellowship to SLC. The authors gratefully acknowledge the staff of the National Resource for Aplysia.
Abbreviations
- AMPA
alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid
- BSC
buccal ganglion S cluster
- CNS
central nervous system
- D-Asp
D-Aspartate
- D-Ser
D-Serine
- L-Glu
L-Glutamate
- NMDA
N-methyl-D-Aspartate
- PVC
pleural ventral caudal
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Antzoulatos EG, Byrne JH. Learning insights transmitted by glutamate. Trends Neurosci. 2004;27:555–560. doi: 10.1016/j.tins.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Audesirk TE. A field study of growth and reproduction in Aplysia californica. Biol Bull. 1979;157:407–421. doi: 10.2307/1541026. [DOI] [PubMed] [Google Scholar]
- Baxter DA, Byrne JH. Feeding behavior of Aplysia: A model system for comparing cellular mechanisms of classical and operant conditioning. Learn Mem. 2006;13:669–680. doi: 10.1101/lm.339206. [DOI] [PubMed] [Google Scholar]
- Brown ER, Piscopo S, Chun JT, Francone M, Mirabile I, D’Aniello A. Modulation of an AMPA-like glutamate receptor (SqGluR) gating by L- and D-aspartic acids. Amino Acids. 2007;32:53–57. doi: 10.1007/s00726-006-0349-3. [DOI] [PubMed] [Google Scholar]
- Capo TR, Fieber LA, Stommes DL, Walsh PJ. Reproductive output in the hatchery-reared California sea hare at different stocking densities. Contemp Topics Lab An Sci. 2003;42:31–35. [PubMed] [Google Scholar]
- Capo TR, Fieber LA, Stommes DL, Walsh PJ. The effect of stocking density on growth rate and maturation time in laboratory-reared California sea hares. Contemp Topics Lab An Sci. 2002;41:25–30. [PubMed] [Google Scholar]
- Carpenter DO, Swann JW, Yarowsky PJ. Effects of curare on responses to dfferent putative neurotransmitters in Aplysia neurons. J Neurobiol. 1977;8:119–132. doi: 10.1002/neu.480080204. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, King WM, McCreery MJ. The role of glutamate reuptake in regulation of glutamate responses in Aplysia neurons. Acta Biol Hung. 1995;46:363–373. [PubMed] [Google Scholar]
- Chen HSV, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- Chin J, Angers A, Cleary LJ, Eskin A, Byrne JH. TGF-beta1 in Aplysia: role in long-term changes in the excitability of sensory neurons and distribution of TbetaR-II-like immunoreactivity. Learn Mem. 1999;6:317–30. [PMC free article] [PubMed] [Google Scholar]
- Croll RP, Chiasson BJ. Postembryonic development of serotonin-like immunoreactivity in the central nervous system of the snail, Lymnaea stagnalis. J Comp Neurol. 1989;280:122–142. doi: 10.1002/cne.902800109. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Lester RAJ. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989;40:143–210. [PubMed] [Google Scholar]
- D’Aniello A, Nardi G, Vetere A, Ferguson GP. Occurrence of free D-aspartic acid in the circumsoesophageal ganglia of Aplysia fasciata. Life Sci. 1993;52:733–736. doi: 10.1016/0024-3205(93)90235-u. [DOI] [PubMed] [Google Scholar]
- D’Aniello A, Lee JM, Petrucelli L, Di Fiore MM. Regional decreases of free D-aspartate levels in Alzheimer’s disease. Neurosci Lett. 1998;250:131–134. doi: 10.1016/s0304-3940(98)00451-0. [DOI] [PubMed] [Google Scholar]
- D’Aniello A. D-Aspartic acid: an endogenous amino acid with an important neuroendocrine role. Brain Res Rev. 2007;53:215–234. doi: 10.1016/j.brainresrev.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fieber LA. The development of excitatory capability in Aplysia californica bag cells observed in cohorts. Devel Brain Res. 2000;122:47–58. doi: 10.1016/s0165-3806(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Fieber LA, Schmale MC, Jordi N, Orbesen E, Diaz GA, Capo TR. Von Bertalanffy growth models for hatchery-reared A. californica. Bull Mar Sci. 2005;76:95–104. [Google Scholar]
- Fiore L, Geppeti L. Neural control of buccal mass activity of Aplysia. In: Salanki J, editor. Neurobiology of invertebrates. London: Pergamon Press; 1981. pp. 201–223. [Google Scholar]
- Fisher G, Petrucelli L, Gardner C, Emory C, Frey WH, D’Aniello A. Free D-amino acids in human cerebrospinal fluid of Alzheimer disease, multiple sclerosis, and healthy control subjects. Molec Chem Neuropath. 1994;23:115–124. doi: 10.1007/BF02815405. [DOI] [PubMed] [Google Scholar]
- Fuchs SA, Berger R, Klomp LWJ, De Koning TJ. D-Amino acids in the central nervous system in health and disease. Mol Gen Metab. 2005;85:168–180. doi: 10.1016/j.ymgme.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Furuchi T, Homma H. Free D-aspartate in mammals. Biol Pharm Bull. 2005;28:1566–1570. doi: 10.1248/bpb.28.1566. [DOI] [PubMed] [Google Scholar]
- Fujii N, Saito T. Homochirality and life. Chem Rec. 2004;4:267–78. doi: 10.1002/tcr.20020. [DOI] [PubMed] [Google Scholar]
- Gerdes R, Fieber LA. Life history and aging of captive-reared California sea hares (Aplysia californica) J Amer Assn Lab Anim Sci. 2006;45:40–47. [PubMed] [Google Scholar]
- Gong XQ, Frandsen A, Lu WY, Wan Y, Zabek RL, Pickering DS, Bai D. D-aspartate and NMDA, but not L-aspartate, block AMPA receptors in rat hippocampal neurons. Br J Pharmacol. 2005;145:449–459. doi: 10.1038/sj.bjp.0706199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson LL, Stoskopf MK, Showers W, Cope G, Eads C, Linnehan R, Kwak TJ, Andersen B, Levine JF. Reference ranges for hemolymph chemistries form Elliptio complanata of North Carolina. Dis Aquat Org. 2005;65:167–176. doi: 10.3354/dao065167. [DOI] [PubMed] [Google Scholar]
- Ha TJ, Kohn AB, Bobkova YV, Moroz LL. Molecular characterization of NMDA-like receptors in Aplysia and Lymnaea: relevance to memory mechanisms. Biol Bull. 2006;210:255–270. doi: 10.2307/4134562. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–52. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Hutton ML, Harvey RJ, Barnard EA, Darlison MG. Cloning of a cDNA that encodes an invertebrate glutamate receptor subunit. FEBS Lett. 1991;292:111–114. doi: 10.1016/0014-5793(91)80846-u. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nichols JG, Martin AR. From Neuron to Brain: A Cellular Approach to the Function of the Nervous System. Sinauer Associates, Inc; Sunderland, MA: 1984. The search for chemical transmitters; pp. 293–320. [Google Scholar]
- Li HL, Huang BS, Vishwasrao H, Sutedja N, Chen W, Jin I, Hawkins RD, Bailey CH, Kandel ER. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron. 2009;61:527–540. doi: 10.1016/j.neuron.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiology. 1987;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Rubakhin SS, Scanlan CR, Wang L, Sweedler JV. D-Aspartate as a putative cell-cell signaling molecule in the Aplysia californica central nervous system. J Neurochem. 2006;97:595–606. doi: 10.1111/j.1471-4159.2006.03791.x. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Brezina V, Weiss KR. AMRP peptides modulate a novel K+ current in pleural sensory neurons of Aplysia. J Neurophysiol. 2002;88:323–332. doi: 10.1152/jn.2002.88.1.323. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, Jezzini S, Lovell P, Iannucculli W, Chen M, Nguyen T, Sheng H, Shaw R, Kalachikov S, Panchin YV, Farmerie W, Russo JJ, Ju J, Kandel ER. Neuronal transcriptome of Aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Gyori JJ, Salanki J. NMDA-like receptors in the CNS of molluscs. Neuroreport. 1993;4:201–204. doi: 10.1097/00001756-199302000-00022. [DOI] [PubMed] [Google Scholar]
- Moroz LL, Kohn AB. Do different neurons age differently? Direct genome-wide analysis of aging in single identified cholinergic neurons. Fron Ag Neuro. 2010 doi: 10.3389/neuro.24.006.2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Homma H, Lee JA, Fukushima T, Santa T, Tashiro K, Iwatsubo T, Imai K. Emergence of D-aspartic acid in the differentiating neurons of the rat central nervous system. Brain Res. 1998;808:65–71. doi: 10.1016/s0006-8993(98)00599-x. [DOI] [PubMed] [Google Scholar]
- Schaeffer EL, Gattaz WF. Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: participation of the phospholipase A2 enzyme. Psychopharmacol. 2008;198:1–27. doi: 10.1007/s00213-008-1092-0. [DOI] [PubMed] [Google Scholar]
- Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29. doi: 10.1016/s0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- Shakhmatova EI, Berger VY, Natochin YV. Cations in molluscan tissues at sharply different hemolymph osmolality. Biology Bull. 2006;33:269–275. [PubMed] [Google Scholar]
- Schell MJ, Cooper OB, Snyder SH. D-aspartate localizations imply neuronal and neuroendocrine roles. Proc Natl Acad Sci USA. 1997;94:2013–2018. doi: 10.1073/pnas.94.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rolf FJ. The principles and Practice of Statistics in Biological Research. W.H. Freeman and Company; New York: 1981. Biometry. [Google Scholar]
- Spinelli P, Brown E, Ferrandino G, Branno M, Montarolo PG, D’Aniello E, Rastogi RK, D’Aniello B, Chieffi G, Fisher GH, D’Aniello A. D-Aspartic acid in the nervous system of Aplysia limacina: Possible role in neurotransmission. J Cell Physiol. 2006;206:672–681. doi: 10.1002/jcp.20513. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Schell MJ. The N-methyl D-aspartate receptor glycine site and D-serine metabolism: an evolutionary perspective. Philos Trans R Soc Lond B Biol Sci. 2004;359:943–964. doi: 10.1098/rstb.2003.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R, Aronoff R, Volkner M, Schmitt B, Mosbach R, Kuner T. Glutamate receptor channel signatures. Trends Pharmacol Sci. 2001;22:7–10. doi: 10.1016/s0165-6147(00)01588-1. [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. I. Response properties and synaptic connections. J Neurophysiol. 1983a;50:1522–1542. doi: 10.1152/jn.1983.50.6.1522. [DOI] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of AplysiaII. Modulation by sensitizing stimulation. J Neurophysiol. 1983b;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- Walters ET, Bodnarova M, Billy AJ, Dulin MF, Díaz-Ríos M, Miller MW, Moroz LL. Somatotopic organization and functional properties of mechanosensory neurons expressing sensorin-A mRNA in Aplysia californica. J Comp Neurol. 2004;471:219–240. doi: 10.1002/cne.20042. [DOI] [PubMed] [Google Scholar]
- Williams SM, Dia CM, Macnab LT, Sullivan RT, Pow DV. Immunocytochemical analysis of D-serine distribution in the mammalian brain reveals novel anatomical compartmentalizations in glia and neurons. Glia. 2006;53:401–411. doi: 10.1002/glia.20300. [DOI] [PubMed] [Google Scholar]
- Xu Y, Cleary LJ, Byrne JH. Identification and characterization of pleural neurons that inhibit tail sensory neurons and motor neurons inAplysia: correlation with FMRFamide immunoreactivity. J Neurosci. 1994;14:3565–3577. doi: 10.1523/JNEUROSCI.14-06-03565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Liu YM. Quantification of D/L-aspartic acids in Aplysia californica central nervous system by beta-cyclodextrin modified micellar electrokinetic chromatography. Biomed Chromatogr. 2001;15:274–279. doi: 10.1002/bmc.72. [DOI] [PubMed] [Google Scholar]